Abstract
There is a strong association between serum antibody responses to toxin A and protection against Clostridium difficile diarrhea. A parenteral C. difficile toxoid vaccine induced very-high-level responses to anti-toxin A immunoglobulin G (IgG) in the sera of healthy volunteers. After vaccination, the concentrations of anti-toxin A IgG in the sera of all 30 recipients exceeded the concentrations that were associated with protection in previous clinical studies. Furthermore, the median concentration of serum anti-toxin A IgG in the test group was 50-fold higher than the previous threshold. These findings support the feasibility of using a vaccine to protect high-risk individuals against C. difficile-associated diarrhea and colitis.
Clostridium difficile is the most commonly known cause of nosocomial infectious diarrhea in industrialized countries (2, 9). Rates of C. difficile infection in high-risk hospital patients receiving antibiotics are as high as 31% (11, 15, 19). The clinical presentation of C. difficile colitis ranges from mild diarrhea to fulminant pseudomembranous colitis (2, 9). However, approximately half of the patients who are colonized by toxin-producing strains of C. difficile are asymptomatic carriers (11, 15).
Two recent clinical studies demonstrated that high concentrations of anti-toxin A immunoglobulin G (IgG) antibody in serum were associated with protection against C. difficile-associated diarrhea and colitis (11, 12). In the first study, infected patients with serum anti-toxin A IgG concentrations of greater than 3 enzyme-linked immunosorbent assay (ELISA) units (EU) were 48-fold more likely to become asymptomatic carriers than patients with lower concentrations of anti-toxin A antibody (11). In the second study, patients who developed high concentrations of serum anti-toxin A IgG during initial episodes of C. difficile-associated diarrhea were 48-fold less likely to suffer a recurrence than those who did not show an anti-toxin A antibody response (12). These findings provide further evidence that active or passive immunization against C. difficile toxins may be effective in preventing C. difficile-associated diarrhea and colitis (1, 3, 5, 10, 13, 17, 20).
Kotloff et al. reported recently their experience using a parenteral C. difficile vaccine containing toxoids A and B administered to healthy adult volunteers (7). To our knowledge, this is the only candidate C. difficile vaccine that has been tested in human studies. The vaccine was produced from a culture filtrate of C. difficile strain ATCC 43255 as previously described (7). Briefly, toxins A and B were partially purified by ammonium sulfate precipitation and application to an S300 Sephacryl size exclusion column, followed by inactivation with formaldehyde. The total protein concentration of the vaccine was 0.52 mg per ml, of which toxins A and B comprised about 44% at a 1.5:1 toxin A-to-toxin B ratio. Both toxoid A and toxoid B were included in the C. difficile vaccine based on preclinical observations which indicated that both toxin A and toxin B may be involved in the pathogenesis of C. difficile-associated diarrhea and in generating protective immunity. Kink and Williams, for example, found that treatment with a mixture of anti-recombinant toxin A (anti-rToxA) and anti-rTox B is required for complete therapeutic (postchallenge) protection against clindamycin-induced C. difficile-associated diarrhea in the hamster challenge model, although anti-rTox A alone is sufficient to prevent C. difficile-associated diarrhea if it is administered prior to challenge (6). Although both of the clinical studies described earlier indicated that high levels of serum anti-toxin A IgG but not serum anti-toxin B IgG are associated with protection, it would be premature to conclude that immune responses to toxin B are immaterial in disease expression and/or immunity (11, 12, 16). This point is highlighted by numerous recent reports of diarrhea and pseudomembranous colitis associated with toxin A-negative, toxin B-positive strains of C. difficile (4, 8, 14, 18). As reported previously, the candidate vaccine was well tolerated and induced a variety of immune responses to both toxins (7). The aim of this collaborative study was to determine whether the concentrations in serum of anti-toxin A IgG that were achieved during vaccination were similar in magnitude to the concentrations that were associated with protection in clinical studies of patients with C. difficile infection (11, 12).
Healthy adults received four intramuscular inoculations of the C. difficile toxoid vaccine on days 1, 8, 30, and 60. The vaccine was administered at a dose of 6.25, 25, or 100 μg, either as soluble toxoids or as toxoids adsorbed to alum (number of subjects in each group, 5). Serum samples collected on days 1 (prevaccination), 8, 15, and 90 were tested for anti-toxin A IgG antibody concentrations by using highly purified C. difficile toxin A as the capture antigen. Results were expressed as ELISA units by reference to a serum standard obtained from patients with high concentrations of serum anti-toxin A IgG antibody as previously described (11, 12).
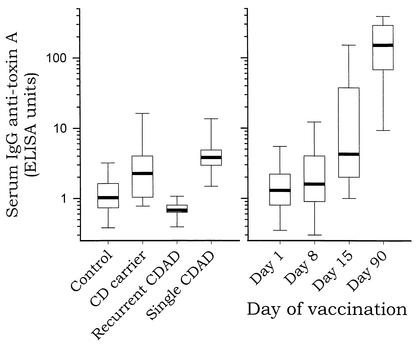
Prior to vaccination (day 1) (Fig. 1 and Table 1), the median concentration of serum anti-toxin A IgG antibody for the 30 healthy adults was low (1.3 EU) and similar to that for control patients not infected by C. difficile (1.0 EU). On day 15, after two inoculations of toxoid, the median concentration of serum anti-toxin A IgG increased to 4.2 EU. This antibody concentration is similar to the median concentration in symptom-free carriers of C. difficile (2.2 EU) or to that in patients who experienced a single episode of C. difficile diarrhea and who did not suffer a recurrence (3.9 EU). On day 90, 30 days after the fourth and final toxoid inoculation, the median concentration of serum anti-toxin A IgG antibody in the vaccine recipients was markedly higher, 151 EU.
FIG. 1.
Serum anti-toxin A IgG concentrations following natural infection with C. difficile (left panel) or following vaccination with C. difficile toxoid (right panel). Results, expressed as ELISA units, are shown for control patients not infected with C. difficile (Control; n = 187), symptom-free carriers of C. difficile (CD carrier; n = 19), patients with recurrent C. difficile-associated diarrhea (Recurrent CDAD; n = 9), patients with a single episode of C. difficile-associated diarrhea (Single CDAD; n = 7), and healthy volunteers at various time points during vaccination with C. difficile toxoid (n = 30). The median values are depicted as bold horizontal lines, the boxes indicate the 25th and 75th percentiles, and the I bars indicate the 10th and 90th percentiles.
TABLE 1.
Serum anti-toxin A IgG concentrations during vaccination with C. difficile toxoid
| Treatment group and toxoid concn (μg/ml)a | Serum anti-toxin A concn (median EU [range]) on treatment dayb
|
|||
|---|---|---|---|---|
| 1 | 8 | 15 | 90 | |
| Soluble toxoids | ||||
| 6.25 | 1.3 (0.4-16) (20) | 1.5 (0.3-29) (20) | 2.3 (0.5-31) (40) | 40 (5.1-424) (100) |
| 25 | 1.7 (0.2-2.9) (0) | 1.7 (0.3-5.4) (40) | 19 (0.7-138) (60) | 144 (3.6-349) (100) |
| 100 | 0.9 (0.7-5.3) (20) | 3.3 (0.9-37) (60) | 80 (1.2-360) (80) | 148 (13-257) (100) |
| Toxoids with alum | ||||
| 6.25 | 0.9 (0.8-1.3) (0) | 1.0 (0.7-1.6) (0) | 2.1 (0.8-21) (20) | 165 (86-288) (100) |
| 25 | 2.1 (0.4-2.8) (0) | 1.6 (0.5-13) (20) | 4.3 (2.0-200) (60) | 309 (120-1688) (100) |
| 100 | 1.4 (0.2-10) (40) | 2.7 (0.1-5.8) (40) | 29 (2.4-165) (80) | 288 (26-327) (100) |
| All groups | 1.3 (0.2-16) (13) | 1.6 (0.1-37) (30) | 4.2 (0.5-360) (57) | 151 (3.6-1688) (100) |
Each group consisted of five subjects. All groups combined consisted of 30 subjects.
The percentage of subjects within each group achieving a serum anti-toxin A IgG level greater than 3 EU is also shown in the second set of parentheses.
Table 1 provides the medians and ranges of serum anti-toxin A IgG concentrations as well as the percentage of subjects within each group who achieved serum anti-toxin A IgG concentrations of greater than 3 EU during vaccination. Prior to vaccination (day 1), only 4 out of 30 subjects (13%) had serum anti-toxin A IgG concentrations of greater than 3 EU. This agrees closely with our previous report that 10% of control, noninfected patients have protective concentrations of serum anti-toxin A IgG (11). On day 8, prior to the second inoculation of toxoid, the number of subjects with serum anti-toxin A IgG antibody concentrations exceeding 3 EU increased to nine (30%). On day 15, prior to the third inoculation, this number increased to 17 subjects (57%). At the highest dose of toxoid vaccine tested (100 μg), 8 of 10 subjects (80%) achieved serum IgG anti-toxin A antibody concentrations of greater than 3 EU by day 15. On day 90, 1 month after completing the vaccination regimen, all 30 subjects had serum anti-toxin A IgG antibody concentrations of greater than 3 EU (median, 151 EU; range, 3.6 to 1,688 EU).
The C. difficile toxoid vaccine also induced high levels of serum-neutralizing activity against toxin A, as evaluated with the tissue culture cytotoxicity assay (7). We therefore measured toxin A-neutralizing activity in sera obtained from asymptomatic carriers of C. difficile as well as in control sera and in sera from patients with C. difficile-associated diarrhea. Only 1 of 18 C. difficile carriers (5.6%) had detectable toxin A-neutralizing activity (at a low reciprocal titer of 5). Ten of 149 control serum samples (6.7%) had detectable toxin A-neutralizing activity (median reciprocal titer of 10, range of 5 to 40). None of 25 subjects with C. difficile-associated diarrhea had detectable toxin A-neutralizing activity. These differences were not statistically significant (P = 0.21, Fisher's exact test). The toxin A-neutralizing activities detected in patient sera were far lower than those measured previously in the sera of subjects who had received the C. difficile toxoid vaccine (median peak toxin A-neutralizing reciprocal titer of 267, range of 10 to 6,827) (7). The finding that toxin A-neutralizing activity was not significantly increased in the sera of individuals protected against C. difficile-associated diarrhea may mean that the neutralization of cytotoxicity as determined with an in vitro tissue culture assay does not correlate closely with the neutralization of the enterotoxic effects of toxin A in vivo. Another possible explanation is that anti-toxin A antibody responses act as a surrogate marker but are not the effectors of a protective immune response.
In this study, a C. difficile toxoid vaccine induced strong serum anti-toxin A IgG antibody responses in human volunteers. After four doses of vaccine, the concentration of serum anti-toxin A IgG antibody in each of 30 subjects exceeded 3 EU, a level that was associated with protection in previous clinical studies. Moreover, the concentrations in the majority far surpassed this level (by a factor of 50). These anti-toxin A antibody responses greatly exceed those observed in patients with C. difficile-associated diarrhea or in asymptomatic carriers of C. difficile (11, 12). The fact that every subject, regardless of the dose received or the formulation of toxoid vaccine used, mounted a substantial antibody response to toxin A indicates that the C. difficile toxoid vaccine is highly immunogenic. Indeed, the uniformly vigorous serum anti-toxin A antibody responses measured in this study suggest that the parenteral toxoid vaccine may be more effective than natural infection in inducing immunity to C. difficile toxin A.
The parenteral C. difficile toxoid vaccine clearly induces vigorous serum anti-toxin A antibody responses in healthy adults. However, whether these vaccine-induced immune responses can confer protective immunity against C. difficile-associated diarrhea and colitis remains to be proven (21). We have initiated clinical trials to determine whether the C. difficile toxoid vaccine induces similarly rapid and robust anti-toxin A antibody responses in elderly individuals and in patients with recurrent C. difficile-associated diarrhea who, as we have shown, fail to mount an appropriate, protective immune response to toxin A during natural challenge with C. difficile (12, 20).
Acknowledgments
We are grateful to Richard Nichols and Robert Schrader of Acambis, Inc., for their assistance in this study.
This work was supported by National Institutes of Health awards AI-45251, AG-00971, and RR-01032 and by Acambis, Inc.
Editor: J. T. Barbieri
REFERENCES
- 1.Aronsson, B., M. Granstrom, R. Mollby, and C. E. Nord. 1985. Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea. Infection 13:97-101. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346:334-339. [DOI] [PubMed] [Google Scholar]
- 3.Johnson, S., D. N. Gerding, and E. N. Janoff. 1992. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J. Infect. Dis. 166:1287-1294. [DOI] [PubMed] [Google Scholar]
- 4.Johnson, S., S. A. Kent, K. J. O'Leary, M. M. Merrigan, S. P. Sambol, L. R. Peterson, and D. N. Gerding. 2001. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 135:434-438. [DOI] [PubMed] [Google Scholar]
- 5.Kelly, C. P. 1996. Immune response to Clostridium difficile infection. Eur. J. Gastroenterol. Hepatol. 8:1048-1053. [DOI] [PubMed] [Google Scholar]
- 6.Kink, J. A., and J. A. Williams. 1998. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 66:2018-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotloff, K. L., S. S. Wasserman, G. A. Losonsky, W. Thomas, Jr., R. Nichols, R. Edelman, M. Bridwell, and T. P. Monath. 2001. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect. Immun. 69:988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuijper, E. J., J. de Weerdt, H. Kato, N. Kato, A. P. van Dam, E. R. van der Vorm, J. Weel, C. van Rheenen, and J. Dankert. 2001. Nosocomial outbreak of Clostridium difficile-associated diarrhoea due to a clindamycin-resistant enterotoxin A-negative strain. Eur. J. Clin. Microbiol. Infect. Dis. 20A:528-534. [DOI] [PubMed] [Google Scholar]
- 9.Kyne, L., R. J. Farrell, and C. P. Kelly. 2001. Clostridium difficile. Gastroenterol. Clin. N. Am. 30:753-777. [DOI] [PubMed] [Google Scholar]
- 10.Kyne, L., and C. P. Kelly. 1998. Prospects for a vaccine for Clostridium difficile. BioDrugs 10:173-181. [DOI] [PubMed] [Google Scholar]
- 11.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342:390-397. [DOI] [PubMed] [Google Scholar]
- 12.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189-193. [DOI] [PubMed] [Google Scholar]
- 13.Leung, D. Y., C. P. Kelly, M. Boguniewicz, C. Pothoulakis, J. T. LaMont, and A. Flores. 1991. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J. Pediatr. 118:633-637. [DOI] [PubMed] [Google Scholar]
- 14.Limaye, A. P., D. K. Turgeon, B. T. Cookson, and T. R. Fritsche. 2000. Pseudomembranous colitis caused by a toxin A− B+ strain of Clostridium difficile. J. Clin. Microbiol. 38:1696-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland, L. V., M. E. Mulligan, R. Y. Kwok, and W. E. Stamm. 1989. Nosocomial acquisition of Clostridium difficile infection. N. Engl. J. Med. 320:204-210. [DOI] [PubMed] [Google Scholar]
- 16.Riegler, M., R. Sedivy, C. Pothoulakis, G. Hamilton, J. Zacherl, G. Bischof, E. Cosentini, W. Feil, R. Schiessel, and J. T. LaMont. 1995. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J. Clin. Investig. 95:2004-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salcedo, J., S. Keates, C. Pothoulakis, M. Warny, I. Castagliuolo, J. T. LaMont, and C. P. Kelly. 1997. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut 41:366-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambol, S. P., M. M. Merrigan, D. Lyerly, D. N. Gerding, and S. Johnson. 2000. Toxin gene analysis of a variant strain of Clostridium difficile that causes human clinical disease. Infect. Immun. 68:5480-5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samore, M. H. 1999. Epidemiology of nosocomial Clostridium difficile diarrhoea. J. Hosp. Infect. 43(Suppl.):183-190. [DOI] [PubMed] [Google Scholar]
- 20.Warny, M., J. P. Vaerman, V. Avesani, and M. Delmee. 1994. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect. Immun. 62:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox, M. H., and J. Minton. 2001. Role of antibody response in outcome of antibiotic-associated diarrhoea. Lancet 357:158-159. [DOI] [PubMed] [Google Scholar]