Abstract
Neonatal immune responses have been associated with the development of atopy in childhood. We assessed in cord blood mononuclear cells (CBMC) whether increased allergen/mitogen-induced lymphoproliferation (LP) is associated with pro-allergic Th2 cytokine IL-13 or Th1 cytokine IFN-γ secretion. We determined whether LP to one allergen is related to heightened lymphocyte function to other allergens/mitogen. CBMC from 135 neonates were stimulated with house dust mite (Derf1), cockroach, ovalbumin, or mitogen. LP to one allergen was associated with significantly increased LP to other allergens/mitogen. Increased Derf1-LP was associated with increased Derf1-induced IL-13 secretion (r = 0.21, p = 0.01). After adjusting for neonatal gender, race, and maternal smoking, Derf1-LP remained associated with Derf1-IL-13 (OR 3.08, 95% CI 1.56–6.10). Increased mitogen-induced proliferation was associated with increased mitogen-induced IL-13 secretion (r = 0.37, p < 0.001). For some individuals, a predisposition to a heightened immune response is already evident at birth. Whether this phenotype results in atopy in childhood warrants further investigation.
Keywords: Allergens, cord blood, immunology, lymphocytes, neonatal
INTRODUCTION
Asthma is the most common chronic disease of childhood in western countries, with an increasing prevalence over the last two decades (1). The development of childhood asthma, a T helper (Th) 2 cell-mediated immune disease, is influenced by genetic, developmental, and environmental factors (2), and immunological phenomena are critical in the differentiation of the early immune system. The search for risk factors and predictors of allergic disease detectable at birth extends beyond the frequently studied relevance of IgE levels in cord blood (3). One factor during the fetal period critical to the development of the immune system is exposure to different allergens, either in utero or postnatally. Antigen-induced proliferation by umbilical cord blood mononuclear cells (CBMCs) in response to a variety of antigens, including allergens, has been acknowledged in a number of studies (4–6) and has been associated with subsequent development of atopic diseases (7, 8).
Besides genetic predisposition, neonatal immune responses may be “primed” by the intrauterine environment, and cytokine responses may be modulated by cytokines present in gestation-associated tissues or in amniotic fluid (9, 10). Specifically, the two cytokines IFN-γ and IL-13, which we analyzed in this study, are of interest. Production of IFN-γ (Th1 cytokine) in unstimulated CBMC was associated with a lower risk of atopic diseases in childhood (11). As a critical Th2 cytokine, IL-13 secretion is increased in the mucosa of atopic and non-atopic asthmatics compared with non-asthmatic controls (12). Further, blockade of IL-13 in murine models of allergic asthma has resulted in decreased airway hyperresponsiveness, mucus production, and eosinophilia in bronchoalveolar lavage (13, 14).
Whether a response to one allergen reflects a heightened response of the neonatal immune system to other allergens or to mitogen and vice versa has not yet been determined. In this study, we hypothesized that increased proliferation of CBMC to one antigen is associated with an increased proliferative response to another antigen and mitogen. Further, we postulated that an increased proliferative response to one allergen is associated with a pro-allergic increase in IL-13 and a decrease in IFN-γ secretion induced by the identical antigen.
METHODS
Human Study Populations
Subjects for this noninterventional study were participants in a pregnancy–birth cohort study from the Boston metropolitan area (15) with enrollment from April 1999 through July 2002. Expectant mothers were approached at their initial prenatal visit for consent. Participants were interviewed and asked to complete questionnaires in the first and second trimester of pregnancy, as well as at the time of delivery. Exclusion criteria included multiple gestation, inability to answer questions in English a plan to move out of the area before delivery, and a gestational age greater than 22 completed weeks at the initial prenatal clinical appointment. Venous umbilical cord blood was obtained at the time of delivery from neonates born as a nonemergency. Subjects were included when data about both proliferation and cytokine secretion were available. The study was approved by the Institutional Review Boards of Brigham and Woman’s Hospital and Harvard Pilgrim Health Care. Informed consent was obtained from mothers including cord blood collection and longitudinal follow-up of their offspring.
Isolation of CBMC and Lymphocyte Proliferation
Cord blood samples (n = 135) were collected from the umbilical vein at delivery and processed as previously described (15, 16). Samples were placed in heparinized tubes and processed within 24 h. Mononuclear cells were isolated by density-gradient centrifugation with Ficoll-Hypaque Plus (Pharmacia, Uppsala, Sweden) after dilution in phosphate-buffered saline (PBS, Sigma Aldrich, St. Louis, MO). Cells were washed in RPMI 1640 and diluted in 10% human serum (Biowhittaker, Walkersville, MD) to a concentration of 5 × 106 cells/ml. For the lymphocyte proliferation (LP) assay, 0.5 × 106 CBMC/well were cultured in quadruplicate in 96-well round-bottom tissue culture plates (Corning, New York, NY) for 72 h, either unstimulated or stimulated with 30 μg/ml Derf1 (Dermatophagoides farinae 1, house dust mite, Indoor Biotechnologies, Charlottesville, VA), 30 μg/ml Blag2 (Blatella germanica 2, cockroach, Indoor Biotechnologies, Charlottesville, VA), 100 μg/ml Ova (Ovalbumin, Sigma Aldrich, St. Louis, MO), or 5 μg/ml PHA (phytohemagglutinin, Sigma Aldrich, St. Louis, MO). The indicated doses and timepoints were chosen after optimization experiments with dose/time–response curves. Allergen-specificity was tested by blockade of lymphoproliferation by anti-MHC II antibodies (10 μg/ml) to the allergen Der f1.
We tested all reagents for endotoxin contamination by Limulus Assay. Endotoxin content was very low (<0.01 EU/ml), did not significantly change lymphocyte proliferation or cytokine secretion in CBMC, and functional ability to stimulate CBMC was not relevant at these concentrations. After stimulation, CBMC were further pulsed with 1 μCi [3H]thymidine for an additional 8 h. Cultures were maintained at 37°C in a humidified 5% CO2 incubation chamber. Cells were harvested with a Tomcat Mach II harvester (Wallac, Turku, Finland) onto filter plates, which were read in a β-counter. Proliferation was quantified by stimulation index (SI), which is calculated as the ratio of mean counts per minute (cpm) of stimulated to unstimulated replicates.
Cytokine Measurements
Cells were cultured in medium alone or with Derf1, Blag2, or PHA as described above and harvested at 72 h of stimulation. Supernatants for determination of IL-13 and IFN-γ secretion were analyzed by ELISA (Endogen, Rockford, IL) according to the manufacturer’s instructions. The lower limit of detection was 7.0 pg/ml for IL-13 and 2.0 pg/ml for IFN-γ.
Definition of Variables
Population characteristics, including smoking history, delivery type, neonate gender, gestational age, birth weight, and race/ethnicity, were determined through written questionnaires, interviews, and review of medical records. Maternal smoking status, both current and prior, and determination of the ethnicity of the neonate based on parental report of race/ethnicity, was assessed on the first trimester questionnaire completed at an average of 10 weeks of gestation. If both parents were white, the child was classified as being white. If either parent was black, the child was classified as being black. If no parent was black but at least one parent was Hispanic, the child was classified as Hispanic. If no parent was black or Hispanic but at least one parent was Asian, the child was classified as Asian. If no parent was black, Hispanic, or Asian but at least one parent was American Indian, the child was classified as being other. Maternal atopy was determined during the first trimester interview and was defined as a history of a doctor’s diagnosis of asthma, hay fever, or eczema.
Definition of Predictor/Outcome Variables and Data Analysis
Lymphocyte proliferation SI was calculated as the ratio of mean cpm in stimulated lymphocytes divided by the mean cpm in unstimulated lymphocytes. Subjects with a PHA SI >3 SD below the geometric mean (PHA SI < 5) (n = 1/135) were excluded from the analyses. Proliferative responses to Derf1 and PHA were natural-log transformed to normalize the distribution of the data. Proliferative responses to Blag2 and Ova did not normalize with natural-log transformation; we used either Pearson’s or Spearman’s correlation testing to assess the correlations between proliferative responses. For Derf1, Blag2, and Ova, an SI > 2 was defined as positive; proliferative responses after stimulation with PHA were dichotomized according to the geometric mean. We evaluated the data in both a linear and dichotomized way.
The cytokines IL-13 and IFN-γ were not normally distributed. Log transformation of the cytokines did not normalize the data. Therefore, cytokines were either analyzed with Spearman’s rank correlation coefficient when reported as a continuous variable (Table III) or dichotomized below or above the geometric mean and analyzed with the chi-square test (Fig. 3). If cytokine levels were nondetectable, data were set to 0.01 and included in the analysis. We used multivariate logistic regression to evaluate the influence of maternal history of asthma, as well as maternal smoking and race/ethnicity. Analyses were performed with Sigma Stat, SPSS, and SAS (Version 8, Cary, NC) software. Statistical significance was defined as p < 0.05.
Table III.
Correlation Between Allergen/Mitogen-Induced SI and Cytokine Levels
| Correlation between allergen/mitogen-induced SI and
|
||||||
|---|---|---|---|---|---|---|
| Stimulated IL-13 levels (n = 134)
|
Stimulated IFN-γ levels (n = 127)
|
Unstimulated IFN-γ levels (n = 128)
|
||||
| Correlation coefficient | p | Correlation coefficient | p | Correlation coefficient | p | |
| Derf1 SI | 0.21 | 0.01 | 0.04 | 0.67 | −0.13 | 0.16 |
| Blag2 SI | 0.48 | 0.58 | −0.06 | 0.50 | −0.16 | 0.07 |
| PHA SI | 0.37 | <0.001 | −0.08 | 0.35 | −0.25 | 0.004 |
Note. n. n = 127–135, depending on number of complete cytokine and proliferation data.
Fig. 3.
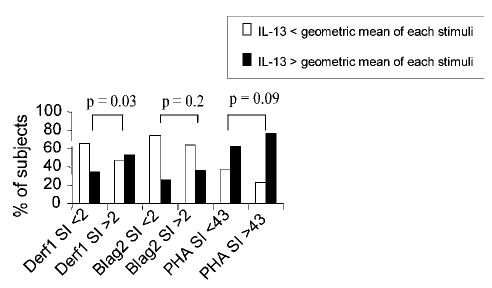
Proliferation and IL-13 secretion in CBMC. Increased lymphoproliferation in response to Derf1 is associated with a higher percentage of neonates with CBMC with IL-13 levels above the geometric mean (p = 0.03). Concentrations of cytokines were determined in supernatants of CBMC and harvested at 72 h after stimulation with Derf1, Blag2, or the mitogen PHA and measured with ELISA (n = 135 subjects).
RESULTS
Population Characteristics
Demographic and medical data for the study population of 135 subjects, including maternal data, are presented in Table I. All subjects had measurements for proliferation following stimulation of CBMCs with the allergens Derf1 and Blag2, Ova, and the mitogen PHA and measurements for cytokine secretions after stimulation with Derf1, Blag2, and the mitogen PHA. Evaluation of maternal characteristics in Table I for potential association or confounding factors for proliferation to Derf1 (log) revealed potential influences of gender, race/ethnicity, and maternal history of ever smoking (p < 0.15) (data not shown). Maternal atopy was no confounding factor for the analysis.
Table I.
Population Characteristics
| Maternal age (years), mean ± SD | 31.5 ± 4.3 |
| Maternal atopy,a n (%) | 49 (36.8) |
| Current smoker, n (%) | 4 (3.3) |
| Delivery type, n (% vaginal delivery) | 109 (80.7) |
| Offspring gender, n (% male) | 75 (55.6) |
| Offspring gestational age (weeks) ± SD | 39.5 ± 1.8 |
| Offspring birthweight (kg) ± SD | 3.52 ± 0.54 |
| Offspring ethnicity, n (%) | |
| White | 95 (70.4) |
| African American | 20 (14.8) |
| Hispanic | 8 (5.9) |
| Asian | 9 (6.7) |
| Other | 3 (2.2) |
Note. n = 135.
Maternal history of atopy was defined as maternal report of one or more of the diagnoses “asthma,” “hay fever,” or “eczema.”
Lymphoproliferative Responses
Lymphoproliferative responses (LP) to Derf1, Blag2, and Ova are presented in Fig. 1 (n = 135). The median (line within the box), 25th and 75th percentile (boundaries of box) and the 90th/10th percentile (whiskers of box) of each stimulus are presented, as well as outlying points. A total of 51.1% of CBMCs responded positively (SI above 2) to stimulation with Derf1, 37% to stimulation with Blag2, and 11.1% to stimulation with Ova. Proliferation in response to PHA was dichotomized by the geometric mean (SI 43, range from SI 6.48 to 458) for PHA stimulation; 71 subjects (52.6%) were above the geometric mean for PHA (data not shown). To determine whether a positive LP to one specific antigen indicated a heightened immune response of neonatal CBMC to another antigen as well, we investigated the correlation between the proliferative responses to different antigens (Table II shows Spearmans/Pearson correlation between LP responses to allergen/mitogen). We also examined the impact of a higher proliferative response to an allergen to the proliferative response to the mitogen PHA to assess whether subjects with higher proliferative responses to allergen also have a heightened T-cell reactivity in general. In addition, we assessed the relevance of a response to a mitogen in relation to allergen-induced proliferation. In analyses expressing LP as a continuous variable, neonates with a higher proliferative response to allergen or mitogen were also more likely to have an increased proliferative response to another allergen or mitogen, respectively (Table II, 1st and 2nd first column of data). We also analyzed proliferation as a dichotomous variable (Table II, 3rd, 4th, and 5th column of data). Neonates with a positive SI to one allergen were more likely to have a positive proliferative response to another allergen, including Derf1 and Blag2, and also to the mitogen PHA (Table II). In reverse, when proliferation in response to PHA was increased, subjects were more likely to have a positive proliferative response to Derf1, Blag2, and Ova.
Fig. 1.
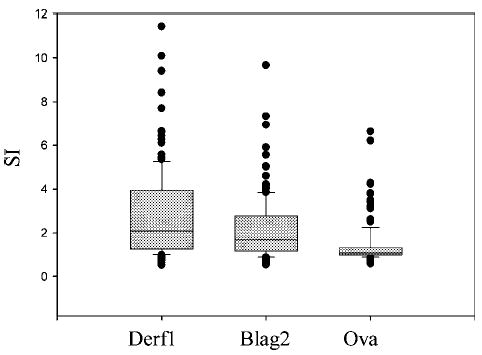
Cord blood proliferation in response to allergens. Lymphocyte proliferation to Der f1, Bla g2, and Ova are presented. The median (line within the box), 25th and 75th percentile (boundaries of box) and the 90th/10th percentile (whiskers of box) are shown, as well as outlying points. Lymphocyte proliferation was determined following stimulation with Derf1, Blag2, or Ova for 72 h by [3H]thymidine uptake as described in Methods section. Proliferation was quantified by stimulation index (SI), which is calculated as the ratio of mean counts per minute (cpm) of stimulated to the mean cpm of unstimulated replicates. A positive stimulation index (SI) was defined as >2 (n = 135 subjects).
Table II.
Correlation Between the Proliferative Responses (SI) to Several Allergens/Mitogen: Analysis of SI as a Continuous (Left) and Dichotomous (Right) Variable
| Spearmans/Pearson correlation coefficient | p | Proliferation (SI) (Median)a of Blag2, Ova, PHA or Derf1 | p (Mann–Whitney rank sum test) | ||
|---|---|---|---|---|---|
| Der f1 SI | Der f1 SI negativeb | Der f1 SI positiveb | |||
| Blag2 SI | 0.78c | <0.001 | 1.16 | 2.47 | <0.001 |
| Ova SI | 0.28c | <0.001 | 1.05 | 1.16 | 0.009 |
| PHA SI | 0.33c | <0.001 | 35.21 | 56.63 | <0.001 |
| 0.30d | <0.001 | ||||
| Blag 2 SI | Blag2 SI negativeb | Blag2 SI positiveb | |||
| Derf1 SI | 0.78c | <0.001 | 1.56 | 4.20 | <0.001 |
| Ova SI | 0.28c | <0.001 | 1.05 | 1.18 | 0.007 |
| PHA SI | 0.34c | <0.001 | 38.83 | 63.80 | 0.003 |
| Ova SI | Ova SI negativeb | Ova SI positiveb | |||
| Derf1 SI | 0.28c | <0.001 | 1.94 | 4.27 | 0.002 |
| Blag2 SI | 0.28c | <0.001 | 1.48 | 2.63 | <0.001 |
| PHA SI | 0.26c | 0.003 | 45.27 | 67.77 | 0.296 |
| PHA SI | PHA SI < 43 | PHA SI > 43 | |||
| Derf1 SI | 0.30d | <0.001 | 1.70 | 2.93 | 0.002 |
| Ova SI | 0.26c | <0.001 | 1.03 | 1.13 | 0.032 |
| Blag2 SI | 0.34c | <0.001 | 1.33 | 1.84 | 0.002 |
Medians refer to allergen-induced SI in the first column.
SI negative: SI <2, SI positive: SI >2.
Spearman rank test.
Pearson correlation coefficient.
Analysis of IL-13 and IFN-γ Secretion in Response to Allergens and Mitogen
The cytokines IL-13 and IFN-γ were frequently undetectable both in unstimulated cells and after stimulation by antigen or mitogen (IL-13 detectable in 43, 31, 99, and 30% of samples after stimulation with Derf1, Blag2, PHA, and in unstimulated cells, respectively; IFN-γ was detectable in 56, 53, 79, and 37% of samples). The distribution of both IL-13 and IFN-γ secretion in unstimulated cells (U) and following stimulation with Derf1 and Blag2 is shown in Fig. 2. To assess the relation between cytokine secretion and proliferative response induced by allergic stimuli and mitogen, we examined IL-13 and IFN-γ as important Th2 and Th1 cytokines, respectively, in relation to allergen- or mitogen-induced proliferation. The data are presented in both a continuous and dichotomous way (Table III, Fig. 3). Increased proliferation in response to Derf1 stimulation was associated with an increase in Derf1-induced secretion of IL-13 but not with any significant change in secretion of IFN-γ (Table III, Fig. 3). Also, increased proliferation in response to the mitogen PHA was associated with increased PHA-induced secretion of IL-13 (Spearman’s) (Table III). However, secretion of IFN-γ induced by PHA was not affected. It is interesting that a higher proliferative response to PHA was associated with a lower secretion of IFN-γ at baseline (Table III) as well as with a lower secretion of IFN-γ in response to Derf1 (data not shown, correlation coefficient, r = −0.25, p = 0.005). Proliferation in response to Blag2 stimulation showed no significant association with the secretion of IL-13 or IFN-γ in unstimulated or stimulated cells.
Fig. 2.
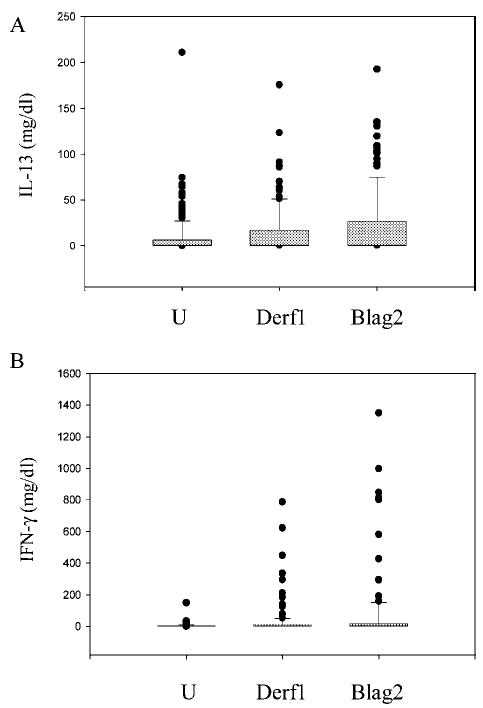
Secretion of IL-13 and IFN-γ following allergen stimulation. Cytokine secretion of IL-13 and IFN-γ are shown. The median (line within the box), 25th/75th percentile (boundaries of box), 90th/10th percentile (whiskers of box) and outliers are demonstrated. Concentrations of cytokines were determined in supernatants of CBMCs and harvested at 72 h after stimulation with Blag2, Derf1, or in unstimulated cells (U) and measured with ELISA (n = 135 subjects).
When secretion of IL-13 was dichotomized below and above the geometric mean, negative Derf1-induced proliferation (SI < 2) was associated with a higher percentage of patients with lower IL-13 levels (below geometric mean), and positive Derf1-induced proliferation (SI > 2) was associated with a higher percentage of patients with higher IL-13 secretion (above geometric mean, p = 0.03, Fig. 3). Evaluation of maternal characteristics in Table I for a potential association or confounding factors for proliferation to Derf1 (log) revealed potential influences of gender, race/ethnicity, and maternal history of ever smoking (p < 0.15) (data not shown). As a maternal history of atopy showed no association with Derf1-induced proliferation or cytokine secretion, respectively, data are not presented in relation to maternal atopy. Logistic regression for univariate analysis showed an OR of 1.88 (95% CI 1.10–3.20) for log Derf1 LP related to Derf1 IL-13 secretion above the geometic mean. After adjusting for gender, race/ethnicity, and smoking, this association strengthened to an OR of 3.08 (95% CI 1.56–6.10, p = 0.001). The interquartile range for 1 unit change in Derf1 (log) SI was 1.2.
The percentage of neonates with Blag2- and PHA-induced IL-13 and IFN-γ secretion above or below the geometric mean was not significantly different when proliferation was dichotomized at 2 (for Blag2) or at the geometric mean (for PHA), respectively (data not shown). Allergen- and mitogen-induced secretion of IFN-γ (Derf1, Blag2, PHA) was not significantly associated with allergen- or mitogen-induced lymphoproliferation (continuous data shown in Table III, dichotomized data not shown).
DISCUSSION
Over the last decade, interest has increased in the neonatal immune system as a model for understanding the role of early immune responses in the development of atopic disease (17–20). This study demonstrates that CBMCs of neonates with an increased proliferative response to one allergen also manifest a higher proliferative response to another allergen, as well as to mitogen. Increased proliferation of CBMC in response to stimulation with dust mite Derf1 is associated with increased Derf1-induced secretion of IL-13. In contrast to the paradigm of an increase in Th2 cytokines (IL-13) and a decrease in Th1 (IFN-γ) cytokines shown in some models of allergic diseases, Derf1-induced secretion of IFN-γ is not reduced in this cohorte. Furthermore, an increased proliferative response to the mitogen PHA is associated with increased secretion of IL-13 in response to PHA, but not with reduced secretion of IFN-γ.
This study also investigated whether these immune phenotypes might be differentially expressed at birth. Evidence that some aspects of allergic disposition are present at birth has arisen from several studies (5, 19, 21–23). Lymphocytes from cord blood are capable of recognizing and proliferating in response to certain allergens such as milk protein and aeroallergens (17–20, 24). Proliferative responses to house dust mite allergen, birch pollen, and rye grass (5, 21, 22) have been observed in mononuclear cells collected at birth and found already to be present at 22 weeks gestation (25). The finding in our study of T cell reactivity in response to several indoor allergens and to mitogen evokes several hypotheses. The ability to demonstrate proliferative responses to allergen at birth (22, 24–26) suggests the potential occurrence of prior intrauterine exposure to allergen leading to the generation of immunological memory. Whether the responses detected by in vitro stimulation of CBMC reflect in vivo primary T cell responses merits further investigation (17, 22, 26–28). Besides a genetic atopic predisposition, several allergens may also be transferred through the placenta. IgG-dependent as well as IgG-independent route of transfer may play a role, as well as alternative routes such as via placental fibroids, paracellular pathways or also endocytic mechanism (29–34). In addition, the possibility of primary in vitro T cell responses or cross-reactivity, rather than specific activation of previously sensitized T cells is another potential interpretation (17, 35–38). The possibility of endotoxin contamination confounding our results was excluded on the basis of low endotoxin detection and functional assays (see Methods section). A positive proliferative response to a number of stimuli may indicate a heightened immune response in the neonate potentially induced in utero. Of interest is the report by several studies of an underlying association between CBMC lymphoproliferation and the development of atopic diseases (6, 17, 24, 35–40), but also no association between proliferation at birth and atopy up to 24 months of age is reported (41).
Increased responses by Th2 lymphocytes to aeroallergens are presumed to be pathogenic in the development of allergic diseases (42). At birth, possibly because of placenta-derived Th2 trophic factors, allergen-induced mononuclear cell responses are skewed toward a Th2-like phenotype (26, 42), and IFN-γ responses are particularly low (43). While IL-13 secretion by CBMCs was correlated with a higher risk of atopic diseases (40, 43), several studies have associated a reduced IFN-γ response at birth and a potential Th1/Th2 imbalance with the development of atopic manifestation in childhood (7, 8, 20, 26, 40, 43–45). The concept of a Th1/Th2 imbalance with the demonstration of decreased Th1 and increased Th2 responses and therefore a pro-allergic phenotype is attractive. However, in one study mitogen-induced cord blood IL-13 responses also appear to be suppressed in children who develop atopic disease (46). In our study, baseline secretion of IL-13 and IFN-γ by CBMCs was low, but detectable. Further, Derf1-induced cytokine secretion of IL-13 was associated with positive LP to Derf1 stimulation. This Der f1-induced Th2 cytokine pattern is consistent with data showing increased Der f-induced IL-5 secretion in relation to SI positivity in response to Derf stimulation (47). It is interesting that SI positivity in our study of 135 patients was not correlated with lower IFN-γ production. In addition, Miller found a positive relationship between IFN-γ production and SI positivity in Derf-stimulated CBMCs in 71 patients (47). Investigations about the role of IFN-γ, a predominant Th1 cytokine, have presented heterogeneous data in studies of atopic disease. Some adult studies report no difference in secretion of IFN-γ in asthmatics compared with controls; others have found lower levels in asthmatics (48). In contrast, increased levels of IFN-γ have been found in the serum and BAL fluid of asthmatics as compared with controls (49). Collectively, our data support the concept that immune maturation evokes a complex array of immune responses that are not solely Th1/Th2-mediated.
Another interesting finding in our study results from the data on mitogen stimulation. Increased lymphocyte proliferation induced by mitogen and associated with allergen-induced lymphocyte proliferation or vice versa may account for a generally activated immune system. We have excluded nonspecific activation by blocking MHC II in allergen-stimulated and mitogen-stimulated CBMC and could confirm allergen-specifity (see Methods section). Positivity in response to more than one stimulus may imply that T-cell reactivity reflects the sensitization to indoor antigens in utero but also that infants with a highly positive response to mitogen may be more prone to allergen-induced T-cell reactivity.
A strength of this study is the investigation of unselected samples, with measures of immune responsiveness at birth: both proliferation and supernatant cytokines levels have been assayed in the cord blood of 135 neonates. Further, the lymphoproliferative profile and cytokine responses were evaluated in freshly stimulated, non-cryopreserved samples both with the allergens Derf1, Blag2, and Ova and with the mitogen PHA. The factor that 36.8% mothers of neonates in this study have a history of atopy merits consideration. One limitation of this study may be that information about skin prick test of parents was not available. However, the entity maternal atopy was not a confounding factor for our results and not major focus of the study. Several factors may be associated with lymphocyte proliferation and cytokine secretion, including maternal atopy, smoking, and birth modus (50). It is interesting that the current literature is diverse indicating either increased proliferative responses in vitro by CBMCs of neonates with a family history of atopic disease (17, 24, 26, 40, 50) in comparison to neonates without parental atopy (4, 27). The logistic multivariate regression revealed that adjusting for potential influencing factors of this study such as gender, race/ethnicity, or smoking further increased the associations between lymphoproliferative responses and cytokine secretion.
CONCLUSION
Taken together, the results of this study show that lymphoproliferative responses to one allergen are associated with increased responses to another allergen and to mitogen. Derf1- and PHA-induced IL-13 secretion are associated with higher proliferative responses but not with lower Derf1-induced IFN-γ secretion. The relevance of allergen-induced proliferation and cytokine secretion in the early immune responses warrants long-term follow-up. Whether neonates with higher proliferative responses to allergens (or mitogen) and additional higher Th2-skewed cytokine secretion will develop an atopic phenotype in the future is currently under investigation.
Acknowledgments
This study was supported by grant 5R01AI045007-04, HD 34568, HL 64925, HL 68041, HL 61907, DFG 997/1-1. We are grateful to the participants and staff of Project Viva and Soma Datta for program assistance.
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ. 2002;51:1. [PubMed] [Google Scholar]
- 2.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158:176. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
- 3.Halonen M, Stern D, Taussig LM, Wright A, Ray CG, Martinez FD. The predictive relationship between serum IgE levels at birth and subsequent incidences of lower respiratory illnesses and eczema in infants. Am Rev Respir Dis. 1992;146:866. doi: 10.1164/ajrccm/146.4.866. [DOI] [PubMed] [Google Scholar]
- 4.Szepfalusi Z, Nentwich I, Gerstmayr M, Jost E, Todoran L, Gratzl R, Herkner K, Urbanek R. Prenatal allergen contact with milk proteins. Clin Exp Allergy. 1997;27:28. [PubMed] [Google Scholar]
- 5.Prescott SL, Macaubes C, Yabuhara A, Venaille TJ, Holt BJ, Habre W, Loh R, Sly PD, Holt PG. Developing patterns of T cell memory to environmental allergens in the first two years of life. Int Arch Allergy Immunol. 1997;113:75. doi: 10.1159/000237512. [DOI] [PubMed] [Google Scholar]
- 6.Miles EA, Warner JA, Jones AC, Colwell BM, Bryant TN, Warner JO. Peripheral blood mononuclear cell proliferative responses in the first year of life in babies born to allergic parents. Clin Exp Allergy. 1996;26:780. [PubMed] [Google Scholar]
- 7.Kondo N, Kobayashi Y, Shinoda S, Takenaka R, Teramoto T, Kaneko H, Fukao T, Matsui E, Kasahara K, Yokoyama Y. Reduced interferon gamma production by antigen-stimulated cord blood mononuclear cells is a risk factor of allergic disorders—6-year follow-up study. Clin Exp Allergy. 1998;28:1340. doi: 10.1046/j.1365-2222.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 8.van der Velden VH, Laan MP, Baert MR, de Waal Malefyt R, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: Risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin Exp Allergy. 2001;31:997. doi: 10.1046/j.1365-2222.2001.01176.x. [DOI] [PubMed] [Google Scholar]
- 9.Schroeter C, Gibbons FK, Finn PW. Development of the early immune system: Impact on allergic diseases. Immunol Allergy Clin N Am. 2002;22:713. [Google Scholar]
- 10.Jones CA, Kilburn SA, Warner JA, Warner JO. Intrauterine environment and fetal allergic sensitization. Clin Exp Allergy. 1998;28:655. doi: 10.1046/j.1365-2222.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 11.Macaubas C, de Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, Sly PD, Holt PG. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 2003;362:1192. doi: 10.1016/s0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- 12.Kroegel C, Julius P, Matthys H, Virchow JC, Jr, Luttmann W. Endobronchial secretion of interleukin-13 following local allergen challenge in atopic asthma: Relationship to interleukin-4 and eosinophil counts. Eur Respir J. 1996;9:899. doi: 10.1183/09031936.96.09050899. [DOI] [PubMed] [Google Scholar]
- 13.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 14.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeter CH, Schaub B, Gold DR, Contreras PJ, Manrique O, Gillman MW, Weiss S, Palmer LJ, Perkins D, Finn PW. Nuclear factor kappa B activation in human cord blood mononuclear cells. Pediatr Res. 2004;56:212. doi: 10.1203/01.PDR.0000132850.33375.D0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn PW, Boudreau JO, He H, Wang Y, Chapman MD, Vincent C, Burge HA, Weiss ST, Perkins DL, Gold DR. Children at risk for asthma: Home allergen levels, lymphocyte proliferation, and wheeze. J Allergy Clin Immunol. 2000;105:933. doi: 10.1067/mai.2000.106546. [DOI] [PubMed] [Google Scholar]
- 17.Devereux G, Barker RN. Studies of cord blood mononuclear cell responses and allergy: Still in their infancy? Clin Exp Allergy. 2002;32:331. doi: 10.1046/j.1365-2222.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 18.Prescott SL, Jones CA. Cord blood memory responses: Are we being naive? Clin Exp Allergy. 2001;31:1653. doi: 10.1046/j.1365-2222.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 19.Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. J Allergy Clin Immunol. 2000;105:S493. doi: 10.1016/s0091-6749(00)90049-6. [DOI] [PubMed] [Google Scholar]
- 20.Martinez FD, Stern DA, Wright AL, Holberg CJ, Taussig LM, Halonen M. Association of interleukin-2 and interferon-gamma production by blood mononuclear cells in infancy with parental allergy skin tests and with subsequent development of atopy. J Allergy Clin Immunol. 1995;96:652. doi: 10.1016/s0091-6749(95)70264-4. [DOI] [PubMed] [Google Scholar]
- 21.Upham JW, Holt BJ, Baron-Hay MJ, Yabuhara A, Hales BJ, Thomas WR, Loh RK, O’Keeffe PT, Palmer L, Le Souef PN, et al. Inhalant allergen-specific T-cell reactivity is detectable in close to 100% of atopic and normal individuals: Covert responses are unmasked by serum-free medium. Clin Exp Allergy. 1995;25:634. doi: 10.1111/j.1365-2222.1995.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 22.Piccinni MP, Mecacci F, Sampognaro S, Manetti R, Parronchi P, Maggi E, Romagnani S. Aeroallergen sensitization can occur during fetal life. Int Arch Allergy Immunol. 1993;102:301. doi: 10.1159/000236541. [DOI] [PubMed] [Google Scholar]
- 23.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Loh R, Holt PG. Reciprocal age-related patterns of allergen-specific T-cell immunity in normal vs. atopic infants Clin Exp Allergy. 1998;28(Suppl 5):39. doi: 10.1046/j.1365-2222.1998.028s5039.x. [DOI] [PubMed] [Google Scholar]
- 24.Piastra M, Stabile A, Fioravanti G, Castagnola M, Pani G, Ria F. Cord blood mononuclear cell responsiveness to beta-lactoglobulin: T-cell activity in ‘atopy-prone’ and ‘non-atopy-prone’ newborns. Int Arch Allergy Immunol. 1994;104:358. doi: 10.1159/000236692. [DOI] [PubMed] [Google Scholar]
- 25.Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol. 1996;7:109. doi: 10.1111/j.1399-3038.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 26.Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, Holt PG. Transplacental priming of the human immune system to environmental allergens: Universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730. [PubMed] [Google Scholar]
- 27.Kondo N, Kobayashi Y, Shinoda S, Kasahara K, Kameyama T, Iwasa S, Orii T. Cord blood lymphocyte responses to food antigens for the prediction of allergic disorders. Arch Dis Child. 1992;67:1003. doi: 10.1136/adc.67.8.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szepfalusi Z. Transplacental priming of the human immune system with environmental allergens can occur early in gestation. J Allergy Clin Immunol. 2000;106:530. doi: 10.1067/mai.2000.108710. [DOI] [PubMed] [Google Scholar]
- 29.Szepfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R. Direct evidence for transplacental allergen transfer. Pediatr Res. 2000;48:404. doi: 10.1203/00006450-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 30.Loibichler C, Pichler J, Gerstmayr M, Bohle B, Kiss H, Urbanek R, Szepfalusi Z. Materno-fetal passage of nutritive and inhalant allergens across placentas of term and pre-term deliveries perfused in vitro. Clin Exp Allergy. 2002;32:1546. doi: 10.1046/j.1365-2222.2002.01479.x. [DOI] [PubMed] [Google Scholar]
- 31.Thornton CA, Vance GH. The placenta: A portal of fetal allergen exposure. Clin Exp Allergy. 2002;32:1537. doi: 10.1046/j.1365-2222.2002.01543.x. [DOI] [PubMed] [Google Scholar]
- 32.Edelbauer M, Loibichler C, Nentwich I, Gerstmayr M, Urbanek R, Szepfalusi Z. Maternally delivered nutritive allergens in cord blood and in placental tissue of term and preterm neonates. Clin Exp Allergy. 2004;34:189. doi: 10.1111/j.1365-2222.2004.01848.x. [DOI] [PubMed] [Google Scholar]
- 33.Edelbauer M, Loibichler C, Witt A, Gerstmayr M, Putschogl B, Urbanek R, Szepfalusi Z. Dose-dependent and preterm-accentuated diaplacental transport of nutritive allergens in vitro. Int Arch Allergy Immunol. 2003;130:25. doi: 10.1159/000068373. [DOI] [PubMed] [Google Scholar]
- 34.Warner JO. The early life origins of asthma and related allergic disorders. Arch Dis Child. 2004;89:97. doi: 10.1136/adc.2002.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramage JM, Young JL, Goodall JC, Gaston JS. T cell responses to heat-shock protein 60: Differential responses by CD4+ T cell subsets according to their expression of CD45 isotypes. J Immunol. 1999;162:704. [PubMed] [Google Scholar]
- 36.Young JL, Daser A, Beverley PC. In vitro proliferative responses of human peripheral blood mononuclear cells to non-recall antigens. J Immunol Methods. 1995;182:177. doi: 10.1016/0022-1759(95)00046-d. [DOI] [PubMed] [Google Scholar]
- 37.Devereux G, Hall AM, Barker RN. Measurement of T-helper cytokines secreted by cord blood mononuclear cells in response to allergens. J Immunol Methods. 2000;234:13. doi: 10.1016/s0022-1759(99)00185-4. [DOI] [PubMed] [Google Scholar]
- 38.Hamelmann E, Wahn U. Immune responses to allergens early in life: When and why do allergies arise? Clin Exp Allergy. 2002;32:1679. doi: 10.1046/j.1365-2222.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 39.Rinas U, Horneff G, Wahn V. Interferon-gamma production by cord-blood mononuclear cells is reduced in newborns with a family history of atopic disease and is independent from cord blood IgE-levels. Pediatr Allergy Immunol. 1993;4:60. doi: 10.1111/j.1399-3038.1993.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 40.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen-Yeung M, Ferguson A, Chan H, Dimich-Ward H, Watson W, Manfreda J, Becker A. Umbilical cord blood mononuclear cell proliferative response to house dust mite does not predict the development of allergic rhinitis and asthma. J Allergy Clin Immunol. 1999;104:317. doi: 10.1016/s0091-6749(99)70373-8. [DOI] [PubMed] [Google Scholar]
- 42.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348. [PubMed] [Google Scholar]
- 43.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 44.Halonen M, Martinez FD. A deficient capacity to produce interferon-gamma: Is it a risk for asthma and allergies? Clin Exp Allergy. 1997;27:1234. [PubMed] [Google Scholar]
- 45.Prescott SL, Holt PG. Abnormalities in cord blood mononuclear cytokine production as a predictor of later atopic disease in childhood. Clin Exp Allergy. 1998;28:1313. doi: 10.1046/j.1365-2222.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 46.Williams TJ, Jones CA, Miles EA, Warner JO, Warner JA. Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J Allergy Clin Immunol. 2000;105:951. doi: 10.1067/mai.2000.106211. [DOI] [PubMed] [Google Scholar]
- 47.Miller RL, Chew GL, Bell CA, Biedermann SA, Aggarwal M, Kinney PL, Tsai WY, Whyatt RM, Perera FP, Ford JG. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med. 2001;164:995. doi: 10.1164/ajrccm.164.6.2011107. [DOI] [PubMed] [Google Scholar]
- 48.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 49.Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 50.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]


