Abstract
Macrophages encode several Toll-like receptors (TLRs) that recognize bacterial components, such as lipoproteins (TLR2) or lipopolysaccharides (TLR4), and activate multiple signaling pathways. Activation of transcription factor NF-κB by TLR2 or TLR4 signaling promotes proinflammatory and cell survival responses. Alternatively, TLR2 or TLR4 signaling can promote apoptosis if the activation of NF-κB is blocked. The gram-negative bacterial pathogen Yersinia pseudotuberculosis secretes into macrophages a protease (YopJ) that inhibits the activation of NF-κB and promotes apoptosis. We show that primary macrophages expressing constitutively active inhibitor κB kinase β (IKKβ) are completely resistant to YopJ-dependent apoptosis, indicating that YopJ inhibits signaling upstream of IKKβ. Apoptosis is reduced two- to threefold in TLR4−/− macrophages infected with Y. pseudotuberculosis, while the apoptotic response of TLR2−/− macrophages to Y. pseudotuberculosis infection is equivalent to that of wild-type macrophages. Therefore, TLR4 is the primary source of apoptotic signaling in Yersinia-infected macrophages. Our results also show that a small percentage of macrophages can die as a result of an apoptotic process that is YopJ dependent but does not require TLR2 or TLR4 signaling.
The ability to recognize and respond to many different types of pathogenic microorganisms is a cornerstone of innate immunity. Pattern recognition receptors (PRRs) recognize conserved elements among many different types of pathogens; these patterns are referred to as pathogen-associated molecular patterns (PAMPs) (15). Toll-like receptors (TLRs) are a subfamily of PRRs. There are at least 10 reported TLR family members in humans (reviewed in reference 13). The gram-negative bacterial cell wall component lipopolysaccharide (LPS) is recognized through TLR4, while bacterial lipoproteins, glycolipids, and certain other gram-positive bacterial surface components activate TLR2. Different TLRs as well as different combinations of TLR heterodimers can be used by cells of the immune system to differentiate among the large number of PAMPs found on pathogens (2).
The signaling pathways that are activated downstream of TLRs are currently being elucidated. TLR family members contain a cytoplasmic Toll-interleukin 1 receptor homology region. After the activation of TLR4 by LPS, a series of events lead to the activation of ubiquitin ligase TRAF6 by a unique self-polyubiquitination reaction (reviewed in reference 1). TRAF6 then activates the TAK1 complex (31). This step leads to the phosphorylation and activation of mitogen-activated protein kinase kinases and the inhibitor κB (IκB) kinase (IKK) complex (18, 31). The IKK complex is comprised of two kinases, IKKα and IKKβ, and a third protein, NEMO (also called IKKγ). Upon activation, IKKβ phosphorylates IκBα, triggering its polyubiquitination and degradation (6, 11). In nonstimulated cells, IκBα interacts with and traps NF-κB in the cytosol. Degradation of IκBα releases NF-κB to translocate into the nucleus and to activate proinflammatory and prosurvival gene expression. Thus, multiple signaling pathways can be activated through TLR4, leading to the production of cytokines and other factors that protect the host against infection.
In addition to activating gene expression, signaling through TLRs may activate a programmed cell death (apoptosis) response in eukaryotic cells. For example, the activation of TLR2 by bacterial lipopeptides can promote apoptosis (3). In this situation, the activation of caspase 8 and, subsequently, the effector caspases leads to the execution of the apoptotic program. The inhibition of NF-κB activation has been shown to potentiate apoptosis (30, 32), indicating that the production of survival factors under the control of NF-κB can counteract apoptotic signaling by TLR2 (3).
In this study, we investigated the role of TLR signaling in the apoptotic response of macrophages to the gram-negative bacterial pathogen Yersinia pseudotuberculosis. Y. pseudotuberculosis is closely related to Yersinia pestis, the agent of plague, and causes a systemic infection in rodents that resembles plague in humans. Previous studies established that Y. pseudotuberculosis stimulates apoptosis in infected macrophages (17). In addition, the ability to trigger macrophage apoptosis is important for systemic infection of mice by this pathogen (16). The toxin YopJ (also known as YopP in Yersinia enterocolitica) is required for apoptosis in macrophages (17). YopJ is one of six toxins secreted by Yersinia into the cytosol of macrophages by a plasmid-encoded bacterial secretion apparatus known as a type III secretion system (5). YopJ is a cysteine protease of the family of ubiquitin-like proteins that inhibits multiple signaling pathways (20). YopJ binds to IKKβ (20), and the translocation of YopJ into macrophages is associated with decreased IKKβ and NF-κB activities (25). It has been suggested, but not demonstrated, that YopJ acts upstream of IKKβ to block its activation (20).
Two models have been proposed to explain how YopJ causes apoptosis in macrophages. In one model, YopJ directly activates a programmed cell death pathway through a process that involves the proteolytic cleavage of the caspase 8 substrate Bid (7). In a second model, YopJ acts to potentiate the apoptotic signaling of LPS by inhibiting the activation of NF-κB (24, 25). Transient overexpression of IKKβ in macrophages reduced apoptosis in response to Yersinia infection by twofold (25). In addition, when a vector producing YopJ was transfected into macrophages, the percentage of cells that underwent apoptosis increased significantly in the presence of LPS (25). However, up to 50% of transfected macrophages not treated with LPS died, suggesting that other mechanisms of YopJ-dependent apoptosis may exist (25). Taken together, these results support the concept that YopJ potentiates the apoptotic signaling of LPS by inhibiting the activation of NF-κB. However, an essential role for TLR4 or IKKβ in YopJ-dependent apoptosis of macrophages has not been established.
We used retroviral transfection of primary macrophages and macrophages from mice deficient in TLR2 or TLR4 (TLR2−/− and TLR4−/−, respectively) to further characterize the mechanism of YopJ-dependent apoptosis. We found that macrophages expressing a constitutively activated derivative of IKKβ are completely protected from YopJ-dependent apoptosis. This result establishes IKKβ as a key target of Yersinia pathogenesis. Although signaling through TLR2 is dispensable for YopJ-dependent apoptosis, signaling through TLR4 contributes significantly to apoptosis but is not absolutely required for this activity.
MATERIALS AND METHODS
Materials.
Polyclonal antibodies to IKKα/β (H-470; sc-7607) and IκBα (C-21; sc-371) were purchased from Santa Cruz Biotechnology. Polyclonal antibodies to c-Jun N-terminal kinase (JNK) (9252), phospho-JNK (9251), and phospho-IκBα (9241) were obtained from Cell Signaling Technology. Anti-FLAG M2 monoclonal antibody and affinity gel and LPS from Escherichia coli O26:B6 were obtained from Sigma. Secondary antibodies conjugated to horseradish peroxidase were obtained from Jackson ImmunoResearch Laboratories. Lipopeptide Pam3CSK4 was obtained from Guenther Jung (University of Tübingen). Protein A- Sepharose CL-4B and glutathione-Sepharose 4B were obtained from Pharmacia Biotech. Tissue culture reagents were obtained from Invitrogen Life Technologies. Tumor necrosis factor alpha (TNF-α) was obtained from the National Institute for Biological Standards and Control. The RetroMax retroviral system was obtained from IMGENEX. A plasmid containing the encephalomyocarditis virus internal ribosome entry site (IRES) was provided by Eckard Wimmer (Stony Brook, N.Y.). Polybrene and plasmids encoding wild-type IKKβ, IKKβ with S177E and S181E mutations, and glutathione S-transferase (GST)-IκBα(1-62) were provided by Kenneth Marcu (Stony Brook, N.Y.). GST- IκBα(1- 62) (where 1-62 is amino acids 1 to 62 of IκBα) was purified from E. coli by using glutathione-sepharose 4B and a procedure recommended by the supplier (Pharmacia Biotech). A protease inhibitor cocktail was obtained from Roche. Plasmid pEGFP-C1, encoding green fluorescent protein (GFP), was obtained from Clontech.
Mice and bone marrow-derived macrophages.
C57BL/6, C3H/HeJ, and C3HeB/FeJ mice were obtained from Jackson ImmunoResearch Laboratories. TLR2−/− mice (derived from 129/SvJ crossed with C57BL/6) (28), TLR4−/− mice (derived from 129/Ola crossed with C57BL/6) (10, 28), and age-matched wild-type control mice were provided by Shizuo Akira (Osaka University) and Ruslan Medzhitov (Yale University). Bone marrow-derived macrophages were obtained as previously described (4).
Retroviral expression of IKKβ in cultured cells.
DNA restriction fragments encoding wild-type or constitutively active IKKβ, the IRES, and GFP were ligated into the EcoRI site in retroviral vector pCLXSN (IMGENEX). The resulting vectors, pCLXSN-IKKβ(wt)-IRES-GFP, pCLXSN-IKKβ(S178E/S181E)-IRES-GFP, and pCLXSN-IRES-GFP, were verified by sequencing. Retroviral particles were collected in the culture supernatants of transfected 293T cells according to the manufacturer's instructions (IMGENEX). To transfect NIH 3T3 cells, the cells were cultured in 1 ml of a 9:1 mixture of Dulbecco modified Eagle medium (DMEM) containing 10% calf serum- 8 μg of Polybrene/ml and 293T-cell supernatant in six-well plates (105 cells/well) for 8 h. The medium was removed, and the cells were cultured in DMEM containing 10% calf serum for 24 h. Macrophages grown for 5 days were used for retroviral transfections. The macrophages were cultured in 0.4 ml of a 3:1 mixture of bone marrow medium (BMM [DMEM containing 20% fetal bovine serum, 30% L-cell-conditioned medium, 2 mM glutamine, and 1 mM pyruvate]) and 293T-cell supernatant on glass coverslips in 24-well dishes (5 × 104 cells/well) for 40 h. The transfected macrophages were washed and incubated in modified BMM (BMM containing 10% fetal bovine serum and 15% L-cell-conditioned medium) 30 min prior to bacterial infection.
Immunoprecipitation and kinase activity assay.
The procedures for immunoprecipitation and the kinase activity assay were those of McKenzie et al. (14). Briefly, transfected NIH 3T3 cells were cultured in DMEM lacking serum overnight. The cells were left untreated or were stimulated with TNF-α for 15 min. The cells were washed twice with ice-cold phosphate-buffered saline containing 1 mM sodium orthovanadate and 10 mM NaF and then incubated in lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 50 mM NaF, 5 mM EDTA, 40 mM β-glycerophosphate, 200 μM sodium orthovanadate, 1% Triton X-100, protease inhibitor cocktail) on ice for 15 min. Lysates clarified by centrifugation were incubated with anti-FLAG M2 affinity gel for 2 h at 4°C. The immune complexes were washed three times with lysis buffer and then twice with kinase assay buffer (20 mM HEPES [pH 7.4], 20 mM MgCl2, 1 mM dithiothreitol, 10 mM p-nitrophenyl phosphate). IKKβ activity was assayed by incubating the immune complexes in 20 μl of kinase assay buffer containing 50 μM ATP and 2.5 μg of GST- IκBα(1- 62). After 10 min at 30°C, an equal volume of 2× Laemmli sample buffer (9a) was added, and the sample was boiled and then analyzed by Western blotting.
Bacterial strains and infection conditions.
Y. pseudotuberculosis strains YP126 (wild type) and YP26 (YopJ−) have been described elsewhere (21). To prepare bacteria for infection, overnight cultures grown at 26°C in Luria broth were diluted to an optical density at 600 nm of 0.1 in Luria broth supplemented with 20 mM MgCl2 and 20 mM sodium oxalate. The cultures were shaken at 26°C for 1 h and at 37°C for 2 h. The bacteria were washed once and resuspended in Hanks balanced salt solution and then used to infect macrophages at a multiplicity of infection of 50. To initiate the infection, the plates were centrifuged for 5 min at 200 × g to bring the bacteria in contact with the macrophages.
Western blot analysis.
Macrophages (5 × 105 cells/well) cultured in 2 ml of fresh modified BMM in six-well plates were stimulated or infected as described in the figure legends. The cells were lysed in boiling 1× Laemmli sample buffer. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% acrylamide). Western blotting with commercial primary antibodies was carried out as suggested by the suppliers. Immunoblots were developed with enhanced chemiluminescence reagents as recommended by the supplier (New England Nuclear).
TUNEL assay.
Macrophages cultured on coverslips (5 × 105 cells/well) were infected for 4 h. Gentamicin was added to a final concentration of 100 μg/ml at the 2-h time point to inhibit further bacterial growth. The cells were then fixed and processed to detect apoptosis by using an in situ cell death detection kit with TMR red or fluorescein (Roche) according to the manufacturer's instructions. The coverslips were mounted in SlowFadeLight antifading medium with glycerol (Molecular Probes) and analyzed by fluorescence microscopy with a Zeiss Axioplan2 microscope. Digital images were captured by using a SPOT camera (Diagnostic Instruments). For transfected macrophages, fluorescence microscopy was used to determine the percentage of transfected macrophages that were terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) positive in at least 10 randomly chosen nonoverlapping fields. For nontransfected macrophages, phase microscopy and fluorescence microscopy were used to determine the percentage of TUNEL-positive cells in at least four randomly chosen nonoverlapping fields.
RESULTS
Constitutively active IKKβ prevents YopJ-dependent apoptosis.
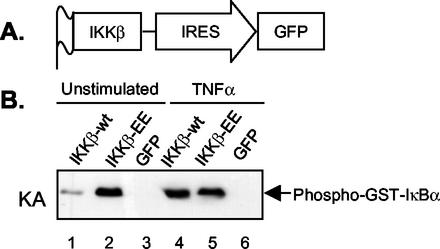
To test whether YopJ inhibits the activation of IKKβ and whether a blockage in IKKβ activation is critical for Yersinia-induced apoptosis, we transiently overexpressed a constitutively active mutant of IKKβ in primary macrophages before infecting them with Y. pseudotuberculosis. We anticipated that the expression of activated IKKβ in macrophages would promote complete protection from YopJ-dependent apoptosis if indeed YopJ inhibits the activation of IKKβ. A gene encoding an epitope (FLAG)-tagged activated form of IKKβ (IKKβ-EE) was inserted into a retroviral vector upstream of sequences specifying an IRES and GFP (Fig. 1A). In IKKβ-EE, two active-site serines have been replaced with glutamic acid (S177E and S181E), resulting in constitutive activation of the kinase (6). The use of this bicistronic vector allowed for the identification of transfected cells by fluorescence microscopy. Similar vectors encoding wild-type IKKβ or GFP alone were constructed for use as controls.
FIG. 1.
Strategy for the expression of wild-type or activated IKKβ in cells. (A) Structure of the bicistronic expression cassette for IKKβ and GFP in the retroviral expression vector. IKKβ contains an N-terminal FLAG epitope tag. The GFP control vector lacks sequences encoding IKKβ (not shown). (B) Kinase assay (KA) of IKKβ activity in transfected NIH 3T3 cells. NIH 3T3 cells transfected with retroviruses expressing wild-type IKKβ (IKKβ-wt) plus GFP, IKKβ-EE plus GFP, or GFP alone were left unstimulated or were stimulated with 25 ng of TNF-α/ml for 10 min after serum starvation. Detergent lysates of the cells were subjected to immunoprecipitation with anti-FLAG antibody. The immune complexes were incubated with purified GST- IκB(1- 62) in kinase assay buffer. Phosphorylation of GST- IκBα(1- 62) was monitored by Western blot analysis with phospho-specific IκBα antibody. The results shown are representative of three independent experiments.
The activities of the kinases encoded by the retroviruses were characterized by using highly transfectable NIH 3T3 cells. At 24 h after the transfection of NIH 3T3 cells with retroviruses, they were starved of serum overnight and then treated or not treated with TNF-α for 10 min to activate IKKβ. The FLAG-tagged IKKβ proteins were immunoprecipitated from detergent extracts of the cells by using anti-FLAG antibody. Purified GST- IκBα(1- 62) was used as a substrate in an in vitro kinase reaction to measure the activities of the immunoprecipitated IKKβ proteins. No IKKβ activity was immunoprecipitated from cells transfected with virus expressing GFP alone (Fig. 1B, lanes 3 and 6). On the other hand, cells transfected with virus expressing IKKβ-EE contained active kinase in the presence or absence of TNF-α stimulation (Fig. 1B, lanes 2 and 5). Cells transfected with virus encoding wild-type IKKβ contained a low but detectable level of kinase activity in the absence of TNF-α (Fig. 1B, lane 1) and a greatly increased level of kinase activity in the presence of TNF-α (lane 4). Thus, the kinases encoded by the retroviruses functioned as expected.
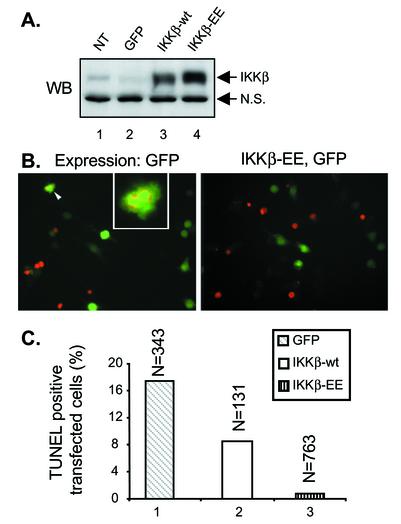
The retroviruses were used to transfect bone marrow-derived macrophages obtained from C57BL/6 mice. At 2 days after transfection, between 15 and 30% of the cells expressed GFP, depending on the individual experiment and the stock of retrovirus used (data not shown). Detergent extracts of the macrophages were prepared and analyzed by immunoblotting to confirm that IKKβ and IKKβ-EE were expressed at similar levels (Fig. 2A).
FIG. 2.
Constitutively active IKKβ protects macrophages from apoptosis induced by Yersinia infection. (A) Western blot (WB) analysis of IKKβ expression in macrophages. Nontransfected macrophages (NT) or macrophages transfected with retroviruses expressing wild-type IKKβ (IKKβ-wt) plus GFP, IKKβ-EE plus GFP, or GFP alone were lysed in detergent. Samples of the lysates were analyzed by Western blotting with anti-IKKα/β antibodies. Nonspecific bands (N.S.) indicate equal loading in the lanes. (B and C) TUNEL assay of Yersinia-induced apoptosis in transfected macrophages. Macrophages transfected with retroviruses expressing IKKβ-wt plus GFP, IKKβ-EE plus GFP, or GFP alone were infected with wild-type Y. pseudotuberculosis strain YP126 for 4 h. Apoptosis was detected by the TUNEL assay. Fluorescence microscopy was used to count the numbers of transfected macrophages (green cells) and the numbers of transfected macrophages that were TUNEL positive (red nuclei) in random nonoverlapping fields. In panel B, representative images of cells expressing GFP alone (left) or IKKβ-EE plus GFP (right) are shown. In the left image of panel B, two adjacent TUNEL-positive transfected macrophages are indicated by the arrowhead and enlarged in the inset. In panel C, the percentages of transfected macrophages that were scored as TUNEL positive are plotted. The data shown are from one representative experiment of three. N, number of transfected cells counted under each condition.
To determine what effect the expression of IKKβ or IKKβ-EE might have on YopJ-dependent apoptosis, transfected macrophages were infected with wild-type Y. pseudotuberculosis for 4 h. The samples were fixed, and apoptotic cells were labeled by the TUNEL reaction. Fluorescence microscopy was used to identify transfected cells (green cells) and to determine the percentages of transfected cells that were labeled by the TUNEL reaction (red nuclei) (Fig. 2B). The results are summarized in Fig. 2C. An initial observation was that the overall level of apoptosis in macrophages infected with Y. pseudotuberculosis was lower in transfected cells than in nontransfected cells. Retroviral infection of cells has been shown to activate NF-κB through a TLR4-dependent pathway (23). The activation of NF-κB prior to Yersinia infection is likely to promote some degree of protection from YopJ-dependent apoptosis. Nevertheless, the impact of IKKβ and IKKβ-EE expression on YopJ-dependent apoptosis was clearly evident: while 17.5% of macrophages expressing GFP alone underwent apoptosis, apoptosis was decreased to 8% in macrophages expressing IKKβ and to a negligible 0.8% in macrophages expressing IKKβ-EE (Fig. 2C). Thus, the expression of IKKβ-EE decreased apoptosis to background levels. The partial protection from apoptosis provided by wild-type IKKβ was consistent with its low level of constitutive activity (Fig. 1B), a result which confirms the findings of Ruckdeschel et al. (25). However, by using a catalytically active form of IKKβ, we further demonstrated that inhibition of the catalytic activity of IKKβ is essential for Yersinia-induced apoptosis. This result suggests that YopJ acts upstream of IKKβ to inhibit its activation.
Role of TLR4 in YopJ-dependent apoptosis.
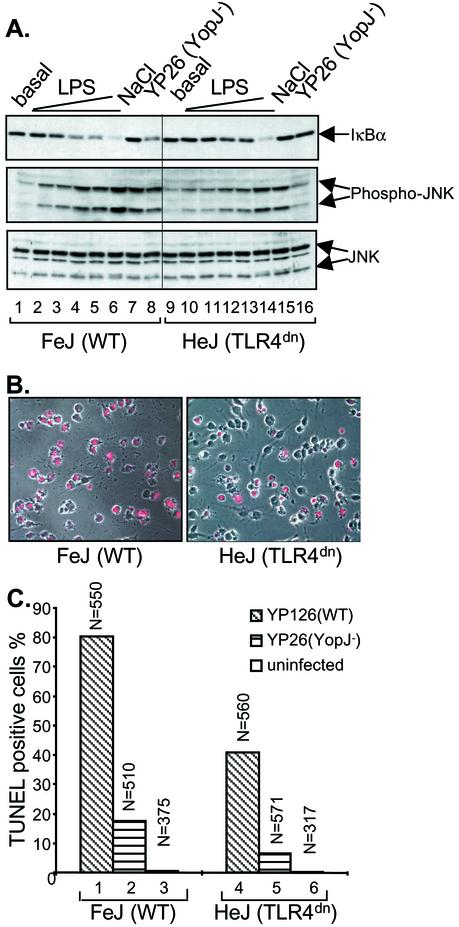
The above data suggest that the inhibition of IKKβ activation by YopJ is required for apoptosis. We next investigated the source of the apoptotic signaling that is normally counteracted by IKKβ. Specifically, we examined whether TLR signaling pathways are required to trigger the apoptotic response. Macrophages deficient in TLR4 function were isolated from C3H/HeJ mice and used in infection assays to test directly the role of this receptor in YopJ-dependent apoptosis. C3H/HeJ mice harbor a naturally occurring codon substitution in Tlr4; this mutation results in a dominant-negative receptor and a TLR4−/− phenotype (22). Initially, we examined the activation of signaling pathways in C3H/HeJ macrophages to confirm that they were hyporesponsive to LPS. Macrophages from C3H/HeJ mice or the LPS-sensitive mouse strain C3HeB/FeJ were stimulated by exposure to osmotic shock or to increasing concentrations of LPS or by infection with a Y. pseudotuberculosis YopJ− mutant for 15 min. The activation of IKKβ or the mitogen-activated protein kinase JNK was examined by immunoblotting. The degradation of IκBα indicates IKKβ activation, while the phosphorylation of JNK is indicative of JNK activation. Both isoforms of JNK (p46 and p54) were activated to equivalent levels in C3H/HeJ and C3HeB/FeJ macrophages following osmotic shock with 0.2 M NaCl (Fig. 3A, middle panel, lanes 7 and 15), confirming that signaling pathways are intact in C3H/HeJ macrophages. Higher levels of LPS were needed to achieve full activation of IKKβ or JNK in C3H/HeJ macrophages than in C3HeB/FeJ macrophages (Fig. 3A, upper and middle panels, compare lanes 1 to 6 with lanes 9 to 14). The fact that C3H/HeJ macrophages responded at all to this preparation of LPS likely is a result of its contamination with lipoproteins, as a previous study showed (8). The activation of IKKβ or JNK in response to infection with the YopJ− mutant was also significantly decreased in C3H/HeJ macrophages compared to C3HeB/FeJ macrophages (Fig. 3A, upper and middle panels, lanes 8 and 16). These results confirm that the C3H/HeJ macrophages that we isolated were hyporesponsive to LPS and that infection of wild-type macrophages with Y. pseudotuberculosis stimulates signaling through TLR4.
FIG. 3.
TLR4 contributes to Yersinia-induced signaling and apoptosis in macrophages. (A) Analysis of IκBα degradation and JNK phosphorylation in C3HeB/FeJ (WT) and C3H/HeJ (TLR4dn) macrophages. Macrophages were left untreated (basal), exposed to LPS (0.03, 0.1, 0.2, 0.3, or 10 μg/ml) or NaCl (0.2 M), or infected with YopJ− Y. pseudotuberculosis (YP26) for 15 min. Cell lysates were subjected to Western blot analysis with anti-IκBα antibody (top), anti-phospho-JNK antibody (middle), or anti-JNK antibody (bottom). Two forms of JNK, p54 and p46, were detected. (B) Analysis of apoptosis by a TUNEL assay and fluorescence microscopy. Representative images show C3HeB/FeJ or C3H/HeJ macrophages stained by TUNEL after infection with wild-type (YP126) Y. pseudotuberculosis for 4 h. Red nuclei indicate a TUNEL-positive reaction. (C) Levels of YopJ-dependent apoptosis in C3HeB/FeJ or C3H/HeJ macrophages. The percentages of TUNEL-positive macrophages left uninfected or infected with wild-type (YP126) or YopJ− (YP26) Y. pseudotuberculosis for 4 h are plotted. The results shown are representative of three independent experiments. N, number of cells counted under each condition.
Macrophages from C3HeB/FeJ or C3H/HeJ mice were infected for 4 h with either wild-type Y. pseudotuberculosis or the YopJ− mutant. The samples were fixed, and the nuclei of apoptotic cells were labeled by the TUNEL reaction. Fluorescence microscopy was used to count TUNEL-positive cells (Fig. 3B). Interestingly, 80.4% of C3HeB/FeJ macrophages underwent apoptosis after infection with wild-type Y. pseudotuberculosis, while only 40.9% of C3H/HeJ macrophages did so (Fig. 3C), a reduction of ∼50%. After infection with the YopJ− mutant, 17.6% of C3HeB/FeJ macrophages underwent apoptosis, while only 6.7% of C3H/HeJ macrophages died, again, a reduction of ∼50%. Extremely low levels of apoptosis occurred in uninfected macrophages of both types. To confirm that TLR4−/− signaling is important for YopJ-dependent apoptosis, we next examined the infection-induced responses of macrophages obtained from mice with null mutations in the Tlr4 gene. Macrophages obtained from mice congenic with the TLR4−/− animals were used as controls. Like CH3/HeJ macrophages, TLR4−/− macrophages showed decreased signaling responses to LPS or YopJ− Y. pseudotuberculosis (Fig. 4A, top and middle panels, compare lanes 2, 3, and 4 with lanes 14, 15, and 16). In addition, YopJ-dependent apoptosis was significantly reduced but not eliminated in TLR4−/− macrophages infected with Y. pseudotuberculosis (71.2% for wild type versus 21.9% for TLR4−/−) (Fig. 4B). These results further demonstrate that TLR4 is the primary source of signaling that triggers YopJ-dependent apoptosis.
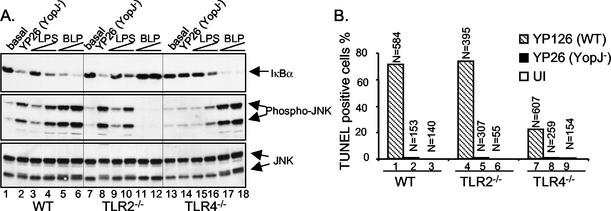
FIG. 4.
TLR2, unlike TLR4, does not contribute to signaling or apoptosis in macrophages infected with Yersinia. (A) Analysis of IκBα degradation and JNK activation in wild-type (WT), TLR2−/−, or TLR4−/− bone marrow-derived macrophages. TLR2−/− mice were derived from 129/SvJ crossed with C57BL/6 (28), and TLR4−/− mice were derived from 129/Ola crossed with C57BL/6 (10, 28). Macrophages were left untreated (basal) or exposed to YopJ− Y. pseudotuberculosis (YP26), LPS (0.1 or 0.3 μg/ml), or synthetic bacterial lipopeptide (BLP) (0.03 or 0.1 μg/ml) for 15 min. Cell lysates were subjected to Western blot analysis with anti-IκBα antibody (top), anti-phospho-JNK antibody (middle), or anti-JNK antibody (bottom). (B) Percentages of TUNEL-positive macrophages left uninfected (UI) or infected with wild-type (YP126) or YopJ− (YP26) Y. pseudotuberculosis for 4 h. The results shown are representative of two experiments. N, number of cells counted.
As a small percentage of TLR4−/− macrophages died of YopJ-dependent apoptosis, it was possible that another signaling pathway stimulated cell death in these cells. The signaling responses of TLR4−/− macrophages to Y. pseudotuberculosis infection were further characterized to explore this possibility. A time course experiment was carried out by using YopJ− Y. pseudotuberculosis to infect wild-type or TLR4−/− macrophages. In wild-type macrophages, robust activation of IKKβ and JNK was observed after 15 min of infection, and the activity appeared to peak between 15 and 30 min (Fig. 5, top panels, lanes 1 to 4 and lanes 6 to 9). The signaling induced by infection was significantly diminished by 60 min (Fig. 5, top panels, lanes 5 and 10). Surprisingly, TLR4−/− macrophages did respond to the infection, although this response was significantly delayed, in that peak activity occurred between 30 and 45 min after infection (Fig. 5, bottom panels, lanes 1 to 10). The delayed response of TLR4−/− macrophages to the infection explains why the activation of IKKβ and JNK was not observed in these cells in the 15-min time-point experiments (Fig. 3 and 4). Thus, Y. pseudotuberculosis infection stimulates macrophage signaling independently of TLR4.
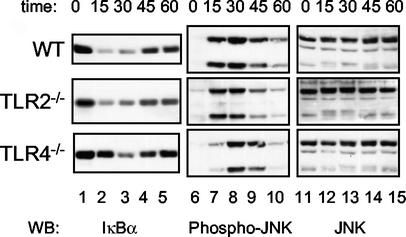
FIG. 5.
Yersinia-induced signaling is delayed but not eliminated in TLR4−/− macrophages. Wild-type (WT), TLR2−/−, or TLR4−/− macrophages were infected with YopJ− Y. pseudotuberculosis (YP26) for the indicated times in minutes. Cell lysates were prepared and subjected to Western blot (WB) analysis with anti-IκBα antibody (left), anti-phospho-JNK antibody (middle), or anti-JNK antibody (right). The results shown are representative of two experiments.
Role of TLR2 in YopJ-dependent apoptosis.
The stimulation of TLR2 by bacterial lipoproteins activates apoptotic signaling in macrophages (3). We wondered whether TLR2 may play a role in the infection-induced signaling and YopJ-dependent apoptosis that we observed for TLR4−/− macrophages. Macrophages were isolated from mice with null mutations in Tlr2 or from their wild-type littermates to examine this possibility. The signaling responses of these macrophages to purified PAMPs or to Yersinia infection were examined initially. The stimulation of wild-type or TLR2−/− macrophages with LPS or by infection with YopJ− Y. pseudotuberculosis for 15 min resulted in similar levels of IKKβ and JNK activation (Fig. 4A, top and middle panels, compare lanes 2, 3, and 4 with lanes 8, 9, and 10). As expected, a synthetic lipopeptide (Pam3CSK4) did not stimulate signaling in TLR2−/− macrophages, while robust responses to the lipopeptide were observed in wild-type macrophages (Fig. 4A, compare lanes 5 and 6 with lanes 11 and 12). Wild-type levels of apoptosis were observed in TLR2−/− macrophages after a 4-h infection with Y. pseudotuberculosis (Fig. 4B). Furthermore, analysis of infection-induced signaling in TLR2−/− macrophages over time indicated that TLR2−/− macrophages displayed wild-type responses to Y. pseudotuberculosis infection (Fig. 5B, middle panels, lanes 1 to 10). These results argue that TLR2 signaling does not contribute to YopJ-dependent apoptosis in macrophages infected with Y. pseudotuberculosis.
DISCUSSION
The results of this study increase the understanding of YopJ-dependent apoptosis and reveal an unexpected complexity in the mechanism by which the innate immune system recognizes a gram-negative pathogen. Our data suggest that the inhibition of IKKβ activation is required for macrophage apoptosis induced by Yersinia infection. We also found that TLR4 signaling contributes to YopJ-dependent macrophage apoptosis; however, another mechanism(s) can also participate in the process.
Our results extend previous conclusions about the relationship between YopJ and IKKβ. It has been shown that the inhibition of NF-κB activation is required for YopJ-dependent apoptosis (25). In addition, the translocation of YopJ into macrophages is associated with decreased IKK activity (25). We show here that macrophages overexpressing a constitutively active mutant of IKKβ are completely protected from YopJ-dependent apoptosis (Fig. 2). These results demonstrate that YopJ acts upstream of IKKβ to block its activation and rule out the possibility that YopJ interferes with the access of activated IKKβ to IκBα. Thus, inhibiting the activation of this single kinase is a key event in Yersinia pathogenesis.
How YopJ inhibits the activation of IKKβ remains unanswered. YopJ is a cysteine protease that appears to cleave ubiquitin or ubiquitin-like modifications from proteins (20). Although YopJ binds to IKKβ and inhibits its activation, there is currently no evidence that IKKβ is modified by ubiquitin or is itself a substrate of YopJ. Since ubiquitination is required for TRAF6 activation (31), it is possible that YopJ acts on TRAF6 to block the downstream activation of IKKβ (20). Recently, Ruckdeschel et al. showed that the transfection of macrophages with a dominant-negative form of TRAF6 increases apoptosis in response to LPS stimulation (26). These findings support the idea that TRAF6, like IKKβ, is important for the expression of survival factors in macrophages exposed to apoptotic stimuli.
Using macrophages deficient in TLR function, we demonstrate that signaling through TLR4 is important for YopJ-dependent apoptosis. This finding confirms the previous finding that LPS stimulation of macrophages can trigger apoptotic signaling (25). How TLR4 signaling triggers apoptosis is not fully understood. YopJ-dependent apoptosis is reduced when dominant-negative forms of MyD88 and FADD are expressed in macrophages (26). Inhibitors of caspase 8 and caspase 9 also significantly reduce YopJ-dependent apoptosis in macrophages (26). It has not been shown that caspase 8 is activated in response to Yersinia infection, although its substrate, Bid, is cleaved (7). These results suggest that apoptotic signaling through TLR4 likely is mechanistically similar to apoptotic signaling mediated by TLR2 (3).
A significant finding of this study is that TLR4−/− macrophages were not completely resistant to YopJ-dependent apoptosis (Fig. 3 and 4). In addition, infection-induced signaling was delayed but not eliminated in TLR4−/− macrophages (Fig. 5). TLR2−/− macrophages displayed wild-type levels of apoptosis and wild-type signaling responses to infection (Fig. 4 and 5). We tentatively conclude that TLR2 signaling is not responsible for the YopJ-dependent apoptosis that we observed in TLR4−/− macrophages. Other studies have failed to demonstrate a role for TLR2 in recognizing gram-negative bacteria as well. For example, Underhill et al. found that macrophages expressing dominant-negative TLR2 produced wild-type levels of TNF-α when challenged with Salmonella (29). Other PAMPs associated with Yersinia could contribute to the signaling responses of TLR4−/− macrophages to infection. For example, Y. pseudotuberculosis produces flagella that would be recognized by TLR5. Another interesting possibility is that a structural component of the type III secretion system could act as a PAMP. Sing et al. recently showed that LcrV stimulates macrophages to produce interleukin 10 independently of TLR4 function (27). They speculated that LcrV is recognized by a TLR on the surface of macrophages (27). Yet another possibility is that intracellular LPS receptors are involved in sensing Yersinia infection. For example, the NOD1 and NOD2 proteins contain a leucine-rich region, a structure found in the TLR4 extracellular domain, which is required for LPS signaling (12). It has been reported that the NOD proteins are able to activate the NF-κB pathway following LPS injection into cells (9, 19). However, it remains unknown how LPS could gain access to the NOD proteins under our infection conditions.
The involvement of receptors other than TLR4 in stimulating infection responses (Fig. 5) is suggestive of a hierarchy in the PRRs of the innate immune system for recognizing gram-negative pathogens. The use of pure PAMPs, such as LPS, has facilitated the identification of TLRs as PRRs and the characterization of TLR signaling pathways. However, new approaches will be needed to discover additional PRRs and to study complex functional relationships between subfamilies of PRRs. The use of live, intact pathogens is one approach that may prove useful in this context. Here, using whole live Yersinia to infect macrophages, we found that the activation of proinflammatory responses can occur in two waves in wild-type macrophages. The fast response is clearly TLR4 dependent, since it was abolished in TLR4−/− cells (Fig. 5). Studies to identify the receptors responsible for the slower response are ongoing.
In summary, our data support the concept that YopJ promotes the apoptotic signaling activity of TLR4 and potentially other PRRs by inhibiting the NF-κB-dependent survival response of macrophages. Even in the absence of YopJ, stimulation by LPS or by infection with Y. pseudotuberculosis can lead to low levels of apoptosis in macrophages. The level of apoptosis that we observed in macrophages infected with YopJ− bacteria varied depending on the genetic background of the macrophages used (Fig. 3 and 4). These results reinforce the idea that the relative strength of the prosurvival response versus the proapoptotic response critically determines whether macrophages live or die when infected by pathogenic bacteria.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (AI43389 to J.B.B.). Y.Z. is supported by a National Institutes of Health postdoctoral fellowship (5T32 CA09176).
We thank members of the laboratory of James B. Bliska for assistance, helpful discussions, and comments on the manuscript. In addition, we thank C. Roy for helpful comments on the retrovirus expression system; S. Akira, R. Medzhitov, K. Marcu, E. Wimmer, and K. Orth for reagents and suggestions; and J. E. Galán and S. Miller for stimulating discussions.
Editor: D. L. Burns
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., R. B. Yang, D. S. Weiss, P. Godowski, and A. Zychlinsky. 2000. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 19:3325-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delhase, M., M. Hayakawa, Y. Chen, and M. Karin. 1999. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 284:309-313. [DOI] [PubMed] [Google Scholar]
- 7.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 8.Faure, E., O. Equils, P. A. Sieling, L. Thomas, F. X. Zhang, C. J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058-11063. [DOI] [PubMed] [Google Scholar]
- 9.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 10.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 11.Hu, Y., V. Baud, M. Delhase, P. Zhang, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science 284:316-320. [DOI] [PubMed] [Google Scholar]
- 12.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Núñez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276:2551-2554. [DOI] [PubMed] [Google Scholar]
- 13.Kimbrell, D. A., and B. Beutler. 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2:256-267. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie, F. R., M. A. Connelly, D. Balzarano, J. R. Muller, R. Geleziunas, and K. B. Marcu. 2000. Functional isoforms of IκB kinase α (IKKα) lacking leucine zipper and helix-loop-helix domains reveal that IKKα and IKKβ have different activation requirements. Mol. Cell. Biol. 20:2635-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 16.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ninomiya-Tsuji, J., K. Kishimoto, A. Hiyama, J. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252-256. [DOI] [PubMed] [Google Scholar]
- 19.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Núñez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 20.Orth, K. 2002. Function of the Yersinia effector YopJ. Curr. Opin. Microbiol. 5:38-43. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, L. E., A. R. Pancetti, S. Greenberg, and J. B. Bliska. 1999. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect. Immun. 67:708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 23.Rassa, J. C., J. L. Meyers, Y. Zhang, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trulzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 26.Ruckdeschel, K., O. Mannel, and P. Schrottner. 2002. Divergence of apoptosis-inducing and preventing signals in bacteria-faced macrophages through myeloid differentiation factor 88 and IL-1 receptor-associated kinase members. J. Immunol. 168:4601-4611. [DOI] [PubMed] [Google Scholar]
- 27.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 29.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 30.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNFα-induced apoptosis by NF-κβ. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 31.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 32.Wang, C.-U., M. W. Mayo, and A. S. Baldwin Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κβ. Science 274:784-787. [DOI] [PubMed] [Google Scholar]