Abstract
Transposon mutagenesis of an encapsulated, virulent strain of Vibrio vulnificus 1003(O) led to the identification of four genetic regions that are essential to capsular polysaccharide (CPS) expression and virulence. Of the four regions, three are believed to be part of a capsule gene locus comprised of biosynthesis, polymerization, and transport genes clustered on a single chromosomal fragment. Genes indicating a Wzy-dependent system of polymerization and transmembrane export are present, suggesting that the CPS of V. vulnificus is lipid linked. The fourth region, while it contains a gene essential for CPS expression, is characteristic of an integron-gene cassette region, similar to the super integron of V. cholerae. It is not believed to be part of a CPS gene locus and is located in a region of the chromosome separate from the putative CPS loci. It is comprised of open reading frames (ORFs) carrying genes of unknown function surrounded by direct repeats. This region also contains IS492, an insertion sequence located numerous times throughout a region of the genome, demonstrating a restriction fragment length polymorphism among an encapsulated and nonencapsulated morphotype of V. vulnificus. Collectively, 22 ORFs were recognized: 13 capsule synthesis genes, 4 insertion sequences, 1 truncated biosynthesis gene, and 4 genes of unknown function. This study has led to the identification of previously unrecognized genetic loci that may help to increase the understanding of capsular genetics and antigenic diversity among V. vulnificus strains.
Vibrio vulnificus has been shown to be the causative agent of two types of human illnesses with distinguishing routes of infections and manifestations (5). The first entails dermal infection via open wound, which can become necrotic in some cases. The second and more severe type results in disease and often death of immunocompromised individuals following the ingestion of raw shellfish. The victim most often has a preexisting condition that results in high levels of serum iron. These high iron levels have been shown to enhance the pathogenicity of V. vulnificus. The iron mouse model was implemented to mimic the septicemic state of a patient with high serum iron levels, demonstrating the increase of virulence potential in the presence of excess iron (36).
While the extent of infection in both disease states is reliant upon the expression of numerous virulence factors, the negatively charged polysaccharide capsule has been shown to protect this organism from the immune system in animal models (31). As a result, the expression of capsular polysaccharide (CPS) is believed to be a primary virulence factor of V. vulnificus that is essential for pathogenicity. Although clinical strains of V. vulnificus are encapsulated, the ability to express CPS is often lost, as witnessed in the laboratory, resulting in a nonencapsulated spontaneous mutant that is no longer virulent and appears translucent. Nomenclature commonly used in the laboratory and herein to describe colony morphology is that of opaque (O) for an encapsulated strain and translucent (T) for a nonencapsulated strain. The event leading to or resulting in the loss of capsule expression is not understood; it is seemingly a spontaneous, random happening that can be visualized on the surface of an agar plate.
The capsule of V. vulnificus has been partially described, both phenotypically and genetically. Multiple capsule types exist, with the carbohydrate composition characteristically acidic, containing many amino sugars, including galactosamine, fucosamine, glucosamine, and quinovosamine (7). The repeating unit is believed to consist of four sugars, with common residues shared among encapsulated strains expressing different capsule types (24). Recently, Wright et al. (37) identified a genetic locus that encodes the transport of a group 1-like CPS in an encapsulated strain of V. vulnificus. Because of these previous studies, it is anticipated that the capsule gene complex of V. vulnificus is comprised of genes that correspond to previously defined characteristics of the CPS, such as carbohydrate composition and grouping.
We proposed to locate the capsule gene complex by using transposon mutagenesis. Selection and genetic analysis of four nonencapsulated transposon mutants has led to the identification of two types of loci. Numerous genes involved in capsular synthesis and expression have been identified which do correspond to possible capsule typing and phenotypic characteristics. In addition, an integron-like region has been located, similar to that of the super integron of V. cholerae.
MATERIALS AND METHODS
Bacterial strains, plasmids, and propagation.
Conjugation, transposon mutagenesis, and cloning experiments were performed as reported previously (40). V. vulnificus 1003(O) is an encapsulated clinical strain. V. vulnificus 1003(T) is a nonencapsulated, translucent strain that was derived spontaneously in the laboratory from the encapsulated strain 1003(O). Transposon pEIS was used for mutagenesis, containing a mini-Tn10/kan transposable element. Plasmid pBluescript SK(−) (Stratagene, La Jolla, Calif.) was used for cloning and was maintained in Escherichia coli DH5α MCR (New England Biolabs, Inc., Beverly, Mass.).
Cloning of essential capsule genes.
The method of Chan and Goodwin (8) was used to isolate genomic DNA, with the exclusion of cetyltrimethylammonium bromide for isolation from nonencapsulated cells. Plasmid DNA was purified by using a Perfect Prep plasmid isolation kit (5 Prime, 3 Prime, Inc., Boulder, Colo.). Transposon insertion into the V. vulnificus chromosome was verified by Southern blotting with a digoxigenin-labeled probe, as described previously (40). Following digestion, the genetic regions harboring the transposon and flanking chromosomal DNA were excised from an agarose gel, purified, and cloned into pBluescript SK(−). Enzymes used for cloning included EcoRI for mutants ABZ1(T), RJS2(T), and TDB3(T) and XbaI for mutant GMB4(T).
Nucleotide sequencing.
The nucleotide sequencing of V. vulnificus chromosomal DNA was determined by using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer, Foster City, Calif.). Following amplification, each sample was purified by using the Centrisep spin columns (Princeton Separation, Adelphia, N.J.). The ABI 310 (Perkin-Elmer) genetic analyzer was utilized for determination of nucleotide sequences.
Analysis of derived sequences.
Translation from nucleotide to amino acid sequences and location of open reading frames (ORFs) was performed by using the Clone Program (Science and Educational Software, Durham, N.C.). As the sequence of each clone (described above) was obtained, the Basic Localization Alignment Search Tool (BLAST) (1) was employed to assess each. Both nucleotide and protein sequences were submitted for analysis and potential identification of each ORF. Prediction of protein transmembrane regions and protein sequence analysis was performed by using Tmpred (15) and NPS@:Network Protein Sequence Analysis (10). Recognition of promoter sequences was performed by using Promoter Locator (27).
Pulsed-field gel electrophoresis.
Pulsed-field gel electrophoresis (PFGE) employed a modified version of that described by Maslanka et al. (19). Digested chromosomal DNA fragments were separated by electrophoresis by using a CHEF MAPPER (Bio-Rad Laboratories). A constant temperature of 14°C was used, and electrophoresis parameters included a voltage of 6 V/cm, an angle of 120, and switch time of 2.91 to 17.33 s for 18 h. Gels were stained with ethidium bromide and were visualized on the Gel Doc 2000 (Bio-Rad). The size of DNA fragments were determined by direct comparison to the CDC PulseNet standard G5244, which was run adjacent to V. vulnificus DNA (data not shown).
Southern blot analysis and chromosomal mapping.
Digoxigenin-labeled probes complementary to selected ORFs were synthesized with the primers listed in Table 1. Each recognized a representative region from each of the four gene clusters. Blotting conditions were identical for each of the probes, with a hybridization temperature of 54°C.
TABLE 1.
Oligonucleotide primer pairs used for amplification and detection of each identified ORF among the 10 serologically diverse V. vulnificus strains
| Primer designation | Oligonucleotide sequence | Tm (°C) applied | ORF amplified |
|---|---|---|---|
| 47B5P | 5′CCGCGAAGAAGATTCTACGA3′ | 60 | wcvI |
| 47B3P | 5′TGCGAATGACCATAGGACGA3′ | ||
| 54T735P | 5′CATATTGTGCCAGAGTAGAT3′ | 55 | wcvF |
| 54T733P | 5′TGCTTGTCTCTCTTCCTGTC3′ | ||
| epiA | 5′ATTGGCAGTGCGACGGCAGA3′ | 65 | wcvA |
| epiB | 5′AACCGAGTATGGCGCAGAGC3′ | ||
| 44XT313P | 5′CCTACATCATCGCCATATCG3′ | 65 | orfI |
| 44XT3-2 | 5′CTGTTCAATAGCGTACGCTG3′ | ||
| VCR1 | 5′TTCAAGAGGGACTTGGCACG3′ | 65 | VCR9 |
| VCR2 | 5′GCAGGCACGCAATACAAAAG3′ | ||
| 44T715P | 5′CCGTATCAACGCCGACATTA3′ | 65 | IS492 |
| 44T713P | 5′GTTCGATTGAACGCTGGCTC3′ |
LD50s.
The protocol utilized to determine the 50% lethal doses (LD50s) of V. vulnificus strains was that of Wright et al. (36). The pathogenicity of each of the nonencapsulated transposon mutants was compared to that of encapsulated 1003(O) in the presence of excess serum iron. Specifically, intraperitoneal injections of V. vulnificus cells were administered immediately, followed by intraperitoneal injections of ferric ammonium citrate into 6- to 8-week-old male mice. Following a 48-h period, mortalities were totaled and the method of Reed and Muench (26) was employed to calculate the LD50 for each strain tested.
Nucleotide sequence accession numbers.
The nucleotide sequences of all putative capsule genes identified in this study have been submitted to GenBank and can be obtained with accession numbers AY097060, AY096104, AY096105, AF499931, AF499932.
RESULTS
Transposon mutagenesis and mutation analysis.
From eight separate conjugation experiments, each yielding approximately 1,500 transformants, 23 nonencapsulated (translucent) mutants were selected. Southern blotting analysis revealed the transposon insertion had occurred and disrupted four distinct genetic regions; each independently resulted in loss of capsule expression. Four mutants, one representative of each transposon insertion, were chosen for further analysis. The insertion site of one mutant, ABZ1(T), was digested with EcoRI, cloned into pBluescript SK(−), and designated pEpiBS. Second, the fragment containing the transposon and flanking chromosomal DNA was cloned from a second mutant, RJS2(T), constructing clone p54BS. Third, clone p47BS was derived from the cloning of EcoRI-digested DNA of mutant TDB3(T). Lastly, the transposon and flanking chromosomal DNA of mutant GMB4(T) was digested with XbaI, cloned in pBluescript SK(−), and designated p44XBS.
Nucleotide sequencing and analysis.
The nucleotide sequence of V. vulnificus chromosomal DNA flanking the transposon insertion was determined for each of the four clones. The T7 and T3 primer sequences of pBluescript SK(−) vector were each utilized for nucleotide sequencing. Subsequent translation and arrangement of ORFs is shown in Fig. 1. Table 2 shows the extent of homology of these V. vulnificus ORFs to genes of heterologous bacterial species.
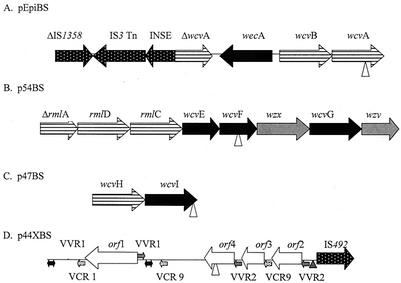
FIG. 1.
Organization of the ORFs recognized in each of the four genetic regions cloned following transposon insertion. Vertical arrowhead symbols in panels A, B, C, and D indicate the sites of transposon insertion to render each mutant nonencapsulated. Grey, black, horizontally striped, white, and spotted arrows indicate processing genes, transferase genes, nucleotide sugar biosynthesis genes, undefined genes, and insertion sequences, respectively. Grey arrows in panel D indicate VVR sequences, and stippled arrows indicate VCR sequences. Black double arrowheads denote stretches homologous to V. cholerae intergenic DNA located on chromosome II. Triangles upstream of IS492 indicate possible sites of insertion (ACAAG).
TABLE 2.
Homology of V. vulnificus ORFs to heterologous bacterial genes involved in polysaccharide synthesis
| Gene | Organism | Putative function | % Amino acid identity | Accession no. | Refer- ence |
|---|---|---|---|---|---|
| wcvA | V. cholerae O139 | Nucleotide-sugar epimerase | 86 | AAC46250 | 38 |
| wcvB | V. cholerae O139 | Nucleotide-sugar dehydrogenase | 87 | AAC46249 | 38 |
| wecA | E. coli O157:H7 | Undecaprenyl phosphate N-acetyl glucosamine 1-phosphate transferase | 48 | AAG58979 | 18 |
| INSE | E. coli | Insertion factor | 58 | AAC73401 | 6 |
| IS3 | E. coli | Insertion sequence transposase | 52 | AAC73402 | 6 |
| IS1358 | V. cholerae O139 | Insertion sequence transposase | 97 | S70960 | 4 |
| rmlA | Actinobacillus actinomycetemcomitans | Glucose-1-phosphate thymidylyl transferase | 82 | BAA94403 | 22 |
| rmlD | A. actinomycetemcomitans | dTDP-4-keto-l-rhamnose reductase | 61 | BAA82534 | 39 |
| rmlC | A. actinomycetemcomitans | dTDP-6-deoxy-d-xylo-4-hexulose 3,5 epimerase | 70 | BAA94405 | 22 |
| wcvE | Staphylococcus aureus | Glycosyltransferase | 29 | AAA64649 | 17 |
| wcvF | K. pneumoniae | Rhamnosyl transferase | 24 | AAC70778 | |
| wzx | E. coli | Flippase | 21 | AAK64374 | 33 |
| wcvG | Bacteroides fragilis | Glycosyltransferase | 18 | AF048749 | 11 |
| wzy | Streptococcus agalactiae | Polymerase | 25 | AAK29654 | |
| wcvH | S. pneumoniae | Unknown | 15 | AAB66523 | 21 |
| wcvI | Lactobacillus delbrueckii | Glycosyltransferase | 27 | AAG44710 | |
| orf1 | None | Unknown | |||
| orf2 | V. cholerae O1 | Unknown | 65 | NP_232690 | 14 |
| orf3 | none | Unknown | |||
| orf4 | Deinococcus radiodurans | Unknown | 26 | NP_295444 | 34 |
| IS492 | P. atlantica | Insertion sequence transposase | 71 | AAA25864 | 3 |
The pEpiBS vector encoded seven (including partial) ORFs and intergenic regions of noncoding DNA (Fig. 1A). This genetic fragment is comprised of a variety of ORFs with functions including biosynthesis, processing, and possible genomic rearrangement. Figure 1B shows p54BS. All oriented in the same direction, this operon-like locus contains numerous genes which are homologous to capsule-type specific genes in a variety of encapsulated bacterial species, shown in Table 2. p47BS is shown in Fig. 1C and is limited to two ORFs. The genetic region of p44XBS identifies five ORFs flanked by imperfect direct repeats. Due to a lack of nucleotide and amino acid sequence homology to sequences compared by using BLAST (1), the functions of three ORFs remains undefined. The schematic in Fig. 1D shows the presence of imperfect direct repeats within this region of the V. vulnificus genome, identifying the position of these stretches of intergenic DNA.
The imperfect direct repeats (VVR, for V. vulnificus repeats) identified in p44XBS are unique to V. vulnificus. The nucleotide sequence of VVR1 is made up of 5′-CATAACGCTGCGTTAAGGGGTGARTGCCGCCTAAACCAASYTTCCGCAGRCCACTTTCACCACTAAAACTCACCGCATACCAAAAATGCCACGCGGCAYGAATCCCACTTGAACGCCTTGTTATGYTTAAGMYTCAAWGGGTTAMGTTTTACSRTA-3′. A second set of VVR, VVR2, is also present in this genetic region. The nucleotide sequence of VVR2 is 5′-ACAAGGCGTTYAAGWGGGATTCATGCCGCGTGGCA TTTTTGGTATGCGGTGAKTWTTGGYGGTGAAAGTG GTCTGCGGAAAGYTGGTTTAKGCGGCATTCACCC CTTAACGCAGCGTTA-3′.
In addition to the VVRs, two Vibrio cholerae repeats (VCR) (13) are also present but are in the opposite orientation of the VVRs. The VCR repeats found in this region, VCR 1 and VCR 9, are also direct repeats. Furthermore, this insert contains repeats of homology, of greater than 90% in most cases, to cassette regions identified as part of super integron regions (14, 30).
IS492 transposition.
Results of Southern blotting with a digoxigenin-labeled IS492-specific probe is demonstrated in Fig. 3. IS492 could be located several times throughout the V. vulnificus chromosome. Also shown are the sites of insertion that differ within the genomes of 1003(O) and 1003(T), resulting in fragment length polymorphisms.
FIG. 3.
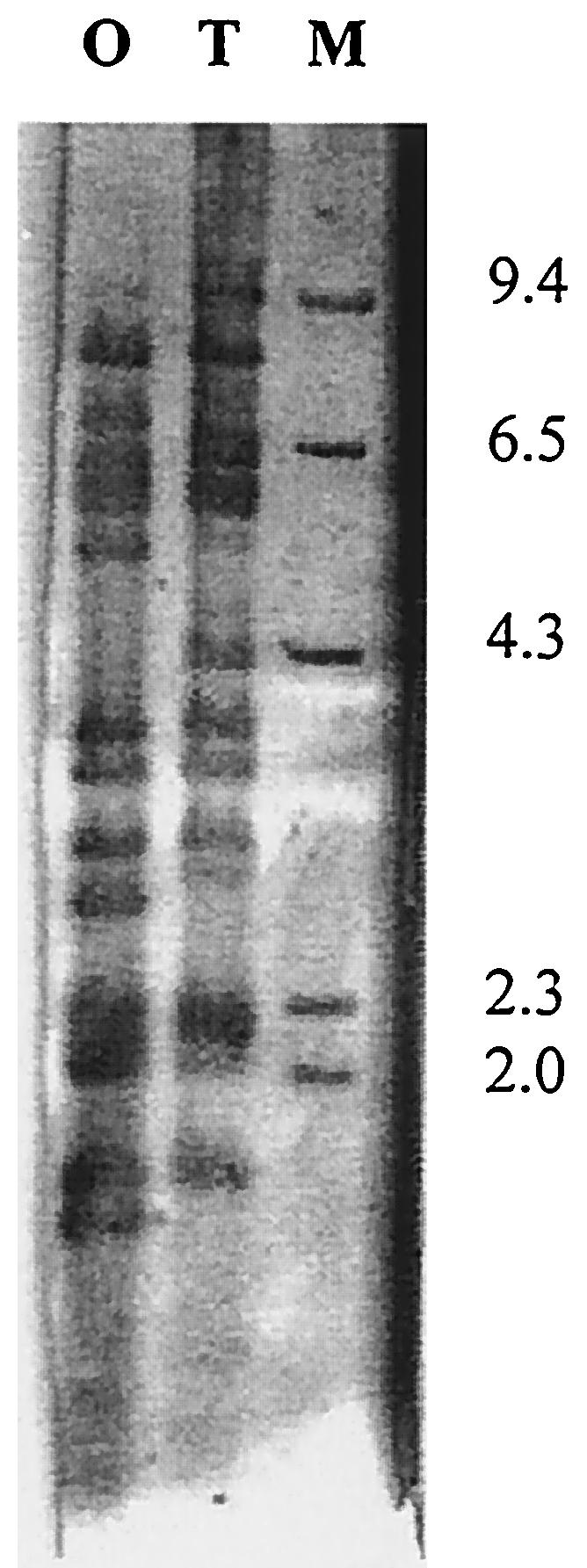
Southern blot of V. vulnificus 1003 chromosomal DNA. The XbaI digested DNA of the encapsulated (O) and nonencapsulated (T) strains are shown following hybridization with IS492 digoxigenin-labeled probe. M indicates the marker lane, λHindIII, with a 23-kb fragment omitted for clarity.
PFGE.
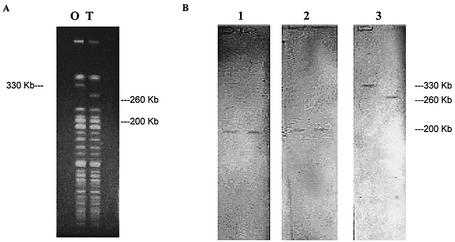
Restriction digestion with NotI resulted in banding patterns that differentiated the genome fragments of 1003(O) and 1003(T), as shown in Fig. 2A. The capsule genes wcvA, wcvI (data not shown), and wcvF, representing three of the V. vulnificus gene clusters, all hybridized to one fragment of approximately 200 Kbp in both 1003(O) and 1003(T) (Fig. 2B). Hybridization of IS492, however, resulted in the recognition of a larger DNA fragment of 330 Kbp that was unique to 1003(O) and absent in 1003(T). The presence of a smaller-size fragment of 260 Kbp was unique to 1003(T); this 260-Kbp fragment was absent in 1003(O). Furthermore, Southern blot analysis showed that IS492, orf1, and VCR 9 are all clustered together and hybridize to the same 330-Kbp fragment that is unique to encapsulated strain 1003(O) (data not shown). These three ORFs remained clustered in the nonencapsulated strain 1003(T) but hybridized to the unique 260-Kbp DNA fragment, discriminating the location of the gene cluster among the encapsulated and nonencapsulated strains.
FIG. 2.
PFGE and Southern blot of V. vulnificus chromosomal DNA. (A) PFGE of V. vulnificus 1003(O) and 1003(T) following digestion with NotI restriction enzyme. (B) Southern blot of PFGE (shown in panel A) following hybridization of probes wcvA (lane 1), wcvF (lane 2), and IS492 (lane 3). Hybridization of wcvI, orf1, and VCR 9 is not shown.
LD50s.
The LD50s of V. vulnificus strains 1003(O), 1003(T), and each of the four translucent mutants ABZ1(T), RJS2(T), TDB3(T), and GMB4(T) was determined by using the iron mouse model to ascertain the role of capsule in virulence. The virulence of the encapsulated strain 1003(O) is highly evident, in contrast to that of the nonencapsulated mutants. Results are shown in Table 3.
TABLE 3.
LD50s of encapsulated and nonencapsulated strains of V. vulnificus with the iron mouse model
| V. vulnificus strain | LD50 (cells) | Reference |
|---|---|---|
| 1003(O) | 0.87 | 40 |
| 1003(T) | >6.5 × 107 | 40 |
| ABZ1(T) | >4.9 × 107 | 40 |
| RJS2(T) | >2.5 × 107 | This study |
| TDB3(T) | >2.4 × 107 | This study |
| GMB(T) | >2.7 × 107 | This study |
DISCUSSION
Nomenclature.
All V. vulnificus genes identified in this study, believed to be involved in capsule polysaccharide synthesis, have been named accordingly as wc**, a nomenclature proposed by Reeves et al. (28). The putative function for each ORF has been assigned on the basis of sequence identity to polysaccharide genes previously described from a variety of encapsulated microorganisms. The wcv locus, described herein, identifies 14 ORFs that correspond to homologues of capsule gene clusters that are involved in the biosynthesis, polymerization, and transport of the polysaccharide units. Furthermore, four ORFs which encode for mobilizable insertion sequences and an additional four undefined ORFs (orf*) with associated repeat regions have also been identified. Collectively, a total of 22 ORFs have been described, four of which are believed to be essential for CPS synthesis of V. vulnificus.
Genes essential for encapsulation and virulence.
The insertion of the mini-Tn10 transposon into wcvA, wcvF, wcvI, and orf4 rendered the wild-type V. vulnificus 1003(O) nonencapsulated, suggesting each is vital to capsule expression. While genes homologous to wcvA that encode nucleotide-sugar epimerase protein products have been linked to polysaccharide precursor synthesis (capsule and O-antigen), it has been previously proven essential to the expression of capsule polysaccharide (40). wcvF, a rhamnosyl transferase (Genbank accession no. AAC70778), is believed to be involved in the serotype-specific synthesis of extracellular polysaccharide capsule. wcvI, a glycosyl transferase, has been reported to be essential for capsule synthesis in Klebsiella pneumoniae (2). Lastly, orf4 remains undefined. While the reason for the loss of capsule expression following inactivation of orf4 is unknown, its location relative to IS492 and direct repeat sequences may provide insight as to its role in regulation of capsule synthesis and expression. Insertional inactivation of each ORF by transposon mutagenesis negated capsular expression directly or indirectly.
Mutation in each of the loci has also been shown to render the mutants avirulent (Table 3). Multiple studies have shown the increase of pathogenicity of V. vulnificus in the presence of excess serum iron, which mimics the usual immunocompromised human host. The ability of V. vulnificus to acquire iron allows the organism to thrive (31) and dramatically reduces the LD50 of a virulent organism. The iron mouse model of Wright et al. (36) is commonly used to demonstrate the enhanced virulence of V. vulnificus in the presence of excess serum iron in vivo. However, herein the iron mouse model has successfully demonstrated that the loss of capsule via transposon mutagenesis corresponds to the loss of virulence, even in the presence of excess iron.
Nucleotide sugar biosynthesis genes.
Six ORFs were identified as being involved in the biosynthesis of activated monosaccharide precursors. The proposed function of each is based on sequence similarity to homologues involved in polysaccharide synthesis of various encapsulated bacteria. The nucleotide sugar epimerase wcvA and nucleotide sugar dehydrogenase wcvB were identified as proximal ORFs of the same orientation. Although it seems likely that these two ORFs could be polycistronic, the Promoter Locator (27) suggests that individual promoter regions and transcriptional start sites for each wcvA and wcvB exist. Therefore, independent expression of each cannot be ruled out.
Three rml genes, rmlA, rmlD, and rmlC, involved in the biosynthesis of l-rhamnose, were identified and located in tandem in the same transcriptional orientation at the 5′ end of p54BS. It is not known whether a promoter region is present upstream of rmlA for transcription of a polycistronic message, but the absence of a promoter in the few bases that separate each of the tightly linked ORFs suggests it is transcribed in such a manner. The presence of these genes is not surprising considering that l-rhamnose was the most common sugar identified as a component of V. vulnificus polysaccharide capsules (7). Because these three genes are known to be part of a four-gene cluster, it is possible that rmlB, while not detected, is most likely positioned upstream and was excised upon being cloned with EcoRI.
wcvH is also believed to mediate the biosynthesis of activated monosaccharide precursors. WcvH in V. vulnificus is homologous to cps19bR, which is encoded by a sequence located within the serotype-specific region of the capsule gene cluster of Streptococcus pneumoniae (21).
Polymerization and processing genes.
In the Wzy-dependent system, a glycosyltransferase (WecA) initiates transfer of activated individual repeat unit precursors, such as GlcNac-1-phosphate, to an undecaprenyl phosphate lipid carrier. This is necessary for building repeating polysaccharide units. Continuing the process, a flippase (Wzx) translocates the repeat units across the cytoplasmic membrane. The repeat sugar units are then polymerized by Wzy. Figure 1 shows the presence of wecA, wzx, and wzy, which suggests the capsule is lipid-linked and Wzy-dependent. GlcNAc and GalNAc are both identified among the four sugar repeating subunits of polysaccharide capsules of V. vulnificus (7, 25), which may be the result of the Wzy-dependent system.
These genes which participate in assembly and transport of repeat sugar units are believed to be serotype specific because they are exacting for the sugars and the glycosidic linkages that join the repeats to confer serological specificity. They are located within the polysaccharide-specific regions of gene clusters in heterologous encapsulated bacteria and are believed to be involved in both K- and O-antigen synthesis (23).
Additional transferase genes.
In addition to the initiating glycosyltransferase (wecA) activity, wcvE, wcvF, wcvG, and wcvI are also believed to encode glycosyltransferase enzymes. Both wcvF and wcvI were disrupted by the transposon, resulting in the loss of capsule expression in V. vulnificus. Since the predicted function of each precise transferase was based on sequence homologies, individual substrates are not known except for that of wcvF, which is believed to encode a rhamnosyl transferase.
Insertion sequences.
Three ORFs homologous to insertion sequences were identified within two regions of the V. vulnificus chromosome. pEpiBS contained both ΔIS1358 and IS3 in tandem. It is possible that IS3 had inserted into this genetic region, rendering both IS1358 and wcvA truncated. However, the ability of IS3 to transpose throughout the V. vulnificus genome remains undetermined.
IS1358 is believed to be involved in homologous recombination of polysaccharide genetic loci (4). Genetic exchange mediated by IS1358 has been hypothesized at an interspecies level among the Vibrio species (32), and it has been shown to transpose at a frequency comparable to those of other insertion sequences (12).
The IS492 element was located in the genetic region cloned in p44XBS (Fig. 1D). IS492 can reversibly inactivate extracellular polysaccharide synthesis in Pseudomonas atlantica, where insertion is site-specific (ACAAG), and excision from the eps gene restores polysaccharide production (3). Analogous to that seen with P. atlantica, Southern blot analysis (Fig. 3) showed that IS492 is found numerous times in the V. vulnificus genome. Blotting also showed that the sites for insertion of IS492 differ among the encapsulated and nonencapsulated morphotypes of V. vulnificus strain 1003. Furthermore, PFGE analysis, shown in Fig. 2B, demonstrates that the repeats of IS492 are confined to a single 330-Kbp chromosomal region in 1003(O) and a single 260-Kbp fragment in 1003(T). These differences in banding patterns of encapsulated versus nonencapsulated strains indicates a genomic rearrangement in V. vulnificus.
Generally, insertion sequence elements have frequently been located near polysaccharide gene loci and are believed to generate polymorphisms seen within a bacterial species due to recombination. Often capsule gene clusters in E. coli strains are flanked by insertion sequence elements. They may mediate genetic transfer among heterologous strains which produces antigenic diversity (23). The presence of recognizable insertion sequences in the V. vulnificus genome suggests that recombination may occur and may explain restriction fragment length polymorphisms observed within the V. vulnificus species.
Undefined ORFs and repeat regions.
The genomic region cloned in p44XBS contained four ORFs that were not homologous to any nucleotide or amino acid sequence when subjected to BLAST (1) analysis. Therefore, no function can be assigned to the four ORFs on the basis of sequence homology. Further analysis of each ORF provided a limited protein profile for each. Orf3 is the only protein of the four analyzed that possesses transmembrane regions. It has two strong transmembrane helices at amino acids 9 to 27 and 50 to 69, with no other notable chemical characteristics. While the region between VCR 9 and orf4 remains unidentified, insertion of the transposon at the 3′ end of orf4 demonstrates an essential role in capsule expression. Orf4 was shown to be hydrophilic in nature, with a predicted alpha helix. The inferred products for the remaining two ORFs possessed no recognizable motifs or characteristics.
The identification of multiple unidentified ORFs in combination with flanking direct repeats is reminiscent of the arrangement of integron-gene cassette regions of super integrons (30). Mazel et al. (19) detected 60 to 100 copies of VCRs within a single locus of the V. cholerae genome, comprising what is thought to be a super integron. VCR cassettes are intergenic regions that have been linked to super integrons located on the smaller of two V. cholerae chromosomes. These repeat regions are thought to be the target of site-specific insertion for acquisition of exogenous DNA, mostly consisting of ORFs with no identified homologues (30), such as orf1. Characteristics of the integron-gene cassette include imperfect direct repeats that flank a single ORF. This mimics the gene cassette array found in antibiotic resistance-encoding integrons and is believed to be a system for acquiring new genes via site-specific recombination in V. cholerae (19). Integrase-mediated recombination between VCRs has been reported previously (9) and results in the acquisition of ORFs with no homologues identified by using database alignment (30). This system was also identified in Vibrio metschnikovii, Vibrio parahaemolyticus, and Vibrio fischeri and appears to be present in V. vulnificus. Regions homologous to the VCR sequences (VCR 1 and VCR 9) which flanked undefined ORFs were located within the V. vulnificus genomic region cloned in p44XBS. The presence of the VCR and VVR direct repeats in combination with the undefined ORF cassettes in p44XBS suggested that the presence of the integron-gene cassette is a possibility. The acquisition of cassette regions is implied by the G+C content of orf1234, which ranges from 36 to 42%, similar to that of the gene-VCR cassettes of V. cholerae (19). Because these values are lower than those of V. vulnificus chromosomal DNA, documented as 45 to 48% (29), it is possible that these ORFs were acquired via site-specific recombination. In addition, similar to the arrangement of the V. cholerae super integron, results of PFGE and Southern blotting positioned the VCR (and VVR) cassettes and undefined ORFs within a single locus of the V. vulnificus chromosome (Fig. 2B). This further enhances the likelihood for the presence of a super integron in V. vulnificus.
Chromosomal linkage.
The V. vulnificus chromosomal DNA that flanked the transposon insertion of each of the four clones is believed to be part of a capsule gene locus. Although the four genetic regions do not overlap to allow chromosomal walking, the relative proximity of each within the V. vulnificus genome was demonstrated by PFGE and Southern blotting analysis. ORFs within clones pEpiBS, p47BS, and p54BS are located on the same 200-Kbp chromosomal fragment (Fig. 2B). The location of genes wcvA, wcvF, and wcvI remained unchanged, even following an unexplained genetic switch observed when comparing the genome of 1003(O) to that of the spontaneously acquired nonencapsulated 1003(T). The constant position of these loci in 1003(O) and 1003(T) indicate that the arrangement of the three capsule gene regions are not affected in the genetic rearrangement of 1003(T).
IS492 is identified as a mobilizable insertion sequence element in P. atlantica. It is capable of inserting into capsule genes to cause the loss of capsule production and then excise itself, permitting the organism to revert back to the encapsulated state (3). PFGE shows that the 330-Kbp chromosomal fragment harboring IS492 in V. vulnificus 1003(O) is shifted in 1003(T) to a 260-Kbp fragment (Fig. 2B). Both orf1 and VCR 9, in conjunction with IS492, were also located on the shifted band of 1003(T) by Southern blot analysis (data not shown). While the cause for the band shift and its relation to loss of capsule expression is unknown, it is logical to recognize the fact that IS492 is located proximal to unidentified ORFs, numerous repeat regions, and stretches of DNA similar to integron cassettes, all located on a single chromosomal fragment. These qualities are characteristic features of the super integrons in V. cholerae and other Vibrio species (19, 30). Collectively, this integron-like region is not believed to be a part of the capsule gene cluster. It follows that the products of this genetic region do not contribute directly to the biosynthesis, polymerization, or transport of the polysaccharide capsule. Instead, disruption of orf4 may have affected capsule expression negatively via an indirect means.
General organization.
Identification of each ORF via sequence similarity to homologues has helped develop an understanding of the organizational arrangement of the capsule genes in V. vulnificus. This arrangement provides insight for classification of the V. vulnificus capsule (K-antigen) according to the four defined capsule groups (35). These are defined by E. coli groups I (IA and IB), II, and, more recently, III (16). A recent reclassification proposed by Whitfield and Roberts (35) placed group IB into a fourth independent group. The polysaccharide genes described in this study place V. vulnificus capsule with both groups 1 and 4 according to the newly described guidelines for the following reasons. The identification of both wzx and wzy indicates that polymerization and transmembrane export is Wzy-dependent, an event and property common to groups 1 and 4. However, the initiating glycosyltransferase involved in this Wzy-dependent system is wecA, which characterizes group 4 (35). With additional properties that distinguish groups 1 and 4 remaining unknown for V. vulnificus, such as regulatory systems and nearby genetic loci, the correct grouping of this capsule gene cluster cannot be determined with certainty at this juncture. The identification of wecA, wzx, and wzy in V. vulnificus has indicated that the polysaccharide capsule of V. vulnificus is lipid linked, analogous to the attachment of lipopolysaccharide synthesis. This concurs with the findings of Wright et al. (37), which identified wza as part of the capsule gene locus and indicates a lipid-linked capsule polysaccharide as well.
Conclusion.
With the capsular carbohydrate composition of V. vulnificus known to be acidic and to contain many amino sugars (7, 25), the regions identified provide a genetic basis for further understanding of the K-antigen. The identification of the Wzy-dependent system suggests the capsule polysaccharide is lipid linked. In addition, the genetic regions of the capsule gene locus of strain 1003(O) may also be used in conjunction with V. vulnificus capsule genes previously identified, such as wza (37).
It should be noted that ORFs known to contribute to CPS synthesis and expression in heterologous bacteria may also contribute to LPS synthesis and expression, as both antigens are comprised of repeating polysaccharide units. The genes involved in polysaccharide biosynthesis, polymerization, and transport may naturally be shared among both processes. However, because virulence studies have shown a correlation of opaque colony morphology and virulence, it is believed that the CPS protects the organism from the host immune system, acting as an essential virulence factor (31). The study herein has illustrated this concept, as translucent mutants were no longer virulent with the iron mouse model. The contribution of the ORFs (Table 2) to LPS expression is a viable possibility and must not be ruled out. Such analysis may provide a more complete understanding of polysaccharide synthesis and expression.
With numerous possibilities for employment of the data presented, the insertion sequence elements, capsule polysaccharide genes, and integron-gene cassette region described in this study can hopefully provide a further understanding of V. vulnificus genetics and antigenic diversity.
Acknowledgments
This research was supported by the Interstate Shellfish Sanitation Conference (ISSC) grant numbers 0996-1197-002 and 0996-117-0102, in addition to the Louisiana Sea Grant College Program, grant number NA86RG0073, and supplemental funding. The Louisiana Sea Grant College Program is a part of the National College Program maintained by NOAA, U.S. Department of Commerce, and the State of Louisiana. We thank the Joe W. and Dorothy Dorsett Brown Foundation for further funding, which was used for the purchase of an ABI 310 Genetic Analyzer.
We thank V. R. Srinivasan, Gregg Pettis, and Richard Cooper for their guidance and support. Many thanks to Chupon Shariffskul and Paul Fiorella for assistance with PFGE protocols.
Editor: D. L. Burns
Footnotes
This paper is dedicated to the memory of Ron Siebeling, an exceptional microbiologist, professor, and mentor.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa, Y., R. Wacharotayankun, T. Nagatsuka, H. Ito, N. Kato, and M. Ohta. 1995. Genomic Organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J. Bacteriol. 177:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, D. H., and M. Silverman. 1989. Nucleotide sequence of IS492, a novel insertion sequence causing variation in extracellular polysaccharide production in the marine bacterium Pseudomonas atlantica. J. Bacteriol. 171:1763-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bik, E. M., A. E. Bunschoten, R. D. Gouw, and F. R. Mooi. 1995. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 14:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublin. 1979. Disease caused by a marine vibrio: clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Block, N. T. Perna, et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Bush, C. A., P. Patel, S. Gunawardena, J. Powell, A. Joseph, J. A. Johnson, and J. G. Morris. 1997. Classification of Vibrio vulnificus strains by the carbohydrate composition of their capsular polysaccharides. Anal. Biochem. 250:186-195. [DOI] [PubMed] [Google Scholar]
- 8.Chan, J. W. Y. F., and P. H. Goodwin. 1995. Extraction of genomic DNA from extracellular polysaccharide-synthesizing gram-negative bacteria. BioTechniques 18:418-422. [PubMed] [Google Scholar]
- 9.Clark, C. A., L. Purins, P. Kaewrakon, T. Focareta, and P. A. Manning. 2000. The Vibrio cholerae O1 chromosomal integron. Genet. Mol. Biol. 146:2605-2612. [DOI] [PubMed] [Google Scholar]
- 10.Combet, C., C. Blanchet, C. Geourjon, ad G. Deleage. 2000. NPS@: network protein sequence analysis. Trends Biochem. Sci. 25:147-150. [DOI] [PubMed] [Google Scholar]
- 11.Comstock, L. E., M. J. Coyne, A. O. Tzianabos, A. Pantosti, A. B. Onderdonk, and D. L. Kasper. 1999. Analysis of a capsular polysaccharide biosynthesis locus of Bacteroides fragilis. Infect. Immun. 67:3525-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumontier, S., P. Trier-Cuot, and P. Berche. 1998. Structural and functional characterization of IS1358 from Vibrio cholerae. J. Bacteriol. 180:6101-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzon, V. L., A. Barker, and P. A. Manning. 1993. Nucleotide sequence encoding the mannose-ducose-resistant hemagglutinin of Vibrio cholerae O1 and construction of a mutant. Infect. Immun. 61:3032-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidelberg, J. F., et al. 2000. DNA Sequence of both chromosomes of the cholera pathogen. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann, K., and W. Stoffel. 1993. Tmbase-a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler. 374:166. [Google Scholar]
- 16.Jann, B., and K. Jann. 1997. Capsules of Escherichia coli. In M. Sussman (ed.), Escherichia coli mechanisms of virulence, p. 113-143. Cambridge University Press, Cambridge, United Kingdom.
- 17.Lin, W. S., T. Cunneen, and C. Y. Lee. 1994. Sequence analysis and molecular characterization of genes required for the biosynthesis of type 1 capsular polysaccharide in Staphylococcus aureus. J. Bacteriol. 176:7005-7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino, K., K. Yokoyama, Y. Kubota, C. H. Yutsudo, S. Kimura, K. Kurokawa, K. Ishii, M. Hattori, I. Tatsuno, H. Abe, T. Iida, K. Yamamoto, M. Ohnishi, T. Hayashi, T. Yasunaga, T. Honda, C. Sasakawa, and H. Shinagawa. 1999. Complete nucleotide sequence of the prophage BT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157:H7 derived from the Sakai outbreak. Genes Genet. Syst. 74:227-239. [DOI] [PubMed] [Google Scholar]
- 19.Maslanka, S. E., J. G. Kerr, G. Williams, J. M. Barbaree, L. A. Carson, J. M. Miller, and B. Swaminathan. 1999. Molecular subtyping of Clostridium perfringens by pulsed-field gel electrophoresis to facilitate food-borne-disease outbreak investigations. J. Clin. Microbiol. 37:2209-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 1998. a distinctive class of integron in the Vibrio cholerae genome. Science 280:605-608. [DOI] [PubMed] [Google Scholar]
- 21.Morona, J. K., R. Morona, and J. C. Paton. 1997. Molecular and genetic characterization of the capsule biosynthesis locus of Streptococcus pneumoniae type 19B. J. Bacteriol. 179:4953-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano, Y., Y. Yoshida, Y. Yamashita, and T. Koga. 1998. A gene cluster for 6-deoxy-L-talan synthesis in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1442:409-414. [DOI] [PubMed] [Google Scholar]
- 23.Rahn, A., J. Drummelsmith, and C. Whitfield. 1999. Conserved organization in the cps gene clusters for expression of Escherichia coli group 1 K antigens: relationship to the colanic acid biosynthesis locus and the cps genes from Klebsiella pneumoniae. J. Bacteriol. 181:2307-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy, G. P., U. Hayat, C. Abeygunawardana, C. Fox, A. C. Wright, D. R. Maneval, Jr., C. A. Bush, and J. G. Morris, Jr. 1992. Purification and determination of the structure of capsular polysaccharide of Vibrio vulnificus M06-24. J. Bacteriol. 174:2620-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy, G. P., U. Hayat, Q. Xu, K. V. Reddy, Y. Wang, K. W. Chiu, J. G. Morris, Jr., and C. A. Bush. 1998. Structure determination of the capsular polysaccharide from Vibrio vulnificus strain 6353. Eur. J. Biochem. 255:279-288. [DOI] [PubMed] [Google Scholar]
- 26.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 27.Reese, M. G. 2001. Application of a time-delay neural network to promoter annotation in the Drosophila melangaster genome. Comput. Chem. 26:51-56. [DOI] [PubMed] [Google Scholar]
- 28.Reeves, P. R., M. Hobbs, M. A. Valvano, M. Skurnik, C. Whitfield, D. Coplin, N. Kido, J. Klena, D. Maskell, C. R. H. Raetz, and P. D. Rick. 1996. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 4:495-503. [DOI] [PubMed] [Google Scholar]
- 29.Reichelt, J. L., P. Baumann, and L. Baumann. 1976. Study of genetic relationships among marine species of the genera Benechea and Photobacterium by means of in vitro DNA/DNA hybridization. Arch. Microbiol. 110:101-120. [DOI] [PubMed] [Google Scholar]
- 30.Rowe-Magnus, D. A., A. Guerout, and D. Mazel. 2001. The evolutionary history of chromosomal super-integron ancestry for multiresistant integrons. Proc. Natl. Acad. Sci. USA 98:652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson, L. M., V. K. White, S. F. Zane, and J. D. Oliver. 1987. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 55:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stroeher, U. H., K. E. Jedani, and P. A. Manning. 1998. Genetic organization of the regions associated with surface polysaccharide synthesis in Vibrio cholerae O1, O139 and Vibrio anguillarum O1 and O2: a review. Gene 223:269-282. [DOI] [PubMed] [Google Scholar]
- 33.Wang, L., C. E. Briggs, D. Rothemund, P. Fratamico, J. B. Luchansky, and P. R. Reeves. 2001. Sequence of the E. coli O104 antigen gene cluster and identification of O104 specific genes. Gene 270:231-236. [DOI] [PubMed] [Google Scholar]
- 34.White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, R. J. Dodson, D. H. Haft, M. L. Gwinn, W. C. Nelson, et al. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 36.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright, A. C., J. L. Powell, J. B. Kaper, and J. G. Morris, Jr. 2001. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 69:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamasaki, S., T. Shimizu, K. Hoshino, S. T. Ho, T. Shimada, G. B. Nair, and Y. Takeda. 1999. The genes responsible for O-antigen synthesis of Vibrio cholerae O139 are closely related to those of Vibrio cholerae O22. Gene 237:321-332. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida, Y., Y. Nakano, N. Suzuki, H. Nakao, Y. Yamashita, and T. Koga. 1999. Genetic analysis of the gene cluster responsible for synthesis of serotype e-specific polysaccharide antigen in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1489:457-461. [DOI] [PubMed] [Google Scholar]
- 40.Zuppardo, A. B., and R. J. Siebeling. 1998. An epimerase gene essential for capsule synthesis of Vibrio vulnificus. Infect. Immun. 66:2601-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]