Abstract
In the present study, we show that in human endothelial cells the tetraspanin CD63/lamp3 distributes predominantly to the internal membranes of multivesicular–multilamellar late endosomes, which contain the unique lipid lysobisphosphatidic acid. Some CD63/lamp3 is also present in Weibel–Palade bodies, the characteristic secretory organelle of these cells. We find that CD63/lamp3 molecules can be transported from late endosomes to Weibel–Palade bodies and thus that CD63/lamp3 cycles between endocytic and biosynthetic compartments; however, movement of CD63/lamp3 is much slower than that of P-selectin, which is known to cycle between plasma membrane and Weibel–Palade bodies. When cells are treated with U18666A, a drug that mimics the Niemann-Pick type C syndrome, both proteins accumulate in late endosomes and fail to reach Weibel–Palade bodies efficiently, suggesting that P-selectin, like CD63/lamp3, cycles via late endosomes. Our data suggest that CD63/lamp3 partitions preferentially within late endosome internal membranes, thus causing its accumulation, and that this mechanism contributes to CD63/lamp3 retention in late endosomes; however, our data also indicate that the protein can eventually escape from these internal membranes and recycle toward Weibel–Palade bodies to be reused. Our observations thus uncover the existence of a selective trafficking route from late endosomes to Weibel–Palade bodies.
INTRODUCTION
Vascular endothelial cells, which play an essential role in blood coagulation and inflammatory processes, are characterized by the presence of specialized rod-shaped secretory granules called Weibel–Palade bodies (Weibel and Palade, 1964). Weibel–Palade bodies store and secrete von Willebrand factor (vWF), an adhesive glycoprotein involved in primary hemostasis (Wagner et al., 1982; Wagner, 1990). These storage granules also contain P-selectin, a membrane protein involved in leukocyte adhesion to the plasma membrane of the endothelium (Wagner, 1993). Although few other membrane proteins of the Weibel–Palade bodies have been identified until now, Vischer and Wagner (1993) showed that these storage granules contain CD63.
CD63 is a well-established component of the late endosomal and lysosomal membranes, also known as lysosome-associated membrane protein 3 (lamp3) or lysosome integral membrane protein 1 (limp1) (Fukuda, 1991; Escola et al., 1998; Kobayashi et al., 1999), which will be referred to here as CD63/lamp3. Unlike lamp1 or lamp2, which are typical type-1 transmembrane proteins, CD63/lamp3 is a member of the tetraspanin superfamily (Metzelaar et al., 1991; Maecker et al., 1997). CD63/lamp3, however, appears to share with lamp1 and lamp2 a cytoplasmic Gly-Tyr motif, which has been reported to serve as a lysosomal targeting signal (Hunziker and Geuze, 1996). In addition, CD63/lamp3 is also present in “secretory lysosomes” (Griffiths, 1996), the secretory granules of cells derived from the hemopoietic lineage that are related to lysosomes. These organelles include azurophilic granules of neutrophils (Cieutat et al., 1998) and α-granules of platelets (Heijnen et al., 1998). Interestingly, the expression of vWF is tissue specific and restricted to Weibel–Palade bodies of endothelial cells and α-granules of megakaryocytes and platelets (Wagner, 1993). Thus, CD63/lamp3 appears to be shared by conventional late endosomes/lysosomes and by specialized, perhaps related secretory organelles.
In the present study, we report that in human umbilical vein endothelial cells (HUVEC), the vast majority of CD63/lamp3 is present within the complex network of internal membranes characteristic for late endosomes in animal cells. CD63/lamp3 is also present in Weibel–Palade bodies, as expected from previous studies. We made use of the fact that CD63/lamp3 can be labeled with endocytosed antibodies to follow the fate of the protein. Our data show that CD63/lamp3 is transported from late endosomes to Weibel–Palade bodies and thus uncover the existence of a trafficking route from late endosomes to this secretory organelle. P-selectin appears to follow the same route, albeit with faster kinetics. Thus, CD63/lamp3, like P-selectin, can cycle between endocytic and exocytic compartments in endothelial cells. Our data also suggest that the CD63/lamp3 residence time in late endosome is relatively long because the protein accumulates within late endosome internal membranes, but that the protein can eventually recycle from these internal membranes to be reused.
MATERIALS AND METHODS
Cells and Reagents
HUVEC were isolated and maintained as described (Vischer and Wollheim, 1998). The monoclonal antibodies against CD63/lamp3 (2C6) (Vischer and Wagner, 1993) and lysobisphosphatidic acid (LBPA) (6C4) (Kobayashi et al., 1998) have been described. Monoclonal anti-CD63/lamp3 antibody was also purchased from Chemicon (Temecula, CA). The monoclonal antibody against human P-selectin was from H.K. Nieuwenhuis (University Hospital Utrecht, The Netherlands). The monoclonal antibody against the human lysosomal-associated membrane protein 2 (lgp 110/lamp2) (H4B4) (Chen et al., 1985) was from the Developmental Studies Hybridoma Bank (Iowa City, IA). Antibodies against giantin (Linstedt and Hauri, 1993) were kindly provided by Hans-Peter Hauri (University of Basel, Basel, Switzerland). Rabbit antibodies against human vWF were from Dako (Glostrup, Denmark). Aminomethylcoumarin acetate (AMCA)-conjugated donkey F(ab′)2 against rabbit antibodies, FITC-conjugated goat anti-mouse antibodies, and rhodamin-conjugated goat anti-rabbit antibodies were from Jackson (West Grove, PA). Oregon Green-labeled antibodies and Alexa 568-labeled antibodies were prepared according to the manufacturer's instruction (Molecular Probes, Eugene, OR). 3β-(2-Diethylaminoethoxy)-androstenone.HCl (U18666A) was from Biomol (Plymouth Meeting, PA).
In Vivo Labeling of Endothelial Cells
HUVEC were grown on gelatin-coated coverslips in RPMI 1640 containing 90 μg/ml heparin (Boehringer Ingelheim, Heidelberg, Germany), 15 μg/ml endothelial cell growth supplement (Upstate Biotechnology, Lake Placid, NY), 10 mM HEPES, 100 U/ml penicillin, and 100 U/ml streptomycin, and supplemented with 10% FCS. mAbs against CD63/lamp3 or mAbs against P-selectin were added to the culture medium, when indicated. At the desired time, cells were washed, fixed, and permeabilized for fluorescence microscopy as described below. Internalized antibodies were either visualized by indirect immunofluorescence using secondary antibodies or after coupling the fluorophore directly to the purified, primary antibody. The intracellular distribution of fluorescent and nonfluorescent antibodies was identical, and no difference was observed when either detection system was used.
Fluorescence Microscopy
HUVEC grown on coverslips were fixed with 4% freshly depolymerized paraformaldehyde in PBS at pH 7.4 (room temperature for 20 min). Cells were washed with PBS, treated for 10 min with 0.27% NH4Cl/0.38% glycine in PBS, and permeabilized for 30 min with 0.05% saponin in PBS in the presence of primary antibodies. Cells were washed and then incubated for 30 min with fluorescent secondary antibodies. After washing, coverslips were mounted in polyvinyl alcohol and examined under a Zeiss Axiophot microscope. Rhodamin, Alexa 568, Oregon green, FITC, and AMCA signal were recorded sequentially using a 63× Plan-NEOFLUAR oil immersion objective. Filipin staining of cholesterol was performed as described (Kobayashi et al., 1999).
Electron Microscopy
HUVEC were prepared for electron microscopy and cryo-sectioned, and then sections were processed for immunogold labeling as described (Griffiths et al., 1984).
RESULTS
CD63/lamp3 Is Present in Both Late Endosomes and Weibel–Palade Bodies in HUVEC
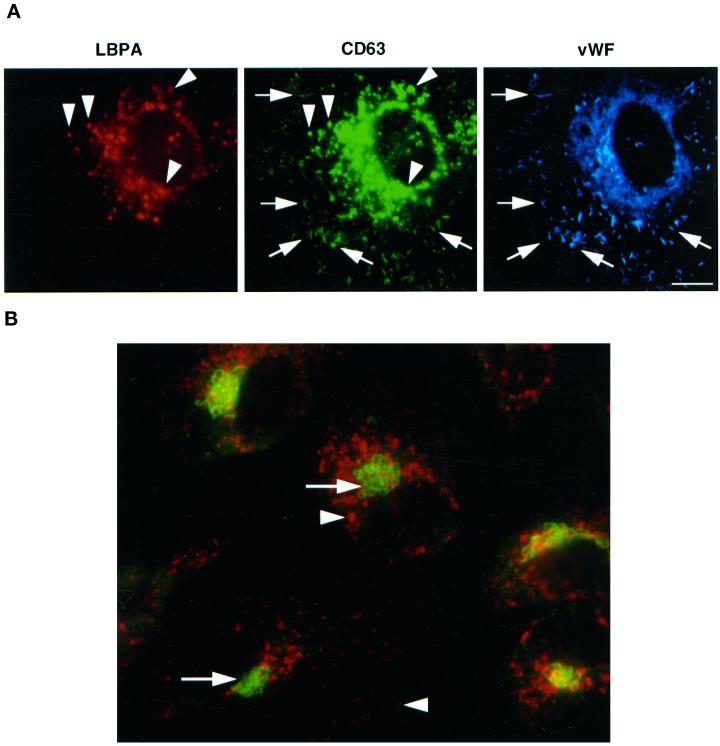
We analyzed the distribution of CD63/lamp3 in HUVEC by immunofluorescence. As expected (Vischer and Wagner, 1993), we found that the protein was present in Weibel–Palade bodies (Figure 1), identified by their characteristic, rod-shaped appearance, and the presence of vWF, the main cargo protein of Weibel–Palade bodies (Wagner, 1990); however, the vast majority of the protein distributed to intracellular structures with a labeling pattern resembling that of endosomes/lysosomes (Figure 1).
Figure 1.
Steady-state distribution of CD63/lamp3 in HUVEC. (A) HUVEC were fixed and triple-labeled with Alexa 568-conjugated monoclonal anti-LBPA antibodies, Oregon Green-conjugated monoclonal anti-CD63/lamp3 antibodies, and rabbit anti-vWF antibodies followed by AMCA-conjugated donkey F(ab′)2 against rabbit antibodies. Arrowheads show LBPA-positive late endosomes, whereas arrows show vWF-positive Weibel–Palade bodies. Bar, 10 μm. (B) HUVEC were double-labeled with Alexa 568-conjugated monoclonal antibody against CD63/lamp3 (arrowheads) and rabbit anti-giantin antibodies (arrows) followed by FITC-conjugated donkey F(ab)2 against rabbit antibodies. CD63/lamp3 can be detected in structures with the typical appearance of late endosomes or Weibel–Palade bodies but not in the Golgi complex. Bar, 10 μm.
We have previously described a late endosome-specific mAb (6C4) that recognizes the poorly degradable phospholipid LBPA (Kobayashi et al., 1998). We found that this lipid is restricted to the complex membrane network that accumulates within multivesicular–multilamellar late endosomes, in all cell types we have tested (Kobayashi et al., 1998; Kobayashi et al., 1999; Schaible et al., 1999). As shown in Figure 1, the numerous structures that contain CD63/lamp3 but are devoid of vWF labeling were efficiently double-labeled with the anti-LBPA mAb. CD63 was not detected in the Golgi complex (Figure 1), which was labeled with antibodies against giantin (Linstedt and Hauri, 1993). These observations demonstrate that some CD63/lamp3 distributes to Weibel–Palade bodies in HUVEC but that the bulk of the protein is present in late endosomes, in agreement with previous observations (Vischer and Wagner, 1993).
CD63/lamp3 Distributes to Late Endosome Internal Membranes in HUVEC
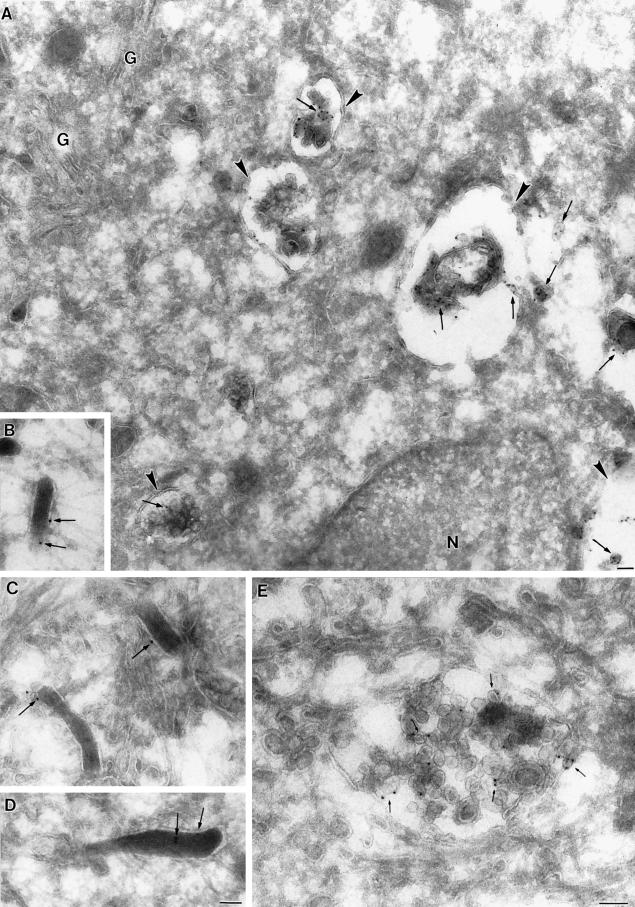
We then investigated the ultrastructure of membranes containing CD63/lamp3 by immunogold labeling of cryosections. As shown in Figure 2, Weibel–Palade bodies, which could be unambiguously identified on cryosections because of their characteristic rod-shaped appearance, were significantly labeled by anti-CD63/lamp3 antibodies. Previously, it was estimated that Weibel–Palade bodies contain ≈5% of the total cellular content of CD63/lamp3 (Vischer and Wagner, 1993). This value is in good agreement with the distribution of gold particles on cryosections. As observed previously (Vischer and Wagner, 1993), CD63/lamp3 was not detected on the plasma membrane by immunofluorescence or immunogold labeling.
Figure 2.
Ultrastructure of compartments containing CD63/lamp3 in HUVEC. Ultrathin frozen sections of HUVEC were labeled with CD63/lamp3 antibodies followed by 10 nm goat anti-mouse gold. (A) Low-power overview showing the general distribution of CD63/lamp3 labeling. Multivesicular late endosomes (arrowheads) show labeling on intralumenal vesicles (arrows). The ER surrounding the nucleus (N) as well as the Golgi complex (G) is not labeled. A representative image of a putative late endosome in which intralumenal vesicles are labeled (arrows) is shown in E. B–D show examples of Weibel–Palade bodies that show significant, albeit low, labeling for CD63/lamp3 (arrows). Bars, 100 nm.
Anti-CD63/lamp3 antibodies labeled structures with the typical multivesicular appearance of late endosomes, in agreement with our immunofluorescence study; however, gold particles exhibited a characteristic distribution: they were mostly observed on the internal membranes of late endosomes (Figure 2), as in other cell types (Escola et al., 1998; Hammond et al., 1998). This distribution is in marked contrast to that of lgp120/lamp1, which is restricted to the limiting membranes of the same organelle (Griffiths et al., 1988; Aniento et al., 1993; Kobayashi et al., 1998). In fact, a quantitative analysis of CD63/lamp3 distribution revealed that 88% of the gold particles present in late endosomes distributed to internal membranes. We recently showed that in HUVEC as in other cells, LBPA distributes to late endosome internal membranes (Galve de Rochemonteix et al., 2000). Our observations thus strongly suggest that CD63/lamp3 partitions preferentially within LBPA-rich membrane domains in the late endosomes of HUVEC.
Internalized Anti-CD63/lamp3 Antibodies Are Transported to Late Endosomes and Weibel–Palade Bodies
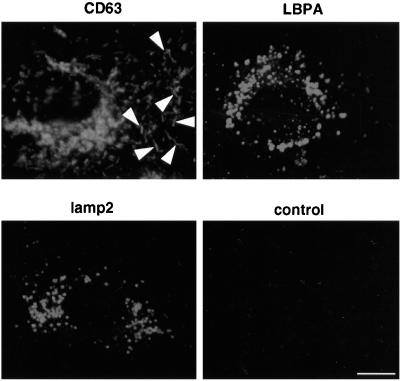
We then made use of the fact that the endosomal pool of CD63/lamp3 is accessible to antibodies internalized from the medium to study the fate of the protein. Because CD63/lamp3 was not detected on the plasma membrane, antibodies were presumably internalized, at least in part, by fluid-phase endocytosis. It is also possible that internalization was facilitated by the presence of low, below detection amounts of the protein cycling at the cell surface. Cells were first incubated for 24 h in the presence of antibodies to label efficiently the intracellular pools of CD63/lamp3, which may intersect with the endocytic pathway. As shown in Figure 3, the internalized antibody accumulated intracellularly, predominantly in late endosomes; however, anti-CD63/lamp3 antibodies were also detected within Weibel–Palade bodies, which could easily be recognized because of their characteristic shape (Figure 3, arrowheads; see also Figure 4). These observations show that endocytosed antibodies against CD63/lamp3 were transported to Weibel–Palade bodies.
Figure 3.
Antibody-tagged CD63/lamp3 is present in both late endosomes and Weibel–Palade bodies. HUVEC were grown in the presence of the monoclonal anti-CD63/lamp3, anti-LBPA, anti-lgp110/lamp 2, or control mouse IgG for 24 h. For optimal detection of internalized antibodies, these were used at a concentration of 50 μg/ml in the medium and revealed by indirect immunofluorescence. Cells were then fixed and stained with FITC-conjugated goat anti-mouse antibodies. Anti-CD63/lamp3 is internalized into both late endosomes and Weibel–Palade bodies, which were recognized by their characteristic rod-shaped structure (arrowheads). Bar, 10 μm.
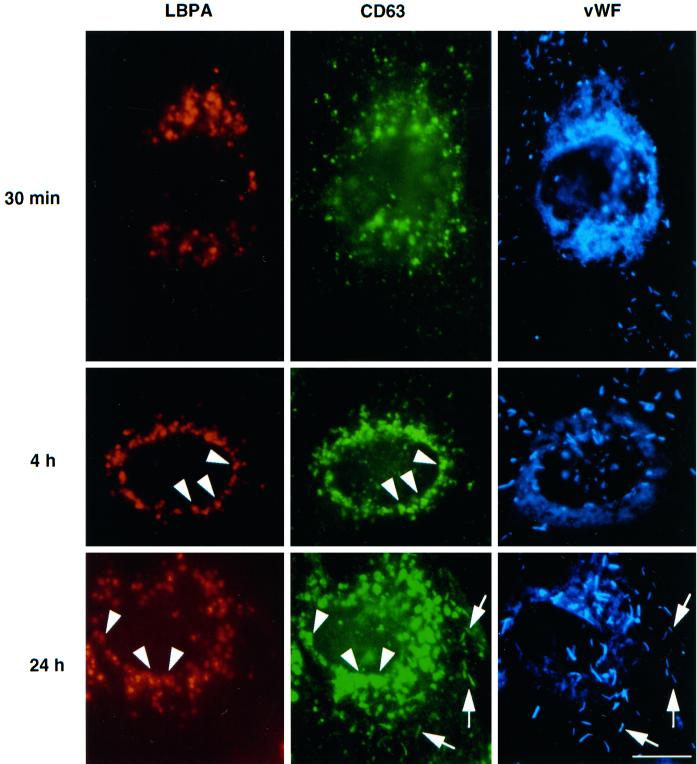
Figure 4.
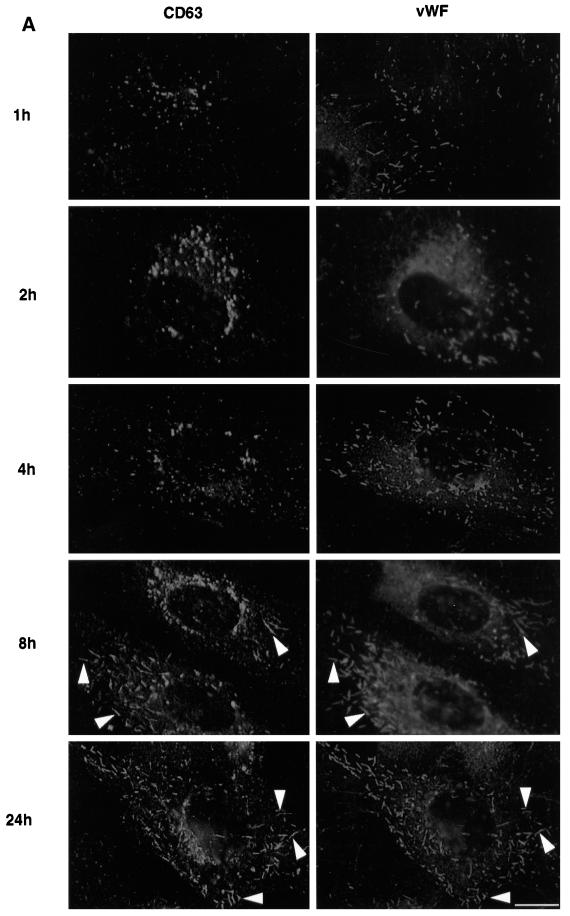
Antibody-tagged CD63/lamp3 is transported to late endosomes and then to Weibel–Palade bodies. HUVEC were grown in the presence of 50 μg/ml Oregon Green-conjugated anti-CD63/lamp3 antibodies. At appropriate time intervals, cells were fixed and triple-labeled with Alexa 568-conjugated anti-LBPA antibodies and rabbit anti-vWF antibodies followed by AMCA-conjugated donkey F(ab′)2 against rabbit antibodies. Arrowheads show LBPA-positive late endosomes, whereas arrows show vWF-positive Weibel–Palade bodies. Amounts of internalized antibodies, hence green fluorescence intensity, increased with incubation time. Exposure time was thus adjusted so that labeled structures could be well resolved. Bar, 10 μm.
When cells were treated under the same conditions with nonrelevant antibodies, these did not accumulate intracellularly, presumably because they were transported to lysosomes and then degraded (Figure 3). We then investigated whether antibodies against other antigens present in late endosomes were also transported to Weibel–Palade bodies. In these experiments, cells were incubated with our mAb against LBPA or with a mAb against lgp110/lamp2. As shown in Figure 3, both antibodies accumulated intracellularly upon binding to their respective antigens, much like antibodies against CD63/lamp3; however, unlike CD63/lamp3, antibodies against LBPA or lgp110/lamp2 remained in late endosomes and were not redistributed to Weibel–Palade bodies. These experiments thus indicate that anti-CD63/lamp3 antibodies were selectively transported to Weibel–Palade bodies while bound to their antigen, and thus that CD63/lamp3 molecules can traffic from endosomes back to Weibel–Palade bodies.
We then followed the movement of internalized, fluorescent anti-CD63/lamp3 antibodies after incubating cells for increasing periods of time (Figure 4, green). After fixation, cells were labeled with antibodies against LBPA (Figure 4, red) and vWF (Figure 4, blue) and then inspected by triple-channel fluorescence. Within 30 min after internalization, anti-CD63/lamp3 antibodies were detected in vesicles at the cell periphery, which did not contain LBPA or vWF, and presumably corresponded to early endosomes. After longer incubation times, antibody molecules accumulated in late endosomes containing LBPA, where they remained for several hours (4 h incubation shown in Figure 3). Then, no antibody could be detected within Weibel–Palade bodies containing vWF. It is only after much longer incubation times that antibodies against CD63/lamp3 eventually redistributed to Weibel–Palade bodies containing vWF (Figure 4, after 24 h; see also Figure 5).
Figure 5.
Kinetics of antibody-tagged P-selectin and CD63/lamp3 transport to Weibel–Palade bodies. Cells were grown in the presence of monoclonal antibodies against CD63/lamp3 (A) or P-selectin (B). At appropriate time intervals, cells were fixed and analyzed by fluorescence microscopy. Anti-CD63/lamp3 antibodies were used at a concentration of 20 μg/ml in the medium, whereas antibodies against P-selectin conjugated to Alexa 568 were used at a concentration of 5 μg/ml. CD63/lamp3 and vWF were revealed by indirect immunofluorescence. Arrowheads show vWF-positive Weibel–Palade bodies. As in Figure 4, exposure times were adjusted so that labeled structures could be well resolved. Bar, 10 μm.
Altogether these experiments show that anti-CD63 antibodies appear sequentially in late endosomes and then in Weibel–Palade bodies and that these two steps are well-separated in time. Because CD63/lamp3 itself distributes predominantly to late endosomes, the simplest interpretation is that CD63/lamp3 molecules tagged with internalized antibodies, perhaps within late endosomes, are transported from late endosomes to Weibel–Palade bodies.
Kinetics of P-Selectin and CD63/lamp3 Movement to Weibel–Palade Bodies
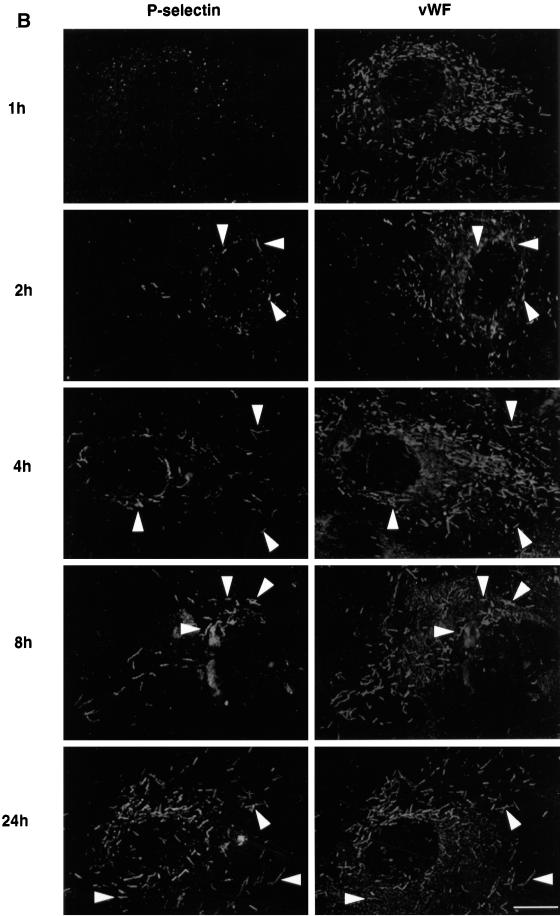
We then compared the transport of CD63/lamp3 to Weibel–Palade bodies with that of P-selectin, an adhesion molecule present in Weibel–Palade bodies (Bonfanti et al., 1989; McEver et al., 1989). Previous studies showed that P-selectin can cycle from the cell surface to Weibel–Palade bodies (Subramaniam et al., 1993).
Cells were incubated with antibodies against P-selectin or CD63/lamp3, as above. After incubation for 1 h, small amounts of either antibody could already be detected intracellularly, presumably within endosomes (Figure 5, A and B, top panels). At that time, no colocalization was observed with vWF, hence antibodies had not reached Weibel–Palade bodies; however, after 2 h of incubation, antibody-tagged P-selectin could already be detected within Weibel–Palade bodies containing the vWF (Figure 5B). Interestingly, only a very small subset of Weibel–Palade bodies contained tagged P-selectin, presumably corresponding to newly formed secretory granules. In marked contrast, antibodies against CD63/lamp3 remained restricted to late endosomes (Figure 5A), consistent with the time-course shown in Figure 4. Eventually, after 8 h incubation, CD63/lamp3 molecules tagged with antibodies started to appear in Weibel–Palade bodies. Only a subset of these granules, however, was labeled at this time, as observed with P-selectin. After 24 h, most Weibel–Palade bodies contained both antibodies, suggesting that granule turnover was complete by this time. We could not detect antibody-tagged CD63 or P-selectin in the Golgi complex/trans-Golgi network (TGN), presumably because transport through this compartment is rapid (Figure 5). It is highly unlikely that differences between P-selectin and CD63/lamp3 reflected differences in antibody endocytosis rates, hence intracellular accumulation. Large amounts of anti-CD63/lamp3 antibodies accumulated intracellularly, when compared with P-selectin antibodies, before CD63/lamp3 movement to Weibel–Palade bodies could be detected. These results thus indicate that the rate of CD63/lamp3 movement from endosomes to Weibel–Palade bodies is slow when compared with P-selectin cycling.
CD63/lamp3 and P-Selectin Cycle from Late Endosomes to Weibel–Palade Bodies
Our time-course experiments suggest that CD63/lamp3 is transported from late endosomes to Weibel–Palade bodies and that P-selectin may follow the same route, but far more efficiently. To investigate this process in more detail, we used the drug U18666A, which causes selective accumulation of low density lipoprotein-derived cholesterol in late endocytic compartments and thereby mimics the genetic disorder Niemann-Pick Type C (Liscum and Klansek, 1998). Recently, we showed that cholesterol accumulates in late endosomes containing LBPA of human fibroblasts and baby hamster kidney cells, and that this accumulation inhibits cycling of the multifunctional receptor (IGF2/MPR) for insulin-like growth factor 2 and ligands bearing mannose 6-phosphate, in particular lysosomal enzymes (Kobayashi et al., 1999). The receptor remains trapped in late endosomes, presumably because cholesterol accumulation interferes with the membrane physicochemical and dynamic properties (Kobayashi et al., 1999). Cholesterol accumulation in late endosomes, however, does not affect TGN38 (Kobayashi et al., 1998; Kobayashi et al., 1999), which rapidly cycles between endosomes and TGN (Reaves et al., 1993). Cholesterol accumulated in late endosomes can easily be detected by light microscopy using filipin as a fluorescent marker (Sokol et al., 1988; Kobayashi et al., 1999).
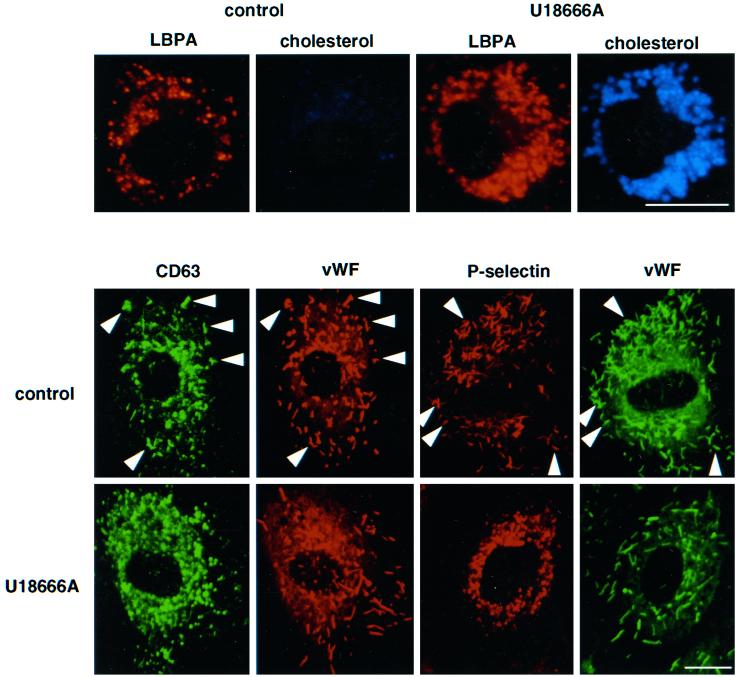
U18666A treatment of HUVEC caused accumulation of cholesterol within late endosomes containing LBPA, as we had observed in human fibroblasts and baby hamster kidney cells (Figure 6). In these experiments, exposure times were adjusted for the detection of accumulated cholesterol and were too short to reveal cholesterol present on the plasma membrane, in early endosomes and in the TGN (Kobayashi et al., 1999). After cholesterol accumulation in U18666A-treated cells, antibodies were endocytosed and reached perinuclear, cholesterol-positive late endosomes; however, antibody-tagged CD63/lamp3 molecules remained trapped in these endosomes and were no longer transported to Weibel–Palade bodies, even after 24 h (Figure 6). When U18666A-treated cells were incubated with control preimmune antibodies, these did not accumulate intracellularly, as in control cells (Figure 1), presumably because the antibodies were degraded in lysosomes. These observations thus demonstrate that cycling of CD63/lamp3 through late endosomes was inhibited after U18666A-mediated cholesterol accumulation in this compartment, much like IGF2/MPR (Kobayashi et al., 1999).
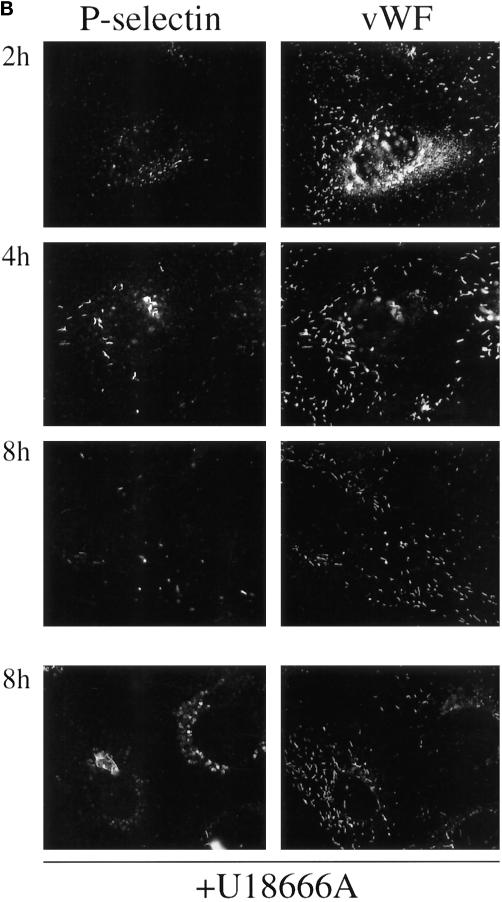
Figure 6.
U18666A inhibits transport of antibody-tagged CD63/lamp3 and P-selectin to Weibel–Palade bodies. Top panel: HUVEC were grown in the absence (control) or presence of 1 μg/ml U18666A for 24 h (U18666A). Cells were then fixed and double-labeled with the anti-LBPA antibody and filipin. Bottom panel: HUVEC were preincubated with 1 μg/ml U18666A for 24 h followed by an additional 24 h incubation with the same concentration of U18666A in the presence of anti-CD63 antibody (20 μg/ml) or Alexa 568-labeled anti-P-selectin (5 μg/ml) antibody. Cells were then fixed and permeabilized; CD63 antibody was revealed with FITC-conjugated goat anti-mouse antibodies, and all coverslips were double-labeled with antibodies against vWF. Bar, 10 μm.
When the experiment was repeated after internalization of anti-P-selectin antibodies, we found that they were also endocytosed and then retained within late endosomes. Antibody-tagged P-selectin did not reach Weibel–Palade bodies, even after 24 h, although these were normally labeled within 2 h in the absence of the drug (Figures 5 and 6). These experiments thus indicate that P-selectin is rapidly transported through late endosomes (Figure 5), consistent with the presence of a sorting motif responsible for its transport from early to late endosomes (Blagoveshchenskaya et al., 1998a,b) and that cycling is inhibited by cholesterol accumulation.
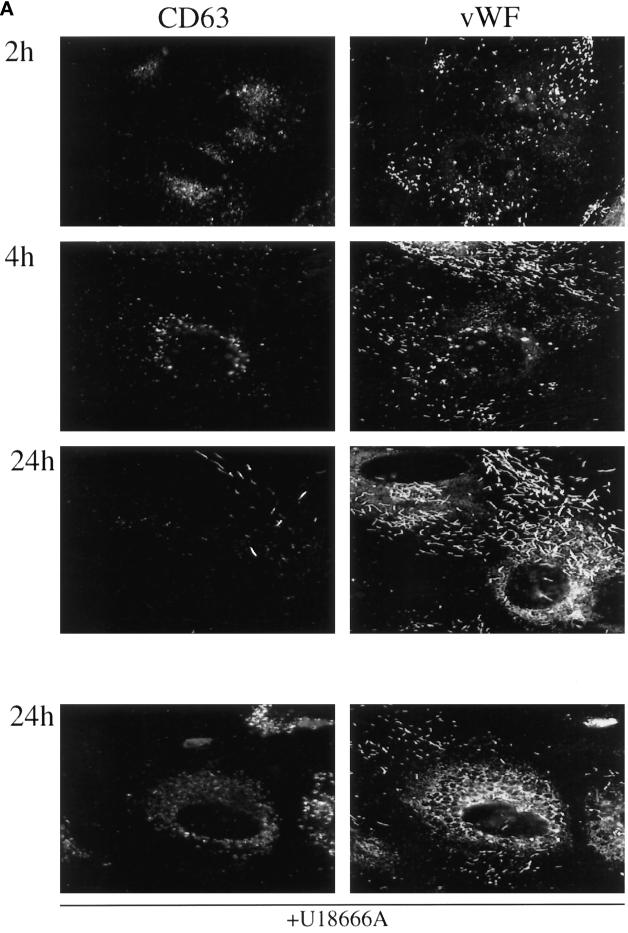
Finally, cells were incubated with antibodies against CD63 for 2 h and then chased for increasing periods of time to follow the trafficking of a pulse of antibody-tagged CD63/lamp3 molecules (Figure 7). Small amounts of antibody were detected intracellularly after the pulse, presumably within early endosomes. Then, antibodies accumulated within late endosomes during the chase, where they remained for several hours (in agreement with Figures 4 and 5A). It is only after long chase time periods that the pulse of antibody-tagged CD63/lamp3 molecules reached Weibel–Palade bodies. As expected, endosome labeling was then significantly lower when compared with that observed after continuous incubation with antibodies for the same time period (Figures 4 and 5A). Also as expected, only fewer Weibel–Palade bodies were labeled after chase when compared with continuous incubations. When cells were treated with U18666A before the experiment, internalized antibodies reached late endosomes, where they accumulated, but subsequent transport to the Weibel–Palade bodies was inhibited significantly, in agreement with Figure 6. These experiments demonstrate that CD63 molecules are transported from late endosomes to Weibel–Palade bodies.
Figure 7.
Pulse–chase analysis of endosome to Weibel–Palade bodies transport. Cells were incubated in the presence of mAbs against CD63/lamp3 for 2 h (A) or P-selectin for 1 h (B) (pulse) and then further incubated in the absence of antibody for the indicated time period (chase). Anti-CD63/lamp3 antibodies were used at a concentration of 20 μg/ml in the medium and revealed by indirect immunofluorescence, whereas antibodies against P-selectin were directly conjugated with Alexa 568 and used at a concentration of 5 μg/ml. Cells were fixed and analyzed by double-immunofluorescence microscopy using antibodies against vWF. As in Figure 4, exposure times were adjusted so that labeled structures could be well resolved. Bar, 10 μm.
Similar pulse–chase experiments with antibodies against P-selectin showed that antibody-tagged P-selectin was detected in endosomes after a 1-h pulse but then rapidly reached Weibel–Palade bodies (Figure 7B), in agreement with data shown in Figure 5B. Again, movement of antibodies to Weibel–Palade bodies during the chase was significantly reduced in cells treated with U18666A (Figure 7B). Altogether, these data indicate that both CD63/lamp3 and P-selectin traffic from late endosomes to Weibel–Palade bodies in human endothelial cells, albeit with very different kinetics.
DISCUSSION
In the present study, we show that the vast majority of the tetraspanin CD63/lamp3 in endothelial cells is present within the complex network of internal membranes rich in LBPA, which is characteristic for late endosomes; however, some CD63/lamp3 is also present within specialized secretory granules, the Weibel–Palade bodies, in agreement with previous studies (Vischer and Wagner, 1993). We find that CD63/lamp3 molecules can be transported from late endosomes to Weibel–Palade bodies and thus that CD63/lamp3 cycles between endocytic and biosynthetic compartments. Kinetics of this transport, however, are slow when compared with P-selectin, which appears to follow the same route. These observations are consistent with the notion that a retention mechanism selective for this tetraspanin exists in late endosomes, perhaps corresponding to CD63/lamp3 preferential partitioning within LBPA-rich internal membrane domains within late endosomes; however, our observations also indicate that the tetraspanin CD63/lamp3 can eventually recycle from internal membranes of late endosomes to be reused in Weibel–Palade body biogenesis.
Intracellular Distribution of CD63
The tetraspanin superfamily contains proteins involved in diverse processes at the cell surface, including proliferation, adhesion, and motility, and can form complexes with integrins and other cell-surface proteins (Maecker et al., 1997); however, this superfamily also contains members that were previously shown to be selectively enriched within internal membranes of multivesicular endosomes in antigen-presenting cells, megakaryocytes, neutrophils, and platelets (Cieutat et al., 1998; Escola et al., 1998; Hammond et al., 1998; Heijnen et al., 1998).
We now find that ≥80% of total CD63/lamp3 in HUVEC localizes to the complex network of LBPA-rich internal membranes present within multivesicular–multilamellar late endosomes. CD63/lamp3 carries an 11-residue cytoplasmic domain, which encodes a C-terminal lysosomal sorting motif (Gly-Tyr-X-X-Met) (Hunziker and Geuze, 1996). Although other lysosomal glycoproteins, including lgp120/lamp1, share this Gly-Tyr motif with CD63/lamp3, lgp120/lamp1 is restricted to the limiting membranes of late endosomes (Griffiths et al., 1988; Aniento et al., 1993; Kobayashi et al., 1998). Thus, this motif cannot be responsible for sorting lgp120/lamp1 and CD63/lamp3 into different endosomal membrane domains. In addition, this motif cannot target CD63/lamp3 to Weibel–Palade bodies, because it is present in lgp120/lamp1 and lgp110/lamp2, which are not found in these granules. Future work will be required to identify the molecular mechanisms regulating CD63/lamp3 targeting.
Relationships between Secretory Lysosomes and Weibel–Palade Bodies
In addition to its endosomal localization, we also find that CD63/lamp3 distributes to Weibel–Palade bodies in HUVEC, as reported previously (Vischer and Wagner, 1993). CD63/lamp3 is also present in the secretory lysosomes of hemopoietic cells (Nieuwenhuis et al., 1987; Griffiths, 1996), including in both multivesicular bodies and α-granules of megakaryocytes and platelets (Heijnen et al., 1998) and azurophilic granules of neutrophils (Cieutat et al., 1998); however, unlike secretory lysosomes (Wagner, 1993), Weibel–Palade bodies do not contain lysosomal hydrolases. In fact, the biogenesis of these secretory granules is not well characterized, and different sorting mechanisms for P-selectin may be used in platelets and endothelial cells (Hartwell et al., 1998). Although the precise relationships between Weibel–Palade bodies and secretory lysosomes clearly remain to be characterized, the presence of the tetraspanin CD63/lamp3 in both types of organelles suggests the existence of some common functional characteristics. This agrees with the recent identification of a common precursor for hemopoietic and endothelial cells (Choi et al., 1998).
Cycling of CD63
Antibodies against CD63/lamp3 are internalized by HUVEC and sequentially appear in early and then late endosomes. Once in late endosomes, antibody molecules are specifically retained, upon binding to their antigen, for several hours. Then, after >8–10 h, CD63/lamp3 tagged with antibodies, but not other lysosomal antigens, is selectively transported from late endosomes to Weibel–Palade bodies. Our data also indicate that P-selectin follows the same route, but with much faster kinetics (≈2 h).
We found that some, but not all, Weibel–Palade bodies contained antibody-tagged CD63/lamp3 and P-selectin at early times in their respective cycle (2 h for P-selectin; 8 h for CD63/lamp3) or after pulse–chase. In contrast, most Weibel–Palade bodies contained both proteins after long time periods of continuous antibody internalization (24 h). Incorporation of P-selectin and CD63 into Weibel–Palade bodies reflects basal granule turnover, because cells were not stimulated in our experiments. Incidentally, from the continuous antibody exposure experiments we can estimate that the basal granule turnover rate is ∼24 h. These observations suggest that proteins tagged with internalized antibody are selectively incorporated into newly formed Weibel–Palade bodies and thus that they are transported from late endosomes to the TGN, presumably following the same pathway as recycling IGF2/MPR molecules (Kornfeld, 1992). Because we failed to detect tagged antibodies in the TGN, transit through this organelle may be comparatively rapid. Our data thus show that in endothelial cells, CD63/lamp3 returns to the storage granules after secretion, like P-selectin (Subramaniam et al., 1993) but less efficiently, to be reused, and that this recycling process occurs via late endosomes.
Determinants responsible for P-selectin trafficking and targeting have been identified. After endocytosis, transport to late endosomes requires a motif present in the cytoplasmic domain (Blagoveshchenskaya et al., 1998a,b), whereas P-selectin targeting to Weibel–Palade bodies depends on both the cytoplasmic domain and the transmembrane region (Koedam et al., 1992; Fleming et al., 1998; Hartwell et al., 1998). In contrast, the motif(s) responsible for CD63/lamp3s targeting to Weibel–Palade bodies is not known, and no sequence homology is observed between P-selectin and CD63/lamp3 cytoplasmic domains; however, in addition to this putative sorting signal, we wish to propose that selective retention of CD63/lamp3 in late endosomes contributes to the sorting process.
Internal Membranes of Late Endosomes: A Sorting Mechanism?
Our quantitative studies show that at steady state most CD63/lamp3 is present within the complex network of LBPA-rich internal membranes, which accumulates within late endosomes. Yet, antibody-tagged CD63/lamp3 can cycle from late endosomes and reach Weibel–Palade bodies (after pulse–chase or continuous internalization of antibodies) and can eventually lead to complete labeling of most secretory granules after 24 h of continuous antibody internalization. The simplest interpretation of these observations is that CD63/lamp3 recycles from late endosome internal membranes toward the secretory pathway.
The fate of proteins present in internal membranes of multivesicular–multilamellar compartments has been the subject of much debate. Because the downregulated epidermal growth factor receptor accumulates in internal membranes of multivesicular endosomes, it was proposed that formation of these internal membranes represents a mechanism used for the lysosomal degradation of both bilayer and cargo (Futter et al., 1996). Liposomes containing LBPA (or other anionic lipids) were also shown to enhance glucosylceramide degradation in vitro (Wilkening et al., 1998). If LBPA-rich membranes can incorporate molecules destined to be degraded, it is unlikely, however, that the bilayer itself becomes degraded, because LBPA is both poorly degradable, because of its unique stereoconfiguration (Brotherus et al., 1974), and very abundant within internal membranes (Kobayashi et al., 1998).
Internal membranes of late endosomes also appear to contain proteins that are destined to be reused. It has been proposed that IGF2/MPR cycles between internal membranes of late endosome (prelysosome) and the TGN (Griffiths et al., 1988). Consistent with this view, we found that IGF2/MPR accumulates within LBPA-rich internal membranes of late endosomes when cycling is inhibited (Kobayashi et al., 1998). In antigen-presenting cells, a specialized pathway allows the direct fusion of multivesicular compartments with the plasma membrane, releasing into the medium internal membranes (exosomes) enriched in some tetraspanins (Escola et al., 1998; Geuze, 1998). As at other transport steps, future studies will be required to determine the precise mechanisms that regulate within these internal membranes the sorting/trafficking of proteins destined to be recycled or degraded; however, some insights can already be gained from this and other studies. Our previous work suggested that LBPA-rich internal membranes function as distribution device for low density lipoprotein-derived cholesterol and contribute to IGF2/MPR sorting/trafficking (Kobayashi et al., 1998,1999). It is attractive to propose that endosome internal and limiting membranes interact in a highly dynamic manner, perhaps via intraluminal fusion/fission events. These dynamic properties may contribute to regulate protein movement, including CD63/lamp3, through the compartment, in combination with yet to be discovered regulatory mechanisms.
The movement of CD63/lamp3 from late endosomes to Weibel–Palade bodies is slow when compared with that of P-selectin, and unlike P-selectin, CD63/lamp3 accumulates in late endosomes. Preferential partitioning of CD63/lamp3 within LBPA-rich membrane domains in late endosomes may thus contribute to an increased CD63/lamp3 residence time in this compartment, hence leading to its accumulation. This mechanism may decrease the number of molecules accessible to transport intermediates forming on the cytoplasmic face of late endosome limiting membrane and thus reduce CD63/lamp3 trafficking rates. In contrast, the cycle of P-selectin is much faster than that of CD63/lamp3, and P-selectin does not accumulate in late endosomes of endothelial cells. P-selectin may have a somewhat reduced tendency to partition within LBPA-rich membranes and thus may be more readily transported. We could not determine the distribution of P-selectin on internal versus limiting membranes of late endosomes in endothelial cells, because amounts of the protein are too low to be detected at steady state; however, this analysis was possible in megakaryocytes and showed that P-selectin is present on both internal and limiting membranes of multivesicular bodies and α-granules, in contrast to CD63, which was enriched on internal membranes (Heijnen et al., 1998). Thus, we propose that the LBPA-rich membrane domain functions as a retention mechanism for CD63/lamp3, as well as perhaps for other molecules, including presumably other tetraspanins, which are enriched within late endosome internal membranes.
ACKNOWLEDGMENTS
We thank Marie-Hélène Beuchat and Angelika Hopkins for expert technical assistance. We also thank Gisou van der Goot for critical reading of the manuscript, and H. K. Nieuwenhuis for the generous gift of the 1.18 antibody against P-selectin. This work was supported by grant 961235 from the National Health and Medical Research Council of Australia (to R.G.P.), as well as grants 31-37296.93 (to J.G.), 3100-0505645.97 (to E.K.), and 3200-052667.97 (to U.V.) from the Swiss National Science Foundation, and grant RG 355/94 from the International Human Frontier Science Program (to J.G., R.G.P., and T.K.).
REFERENCES
- Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin. J Biol Chem. 1998a;273:27896–27903. doi: 10.1074/jbc.273.43.27896. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Norcott JP, Cutler DF. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J Biol Chem. 1998b;273:2729–2737. doi: 10.1074/jbc.273.5.2729. [DOI] [PubMed] [Google Scholar]
- Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel–Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- Brotherus J, Renkonen O, Herrmann J, Fisher W. Novel stereochemical configuration in lysobisphosphatidic acid of cultured BHK cells. Chem Phys Lipids. 1974;13:178–182. doi: 10.1016/0009-3084(74)90034-6. [DOI] [PubMed] [Google Scholar]
- Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Cieutat AM, Lobel P, August JT, Kjeldsen L, Sengelov H, Borregaard N, Bainton DF. Azurophilic granules of human neutrophilic leukocytes are deficient in lysosome-associated membrane proteins but retain the mannose 6-phosphate recognition marker. Blood. 1998;91:1044–1058. [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Berger G, Guichard J, Cramer EM, Wagner DD. The transmembrane domain enhances granular targeting of P-selectin. Eur J Cell Biol. 1998;75:331–343. doi: 10.1016/s0171-9335(98)80066-6. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve de Rochemonteix B, Kobayashi T, Rosnoblet C, Lindsay M, Parton RG, Reber G, de Maistre E, Wahl D, Kruithoff BKO, Gruenberg J, de Moerlosse P. Interaction of anti-phospholipid antibodies with late endosomes of human endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:563–574. doi: 10.1161/01.atv.20.2.563. [DOI] [PubMed] [Google Scholar]
- Geuze HJ. The role of endosomes and lysosomes in MHC class II functioning. Immunol Today. 1998;19:282–287. doi: 10.1016/s0167-5699(98)01269-9. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G, McDowall A, Back R, Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984;89:65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- Griffiths GM. Secretory lysosomes: a special mechanism of regulated secretion in hemopoietic cells. Trends Cell Biol. 1996;6:329–332. doi: 10.1016/0962-8924(96)20031-5. [DOI] [PubMed] [Google Scholar]
- Hammond C, Denzin LK, Pan M, Griffith JM, Geuze HJ, Cresswell P. The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. J Immunol. 1998;161:3282–3891. [PubMed] [Google Scholar]
- Hartwell DW, Mayadas TN, Berger G, Frenette PS, Rayburn H, Hynes RO, Wagner DD. Role of P-selectin cytoplasmic domain in granular targeting in vivo and in early inflammatory responses. J Cell Biol. 1998;143:1129–1141. doi: 10.1083/jcb.143.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. BioEssays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat M-H, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Koedam JA, Cramer EM, Briend E, Furie B, Furie BC, Wagner DD. P-selectin, a granule membrane protein of platelets and endothelial cells, follows the regulated secretory pathway in AtT-20 cells. J Cell Biol. 1992;116:617–625. doi: 10.1083/jcb.116.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose-6-phosphate/insulin-like growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri HP. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L, Klansek JJ. Niemann-Pick disease type C. Curr Opin Lipidol. 1998;9:131–135. doi: 10.1097/00041433-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel–Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzelaar MJ, Wijngaard PL, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991;266:3239–3245. [PubMed] [Google Scholar]
- Nieuwenhuis HK, van Oosterhout JJ, Rozemuller E, van Iwaarden F, Sixma JJ. Studies with a monoclonal antibody against activated platelets: evidence that a secreted 53,000-molecular weight lysosome-like granule protein is exposed on the surface of activated platelets in the circulation. Blood. 1987;70:838–845. [PubMed] [Google Scholar]
- Reaves B, Horn M, Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Schlesinger PH, Steinberg TH, Mangel WF, Kobayashi T, Russell DG. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J Cell Sci. 1999;112:681–693. doi: 10.1242/jcs.112.5.681. [DOI] [PubMed] [Google Scholar]
- Sokol J, Blanchette-Mackie J, Kruth HS, Dwyer NK, Amende LM, Butler JD, Robinson E, Patel S, Brady RO, Comly ME. Type C Niemann-Pick disease. Lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J Biol Chem. 1988;263:3411–3417. [PubMed] [Google Scholar]
- Subramaniam M, Koedam JA, Wagner DD. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol Biol Cell. 1993;4:791–801. doi: 10.1091/mbc.4.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer UM, Wagner DD. CD63 is a component of Weibel–Palade bodies of human endothelial cells. Blood. 1993;82:1184–1191. [PubMed] [Google Scholar]
- Vischer UM, Wollheim CB. Purine nucleotides induce regulated secretion of von Willebrand factor: involvement of cytosolic Ca2+ and cyclic adenosine monophosphate-dependent signaling in endothelial exocytosis. Blood. 1998;91:118–127. [PubMed] [Google Scholar]
- Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- Wagner DD. The Weibel–Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993;70:105–110. [PubMed] [Google Scholar]
- Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel–Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening G, Linke T, Sandhoff K. Lysosomal degradation on vesicular membrane surfaces. Enhanced glucosylceramide degradation by lysosomal anionic lipids and activators. J Biol Chem. 1998;273:30271–30278. doi: 10.1074/jbc.273.46.30271. [DOI] [PubMed] [Google Scholar]