Abstract
The observation that E-cadherin is the principal epithelial receptor for the bacterial pathogen Listeria monocytogenes led us to investigate whether N-terminal fragments of E-cadherin containing the L. monocytogenes binding domain could inhibit entry of the bacteria into cultured epithelial cells. Here we demonstrate that a conditioned medium from a gastric cancer cell line (Kato III) that carries a truncating CDH-1 mutation 3′ of the L. monocytogenes binding domain can inhibit the uptake of the bacteria into Caco-2 cells. The inhibitory activity of the Kato III conditioned medium could be mimicked by incubation of the bacteria with a recombinant 26-kDa N-terminal E-cadherin peptide prior to infection. Furthermore, these data suggest that cleavage of the 80-kDa extracellular domain of E-cadherin from the cell surface may provide an innate form of pathogen defense by acting as a decoy receptor for L. monocytogenes.
Listeria monocytogenes is the causal agent of listeriosis, a severe food-borne disease that has a high infant mortality rate, induces miscarriage, and promotes meningitis in immunocompromised individuals (23). It is internalized into epithelial cells by a process that requires binding of the bacterial protein internalin A (InlA) to the homophilic cell to cell adhesion protein E-cadherin (16). The location of the InlA binding domain in the extracellular domain of E-cadherin (11) would suggest that E-cadherin peptides containing this site may be capable of inhibiting the L. monocytogenes infection process.
To investigate this question, we first examined the inhibitory effect of conditioned medium from the gastric cancer cell line Kato III. Kato III carries a G-to-A transversion at position 1008 of CDH-1. This frameshift mutation leads to splicing at an intronic cryptic splice sequence (19) that would be predicted to give rise to a secreted 25-kDa N-terminal E-cadherin peptide. Since the InlA binding site (11) is contained within the predicted 25-kDa E-cadherin fragment (corresponding to amino acids 1 to 178 of the mature protein), we hypothesized that this fragment would retain affinity for L. monocytogenes and be able to inhibit the infection process.
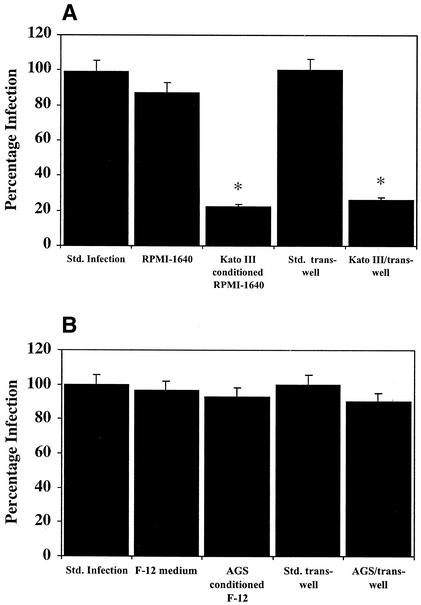
To examine this hypothesis, we used the in vitro infection model described by Mengaud et al. (16) to investigate the effect of the Kato III conditioned medium on the entry of L. monocytogenes (NCTC 7973/ATCC 35152, serotype 1a; 10 bacteria/cell) into Caco-2, a colorectal cancer cell line (ATCC CRL-2102 [5]) that strongly expresses E-cadherin (16). When the Caco-2 culture medium was changed from the standard fetal bovine serum-supplemented (10%) Dulbecco modified Eagle medium (DMEM) medium (Gibco-BRL) to conditioned serum-free RPMI 1640 medium (Gibco-BRL) from Kato III cells, the numbers of L. monocytogenes successfully invading the Caco-2 cells was reduced to 22% ± 8.6% of the control levels obtained in DMEM medium (Fig. 1A and Table 1). The infection levels were not significantly affected by changing the DMEM medium to RPMI 1640 medium. To confirm these results, Kato III cells (a kind gift from Kazuya Shinmura) were cocultured in RPMI 1640 medium in trans-well chambers above Caco-2 cells at a 1:1 ratio and a density of 105 cells/ml. These cells were allowed to equilibrate overnight in serum-free RPMI 1640 medium prior to inoculation of L. monocytogenes into the Caco-2 compartment below the trans-well insert. Under these conditions, the level of infection of the Caco-2 cells was reduced to 26% ± 7.2% (P < 0.01) of the infection level observed when the Caco-2 cells were cultured in the absence of Kato III cells. The Kato III conditioned medium had no significant effect on the viability of the bacteria (results not shown). In contrast to the inhibitory effect of the Kato III medium, the addition of conditioned serum-free medium from an unrelated cell line (gastric cancer cell line AGS; ATTC CRL-1739 [1]) to the Caco-2 cells or coculture with AGS cells in trans-well chambers had no effect on the uptake of bacteria into the Caco-2 cells (Fig. 1B).
FIG. 1.
(A) Kato III conditioned medium blocks uptake of L. monocytogenes into Caco-2. For the standard infection, L. monocytogenes infection of Caco-2 monolayer took place in serum-free DMEM. The level of infection was normalized to represent 100%. Remaining columns: RPMI 1640 medium, infection was carried out as in the standard infection but in serum-free RPMI 1640 medium; Kato III conditioned medium, infection of Caco-2 monolayer in serum-free Kato III-conditioned RPMI 1640 medium; standard trans-well, bacteria were added to a Caco-2 monolayer grown in a trans-well chamber and equilibrated in serum-free RPMI 1640 medium; Kato III/trans-well, Kato III cells were cultured in trans-well inserts above a Caco-2 cell monolayer at a 1:1 ratio. Both cell layers were equilibrated overnight in serum-free RPMI 1640 before addition of L. monocytogenes to the Caco-2 layer. An asterisk denotes a significant difference at P < 0.01. (B) Effect of AGS-conditioned medium on the uptake of L. monocytogenes into Caco-2. Infection conditions were as described in panel A except that the RPMI 1640 medium and the Kato III-conditioned RPMI 1640 medium were substituted with F-12 medium and AGS conditioned F-12 medium, respectively. Std., standard.
TABLE 1.
Inhibition of entry of L. monocytogenes into epithelial cell lines by conditioned Kato III medium
| Cells and culture conditions | Avg no. of intracellular bacteria/cella | % Cells infected (range over replicates)a | Relative infection (%)b |
|---|---|---|---|
| Caco-2 | |||
| Standard conditions | 5.34 ± 1.26 | 41-66 | 100 |
| Kato III conditioned plus RPMI 1640 medium | 1.18 ± 0.46 | 7-16 | 22 |
| RPMI 1640 medium alone | 4.58 ± 0.35 | 35-65 | 92 |
| AGS | |||
| Standard conditions | 0.35 ± 0.18 | 1.8-5.4 | 100 |
| Kato III conditioned plus RPMI 1640 medium | 0.06 ± 0.03 | 0.3-0.9 | 17 |
| RPMI 1640 alone | 0.26 ± 0.04 | 1.3-5.0 | 83 |
Determined from a minimum of nine replicate experiments.
That is, with respect to standard conditions.
Infection studies were also carried out with AGS cells (using serum-free F-12 medium), a cell line that expresses low levels of E-cadherin (1, 9; unpublished observations). The number of intracellular L. monocytogenes recovered from AGS cells was 15- to 20-fold lower than were recovered from Caco 2 cells (see Table 1). Nevertheless, the addition of Kato III conditioned serum-free RPMI 1640 medium reduced the level of infection to 17% ± 8.3% of the standard infection compared to a reduction to 83% ± 11.2% when the infection was carried out in RPMI 1640 medium alone.
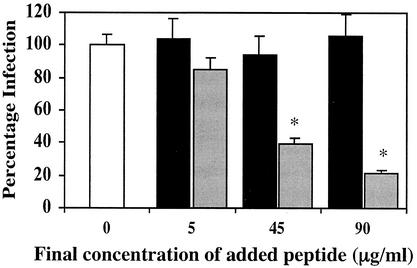
To further test the hypothesis, we constructed a recombinant 26-kDa E-cadherin peptide (EC-26) corresponding to amino acids 1 to 192 of the mature protein. EC-26 was constructed by PCR amplifying human cDNA with the primers FER1f (5′-AGCACGTCGACTAACTCCTCTCCTGGCCTCAGA-3′) and FER1r (5′-GAGTCTAGAAAGGTCAGCAGCTTGAACCAC-3′) and ligating the product into the His6-tagged vector pPROEX-1; the recombinant protein was purified from transformed DH5-α cells by using nickel-nitrilotriacetic acid agarose resin (Qiagen) and then gel purified (22). The addition of increasing concentrations (5, 45, and 90 μg) of EC-26 to L. monocytogenes suspensions prior to infection of Caco-2 cells led to a concentration-dependent decrease in invasion of Caco-2 cells by L. monocytogenes (Fig. 2). Thus, 90 μg of EC-26 decreased the infection to 22% of the control infection level. A mutant EC-26 peptide (EC-26M) carrying a proline-to-glutamate amino acid substitution at position 16 of the mature E-cadherin protein was also constructed. This change has been shown to abrogate the binding of E-cadherin to InlA (11). The EC-26 M expression construct was derived from the EC-26 construct by using the QuickChange site-directed mutagenesis kit (Stratagene) and oligonucleotides FER2f (5′-GCTGCCCAGAAAATGAAAAAGGCGAATTTCCTAAAAACCTGGTTCAG-3′) and FER2r (5′-CTGAACCAGGTTTTTAGGAAATTCGCCTTTTTCATTTTCTGGGCAGC-3′). Incubation of L. monocytogenes with increasing amounts of EC-26M failed to significantly inhibit the infection of Caco-2 cells (Fig. 2).
FIG. 2.
Effect of EC-26 (░⃞) and EC-26M (▪) peptides on the uptake of L. monocytogenes into Caco-2. L. monocytogenes was incubated with increasing amounts of EC-26 and EC-26M peptides for 1 h at 37°C prior to infection of Caco-2 monolayers in DMEM medium (an asterisk denotes a significant difference at P < 0.01). □, no added peptide.
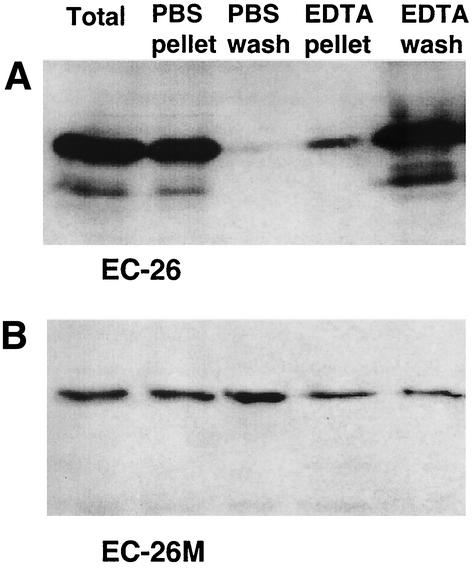
To provide further evidence that the inhibitory effect of the EC-26 peptide was a consequence of a specific interaction with InlA, an in vitro binding assay was developed. In this assay aliquots of L. monocytogenes were incubated with either EC-26 or EC-26M in serum-free RPMI 1640 medium containing 2 mM Ca2+. The bacteria were then pelleted and washed consecutively in phosphate-buffered saline (PBS) and 10 mM EDTA. Since chelation of Ca2+ by EDTA disrupts the interaction between E-cadherin and InlA (15, 16), it was predicted that if the interaction between EC-26 and L. monocytogenes was mediated by InlA, it would be disrupted by EDTA. After incubation of EC-26 with L. monocytogenes, aliquots were removed from the suspension, the pellet, and the wash fractions (Fig. 3A). These aliquots were then analyzed by Western hybridization with an anti-His antibody. The EDTA wash released the majority of EC-26 from the bacterial pellet, whereas washing with PBS had little effect. In comparison, when EC-26M was incubated with the bacteria the EDTA wash was no more efficient at eluting the peptide from the bacteria than was the PBS wash (Fig. 3B). This observation is consistent with the calcium dependency of the interaction between L. monocytogenes InlA and E-cadherin (15, 16).
FIG. 3.
EC-26 but not EC-26M binds L. monocytogenes with high efficiency. EC-26 (A) and EC-26M (B) peptides were bound to L. monocytogenes in DMEM in the presence of 2 mM added Ca2+ and then washed consecutively in PBS and 0.2 M EDTA. The presence of the peptides in the wash and pellet fractions was visualized by using the mouse monoclonal Penta-anti-His antibody.
Taken together, these results provide strong evidence that E-cadherin peptides containing the InlA binding site are able to bind L. monocytogenes in a calcium-dependent manner and inhibit the entry of the bacteria into epithelial cells. It is notable that E-cadherin tends to undergo cleavage by metalloproteases (18), resulting in the release of a soluble 80-kDa extracellular domain fragment (2, 7, 18, 24). It is probable that the 80-kDa fragment also retains the ability to inhibit L. monocytogenes infection and therefore may act as a decoy receptor for L. monocytogenes. Since the expression of metalloproteinases can be induced by nonspecific inflammatory reactions and a broad range of pathogenic virulence factors (4, 6, 12-14, 17, 20, 21), the shedding of the 80-kDa E-cadherin fragment from the surface of epithelial cells may also be stimulated during the early stages of L. monocytogenes infection. It is also notable that a germ line CDH-1 mutation (1008G→T) equivalent to the Kato III CDH-1 mutation has been described in a New Zealand Maori family with the familial cancer syndrome hereditary diffuse gastric cancer (8). It will be intriguing to determine whether this mutation and other germ line CDH-1 mutations occurring 3′ of the InlA binding site (3, 10) could have provided carriers with an evolutionary advantage that has outweighed the increased cancer risk.
Acknowledgments
This research was supported by a grant from the New Zealand Marsden Fund.
Editor: J. T. Barbieri
REFERENCES
- 1.Barranco, S. C., C. M. Townsend, C. Casartelli, B. G. Macik, N. L. Burger, W. R. Boewinkle, and W. K. Gourley. 1983. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 43:1703-1709. [PubMed] [Google Scholar]
- 2.Boterberg, T., K. M. Vennekens, M. Thienpont, M. M. Mareel, and M. E. Bracke. 2000. Internalization of the E-cadherin/catenin complex and scattering of human mammary carcinoma cells MCF-7/AZ after treatment with conditioned medium from human skin squamous carcinoma cells COLO 16. Cell Adhesion Commun. 7:299-310. [DOI] [PubMed] [Google Scholar]
- 3.Dunbier, A., and P. Guilford. 2001. Hereditary diffuse gastric cancer. Adv. Cancer Res. 83:55-65. [DOI] [PubMed] [Google Scholar]
- 4.Firth, J. D., E. E. Putnins, H. Larjava, and V. J. Uitto. 1997. Bacterial phospholipase C upregulates matrix metalloproteinase expression by cultured epithelial cells. Infect. Immun. 65:4931-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogh, J., J. M. Fogh, and T. Orfeo. 1977. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 59:221-226. [DOI] [PubMed] [Google Scholar]
- 6.Gooz, M., P. Gooz, and A. J. Smolka. 2001. Epithelial and bacterial metalloproteinases and their inhibitors in Helicobacter pylori infection of human gastric cells. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G823-G832. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths, T. R., I. Brotherick, R. I. Bishop, M. D. White, D. M. McKenna, C. H. Horne, B. K. Shenton, D. E. Neal, and J. K. Mellon. 1996. Cell adhesion molecules in bladder cancer: soluble serum E-cadherin correlates with predictors of recurrence. Br. J. Cancer 74:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilford, P., J. Hopkins, J. Harraway, M. McLeod, N. McLeod, P. Harawira, H. Taite, R. Scoular, A. Miller, and A. E. Reeve. 1998. E-cadherin germline mutations in familial gastric cancer. Nature 392:402-405. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh, M. C., C. W. Wu, L. H. Wu, W. Y. Lui, K. P'Eng, F., and C. L. Yu. 1996. Heat shock and cytokines modulate the expression of adhesion molecules on different human gastric-cancer cell lines. Int. J. Cancer 67:690-694. [DOI] [PubMed] [Google Scholar]
- 10.Humar, B., T. Toro, F. Graziano, H. Müller, Z. Dobbie, H. Kwang-Yang, C. Eng, H. Hampel, D. Gilbert, I. Winship, S. Parry, R. Ward, M. Findlay, A. Christian, M. Tucker, K. Tucker, T. Merriman, and P. Guilford. 2002. Novel germline CDH1 mutations in hereditary diffuse gastric cancer families. Hum. Mutat. 19:518-525. [DOI] [PubMed] [Google Scholar]
- 11.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Boado, Y. S., C. L. Wilson, L. V. Hooper, J. I. Gordon, S. J. Hultgren, and W. C. Parks. 2000. Bacterial exposure induces and activates matrilysin in mucosal epithelial cells. J. Cell Biol. 148:1305-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Boado, Y. S., C. L. Wilson, and W. C. Parks. 2001. Regulation of matrilysin expression in airway epithelial cells by Pseudomonas aeruginosa flagellin. J. Biol. Chem. 276:41417-41423. [DOI] [PubMed] [Google Scholar]
- 14.Mauviel, A. 1993. Cytokine regulation of metalloproteinase gene expression. J. Cell Biochem. 53:288-295. [DOI] [PubMed] [Google Scholar]
- 15.Mengaud, J., M. Lecuit, M. Lebrun, F. Nato, J.-C. Mazie, and P. Cossart. 1996. Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E-cadherin. Infect. Immun. 64:5430-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mengaud, J., H. Ohayon, P. Gounon, R.-M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 17.Miyajima, S., T. Akaike, K. Matsumoto, T. Okamoto, J. Yoshitake, K. Hayashida, A. Negi, and H. Maeda. 2001. Matrix metalloproteinases induction by pseudomonal virulence factors and inflammatory cytokines in vitro. Microb. Pathog. 31:271-281. [DOI] [PubMed] [Google Scholar]
- 18.Noe, V., B. Fingleton, K. Jacobs, H. C. Crawford, S. Vermeulen, W. Steelant, E. Bruyneel, L. M. Matrisian, and M. Mareel. 2001. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J. Cell Sci. 114:111-118. [DOI] [PubMed] [Google Scholar]
- 19.Oda, T., Y. Kanai, T. Oyama, K. Yoshiura, Y. Shimoyama, W. Birchmeier, T. Sugimura, and S. Hirohashi. 1994. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc. Natl. Acad. Sci. USA 91:1858-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagenstecher, A., A. K. Stalder, C. L. Kincaid, B. Volk, and I. L. Campbell. 2000. Regulation of matrix metalloproteinases and their inhibitor genes in lipopolysaccharide-induced endotoxemia in mice. Am. J. Pathol. 157:197-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiding-Jarbrink, M., D. A. Smith, and G. J. Bancroft. 2001. Production of matrix metalloproteinases in response to mycobacterial infection. Infect. Immun. 69:5661-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Retamal, C. A., P. Thiebaut, and E. W. Alves. 1999. Protein purification from polyacrylamide gels by sonication extraction. Anal. Biochem. 268:15-20. [DOI] [PubMed] [Google Scholar]
- 23.Schuchat, A., B. Swaminathan, and C. V. Broome. 1991. Epidemiology of human listeriosis. Clin. Microbiol. Rev. 4:169-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheelock, M. J., C. A. Buck, K. B. Bechtol, and C. H. Damsky. 1987. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J. Cell Biochem. 34:187-202. [DOI] [PubMed] [Google Scholar]