Abstract
Amebiasis is a major cause of morbidity and mortality worldwide. Invasion by Entamoeba histolytica trophozoites causes secretion of proinflammatory cytokines from host epithelial cells, leading to a local acute inflammatory response, followed by lysis of colonic cells. Extracellular cysteine proteinases from amebic trophozoites are key virulence factors and have a number of important interactions with host defenses, including cleavage of immunoglobulin G (IgG), IgA, and complement components C3 and C5. Amebic lysates have also been shown to activate the precursor to interleukin 1-beta (proIL-1β), mimicking the action of caspase-1. IL-18 is also a central cytokine, which induces gamma interferon (IFN-γ) and activates macrophages, one of the main host defenses against invading trophozoites. Because proIL-18 is also activated by caspase-1, we evaluated whether amebic proteinases had a similar effect. Instead, we found that recombinant proIL-18 was cleaved into smaller fragments by the complex of surface-associated and released amebic proteinases. To evaluate the function of an individual proteinase from the complex pool, we expressed an active surface proteinase, EhCP5, which is functional only in E. histolytica. Recombinant EhCP5 expressed in Pichia pastoris had kinetic properties similar to those of the native enzyme with respect to substrate specificity and sensitivity to proteinase inhibitors. In contrast to the activation of proIL-1β by amebic lysates, the purified proteinase cleaved proIL-18 and mature IL-18 to biologically inactive fragments. These studies suggest that the acute host response and amebic invasion result from a complex interplay of parasite virulence factors and host defenses. E. histolytica may block the host inflammatory response by a novel mechanism, inactivation of IL-18.
Entamoeba histolytica is an enteric protozoan parasite that causes amebic dysentery and liver abscesses. Trophozoites invade the bowel by attaching to the epithelium through a galactose-inhibitable lectin (21), degrading the extracellular matrix by the action of neutral cysteine proteinases (16), lysing epithelial cells via an amebapore (20), and penetrating into the mucosa. During this process, multiple potent chemoattractant and proinflammatory cytokines are released by host epithelial cells (10), initiating an acute inflammatory response, which is seen in animal models (6) and human intestinal xenografts (29). Multiple factors enter in the control of the acute inflammatory response. Interleukin 8 (IL-8) and growth-related oncogene alpha released from epithelial cells act as chemoattractants and activators of neutrophils (2), while neutral cysteine proteinases from the amebae degrade the anaphylatoxins C3a and C5a (25).
IL-18, which is also expressed in intestinal epithelial cells (8), is a coinducer of the Th1 response. The resulting stimulation of gamma interferon (IFN-γ) then activates macrophages, the major cell capable of killing E. histolytica trophozoites (27). Unlike most other cytokines, IL-18 and IL-1β lack a signal peptide and are first synthesized as biologically inactive precursors (proIL-18 and proIL-1β). These precursors are cleaved by caspase-1 (IL-1β-converting enzyme [ICE]), after an aspartic acid residue in the P1 position. The resulting mature cytokines are subsequently released from cells (8, 19). Amebic cysteine proteinases also possess ICE-like activity, which cleaves proIL-1β to produce the active proinflammatory cytokine IL-1β (33). Studies in a human intestinal xenograft model of disease indicated that E. histolytica trophozoites that were transfected with an antisense plasmid to the ehcp5 gene failed to induce intestinal epithelial cell production of the inflammatory cytokines IL-1β and IL-8 and caused significantly less intestinal inflammation and tissue damage (33). We asked whether amebic proteinases could also act as an IL-18 activator in vitro.
Cysteine proteinases are the major extracellular enzymes responsible for in vitro cytopathology and degradation of the extracellular matrix during the first steps of bowel invasion (16, 23-24). To date, seven genes encoding cysteine proteinases have been identified in E. histolytica (4, 11, 24). One particular cysteine proteinase (designated EhCP5) is located on the surface of E. histolytica trophozoites and is not expressed in the closely related but noninvasive species, Entamoeba dispar (15). We now report the expression of active recombinant EhCP5 cysteine proteinase in the Pichia pastoris yeast and the characterization of the purified active enzyme. We show that rEhCP5 inactivates both pro- and mature IL-18, potentially limiting the host immune defenses.
MATERIALS AND METHODS
Expression and purification of proIL-18.
For prokaryotic expression of human proIL-18, the human proIL-18 coding sequence was cloned in-frame into the pProExHTa expression vector (Invitrogen, Carlsbad, Calif.) using BamHI and EcoRI sites (19). The proIL-18 was expressed in Escherichia coli as an N-terminally His6-tagged recombinant protein and purified on a nickel-nitrilotriacetic acid (Ni-NTA) agarose resin column. The pProExHTa/IL-18 plasmid was transformed into the competent E. coli strain TOP10 (Invitrogen). An overnight culture of 25 ml from a fresh, single E. coli colony transformed with the plasmid pProExHTa/IL-18 was added to 450 ml of Luria-Bertani medium containing 100 μg of ampicillin/ml and grown until it reached an optical density of 0.6 to 1.0. Protein expression was then induced by adding isopropylthiogalactoside (0.3 mM), and incubation continued at 37°C with shaking for 4 h. Bacteria were harvested, and the pellet was suspended in 20 ml of lysis buffer (19) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), a serine protease inhibitor. Cells were lysed by mild sonication (two times, 30-s bursts) on ice. The soluble protein was applied to a 3-ml mini-Ni-NTA column. The Ni-NTA column was washed with 30 bed volumes of wash buffer and then eluted with 6 ml of 250 mM imidazole in wash buffer. The elute was dialyzed against 50 mM Tris buffer, pH 7.5, at 4°C for 20 h and concentrated by ultrafiltration (Centricon-10; Amicon, Bedford, Mass.) and stored at −70°C.
Amebic cultures and released proteinases.
E. histolytica strain HM1:IMSS was grown axenically in TYI-S-33 medium (7) and subcultured every 48 to 72 h. To obtain released proteinases, purified trophozoites were incubated in minimal essential medium-HEPES buffer solution with cysteine (MEM-CH) (Difco, Detroit, Mich.) at a concentration of 5 × 106/ml for an hour at 37°C and the supernatant was collected. Purified proIL-18 (80 ng) was incubated with 4 μl of released proteinases (5 pmol of 7-amino-4-methyl coumarin [AMC] cleaved/min/μl) (24) at 37°C, and aliquots were removed at timed intervals. Samples were boiled in the presence of Complete proteinase inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany) plus 100 μM E-64 and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to nitrocellulose, and the resulting immunoblot was incubated with a 1:1,000 dilution of anti-human IL-18 (US Biological, Swampscott, Mass.) followed by goat anti-mouse conjugate (ZyMed, San Francisco, Calif.) and developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) buffer solution (ZyMed).
Antibodies.
EhCP5 antibodies were made by immunizing rabbits with inactive rEhCP5 expressed in pBAD/Thio-TOPO (Invitrogen) and purified using His-trap columns under denaturing conditions, according to kit instructions. Rabbits were immunized with 100 μg of purified protein with Titermax (Sigma, St. Louis, Mo.) and boosted once. The immunoglobulin G (IgG) fraction was purified over a protein G column (Amersham Pharmacia Biotech, Piscataway, N.J.).
Expression and purification of Pichia-expressed EhCP5 proteinase.
Recombinant EhCP5 was expressed in P. pastoris under the control of a methanol-inducible, alcohol oxidase (AOX1) promoter and the yeast α-factor secretion signal (Invitrogen). The coding region of the amebic EhCP5 gene was isolated from genomic DNA of E. histolytica by PCR. The sense primer (5′-GTG CTC GAG AAA AGA ACA AAT TTC AAT ACT TGG GTT G-3′) encoded the seven amino acids at the amino terminus of the pro-EhCP5 and contained an XhoI site (italics) and a Kex2 cleavage site (EKR, underlined) at the 5′ end. The reverse primer (5′-ATA GCG GCC GCT TAA GCA TCA GCA ACC CCA ACT-3′) encoded six amino acids at the carboxyl terminus of the EhCP5 proteinase and a NotI site (italics). The pPICZα-CP5 plasmid was made by inserting the pro-EhCP5 gene into the XhoI and NotI sites of the Pichia vector, pPICZαA, in frame and downstream of the α-factor signal sequence and the Kex2 cleavage site. Pichia pastoris strain X33 was transformed by electroporation with the linearized recombinant plasmid pPICZα-CP5 (10 μg) to target integration into the chromosomal 5′-AOX1 locus via homologous recombination. Procedures to express rEhCP5 in the X33 strain of P. pastoris were carried out as detailed by the manufacturer (Invitrogen). Briefly, Pichia colonies growing under Zeocin selection were first screened for proteinase activity in small-scale cultures (10 ml). Single colonies expressing the highest level of proteinase activity were inoculated in larger-scale cultures of yeast extract-peptone-dextrose medium (600 ml) for 2 days at 28°C and then in buffered minimal medium (containing 1% methanol as the sole carbon source to activate the AOX1 promoter). The culture supernatant was clarified through a 0.45-μm-pore-size filter, lyophilized, and stored at −20°C. Lyophilized culture media containing rEhCP5 proteinase were resuspended to a volume one-tenth of that the original and dialyzed in Tris buffer, pH 7.5, for 8 h with several buffer exchanges. Recombinant EhCP5 was purified by ion exchange chromatography on a Mono-Q column (FPLC System; Amersham Pharmacia Biotech), and eluted with a linear gradient of 0 to 500 mM NaCl in 25 mM Tris-2 mM dithiothreitol (DTT), pH 7.4. Column fractions were tested for proteolytic activity against the fluorogenic peptidyl substrate (Z-arginine-arginine-AMC; Enzyme System Products, Livermore, Calif.) in 96-well microtiter plates. Fractions containing the highest level of activity were pooled and concentrated in Centricon-10 centrifugation units (10-kDa cutoff; Millipore, Bedford, Mass.) and stored at −70°C until analysis.
Substrate specificity and kinetic studies.
The activity of rEhCP5 was assayed by the liberation of the fluorescent leaving group, AMC, from the peptide substrates, Z-Arg-Arg-AMC, Z-Ala-Arg-Arg-AMC, Z-Phe-Arg-AMC, and Z-Phe-Ala-Arg-AMC (Enzyme System Products). Assays were performed in black 96-well microtiter plates and contained 4 nM enzyme that had been preincubated for 5 min in 100 μl of buffer consisting of 50 mM Tris, 2 mM EDTA, and 2 mM DTT (pH 7.5). Peptidyl substrates (8 μM) in 100 μl of the same buffer were then added to the reaction. Fluorescence was measured up to 10 min in a Labsystems Fluoroskan II plate reader at excitation and emission wavelengths of 355 and 460 nm, respectively (22). The initial velocities were calculated by linear regression of the substrate hydrolysis curves. The kinetic parameters Km and kcat were extrapolated from Lineweaver-Burk plots.
pH optimum and stability.
The pH profile of the purified rEhCP5 was determined by assaying cleavage of the preferred synthetic peptide substrate Z-Arg-Arg-AMC in buffers with pHs ranging from 4.0 to 10.0 using 50 mM acetate for pH 4 to 6 and 50 mM Tris for pH 6 to 10. Stabilities of the purified enzymes were evaluated by incubation in 50 mM Tris-2 mM EDTA, pH 8.0, at 37°C. At timed intervals, aliquots were removed and the cleavage of synthetic substrates was measured.
Enzyme and inhibition assays.
The inhibitor profile of rEhCP5 was determined by monitoring inhibition of the cleavage of the preferred substrate, Z-Arg-Arg-AMC (4 μM), in the presence of inhibitor. Inhibitors at various concentrations were preincubated with the enzyme (4 nM) for 5 min prior to addition of the substrate. Enzyme activity was expressed as percent residual activity compared with an uninhibited control and was plotted versus increasing inhibitor concentrations to calculate the 50% inhibitory concentration (IC50). The reversible hydrazide inhibitors (ZL-compounds, ZLIII115A), irreversible vinyl sulfone inhibitors (K-compounds, K002 and K777), and Z-Phe-Arg-fluoromethyl ketone (Z-FR-FMK) were also tested against rEhCP5. Inhibitors were prepared as 10 mM stocks in dimethyl sulfoxide and stored at −20°C. (For structures, see the URL http://itsa.ucsf.edu/∼schisto/).
Cytokines.
Bioactive, mature rIL-18 was obtained from Medical and Biological Laboratory (Nagoya, Japan), and IL-12 was obtained from PeproTech (Rocky Hill, N.J.).
Enzymatic cleavage of pro- and mature IL-18 by rEhCP5.
The affinity-purified proIL-18 or mature IL-18 was incubated with the rEhCP5 enzyme at 37°C for 3 h in the presence or absence of E-64 in a total volume of 12.5 μl of reaction buffer (50 mM Tris, 2 mM EDTA, 2 mM DTT [pH 7.5]). At timed intervals, aliquots were removed and resolved on reducing SDS-PAGE gels followed by immunoblots. N-terminal protein sequence analysis was performed on individual protein bands, obtained by SDS-15% PAGE under reducing conditions, followed by electroblotting to a polyvinylidene difluoride membrane using a semidry blotting apparatus. The proteins were visualized by Coomassie brilliant blue staining and excised for direct NH2-terminal sequencing at the University of California—San Francisco Protein Sequencing Core. For immunoblots, gels were transferred to nitrocellulose membranes and then incubated with primary monoclonal anti-human IL-18 antibody (1:1,000 dilution; US Biological). After 1.5 h, goat anti-mouse conjugate (ZyMed) was added and developed with an NBT-BCIP buffer solution (ZyMed).
NKO cell line assays for IL-18.
IL-18 activity was assessed by its ability to produce IFN-γ in human NKO cells upon costimulation with IL-12 (18). Briefly, NKO cells were seeded into 96-well culture plates (2 × 105 per well) and stimulated with 0.5 to 1.0 ng of IL-12/ml and various concentrations of IL-18 cleavage products (final volume, 250 μl) with or without E-64 (20 μM). After 24 h at 37°C in humidified air with 5% CO2, the culture supernatant was collected for IFN-γ assay by liquid-phase electrochemiluminescence (Igen, Gaithersburg, Md.) (18).
RESULTS
Cleavage of human pro-IL-18 by released amebic proteinases.
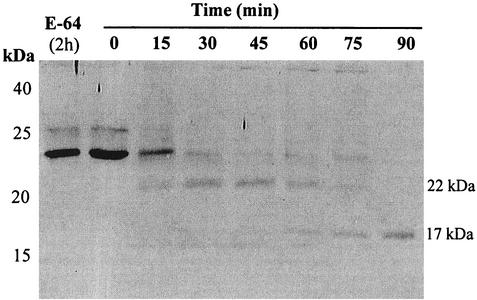
To determine whether proIL-18 was activated by amebic cysteine proteinases, we first expressed recombinant proIL-18 and evaluated its cleavage by released amebic cysteine proteinases. The His6 residue tag was expressed at the N terminus of proIL-18, allowing purification by a nickel-NTA affinity column. Fusion of proIL-18 to the His tag at its N terminus did not adversely affect the overall folding of proIL-18, as was evident from the substantial biological activity observed following cleavage from previous studies (18). Following induction, a 24-kDa band, corresponding in size to the fusion protein, was detected in bacterial extracts by SDS-PAGE. When proIL-18 was incubated with released amebic proteinases, proIL-18 was cleaved into 22- and 17-kDa fragments, which were further degraded into fragments smaller than mature IL-18 (18 kDa) (Fig. 1).
FIG. 1.
Cleavage of proIL-18 by released amebic proteinases. Recombinant proIL-18 (10 ng) was incubated with released amebic proteinases (2.5 U) at 37°C at designated times and analyzed by SDS-12% PAGE and blotted with anti-IL-18 monoclonal antibody.
Expression of enzymatically active EhCP5 proteinase in the P. pastoris.
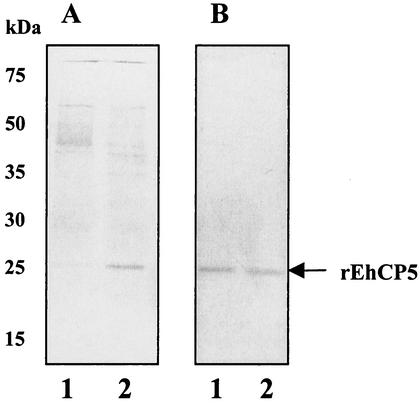
Because the crude preparation of released proteinases likely contains multiple enzymes, we evaluated the cleavage of proIL-18 by a single, purified amebic cysteine proteinase. We chose to express recombinant EhCP5, a surface proteinase which is present only in invasive E. histolytica (15). The construct generated a fusion product of proEhCP5 under the control of the yeast α-factor promoter (3, 13). This system allows removal of the α-factor signal peptide by the yeast Kex2 enzyme as the fusion protein enters the secretory pathway. The rEhCP5 expressed in Pichia X33 was found as an active mature enzyme in the culture supernatant. After further purification by ion exchange chromatography on a Mono-Q column, the specific activity was enhanced approximately 40 times. Silver-stained SDS-PAGE gels of purified rEhCP5 showed a band with an apparent molecular mass of 25 kDa in the last step of purification (Fig. 2A). A band of 25 kDa was also identified by Western blotting using a rabbit antiserum raised against rEhCP5 (Fig. 2B). Heterologous expression of EhCP5 in Pichia produced an active cysteine proteinase at levels of up to 1.5 mg/liter in culture medium. Purified rEhCP5 was highly stable, retaining greater than 95% of its activity after storage in 50% glycerol at −20°C for more than 6 months.
FIG. 2.
Expression of recombinant EhCP5 by Pichia transformed with the vector pPICZα-CP5. (A) Silver staining of the fraction containing cysteine proteinase activity from the culture supernatant of pPICZα-CP5-transformed Pichia cells (10-fold concentrated) (lane 1) or purified rEhCP5 proteinase (lane 2). (B) Western blot of rEhCP5 using anti-EhCP5 antiserum.
Substrate specificity and kinetic properties of rEhCP5.
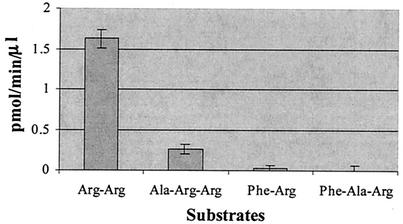
To investigate the peptide substrate specificity of rEhCP5, the cleavage of small chromogenic peptide substrates was compared. The preferred substrates of rEhCP5 contained an arginine residue at the P1 and P2 positions, similar to findings with the native enzyme (15). The rEhCP5 showed a strong preference for the cathepsin B substrate (Z-Arg-Arg-AMC) over the cathepsin L (Z-Phe-Arg-AMC) (Fig. 3). Although rEhCP5 is structurally closely related to mammalian cathepsin L, rEhCP5 has the substrate specificity of cathepsin B enzymes. The specific activity was 65-fold higher with the Arg-Arg substrate than with the Phe-Arg substrate. The kinetic constants for the hydrolysis of Z-Arg-Arg-AMC are the following: Km 7.29 μM; and kcat, 46.65 s−1.
FIG. 3.
Substrate specificity of rEhCP5. The substrate specificity was measured using different peptide substrates with 7-AMC as the leaving group and following the initial velocity of the reaction.
pH optimum and inhibition profiles.
The rEhCP5 was active in a broad pH range of 4.5 to 10 and had a pH optimum between 8.5 and 9.0. Activity against the substrate Z-Arg-Arg-AMC was maximal at alkaline pH (pH 8.8) and was significantly stimulated by the reducing agent DTT. rEhCP5 showed greater stability at alkaline pH values than native enzymes. Significantly, native enzymes were almost completely inactivated after 10 min of incubation at pH 11 (X. Que, unpublished observation), while rEhCP5 still displayed 76% of its initial activity. The greater stability of rEhCP5 at extreme alkaline pH values (pH 11) may stem from the secondary structure in the central domain of recombinant protein. The rEhCP5 was stable at room temperature (22°C) and retained 75% activity after 24 h. However, following incubation at 37°C for 4 and 24 h, the rEhCP5 lost 60 and 87% of its activity, respectively.
The proteinase activity of rEhCP5 was not affected by EDTA (10 mM) or EGTA (10 mM). Very little effect on enzymatic activity was measured with 1 mM PMSF (serine proteinase inhibitor) or 0.1 mM pepstatin (aspartic proteinase inhibitor) (Table 1). Consistent with previous studies of native amebic proteinases, rEhCP5 was sensitive to E-64 (IC50 = 6 μM), a specific papain-like cysteine proteinase inhibitor (Table 2). Both reversible and irreversible cysteine proteinase inhibitors can effectively target rEhCP5 of E. histolytica in a dose-dependent manner. However, the reversible hydrazide inhibitor (ZL compounds, ZLIII115A; IC50 = 20 μM) and irreversible vinyl sulfone (K002 [IC50 = 15 μM] and K777 [IC50 = 8.5 μM]) inhibitors (Table 2), which effectively blocked the activity of Leishmania cpB and cpL proteases (28), less effectively targeted rEhCP5. E-64 inhibited both the native (15) and recombinant enzymes (IC50 = 6 μM), and peptidyl fluoromethyl ketone inhibitors (Z-FR-FMK) were effective as well.
TABLE 1.
Effect of different classes of inhibitors on rEhCP5 activity
| Inhibitor | Class | Concn | Inhibition (%) |
|---|---|---|---|
| EDTA | Metallo | 20 mM | 0 |
| PMSF | Serine | 1 mM | 7.1 |
| Pepstatin | Aspartic | 0.1 mM | 8.6 |
| TLCK | Serine/cysteine | 10 μM | 78.9 |
| E-64 | Cysteine | 10 μM | 84.3 |
| Z-FR-FMK | Serine/cysteine | 10 μM | 98.1 |
TABLE 2.
Inhibition of rEhCP5 with cysteine proteinase inhibitors
| Inhibitor | IC50 (<5 μM) | IC50 (>5 μM) |
|---|---|---|
| MC357 | 5 | |
| SKB439903 | 4.5 | |
| SKB331750 | 4 | |
| WRR204 | 1.5 | |
| WRR313 | 30 | |
| WRR276 | 15 | |
| ZLIII115A | 20 | |
| K777 | 8.5 | |
| TF1-75 | 6 | |
| DU5-17 | 70 | |
| E-64 | 6 |
Cleavage of pro- and mature IL-18 by rEhCP5 generates biologically inactive fragments.
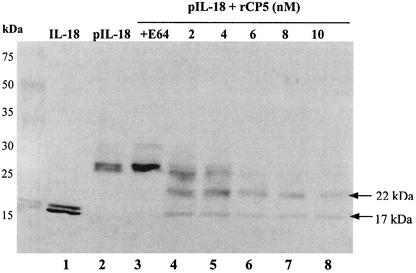
ProIL-18 was digested with rEhCP5 and analyzed by SDS-PAGE for the presence of cleavage fragments. Two protein bands (22 and 17 kDa) were detected (Fig. 4). A 22-kDa band, corresponding to the incompletely digested proIL-18 without the His6 tag, was still present in the cleavage mixture. Upon prolonged exposure, rEhCP5 further degrades IL-18 to fragments with masses of less than 18 kDa, the size of mature IL-18.
FIG. 4.
Cleavage of proIL-18 by rEhCP5. Recombinant proIL-18 was incubated with increasing concentrations of rEhCP5 (0 to 10 nM) at 37°C for 3 h. Controls included proIL-18 and IL-18 in buffer alone for 3 h and proIL-18 incubated with rCP5 (8 nM) that was inhibited with 20 μM E-64. The cleavage mixtures were analyzed by SDS-12% PAGE and blotted with anti-IL-18 monoclonal antibody. Lane 1, mature IL-18 (18.3 kDa); lane 2, recombinant proIL-18 (10 ng); lane 3, proIL-18 (20 ng) plus rEhCP5 (8 nM) plus E-64 (20 μM); lanes 4 to 8, proIL-18 incubated with the indicated amount of rEhCP5.
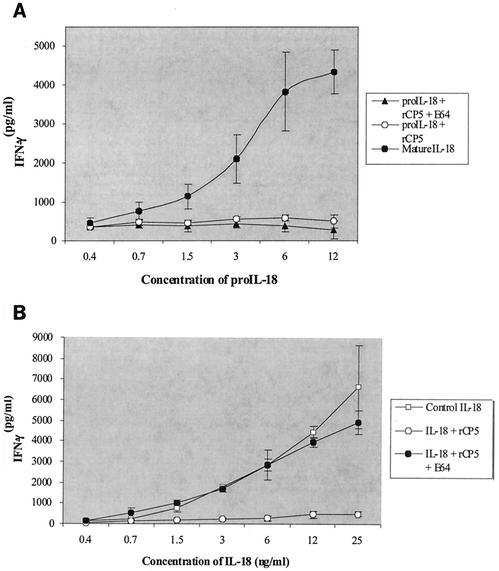
We measured the bioactivity of the proIL-18 cleavage fragments with an assay based on induction of IFN-γ in NKO cells in the presence of low-dose IL-12. Cells were incubated with different concentrations of the cleavage products together with 0.5 to 1 ng of IL-12/ml. Neither uncleaved proIL-18 (rEhCP5 plus E-64) nor cleaved proIL-18 (plus rEhCP5) had biological activity (Fig. 5A), in contrast to mature IL-18 (caspase derived), which induced IFN-γ in a dose-dependent manner (Fig. 5B) (P < 0.001). Recombinant CP5 alone had no effect on NKO cells (data not shown).
FIG. 5.
Bioassays of the pro- and mature IL-18 cleaved products. NKO cells were stimulated with proIL-18 (A) and mature IL-18 (B) that was cleaved, uncleaved, or cleaved in the presence of inhibitor (E-64) samples at concentrations shown under the horizontal axis in the presence of IL-12 (0.5 to 1.0 ng/ml). After 24 h, IFN-γ was measured at the concentration shown to the left of the vertical axes. The data represent means ± standard errors of the means (n = 3).
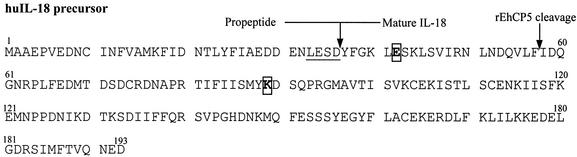
The lack of activity of proteinase-cleaved proIL-18 was explained by peptide sequencing of the resulting fragment, which revealed that rEhCP5 cleaved the proIL-18 between Phe57 and Ile58 to a 17-kDa fragment, 21 residues less at its amino terminus than mature IL-18 (Fig. 6). Therefore, we tested whether rEhCP5 could also inactivate mature IL-18. Once cleaved by recombinant EhCP5, mature IL-18 also lost biological activity in NKO cells (Fig. 5B). When the cysteine proteinase inhibitor (E-64) was added to block the cleavage of mature IL-18 by rEhCP5, the protected mature IL-18 was still highly active in NKO cells (Fig. 5B) (P < 0.001).
FIG. 6.
Site of cleavage of proIL-18 by rEhCP5. The underlined area indicates the ICE cleavage site, and the amino acids in bold are critical residues for biological activity of IL-18.
DISCUSSION
Successful invasion by E. histolytica trophozoites requires a fine balance between expression of parasite virulence factors and host defenses. Only a minority of asymptomatic carriers of potentially invasive E. histolytica develop clinical amebiasis (12). Yet when invasion is successful, severe amebic colitis or liver abscesses can result in a mortality rate second only to those of malaria and schistosomiasis among parasitic diseases (31). Thus, further understanding of the host-parasite interactions is key to analyzing the pathogenesis of invasive amebiasis.
The epithelial layer of the intestine is both a physical barrier against entry of invasive E. histolytica and the first cellular target. A complex signaling system of cytokines is triggered by pathogen invasion (9). We have shown that E. histolytica trophozoites cocultured with human cell lines cause release of proinflammatory cytokines (10), which results in early neutrophil infiltration (29). The cellular response is also affected by the ability of amebic cysteine proteinases to cleave proIL-1β to the active cytokine (33). Because both proIL-1β and proIL-18 are activated by caspase-1, we hypothesized that a similar activation of proIL-18 by amebic cysteine proteinases might occur.
Cysteine proteinases are central to many of the parasite's interactions with immune and nonimmune host defenses. They degrade components of the extracellular matrix, causing the initial separation of epithelial cells leading to invasion (16). They are able to interrupt humoral immunity by degrading IgA (17) and IgG (30). Extracellular cysteine proteinases also activate the alternative pathway of complement and degrade the anaphylatoxins C3a and C5a (25). We first investigated whether released cysteine proteinases could activate proIL-18. We found that proIL-18 was cleaved into a 17-kDa fragment, smaller than mature IL-18 (Fig. 1).
IL-18 is a key proinflammatory cytokine and an important mediator of the Th1 immune response. IL-18 has structural similarities to the IL-1 family of proteins and is synthesized as an inactive precursor molecule with no signal peptide sequence. Proteolytic cleavage by caspase-1, an intracellular cysteine proteinase also known as ICE, is required for IL-18 maturation (8, 19).
Because E. histolytica has at least five expressed cysteine proteinases (4, 11, 24), any studies using native enzymes are likely to contain a mixed population of proteinases. Therefore, we chose to express a single recombinant enzyme, EhCP5, which is a surface-associated proteinase on E. histolytica trophozoites but absent from noninvasive E. dispar (15). We expressed a functional recombinant EhCP5 proteinase in Pichia transformed with the ehcp5 cDNA. When recombinant pro-cathepsin S or pro-papain were expressed in Saccharomyces, the enzymes accumulated in the intracellular milieu as the pro-form and required acidic pH and the presence of reducing agents to undergo self-maturation in vitro (3, 13). In contrast, we readily detected active enzyme in the culture supernatant. The conditions used for pro-enzyme maturation, 24 h of incubation at acidic pH at 37°C, failed to enhance the yield of activity found in Pichia extracts. These finding suggest that the precursor EhCP5 processed to mature enzyme within the Pichia cell. The enzyme was a major component of the extracellular culture medium and was purified to homogeneity by Mono Q chromatographic steps (Fig. 2). Enzyme kinetic data showed that Pichia-expressed EhCP5 and native amebic cysteine proteinases have the same specificity for substrates with positively charged residues in the P2 position. To date, all purified recombinant proteinases, including ACP1, ACP2 (22), and EhCP5, have the same peptide substrate specificity. Potential differences in activity against physiologic substrates remain to be defined.
We next investigated cleavage of proIL-18 by rEhCP5. When recombinant proIL-18 was incubated with rEhCP5, the proIL-18 was cleaved into a 17-kDa fragment, which was further degraded (Fig. 4) with a loss of biological activity after 3 h of incubation at 37°C (Fig. 5A). Peptide sequencing demonstrated that the cleavage site was 21 residues from the start of mature IL-18 (Fig. 6). The cleavage by rEhCP5 removed Glu-42, a key residue for biological activity of IL-18 (18), and therefore, the cleavage products had no biological activity (Fig. 5B). Recombinant EhCP5 cleaved pro- or mature IL-18 between phenylalanine and isoleucine. Molecular modeling of EhCP5 identified a glycine at the base of the S2 pocket of EhCP5, which might give the enzyme a broader substrate specificity (22). The secondary structure of IL-18 may also contribute to the observed cleavage site, which does not directly correlate with peptide substrate specificities. ProIL-1β was also cleaved by amebic proteinases, but only five residues from the caspase site, resulting in a smaller fragment which still retained biological activity (33).
T-cell immunity is the major host defense against amebiasis. IFN-γ-activated macrophages can kill E. histolytica through both oxidative and nonoxidative mechanisms (5). Nitric oxide is the major mediator of amebicidal activity of macrophages (27). T cells incubated with the amebic Gal/GalNAc lectin stimulate contact-dependent cytolysis of trophozoites (26). Therefore, the Th1 response, initiated by IL-18, is required for effective immunity against amebiasis. Other pathogens have targeted this arm of the immune response. Poxvirus makes a homologue to IL-18 binding protein which blocks active IL-18 (32). To date, no other parasite directly targets cytokines. E. histolytica may also act indirectly by upregulating caspase-3 in host cells (14), the natural regulator of IL-18 activity (1). We have shown that an amebic surface proteinase is capable of preventing activation of proIL-18 and degrading mature IL-18, a novel mechanism for blocking the host immune response against pathogenic amebae. Thus, specific inhibitors which block the action of cysteine proteinases both on initial invasion (25) and on the host immune response may prove to have therapeutic potential in the future.
Acknowledgments
This work was funded in part by National Institutes of Health grants AI-28035, AI-49531, and DK-35108 (S.R.), AI-35707 (J.H.M.), and AI-15614 (C.A.D.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akita, K., T. Ohtsuki, Y. Nukada, T. Tanimoto, M. Namba, T. Okura, R. Takakura-Yamamoto, K. Torigoe, Y. Gu, M. S. Su, M. Fujii, M. Satoh-Itoh, K. Yamamoto, K. Kohno, M. Ikeda, and M. Kurimoto. 1997. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP0.1 cells. J. Biol. Chem. 272:26595-26603. [DOI] [PubMed] [Google Scholar]
- 2.Baggliolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and released chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 3.Bromme, D., P. R. Bonneau, P. Lachance, B. Wiederanders, H. Kirschke, C. Peters, D. Y. Thomas, A. C. Store, and T. Vernet. 1993. Expression of functional papain precursor in Saccharomyces cerevisiae: rapid screening of mutants. Protein Eng. 6:213-219. [DOI] [PubMed] [Google Scholar]
- 4.Bruchhaus, I., T. Jacobs, M. Leippe, and E. Tannich. 1996. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol. Microbiol. 22:255-263. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, D., and K. Chadee. 1997. Survival strategies of Entamoeba histolytica: modulation of cell-mediated immune responses. Parasitol. Today 13:185-190. [DOI] [PubMed] [Google Scholar]
- 6.Chadee, K., and E. Meerovitch. 1985. The pathology of experimentally induced cecal amebiasis in gerbils (Meriones unguiculatus). Am. J. Pathol. 119:485-494. [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello, C. A. 1999. Interleukin-18. Methods 19:121-132. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann, L., H. C. Jung, C. Schurer-Maly, A. Panja, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1993. Differential cytokine expression by human intestinal epithelial cells: regulated expression of interleukin-8. Gastro 105:1689-1697. [DOI] [PubMed] [Google Scholar]
- 10.Eckmann, L., S. L. Reed, J. R. Smith, and M. F. Kagnoff. 1995. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1α. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Rivera, G., M. A. Rodriguez, R. Ocadiz, M. C. Martinez-Lopez, R. Arroyo, A. Gonzalez-Robles, and E. Orozco. 1999. Entamoeba histolytica: a novel cysteine protease and an adhesin form the 112 kDa surface protein. Mol. Microbiol. 33:556-568. [DOI] [PubMed] [Google Scholar]
- 12.Gathiram, V., and T. F. H. G. Jackson. 1987. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. S. Afr. Med. J. 72:669-672. [PubMed] [Google Scholar]
- 13.Hasnain, S., T. Hirama, A. Tam, and J. S. Mort. 1992. Characterization of recombinant rat cathepsin B and non-glycosylated mutants expressed in yeast. New insights into pH dependence of cathepsin B-catalyzed hydrolyses. J. Biol. Chem. 267:4713-4721. [PubMed] [Google Scholar]
- 14.Huston, C. D., E. R. Houpt, B. J. Mann, C. S. Hahn, and W. A. Petri. 2000. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell. Microbiol. 2:617-625. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, T., I. Bruchhaus, T. Dandekar, E. Tannich, and M. Leippe. 1998. Isolation and molecular characterization of a surface-bound proteinase of Entamoeba histolytica. Mol. Microbiol. 27:269-276. [DOI] [PubMed] [Google Scholar]
- 16.Keene, W. E., M. G. Pettit, S. Allen, and J. H. McKerrow. 1986. The major neutral proteinase of Entamoeba histolytica. J. Exp. Med. 163:536-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelsall, B. L., and J. I. Ravdin. 1993. Degradation of human immunoglobulin A by Entamoeba histolytica. J. Infect. Dis. 168:1319-1322. [DOI] [PubMed] [Google Scholar]
- 18.Kim, S. H., T. Azam, D. Novick, D. Y. Yoon, L. L. Reznikov, P. Bufler, M. Rubinstein, and C. A. Dinarello. 2002. Identification of amino acid residues critical for biological activity in human interleukin-18. J. Biol. Chem. 277:10998-11003. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S. H., T. Azam, D. Y. Yoon, L. L. Reznikov, D. Novick, M. Rubinstein, and C. A. Dinarello. 2001. Site-specific mutations in the mature form of human IL-18 with enhanced biological activity and decreased neutralization by IL-18 binding protein. Proc. Natl. Acad. Sci. 98:3304-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leippe, M., E. Sebastian, O. L. Schoenberger, R. D. Horstmann, and H. J. Muller-Eberhard. 1991. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 88:7659-7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCoy, J. J., B. J. Mann, and W. A. Petri. 1994. Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect. Immun. 62:3045-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Que, X., L. S. Brinen, P. Perkins, D. S. Herdman, K. Hirata, B. E. Torian, H. M. J. H. Rubin, and S. L. Reed. 2002. Cysteine proteinases from distinct cellular compartments are recruited to phagocytic vesicles by Entamoeba histolytica. Mol. Biochem. Parasitol. 119:23-32. [DOI] [PubMed] [Google Scholar]
- 23.Que, X., and S. L. Reed. 1997. The role of extracellular cysteine proteinases in pathogenesis of Entamoeba histolytica invasion. Parasitol. Today 13:190-194. [DOI] [PubMed] [Google Scholar]
- 24.Reed, S. L., J. Bouvier, A. S. Pollack, J. C. Engel, M. Brown, K. Hirata, X. Que, A. Eakin, P. Hagblom, F. D. Gillin, and J. H. McKerrow. 1993. Cloning of a virulence factor of Entamoeba histolytica: pathogenic strains possess a unique cysteine proteinase gene. J. Clin. Investig. 91:1532-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed, S. L., J. A. Ember, D. S. Herdman, R. G. DiScipio, T. E. Hugli, and I. Gigli. 1995. The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a. J. Immunol. 155:266-274. [PubMed] [Google Scholar]
- 26.Schain, D. C., R. A. Salata, and J. I. Ravdin. 1992. Human T-lymphocyte proliferation, lymphokine production, and amebicidal activity elicited by the galactose-inhibitable adherence protein of Entamoeba histolytica. Infect. Immun. 60:2143-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seguin, R., B. J. Mann, K. Keller, and K. Chadee. 1997. The tumor necrosis factor alpha-stimulating region of galactose-inhibitable lectin of Entamoeba histolytica activates gamma interferon-primed macrophages for amebicidal activity mediated by nitric oxide. Infect. Immun. 65:2522-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selzer, P. M., S. Pingel, I. Hsieh, B. Ugele, V. J. Chan, J. C. Engel, M. Bogyo, D. G. Russell, J. A. Sakanari, and J. H. McKerrow. 1999. Cysteine protease inhibitors as chemotherapy: lessons from a parasite target. Proc. Natl. Acad. Sci. USA 96:11015-11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seydel, K. B., E. Li, P. E. Swanson, and S. L. Stanley. 1997. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect. Immun. 65:1631-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran, V. Q., D. S. Herdman, B. E. Torian, and S. L. Reed. 1998. The neutral cysteine proteinase of Entamoeba histolytica degrades IgG and prevents its binding. J. Infect. Dis. 177:508-511. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization/Pan America Health Organization Expert Consultation on Amoebiasis. 1997. Amoebiasis. Wkly. Epidemiol. Rec. 72:97-100.9100475 [Google Scholar]
- 32.Yiang, Y., and B. Moss. 1999. IL-18 binding and inhibition of interferon gamma induction by human poxvirus-encoded proteins. Proc. Natl. Acad. Sci. USA 96:11537-11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, Z., L. Yan, L. Wang, K. B. Seydel, E. Li, S. Ankri, D. Mirelman, and S. L. Stanley. 2000. Entamoeba histolytica cysteine proteinases with interleukin-1 beta converting enzyme (ICE) activity cause intestinal inflammation and tissue damage in amoebiasis. Mol. Microbiol. 37:5442-5548. [DOI] [PubMed] [Google Scholar]