Abstract
The cocrystal structure of streptococcal pyrogenic exotoxin C (SPE C) with HLA-DR2a (DRA*0101,DRB5*0101) revealed a zinc-dependent interaction site through residues 167, 201, and 203 on SPE C and residue 81 on the β-chain of HLA-DR2a (DRA*0101,DRB5*0101). Mutation of these SPE C residues resulted in dramatically reduced biological activities. Thus, the zinc-dependent major histocompatibility complex II binding site is critical for maximal biological function of SPE C.
Group A streptococci possess multiple secreted virulence factors that contribute to a range of human diseases. The most potent of these factors are the streptococcal pyrogenic exotoxins (SPEs; scarlet fever toxins), which belong to a large family of pyrogenic toxin superantigens (PTSAgs). PTSAgs activate large populations of T lymphocytes bearing certain variable regions of the β-chain of the T-cell receptor (VβTCR) by direct PTSAg cross-linking of the major histocompatibility complex (MHC) class II molecules on antigen-presenting cells, outside of the antigenic peptide binding groove, with the VβTCR. SPE C activates T cells bearing VβTCR 1, 2, 5.1, and 10 (15). This widespread superantigen-induced T-cell activation results in the massive release of cytokines, both from T cells and macrophages, which is believed to be important for pathogen immune response evasion through several proposed mechanisms and causation of toxic shock syndrome (TSS) (4).
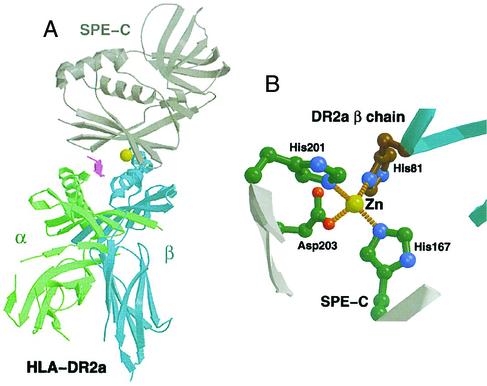
There is variation in the orientation of the PTSAg interaction with MHC class II molecules. For example, staphylococcal enterotoxin B and TSS toxin 1 interact with the α-chain of MHC class II molecules through N-terminal amino acid residues (3, 9). Alternately, the zinc-binding site of SPE C interacts with the β-chain of MHC class II molecules through a zinc ion bound to residues in the C-terminal domain as observed in structural studies of SPE C and MHC class HLA-DR2a (DRA*0101,DRB5*0101) (Fig. 1) (6). This structural study showed an interaction between HLA-DR2a (DRA*0101,DRB5*0101) β-chain, His-81, through a zinc ion that is coordinated with the SPE C residues His-167, His-201, and Asp-203 in the C-terminal β-grasp fold of SPE C. There are additional interactions between SPE C and HLA-DR2a (DRA*0101,DRB5*0101), as well as interactions with the antigenic peptide loaded in the peptide-binding groove of HLA-DR2a (DRA*0101,DRB5*0101). The goal of this work was to determine the role in biological activity of the zinc-binding residues on SPE C through the use of mutants in these residues.
FIG. 1.
Ribbon diagram of cocrystal structure of SPE C complexed with HLA-DR2a (DRA*0101,DRB5*0101) (A) and residues interacting with zinc (B). (Reprinted from reference 6 with permission from Elsevier Science.)
Residues in SPE C were chosen for mutation based on their defined interaction with zinc in the MHC class II:SPE C cocrystal structure (Fig. 1B) (6). For the present study, mutant SPE C molecules will be described as in this example; H167A, meaning histidine at position 167 in the mature protein changed to alanine. The wild-type SPE C gene was subjected to site-directed mutagenesis (7) to generate three single-site mutants (H167A, H201A, and D203A), four double-site mutants (H167A/H201A, H167A/D203A, H201A/D203A, and N38D/D203A), and a triple mutant (H167A/H201A/D203A). The proteins were expressed and purified from Escherichia coli BL21(DE3) (Novagen, Madison, Wis.) as in a previous study (7).
One of the defining characteristics of PTSAgs is their ability to cause fever in rabbits (8). Fever has been defined as an average increase in body temperature of greater than 0.5°C over a 4-h period (1). The single-site SPE C mutants N38D, H35A, H167A, H201A, and D203A and a known nonpyrogenic mutant (Y15A/H35A/N38D) (7) were assayed for pyrogenicity (Table 1) (all animals were used in compliance with federal and institutional animal care and use committee guidelines). Rabbits that received wild-type SPE C showed an average increase of 1.67°C over 4 h. The H167A and H35A mutants gave average increases of 0.9 and 0.73°C, respectively, and thus retained significant pyrogenic activity. The other single-site mutants and the control triple mutant were nonpyrogenic.
TABLE 1.
Biological activities of SPE C mutants
| Protein tested | Pyrogenicitya (Δ4h°C) | No. of rabbits surviving/total no. of animals
|
Half-maximum superantigen activity (μg/well)d | |
|---|---|---|---|---|
| Endotoxin enhancementb | Miniosmotic pump lethalityc | |||
| Wild type | Pyrogenic (1.67) | 0/6 | 0/6 | 10−8 |
| H167A | Pyrogenic (0.90) | 1/3 | 3/3 | 10−4 |
| H201A | Nonpyrogenic (0.30) | 3/3 | 3/3 | 10−2 |
| D203A | Nonpyrogenic (0.37) | 3/3 | 3/3 | 10−2 |
| H35A | Pyrogenic (0.73) | 0/3 | NDe | 10−5 |
| N38D | Nonpyrogenic (−0.30) | 3/3 | ND | 10−3 |
| Y15A | ND | ND | ND | >1 |
| N38D/D203A | ND | ND | ND | 10−2 |
| H167A/H201A/D203A | ND | ND | ND | >1 |
| Y15A/H167A/H201A/D203A | ND | ND | ND | >1 |
| Y15A/H35A/N38D | Nonpyrogenic (0.27) | 3/3 | ND | >1 |
Δ4h°C, average change in rabbit temperature (in degrees centigrade) at 4 h after intravenous injection.
Five micrograms of protein/kg/rabbit was given intravenously to each rabbit at time 0 followed by 10 μg of endotoxin/kg at 4 h. Animals were monitored for lethality for 2 days.
Five hundred micrograms of protein/kg/rabbit was administered via subcutaneous minosmotic pumps. Mortality was monitored for 15 days.
Rabbit splenocytes were incubated with protein in 96-well plates. After 3 days of incubation, 3H-labeled thymidine was added, and the plates were incubated an additional day. DNA was harvested, and radioactivity was measured. Half-maximum activity is reported as the quanity of protein required to give half the cpms of the most-active concentration of the same protein.
ND, not determined.
PTSAgs also have the ability to enhance the lethal effects of endotoxin. The 50% lethal dose (LD50) of lipopolysaccharide (LPS) alone is ca. 500 μg/kg in rabbits (5). Pretreatment with PTSAgs can reduce the LD50 of LPS by as much as 50,000-fold (13). Rabbits that received wild-type SPE C (5 μg/kg) showed enhanced susceptibility to the lethal effects of endotoxin (10 μg/kg) (Table 1). At these same SPE C and endotoxin doses, the H35A mutant retained wild-type activity, the H167A mutant retained partial lethal activity, but the N38D, H201A, and D203A mutants were not lethal. The Y15A/H35A/N38D triple mutant, included as a negative control, displayed no lethal activity.
The miniosmotic pump model of TSS uses an implanted device that releases toxin subcutaneously over 7 to 8 days (10). All rabbits treated with wild-type SPE C succumbed in such tests (Table 1), whereas none of the mutant toxins evaluated were lethal at doses of 500 μg/rabbit.
The proliferation of rabbit splenocytes was used as an in vitro measure of PTSAg-induced superantigenicity (11). Table 1 shows the activity of all of the mutants; it is convenient to describe the activity of a protein in terms of the quantity required to reach half of its maximum activity. The SPE C H201A and D203A mutants reached the half maximum activity point at 0.01 μg/well. Wild-type SPE C, H35A, and H167A reach the half-maximum activity point at 10−8, 10−5, and 10−4 μg/well, respectively. In a second assay, the single-site mutant N38D from previous work was included. Its half-maximum activity point was at 10−3 μg/well. The Y15A mutation of SPE C is known to be a critical residue in TCR binding (14). This mutant was completely inactive in this assay.
Table 1 also shows the activity of four multiple site mutants constructed in this work. The triple mutant H167A/H201A/D203A reached half its maximal activity at >1 μg. The Y15A mutation was combined with the triple mutant to create the quadruple Y15A/H167A/H201A/D203A mutant. This mutant was also completely inactive. The N38D/D203A double mutant had half-maximal activity at 0.01 μg/well. Finally, the previously tested nontoxic mutant, Y15A/H35A/N38D, was inactive.
The results from the present study are conclusive in demonstrating that His-167, His-201, and Asp-203 in SPE C are critical for full biological activity, as predicted by the crystal structure determination. Mutations in these residues resulted in toxins that showed reduced activity when tested in two rabbit models for TSS, in assays for pyrogenicity, and in superantigenicity assays. However, mutations at positions 201 and 203 had a greater impact on activity than did the mutation at position 167. These residues comprise three parts of the zinc coordination site, as determined from the three-dimensional structure, giving rise to a high-affinity MHC II binding site.
The results also suggest that there may be a second, low-affinity MHC II binding site in this toxin. This view is based on the reduced activity demonstrated by the N38D mutant. This would be consistent with that proposed for staphylococcal enterotoxin A, which has been suggested to have both sites (2). Our studies suggest that, if this low-affinity site is present, the His-35 site is not individually critical, since mutations in that residue did not block activity. It is interesting that the three-dimensional structure of SPE C complexed with HLA-DRA*0101,DRB5*0101 was determined through use of the H35A mutant. It has previously been suggested that SPE C may form dimers to a limited extent, in part dependent on this residue, and that such dimerization may interfere with structure determination (6, 12).
Finally, the present study revealed a direct correlation between the half-maximum activity of toxins in the superantigenicity assay and lethality in the endotoxin enhancement model. Toxins reaching their half-maximum activity at <10−4 μg, when tested in superantigenicity assays, were not lethal in the endotoxin enhancement model. The H167A mutant had a half-maximum activity at 10−4 μg, and the protein was intermediate in lethality. Any toxin with a lower half-maximum activity superantigen activity was completely lethal. Therefore, these data suggest that SPE C effects on T cells and induction of lethality by the toxin are not separable.
Acknowledgments
This study was supported by U.S. Public Health Service research grant HL36611 from the National Heart, Lung, and Blood Institute.
We thank Timothy Leonard for help with the artwork.
Editor: D. L. Burns
REFERENCES
- 1.Anonymous. 2002. The United States Pharmacopeia. U.S. Pharmacopeial Convention, Rockville, Md..
- 2.Hudson, K. R., R. E. Tiedemann, R. G. Urban, S. C. Lowe, J. L. Strominger, and J. D. Fraser. 1995. Staphylococcal enterotoxin A has two cooperative binding sites on major histocompatibility complex class II. J. Exp. Med. 182:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim, J., R. G. Urban, J. L. Strominger, and D. C. Wiley. 1994. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science 266:1870-1874. [DOI] [PubMed] [Google Scholar]
- 4.Kotb, M. 1995. Bacterial pyrogenic exotoxins as superantigens. Clin. Microbiol. Rev. 8:411-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee, P. K., J. R. Deringer, B. N. Kreiswirth, R. P. Novick, and P. M. Schlievert. 1991. Fluid replacement protection of rabbits challenged subcutaneous with toxic shock syndrome toxins. Infect. Immun. 59:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, Y., H. Li, N. Dimasi, J. K. McCormick, R. Martin, P. Schuck, P. M. Schlievert, and R. A. Mariuzza. 2001. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity 14:93-104. [DOI] [PubMed] [Google Scholar]
- 7.McCormick, J. K., T. J. Tripp, S. B. Olmsted, Y. V. Matsuka, P. J. Gahr, D. H. Ohlendorf, and P. M. Schlievert. 2000. Development of streptococcal pyrogenic exotoxin C vaccine toxoids that are protective in the rabbit model of toxic shock syndrome. J. Immunol. 165:2306-2312. [DOI] [PubMed] [Google Scholar]
- 8.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 9.Papageorgiou, A. C., H. S. Tranter, and K. R. Acharya. 1998. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 Å resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J. Mol. Biol. 277:61-79. [DOI] [PubMed] [Google Scholar]
- 10.Parsonnet, J., Z. A. Gillis, A. G. Richter, and G. B. Pier. 1987. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect. Immun. 55:1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poindexter, N. J., and P. M. Schlievert. 1985. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J. Infect. Dis. 151:65-72. [DOI] [PubMed] [Google Scholar]
- 12.Roussel, A., B. F. Anderson, H. M. Baker, J. D. Fraser, and E. N. Baker. 1997. Crystal structure of the streptococcal superantigen SPE-C: dimerization and zinc binding suggest a novel mode of interaction with MHC class II molecules. Nat. Struct. Biol. 4:635-643. [DOI] [PubMed] [Google Scholar]
- 13.Schlievert, P. M. 1982. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect. Immun. 36:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundberg, E. J., H. Li, A. S. Llera, J. K. McCormick, J. Tormo, P. M. Schlievert, K. Karjalainen, and R. A. Mariuzza. 2002. Structures of two streptococcal superantigens bound to TCRβ chains reveal diversity in the architecture of T cell signaling complexes. Structure 10:687-699. [DOI] [PubMed] [Google Scholar]
- 15.Tomai, M. A., P. M. Schlievert, and M. Kotb. 1992. Distinct T-cell receptor V β gene usage by human T lymphocytes stimulated with the streptococcal pyrogenic exotoxins and pep M5 protein. Infect. Immun. 60:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]