Abstract
Placenta-sequestered Plasmodium falciparum parasites that cause pregnancy-associated malaria (PAM) in otherwise clinically immune women express distinct variant surface antigens (VSAPAM) not expressed by parasites in nonpregnant individuals. We report here that parasites from the peripheral blood of clinically immune pregnant women also express VSAPAM, making them a convenient source of VSAPAM expressors for PAM vaccine research.
Pregnancy-associated malaria (PAM) caused by the accumulation of Plasmodium falciparum parasites in the placental intervillous space is a major cause of maternal anemia, low birth weight, and infant mortality (8-10, 20). In areas of intense parasite transmission where adults are generally clinically immune to malaria, primigravid women are highly susceptible to PAM and this susceptibility rapidly decreases with increasing parity (2). These observations suggest that parasites involved in the pathogenesis of PAM are antigenically distinct from parasites causing disease in nonpregnant individuals and that protective immunity to PAM is rapidly developed following exposure to such parasites. Experimental evidence supports this scenario. Parasites isolated at delivery from the placentas of women with PAM can adhere to chondroitin sulfate A (CSA), an adhesive phenotype rarely seen among other parasites (1, 5, 17). This adhesion is mediated by particular parasite-encoded variant surface antigens (VSA) expressed on the surfaces of infected red blood cells, which confer unique phenotypic characteristics upon the infected cells (16, 19). Thus, malaria-exposed males and women who have never been pregnant do not possess immunoglobulin G (IgG) specific for VSA expressed by placental parasites (VSAPAM) (T. Staalsoe, C. E. Shülman, J. M. Bülmer, K. Kawuondo, K. Marsh, and L. Hviid, unpublished data). Furthermore, levels of adhesion-blocking VSAPAM-specific IgG increase with increasing parity and these antibodies protect against maternal anemia and low birth weight (7, 16, 19; T. Staalsoe et al., submitted for publication).
Much effort is currently being invested in the characterization and molecular identification of VSAPAM. However, this effort is complicated by the logistic and technical difficulties of bringing placental parasites into continuous in vitro culture. Pregnant women living in areas where malaria is highly endemic often have only scant peripheral parasitemia despite a substantial placental parasite load (11, 12), and it has been proposed that this may be because the entire life cycle of PAM-related parasites takes place within the placenta (15). We undertook the present study to investigate the relationship between peripheral and placental parasites by taking advantage of the gender-specific and parity-dependent IgG recognition of PAM-related parasites (16, 19).
We collected peripheral blood samples from eight pregnant women with microscopically detectable asexual P. falciparum parasitemia at the time of their enrollment in a trial of early-versus-late supervised monthly antimalarial treatment (SMAT II) conducted in the Ejisu-Juaben district, Kumasi, Ghana (E. N. L. Browne et al., unpublished data). Aliquots of red blood cells were cryopreserved in liquid N2 on site and subsequently adapted to continuous in vitro culture as described previously (13). Parasites isolated from a Ghanaian child as part of a previous study were also analyzed (13). On the day of analysis, red blood cells infected with hemozoin-containing late trophozoites and schizonts were isolated by exposure to a strong magnetic field, processed, and analyzed by flow cytometry as described previously (18). For each isolate, we measured VSA-specific IgG levels in pools of plasma from Danish adults without parasite exposure; from Ghanaian parasite-exposed men and women; from Ghanaian third-trimester pregnant women with high VSAPAM-specific IgG reactivity; and in individual plasma samples from parasite-exposed primigravidae (n = 47), secundigravidae (n = 28), multigravidae (parity, 3 to 4; n = 18), and grand-multigravidae (parity, 5 to 10; n = 13) from Ghana. The Ethics Committee of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, approved the study, and the Ministry of Health, Accra, Ghana, gave administrative clearance. All study women gave informed consent (thumb-printed consent form) in the presence of a witness after explanation of the study in Asante Twi.
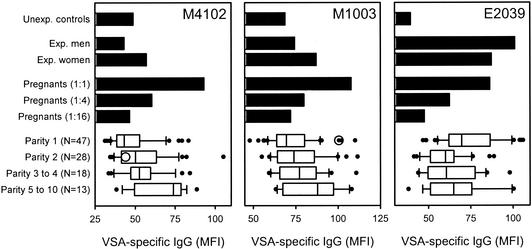
All the parasite isolates obtained from pregnant women that were tested showed the characteristic gender-specific and strongly parity-dependent IgG recognition diagnostic of PAM-related P. falciparum isolates (16, 19; T. Staalsoe et al., unpublished data) (Fig. 1, left and center panels, and Table 1). As such, our data do not support the hypothesis of a noncirculating, cryptic life cycle of PAM-related parasites (15). Three of these isolates (M4102, M4129, and M7201) did not express VSA that could be recognized by IgG present in pooled plasma from P. falciparum-exposed men (Fig. 1, left panel, and Table 1), in line with previous observations of CSA-adhering P. falciparum isolates (16, 19). This finding strongly indicates that these parasites originated exclusively from ongoing placental infections in the donors. Some IgG with specificity for the remaining three isolates (M1003, M1106, and M5201) were present in pooled plasma from exposed men but always at lower levels than in pooled plasma from exposed women (Fig. 1, center panel, and Table 1). This result is in contrast to what is essentially always seen with parasites not related to PAM (Fig. 1, right panel, and see references 16 and 19). The explanation for this finding may be that these women had at least two concomitant infections, with foci both in the placenta and elsewhere. Previous studies have reported the occurrence of mixtures of CSA-adhering and CD36-adhering parasites in the peripheral blood of pregnant women, supporting this possibility (1, 5). Alternatively, the non-VSAPAM-expressing subpopulations in these isolates may have arisen from in vitro switching away from VSAPAM, although VSA expression is generally quite stable in vitro in the absence of selection pressure (4, 13). Whatever their source, the fact is that these isolates took longer to adapt to in vitro culture than those exclusively expressing PAM-type VSA, leaving time for some outgrowth of minor subpopulations.
FIG. 1.
Plasma IgG recognition of VSA expressed by P. falciparum parasites isolated from the peripheral blood of pregnant women exposed to malaria (strain M1003 or M4102) and a child exposed to malaria (strain E2039) as measured by flow cytometry. The bar graphs show levels of IgG in pools of plasma from unexposed individuals (Unexp. controls), from P. falciparum-exposed men (Exp. men) and women (Exp. women), and in a fourfold-titrated pool of plasma from pregnant women with high levels of IgG specific for VSA expressed by parasites associated with pregnancy-associated malaria (Pregnants). The diagrams show VSA-specific IgG levels in individual plasma samples from primigravidae (Parity 1), secundigravidae (Parity 2), multigravidae (Parity 3 to 4), and grand-multigravidae (Parity 5 to 10). In each diagram, the vertical line indicates the median, the box indicates the 25 to 75% interquartile range, the horizontal “I” bar indicates the 10 to 90% interpercentile range, and the filled circles indicate all individual data points outside this range. The level of VSA-specific IgG in the autologous plasma sample is shown as an open circle.
TABLE 1.
Isolate and donor characteristics
| Isolate | Donor status | Gender-specific IgG recognition of VSAa | Parity-dependent antibody recognition of VSAb | Presence of autologous VSA IgG (reference) |
|---|---|---|---|---|
| M1002 | Pregnant | Complete | NDc | No |
| M1003 | Pregnant | Yes | Yes (P = 0.004) | Yes |
| M1106 | Pregnant | Yes | Yes (P = 0.004) | Yes |
| M4102 | Pregnant | Complete | Yes (P = 0.0005) | No |
| M4129 | Pregnant | Complete | Yes (P < 0.0001) | ND |
| M4608 | Pregnant | Complete | ND | Yes |
| M5201 | Pregnant | Yes | Yes (P = 0.0007) | ND |
| M7201 | Pregnant | Complete | Yes (P < 0.0001) | ND |
| E2039 | Child | No | No (P = 0.07)d | No (14) |
Complete, complete lack of recognition of VSA by IgG in plasma from exposed men; yes, VSA-specific IgG was present in plasma from exposed males but at lower levels than in plasma from exposed women; no, VSA-specific IgG was present in plasma from both exposed men and exposed women (levels were higher in plasma from men).
P values indicate the statistical significance of the correlation between VSA-specific IgG levels and the parity of the plasma donor (Pearson product moment correlation).
ND, not done.
Trend towards decreasing recognition with increasing parity.
In the four cases where we could test in plasma levels of IgG specific for VSA expressed by the autologous parasites, two isolates (M1002 and M4102) (Fig. 1, left panel) were not recognized by autologous IgG. This result is in line with the “hole in the VSA antibody repertoire” hypothesis, according to which parasites causing malaria express VSA to which the patient has no preexisting specific antibody response (3, 13). However, two women had appreciable levels of IgG specific for the VSA expressed by an autologous parasite isolate (M1003 or M1106) (Fig. 1, center panel, and Table 1). The most likely explanations are that these women had harbored a placental infection for some time and that the parasites collected represented the tail end of an infection in the process of being controlled by an acquired, VSA-specific immune response. Detailed longitudinal studies will be necessary to resolve this issue.
In contrast to the isolates from the peripheral blood of Ghanaian pregnant women, the child isolate (E2039) did not show any evidence of gender-specific and parity-dependent IgG recognition (Fig. 1, right panel), in line with previous data (16, 19). Furthermore, we have never observed the above-mentioned gender-specific and parity-dependent antibody recognition of VSA in studies of more than 10 P. falciparum isolates from children and nonpregnant adults (our unpublished data). Taken together, these findings support the concept that PAM-related parasites express a distinct set of VSA (VSAPAM) not expressed by parasites in nonpregnant individuals (5, 6, 14, 16, 19).
In conclusion, we have shown that peripheral parasites obtained from pregnant women living in an area of intense parasite transmission generally express VSA with an antibody recognition profile that strongly suggests that they are derived from an infection with a placental focus. This finding is of considerable practical importance for studies of immune responses to VSAPAM, in particular, longitudinal studies that have been hampered by the problems of adapting placental isolates to long-term in vitro culture.
Acknowledgments
We are indebted to the donors of blood samples used in this study and to all personnel involved in the SMAT II trial.
This study received financial support from the Enhancement of Research Capacity in Developing Countries (ENRECA) program of Danish International Development Assistance (Danida) (grant 14.Dan.8.L.306) and from the PAMVAC consortium sponsored by the Commission of the European Communities (grant QLK2-CT-2001-01302). The SMAT II trial was supported by the Danish Research Council for Development Research (RUF, grant 90982).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 3.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsyth, K. P., G. Philip, T. Smith, E. Kum, B. Southwell, and G. V. Brown. 1989. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am. J. Trop. Med. Hyg. 41:259-265. [PubMed] [Google Scholar]
- 5.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulphate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 6.Fried, M., and P. E. Duffy. 1998. Maternal malaria and parasite adhesion. J. Mol. Med. 76:162-171. [DOI] [PubMed] [Google Scholar]
- 7.Fried, M., F. Nosten, A. Brockman, B. T. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 8.Gilles, H. M., J. B. Lawson, M. Sibelas, A. Voller, and N. Allan. 1969. Malaria, anaemia and pregnancy. Ann. Trop. Med. Parasitol. 63:245-263. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt, H. L., and R. W. Snow. 2001. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans. R. Soc. Trop. Med. Hyg. 95:569-576. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt, H. L., and R. W. Snow. 2001. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 64:36-44. [DOI] [PubMed] [Google Scholar]
- 11.Leke, R. F., R. R. Djokam, R. Mbu, R. J. Leke, J. Fogako, R. Megnekou, S. Metenou, G. Sama, Y. Zhou, T. Cadigan, M. Parra, and D. W. Taylor. 1999. Detection of the Plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J. Clin. Microbiol. 37:2992-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mockenhaupt, F. P., B. Rong, H. Till, T. A. Eggelte, S. Beck, C. Gyasi-Sarpong, W. N. Thompson, and U. Bienzle. 2000. Submicroscopic Plasmodium falciparum infections in pregnancy in Ghana. Trop. Med. Int. Health 5:167-173. [DOI] [PubMed] [Google Scholar]
- 13.Ofori, M. F., D. Dodoo, T. Staalsoe, J. A. L. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neill-Dunne, I., R. N. Achur, S. T. Agbor-Enoh, M. Valiyaveettil, R. S. Naik, C. F. Ockenhouse, A. Zhou, R. Megnekou, R. Leke, D. W. Taylor, and D. C. Gowda. 2001. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 69:7487-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouvelle, B., P. A. Buffet, C. Lepolard, A. Scherf, and J. Gysin. 2000. Cytoadhesion of Plasmodium falciparum ring-stage-infected erythrocytes. Nat. Med. 6:1264-1268. [DOI] [PubMed] [Google Scholar]
- 16.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 17.Rogerson, S. J., R. Tembenu, C. Dobaño, S. Plitt, T. E. Taylor, and M. E. Molyneux. 1999. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am. J. Trop. Med. Hyg. 61:467-472. [DOI] [PubMed] [Google Scholar]
- 18.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 19.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 20.Yamada, M., R. Steketee, C. Abramowsky, M. Kida, J. Wirima, D. Heymann, J. Rabbege, J. Breman, and M. Aikawa. 1989. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am. J. Trop. Med. Hyg. 41:161-168. [DOI] [PubMed] [Google Scholar]