Abstract
Although previous studies demonstrated a requirement for CD40-CD40 ligand (CD40L) interaction in the development of resistance to Leishmania infection, we recently showed that mice lacking the gene for CD40L (CD40L−/− mice) can control Leishmania major infection when they are infected with reduced numbers of parasites. In this study, we examine the cytokine pattern in healing versus nonhealing CD40L−/− mice and investigated whether CD40 activation is required for resistance to reinfection. We observed that CD4+ cells in healed CD40L−/− mice produce high levels of gamma interferon compared to cells from nonhealing, high-dose-inoculated mice. In addition, we observed a higher frequency of interleukin-12 (IL-12)- producing cells and a reduced number of IL-4-producing cells in mice infected with reduced numbers of parasites. Importantly, we found that healed CD40L−/− mice are highly resistant to reinfection with a large parasite inoculum. In addition, by comparing the cytokine patterns at an early and late stage of infection in nonhealing CD40L−/− mice, we demonstrated that nonhealing CD40L−/− mice produce a weak Th1-type response during the early stage of infection, but this response wanes as a Th2-type response emerges during late stages of infection. Anti-IL-4 antibody treatment, starting either at the beginning of infection or at week 4 postinfection enabled CD40L−/− mice to control a high-dose infection. Together, these results show that CD40-CD40L interaction, although important for IL-12 production in high-dose infections, is not required for either the development or maintenance of resistance in mice infected with reduced numbers of parasites.
Cutaneous infection with Leishmania major is a self-limiting disease in many inbred mice, including the C57BL/6, CBA, C3H, and 129/J strains. In contrast, BALB/c mice are highly susceptible to L. major and develop progressive, nonhealing cutaneous lesions (9, 16). Resistance or susceptibility to L. major is dependent upon the type of the immune response the mice develop during infection. Resistant mice develop an interleukin-12 (IL-12)-dependent Th1-type response in which parasite-specific CD4+ cells produce gamma interferon (IFN-γ) that induces the production of nitric oxide and parasite elimination by macrophages. IL-12 is essential for both the induction of protective immunity against L. major and maintenance of resistance (13, 26, 27, 32). In contrast, susceptible mice develop a dominant Th2-type response characterized by the production of high levels of IL-4 and other Th2 cytokines, but little IFN-γ. It is believed that early IL-4 production in BALB/c mice down-regulates IL-12 receptor beta 2 (IL-12Rβ2) chain expression, resulting in progressive unresponsiveness to IL-12 (14, 15, 18). Susceptible BALB/c mice treated with anti-IL-4 antibody or mice deficient in the gene for IL-4 or IL-4R are resistant to infection, consistent with a role for IL-4 in favoring Th2 response (21, 24, 29). In addition to IL-12 and IL-4, several other cytokines have marked effects on infection with L. major in mice. For instance, tumor necrosis factor alpha and IFN-α/β induce the activation of macrophages to produce nitric oxide to kill L. major and promote the development of a protective Th1/IFN-γ response to infection (3, 34). In contrast, transforming growth factor β, IL-10, and IL-13 favor a Th2-type response (2, 20, 22). The immune response to L. major is also influenced by the size of the parasite dose used to initiate infection, as evidenced by the observation that BALB/c mice develop a stable Th1 response and control infection if inoculated with <1,000 stationary-phase promastigotes and are resistant to a pathological challenge infection with L. major (4). Recently, Compton and Farrell (8) demonstrated that BALB/c mice deficient in CD28 molecule can resolve an infection following inoculation of low numbers of promastigotes which produce progressive disease in wild-type BALB/c mice.
In the past few years, a number of studies have examined the role of costimulatory molecules in the development of immune response to a variety of infections. It has been shown that CD40-CD40 ligand (CD40L) interactions are important for induction of protective cell-mediated immunity to both L. major and the related protozoan parasite Leishmania amazonensis (5, 19, 31). CD40−/− or CD40L−/− mice on a resistant C57BL/6 × 129/J background are susceptible to L. major or L. amazonensis and are markedly impaired in their ability to produce IL-12 and IFN-γ during infection. Susceptibility to Leishmania infection can be overcome by treatment of CD40L−/− mice with exogenous IL-12, suggesting that the CD40-CD40L costimulatory pathway is critical to IL-12 production. However, we recently showed that CD40L−/− mice control L. major infection following inoculation with low numbers of parasites (25), which suggests that although CD40-CD40L interactions are important, they are not absolutely required for the development of a protective response.
In this study, we analyzed in detail the cytokine patterns in nonhealing CD40L−/− mice at early and late stages of L. major infection and compared them to those in resistant C57BL/6 mice. We also examined the cytokine patterns in healing, low-dose-infected versus nonhealing, high-dose-infected CD40L−/− mice. Together, our results show that cells from CD40L−/− mice produce sufficient IL-12 to promote a dominant Th1-type response following infection with low numbers of parasites and that resistance to a high-dose challenge infection in healed mice is independent of CD40 activation. In addition, we show that production of IL-4 is associated with the inability of CD40L−/− mice to resolve infection following inoculation of a high dose of parasites and that susceptibility can be reversed following in vivo neutralization of IL-4.
MATERIALS AND METHODS
Parasites and animals.
Female C57BL/6 and CD40L−/− mice (B6;129S-Tnfsf5tm1Imx) were purchased from the Jackson Laboratory (Bar Harbor, Maine). All mice were 6 to 10 weeks old at the time of infection. L. major (WHO MHOM/IL/80/Friedlin) was maintained in Grace's insect cell culture medium (Life Technologies, Grand Island, N.Y.) containing 20% fetal bovine serum (FBS), 2 mM l-glutamine, 100 μg of streptomycin, and 100 U of penicillin G sodium per ml.
Infections.
Mice were inoculated by injecting promastigotes into one hind footpad. Either 2 × 105(high-dose) or 2 × 104 (low-dose) L. major metacyclic promastigotes, isolated from stationary-phase cultures by negative selection using peanut agglutinin (Sigma) as described previously (28), were used. The metacyclic promastigotes were in a volume of 50 μl, and lesion size was measured weekly with a vernier caliper and expressed as the difference in thickness between the infected and uninfected contralateral footpad. Parasites were enumerated by a limiting dilution assay as described previously (1). In brief, the homogenates of infected lesions or cells from lymph nodes were serially diluted in Grace's insect culture medium plus 20% FBS and observed 5 to 7 days later for growth of promastigotes. Parasite numbers are expressed as the negative log10 dilution at which promastigote growth was observed.
Anti-IL-4 MAb treatment protocol.
One group of CD40L−/− mice was treated intraperitoneally, starting at the beginning of infection with anti-IL-4 monoclonal antibody (MAb) (11B11; Harlan, Madison, Wis.) on day 1 (5.0 mg/mouse) and then on days 8 and 15 (3.0 mg/mouse) of infection. In other groups of CD40L−/− mice, the treatment was started at week 4 postinfection and performed at weekly intervals for three consecutive weeks with doses of anti-IL-4 MAb similar to the initial doses.
RNase protection assay.
Total RNA was isolated from popliteal draining lymph nodes using RNA STAT-60 (Tel-Test B, Friendswood, Tex.) as directed by the manufacturer. mRNA was quantified by a RNase protection assay using a Riboquant kit (PharMingen, San Diego, Calif.) as directed by the manufacturer. A custom probe from PharMingen was prepared using [32P]UTP and hybridized to 15 μg of each sample RNA. The protected probe was purified and resolved on 5% denaturing polyacrylamide gels using Ultra Pure Sequagel reagents (National Diagnostics, Atlanta, Ga.). Dried gels were exposed to a phosphorimaging screen, and protected fragments were visualized using a Phospho-Imager GS-525 Molecular Imager System (Bio-Rad, Richmond, Calif.).
ELISPOT assay.
The numbers of IL-12p40- and IL-4-secreting cells in splenocyte suspensions were determined by using an enzyme-linked immunospot (ELISPOT) assay as previously described (22, 33). MAbs C17.8 and biotinylated C15.6, generously provided by Christopher Hunter at the University of Pennsylvania (Philadelphia), were used for IL-12 ELISPOT assay. MAbs 11B11 (Harlan) and biotinylated BVD-6 were used to detect IL-4-secreting cells. IL-12- and IL-4-secreting cells were determined in cell cultures following overnight stimulation with 50 μg of soluble leishmanial antigen (SLA) per ml prepared as described previously (30).
Cell culture and ELISA.
Single-cell suspensions of spleens were cultured at 5 × 106 cells/ml in Dulbecco modified Eagle medium containing 10% FBS, 2 mM glutamine, 100 U of penicillin G sodium/ml, 100 μg of streptomycin sulfate/ml, and 5 × 10−5 M 2-mercaptoethanol in the presence of 50 μg of SLA per ml. Supernatants were collected at 72 h and tested for IFN-γ and IL-10 in an enzyme-linked immunosorbent assay (ELISA). The ELISA for IFN-γ was performed as previously described (23). Recombinant IFN-γ (generously provided by Phillip Scott, University of Pennsylvania) was used as the standard. The reagents for IL-10 ELISA were obtained from PharMingen, and the assay was run as directed by the manufacturer.
Flow cytometric analysis.
For intracellular detection of IFN-γ, purified splenocytes were plated in a 96-well plate (Costar) at a density of 4 × 105 cells/well in a final volume of 200 μl. Cells were stimulated with SLA (50 μg/ml) for 36 h, and then phorbol myristate acetate (50 ng/ml), ionomycin (500 ng/ml), and brefeldin A (10 μg/ml; Sigma-Aldrich) were added to cultures during the last 5 h of stimulation. Cells were harvested, washed, resuspended in fluorescence-activated cell sorter (FACS) buffer (1× phosphate-buffered saline, 0.2% bovine serum albumin fraction V, 4 mM sodium azide) and then preincubated with saturating concentrations of Fc block for 20 min on ice and stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 or anti-CD8 (PharMingen) for 30 min on ice. Cells were then washed with FACS buffer, fixed with 4% (wt/vol) paraformaldehyde, washed again, and permeabilized with 0.1% (wt/vol) saponin in FACS buffer. After permeabilization, cells were stained with allophycocyanin-conjugated anti-IFN-γ (PharMingen) for 30 min on ice. Cells were washed once with 0.1% saponin buffer and then with FACS buffer. Analysis of the cells was performed using a FACSCalibur flow cytometer (BD Biosciences). Results were analyzed using CellQuest software (BD Biosciences). The recommended antibody concentrations were used to give optimal staining for flow cytometric analyses.
Statistical analysis.
Statistically significant differences between groups were determined using the unpaired Student's t test. Significance was assumed if P was <0.05.
RESULTS
Susceptibility of CD40L−/− mice to L. major is determined by the challenge dose.
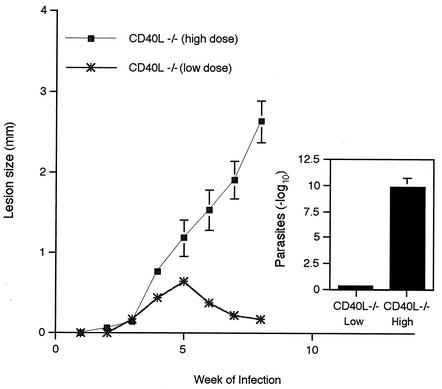
We initially compared the course of infection in CD40L−/− mice inoculated with high and low parasite doses. CD40L−/− mice were inoculated with either 2 × 105 (high-dose) or 2 × 104 (low-dose) metacyclic promastigotes, and footpad size was measured weekly to assess the course of infection. As can be seen in Fig. 1, as expected, high-dose-inoculated CD40L−/− mice failed to control the infection, as evidenced by continued expansion of lesion size through week 9. In contrast, mice inoculated with the lower parasite dose effectively controlled infection, as evidenced by resolution of footpad lesions, confirming our previous report (25). When lesion parasite numbers were determined at week 9, low-dose-inoculated mice had ∼10-log-units-fewer parasites than the nonhealing, high-dose-inoculated mice (Fig. 1, insert).
FIG. 1.
Susceptibility of CD40L−/− mice to L. major is determined by the challenge dose. CD40L−/− mice were inoculated with either 2 × 105 (high-dose) or 2 × 104 (low-dose) metacyclic promastigotes, and the infection was monitored for 9 weeks. Lesion size was expressed as the difference in thickness between the infected and uninfected contralateral footpads. The numbers of lesion parasites at week 9 of infection were determined by limiting dilution assay and expressed as the negative log10 dilution at which promastigote growth was observed. Values are the means ± standard errors (error bars), with four or five mice in each group.
Healing in low-dose-inoculated CD40L−/− mice is associated with increased IL-12 and IFN-γ production.
We have previously shown that the levels of IFN-γ produced by cells from healing, low-dose-inoculated CD40L−/− mice are similar to those from infected C57BL/6 mice (25). In order to determine the cytokine patterns in healing versus nonhealing CD40L−/− mice, we analyzed the frequency of IL-12- and IL-4-secreting cells in the spleens of the low-dose- or high-dose-infected groups at week 9 postinfection using an ELISPOT assay and also measured IFN-γ production by antigen-stimulated spleen cells by ELISA. As can be seen in Fig. 2, the number of IL-12p40-secreting cells and the level of IFN-γ production were significantly higher in low-dose-infected CD40L−/− mice than in high-dose-infected mice. In contrast, the number of IL-4-producing cells was significantly reduced in the low-dose-infected group. Together, these results demonstrate that reducing the parasite inoculum from 2 × 105 to 2 × 104 promastigotes enabled mice to develop a protective Th1-type response.
FIG. 2.
Cytokine production in healing versus nonhealing CD40L−/− mice. Spleen cells from the mice in Fig. 1 were assayed for IL-4, IL-12p40, and IFN-γ production. (A) IL-4 and IL-12p40 production was measured by direct ELISPOT assay following overnight stimulation of cells with SLA (50 μg/ml). The data are expressed as mean frequencies per 106 splenocytes ± standard errors (error bars), with three or more mice in each group. (B) IFN-γ production was measured in cell supernatants by ELISA following in vitro stimulation of cells with SLA (50 μg/ml) for 72 h. Values are the means ± standard errors (error bars), with three mice in each group.
Resistance to reinfection is independent of CD40 activation.
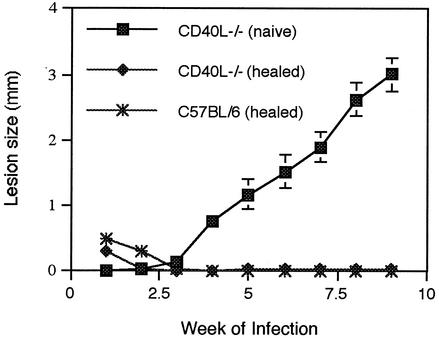
Next, we examined whether CD40-CD40L interactions are required for the maintenance of protective immunity. CD40L−/− and C57BL/6 mice were inoculated with 2 × 104 parasites and allowed to heal. At week 18 of primary infection, healed CD40L−/− and C57BL/6 mice, as well as naive CD40L−/− mice, were challenged with a high parasite dose (2 × 105 metacyclic promastigotes) delivered into the contralateral footpad, and lesion size was monitored weekly to assess the course of disease. As can be seen in Fig. 3, healed CD40L−/− and C57BL/6 mice were highly resistant to the challenge infection and exhibited a dominant Th1 response (Fig. 4). Parasites were not detected within lesions of healed CD40L−/− and C57BL/6 mice at week 10 after challenge, confirming that these mice were highly resistant to reinfection (data not shown).
FIG. 3.
Resistance to reinfection is independent of CD40 activation. CD40L−/− and C57BL/6 mice were inoculated with 2 × 104 L. major metacyclic promastigotes and allowed to heal. Healed CD40L−/− and C57BL/6 mice and naive CD40L−/− were then rechallenged with 2 × 105 promastigotes, and lesion size was monitored for 9 weeks. Values are the means ± standard errors (error bars), with four mice in each group.
FIG. 4.
IFN-γ production by spleen cells from rechallenged or naive CD40L−/− and C57BL/6 mice. At week 9, cells from infected mice were stimulated in vitro with SLA, and cell supernatants were harvested at 72 h and assayed for IFN-γ. Values are the means ± standard errors (error bars), with four mice in each group.
CD4+ cells are the primary IFN-γ-producing cells during L. major infection in CD40L−/− mice.
To determine the type of the cells responsible for resistance during a challenge infection, splenocytes were harvested at week 10 following reinfection of healed CD40L−/− and C57BL/6 mice. Cells were restimulated with SLA (50 μg/ml) for 36 h, followed by another 5 h in the presence of phorbol myristate acetate, ionomycin, or brefeldin A. Surface and intracellular staining were performed as described above in Materials and Methods at the recommended concentrations for the antibodies and analyzed using a FACSCalibur flow cytometer. Nonhealing CD40L−/− mice infected with the high parasite dose were used as controls. Analysis of cytokine production by week 10 after challenge showed that CD4+ T cells were the dominant IFN-γ-secreting cell in healing mice (Table 1). IFN-γ-secreting CD8 cells were also detected, but the numbers of these cells were similar in healing and nonhealing CD40L−/− mice.
TABLE 1.
IFN-γ-producing CD4+ and CD8+ cells in two C57BL/6 and CD40L−/− mice
| Micea | % CD4+ cells | % CD8+ cells |
|---|---|---|
| Rechallenged C57BL/6 | ||
| Mouse 1 | 6.4 | 2.8 |
| Mouse 2 | 7.26 | 2.5 |
| Rechallenged CD40L−/− | ||
| Mouse 1 | 13 | 2.92 |
| Mouse 2 | 8.4 | 4.86 |
| Naive CD40L−/− (control) | ||
| Mouse 1 | 1.45 | 3.47 |
| Mouse 2 | 1.47 | 6.37 |
Rechallenged C57BL/6 and CD40L−/− mice were healed, low-dose-infected mice, and naive CD40L−/− mice were inoculated with high parasite doses.
Nonhealing CD40L−/− mice produce a weak Th1-type response during early stages of L. major infection.
Our findings that mice can produce IL-12 and mount a protective Th1-type response in the absence of CD40 signaling suggest that CD40-CD40L interactions are not indispensable for induction of a protective Th1-type response to L. major infection. Although previous studies have observed a defect in IL-12 production in nonhealing CD40L−/− mice, it is possible that these mice can produce sufficient IL-12 to mount a protective Th1-type response following in vivo blockade of IL-4. Prior to treatment with anti-IL-4 antibody, we first examined cytokine message levels in CD40L−/− and C57BL/6 mice following inoculation of a high dose (2 × 105) of parasites. As evidenced in Fig. 5, the cytokine patterns at week 4 in CD40L−/− and C57BL/6 mice were very similar with detectable message levels for IL-12p40 and IL-12Rβ2. However, the level for IFN-γ message was lower in CD40L−/− mice than in C57BL/6 mice, and IL-4 message, which could not be detected in C57BL/6 mice, was observed in the CD40L−/− mice. In contrast to week 4, a pattern of Th2 dominance was clearly present by week 9 in nonhealing CD40L−/− mice, as evidenced by decreased levels of message for IFN-γ, IL-12, and IL-12β2 and increased mRNA levels for IL-4 (Fig. 5).
FIG. 5.
CD40L−/− mice exhibit a Th2-type cytokine profile by week 9 of infection. Cytokine and cytokine receptor mRNA levels in the lymph nodes of CD40L−/− and C57BL/6 mice were compared at weeks 4 and 9 following infection with 2 × 105 L. major metacyclic promastigotes (high dose). For the week 4 gel, the levels of cytokine and cytokine receptor mRNA from a naive mouse (lanes 1) and two individual infected mice (lanes 2 and 3) are shown. For the week 9 gel, the levels of cytokine and cytokine receptor mRNA from two individual infected mice (lanes 1 and 2) are shown. MIF, migration inhibitory factor.
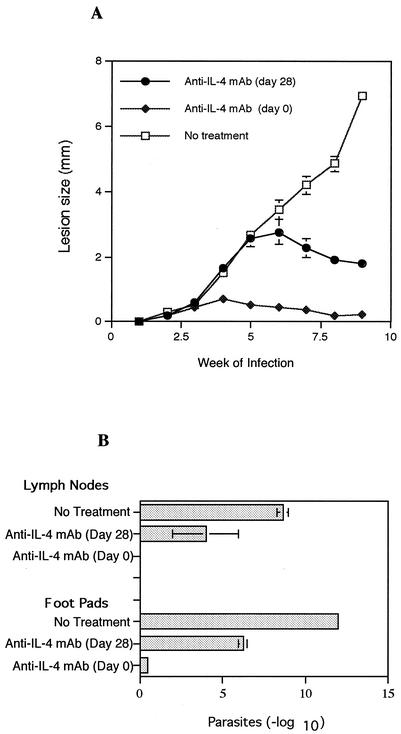
On the basis of these observations, we predicted that treatment with anti-IL-4 antibody would promote healing in high-dose-infected CD40L−/− mice and that this treatment could be delayed until several weeks after infection. CD40L−/− mice were infected with the high parasite dose and treated with anti-IL-4 MAb starting either at the beginning of infection or at week 4 postinfection. The results in Fig. 6A demonstrate that treatment with anti-IL-4 antibody starting either at the beginning or at week 4 postinfection promoted control of infection in CD40L−/− mice, although lesions in mice treated at the start of infection were significantly smaller and resolved more rapidly than those in mice receiving delayed anti-IL-4 antibody treatment. Analysis of parasite numbers confirmed the protective effects of in vivo IL-4 neutralization (Fig. 6B). We also examined cytokine production in these mice and observed that spleen cells from CD40L−/− mice treated with anti-IL-4 antibody produced more IFN-γ and less IL-10 (Fig. 7) than the cells from nonhealing control mice. Together, these results suggest that CD40L−/− mice are fully capable of developing a Th1-type response following infection with a high parasite dose, provided that in vivo levels of IL-4 remain low.
FIG. 6.
Effect of anti-IL-4 MAb treatment on the course of L. major infection in high-dose-infected CD40L−/− mice. CD40L−/− mice were infected with 2 × 105 L. major metacyclic promastigotes (high dose) and treated with anti-IL-4 MAb starting either at the beginning of infection or at week 4 postinfection. (A) Lesion size was expressed as the difference in thickness between the infected and uninfected contralateral footpads. (B) The numbers of parasites in the lesions and lymph nodes at week 9 of infection were determined by limiting dilution assay and expressed as the negative log10 dilution at which promastigote growth was observed. Values are the means ± standard errors (error bars), with four mice in each group.
FIG. 7.
IL-10 and IFN-γ production at week 9 of L. major infection in CD40L−/− mice treated with anti-IL-4 MAb. Spleen cells from mice treated with anti-IL-4 MAb or untreated CD40L−/− mice were stimulated in vitro with SLA, and cell supernatants were harvested at 72 h and assayed for IFN-γ by ELISA. Values are the means ± standard errors (error bars), with four mice in each group.
DISCUSSION
In this study, we examined how the inoculation of low numbers of parasites influences the course of L. major infection in CD40L−/− mice and also investigated whether CD40-CD40L interaction is essential for resistance to reinfection. Over the past few years, a number of studies have examined how signaling through costimulatory molecules affects cytokine production and subsequent development of resistance to cutaneous leishmaniasis. These studies have shown that CD40-CD40L interaction appears to be essential for induction of protective cell-mediated immunity to both L. major and the related protozoan parasite L. amazonensis, as evidenced by the observations the mice lacking the genes for CD40 or CD40L are highly susceptible to infection with these parasites (5, 19, 31). Additional studies have shown that transient treatment with agonistic antibody to CD40 can also induce resistance in susceptible BALB/c mice (10), whereas treatment with a neutralizing antibody to CD40L exacerbates the infection in a resistant mouse strain (12). Susceptibility to infection in these mice was associated with decreased production of IL-12 and could be overcome by treatment of CD40L−/− mice with exogenous IL-12, suggesting that the CD40-CD40L costimulatory pathway is a major stimulus for IL-12 production by dendritic cells (DCs) or macrophages. A recent vaccine study combining CD40L with leishmanial antigen provided additional evidence that CD40 ligation is a potent inducer of in vivo IL-12 production (7). Although these experiments demonstrate the importance of CD40-CD40L interactions in promoting resistance to Leishmania infection, they all used a high parasite inoculum to initiate infection, whereas we have recently shown that CD40L−/− mice can effectively control L. major infection when inoculated with reduced numbers of parasites (25). In this study, we analyzed in greater detail the healing versus nonhealing patterns in CD40L−/− mice and confirm that CD40L−/− mice inoculated with low parasite numbers control the infection efficiently and developed a protective Th1 response.
Our observation that low-dose-infected CD40L−/− mice heal and develop resistance to reinfection with high parasite dose suggests that protective Th1 immunity can be achieved against L. major, independent of CD40 activation. This was confirmed by our demonstration that cells from low-dose-inoculated CD40L−/− mice produced high levels of IFN-γ and that spleens from infected mice had significantly higher numbers of IL-12-secreting cells and fewer IL-4-secreting cells compared to mice inoculated with a high parasite dose. We also found that CD4+ T cells are the dominant IFN-γ-secreting cells in both resistant C57BL/6 and CD40L−/− mice, suggesting that a classical Th1 type response developed in the absence of CD40L costimulation. These results are in contrast to previous reports, suggesting that CD4+ cells are defective in the absence of CD40-CD40L interaction and fail to differentiate into IFN-γ-producing Th1-type cells (11, 17).
Since CD40L−/− mice infected with the higher parasite dose appeared to ultimately develop a dominant Th2-type response, we further analyzed the response in these mice by examining cytokine mRNA levels during infection. At week 4 of infection, levels of IFN-γ message were lower in CD40L−/− mice than in C57BL/6 mice, and low levels of IL-4 message, which could not be detected in C57BL/6 mice, were observed in the CD40L−/− mice. However, message levels for IL-12p40 and IL-12Rβ2 in these mice were comparable. However, by week 9, message levels for IFN-γ, IL-12p40, and IL-12Rβ2 decreased, while those for IL-4 were elevated, suggesting that Th1-type cytokine production during early stages of infection wanes as a Th2-type response emerges during later stages of infection. Interestingly, treatment of CD40L−/− mice with anti-IL-4 MAb as late as week 4 postinfection led to control of infection, suggesting that blockade of IL-4 can reverse the effects of developing a Th2-type response. The capacity of delayed anti-IL-4 treatment to promote healing in CD40L−/− mice differentiates these mice from BALB/c mice in which anti-IL-4 antibody must be administered at the start of infection in order to promote resistance (6), although the disease progression was substantially inhibited. A possible reason for these differing results may be the different nature of the Th1 or Th2 phenotype of the associated immune response at the time of initiating treatment and the different genetic backgrounds of the mice used in these two studies. In genetically susceptible BALB/c mice, early IL-4 production quickly down-regulates IL-12Rβ2 chain expression on CD4+ T cells, resulting in a state of unresponsiveness to IL-12 required for Th1-type response during L. major infection (14, 15, 18), whereas lower levels of IL-4 production combined with enhanced levels of IL-12Rβ2 chain expression in CD40L−/− mice enable IL-4 blockade to halt the development of a dominant Th2-type response.
In summary, we demonstrate here that CD40-CD40L interactions, although important, are not absolutely essential for IL-12 production and initiation of Th1-type response against L. major infection, provided that mice are not inoculated with a high dose of parasites. In addition, we show that resistance to reinfection is independent of CD40 activation. Given the importance of IL-12 in the induction of a Th1 response as well as maintenance of resistance to infection with L. major, our results suggest that alternative pathways of IL-12 production occur in the absence of CD40-CD40L interaction. Whether L. major directly induced IL-12 production by antigen-presenting cells or whether other costimulatory interactions are important for in vivo IL-12 production has yet to be determined. However, it is clear that a reduction in levels of infection may reveal additional components of the immune response that are obscured following inoculation of high numbers of parasites.
Acknowledgments
This work was supported in part by National Institutes of Health grant AI-27828.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Afonso, L. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. [DOI] [PubMed] [Google Scholar]
- 2.Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher, P. A., G. Wei, J. N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257:539-542. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, K. A., P. J. Ovendale, M. K. Kennedy, W. C. Fanslow, S. G. Reed, and C. R. Maliszewski. 1996. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4:283-289. [DOI] [PubMed] [Google Scholar]
- 6.Chatelain, R., K. Varkila, and R. L. Coffman. 1992. IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 148:1182-1187. [PubMed] [Google Scholar]
- 7.Chen, G., P. A. Darrah, and D. M. Mosser. 2001. Vaccination against the intracellular pathogens Leishmania major and L. amazonensis by directing CD40 ligand to macrophages. Infect. Immun. 69:3255-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton, H. L., and J. P. Farrell. 2002. CD28 costimulation and parasite dose combine to influence the susceptibility of BALB/c mice to infection with Leishmania major. J. Immunol. 168:1302-1308. [DOI] [PubMed] [Google Scholar]
- 9.DeTolla, L. J., P. A. Scott, and J. P. Farrell. 1981. Single gene control of resistance to cutaneous leishmaniasis in mice. Immunogenetics 14:29-39. [DOI] [PubMed] [Google Scholar]
- 10.Ferlin, W. G., T. von der Weid, F. Cottrez, D. A. Ferrick, R. L. Coffman, and M. C. Howard. 1998. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur. J. Immunol. 28:525-531. [DOI] [PubMed] [Google Scholar]
- 11.Grewal, I. S., and R. A. Flavell. 1996. The role of CD40 ligand in costimulation and T-cell activation. Immunol. Rev. 153:85-106. [DOI] [PubMed] [Google Scholar]
- 12.Heinzel, F. P., R. M. Rerko, and A. M. Hujer. 1998. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell. Immunol. 184:129-142. [DOI] [PubMed] [Google Scholar]
- 13.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himmelrich, H., P. Launois, I. Maillard, T. Biedermann, F. Tacchini-Cottier, R. M. Locksley, M. Rocken, and J. A. Louis. 2000. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164:4819-4825. [DOI] [PubMed] [Google Scholar]
- 15.Himmelrich, H., C. Parra-Lopez, F. Tacchini-Cottier, J. A. Louis, and P. Launois. 1998. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 161:6156-6163. [PubMed] [Google Scholar]
- 16.Howard, J. G., C. Hale, and W. L. Chan-Liew. 1980. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 2:303-314. [DOI] [PubMed] [Google Scholar]
- 17.Howland, K. C., L. J. Ausubel, C. A. London, and A. K. Abbas. 2000. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J. Immunol. 164:4465-4470. [DOI] [PubMed] [Google Scholar]
- 18.Jones, D., M. M. Elloso, L. Showe, D. Williams, G. Trinchieri, and P. Scott. 1998. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major. Infect. Immun. 66:3818-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamanaka, M., P. Yu, T. Yasui, K. Yoshida, T. Kawabe, T. Horii, T. Kishimoto, and H. Kikutani. 1996. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity 4:275-281. [DOI] [PubMed] [Google Scholar]
- 20.Matthews, D. J., C. L. Emson, G. J. McKenzie, H. E. Jolin, J. M. Blackwell, and A. N. McKenzie. 2000. IL-13 is a susceptibility factor for Leishmania major infection. J. Immunol. 164:1458-1462. [DOI] [PubMed] [Google Scholar]
- 21.Mohrs, M., C. Holscher, and F. Brombacher. 2000. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect. Immun. 68:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris, S. C., K. B. Madden, J. J. Adamovicz, W. C. Gause, B. R. Hubbard, M. K. Gately, and F. D. Finkelman. 1994. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J. Immunol. 152:1047-1056. [PubMed] [Google Scholar]
- 23.Mosmann, T. R., and T. A. Fong. 1989. Specific assays for cytokine production by T cells. J. Immunol. Methods 116:151-158. [DOI] [PubMed] [Google Scholar]
- 24.Noben-Trauth, N., W. E. Paul, and D. L. Sacks. 1999. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 162:6132-6140. [PubMed] [Google Scholar]
- 25.Padigel, U. M., P. J. Perrin, and J. P. Farrell. 2001. The development of a Th1-type response and resistance to Leishmania major infection in the absence of CD40-CD40L costimulation. J. Immunol. 167:5874-5879. [DOI] [PubMed] [Google Scholar]
- 26.Park, A. Y., B. Hondowicz, M. Kopf, and P. Scott. 2002. The role of IL-12 in maintaining resistance to Leishmania major. J. Immunol. 168:5771-5777. [DOI] [PubMed] [Google Scholar]
- 27.Park, A. Y., B. D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896-902. [DOI] [PubMed] [Google Scholar]
- 28.Sacks, D. L., and P. V. Perkins. 1984. Identification of an infective stage of Leishmania promastigotes. Science 223:1417-1419. [DOI] [PubMed] [Google Scholar]
- 29.Sadick, M. D., F. P. Heinzel, B. J. Holaday, R. T. Pu, R. S. Dawkins, and R. M. Locksley. 1990. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J. Exp. Med. 171:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott, P., E. Pearce, P. Natovitz, and A. Sher. 1987. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J. Immunol. 139:221-227. [PubMed] [Google Scholar]
- 31.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, Jr., N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 32.Sypek, J. P., C. L. Chung, S. E. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, Z. E., S. L. Reiner, S. Zheng, D. K. Dalton, and R. M. Locksley. 1994. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J. Exp. Med. 179:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm, P., U. Ritter, S. Labbow, N. Donhauser, M. Rollinghoff, C. Bogdan, and H. Korner. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J. Immunol. 166:4012-4019. [DOI] [PubMed] [Google Scholar]