Abstract
Speed of drug onset is assumed to be an important determinant of the abuse liability of a drug. Studies in human and non-human primates suggest that the subjective and reinforcing effects of cocaine can be influenced by route of administration and/or speed of intravenous injection. Sensitization to the reinforcing effects of cocaine was studied in rats and the effects of various injection durations (i.e. speed of injection) on the development of sensitization was examined using a progressive ratio schedule. In addition, the effects of cocaine dose on sensitization and the effects of injection duration on the acute reinforcing effects of cocaine were examined. The initial study demonstrated that the development of sensitization (i.e. progressive increases in breakpoints) was dose-dependent. A robust sensitization of the reinforcing effects of cocaine was replicated in animals receiving cocaine at the highest rate (i.e. shortest duration; 5 s), but not in animals receiving the same dose over 25 or 50 s. Subsequent testing revealed that injection duration did not have profound effects on the acute reinforcing effects of cocaine (assessed by breakpoints or rate of responding on a fixed ratio schedule). These findings are similar to recent studies demonstrating that the development of sensitization, but not the acute responsivity, to cocaine’s locomotor-activating effects are influenced by rate of intravenous injection. Taking these findings together, we hypothesize that the process of drug addiction involves both the acute reinforcing effects and the development of sensitization.
Keywords: abuse liability, infusion duration, locomotor activity, motivation, pharmacokinetics
Introduction
Speed of drug onset is assumed to be an important determinant of the abuse liability of a drug (for discussion see Zernig et al., 2003). For any particular drug, a route of administration that produces a more efficient or rapid drug onset is generally associated with a greater level of abuse liability (O’Brien, 2001). For example, epidemiological evidence suggests that, compared with intranasal use, smoking or injecting cocaine is associated with greater escalation in drug intake, a higher level of dependence, a more rapid progression from initial to regular use, a greater number of psychosocial disruptions, and a faster switch from recreational use to obsessive drug taking and seeking (Siegel, 1979; Brower et al., 1986; Gawin & Ellinwood, 1988; Gossop et al. 1992,1994; Hatsukami & Fischman, 1996). Several experimental studies have examined the idea that speed of drug onset can influence the subjective effects of cocaine. For example, smoking cocaine has been demonstrated to be slightly more intense than injecting cocaine (Perez-Reyes et al., 1982) and higher speeds of injection (and presumably faster rates of drug onset) are associated with higher scores on various subjective ratings (Abreu et al., 2001). A related idea is that the abuse liability of a drug is enhanced by quick drug onset, because of stronger associations established between drug-taking and subsequent drug effects (see O’Brien, 2001).
Studies with non-human primates have provided evidence that the speed of injection can influence the reinforcing effects of cocaine (Balster & Schuster, 1973; Kato et al., 1987; Panlilio et al., 1998; Woolverton & Wang, 2004). In rats, however, relationships between speed of injection and the reinforcing effects of cocaine have been more difficult to demonstrate. For example, rate of acquisition, rate of intake on a fixed ratio schedule and breakpoints maintained on a progressive ratio (PR) schedule do not differ when the injection speed ranges from 5 to 100 s (Pickens et al., 1969; Crombag et al., 2003a). Interestingly, recent studies demonstrate that speed of injection has profound effects on the development of sensitization to the locomotor effects of cocaine, a phenomenon thought to be important in the addiction process (Robinson & Berridge 1993, 2001). Samaha et al. (2002, 2004) reported that faster cocaine injections (i.e. shorter durations) produced sensitization of stimulant-induced rotational behavior, whereas lower rates of administration with the same dose failed to produce sensitization. These rapid infusions enhanced the ability of cocaine to suppress dopamine uptake, and produced differential changes in c-fos and arc mRNA expression in certain brain regions. These experiments highlight the fact that the speed of drug administration can affect certain forms of neuroadaptations, including those associated with sensitization.
The relationship between sensitization of the locomotor-activating effects of stimulants and the reinforcing effects has been studied in several ways; these include an examination of the effects of cocaine self-administration on locomotor behavior (Hooks et al., 1994; Phillips & Di Ciano, 1996; Zapata et al., 2003; Ben-Shahar et al., 2004), and conversely the effects of experimenter-administered stimulants on subsequent self-administration (Horger et al.1990, 1992; Schenk et al., 1991; Mendrek et al., 1998; Lorrain et al., 2000; Covington & Miczek, 2001; Suto et al.2002,2003). Our laboratory has been interested in identifying experimental conditions in which cocaine self-administration produces sensitization of the reinforcing effects (e.g. Morgan et al. 2002,2005b; Morgan & Roberts, 2004). We have recently reported that sensitization can be demonstrated during an early phase of self-administration (Liu et al., 2004; Morgan et al., 2005a). In the present series of studies, the effect of manipulating the unit injection dose and speed of injection on the development of sensitization of the reinforcing effects of cocaine was further examined.
Methods
Animals and surgery
Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) were used as subjects and housed individually in cages under a 12-h light/dark cycle (lights off at 14:00 h) throughout the experiment with food and water continuously available. The animals had at least 3 days of habituation after their arrival in the laboratory, and weighed approximately 350 g at the start of experiment. Each rat was anesthetized with an i.p. injection of a ketamine (100 mg/kg) and xylazine (8 mg/kg) combination and implanted with a chronic indwelling Silastic cannula into the right jugular vein that exited through the skin on the dorsal surface in the region of the scapulae (Roberts & Goeders, 1989). The cannula was connected to an infusion pump via tygon tubing through a spring tether, allowing drug delivery and free movement within the chamber. Following surgery, rats were individually placed in 30 × 30 × 30-cm operant chambers. Catheters were flushed daily with heparinized saline to help maintain patency. All experimental procedures described in this report were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were reviewed and approved by the Wake Forest University Animal Care and Use Committee.
Self-administration procedure
Daily sessions started at the beginning of the dark cycle by extending a retractable lever into the test chamber. Each lever press resulted in the delivery of 1.5 mg/kg per injection; this equates to 0.1 mL of 5mg/mL cocaine delivered over 5 s for a 450-g rat, with the injection duration adjusted based on body weight. For ease of discussion, this speed/duration is referred to as ‘5 s’, with the groups receiving cocaine at five or ten times lower speeds referred to as ‘25 s’ and ‘50 s’, respectively. Following each response, the lever was retracted for a post-response time-out period. The time-out duration was determined by whichever interval was longer - either 20 s (our standard time-out period) or the actual duration of the injection, i.e. the 5-, 25- and 50-s groups had 20-, 25- and 50-s time-out periods, respectively. Daily ‘acquisition’ sessions were conducted until 20 reinforcers had been self-administered and a ‘regular’ pattern of self-administration emerged (5-10-min inter-response intervals) (see Liu et al., 2005; Morgan et al., 2005a). Following acquisition of the drug-reinforced response, animals were placed into different experimental groups as described below.
Assessment of the reinforcing effects of cocaine using a PR procedure
Under a PR schedule of reinforcement, delivery of intravenous cocaine injections was contingent on an increasing number of responses incremented through the following progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603 ... (for details see Richardson & Roberts, 1996).
Experiment 1. Effects of dose on the development of sensitization
Twenty-four animals were used in this set of experiments. Following acquisition of cocaine self-administration, animals were randomly assigned to different dose groups (0.38, 0.75, 1.5 and 3 mg/kg per injection). Breakpoints maintained by different doses of cocaine were determined for 21 days.
Experiment 2. Effects of injection duration on the development of sensitization
Three groups of animals (n = 6-7 per group) were used in the current experiment. Following acquisition, animals self-administered ten injections of 1.5 mg/kg per injection cocaine daily on an fixed ratio 1 (FR1) schedule for an additional 5 days. Then, breakpoints maintained by cocaine on a PR schedule were assessed for 15 days. On the first day, cocaine at 1.5 mg/kg per injection was delivered over a 5-s period. During the subsequent 14 days, animals received the same dose of drug delivered at one of three speeds (1.2, 0.24 and 0.12 mL/min) with approximate durations of 5, 25 and 50 s, respectively.
Experiment 3. Effects of injection duration on the acute reinforcing effects of cocaine
The animals from Experiment 2 were used in this experiment. Following the 14-day test period described above, the effects of various injection durations (5, 25 and 50 s) on breakpoints and rates of responding on an FR1 schedule were studied in each animal. The average breakpoints for each rate of infusion were obtained from 3 days of stable performance (i.e. breakpoints stayed in a range of three increments). Subsequently, animals responded on an FR1 schedule with 20 reinforcers available until stable responding was observed. Response rates (the number of injections per hour) were measured.
Drug
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA) and dissolved in a solution of physiological saline. The effects of different cocaine doses were studied by altering the concentration of cocaine. The effects of different injection speeds were studied by altering the infusion pump motor and/or the concentration of cocaine.
Data analysis
The main dependent variables were breakpoints on a PR schedule (i.e. the final ratio completed) and rate of intake (injections/h) on the FR1 schedule. Differences or changes in these measures were examined using an ANOVA. Repeated-measures analyses were used to examine changes across days of cocaine access, for comparisons of breakpoints among three speeds of injection (5, 25 and 50 s), and for the ‘dose’ factor of the dose-effect curve analysis. P-values less than 0.05 were considered statistically significant. Newman-Keuls post-hoc analyses were used to identify differences between groups or time points. Analyses were conducted on data subjected to a logarithmic transformation to increase homogeneity of variance.
Results
Development of sensitization is dose-dependent
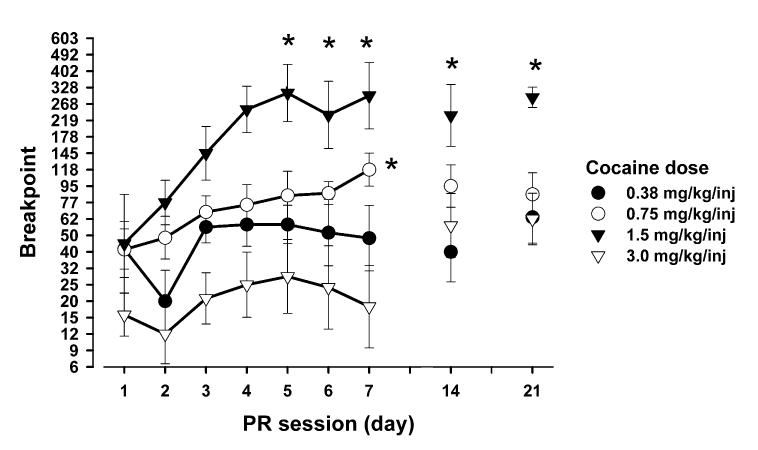
To examine whether development of sensitization of the reinforcing effects of cocaine in self-administering animals (see Morgan et al., 2005a) is dose-dependent, responding on a PR schedule was maintained by different doses of cocaine (0.38, 0.75, 1.5 and 3 mg/kg per injection). Increases in breakpoints across days occurred in a dose-dependent fashion (day: F6,150 = 37.25, P < 0.0001; dose: F3,24 = 11.99, P = 0.0001; interaction: F18,126 = 5.41, P < 0.05; see Fig. 1). Newman-Keuls tests (P< 0.05) show that the progressive increases in breakpoints were observed with 0.75 or 1.5 mg/kg cocaine per injection; by contrast, breakpoints maintained by 0.38 or 3.0 mg/kg per injection remained stable across days. Taken together, the development of sensitization to the reinforcing effects of cocaine was dose-dependent, and the most robust sensitization occurred with 1.5 mg/kg per injection.
Fig. 1.
Effects of cocaine dose on the development of sensitization of the reinforcing effects. Animals with limited initial exposure were tested on the PR schedule with different cocaine doses (n = 6-7 per group). Data are expressed as mean (± SEM) breakpoints. Asterisks indicate significant difference from the first day of testing. Breakpoints maintained by 0.75 and 1.5 mg/kg per injection increased across days (P < 0.05); by contrast, 0.38 or 3.0 mg/kg per injection failed to produce such sensitization.
Injection duration and the development of sensitization
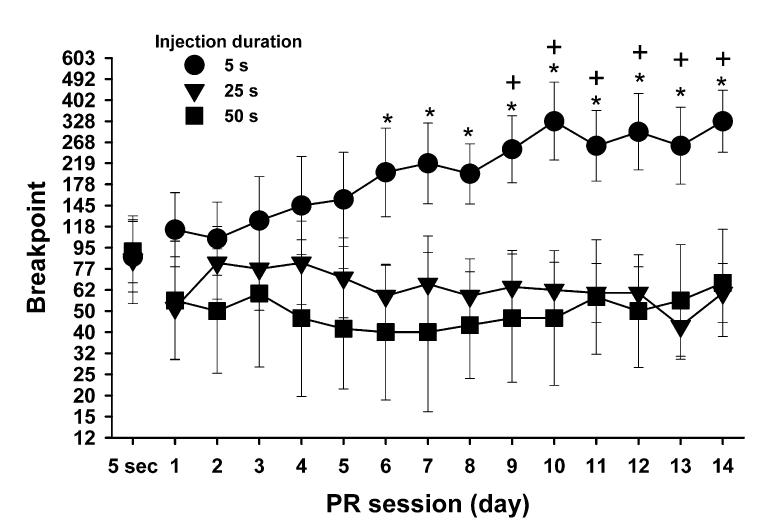
Following acquisition and 5 days of self-administration on an FR1 schedule, schedule contingencies were changed to a PR schedule maintained by cocaine (1.5 mg/kg per injection) delivered over 5 s for 1 day. Then the injection durations were altered to 5, 25 or 50 s, and responding on a PR schedule was examined for 14 days (see Fig. 2). Baseline breakpoints (Day 1) were not different across groups (Newman-Keuls test, P > 0.05). Rats receiving cocaine delivered at the highest speed (shortest duration) showed a progressive increase in breakpoints across days (speed-day interaction: F28,224 = 16.18, P < 0.05). By contrast, cocaine delivery over 25 or 50 s duration failed to result in a progressive increase in breakpoints (Newman-Keuls tests, P > 0.05). These data demonstrate that cocaine delivered at the highest speed produced sensitization to self-administered cocaine, whereas lower rates of infusions did not support the development of sensitization.
Fig. 2.
Effects of injection speed on the development of sensitization to the reinforcing effects of cocaine. Animals responded on a PR schedule maintained by cocaine (1.5 mg/kg per injection) delivered over 5 s for one session (data points over ‘5 s’), and over the subsequent 14 sessions; responding was maintained by cocaine delivered over different durations (5, 25 or 50 s; n = 6-7 per group). Symbols represent mean (± SEM) breakpoints. Asterisks indicate significant differences between 5-s and the 25- and 50-s groups; ‘+’ symbols represent significant differences from the first day of PR testing (P < 0.05). The highest injection speed (i.e. shortest duration) resulted in sensitization, whereas longer durations failed to result in sensitization.
Effects of injection duration on the acute reinforcing effects of cocaine
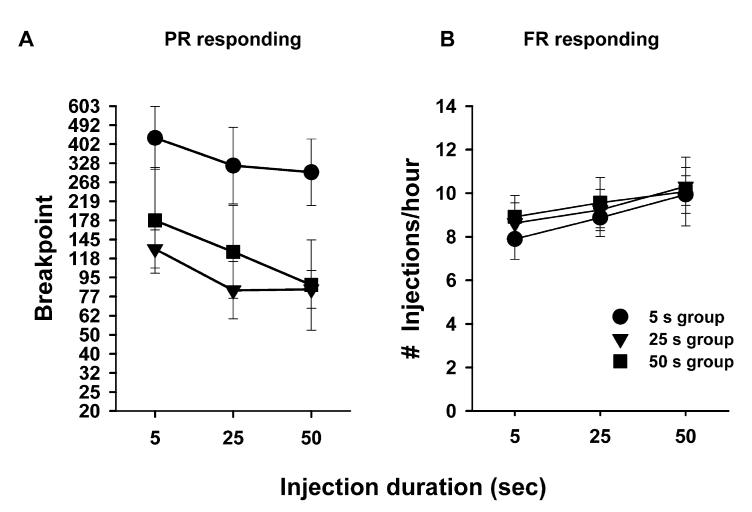
Following this 14-day period of responding on a PR schedule, the effects of injection duration were assessed on breakpoints and response rate in the three groups of animals. Overall, animals showing sensitization (5-s group) produced higher breakpoints than animals not exhibiting sensitization (25- or 50-s groups) regardless of injection duration (group: F2,55 = 12.84, P < 0.05; speed: F2,112 = 8.79, P < 0.05). By contrast, although there were small but significant duration-dependent effects on responding maintained on an FR schedule (speed: F2,34 = 3.83, P < 0.05), performance across groups of rats were not significantly different (group: F2,16 = 0.11, P > 0.05).
Discussion
The present studies demonstrate that the unit injection dose and speed of injection are important contributors to the development of sensitization of the reinforcing effects of cocaine. These experiments show that the development of sensitization is dose-dependent, with the most robust sensitization occurring with a dose of 1.5 mg/kg per injection. This dose was selected to study the influence of injection speed. Consistent with previous reports, rate of drug injection failed to dramatically affect the acute reinforcing strength of cocaine measured by breakpoints on a PR schedule. Importantly, however, it was found that rate of drug injection significantly affected the development of sensitization to the reinforcing effects of cocaine. The shortest injection duration (5 s) resulted in time-dependent progressive increases in breakpoints maintained by cocaine, whereas longer durations (25 and 50 s) failed to result in sensitization. These observations replicate and extend previous studies examining the critical self-administration parameters that produce sensitization to the reinforcing effects of cocaine (Morgan et al.,2002,2005a,b; Morgan & Roberts, 2004).
The present findings showing that speed of injection influences sensitization of cocaine-reinforced breakpoints are consistent with recent studies examining sensitization of locomotor activity. Samaha et al. (2002, 2004) reported that the rate of intravenous cocaine delivery-although having no effect on the acute drug response-significantly affected sensitization of cocaine-induced locomotor activity and rotational behavior. Taken together, the results suggest that relatively rapid intravenous injections are necessary to produce sensitization, whereas injections lasting longer than 25 s fail to produce sensitization. Importantly, Samaha et al. (2004) have shown that neurochemical markers associated with locomotor sensitization (e.g. expression of the immediate early genes c-fos and arc mRNA) are also affected by the rapidity of drug onset.
Interestingly, the speed of injection did not affect acutely determined cocaine-reinforced breakpoints (i.e. the reinforcing strength of acutely administered cocaine). That is, following the 14-day self-administration protocol that resulted in differential break-points (see Fig. 2), examination of the effects of injection speed failed to reveal a profound influence regardless of ‘baseline’ breakpoint (see Fig. 3). These findings are consistent with unpublished data from our laboratory showing that in animals with relatively extensive histories of cocaine self-administration, there is generally no effect on the cocaine dose-effect curve assessed with a PR schedule when the injection speed is manipulated. Similarly, Crombag et al. (2003b) have demonstrated with cocaine and amphetamine self-administration that the rate of acquisition, response rates maintained using an FR schedule, breakpoints on a PR schedule and reinstatement of extinguished responding are not affected by different injection speeds (i.e. 5 vs. 100 s). In the locomotor sensitization papers described above (Samaha et al.2002,2004), although the development of sensitization was dependent on injection speed, the baseline locomotor response (on Day 1) was similar regardless of injection duration. Taken together, these findings demonstrate that the acute locomotor and reinforcing effects of cocaine are not dependent on speed of injection whereas sensitization of the response is.
Fig. 3.
Effects of injection speed on cocaine’s acute reinforcing effects in animals showing or not showing sensitization. Following testing on a PR schedule for 14 days at a particular injection speed (see Fig. 2), animals were tested with each injection duration during PR and FR sessions. (A) Breakpoints maintained in animals with a history of 5-s injection durations (i.e. sensitized animals) were higher than those in the 25- and 50-s groups, regardless of the current injection speed conditions (F2,55 = 12.84, P < 0.05). Data are represented as mean (± SEM) breakpoints. (B) Rate of intake (injections per hour) increased as the speed of injection decreased across all groups. There were no differences among these curves across groups (speed: F2,34 = 3.83, P < 0.05; group: F2,16 = 0.11, P > 0.05).
The present data suggest that sensitization may be an important issue in determining a drug’s abuse liability. We have previously reported the dose-response relationship for various psychostimulant drugs on a PR schedule (Richardson & Roberts, 1996; Roberts et al., 1999). These curves allowed comparisons between drugs on such factors as relative reinforcing potency and efficacy. With our recent observations that the cocaine dose-effect curve can be shifted leftward and/or upward (i.e. sensitized) by a number of exposure regimens (Morgan & Roberts, 2004; Morgan et al.2005a,b) it would appear that acutely determined dose-effect curves may underestimate the reinforcing efficacy of cocaine and perhaps other drugs. If the maximal breakpoint is taken to reflect abuse liability, then clearly the values following sensitization should be taken as the best estimate. Whether all reinforcing drugs will show sensitization remains to be determined; however, the present demonstration regarding speed of injection suggests that differences in abuse liability between drugs (or routes of administration) might be accounted for by rates of sensitization in addition to differences in acute reinforcement.
The initial experiment described here demonstrated that the sensitization of cocaine-reinforced breakpoints is dose-dependent. In a previous paper (Morgan et al., 2005a) we reported that robust sensitization occurs when the animals are given limited histories of self-administration (e.g. 1-5 days of self-administration with access to a limited number of injections per day) and tested with 1.5 mg/kg per injection (our typical training dose). In animals with more extended histories, this sensitization appears to be suppressed. Interestingly, the same high level of cocaine intake that blocks the development of sensitization failed to alter the high breakpoints obtained in animals that were already sensitized (i.e. it did not block the expression of sensitization). Following this sensitization of cocaine-reinforced breakpoints, the dose-effect curve for cocaine is shifted leftward and upward relative to non-sensitized animals (Morgan et al., 2005a). The present findings replicate the sensitization observed with 1.5 mg/kg per injection and show that lower doses produce less robust (0.75 mg/kg per injection) or no (0.38 mg/kg per injection) sensitization. Somewhat surprisingly, the highest dose (3.0 mg/kg per injection) supported low breakpoints across the first 7 days. In animals with relatively extensive histories of self-administration, this dose can support the highest breakpoints in our studies (Roberts et al., 2002; Liu et al., 2005; Morgan et al., 2005b). Given that animals in the present experiments had limited exposure before being tested with this relatively high dose, the finding that breakpoints were very low might implicate aversive effects of cocaine to which the animals would typically become tolerant.
In summary, the present experiments show that the development of sensitization to the reinforcing effects of cocaine, measured as increases in breakpoints maintained on a PR schedule of reinforcement, is influenced by unit injection dose and by speed of intravenous injection. This is in contrast to the findings that the acute reinforcing effects of cocaine are not dramatically influenced by the injection duration. We suggest that the process of drug addiction involves both of these factors, the acute reinforcing effects and a transition into a state where cocaine comes to function as a stronger reinforcer (assessed by progressive increases in breakpoints), and that these appear to be dissociable phenomena. These data suggest that behavioral, pharmacological and neurobiological studies investigating the development of drug addiction in animal models should incorporate both of these factors.
Acknowledgements
This research was supported by National Institute of Health grants P50DA06643 and R01DA14030 to D.C.S.R., and K01DA13957 to D.M.
Footnotes
- FR
- fixed ratio
- PR
- progressive ratio.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl.) 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Balster R, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J. Exp. Anal. Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Hierholzer R, Maddahian E. Recent trends in cocaine abuse in a VA psychiatric population. Hosp. Community Psychiatry. 1986;37:1229–1234. doi: 10.1176/ps.37.12.1229. [DOI] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration ‘binges’. Psychopharmacology (Berl.) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Farrario C, Myc PP, Robinson TE. The rate of intraveneous drug infusion does not affect psychomotor stimulant-taking or seeking. Behav. Pharmacol. 2003a;14:s56–s56. [Google Scholar]
- Crombag HS, Ferrario C, Piotr MP, Robinson TE. The rate of intravenous drug delivery does not affect psychostimulant-taking or seeking. Soc. Neurosci. Abstr. 2003b Abstract number 424.1. [Google Scholar]
- Gawin FH, Ellinwood EH., Jr Cocaine and other stimulants. Actions, abuse, and treatment. N. Engl. J. Med. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br. J. Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Cocaine: patterns of use, route of administration, and severity of dependence. Br. J. Psychiatry. 1994;164:660–664. doi: 10.1192/bjp.164.5.660. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality. JAMA. 1996;276:1580–1588. [PubMed] [Google Scholar]
- Hooks MS, Duffy P, Striplin C, Kalivas PW. Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology (Berl.) 1994;115:265–272. doi: 10.1007/BF02244782. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl.) 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Horger BA, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol. Biochem. Behav. 1990;37:707–711. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- Kato S, Wakasa Y, Yanagita T. Relationship between minimum reinforcing doses and injection speed in cocaine and pentobarbital self-administration in crab-eating monkeys. Pharmacol. Biochem. Behav. 1987;28:407–410. [PubMed] [Google Scholar]
- Liu Y, Roberts DCS, Morgan D. Sensitization to the reinforcing effects of cocaine in rats. Soc. Neurosci. Abstr. 2004 Abstract number 688.12. [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl.) 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav. Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl.) 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Morgan D, Brebner K, Lynch WJ, Roberts DC. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav. Pharmacol. 2002;13:389–396. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Morgan D, Liu Y, Roberts D. Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology. 2005a doi: 10.1038/sj.npp.1300773. in press. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci. Biobehav. Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Morgan D, Smith MA, Roberts DC. Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl.) 2005b;178:309–316. doi: 10.1007/s00213-004-1992-6. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Drug addiction and drug abuse. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 10th edn McGraw-Hill; New York: 2001. pp. 621–624. [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl.) 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Di Guiseppi S, Ondrusek G, Jeffcoat AR, Cook CE. Free-base cocaine smoking. Clin. Pharmacol. Ther. 1982;32:459–465. doi: 10.1038/clpt.1982.189. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Di Ciano P. Behavioral sensitization is induced by intravenous self-administration of cocaine by rats. Psychopharmacology (Berl.) 1996;124:279–281. doi: 10.1007/BF02246669. [DOI] [PubMed] [Google Scholar]
- Pickens R, Dougherty J, Thompson T. Minutes of the Meeting of the Committee on Problems of Drug Dependence. NAS-NRC; Washington, DC: 1969. Effects of Volume and duration of infusion on cocaine reinforcement with concurrent activity recording; pp. 5805–5811. [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Goeders NE. Drug self-administration: experimental methods and determinants. In: Boulton AA, Baker GB, Greenshaw AJ, editors. Neuromethods: Psychopharmacology. Humana; Clifton: 1989. pp. 349–398. [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. Self-administration of cocaine analogs by rats. Psychopharmacology (Berl.) 1999;144:389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J. Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J. Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Snow S, Horger BA. Pre-exposure to amphetamine but not nicotine sensitizes rats to the motor activating effect of cocaine. Psychopharmacology (Berl.) 1991;103:62–66. doi: 10.1007/BF02244075. [DOI] [PubMed] [Google Scholar]
- Siegel RK. Cocaine smoking. N. Engl. J. Med. 1979;300:373. doi: 10.1056/NEJM197902153000731. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–979. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner. Neuropsychopharmacology. 2003;28:629–639. doi: 10.1038/sj.npp.1300075. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur. J. Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Zapata A, Chefer VI, Ator R, Shippenberg TS, Rocha BA. Behavioural sensitization and enhanced dopamine response in the nucleus accumbens after intravenous cocaine self-administration in mice. Eur. J. Neurosci. 2003;17:590–596. doi: 10.1046/j.1460-9568.2003.02491.x. [DOI] [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: drug users’ self-reports versus researchers’ perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]