Abstract
Anthrax, a disease usually associated with herbivores, is caused by the bacterium Bacillus anthracis. The current vaccine licensed for human use requires a six-dose primary series and yearly boosters and causes reactogenicity in up to 30% of vaccine recipients. A minimally reactogenic vaccine requiring fewer inoculations is warranted. Venezuelan equine encephalitis (VEE) virus has been configured for use as a vaccine vector for a wide variety of immunogens. The VEE vaccine vector is composed of a self-replicating RNA (replicon) containing all of the VEE virus nonstructural genes and a multiple-cloning site in place of the VEE structural genes. Four different anthrax vaccines were constructed by cloning the protective antigen (PA) gene from B. anthracis into the VEE vaccine vector. The anthrax vaccines were produced by assembling the vectors into propagation-deficient VEE replicon particles in vitro. A/J mice inoculated subcutaneously with three doses of the mature 83-kDa PA vaccine were completely protected from challenge with the Sterne strain of B. anthracis. Similar results were obtained with vaccines composed of the PA gene fused to either the B. anthracis secretory sequence or to a tissue plasminogen activator secretory sequence in three additional mouse strains. Mice were unprotected from challenge after inoculation with the carboxy-terminal 63-kDa PA vaccine. These results suggest that these VEE-vectored vaccines may be suitable as candidate vaccines against anthrax.
Yearly outbreaks of anthrax in herbivores occur worldwide, with human infection resulting from handling contaminated meat and animal products (4). Anthrax in animals is hyperendemic in areas such as Iran, Turkey, Iraq, Pakistan, and sub-Saharan Africa, although the organism can be found in most other areas, including the United States. Inhalation, gastrointestinal, and cutaneous anthrax can result from inhaling spores during the processing of animal products, ingesting spores in contaminated meat, or by exposing an open wound to spores, respectively. Untreated inhalation or gastrointestinal anthrax has a case fatality rate of essentially 100% while untreated cutaneous anthrax has a case fatality rate of up to 25%. Early and aggressive antibiotic treatment can prevent disease-associated morbidity and mortality. The current human vaccine used in the United States, anthrax vaccine absorbed (AVA), consists of an aluminum hydroxide-precipitated Bacillus anthracis cell-free filtrate and was licensed in 1970 by the Food and Drug Administration. The vaccine requires a primary series of three inoculations (given at 0, 14, and 28 days) followed by three booster inoculations (given at 6, 12, and 18 months) and yearly boosters. A less reactogenic vaccine requiring fewer inoculations and boosters would be more beneficial and easier to administer to at-risk personnel.
Venezuelan equine encephalitis (VEE) virus, a member of the Alphavirus genus and Togaviridae family, has been developed as a vaccine vector for the expression of vaccine-related genes (11). The system is composed of a self-replicating RNA expression vector (replicon) containing all of the VEE virus nonstructural genes and a vaccine gene in place of the VEE structural genes. Cotransfection (by electroporation) of cells in vitro with a recombinant VEE replicon and two helper RNA molecules, the latter encoding all of the VEE structural proteins, results in the production of propagation-deficient VEE replicon particles (VRPs). When administered to an animal, the VRPs infect host cells and lead to the production of immunogens that stimulate an immune response. As the VRPs lack any structural genes, the infected cells do not produce progeny viral particles. Previous studies demonstrated the ability of the VRPs to elicit potent immune responses and protective immunity against bacterial toxins and viruses in mice, guinea pigs, and nonhuman primates (2, 7, 10). Previous research showed that purified protective antigen (PA) produced by B. anthracis can protect animals from a B. anthracis challenge (3, 12, 14). In this study, we evaluated the VEE replicon expressing the PA gene from B. anthracis for immunogenicity and protective efficacy in mice.
MATERIALS AND METHODS
Replicons.
Construction, safety data, and discussions of possible recombination events with the VEE replicon, capsid 3014 (C-) helper, and glycoprotein 3014 (GP-) helper RNA, which contains attenuating mutations, were previously published (11). The Lassa virus nucleocapsid replicon (Lassa N-replicon) (11) or the mSEB (mutagenized staphylococcal enterotoxin B) replicon (6) was constructed as previously described and used as a negative control replicon. The tissue plasminogen activator (TPA)-PA replicon contained the 83-kDa full-length PA gene fused with the TPA secretory signal sequence (the TPA-PA gene was a gift from Connie Schmaljohn, U.S. Army Medical Research Institute for Infectious Diseases, Fort Detrick, Md.). The TPA-PA gene was PCR cloned by using ClaI restriction enzyme recognition sequence gene-specific primers. The b-PA replicon contained the 83-kDA PA gene fused to the B. anthracis secretory signal sequence. The b-PA gene was cloned into the VEE replicon plasmid as a BamHI fragment by using a shuttle vector. The MAT-PA replicon contained only the mature 83-kDa PA gene (no secretory signal sequence), whereas the PA63 replicon contained the 63-kDa carboxy-terminal portion of the PA protein. Both genes were PCR cloned into the replicon by using ClaI restriction enzyme recognition sequence gene-specific primers.
Preparation and characterization of VRP.
The replicons were assembled into VRPs as previously described (7, 11). Expressed proteins were analyzed by Western blotting as previously described (7) with modifications. Cell lysates from cells infected with the different PA VRPs were prepared 13 and 19 h postinfection. Proteins were separated on a NuPAGE 4 to 12% Bis-Tris gradient gel with NuPAGE morpholinepropanesulfonic acid sodium dodecyl sulfate (SDS) running buffer (Invitrogen, Inc., Carlsbad, Calif.), and PA protein was detected by using rabbit anti-PA antisera (a gift from Stephen Little, U.S. Army Medical Research Institute for Infectious Diseases) or by using antisera prepared from rabbits hyperimmunized with AVA, horseradish peroxidase-conjugated goat anti-rabbit antibody, and 3,3′,5,5′-tetramethylbenzidine peroxidase membrane substrate according to the manufacturer's instructions (Kirkegaard and Perry Laboratories [KPL], Inc., Gaithersburg, Md.). Deglycosylation of the TPA-PA replicon-expressed protein (19 h postinfection) was accomplished with the peptide N-glycosidase F (PNGase F) according to the manufacturer's instructions (New England Biolabs, Inc., Beverly, Mass.) and analyzed by Western blotting. Titration and immunofluorescence analysis of whole cells infected with the TPA-PA or MAT-PA replicons with rabbit anti-PA or anti-AVA antisera and fluorescein isothiocyanate-conjugated goat anti-rabbit antibody (KPL, Inc.) was as previously described (7). Cell nuclei were stained with 1 μg of 4′,6-diamidino-2-phenylindole (DAPI)/ml in VectaShield mounting medium (Vector Labs, Inc., Burlingame, Calif.). The VRP titers are expressed in focus-forming units (FFU) where 1 FFU is equivalent to 1 infectious unit (iu). VRP preparations were monitored for the generation of replication-competent VEE virus by using a standard plaque assay; no PFU were found in any VRP preparations.
Capture enzyme-linked immunosorbent assay (ELISA) of secreted PA.
The amount of PA protein secreted into the cell culture supernatant by cells infected with different VRPs was determined at 13 and 19 h postinfection. Microtiter plates (Thermal Lab Systems, Inc., Chantilly, Va.) were coated with a mixture of three different monoclonal antibodies (1-μg/ml concentrations [each] of antibodies 3F3, 6E11, and 5G3; gifts from Stephen Little) in 100 μl of phosphate-buffered saline (PBS) and allowed to adsorb overnight at 4°C. After washing the plates five times with wash buffer (PBS containing 0.1% Tween 20), we added 100 μl (per well) of cell culture supernatant or standard PA protein diluted in complete medium and incubated the plates at 37°C for 1 h. After washing, 100 μl of rabbit anti-PA antisera in blocking buffer (diluted 1:1,000; PBS, 0.1% Tween 20, and 5% dried nonfat milk) was added to the plates and incubated for an additional 1 h at 37°C. After washing, 100 μl of horseradish peroxidase-conjugated goat anti-rabbit secondary antibody in blocking buffer (diluted 1:1,000; KPL, Inc.) was added to the plates and incubated for an additional 1 h at 37°C. After washing, bound antibody was detected colorimetrically by using 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulfonate) (ABTS) as a substrate (KPL, Inc.). The amount of secreted PA was determined for each sample from the standard curve.
Vaccination and challenge of mice.
Mice were inoculated subcutaneously (s.c.) with 105, 106, or 107 iu of VRPs (in 200 μl of PBS), two, three, or four times at 28- to 35-day intervals. Positive-control mice were inoculated s.c. with 0.1 to 0.2 ml of AVA, and negative-control mice were inoculated s.c. with 107 iu of the Lassa N VRP or mSEB VRP. Sera for ELISA were obtained 1 to 2 days before each inoculation and 2 to 5 days before challenge. The mice were challenged s.c., 28 to 35 days after the last inoculation, with heat-shocked spores of the Sterne strain of B. anthracis. The Sterne strain is a capsule-negative (pXO2−), toxin-positive (pXO1+) strain.
Serum ELISA.
The quantity of antibody present in the serum of vaccinated animals was measured by ELISA as previously described (7). Microtiter plates were coated with Escherichiacoli-expressed PA protein (1 μg/ml) in PBS. Titers are defined as the reciprocal of the last dilution with an A405 of ≥0.1 after correction for background. Titers below 2.00 log10 and above 5.61 log10 were estimated. Sera from individual animals were assayed in duplicate and used to calculate a geometric mean titer for the group.
Statistical analysis.
The Fisher exact test was used to determine statistical differences in survival between groups that received the different PA VRP vaccines and the negative-control groups.
Research was conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
RESULTS
Packaging and expression of the PA replicons.
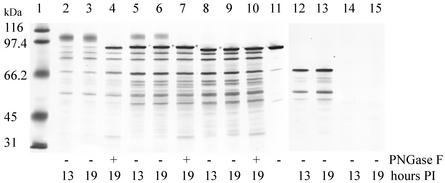
The PA replicons were assembled into VRPs by using the double-helper system originally developed by Pushko et al. (11). The titers of VRPs obtained after purification ranged from 9.6 × 107 iu/ml to 7.1 × 108 iu/ml, depending on the replicon used. No replication-competent virus was detected in the medium from cotransfected BHK cell cultures. A Western blot of cell lysates prepared from BHK cells infected with the different PA VRPs is shown in Fig. 1. The Western blot was heavily stained to better visualize the weakly staining glycosylated expression products, which also overemphasized the degradation products. The majority of the degradation products were created from harvesting the cells and not from intracellular degradation (data not shown), except for the PA63 band that may represent further intracellular processing of PA83 by cell-associated furin-like proteases (8). The amount of PA protein present in the lysates at 13 h was similar to the amount of protein present at 19 h. The TPA-PA replicon expression product migrated as a diffuse band with a greater molecular mass than the PA standard on SDS-polyacrylamide gel electrophoresis (PAGE). To investigate the nature of the shifted product, the presence of sugar residues on TPA-PA were determined by treating the product with different glycosidases. Treatment of cell lysate containing TPA-PA (19 h postinfection) with PNGase F (Fig. 1) or with endoglycosidase H (data not shown) produced a product that comigrated with the PA standard on SDS-PAGE. Western blot analysis of the b-PA replicon expression product showed that it migrated as a slightly larger protein than the PA standard because the b-PA protein contained a 29-amino-acid prokaryotic secretory signal, which was not cleaved off during expression in the eukaryotic BHK cells (Fig. 1). A minor band of a higher-molecular-mass protein, indicating glycosylated protein, was also present. Treating the b-PA expression product (19 h postinfection) with PNGase F produced a product that comigrated with the major band (Fig. 1). Expression of MAT-PA and PA63 replicons in BHK cells produced products that migrated on SDS-PAGE at the expected molecular masses and without any evidence of glycosylated products. Treating either of the expression products with PNGase F (Fig. 1 and data not shown) resulted in no change in the migration pattern of either product.
FIG. 1.
Western blot analysis of PNGase F-treated (+) and untreated (−) cell lysates from BHK cells infected with different PA VRPs. The blot was heavily stained to visualize the weakly staining glycosylated proteins. Lane 1, molecular mass markers; lane 2, TPA-PA VRP cell lysate at 13 h postinfection (PI); lane 3, TPA-PA VRP cell lysate at 19 h PI; lane 4, PNGase F-treated TPA-PA VRP cell lysate at 19 h PI; lane 5, b-PA VRP cell lysate at 13 h PI; lane 6, b-PA VRP cell lysate at 19 h PI; lane 7, PNGase F-treated b-PA VRP cell lysate at 19 h PI; lane 8, MAT-PA VRP cell lysate at 13 h PI; lane 9, MAT-PA VRP cell lysate at 19 h PI; lane 10, PNGase F-treated MAT-PA VRP cell lysate at 19 h PI; lane 11, PA standard; lane 12, PA63 VRP cell lysate at 13 h PI; lane 13, PA63 VRP cell lysate at 19 h PI; lane 14, uninfected cell lysate at 13 h PI; lane 15, uninfected cell lysate at 19 h PI.
The amount of PA protein secreted by BHK cells infected with different PA VRPs is shown in Table 1. Because TPA-PA contained a eukaryotic secretion signal, 6- and 35-fold-more PA protein was detected in the cell culture supernatant 19 h postinfection than of b-PA, which contained a bacterial secretory signal, and MAT-PA, which contained no secretory signal, respectively.
TABLE 1.
Capture ELISA of PA from BHK cells infected with VRPs
| VRP treatment | Amt of secreted PA protein (ng/ml)a present in the cell culture supernatant at time (h) postinfection
|
|
|---|---|---|
| 13 | 19 | |
| TPA-PA | 61 ± 9 | 106 ± 9 |
| b-PA | 9 ± 1 | 19 ± 3 |
| MAT-PA | 3 ± 1 | 4 ± 0 |
| PA63 | 2 ± 0 | 3 ± 0 |
| None | 0 ± 0 | 0 ± 0 |
Values are means ± standard deviations (three measurements per supernatant).
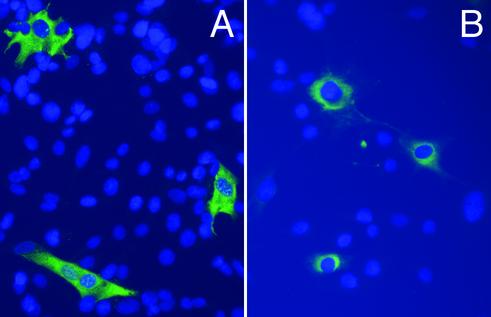
Immunofluorescence analysis of BHK cells infected with either TPA-PA VRPs or MAT-PA VRPs showed perinuclear staining (consistent with secreted proteins) or diffuse cytoplasmic staining (consistent with nonsecreted proteins), respectively (Fig. 2). BHK cells infected with either b-PA or PA63 VRPs showed diffuse cytoplasmic staining (data not shown) similar to stained MAT-PA VRP-infected BHK cells.
FIG. 2.
Indirect immunofluorescence of BHK cells infected with MAT-PA VRP (A) or TPA-PA VRP (B). PA antigen-positive cells appear green and DAPI-stained cell nuclei appear blue.
Dose effect of b-PA VRPs on protection against B. anthracis (Sterne) challenge.
Prechallenge anti-PA antibody titers and survival of A/J mice inoculated with 105, 106, or 107 iu of b-PA VRPs are shown in Table 2. Doses of 105 or 106 did not stimulate strong antibody responses and did not significantly protect the mice from challenge. A dose of 107 was necessary to protect 30, 70, or 90% of the A/J mice after two, three, or four inoculations, respectively, from a B. anthracis (Sterne strain) challenge. The geometric mean of the serum antibody titers against PA in A/J mice given two, three, or four doses of 107 iu of b-PA VRP were 3.57 log, 4.38 log, and 4.91 log, respectively, compared to less than 2.39 log for mice that were inoculated with the negative-control replicon. As expected, none of the mice that were inoculated with the negative-control replicon survived the challenge.
TABLE 2.
Antibody responses and survival of A/J mice inoculated with various doses of b-PA VRPs
| Inoculuma | Dose (iu or ml)b | No. of doses | Prechallenge GMT (log10) (SD)c | No. survived/ no. totald | Challenge dose (LD50)e | MTD, (days)f |
|---|---|---|---|---|---|---|
| b-PA VRP | 105 | 2 | 1.93 (0.19) | 0/10 | 10.0 | 2.6 |
| 105 | 3 | 1.69 (0.43) | 0/10 | 25.6 | 4.0 | |
| 105 | 4 | 2.00 (0.00) | 0/10 | 18.0 | 3.1 | |
| 106 | 2 | 2.50 (1.12) | 0/10 | 10.0 | 3.0 | |
| 106 | 3 | 1.91 (0.63) | 1/10g | 25.6 | 3.1 | |
| 106 | 4 | 2.16 (0.29) | 1/10g | 18.0 | 3.1 | |
| 107 | 2 | 3.57 (1.67) | 3/10h | 10.0 | 3.4 | |
| 107 | 3 | 4.38 (1.16) | 7/10i | 25.6 | 3.0 | |
| 107 | 4 | 4.91 (1.04) | 9/10i | 18.0 | 5.0 | |
| AVA | 0.2 | 2 | 6.21 (0.00) | 10/10i | 10.0 | |
| 0.2 | 3 | 6.21 (0.00) | 10/10i | 25.6 | ||
| 0.2 | 4 | 6.21 (0.00) | 10/10i | 18.0 | ||
| Negative control | 107 | 2 | 2.16 (0.29) | 0/10 | 10.0 | 2.9 |
| 107 | 3 | 1.55 (0.29) | 0/10 | 25.6 | 3.2 | |
| 107 | 4 | 2.39 (0.41) | 0/10 | 18.0 | 3.2 |
Inoculations were given s.c. 28 days apart. Negative-control groups were inoculated with an unrelated Lassa N VRP.
Infectious units were used to measure VRP and negative-control inocula, and milliliters were used to measure AVA inocula.
Serum obtained 2 days before challenge from individual animals was assayed in duplicate and used to calculate the log10 geometric mean titer (GMT) for each group. Titers greater than 5.61 log10 and less than 2 log10 were estimated.
Unless indicated, surviving mice displayed no disease symptoms.
Mice were challenged s.c. with 12 to 30 50% lethal doses (LD50) (1.3 × 104 to 3.3 × 104) of heat-shocked Sterne strain spores 28 days after the last inoculation.
MTD, mean time to death.
The surviving mouse displayed mild disease symptoms.
One surviving mouse displayed mild disease symptoms; the remaining two mice did not.
Statistically significant, P < 0.05, compared to the corresponding negative control.
Table 3 shows the primary and booster antibody responses for C57BL/6 mice inoculated with two, three, or four doses of 107 iu of b-PA VRP. Mice that received two or three inoculations of the b-PA VRP were marginally protected, whereas 90% of the C57BL/6 mice that received four inoculations were protected from an otherwise lethal challenge of the Sterne strain. The geometric mean antibody titers to PA stimulated in the C57BL/6 mice given b-PA VRP were 2.47 log, 3.43 log, 4.35 log, and 4.78 log measured 26 days after the first, second, third, and fourth inoculations, respectively. The antibody titers determined for mice inoculated with the negative-control replicon were less than 2.05 log, and none of the mice survived challenge. Positive-control A/J and C57BL/6 mice vaccinated with AVA were completely protected from a Sterne strain challenge.
TABLE 3.
Antibody responses and survival of C57BL/6 mice inoculated with b-PA VRP vaccine
| Inoculuma | Dose (iu or ml)b | No. of doses | Geometric mean titer (log10) (SD)c on day(s):
|
Day of challenge | No. survived/ no. total (%)d | Challenge dose (LD50)e | MTD (days)f | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 54-56 | 82-85 | 112 | |||||||
| b-PA VRP | 107 | 2 | 2.27 (0.43) | 3.24 (1.03) | 59 | 3/10 (30)g | 10.0 | 5.3 | ||
| 107 | 3 | 2.93 (0.75) | 4.11 (1.37) | 4.01 (1.53) | 88 | 8/11 (73)g,h | 25.6 | 4.3 | ||
| 107 | 4 | 2.47 (0.98) | 3.43 (1.84) | 4.35 (1.26) | 4.78 (1.20) | 115 | 8/9 (89)g,h | 18.0 | 4.0 | |
| AVA | 0.2 | 2 | 6.15 (0.19) | 6.21 (0.00) | 59 | 10/10 (100)h | 10.0 | |||
| 0.2 | 3 | 5.96 (0.42) | 6.21 (0.00) | 6.21 (0.00) | 88 | 10/10 (100)h | 25.6 | |||
| 0.2 | 4 | 6.09 (0.25) | 6.21 (0.00) | 6.21 (0.00) | 6.21 (0.00) | 115 | 10/10 (100)h | 18.0 | ||
| Negative control | 107 | 2 | 1.57 (0.60) | 1.90 (0.41) | 59 | 0/10 (0) | 10.0 | 4.8 | ||
| 107 | 3 | 1.07 (0.17) | 1.88 (0.42) | 1.50 (0.25) | 88 | 0/10 (0) | 25.6 | 5.3 | ||
| 107 | 4 | 1.61 (0.32) | 2.05 (0.20) | 1.62 (0.50) | 2.00 (0.00) | 115 | 0/10 (0) | 18.0 | 4.0 | |
Inoculations were given s.c. on days 0, 28, 56, and 84. Negative-control groups were inoculated with an unrelated Lassa N VRP.
Infectious units were used to measure VRP and negative-control inocula, and milliliters were used to measure AVA inocula.
Serum obtained before challenge from individual animals was assayed in duplicate and used to calculate the log10 geometric mean titer for each group. Titers greater than 5.61 log10 and less than 2 log10 were estimated.
Unless indicated, surviving mice displayed no disease symptoms.
Mice were challenged s.c. with 18 to 32 50% lethal doses (LD50) (1.8 × 107 to 3.2 × 107) of heat-shocked Sterne strain spores.
MTD, mean time to death.
One surviving mouse from each group displayed mild disease symptoms; the remaining mice in each group displayed no symptoms.
Statistically significant, P < 0.05, compared to the corresponding negative control.
Mouse strain-dependent antibody responses and protection after vaccination with different PA VRPs.
Table 4 shows the prechallenge geometric mean antibody titers and survival for four different mouse strains inoculated with 107 iu of either TPA-PA VRP, MAT-PA VRP, or PA63 VRP. Three inoculations of MAT-PA VRPs induced the highest prechallenge antibody titers in A/J and CBA/J mice, with titers of 5.43 log10 and 5.79 log10, respectively. The antibody titers from the Swiss mice inoculated with the same VRPs were 4.53 log10 while the lowest antibody titers were from BALB/c at 2.30 log10. TPA-PA VRPs stimulated strong antibody responses in A/J mice after two inoculations, with a titer of 5.24 log10 (6 of 10 were protected from challenge), and in CBA/J mice after three inoculations, with a titer of 5.97 log10 (9 of 10 were protected from challenge). Complete protection against challenge with the Sterne stain was observed for A/J mice (28-day inoculation schedule) and CBA/J mice (35-day inoculation schedule) inoculated with three doses of MAT-PA VRPs. Challenge of the Swiss and BALB/c mice was not possible due to their high resistance to the Sterne strain. CBA/J mice inoculated with PA63 VRPs produced low antibody titers of 2.72 log10 and failed to survive challenge. All positive control mice vaccinated with AVA produced high levels of anti-PA antibody in excess of 5.97 log10, and all of the A/J and CBA/J mice were protected (except one CBA/J mouse and eight mice in the PA63 VRP evaluation experiment) from a B. anthracis (Sterne strain) challenge. The reason why the eight animals died is not clear. The majority of the animals inoculated with negative-control VRPs failed to survive challenge, and those that did survive displayed signs and symptoms of disease.
TABLE 4.
Survival and antibody responses of different mouse strains inoculated with PA VRP vaccines
| Inoculuma | Dose (iu or ml)b | No. of doses | Mouse strain | Prechallenge GMT (log10) (SD)c | No. survived/ no. totald | Challenge dose (LD50)e | MTD (days)f |
|---|---|---|---|---|---|---|---|
| AVA | 0.1 | 2 | A/J | 6.82 (0.00) | 10/10l | 30 | |
| Negative control | 107 | 2 | A/J | 2.00 (0.00) | 0/10 | 30 | 3.6 |
| MAT-PA VRP | 107 | 2 | A/J | 4.57 (1.21) | 8/10l | 30 | 5.0 |
| TPA-PA VRP | 107 | 2 | A/J | 5.24 (0.30) | 6/10l | 30 | 4.5 |
| AVA | 0.1 | 3 | A/J | 6.51 (0.32) | 5/5l | 19.8 | |
| Negative control | 107 | 3 | A/J | 2.45 (0.51) | 0/5 | 19.8 | 3.6 |
| MAT-PA VRP | 107 | 3 | A/J | 5.43 (1.14) | 10/10l | 19.8 | |
| AVA | 0.1 | 3 | CBA/J | 6.82 (0.00) | 9/10h,l | 2.85 | 4.0 |
| Negative control | 107 | 3 | CBA/J | 2.06 (0.19) | 2/10i | 2.85 | 3.6 |
| MAT-PA VRP | 107 | 3 | CBA/J | 5.79 (0.73) | 8/10l | 2.85 | 5.0 |
| TPA-PA VRP | 107 | 3 | CBA/J | 5.97 (0.30) | 9/10j,l | 2.85 | 5.0 |
| AVA | 0.1 | 3g | CBA/J | 6.12 (0.29) | 10/10l | 10 | |
| Negative control | 107 | 3g | CBA/J | 1.70 (0.31) | 2/10k | 10 | 3.5 |
| MAT-PA VRP | 107 | 3g | CBA/J | 5.16 (0.73) | 10/10l | 10 | |
| AVA | 0.1 | 4 | CBA/J | 6.76 (0.30) | 2/10 | 10 | 4.0 |
| Negative control | 107 | 4 | CBA/J | 1.48 (0.47) | 0/10 | 10 | 3.2 |
| PA63 VRP | 107 | 4 | CBA/J | 2.72 (0.77) | 0/10 | 10 | 3.2 |
| AVA | 0.1 | 3 | BALB/c | 5.67 (0.33) | NC | ||
| Negative control | 107 | 3 | BALB/c | 2.22 (0.45) | NC | ||
| MAT-PA VRP | 107 | 3 | BALB/c | 2.45 (0.38) | NC | ||
| AVA | 0.1 | 3g | Swiss | 6.39 (0.34) | NC | ||
| Negative control | 107 | 3g | Swiss | 2.20 (0.41) | NC | ||
| MAT-PA VRP | 107 | 3g | Swiss | 4.53 (0.54) | NC |
Inoculations were given s.c. 28 days apart (except as noted below). Negative-control groups were inoculated with an unrelated Lassa N VRP or mSEB VRP.
Infectious units were used to measure VRP and negative-control inocula, and milliliters were used to measure AVA inocula.
Serum obtained 2 to 5 days before challenge (26 to 33 days after the last inoculation) from individual animals was assayed in duplicate and used to calculate the log10 geometric mean titer (GMT) for each group. Titers greater than 5.61 log10 and less than 2 log10 were estimated.
Unless indicated, surviving mice displayed no disease symptoms.
A/J and CBA/J mice were challenged s.c. with 12 to 30 50% lethal doses (LD50) (1.3 × 104 to 3.3 × 104) or 2.9 to 10 LD50 (6.0 × 107 to 2.1 × 108) of heat-shocked Sterne strain spores, respectively, 28 to 35 days after the last inoculation. NC, not challenged.
MTD, mean time to death.
Inoculations were given s.c. 35 days apart.
The titer of the mouse that failed to survived challenge was 6.82 log10.
Surviving mice displayed mild disease symptoms.
The titer of the mouse that failed to survived challenge was 5.61 log10.
Surviving mice displayed severe disease symptoms.
Statistically significant, P < 0.05, compared to the corresponding negative control.
DISCUSSION
The licensed human anthrax vaccine, AVA, contains mostly PA protein and is presumed to protect humans by eliciting an antibody response that can neutralize the PA component of the anthrax toxins produced by invading B. anthracis (9). After neutralization of the toxin, the immune system destroys the invading bacteria. In order to model the human disease, we chose to use mice and the Sterne strain of B. anthracis. The Sterne strain produces anthrax toxin (encoded on the pXO1 plasmid) but lacks a capsule (encoded on the pXO2 plasmid). Mice exposed to Sterne strain spores in excess of the lethal dose, which varies from 103 to 108 spores, depending on the susceptibility of the mouse strain, succumb to infection in a manner resembling that associated with infection with a fully virulent strain (15, 16). We hypothesized that by vaccinating mice with a replicon-expressing PA, thereby inducing neutralizing antibodies to the PA component of the toxins produced by a Sterne challenge inoculum, that the mice would be protected. Because vaccinating mice with PA VRPs protected them from a Sterne strain challenge, the use of mice and the Sterne strain looks promising as a model system for studying the immunogenicity and efficacy of replicon-based anthrax vaccines.
VEE replicons were constructed to express different configurations of the PA gene from B. anthracis and were evaluated for in vitro expression, in vivo immunogenicity, and protective efficacy. Initial experiments focused on vaccinating mice with VRPs containing the b-PA replicon. The b-PA replicon was constructed by using the entire B. anthracis PA gene, which contained the PA gene and its associated bacterial secretory signal sequence. Western blot analysis of lysates from BHK cells infected with b-PA VRP revealed low levels of glycosylated protein. Because the bacterial secretory signal only contained marginal amino acid sequence homology to eukaryotic secretion signals, the PA protein was minimally glycosylated in and secreted from the eukaryotic BHK cells. Doses of 105 and 106 iu of b-PA VRPs failed to protect the mice from challenge. Even doses of 107 iu of b-PA VRPs did not stimulate a strong primary antibody response but did stimulate better booster antibody responses in the mice. Considering that four inoculations were necessary to achieve 90% protection in A/J and C57BL/6 mice, other replicons were constructed with the aim of increasing immunogenicity with fewer doses.
Expression of vaccine-related genes from the replicon usually occurs in the cytoplasm of VRP-infected cells. To investigate the difference in immunogenicity between intracellularly retained proteins and secreted proteins and their ability to stimulate protective immune responses, two replicons were constructed. The first replicon contained the entire 83-kDa PA gene fused to the eukaryotic secretory signal sequence from TPA. The wild-type 83-kDa PA protein contains 11 possible N-linked glycosylation sites consisting of either asparagine-any amino acid-serine (N-X-S) or asparagine-any amino acid-threonine (N-X-T). The secreted PA protein would be expected to elicit a humoral immune response after uptake, processing, and display of the PA peptides by antigen-presenting cells in the vaccinated animal. The second replicon, MAT-PA, did not contain a signal sequence. The MAT-PA replicon would therefore express PA protein in the cytoplasm of a cell and would appear to the immune system as a virus-infected cell, thus stimulating a more-cellular immune response. Previous studies with the mSEB replicon provided evidence that even though the intracellularly expressed mSEB protein stimulated a cellular immune response, antibodies were produced in vaccinated animals that could neutralize a toxin challenge (6). By comparing the antibody responses and protection in animals after vaccination with either TPA-PAVRPs or MAT-PA VRPs, the best method for expressing PA protein from the replicon could be determined. That is, does secreted protein stimulate better humoral immunity or does glycosylation of the antigen interfere with immune recognition of the antigen.
PNGase F or endoglycosidase H treatment of lysate from TPA-PA VRP-infected BHK cells showed that the TPA-PA expression product contained all N-linked sugars and that those sugars were composed of a high proportion of mannose residues. Even though the TPA-PA expression product was found to be glycosylated, the N-linked sugars did not interfere with the immunogenicity of PA. Since both the TPA-PA and MAT-PA VRPs produced similar immune responses and protected similar numbers of animals, the mechanisms for stimulating the immunity must have been related. VRP and wild-type alphavirus infection of vertebrate cells eventually leads to cell death by apoptosis (13). Before cell death, the TPA-PA VRP-infected cells would have secreted PA protein into the extracellular space, resulting in stimulation of a humoral immune response, whereas the MAT-PA VRP-infected cells would not. During or after cell death, leakage of PA protein or PA protein contained in the cellular debris from TPA-PA or MAT-PA VRP-infected cells would have also stimulated a humoral immune response. The result showed that secretion of PA did not enhance antibody production or protection in mice. Further work is needed to evaluate the mechanism by which VEE replicon-expressed secreted proteins versus intracellularly retained proteins stimulate humoral immunity and how these different expression products influence cellular immunity.
As an initial investigation into the region of PA responsible for inducing toxin-neutralizing antibodies, a replicon was constructed that expressed the 63-kDa PA protein (PA63) normally produced after in vivo cleavage of the 83-kDa PA protein by a cell-associated furin protease (8). After cleavage, the PA63 protein forms a complex with six other PA63 molecules and functions by transferring edema factor or lethal factor into host cells. Western blot analysis of BHK cells infected with PA63 VRPs showed a high level of expression of this protein. Subsequent inoculation of PA63 VRPs into CBA/J mice failed to stimulate an adequate immune response and failed to protect the mice from challenge. Multimerization of PA63 may have prevented proper antigen processing, thus preventing proper display of epitopes and recognition by other immune effector cells. Additional vaccine studies utilizing the crystal structure domains of PA (8) cloned into the replicon are in progress. Previously published vaccine studies involving the PA domains fused to glutathione S-transferase protein or adsorbed onto aluminum hydroxide reported protective immunity (1, 5). No data were reported for untreated native PA63.
In the studies reported here, we used the VEE replicon as a vector to express the prokaryotic PA gene in eukaryotic cells and to develop new vaccine candidates against anthrax. The different PA VRPs were effective at protecting animals against anthrax with as few as two doses without the need for adjuvants or formulations with formaldehyde. Since the VEE replicon did not produce antibody titers in mice as high as AVA, we are currently developing ways of improving expression and delivery of the replicons. For example, improvements that are already under way include changing the native PA gene, which contains approximately 70% adenosine or thymidine bases, to a synthetic gene that contains codons more-frequently used by mammalian cells. The VEE replicon is a vaccine vector capable of expressing either prokaryotic or eukaryotic genes and is effective at stimulating protective immune responses in animals.
Acknowledgments
We thank S. F. Little and J. W. Farchaus for supplying recombinant PA protein and C. S. Schmaljohn for supplying the TPA-PA gene.
The views in this report are those of the authors and should not be considered Department of the Army positions.
Editor: D. L. Burns
REFERENCES
- 1.Flick-Smith, H. C., N. J. Walker, P. Gibson, H. Bullifent, S. Hayward, J. Miller, R. W. Titball, and E. D. Williamson. 2002. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect. Immun. 70:1653-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hevey, M., D. Negley, P. Pushko, J. Smith, and A. Schmaljohn. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28-37. [DOI] [PubMed] [Google Scholar]
- 3.Iacono-Connors, L. C., S. L. Welkos, B. E. Ivins, and J. M. Dalrymple. 1991. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect. Immun. 59:1961-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 5.Ivins, B., and S. L. Welkos. 1988. Recent advances in the development of an improved, human anthrax vaccine. Eur. J. Epidemiol. 4:12-19. [DOI] [PubMed] [Google Scholar]
- 6.Lee, J. S., B. K. Dyas, S. S. Nystrom, C. M. Lind, J. F. Smith, and R. G. Ulrich. 2002. Immune protection against staphylococcal enterotoxin-induced toxic shock by vaccination with a Venezuelan equine encephalitis virus replicon. J. Infect. Dis. 185:1192-1196. [DOI] [PubMed] [Google Scholar]
- 7.Lee, J. S., P. Pushko, M. D. Parker, M. T. Dertzbaugh, L. A. Smith, and J. F. Smith. 2001. Candidate vaccine against botulinum neurotoxin serotype A derived from a Venezuelan equine encephalitis virus vector system. Infect. Immun. 69:5709-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-838. [DOI] [PubMed] [Google Scholar]
- 9.Pitt, M. L. M., S. Little, B. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalation anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 10.Pushko, P., M. Parker, J. Geisbert, D. Negley, A. Schmaljohn, P. Jahrling, and J. Smith (ed.). 1997. Venezuelan equine encephalitis virus replicon vector: immunogenicity studies with ebola NP and GP genes in guinea pigs. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 12.Singh, Y., B. Ivins, and S. H. Leppla. 1998. Study of immunization against anthrax with the purified recombinant protective antigen of Bacillus anthracis. Infect. Immun. 66:3447-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welkos, S. L., and A. Friedlander. 1988. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb. Pathog. 5:127-139. [DOI] [PubMed] [Google Scholar]
- 15.Welkos, S. L., and A. M. Friedlander. 1988. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb. Pathog. 4:53-69. [DOI] [PubMed] [Google Scholar]
- 16.Welkos, S. L., N. J. Vietri, and P. H. Gibbs. 1993. Non-toxigenic derivatives of the Ames strain of Bacillus anthracis are fully virulent for mice: role of plasmid pX02 and chromosome in strain-dependent virulence. Microb. Pathog. 14:381-388. [DOI] [PubMed] [Google Scholar]