Abstract
Moraxella catarrhalis is a common cause of lower respiratory tract infection in adults with chronic obstructive pulmonary disease (COPD). The antibody response to outer membrane protein (OMP) CD, a highly conserved surface protein of M. catarrhalis under consideration as a vaccine antigen, was studied in adults with COPD following 40 episodes of infection or colonization. Following infection or colonization, 9 of 40 patients developed new serum immunoglobulin G (IgG) to OMP CD, as measured by enzyme-linked immunosorbent assay. Adsorption assays revealed that a proportion of the serum IgG was directed toward surface-exposed epitopes on OMP CD in six of the nine patients who developed new IgG to OMP CD. Immunoblot assays with fusion peptide constructs indicated that the new antibodies that developed after infection or colonization recognized conformational epitopes, particularly in the carboxy region of the protein. Three of 28 patients developed new mucosal IgA to OMP CD in sputum supernatants. This study establishes that OMP CD is a target of a systemic and mucosal immune response following infection and colonization in some patients with COPD.
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States and in the world (1, 29, 39). The course of COPD is characterized by intermittent exacerbations of the disease, and many of these exacerbations are caused by bacterial infection (35). Bacterial infection of the respiratory tract is associated with substantial morbidity and mortality in adults with COPD, and strategies to prevent these infections would have an important impact on the course of the disease (27). One such strategy is the development of vaccines. Elucidating human immune responses to bacteria which cause exacerbations of COPD will serve as a guide for the development of vaccines to prevent bacterial infection in patients with COPD.
Several lines of evidence implicate Moraxella catarrhalis as an important cause of exacerbations of COPD. (i) A subset of patients with exacerbations have sputum smears which show a predominance of gram-negative diplococci on Gram staining and yield nearly pure cultures of M. catarrhalis (6, 22, 30). (ii) Pure cultures of M. catarrhalis are recovered from samples collected from patients experiencing exacerbations by using methods which reliably reflect lower airway bacteriology (13, 14, 23, 31, 38, 40). (iii) Clinical improvement following administration of specific antibiotic therapy is seen in patients with exacerbations and sputum cultures which are positive for M. catarrhalis (22, 30). (iv) The development of new antibodies to the homologous patient-infecting isolate of M. catarrhalis occurs following exacerbations (2, 6). (v) Increased airway inflammation is associated with the isolation of M. catarrhalis from the sputum of patients experiencing exacerbations of COPD (17, 36). (vi) A prospective study of COPD found that acquisition of a strain of M. catarrhalis new to a patient with COPD is strongly associated with the occurrence of an exacerbation (33). Taken together, these lines of evidence indicate that a proportion of exacerbations of COPD are caused by M. catarrhalis.
Outer membrane protein (OMP) CD is a 45-kDa protein which has been the subject of investigation as a potential vaccine antigen for M. catarrhalis. OMP CD has several characteristics to suggest that it will be an effective vaccine. It is present in all strains of M. catarrhalis and has epitopes that are present on the surface of the intact bacterium (24, 32). The presence of surface-exposed epitopes suggests that potentially protective antibodies would be able to bind OMP CD on the whole bacterial cell. OMP CD is highly conserved among strains of M. catarrhalis (18, 25). Three lines of evidence suggest that immunization with OMP CD will induce protective antibodies. First, immunization of experimental animals with OMP CD induces bactericidal antibodies (41). Second, both mucosal and systemic immunization with recombinant OMP CD enhance pulmonary clearance of M. catarrhalis in a mouse pulmonary challenge model (26). Finally, the level of serum antibodies to OMP CD in infants and children is inversely correlated with the severity of otitis media with effusion, suggesting that antibodies to OMP CD play a protective role (15).
In a previous study levels of immunoglobulin to OMP CD were measured in serum and sputum samples from three groups, including 10 healthy adults, 10 adults with COPD who were free of colonization by M. catarrhalis, and 10 adults with COPD who experienced exacerbations due to M. catarrhalis (24). The concentration of serum immunoglobulin G (IgG) to OMP CD was significantly higher in the COPD group with exacerbations than in the COPD group without colonization and the healthy controls. A clear-cut rise in levels of immunoglobulin to OMP CD was not observed following exacerbation in the 10 patients studied.
The goal of the present study was to characterize more rigorously the human immune response to OMP CD in patients with COPD by studying a large number of patients who experienced episodes of exacerbation or colonization due to M. catarrhalis and by using an enzyme-linked immunosorbent assay (ELISA) designed to detect new antibodies to OMP CD by directly comparing samples from COPD patients before exacerbation with samples from COPD patients after exacerbation. The proportion of human antibodies to CD which are directed at surface-exposed epitopes was elucidated, and the regions of the OMP CD molecule which are targets of human antibodies were studied. Such studies will be important in elucidating the human immune response to M. catarrhalis in adults with COPD and will also contribute to further evaluating OMP CD as a potential vaccine antigen.
MATERIALS AND METHODS
COPD Study Clinic.
All bacterial isolates, serum samples, and sputum supernatants were recovered from adults who were enrolled in the COPD Study Clinic at the Buffalo Veterans Affairs Medical Center. The COPD Study Clinic has been described previously (2, 28, 33). Briefly, to be included in the study, each patient had to have a significant history of smoking and chronic bronchitis as defined by the American Thoracic Society, i.e., recurrent productive cough on most days for a minimum of 3 months in each of two consecutive years (1). In addition, the patient had to be willing to attend a monthly study clinic where progress was assessed and sputum and serum samples were collected. Patients with asthma, known bronchiectasis, malignancies, or other immunocompromising illnesses were excluded.
Patients were seen in the study clinic every month and at the time of suspected exacerbations. At each clinic visit, a clinical evaluation was performed by a study nurse. In addition, one of the study physicians evaluated each patient who was suspected of having an exacerbation. The examiner asked six questions regarding overall well-being, dyspnea, cough, sputum production, sputum viscosity, and sputum purulence. Emphasis was placed on grading three cardinal symptoms relative to the baseline: volume of sputum production, sputum purulence (color), and dyspnea. The patient was then evaluated with regard to overall appearance, respiratory rate, wheezes, and rales. On the basis of this information, a clinical determination of whether the patient was clinically stable or was having an exacerbation was recorded. Each patient brought an expectorated sputum sample produced the morning of the clinic visit.
Bacteriologic methods.
An equal volume of dithiothreitol (Sputolysin; Calbiochem, La Jolla, Calif.) was added to the sputum sample, and the sample was mixed by vortexing and incubated at 37°C for 15 min. Cultures were performed by plating aliquots of the sputum samples onto blood agar and chocolate agar plates. Identification of bacteria was done by standard techniques. The identity of an isolate as M. catarrhalis was confirmed by colony morphology and the presence of butyrate esterase by using the Catarrhalis Test Disk (Remel).
Serum and sputum supernatant samples.
Blood samples were obtained by venipuncture and allowed to clot. Serum samples were obtained by centrifugation and stored at −80°C. After an aliquot of the homogenized sputum sample was removed for culture as described above, sputum supernatants were obtained by centrifugation at 27,000 × g for 30 min at 4°C. The supernatants were saved by storage at −80°C. Preexacerbation serum and sputum samples were obtained approximately 4 weeks prior to the exacerbation (range, 2 to 8 weeks). Postexacerbation samples were obtained 4 to 8 weeks following the exacerbation. Serum samples from two patients in the present study were included in a previous study (24).
Purification of recombinant OMP CD.
The construction and method for purification of recombinant OMP CD containing a six-histidine amino-terminal tag have been described previously (26). This plasmid (pCDSA), which expresses an OMP CD of M. catarrhalis strain 25240, was engineered to remove the histidine tag, yielding plasmid pLP137. To express recombinant OMP CD which lacks a histidine tag, Terrific broth supplemented with 0.3 mg of carbenicillin per ml was inoculated with various dilutions of Escherichia coli containing plasmid pLP137 and the culture was incubated overnight. The next morning, an aliquot of culture with an optical density at 600 nm (OD600) of <1.0 was diluted to achieve an OD600 of 0.05 and this dilution was used to inoculate 50 ml of broth. The culture was grown to an OD600 of 1.0, and IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 1 mM. After 10 min, rifampin was added to a final concentration of 0.15 mg/ml and the culture was incubated for an additional 3 h. Cells were harvested by centrifugation at 6,000 × g for 15 min at 4°C. The pellet was suspended in 25 ml of phosphate-buffered saline (PBS) containing 1% Triton X-100. The cell suspension was sonicated six times for 10 s each time with a Branson sonicator with a medium tip at a setting of 7. Inclusion bodies containing recombinant OMP CD were collected by centrifugation at 45,000 × g for 20 min. The resulting pellet was suspended in 4 ml of 0.1 M Tris (pH 8) containing 6 M urea and rocked for 2 h at 4°C. The suspension was centrifuged to remove particulate material. The supernatant contained solubilized OMP CD.
The recombinant OMP CD was diluted with an equal volume of 0.2% Triton X-100 in water to yield a buffer consisting of 0.05 M Tris (pH 8), 3 M urea, and 0.1% Triton X-100 (buffer A). The solution was applied to a TMAE column (Fractogel EMD TMAE 650(s); EM Industries, Inc., Gibbstown, N.J.) equilibrated in buffer A. The column was washed with 95% buffer A-5% buffer B (buffer B is buffer A plus 0.5 M NaCl). Recombinant OMP CD was eluted with 80% buffer A-20% buffer B. The eluted protein was then dialyzed against PBS containing 0.1% Triton X-100. Coomassie blue staining and silver staining of aliquots of purified OMP CD subjected to polyacrylamide gel electrophoresis revealed a single band and the absence of detectable lipooligosaccharide.
Immunoassays with recombinant OMP CD purified from both plasmids, including pCDSA which contains an amino-terminal histidine tag and pLP137 which has no histidine tag, yielded identical results with selected human samples in this study.
Construction and purification of fusion peptides of OMP CD.
Two new constructs were made in pGEX4T3 (Promega Corporation, Madison, Wis.) for this study by using previously described methods (24). Plasmid Px1-261 contained a fragment of the gene that encodes amino acids 1 through 261, and plasmid Px261-427 contains a fragment of the gene that encodes amino acids 261 through 427. Peptides corresponding to six selected regions spanning the gene which encodes OMP CD were expressed as glutathione S-transferase (GST) fusion proteins and purified as previously described (24).
ELISA.
Wells of a 96-well microtiter plate (Immulon 4; Dynatech) were coated overnight at room temperature with 1 μg of recombinant OMP CD per ml in 0.1 M sodium carbonate-0.1 M sodium bicarbonate (pH 9.6). The wells were washed three times between each step with PBS containing 0.05% Tween 20 (PBS-Tween). The wells were blocked by adding 3% nonfat dry milk in PBS-Tween and incubating them at room temperature for 1 h. After the wells were washed, serum or sputum supernatant diluted in 1% nonfat dry milk in PBS-Tween was added to the wells and they were incubated at 37°C for 2 h. To measure serum IgG bound to OMP CD, horseradish peroxidase-conjugated rabbit anti-human IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), diluted 1:3,000 in 3% goat serum, was added to the wells and the wells were incubated for 1 h at room temperature. To measure sputum supernatant IgA bound to OMP CD, horseradish peroxidase-conjugated rabbit anti-human IgA (Kirkegaard & Perry Laboratories), diluted 1:3,000 in 3% goat serum, was used. After the wells were washed, color was developed by adding 0.1 mg of 3,3′,5,5′-tetramethylbenzidine-dimethyl sulfoxide-0.02% hydrogen peroxide per ml in 0.1 M sodium acetate adjusted to pH 4.5 with citric acid. After 15 min of incubation, the reaction was stopped by the addition of 4 N H2SO4. The OD450 was read.
All samples were run in duplicate. A negative control included wells coated with OMP CD and assayed with buffer instead of serum. A second negative control was run with each sample. Wells were sham coated with buffer instead of OMP CD, and the wells were assayed with each dilution of serum. The values determined for these wells were subtracted from the values obtained with the wells assayed with OMP CD for each dilution of serum and sputum supernatant. To control for day-to-day variability in assays with serum samples, duplicate wells were coated with OMP CD and assayed with a serum known to yield a specific OD. Assay results in which the value of this control differed by >15% from the predicted value were excluded. The percent change in OD between the preexacerbation samples and postexacerbation samples for each dilution was calculated with the following formula: [(OD of postexacerbation serum) − (OD of preexacerbation serum)/(OD preexacerbation serum)] × 100.
Adsorption of serum samples with whole bacterial cells.
All adsorption assays were performed with the homologous patient isolate of M. catarrhalis. A volume of 50 ml of brain heart infusion broth was inoculated with colonies from an agar plate and grown with shaking to late logarithmic phase (OD600, ∼0.75). Bacteria were harvested by centrifugation at 1,000 × g for 15 min at 4°C. After being washed once with PBS, the pellet was suspended in 1 ml of serum diluted 1:10 in PBS containing 0.15 mM CaCl2 and 0.5 mM MgCl2 and incubated for 30 min at 4°C. Bacteria were removed by centrifugation at 16,000 × g for 10 min at 4°C. The supernatant was filter sterilized and saved at 4°C.
RESULTS
Characteristics of clinical samples.
As part of a prospective study, M. catarrhalis was isolated from sputum samples collected during 21 exacerbations in 18 adults with COPD over a 4-year period (2). In previous work, new IgG was detected in 12 of the 21 postexacerbation serum samples by using a whole-cell ELISA with the homologous infecting isolate in each assay (2). These 12 sets of samples were analyzed in the present study for the presence of antibodies to OMP CD.
In addition to the 21 exacerbations, 19 episodes of colonization (defined as isolation of M. catarrhalis from sputum during clinically stable periods) in 16 adults with COPD were identified over the same 4-year period. New serum IgG to the homologous isolate was detected by a whole-cell ELISA in serum samples obtained after clearance of the strain from the sputum following 6 of the 19 episodes of colonization. These 18 sets of serum samples (12 from exacerbations and 6 from colonizations) from patients who developed new serum IgG to M. catarrhalis were the samples used in the present study to characterize the serum IgG response to OMP CD.
To study the development of new mucosal IgA to OMP CD, paired sputum supernatants from the same set of patients were studied. Sputum supernatants were available from 13 of the 21 exacerbations and from 15 of the 19 episodes of colonization. These 28 pairs were assayed for IgA to OMP CD.
Serum IgG response to OMP CD determined by ELISA.
To determine whether some of the new antibodies in samples from patients who developed new serum IgG to M. catarrhalis in a whole-cell ELISA were directed at OMP CD, an ELISA using purified recombinant OMP CD as the antigen was performed. To determine the cutoff value for a significant percent change in serum IgG levels between samples, 12 pairs of control serum samples collected 2 months apart from subjects in the COPD Study Clinic who had never been colonized with M. catarrhalis were subjected to ELISAs with purified recombinant OMP CD. These paired control serum samples demonstrated a 6.89% ± 5.77% (mean ± standard deviation) change when tested with purified OMP CD. A change in OD of 21.75% represented the upper limit of the 99% confidence interval for the control samples. Therefore, any change in OD of >21.75% between pre- and postexacerbation or pre- and postcolonization serum samples was regarded as a significant change. Figure 1 shows that when serum samples collected 1 month before infection or colonization and 1 month following infection or clearance of colonization were tested, new serum IgG to OMP CD was detected in 9 of 18 sets of the samples. These 9 samples included 6 of the 12 exacerbation samples and 3 of the 6 samples from episodes of colonization.
FIG. 1.
Results of ELISAs measuring the percent change in levels of IgG to OMP CD between pre- and postexacerbation serum samples. The horizontal line at 21.75% shows the cutoff for significant change. The x axis refers to episodes of exacerbation or colonization.
Proportion of new serum IgG directed at surface-exposed epitopes of OMP CD.
To determine whether any of the new serum IgG antibodies to OMP CD were directed at epitopes on the bacterial surface, adsorption experiments were performed. Aliquots of serum were adsorbed with logarithmic-phase cells of the homologous infecting isolate of M. catarrhalis. An ELISA to detect antibodies to OMP CD was performed simultaneously on aliquots of adsorbed and unadsorbed serum. The percent adsorption was calculated with the following formula: [(OD450 of unadsorbed serum) − (OD450 of adsorbed serum)/(OD450 of unadsorbed serum)] × 100.
To assess the specificity of the adsorption method, an aliquot of each serum sample was adsorbed with logarithmic-phase cells of E. coli HB101 (an irrelevant control bacterium in these studies) and an ELISA with OMP CD as the antigen was performed with the aliquots of unadsorbed and E. coli-adsorbed serum. The mean ± standard deviation of the adsorption value obtained for the nine serum samples adsorbed with E. coli was 2.2% ± 7.14%. A change of 20.6% in the percent adsorption value represented the 99% confidence interval for these control values. Therefore, a change in the percent adsorption of ≥20.6% was considered significant.
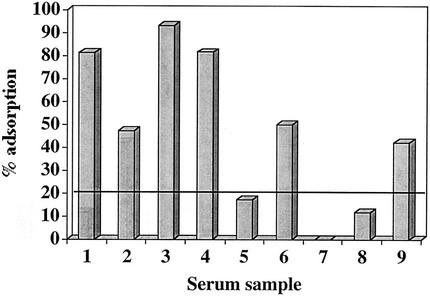
Figure 2 shows the results of assays using the nine postinfection or postcolonization serum samples adsorbed with their homologous strains of M. catarrhalis. Six of the nine serum samples showed a significant reduction in IgG to OMP CD following adsorption with whole bacterial cells, indicating that at least a proportion of the antibodies to OMP CD were directed at epitopes which are exposed on the bacterial surface.
FIG. 2.
Results of adsorption assays with postexacerbation and postcolonization serum samples. Serum samples were adsorbed with homologous whole bacterial cells, and an ELISA was performed with adsorbed and unadsorbed aliquots of serum to measure the levels of IgG to OMP CD in serum to determine the proportion of antibodies directed at surface-exposed epitopes on OMP CD. The y axis shows the percent adsorption calculated with the formula given in Results. The x axis indicates the nine serum samples that contain new IgG to OMP CD. The horizontal line at 20.6% indicates the 99% confidence interval for significant adsorption (see the text).
Identification of epitopes recognized by human serum IgG.
To elucidate the important parts of the OMP CD molecule with regard to the human antibody response, GST fusion peptides representing the amino-terminal 261-amino-acid portion of OMP CD and the carboxy-terminal 166-amino-acid-portion of OMP CD were engineered, expressed, and purified. Ongoing studies of the structure of OMP CD indicate that the 261 amino acids in the amino region of the molecule contain a beta-barrel structure followed by a hinge motif. The two constructs include regions of the protein upstream and downstream of the hinge motif. Immunoblot assays were performed with each set of the pre- and postexacerbation and pre- and postcolonization serum samples. Figure 3 shows the results of four such pairs with the GST construct that includes amino acids 261 through 427. Five of the nine serum samples showed the development of new antibodies to an epitope(s) on the carboxy-terminal 166 amino acids of OMP CD, as indicated by the appearance of a new band in the postexacerbation serum sample or by an unequivocal increase in the intensity of the band compared to that of the preexacerbation serum sample (Fig. 4). Two of the nine sets of serum samples showed the development of new antibodies to the amino-terminal 261 amino acids (Fig. 4).
FIG. 3.
Immunoblot assays of preexacerbation and postexacerbation serum samples to detect serum IgG. All sera were tested at a dilution of 1:100. Each lane has a GST fusion peptide containing amino acids 261 to 427 (arrow). The band at ∼80 kDa is an E. coli protein that copurifies with recombinant GST fusion peptides. Molecular masses are noted in kilodaltons on the right.
FIG. 4.
Summary of results of immunoblot assays with pre- and postexacerbation and pre- and postcolonization serum samples against GST fusion peptides corresponding to the amino acids of OMP CD as noted at the top. Serum sample identification numbers are given on the left. A plus indicates that a new band or a clear-cut increase in the intensity of a band was observed in the postexacerbation or postcolonization serum sample in comparison to that of the preexacerbation or precolonization serum sample. A minus indicates the absence of new band development in postexacerbation or postcolonization serum sample compared to that in preexacerbation or precolonization serum sample.
In an effort to further identify the portions of OMP CD recognized by human antibodies, a series of six GST fusion proteins spanning OMP CD were purified as previously described (24). The peptides ranged in size from 37 to 105 amino acids. Aliquots of the nine postexacerbation and postcolonization serum samples which showed new IgG to OMP CD were studied in immunoblot assays with each GST fusion peptide and recombinant OMP CD. Four of the serum samples showed reactivity with selected constructs, and five of the serum samples contained no detectable antibodies to the six smaller GST fusion proteins in the immunoblot assay. Each of the four serum samples that contained antibodies to GST fusion peptides in the immunoblot assay were tested with their corresponding preexacerbation sera to determine whether these antibodies were made in response to infection. In all four cases, the antibodies to the fusion peptides were present in the preexacerbation sera, indicating that these were preexisting antibodies. Based on these results, we conclude that new antibodies formed to OMP CD recognize conformational epitopes which are not present in fusion proteins consisting of peptides that range in size from 37 to 105 amino acids.
Mucosal immune response to OMP CD.
In previous work, ELISAs performed with sputum supernatants on whole bacterial cells showed a high degree of variability (2). To determine whether ELISAs with sputum supernatants against purified OMP CD would reliably measure IgA, six paired sputum supernatants recovered 2 months apart from study clinic patients who had never been colonized with M. catarrhalis and who were not having exacerbations were studied as negative controls. None of the 12 samples had detectable IgA to OMP CD. To further assess the reliability of the assays, a sputum supernatant in which IgA was detected was run on each plate to control for day-to-day variability. Analysis of the OD450 results of eight assays on different days revealed that all values were within 16.2% of the mean value.
Twenty-eight paired sputum supernatants, including 13 from patients with exacerbations and 15 from patients with colonization, were assayed for IgA by ELISA with OMP CD. Three pairs from the exacerbation group showed increases in levels of IgA to OMP CD of 227, 147, and 113% from pre- to postexacerbation. None of the pairs from the colonization group showed increases. Four additional sets of sputum supernatants (two from exacerbations and two from colonizations) had detectable IgA to OMP CD but showed no change from pre- to postexacerbation. Of the three patients who developed new IgA to OMP CD in their sputa following exacerbation, two also developed new IgG to OMP CD in their sera and one did not.
DISCUSSION
Adults with COPD who experience respiratory tract infections due to M. catarrhalis develop serum IgG and mucosal IgA responses following infection (2, 6, 8, 9). In addition, children develop serum and mucosal antibodies to M. catarrhalis following otitis media (11, 12, 19-21). The goal of the present study was to elucidate the immune response specifically to OMP CD, a highly conserved protein on the bacterial surface, by using serum and sputum samples collected prospectively from a well-characterized population of patients with COPD. Whole-cell ELISAs were performed on paired serum samples following 21 exacerbations and 19 episodes of colonization due to M. catarrhalis (2). Eighteen of these episodes were associated with the development of new serum IgG to the homologous isolates, and these 18 sets of serum samples were used in the present study. Nine of the 18 (50%) developed new serum IgG to OMP CD. An important consideration in assessing the potential protective capacity of antibodies is to determine whether or not the antibodies are directed at epitopes that are exposed on the bacterial surface. Antibodies which bind to the intact bacterial cell have the potential to opsonize, mediate bactericidal killing, or block adherence, whereas antibodies directed at epitopes that are not available on the bacterial surface are unlikely to mediate such protective immune responses. Adsorption assays to determine the proportion of antibodies to OMP CD directed at epitopes that are exposed on the surface of the intact bacterium revealed that serum samples from six of the nine patients who developed new serum IgG contained antibodies to surface epitopes on OMP CD (Fig. 2).
In an effort to identify the portions of the OMP CD molecule to which human antibodies bind, fusion peptides corresponding with two regions of OMP CD separated by a hinge motif were constructed and tested in an immunoblot assay. Five of the nine patients developed new IgG to the carboxy region of the molecule in their sera, and two developed new IgG to the amino region of the protein in their sera (Fig. 4). To further address this question, six fusion peptide constructs ranging in size from 37 to 105 amino acids that spanned the OMP CD molecule were engineered and tested in an immunoblot assay with serum samples from the nine patients who developed new serum IgG to OMP CD. Postexacerbation serum samples from four patients contained IgG to fusion peptides as noted previously (24). Interestingly, although antibodies to the smaller fusion peptides were detected in postexacerbation serum samples from four patients, the antibodies were also present in the corresponding preexacerbation serum samples. Therefore, none of the nine patients showed evidence of new antibody responses to the six constructs in the immunoblot assay in spite of the unequivocal development of new serum IgG to OMP CD determined by ELISA. Furthermore, three patients had no evidence of new antibodies to the two larger constructs in the immunoblot assay in spite of new antibody detected by ELISA (Fig. 4). These results are consistent with the development of antibodies that recognize conformational epitopes on OMP CD. We conclude that human antibodies that are formed in response to M. catarrhalis infection recognize conformational epitopes on OMP CD, particularly in the carboxy region of the protein.
M. catarrhalis is a mucosal pathogen, suggesting that mucosal immune responses may be important in protection against infection. Previous work with this patient population revealed that a mucosal immune response detected by flow cytometry with sputum supernatant occurs following infection in many patients and that the mucosal immune response occurs independently of the systemic immune response (2). Three of 28 pairs of sputum supernatants showed the development of new antibodies following exacerbations. One must be cautious in drawing firm conclusions about the frequency with which adults with COPD develop mucosal immune responses to OMP CD because of the inherent limitations in studying sputum samples. Sputum supernatants show more variability in results and a higher background in ELISA than do serum samples. This observation is likely due to several factors, including the quality of the sputum sample, contamination of sputum with saliva to various degrees, loss of some antibodies during centrifugation, proteolytic degradation of antibodies in the samples, and other factors. We conclude that some adults with COPD make new mucosal IgA to OMP CD following exacerbations, but we cannot draw conclusions about the frequency of this immune response.
M. catarrhalis is an exclusively human pathogen, which emphasizes the importance of using human samples to elucidate the immune response to the bacterium. Several surface antigens of M. catarrhalis have been identified as targets of the human immune response, including UspA1, UspA2, CopB, TbpB (OMP B1), LbpB, OMP E, and lipooligosaccharide (2-5, 7, 8, 16, 34, 42). The present study establishes that OMP CD is one of the surface antigens to which human antibodies are directed following infection and colonization with M. catarrhalis. Christensen (8) and Mathers et al. (21) demonstrated the development of new antibodies to a 60-kDa band following M. catarrhalis infection in children and adults. These observations are consistent with the development of antibodies to OMP CD, since the apparent molecular mass of OMP CD as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis is ∼60 kDa.
A key question generated by this study showing a human immune response to OMP CD is whether these antibodies are capable of protecting against infection and/or colonization. Recurrent exacerbations caused by the same strain of M. catarrhalis have not been observed in the COPD Study Clinic (our unpublished observations), suggesting that protective immune responses occur following exacerbations. Bactericidal antibodies in serum are associated with protection against infection by nontypeable Haemophilus influenzae (10, 37). No such correlation has been observed for M. catarrhalis. Indeed, serum samples from patients in the COPD Study Clinic do not have bactericidal activity for the clinical isolates of M. catarrhalis in our hands. The present study demonstrates that adults make new antibodies to OMP CD epitopes which are present on the bacterial surface. Such antibodies may be capable of opsonizing M. catarrhalis or blocking adherence to mucosal surfaces. Prospective studies will allow conclusions regarding the protective capacity of these antibodies to be drawn. It is not possible to draw conclusions regarding the protective capacity of antibodies to OMP CD from the present study because of the relatively small number of patients studied.
OMP CD has many characteristics indicating that it may be an effective vaccine antigen. The present study establishes that OMP CD is a target of a human systemic and mucosal immune response. Future work will address whether inducing an immune response to this highly conserved surface protein will be protective.
Acknowledgments
This work was supported by NIH grants AI 28304 and AI 46422 and by the Department of Veterans Affairs.
We thank Aimee Brauer for expert technical assistance, Steven Baker and John McMichael for providing plasmid pLP137, and Anthony Campagnari and Thomas Russo for critically reviewing the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.American Thoracic Society. 1987. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am. Rev. Respir. Dis. 136:225-244. [DOI] [PubMed] [Google Scholar]
- 2.Bakri, F., A. L. Brauer, S. Sethi, and T. F. Murphy. 2002. Systemic and mucosal antibody response to Moraxella catarrhalis following exacerbations of chronic obstructive pulmonary disease. J. Infect. Dis. 185:632-640. [DOI] [PubMed] [Google Scholar]
- 3.Bonnah, R. A., R.-H. Yu, H. Wong, and A. B. Schryvers. 1998. Biochemical and immunological properties of lactoferrin binding proteins from Moraxella (Branhamella) catarrhalis. Microb. Pathog. 24:89-100. [DOI] [PubMed] [Google Scholar]
- 4.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 64:3920-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnari, A. A., K. L. Shanks, and D. W. Dyer. 1994. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect. Immun. 62:4909-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman, A. J., D. M. Musher, S. Jonsson, J. E. Clarridge, and R. J. Wallace. 1985. Development of bactericidal antibody during Branhamella catarrhalis infection. J. Infect. Dis. 151:878-882. [DOI] [PubMed] [Google Scholar]
- 7.Chen, D., V. Barniak, K. R. VanDerMeid, and J. C. McMichael. 1999. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect. Immun. 67:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, J. J. 1999. Moraxella (Branhamella) catarrhalis: clinical, microbiological, and immunological features in lower respiratory tract infections. APMIS 107(Suppl.):1-36. [PubMed] [Google Scholar]
- 9.Christensen, J. J., N. Q. Hansen, and B. Bruun. 1996. Serum antibody response to outer membrane proteins of Moraxella (Branhamella) catarrhalis in patients with bronchopulmonary infection. Clin. Diagn. Lab. Immunol. 3:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faden, H., J. Bernstein, L. Brodsky, J. Stanievich, D. Krystofik, C. Shuff, J. J. Hong, and P. L. Ogra. 1989. Otitis media in children. I. The systemic immune response to nontypable Haemophilus influenzae. J. Infect. Dis. 160:999-1004. [DOI] [PubMed] [Google Scholar]
- 11.Faden, H., J. Hong, and T. Murphy. 1992. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect. Immun. 60:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faden, H., J. J. Hong, and N. Pahade. 1994. Immune response to Moraxella catarrhalis in children with otitis media: opsonophagocytosis with antigen-coated latex beads. Ann. Otol. Rhinol. Laryngol. 103:522-524. [DOI] [PubMed] [Google Scholar]
- 13.Fagon, J.-Y., J. Chastre, J.-L. Trouillet, Y. Domart, M.-C. Dombret, M. Bornet, and C. Gibert. 1990. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Am. Rev. Respir. Dis. 142:1004-1008. [DOI] [PubMed] [Google Scholar]
- 14.Hager, H., A. Verghese, S. Alvarez, and S. L. Berk. 1987. Branhamella catarrhalis respiratory infections. Rev. Infect. Dis. 9:1140-1149. [DOI] [PubMed] [Google Scholar]
- 15.Harabuchi, Y., H. Murakata, M. Goh, H. Kodama, A. Kataura, H. Faden, and T. F. Murphy. 1998. Serum antibodies specific to CD outer membrane protein of Moraxella catarrhalis, P6 outer membrane protein of nontypeable Haemophilus influenzae and capsular polysaccharides of Streptococcus pneumoniae in children with otitis media with effusion. Acta Otolaryngol. 118:826-832. [DOI] [PubMed] [Google Scholar]
- 16.Helminen, M. E., I. Maciver, J. L. Latimer, J. Klesney-Tait, L. D. Cope, M. Paris, G. H. McCracken, Jr., and E. J. Hansen. 1994. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170:867-872. [DOI] [PubMed] [Google Scholar]
- 17.Hill, A. T., E. J. Campbell, S. L. Hill, D. L. Bayley, and R. A. Stockley. 2000. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am. J. Med. 109:288-295. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao, C. B., S. Sethi, and T. F. Murphy. 1995. Outer membrane protein CD of Branhamella catarrhalis: sequence conservation in strains recovered from the human respiratory tract. Microb. Pathog. 19:215-225. [DOI] [PubMed] [Google Scholar]
- 19.Karjalainen, H., M. Koskela, J. Luotonen, E. Herva, and P. Sipila. 1991. Occurrences of antibodies against Streptococcus pneumoniae, Haemophilus influenzae, and Branhamella catarrhalis in middle ear effusion and serum during the course of acute otitis media. Acta Otolaryngol. 111:112-119. [DOI] [PubMed] [Google Scholar]
- 20.Karjalainen, H., M. Koskela, J. Luotonen, and P. Sipila. 1991. Secretory antibodies specific to Streptococcus pneumoniae, Haemophilus influenzae, and Branhamella catarrhalis in middle ear effusion during acute otitis media. Acta Otolaryngol. 111:524-529. [DOI] [PubMed] [Google Scholar]
- 21.Mathers, K., M. Leinonen, and D. Goldblatt. 1999. Antibody response to outer membrane proteins of Moraxella catarrhalis in children with otitis media. Pediatr. Infect. Dis. J. 18:982-988. [DOI] [PubMed] [Google Scholar]
- 22.McLeod, D. T., F. Ahmad, S. Capewell, M. J. Croughan, M. A. Calder, and A. Seaton. 1986. Increase in bronchopulmonary infection due to Branhamella catarrhalis. Br. Med. J. 292:1103-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monso, E., J. Ruiz, A. Rosell, J. Manterola, J. Fiz, J. Morera, and V. Ausina. 1995. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am. J. Respir. Crit. Care Med. 152:1316-1320. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, T. F., C. Kirkham, E. DeNardin, and S. Sethi. 1999. Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis. Infect. Immun. 67:4578-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy, T. F., C. Kirkham, and A. J. Lesse. 1993. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol. Microbiol. 10:87-98. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, T. F., J. M. Kyd, A. John, C. Kirkham, and A. W. Cripps. 1998. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J. Infect. Dis. 178:1667-1675. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, T. F., and S. Sethi. 1997. A national strategy for research in chronic obstructive pulmonary disease. JAMA 277:1596. [PubMed] [Google Scholar]
- 28.Murphy, T. F., S. Sethi, K. L. Klingman, A. B. Brueggemann, and G. V. Doern. 1999. Simultaneous respiratory tract colonization by multiple strains of nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease: implications for antibiotic therapy. J. Infect. Dis. 180:404-409. [DOI] [PubMed] [Google Scholar]
- 29.National Heart, Lung, and Blood Institute. 1998. Morbidity and mortality chartbook on cardiovascular, lung, and blood diseases. National Institutes of Health, Bethesda Md.
- 30.Nicotra, B., M. Rivera, J. I. Luman, and R. J. Wallace. 1986. Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch. Intern. Med. 146:890-893. [PubMed] [Google Scholar]
- 31.Ninane, G., J. Joly, and M. Kraytman. 1978. Bronchopulmonary infection due to Branhamella catarrhalis: 11 cases assessed by transtracheal puncture. Br. Med. J. 1:276-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarwar, J., A. A. Campagnari, C. Kirkham, and T. F. Murphy. 1992. Characterization of an antigenically conserved heat-modifiable major outer membrane protein of Branhamella catarrhalis. Infect. Immun. 60:804-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi, S., N. Evans, B. J. B. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465-471. [DOI] [PubMed] [Google Scholar]
- 34.Sethi, S., S. L. Hill, and T. F. Murphy. 1995. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect. Immun. 63:1516-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sethi, S., and T. F. Murphy. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sethi, S., K. Muscarella, N. Evans, K. L. Klingman, B. J. B. Grant, and T. F. Murphy. 2000. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 118:1557-1565. [DOI] [PubMed] [Google Scholar]
- 37.Shurin, P. A., S. I. Pelton, I. B. Tazer, and D. L. Kasper. 1980. Bactericidal antibody and susceptibility to otitis media caused by nontypeable strains of Haemophilus influenzae. J. Pediatr. 97:364-369. [DOI] [PubMed] [Google Scholar]
- 38.Soler, N., A. Torres, S. Ewig, J. Gonzalez, R. Celis, M. El-Ebiary, C. Hernandez, and R. Rodriguez-Roisin. 1998. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am. J. Respir. Crit. Care Med. 157:1498-1505. [DOI] [PubMed] [Google Scholar]
- 39.Vollmer, W. M., M. L. Osborne, and A. S. Buist. 1998. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am. J. Respir. Crit. Care Med. 157:1079-1084. [DOI] [PubMed] [Google Scholar]
- 40.West, M., S. L. Berk, and J. K. Smith. 1982. Branhamella catarrhalis pneumonia. South. Med. J. 75:1021-1023. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Y.-P., L. E. Myers, U. McGuinness, P. Chong, Y. Kwok, M. H. Klein, and R. E. Harkness. 1997. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol. Med. Microbiol. 17:187-199. [DOI] [PubMed] [Google Scholar]
- 42.Yu, R.-H., R. A. Bonnah, S. Ainsworth, and A. B. Schryvers. 1999. Analysis of the immunological responses to transferrin and lactoferrin receptor proteins from Moraxella catarrhalis. Infect. Immun. 67:3793-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]