Abstract
Brucella species are gram-negative, facultative intracellular bacteria that infect humans and animals. These organisms can survive and replicate within a membrane-bound compartment inside professional and nonprofessional phagocytic cells. Inhibition of phagosome-lysosome fusion has been proposed as a mechanism for intracellular survival in both cell types. However, the molecular mechanisms and the microbial factors involved are poorly understood. Smooth lipopolysaccharide (LPS) of Brucella has been reported to be an important virulence factor, although its precise role in pathogenesis is not yet clear. In this study, we show that the LPS O side chain is involved in inhibition of the early fusion between Brucella suis-containing phagosomes and lysosomes in murine macrophages. In contrast, the phagosomes containing rough mutants, which fail to express the O antigen, rapidly fuse with lysosomes. In addition, we show that rough mutants do not enter host cells by using lipid rafts, contrary to smooth strains. Thus, we propose that the LPS O chain might be a major factor that governs the early behavior of bacteria inside macrophages.
Brucella species are gram-negative, facultative intracellular bacteria that infect humans and animals. These organisms can survive and replicate within a membrane-bound compartment inside professional (5, 14, 17, 28) and nonprofessional (9, 25, 26) phagocytic cells. Usually, phagosomes mature gradually into phagolysosomes which are capable of degrading proteins by lysosomal enzymes. In the case of Brucella, inhibition of phagosome-lysosome fusion has been proposed as a mechanism of intracellular survival of bacteria in both types of cells (2, 12, 14, 17, 22, 25, 26). In nonprofessional phagocytic cells, it has been found that virulent Brucella abortus escapes lysosomal fusion by invading endoplasmic reticulum-like structures (9, 25, 26). In macrophages, this phenomenon is not observed (2), although it has been shown that phagosomal membrane modifications may be responsible for phagosome-lysosome fusion inhibition (20). Another more recent hypothesis has been proposed to explain fusion inhibition: Brucella spp. enter host cells via lipid rafts (21, 36). This route of entry may determine the intracellular fate of bacteria, as it involves an endocytic pathway which avoids fusion with lysosomes (21, 36) and suggests that fusion inhibition is restricted by the structure of the phagosomal membrane (20). However, the cellular receptor involved in bacterial uptake remains unknown. Similarly, a bacterial ligand has to be identified. As the route of entry and the phagosomal membrane appear essential in avoiding phagosome-lysosome fusion, the receptor could be a virulence factor present on the bacterial surface and therefore involved in the fate of the bacteria. One of these surface molecules that shares these properties is lipopolysaccharide (LPS), which is a major component of the outer membrane of gram-negative bacteria and has been reported to be an important virulence factor of Brucella spp. (11, 18), although its precise role in pathogenesis is not yet clear. LPS is composed of three domains: the lipid A, the core oligosaccharide, and the O antigen or O side chain. The presence of the O side chain in LPS determines the smooth phenotype, it is absent from LPS found in the rough phenotype. Generally, rough mutants are less virulent than the wild type (29). Also, rough mutants are generally more sensitive to lysis mediated by complement, which probably explains why most rough variants have an avirulent phenotype in animal models. Some rough mutants of Brucella spp. which fail to express the O antigen have recently been identified and genetically characterized: transposon-derived B. abortus rough mutants (one of them has a mutation in the gene for phosphomannomutase) (1); a Brucella melitensis mutant, designated B3B2, with a mutation in the perosamine synthetase gene (13); a B. abortus mutant with a mutation in the glycosyltranferase gene (19); and a B. abortus mutant with a mutation in the phosphoglucomutase gene (pgm) (34). All of these mutants are unable to survive in mice. To date, the replication of rough mutants inside macrophages is not definitively understood. There are some discrepancies in the analysis of the different studies published. For example, the phophomannomutase mutants described by Allen et al. (1) exhibit reduced intracellular survival at 12 h postinfection, the B3B2 mutant grows intracellularly at a rate similar to that of the parental strain (13), and the pgm mutant, recently described by Ugalde et al. (34), exhibits delayed intracellular replication in comparison to the wild type. Three rough mutants of Brucella suis have been also described as replicating poorly in cultured macrophages (10). However, in all of these studies, rough mutants were studied for their survival in mice or cells (1, 13, 34) or for their sensitivity to individual killing mechanisms such as sensitivity to complement-mediated lysis (1) or polymyxin B (1, 13, 34). They have never been studied for their intracellular fate, in contrary to virB mutants (6, 7) and bvrR and bvrS mutants (33).
In the present work, we analyzed interactions between different Brucella strain-containing phagosomes and lysosomes in murine macrophages and, especially, we compared smooth and rough strains to study the involvement of the O antigen in fusion inhibition. In addition, we studied the involvement of lipid rafts in the entry and short-term survival of rough mutants in comparison to smooth mutants to determine the function of the LPS O antigen. In smooth strains, the presence of the O side chain is essential for the early inhibition of phagosome-lysosome fusion, and contrary to smooth strains, rough mutants, which lack the O antigen, do not enter host cells via lipid rafts and rapidly undergo phagosome-lysosome fusion.
MATERIALS AND METHODS
Reagents.
N-Hydroxysuccinimidyl ester 5-(and-6)-carboxyfluorescein and Texas red-dextran (TRD) (molecular weight of 70,000) were purchased from Molecular Probes (Eugene, Oreg.). Filipin, β-methyl cyclodextrin, and cholera toxin B subunit were obtained from Sigma-Aldrich (St. Quentin Fallavier, France).
Cell culture.
J774.A1 cells (from a murine macrophage-like cell line) were grown in RPMI 1640 medium with glutamax I (Gibco/BRL) containing 10% heat-inactivated fetal calf serum (complete medium) at 37°C and 5% CO2. Cells were resuspended at a concentration of 2 × 105 cells/ml and cultured for 1 day in 24-well plates on glass coverslips for microscopy.
Bacterial strains, culture, and preparation.
Bacterial strains used throughout the experiments are summarized in Table 1. Brucella canis, B. suis manB, B. melitensis B3B2, and B. suis virB5 constitutively expressed green fluorescent protein (GFP) and were prepared as described previously for B. suis 1330 p/sog (16, 24). B. suis 18E10 and B. suis 20C2 were obtained by transposon mutagenesis. GFP was directly integrated on the genome, and the resulting fluorescent B. suis was the acceptor strain for transposon Tn5 (15). Heat-killed B. suis was labeled with fluorescein as described previously (27). Bacteria were grown in tryptic soy broth (Difco Laboratories) at 37°C overnight to stationary phase. Killed bacteria were obtained either by incubation at 65°C for 30 min or by treatment at 37°C for 1 h with gentamicin (1.2 mg/ml). Smooth strains (B. suis and B. suis virB5) were opsonized with polyclonal murine anti-Brucella antibodies for 30 min at 37°C.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristic(s) | Source or reference(s) |
|---|---|---|
| B. suis 1330a | Wild-type virulent strain | ATCC 23444 |
| B. suis p/soga | Wild-type expressing GFP on a plasmidb | 14, 22 |
| B. suis manBa | manB mutant (phosphomanomutase) (rough) | 9 |
| B. melitensis B3B2 | rfbE mutant (perosamine synthase) (rough) | 12 |
| B. suis 18E10a | wbdA mutant (manosyl transferase) (rough) | 15 |
| B. suis 20C2a | lpsA mutant (glycosyl transferase) (rough) | 15 |
| B. suis virB5a | virB5 mutant (type IV secretion) (avirulent) | 21, 15 |
| B. canis RM6/66 | Naturally rough strain | ATCC 23365 |
Strain is isogenic to ATCC 23444.
Other GFP-expressing bacteria were obtained by transferring the GFP-containing plasmid.
Lysosome labeling.
Lysosomes were labeled by fluid-phase pinocytosis with 10 μg of TRD/ml, pulsed for 16 h, and chased for 1.5 h before infection with bacteria.
Treatment of cells with drugs prior to infection.
To investigate the role of cholesterol- and glycosphingolipid-enriched microdomains, cells were incubated at 37°C for 30 min in complete medium containing cholesterol-binding (filipin) or -depleting (β-methyl cyclodextrin) molecules or with a ganglioside GM1-binding molecule (cholera toxin B subunit) prior to infection. After treatment, cells were washed once with phosphate-buffered saline (PBS). As a control, the viability of the cells following treatment was assessed with dextran blue staining. We also ensured that the viability of bacteria was not affected by drug treatment.
Infection of cells.
Cells were infected with Brucella at a multiplicity of infection of 100 bacteria per cell for 45 min at 37°C. After three washes with PBS, cells were reincubated in culture medium containing gentamicin at a concentration of 30 μg/ml for various times.
Phagosome-lysosome fusion measurement.
Measurements were taken following lysosomal labeling and infection. Cells were fixed for 20 min with 3% paraformaldehyde and washed in PBS. Coverslips were mounted in Mowiol medium and examined by confocal fluorescence microscopy with a Leica DM RB microscope. Fusion was evaluated by the colocalization of both markers, GFP or fluorescein and TRD. To determine the percentage of fusion, 100 to 200 bacteria were analyzed for each strain at each time point. Results were calculated from two independent experiments.
Phagocytosis measurement by fluorescence microscopy.
Following treatment with the relevant drug, infection with Brucella spp., and postinfection incubation for 1 h, cells were fixed and washed twice in PBS. Then coverslips were mounted in Mowiol medium and examined by classical fluorescence microscopy with an inverted Leica DM IRB microscope.
Intracellular viability assay of Brucella in macrophages.
Following infection with Brucella spp. and postinfection incubation for various times, cells were washed once with PBS and lysed in 0.2% Triton X-100. Bacterial counts (in CFU) were determined by plating appropriate dilutions on tryptic soy agar and incubating at 37°C for 3 days.
RESULTS
Association of B. suis- and latex bead-containing vacuoles with the fluid-phase marker TRD.
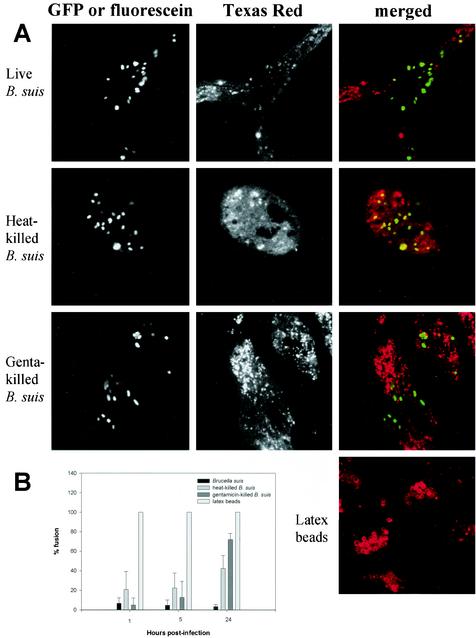
We evaluated interactions between B. suis-containing vacuoles and lysosomes inside the murine macrophage cell line J774 by confocal fluorescence microscopy. Lysosomes were first labeled by fluid-phase pinocytosis of TRD and then infected with bacteria which constitutively express a GFP or labeled with fluorescein. Cells were fixed at different time points following infection and examined. Fusion was evaluated by the colocalization of both markers. In some cases, we observed a TRD ring around the internalized particle, and in other cases, phagosomes were only partially associated with TRD. We considered fusion to have occurred in either case.
Association of TRD with phagosomes containing live, heat-killed, and gentamicin-killed B. suis was observed at 1 h postinfection and compared to that of latex bead-containing vacuoles (Fig. 1A). Then fusion was quantified at different time points following infection (Fig. 1B). Live B. suis-containing phagosomes never fused significantly with lysosomes. An insignificant level of colocalization was observed at all time points: 6.71% ± 5.63% fusion at 1 h, 4.65% ± 5.50% fusion at 5 h, and 3.48% ± 2.24% fusion at 24 h. In contrast, latex bead-containing vacuoles were labeled very rapidly (100% fusion at 1 h), we quantitatively observed a TRD ring around each bead. As dextran is not degradable, we still observed the same ring 24 h after internalization. Phagosomes containing heat-killed bacteria exhibited a more-complex pattern. Vacuoles were sparingly associated with TRD at 1 h postinfection (20.65% ± 18.63% fusion) and at 5 h postinfection (22.35% ± 15.34%), but at 24 h, fusion increased with a value of 42.36% ± 13.35%. Gentamicin-killed bacterium-containing vacuoles yielded similar behavior in fusion events: 5 ± 7.07 at 1 h, 12.75 ± 16.41 at 5 h, and 71.87 ± 6.25 at 24 h. Heat-killed and gentamicin-killed bacteria clearly showed a fusion delay compared to latex beads. We considered that some components of the bacterial membrane, particularly LPS structure, were not modified by gentamicin treatment nor by heating, and therefore, fusion delay may be correlated with its presence. To test this hypothesis, we performed the same kind of analysis on rough mutants.
FIG. 1.
Interaction of live or killed B. suis- and latex bead-containing phagosomes with lysosomes in murine macrophages. Lysosomes were labeled with TRD. Live and gentamicin (Genta)-killed B. suis expressed GFP; heat-killed B. suis were labeled with fluorescein. Bacterium and latex bead internalization was performed for 45 min. Cells were fixed at different time points after infection. (A) Confocal images of cells containing live or heat- or gentamicin-killed B. suis or latex beads obtained at 1 h postinfection. (B) Percentage of phagosome-lysosome fusion at different time points after infection. Fusion was evaluated by the colocalization of both markers, GFP or fluorescein and TRD. To determine the percentage of fusion, 100 to 200 bacteria were analyzed at each time point. Results were calculated from two independent experiments. Values are given as mean percentages of fusion ± standard deviations.
Association of rough Brucella strain-containing phagosomes with TRD.
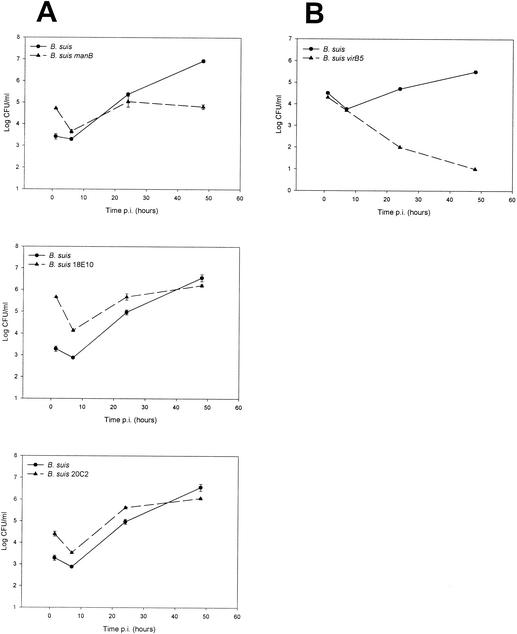
We decided to analyze phagosome-lysosome interactions with rough mutants. We used B. suis manB (10), B. melitensis B3B2 (13), B. suis 18E10, and B. suis 20C2 (15). We also included B. canis because it is a naturally occurring rough strain, although the exact nature of its mutation is still unknown. There are some discrepancies in the literature about interpretation of the capacity of the rough mutants to replicate in macrophagic cell lines. However, it is generally admitted that rough mutants develop poorly inside macrophages. For instance, it has been reported that B. canis is able to survive but not to multiply inside macrophages (5). B. melitensis B3B2 viability is similar to that of the parental strain in bovine macrophages (13). The phophomannomutase mutants described by Allen et al. (1) exhibit reduced intracellular survival at 12 h postinfection. Growths of the B. suis rough mutants used in our study (B. suis manB, B. suis 18E10, and B. suis 20C2) are shown in Fig. 2. In murine macrophages, we observed a significant uptake of rough organisms (1 or 2 log higher than that of wild-type strain B. suis). Then we observed a rapid decrease in viability (between 1 and 2 log) followed by a slow replication. Simple comparison of the number of living rough Brucella bacteria at the beginning and at the end of infection (48 h) led to the conclusion that the bacteria did not replicate. However, it was clear that after this drastic decrease, a slow increase was evidenced by the growth curves. This result was confirmed by microscopy experiments: while all the cells were infected by rough bacteria at 1 h postinfection (100%), some bacteria escaped to cell defense mechanisms and replicated in a small number of cells (2.4%), as observed at 48 h postinfection (data not shown).
FIG. 2.
Growth of B. suis (wild-type strain) and B. suis mutants in murine macrophages. (A) Rough mutants: B. suis manB (manB mutant), B. suis 18E10 (wbdA mutant), and B. suis 20C2 (lpsA mutant). (B) VirB mutant: B. suis virB5 (virB5 mutant). Cells were infected, and at the indicated times postinfection (p.i.), the number of intracellular bacteria was determined as described in Materials and Methods. The data points are the means ± standard deviations of two wells. These data sets are representative of at least two independent experiments with similar results.
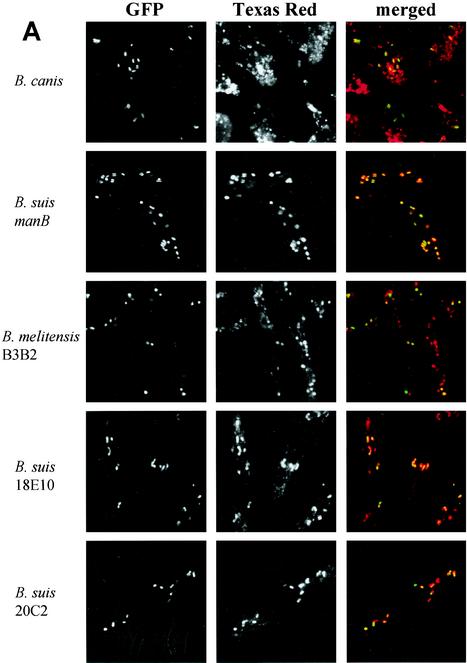
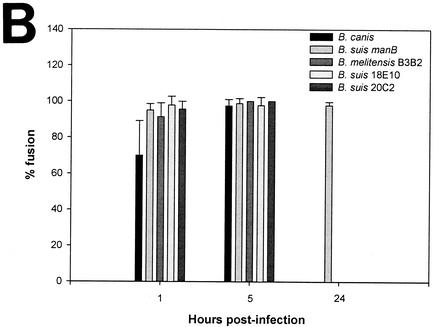
Phagosome-lysosome fusion was studied and quantified at different time points after infection as described for the smooth strains. B. canis-containing phagosomes fused rapidly with lysosomes: 69.95% ± 19.18% fusion at 1 h and 97.50% ± 3.57% at 5 h (Fig. 3). The 4 mutants led to an immediate and very significant fusion between phagosomes and lysosomes: (i) B. suis manB, 95.07% ± 3.56% fusion at 1 h, 98.76% ± 2.77% at 5 h, and 97.73% ± 2.00% at 24 h; (ii) B. melitensis B3B2, 91.33% ± 7.71% at 1 h and 100% ± 0% at 5 h; (iii) B. suis 18E10, 97.76% ± 5.00% at 1 h and 97.72% ± 4.55% at 5 h; (iv) B. suis 20C2, 95.62% ± 4.25% at 1 h and 100% ± 0% at 5 h. For these 4 mutants, all of the TRD rapidly colocalized around the bacteria in the infected cells as observed by the very clear TRD ring around the bacteria. In the absence of the LPS O antigen, the phagosome-lysosome fusion was immediate and complete. We conclude that the native LPS is essential for early avoidance of fusion. The delay observed in the fusion between heat-killed or gentamicin-killed B. suis with lysosomes (Fig. 1B) may be due to the presence of an intact O antigen on the bacterial membrane. On the other hand, despite the fusion, the B. suis manB and heat-killed or gentamicin-killed B. suis were resistant to degradation by the proteolytic enzymes and the antimicrobial peptides present in lysosomes. At 24 h postinfection, intact Brucella bacteria could still be observed, which was in sharp contrast to Escherichia coli, which was rapidly degraded in small bacterial fragments (data not shown). These observations were in agreement with the resistance of the outer membranes of Brucella spp. to bactericidal cationic peptides observed in vitro (11, 18). Furthermore, it should also be stated that the significant decrease observed with rough bacteria during the first hours postinfection was probably due to the rapid phagosome-lysosome fusion.
FIG. 3.
Interaction of Brucella rough strain-containing phagosomes with lysosomes in murine macrophages. Lysosomes were labeled with TRD. Bacteria expressed GFP. Cells were infected for 45 min and fixed at different time points after infection. (A) Confocal images of cells containing B. canis, B. suis manB (manB mutant), B. melitensis B3B2 (rfbE mutant), B. suis 18E10 (wbdA mutant), and B. suis 20C2 (lpsA mutant) obtained at 1 h postinfection. (B) Percentage of phagosome-lysosome fusion for each strain at different time points after infection. Fusion was evaluated by the colocalization of the both markers, GFP and TRD. To determine the percentage of fusion, 100 to 200 bacteria were analyzed for each strain at each time point. Values are given as mean percentages of fusion ± standard deviations.
Association of smooth avirulent Brucella mutant-containing phagosomes with TRD.
The rapid fusion between phagosomes containing rough mutants and lysosomes suggests an essential role for the O side chain in avoiding the lysosomal pathway. However, it is clearly established that multiplication of Brucella depends on many virulence genes coding for bacterial proteins (3). Some of them have been shown to be involved in Brucella trafficking inside the epithelial HeLa cells (6, 7, 33).
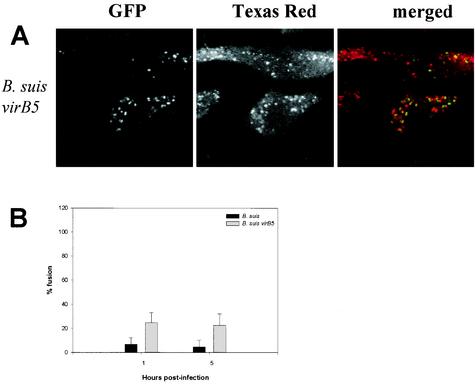
We chose to study the type IV secretion system, VirB, that has been described for B. suis (23). The proteins encoded by the VirB system are essential for survival and multiplication of Brucella in macrophages. We used a B. suis virB5 mutant which possesses a smooth LPS. Growth of this mutant is shown in Fig. 2B. The uptake and the decrease in viability during the first hours postinfection were quite similar for both the mutant and the wild-type strains, and then the viability of the mutant decreased rapidly. This would mean that the virB system was not involved in the early steps of infection. Phagosome-lysosome interactions were performed as before and quantified at different time points after infection. B. suis virB5 mutant-containing vacuoles were partly associated with TRD at 1 h postinfection (24.86% ± 8.12% fusion) and at 5 h postinfection (22.47% ± 9.58%) (Fig. 4). We observed a rapid fusion for some bacteria which was comparable to the one noted for heat-killed and gentamicin-killed bacteria. We concluded that the VirB system was not directly implicated in the early steps of the vacuole formation. The intracellular fate of three virB mutants of B. melitensis (virB2, virB4, and virB9) has been examined in HeLa cells by immunofluorescence (7). The three VirB proteins are not necessary for phagosome-lysosome fusion inhibition during the first hours postinfection. In agreement with our observations, these results support our hypothesis that the first determinant in fusion inhibition may be the LPS O antigen, which is intact in the Brucella virB5 mutant.
FIG. 4.
Interaction of smooth avirulent B. suis mutant-containing phagosomes with lysosomes in murine macrophages. Lysosomes were labeled with TRD, and bacteria expressed GFP. Cells were infected for 45 min and fixed at different time points after infection. (A) Confocal images of cells containing B. suis virB5 (virB5 mutant) obtained at 1 h postinfection. (B) Percentage of phagosome-lysosome fusion at different time points after infection. Fusion was evaluated by the colocalization of both markers, GFP and TRD. To determine the percentage of fusion, 100 to 200 bacteria were analyzed for each strain at each time point. Values are given as mean percentages of fusion ± standard deviations.
Role of cholesterol and the ganglioside GM1 in entry of B. suis manB in murine macrophages.
The results described above suggested that the LPS O side chain is an important determinant of the fusion between phagosomes and lysosomes, at least during the first hours postinfection. Two possible hypotheses can explain these results: either the O antigen is involved in the dynamics of entry of the pathogen or, alternatively, the O chain is used by Brucella to avoid fusion. Recent studies from our laboratory have revealed that cholesterol and the ganglioside GM1, two components of lipid rafts, may be implicated in the entry of the smooth strain, B. suis, into murine macrophages (21), and the results have been confirmed with B. abortus (36).
Hence, we investigated the role of lipid rafts in the entry of the rough B. suis manB (10). The role of cholesterol- and glycosphingolipid-enriched microdomains was studied by treating cells with cholesterol-binding (filipin) or -depleting (β-methyl cyclodextrin) molecules or the ganglioside GM1-binding molecule (cholera toxin B subunit). One hundred percent of the cells were infected in the absence as well as in the presence of drugs for all of the tested concentrations of filipin (2, 5, and 8 μg/ml), β-methyl cyclodextrin (2, 5, and 10 mM), and cholera toxin B subunit (10, 20, and 40 μg/ml). Approximately 200 to 300 cells were examined for each condition, with an average of 11.06 ± 2.13 (mean ± standard deviation) bacteria per cell, as observed by fluorescent microscopy. Phagocytosis of B. suis manB, in contrast to that of the smooth strain B. suis (21) was clearly not affected by drug treatment and thus did not involve lipid rafts.
Role of cholesterol and the ganglioside GM1 in short-term survival of B. suis manB in murine macrophages.
In a previous work, a strong inhibition of short-term survival (1 h) of the smooth B. suis strain was observed when cells were pretreated with cholesterol-binding or -depleting molecules or with a ganglioside GM1-binding molecule (21). Therefore, we speculated that bacteria must associate with lipid rafts to escape phagosome-lysosome fusion and survive inside the cell. As rough bacterium-containing phagosomes rapidly fused with lysosomes, we examined the effects of drugs on the short-term survival of rough bacteria inside macrophages compared to that of smooth bacteria. As previously described (21), pretreatment with the drugs filipin (8 μg/ml), β-methyl cyclodextrin (10 mM), and cholera toxin B subunit (40 μg/ml) strongly inhibited short-term intracellular survival of nonopsonized or opsonized smooth B. suis (Fig. 5). In contrast, short-term survival of the rough mutant B. suis manB was not affected after drug treatment (Fig. 5) and thus did not involve lipid rafts. Moreover, we observed above that B. suis manB-containing phagosomes strongly fused with lysosomes. These results support the hypothesis that lipid rafts may be implicated only in the entry of smooth Brucella into host cells, through an endocytic pathway that avoids fusion with lysosomes.
FIG. 5.
Effect of filipin, β-methyl cyclodextrin, and cholera toxin B subunit on short-term survival of nonopsonized and opsonized B. suis and B. suis manB (manB mutant) inside murine macrophages. Cells were pretreated with cholesterol-binding (filipin, 8 μg/ml) or -depleting (β-methyl cyclodextrin, 10 mM) molecules or the ganglioside GM1-binding molecule (cholera toxin B subunit, 40 μg/ml). Following treatment with the relevant drug, infection with Brucella spp., and postinfection incubation for 1 h, cells were lysed in Triton X-100 and intracellular viability at this short time (1 h) was measured as described in Materials and Methods. For each strain or condition, the control (100% value) was obtained in the absence of drug treatment. Experiments were performed in triplicate, and the values represent means ± standard deviations. The experiments were repeated at least four times.
DISCUSSION
Our results have clearly shown that the LPS O chain, which has long been recognized as a major virulence factor, is involved in the avoidance of phagosome-lysosome fusion, at least during the first few hours after phagocytosis. Phagosomes containing live B. suis with a smooth LPS never fuse with lysosomes. Smooth strains of Brucella unable to replicate (i.e., killed B. suis or the avirulent mutant B. suis virB5) exhibit delayed phagosome-lysosome fusion. Phagosomes containing bacteria with rough LPS fuse very rapidly with lysosomes (B. canis, B. suis manB, B. melitensis B3B2, B. suis 18E10, and B. suis 20C2).
It was shown previously (21) that lipid rafts may provide a portal for entry of the nonopsonized smooth strains of B. suis into murine macrophages and that they are also implicated in the short-term survival of nonopsonized and opsonized B. suis (21). These observations were recently confirmed for B. abortus (36). In the present work, we observed that entry and short-term survival of the rough strain in murine macrophages did not involve lipid rafts and that rough strains rapidly fused with lysosomes, whereas smooth strains did not. This suggests that the LPS O antigen might govern entry via the lipid rafts and determine the genesis of phagosomes. We might suppose that the LPS O chain may be involved in an interaction between B. suis and the lipid rafts of the cell membrane, either directly or, more likely, by an unknown receptor. For example, bacterial LPS binds to CD14 in low-density domains of the monocyte-macrophage plasma membrane, a characteristic of lipid rafts (35). After vacuole formation, the interactions between B. suis and the lipid rafts in the phagosomal membrane remained strong enough to impair phagosome-lysosome fusion. Therefore, these results support the hypothesis that lipid rafts may be implicated in the entry of some microorganisms into host cells, resulting in an endocytic pathway which avoids rapid fusion with lysosomes (30, 31).
Taken together, these observations suggest that bacterial entry by way of lipid rafts is essential for the maturation of the nascent vacuole. It is known that following uptake, B. suis-containing phagosomes rapidly become acidic (27). It has been shown that subunits of the proton pump V-ATPase concentrate inside the raft structure (8). Thus, it is possible that the association with lipid rafts is responsible for the rapid decrease of pH. This early acidification is necessary for survival and replication of the bacteria within the macrophage (27) and particularly for the induction of virulence genes, such as virB, in the phagosome at 3 to 4 h postinfection (4). Thus, the type IV secretion system VirB (23) was not directly involved in the early steps of vacuole formation but may be implicated in later steps of maturation by an unknown mechanism. These results did not appear consistent with the results of Watarai et al. (36) with B. abortus, in which an obvious effect of virB mutations was immediately observed during phagocytosis. But the virB genes of B. abortus are expressed at the beginning of the stationary phase, outside the cell (32), and so are present at the moment of infection. In contrast, in B. suis, VirB is not present on the surface and is expressed only after acidification of the phagosome (4). This can explain the significant difference observed in the uptake of wild-type B. abortus and the B. abortus VirB mutant.
We therefore suggest that the LPS O antigen of B. suis is involved in virulence by impairing or delaying phagosome-lysosome fusion and thus allows expression of genuine virulence genes involved in building a definitive replication niche. However, the presence of the LPS O antigen was not sufficient to explain virulence since some strains, such as Brucella ovis and B. canis, are rough and virulent, at least in their natural hosts. In addition, some genetically defined rough mutants, such as B. melitensis B3B2 (13), B. abortus pgm (34), B. suis manB (10), and B. suis rough mutants in this study, exhibit late and slow intracellular replication. Analysis of these results in the literature are only apparently contradictory. These discrepancies are mainly due to the definition of multiplication inside the macrophage. For example, intracellular survival of the phophomannomutase mutants described by Allen et al. (1), studied at 12 h postinfection was reduced relative to that of the parental smooth strain. Analysis of the results shows that in the first hours following phagocytosis, the number of viable rough bacteria is far less than immediately after internalization (see reference 5 for B. canis, reference 13 for the B. melitensis rough mutant, and this paper for B. suis rough mutants). It seems that after 24 h some bacteria have had the opportunity to escape killing and, due to activation of virulence genes, are able to multiply inside the cell in their definitive niche. In animals, rough mutants are always attenuated (see reference 1 for a well-documented example).
In conclusion, this study clearly shows that the LPS O side chain is involved in the route of B. suis uptake into murine macrophages by targeting lipid rafts and results in nonfusion of phagosomes and lysosomes during the first hours after infection. Thus, the LPS O antigen may be a major factor that governs the early behavior of bacteria inside macrophages and a major virulence determinant directly involved in the entry and fate of B. suis in the macrophage host cell.
Acknowledgments
We thank S. Kohler for critical reading of the manuscript, J. J. Letesson for B. melitensis B3B2, V. Foulongne for B. suis manB, S. Köhler for B. suis 18E10 and 20C2, and D. O'Callaghan for B. suis virB5.
A.N. was supported by a fellowship from the French government and by a grant from the Fondation pour La Recherche Médicale. This work was supported by grant QLK2-CT-1999-00014 from the European Union.
Editor: D. L. Burns
REFERENCES
- 1.Allen, C. A., L. G. Adams, and T. A. Ficht. 1998. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenas, G. N., A. S. Staskevich, A. Aballay, and L. S. Mayorga. 2000. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect. Immun. 68:4255-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boschiroli, M. L., V. Foulongne, and D. O'Callaghan. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4:58-64. [DOI] [PubMed] [Google Scholar]
- 4.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 99:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron, E., J. P. Liautard, and S. Kohler. 1994. Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J. Leukoc. Biol. 56:174-181. [DOI] [PubMed] [Google Scholar]
- 6.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3:159-168. [DOI] [PubMed] [Google Scholar]
- 7.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 8.Dermine, J. F., S. Duclos, J. Garin, F. St-Louis, S. Rea, R. G. Parton, and M. Desjardins. 2001. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 276:18507-18512. [DOI] [PubMed] [Google Scholar]
- 9.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58:2320-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulongne, V., G. Bourg, C. Cazevieille, S. Michaux-Charachon, and D. O'Callaghan. 2000. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect. Immun. 68:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freer, E., E. Moreno, I. Moriyon, J. Pizarro-Cerda, A. Weintraub, and J. P. Gorvel. 1996. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J. Bacteriol. 178:5867-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frenchick, P. J., R. J. Markham, and A. H. Cochrane. 1985. Inhibition of phagosome-lysosome fusion in macrophages by soluble extracts of virulent Brucella abortus. Am. J. Vet. Res. 46:332-335. [PubMed] [Google Scholar]
- 13.Godfroid, F., B. Taminiau, I. Danese, P. Denoel, A. Tibor, V. Weynants, A. Cloeckaert, J. Godfroid, and J. J. Letesson. 1998. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect. Immun. 66:5485-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmon, B. G., L. G. Adams, and M. Frey. 1988. Survival of rough and smooth strains of Brucella abortus in bovine mammary gland macrophages. Am. J. Vet. Res. 49:1092-1097. [PubMed] [Google Scholar]
- 15.Kohler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J.-P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler, S., S. Ouahrani-Bettache, M. Layssac, J. Teyssier, and J. P. Liautard. 1999. Constitutive and inducible expression of green fluorescent protein in Brucella suis. Infect. Immun. 67:6695-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liautard, J. P., A. Gross, J. Dornand, and S. Kohler. 1996. Interactions between professional phagocytes and Brucella spp. Microbiologia 12:197-206. [PubMed] [Google Scholar]
- 18.Martinez de Tejada, G., J. Pizarro-Cerda, E. Moreno, and I. Moriyon. 1995. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect. Immun. 63:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McQuiston, J. R., R. Vemulapalli, T. J. Inzana, G. G. Schurig, N. Sriranganathan, D. Fritzinger, T. L. Hadfield, R. A. Warren, L. E. Lindler, N. Snellings, D. Hoover, S. M. Halling, and S. M. Boyle. 1999. Genetic characterization of a Tn5-disrupted glycosyltransferase gene homolog in Brucella abortus and its effect on lipopolysaccharide composition and virulence. Infect. Immun. 67:3830-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naroeni, A., N. Jouy, S. Ouahrani-Bettache, J. P. Liautard, and F. Porte. 2001. Brucella suis-impaired specific recognition of phagosomes by lysosomes due to phagosomal membrane modifications. Infect. Immun. 69:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naroeni, A., and F. Porte. 2002. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 70:1640-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberti, J., R. Caravano, and J. Roux. 1981. Attempts of quantitative determination of phagosome-lysosome fusion during infection of mouse macrophages by Brucella suis. Ann. Inst. Pasteur Immunol. 132D:201-206. [Google Scholar]
- 23.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 24.Ouahrani-Bettache, S., F. Porte, J. Teyssier, J. P. Liautard, and S. Kohler. 1999. pBBR1-GFP: a broad-host-range vector for prokaryotic promoter studies. BioTechniques 26:620-622. [DOI] [PubMed] [Google Scholar]
- 25.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizarro-Cerda, J., E. Moreno, V. Sanguedolce, J. L. Mege, and J. P. Gorvel. 1998. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 66:2387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porte, F., J. P. Liautard, and S. Kohler. 1999. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price, R. E., J. W. Templeton, R. Smith III, and L. G. Adams. 1990. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect. Immun. 58:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roantree, R. J. 1971. The relationship of lipopolysaccharide to bacterial virulence, p. 1-37. In S. K. G. Weinbaum and S. J. Ajl (ed.), Microbial toxins: a comprehensive treatise, vol. 5. Academic Press, New York, N.Y.
- 30.Shin, J., and S. N. Abraham. 2001. Caveolae as portals of entry for microbes. Microbes Infect. 3:755-761. [DOI] [PubMed] [Google Scholar]
- 31.Shin, J. S., and S. N. Abraham. 2001. Co-option of endocytic functions of cellular caveolae by pathogens. Immunology 102:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sola-Landa, A., J. Pizarro-Cerda, M. J. Grillo, E. Moreno, I. Moriyon, J. M. Blasco, J. P. Gorvel, and I. Lopez-Goni. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29:125-138. [DOI] [PubMed] [Google Scholar]
- 34.Ugalde, J. E., C. Czibener, M. F. Feldman, and R. A. Ugalde. 2000. Identification and characterization of the Brucella abortus phosphoglucomutase gene: role of lipopolysaccharide in virulence and intracellular multiplication. Infect. Immun. 68:5716-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, P., R. Kitchens, and R. Munford. 1995. Bacterial lipopolysaccharide binds to CD14 in low-density domains of the monocyte-macrophage plasma membrane. J. Inflamm. 47:126-137. [PubMed] [Google Scholar]
- 36.Watarai, M., S. Makino, Y. Fujii, T. Okamoto, and T. Shirahata. 2002. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell. Microbiol. 4:341-355. [DOI] [PubMed] [Google Scholar]