Abstract
Neuronal Wiskott-Aldrich syndrome protein (N-WASP) and the actin-related protein 2/3 (Arp2/3) complex have emerged as critical host proteins that regulate pathogen actin-based motility. Actin tail formation and motility in Listeria monocytogenes require the Arp2/3 complex but bypasses N-WASP signaling. Motility of Shigella flexneri and vaccinia virus requires both N-WASP and the Arp2/3 complex. Functional roles for these cytoskeletal regulatory proteins in actin-based motility of Rickettsia rickettsii have not been established. In this study, functional domains of N-WASP tagged with green fluorescent protein that have characterized effects on Shigella and vaccinia virus actin-based motility were ectopically expressed in HeLa cells infected with R. rickettsii to assess their effects on rickettsial motility. S. flexneri-infected cells were used as a control. Expressed N-WASP domains did not localize to R. rickettsii or their actin tails. Expression of N-WASP missing the VCA domain (for “verprolin homology, cofilin homology, and acidic domains”), which acts as a dominant-negative form of N-WASP, completely inhibited actin-based motility of S. flexneri while only moderately inhibiting motility of R. rickettsii. Similarly, expression of the VCA domain, which acts as a dominant-negative with respect to Arp2/3 complex function, severely inhibited actin-based motility of S. flexneri (no motility observed in the majority of expressing cells) but only moderately inhibited R. rickettsii motility. These results, taken together with the differential effects on motility observed upon expression of other N-WASP domains, suggest that actin-based motility of R. rickettsii is independent of N-WASP and the Arp2/3 complex.
Rickettsia spp. are obligate intracellular bacteria that are transmitted to mammalian hosts exclusively by arthropods (40). Pathogenic rickettsiae have a tropism for the endothelium, where they replicate and spread to cause increased vascular permeability. A likely rickettsial virulence mechanism that aids in intracellular dissemination is actin-based motility (ABM) (11-13, 36). The propulsive force provided by rickettsia-driven polarized actin polymerization propels the organism into membrane protrusions that are engulfed by neighboring cells, thereby facilitating direct cell-to-cell spread. With the exception of the typhus group organism Rickettsia typhi, ABM is confined to members of the spotted fever group of rickettsiae such as Rickettsia rickettsii and Rickettsia conorii, the causative agents of Rocky Mountain spotted fever and Mediterranean spotted fever, respectively (13, 36).
There is a paucity of information on regulators of R. rickettsii ABM. However, the actin polymerization machinery that promotes motility of Listeria, Shigella, and vaccinia virus is reasonably well described and is comprised of both pathogen and host proteins (reviewed in references 9 and 10). Neuronal Wiskott-Aldrich syndrome protein (N-WASP) and the actin-related protein 2/3 (Arp2/3) complex of proteins are critical host proteins that regulate ABM (10). The Arp2/3 complex consists of two actin-related proteins (Arp2 and Arp3) and five additional protein subunits and is the only known cellular factor that nucleates polymerization of actin filaments that grow from their fast-growing barbed ends (16, 29). N-WASP, which normally couples surface receptor stimulation with actin polymerization, is a member of the WASP family of proteins that in mammals also includes WASP and three isoforms of the suppressor of cyclic AMP receptor (Scar) (3, 18, 28). The actin assembly activity of the Arp2/3 complex is activated upon binding of activated N-WASP (18, 28). N-WASP contains a number of functional domains, some of which bind effector molecules involved in N-WASP activation. A centrally located Cdc42/Rac interactive binding motif (CRIB) and a basic region adjacent to CRIB act as ligands for Cdc42 and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], respectively (28, 32). The C-terminal VCA domain (for “verprolin homology, cofilin homology, and acidic domains”; called WA for WASP homology 2 and acidic domains in Scar) of unfolded N-WASP binds the Arp2/3 complex and stimulates its actin-nucleating activity (18, 41). Additional functional domains of N-WASP include a proline-rich central domain, which binds profilin and Src homology domain 3-containing proteins such as the adaptor protein Nck1 (9, 26), and the N-terminal Wiskott-Aldrich homology 1 (WH1) domain, which mediates interactions with calmodulin, PI(4,5)P2, actin filaments, and a WASP-regulating protein termed WASP-interacting protein (5, 19, 23, 31). N-WASP is synergistically activated by binding PI(4,5)P2 and GTP-bound Cdc42 (32). Binding relieves the intramolecular interactions between the C-terminal VCA domain and the basic-region, CRIB, and WH1 domains, promoting a conformational change from an autoinhibited, folded state to an active, unfolded state (32).
N-WASP is directly recruited to Shigella by the bacterial surface protein IcsA (35). Binding to IcsA causes N-WASP to undergo a conformational change to an active form reminiscent of Cdc42 activation (3, 5). Tyrosine phosphorylation of the vaccinia virus protein A36R results in stepwise recruitment of the SH2/SH3 adaptor protein Nck1, WASP-interacting protein, and N-WASP (8, 26), with recruitment of the adaptor Grb2 downstream of N-WASP also occurring (33). In each case, activated N-WASP recruits the Arp2/3 complex and activates its actin-nucleating activity, with actin tail formation and motility ensuing. Listeria circumvents a need for N-WASP by synthesizing ActA, which can directly bind and activate the Arp2/3 complex in an N-WASP-like manner (2, 38, 39). Thus, these three pathogens employ unique forms of “molecular mimicry” to activate critical regulators of the actin cytoskeleton to promote ABM: Shigella mimics Cdc42 activation of N-WASP, Listeria mimics N-WASP activation of the Arp2/3 complex, and vaccinia virus mimics receptor tyrosine kinase signaling (9).
A previous study by Gouin and coworkers (11) demonstrated by immunofluorescence that the related organism R. conorii also does not recruit N-WASP or Arp3. A potential caveat is that N-WASP and Arp2/3 complex may be recruited by rickettsiae but are beyond the limit of immunofluorescent detection (4). Given the universal requirement for N-WASP (or an N-WASP mimic such as ActA) and the Arp2/3 complex in pathogen ABM, we wished to further investigate similar requirements in R. rickettsii motility using a functional assay. In this study, functional domains of N-WASP tagged with green fluorescent protein (GFP) that have characterized effects on Shigella and vaccinia virus ABM were expressed in HeLa cells infected with R. rickettsii (26). The effect of expression on rickettsial ABM was determined by comparing the rate of motility in expressing cells to that in nonexpressing cells.
R. rickettsii (HLP strain) and Shigella flexneri 2457T (a generous gift from Anthony Maurelli, Uniformed Services University of the Health Sciences) were used in these studies. Transfections were conducted with infected HeLa cells (CCL-2.1; American Type Culture Collection) because in our hands they are easily transfected and infected with both Shigella and Rickettsia. Petri dishes (35-mm-coverslip bottom; MatTek Corp., Ashland, Mass.) were seeded with HeLa cells to semiconfluence and cultivated overnight at 37°C in M199 medium (Life Technologies, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS) (Life Technologies) and 10 μg of gentamicin (Life Technologies) per ml. Rickettsiae suspended in 3.7% brain heart infusion broth (Difco Laboratories, Detroit, Mich.) were used to infect monolayers at a multiplicity of infection of 0.1 to 1.0 for 45 min. The inoculum was removed, cells were washed once with Hanks' buffered saline solution (Life Technologies), M199 medium supplemented with 2% FBS was added, and incubation was continued at 34°C. For infection of HeLa cells with S. flexneri, organisms were cultivated overnight at 38.5°C in tryptic soy broth (TSB; Difco Laboratories), diluted 100-fold in TSB, and incubated for an additional 2 to 3 h at 38.5°C. Five hundred microliters of suspended bacteria was added to petri dishes, and the dishes were then centrifuged at 900 × g for 12 min at room temperature to facilitate infection. Infected HeLa cells were incubated for 1 h, washed three times with Hanks' buffered saline solution, M199 containing 2% FBS and gentamicin sulfate (75 μg/ml; to kill extracellular shigellae) was added, and incubation was continued at 37°C.
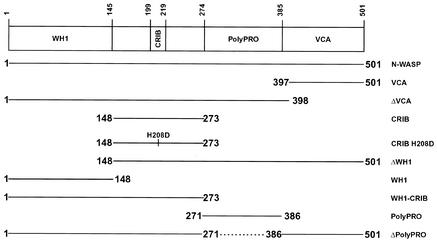
Transfections were conducted by using Lipofectamine and methods suggested by the supplier (Life Technologies). Due to the low growth rate of rickettsia, HeLa cells were infected with R. rickettsii for 2 to 4 days prior to transfection. Rickettsial infection and expression of GFP constructs were then allowed to proceed for an additional 1 to 2 days before microscopy. HeLa cells were transfected 24 h before infection with Shigella, which was continued for 3 h before microscopy. A schematic of the N-WASP-encoding plasmids used in this study is presented in Fig. 1. Their construction has been described elsewhere (26).
FIG. 1.
Schematic representation of human N-WASP GFP constructs and functional domains (26). All proteins are N-terminally fused to the C terminus of GFP. Dashed lines indicate internal deleted regions.
Live infected cells expressing GFP constructs were located by epifluorescence and imaged by time-lapse video phase-contrast microscopy to document movements of intracellular R. rickettsii and S. flexneri. Images were captured using a Nikon Diaphot inverted microscope with an integrated Dage cooled charge-coupled device video camera (MTI, Inc., Michigan City, Ind.) and QED imaging software (QED Imaging Inc., Pittsburgh, Pa.). Digital time-lapse videos of transfected and neighboring nontransfected infected cells were recorded with images collected at 15-s intervals. The distance moved by intracellular bacteria was measured with ImageJ software for intracellular bacteria that could be tracked for at least 2 min and where forward movement was not impeded by cellular structures (e.g., the plasma membrane). The rate of bacterial movement in archived digital video was calculated by tracking the pixel position of the actin-polymerizing end of individual bacteria over time and is expressed as mean rate (micrometers per minute) ± standard deviation. A minimum of 12 transfected and nontransfected infected cells were analyzed for each GFP construct on at least two separate occasions. Statistical analysis was conducted with MINITAB 12.1 software (Minitab Inc., State College, Pa.) using the independent two-sample t test with a 95% confidence interval. Staining for Arp3 and F actin was conducted as previously described (13). Rabbit anti-Arp3 antibody and Alexa Fluor 568 phalloidin were purchased from Upstate Biotechnology (Lake Placid, N.Y.) and Molecular Probes, Inc. (Eugene, Oreg.), respectively. Epifluorescence and phase-contrast micrographs depicting the localization of GFP constructs and Arp3 were processed with Adobe Photoshop 5.0.
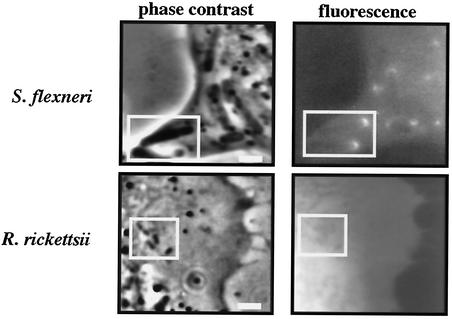
As reported by others (26), GFP fused to full-length N-WASP localized to one pole of intracellular S. flexneri organisms (Fig. 2). Conversely, this protein was not recruited by R. rickettsii (Fig. 2), which was also the case for all N-WASP constructs employed in this study (data not shown). Expression moderately inhibited the rate of ABM of both pathogens, with Shigella and Rickettsia organisms moving at 75 and 87%, respectively, of the rate of bacteria in nonexpressing cells. (The effects of expression of all N-WASP functional domains on Shigella and Rickettsia ABM are summarized in Table 1.) Inhibition of Shigella actin tail formation by expression of GFP fused to wild-type N-WASP has been reported (26).
FIG. 2.
N-WASP is recruited by S. flexneri but not R. rickettsii. Full-length N-WASP N-terminally fused to the C terminus of GFP was ectopically expressed in HeLa cells infected with S. flexneri or R. rickettsii. Parallel fluorescence and phase-contrast images of live cells were captured. Two shigellae (boxed region) show obvious polar recruitment of GFP-N-WASP. In contrast, four rickettsiae (boxed region; one organism is undergoing binary fission) do not show recruitment of GFP-N-WASP. Bars, 2 μm.
TABLE 1.
Effects of expression of N-WASP domains on ABM of S. flexneri and R. rickettsii
| Domain |
S. flexneri
|
R. rickettsii
|
||||||
|---|---|---|---|---|---|---|---|---|
| ABM velocity (μm/min) in:
|
Ratio | Recruit- menta | ABM velocity (μm/min) in:
|
Ratio | Recruit- menta | |||
| Nonexpressing cells (n) | Expressing cells (n) | Nonexpressing cells (n) | Expressing cells (n) | |||||
| N-WASP | 4.60 ± 1.85 (69) | 3.44 ± 1.75 (41) | 0.75b | + | 3.43 ± 1.20 (83) | 2.98 ± 1.21 (92) | 0.87b | − |
| WH1 | 4.61 ± 2.32 (50) | 5.51 ± 2.67 (33) | 1.20 | + | 3.17 ± 0.64 (59) | 3.69 ± 1.07 (62) | 1.16b | − |
| ΔWH1 | 5.09 ± 2.79 (29) | 4.35 ± 2.57 (34) | 0.86 | + | 3.04 ± 1.18 (48) | 1.37 ± 0.45 (72) | 0.45b | − |
| CRIB | 5.08 ± 3.17 (54) | 2.48 ± 2.14c (11) | 0.49b | + | 2.95 ± 0.65 (75) | 2.56 ± 0.48 (43) | 0.87b | − |
| CRIB H208D | 5.13 ± 3.23 (100) | 2.07 ± 1.45d (10) | 0.40b | + | 3.93 ± 0.91 (95) | 3.68 ± 0.94 (99) | 0.96 | − |
| WH1-CRIB | 5.60 ± 2.98 (48) | 4.15 ± 2.37e (18) | 0.74b | + | 3.51 ± 0.83 (71) | 3.26 ± 0.72 (66) | 0.93 | − |
| VCA | 6.49 ± 3.02 (38) | 2.66 ± 2.25f (23) | 0.41b | − | 2.77 ± 0.70 (88) | 1.85 ± 0.80 (49) | 0.67b | − |
| ΔVCA | 5.90 ± 3.01 (55) | 0g | 0 | + | 3.23 ± 0.84 (69) | 1.99 ± 1.06 (53) | 0.61b | − |
| Poly-Pro | 4.38 ± 2.32 (41) | 4.85 ± 3.07 (38) | 1.11 | − | 3.25 ± 0.70 (52) | 3.42 ± 0.93 (95) | 1.05 | − |
| Δpoly-Pro | 4.92 ± 3.02 (58) | 2.75 ± 1.91 (43) | 0.66b | + | 3.05 ± 0.55 (57) | 2.92 ± 0.57 (79) | 0.96 | − |
Recruitment to bacteria and/or their actin tails.
P ≤ 0.05.
ABM not detected in 69.2% of transfected infected cells.
ABM not detected in 76.9% of transfected infected cells.
ABM not detected in 50.0% of transfected infected cells.
ABM not detected in 78.9% of transfected infected cells.
ABM not detected in 100% of transfected infected cells.
We next expressed specific N-WASP functional domains having dominant-negative effects to substantiate a lack of N-WASP involvement in rickettsial ABM and to provide insight into possible roles for N-WASP effector proteins that may regulate rickettsial motility in an N-WASP-independent fashion. We first investigated the effect of expression of N-WASP missing the VCA domain (ΔVCA). This truncated form of N-WASP is recruited like the wild-type protein but is unable to bind the Arp2/3 complex and activate its actin-nucleating activity. It consequently behaves as a dominant-negative form of N-WASP (6, 26). Expression of ΔVCA inhibits both N-WASP-mediated endocytosis of invasive bacteria (1, 6, 22) and actin tail formation of vaccinia virus and Shigella (26). Ectopically expressed ΔVCA was recruited by Shigella (data not shown) and completely inhibited ABM in all expressing cells. In contrast, a moderate inhibitory effect on R. rickettsii motility was observed, with organisms in expressing cells moving at 61% of the rate of organisms in nonexpressing cells. Because R. rickettsii does not recruit N-WASP or ΔVCA, the moderate inhibition of rickettsial ABM by both proteins may be due to sequestration of N-WASP binding proteins that regulate rickettsial motility in an N-WASP-independent fashion.
Expression of the N-WASP CRIB region also severely inhibited the rate of Shigella ABM, with no motility observed in 69.2% of expressing cells. In the remaining expressing cells, Shigella moved at 49% of the rate of organisms in nonexpressing cells. The null-to-partial Shigella motility phenotype associated with this and other N-WASP constructs was likely due to variable expression of the transgene between transfected cells (26). A similar effect was observed upon expression of CRIB containing an H-to-D mutation at amino acid 208 (CRIB H208D), which does not bind activated GTP-bound Cdc42 (24). No motility was observed in 76.9% of expressing cells, while in the remaining expressing cells Shigella moved at 40% of the rate of organisms in nonexpressing cells. Expression of WH1-CRIB inhibited Shigella ABM to a lesser degree than CRIB and CRIB H208D, with no motility observed in 50.0% of expressing cells. In the remaining expressing cells, Shigella moved at 74% of the rate of organisms in nonexpressing cells. CRIB, CRIB H208D, and WH1-CRIB were recruited by Shigella (data not shown), presumably via IcsA-CRIB interactions (26). Their inhibitory effects have been attributed to inhibition of recruitment of endogenous N-WASP (15, 26). Because CRIB also contains a binding motif for the N-WASP effector Cdc42, a Rho-family GTPase involved in N-WASP activation, cytosolic expression of CRIB or WH1-CRIB could also conceivably inhibit Shigella ABM by sequestering Cdc42. However, a growing body of evidence suggests that Cdc42 activation of N-WASP is not required for Shigella ABM (14, 15, 27, 34). Expression of CRIB, but not of CRIB H208D or WH1-CRIB, moderately inhibited the rate of rickettsial ABM, with organisms in expressing cells moving at 87% of the rate of those in nonexpressing cells. The observation that CRIB is not recruited by Rickettsia, in conjunction with the lack of inhibition by CRIB H208D, may suggest a role for Cdc42 signaling in rickettsial ABM, although additional studies are needed to define functions for this and other Rho-family GTPases.
The rate of Shigella and Rickettsia ABM was not significantly inhibited by expression of the N-WASP polyproline-rich region (poly-Pro), nor was the protein recruited by either bacterium. This proline-rich motif can act as a ligand for the actin monomer-sequestering protein profilin and Src homology domain 3-containing proteins (9). While not essential for Shigella ABM, profilin increases the rate of movement in cell-free reconstitution assays (14). A functional role for profilin in rickettsial ABM has not been established, although the protein localizes throughout the rickettsial actin tail (37). Ectopically expressed poly-Pro would be expected to sequester profilin and make it less accessible for ABM. In fact, microinjection of the oligoproline peptide (GPPPPP)3 corresponding to the profilin binding motif of VASP inhibits Shigella ABM in PtK2 cells, presumably by sequestering profilin (42). We anticipated that cytosolic expression of poly-Pro would have a similar effect. The lack of inhibition of both Shigella and Rickettsia ABM may indicate that this GFP fusion protein does not efficiently sequester profilin.
In contrast to results with poly-Pro, N-WASP missing the proline-rich domain (Δpoly-Pro) was recruited by Shigella, and expression moderately inhibited ABM, with organisms in expressing cells moving at 66% of the rate of those in nonexpressing cells. These findings are in keeping with recent studies that demonstrated recruitment by Shigella of similar N-WASP poly-Pro constructs that result in deficient actin tail formation (15, 25). In these studies, the actin tail deficiency was attributed to the lack of profilin recruitment (15, 25), although a recent report suggests that profilin augmentation of Shigella ABM is independent of binding to the N-WASP polyproline region (5). Expression of Δpoly-Pro did not inhibit rickettsial ABM, nor was the protein recruited by Rickettsia. Unless activated, Δpoly-Pro should reside in a folded, autoinhibited state that does not activate the Arp2/3 complex.
Expression of the WH1 domain of N-WASP did not significantly inhibit the rate of Shigella or Rickettsia ABM. However, the protein was recruited by Shigella (data not shown). WH1 recruitment by Shigella has been described, and the domain has been speculated to augment CRIB-mediated binding to IcsA (15).
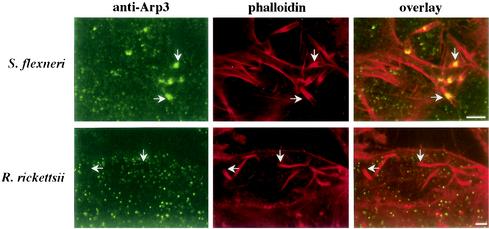
When ectopically expressed, the VCA domain of WASP family proteins sequesters the Arp2/3 complex to result in delocalized activation of its actin-nucleating activity. It therefore has a dominant-negative effect with respect to Arp2/3 complex function by preventing recruitment and focalized activation by endogenous WASP family members (17, 18, 26). Expression of VCA inhibits actin tail formation of Shigella and vaccinia virus (26) and completely inhibits intracellular motility of Listeria (21). VCA also inhibits FcγR- and CR3-promoted phagocytosis (20) as well as parasite-directed endocytosis (1, 6, 22). In keeping with previous work (26), expression of VCA had a severe inhibitory effect on Shigella ABM, with no motility observed in 78.9% of expressing cells. In the remaining expressing cells, Shigella organisms moved at 41% of the rate of organisms in nonexpressing cells. Recruitment of VCA by Shigella was not observed (data not shown). A moderate inhibitory effect on the rate of R. rickettsii ABM was observed in HeLa cells expressing VCA, with organisms in expressing cells moving at 67% of the rate of those in nonexpressing cells. This inhibitory effect of VCA was likely indirect and not due to inhibition of recruitment of the Arp2/3 complex by rickettsia. Rather, the actin-nucleating machinery associated with rickettsial ABM is likely deficient in competing for the monomeric actin pool with delocalized and activated Arp2/3 complex, thereby lowering the rate of tail formation and movement (17, 20). Consistent with these results is the observation that Arp3 localized to the bacterium-tail interface of S. flexneri but not R. rickettsii (Fig. 3).
FIG. 3.
Arp3 is recruited by S. flexneri but not R. rickettsii. Immunofluorescence localization of the cytoskeletal signaling protein Arp3 in HeLa cells infected with S. flexneri or R. rickettsii is shown. Arp3 (green) was localized by indirect immunofluorescence using an anti-Arp3 polyclonal antibody. Actin tails and cellular F actin (red) were counterstained with Alexa Fluor 568 phalloidin. Fixed cells were viewed by laser scanning confocal microscopy. Arp3 localized to the bacterium-tail interface of S. flexneri but not R. rickettsii (arrows). Bars, 5 μm.
In contrast to the VCA domain, expression of N-WASP missing the WH1 domain (ΔWH1) did not significantly inhibit ABM of Shigella. This N-WASP deletion protein activates the Arp2/3 complex in a fashion similar to VCA and therefore likely exists in an unfolded, activated state (18, 26). The lack of ΔWH1 inhibition of Shigella motility is attributable to CRIB-mediated recruitment and utilization of this protein by Shigella in a fashion similar to wild-type N-WASP (15). Consistent with this hypothesis is the observation that ΔWH1, like full-length N-WASP and all CRIB-containing proteins, localized to the actin-polymerizing pole of intracellular Shigella (data not shown). Moreover, Lommel et al. (15) demonstrated that a similar N-WASP construct rescues actin tail formation in an N-WASP-deficient cell line. As for VCA, expression of ΔWH1 moderately inhibited ABM of Rickettsia, with organisms in expressing cells moving at 45% of the rate of those in nonexpressing cells. The greater inhibition of rickettsial motility by ΔWH1 over the VCA domain may be due to sequestration of other N-WASP effector molecules (e.g., Cdc42), in addition to delocalized Arp2/3 activation and depletion of the monomer actin pool.
In summary, the results of this study suggest that maintenance of R. rickettsii ABM does not require N-WASP or Arp2/3 complex activity. Independence from N-WASP signaling is most clearly supported by the lack of recruitment of all N-WASP domains, in addition to the moderate inhibition of rickettsial ABM by ΔVCA, the dominant-negative form of N-WASP, which completely inhibits Shigella ABM. The mechanism of moderate inhibition of rickettsial ABM by ΔVCA is unknown, but it may involve sequestration of N-WASP binding proteins, such as Cdc42 and profilin, that affect rickettsial motility in an N-WASP-independent fashion. Future studies using null cell lines and specific inhibitors of N-WASP effector molecules should further define the host cell requirements for rickettsial ABM.
Independence of R. rickettsii ABM from Arp2/3 complex actin-nucleating activity is supported by the moderate inhibition of rickettsial ABM by VCA, which severely and completely inhibits motility of Shigella (this study) and Listeria (21), respectively. Moreover, R. rickettsii does not recruit Arp3. The inhibition of rickettsial ABM by VCA is likely due to depletion of the polymerizable monomer actin pool by untargeted and activated Arp2/3 complex (17, 20). The actin-nucleating machinery associated with rickettsial motility may be deficient in competing for the monomeric actin pool, thereby slowing tail formation and movement. This hypothesis is consistent with the fact that rickettsiae move substantially more slowly than shigellae and listeriae (10) and are therefore likely to be less efficient at nucleating actin than these pathogens. Further evidence against a role for the Arp2/3 complex in rickettsial ABM is that actin filaments of R. rickettsii and R. conorii actin tails lack the dendritic side branching characteristic of Arp2/3 complex activity (11, 16, 37). Zyxin and the formans have recently been shown to nucleate the production of unbranched filaments (7, 30). Whether either protein is involved in rickettsial ABM remains to be determined. The possibility that Rickettsia produces an actin nucleator that promotes the synthesis of unbranched filaments also exists.
Acknowledgments
We thank Shelly Robertson for review of the manuscript and Lorraine Barrows and Nicole Lindstrom for technical assistance.
This work was supported by National Institutes of Health grant AI-43502 (R.A.H.).
Editor: F. C. Fang
REFERENCES
- 1.Alrutz, M. A., A. Srivastava, K. W. Wong, C. D'Souza-Schorey, M. Tang, L. E. Ch'Ng, S. B. Snapper, and R. R. Isberg. 2001. Efficient uptake of Yersinia pseudotuberculosis via integrin receptors involves a Rac1-Arp 2/3 pathway that bypasses N-WASP function. Mol. Microbiol. 42:689-703. [DOI] [PubMed] [Google Scholar]
- 2.Boujemaa-Paterski, R., E. Gouin, G. Hansen, S. Samarin, C. Le Clainche, D. Didry, P. Dehoux, P. Cossart, C. Kocks, M. F. Carlier, and D. Pantaloni. 2001. Listeria protein ActA mimics WASp family proteins: it activates filament barbed end branching by Arp2/3 complex. Biochemistry 40:11390-11404. [DOI] [PubMed] [Google Scholar]
- 3.Caron, E. 2002. Regulation of Wiskott-Aldrich syndrome protein and related molecules. Curr. Opin. Cell. Biol. 14:82-87. [DOI] [PubMed] [Google Scholar]
- 4.Cossart, P. 2000. Actin-based motility of pathogens: the Arp2/3 complex is a central player. Cell. Microbiol. 2:195-205. [DOI] [PubMed] [Google Scholar]
- 5.Egile, C., T. P. Loisel, V. Laurent, R. Li, D. Pantaloni, P. J. Sansonetti, and M. F. Carlier. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott, D. A., D. J. Coleman, M. A. Lane, R. C. May, L. M. Machesky, and D. P. Clark. 2001. Cryptosporidium parvum infection requires host cell actin polymerization. Infect. Immun. 69:5940-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fradelizi, J., V. Noireaux, J. Plastino, B. Menichi, D. Louvard, C. Sykes, R. M. Golsteyn, and E. Friederich. 2001. ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat. Cell Biol. 3:699-707. [DOI] [PubMed] [Google Scholar]
- 8.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401:926-929. [DOI] [PubMed] [Google Scholar]
- 9.Frischknecht, F., and M. Way. 2001. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 11:30-38. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg, M. B. 2001. Actin-based motility of intracellular microbial pathogens. Microbiol. Mol. Biol. Rev. 65:595-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouin, E., H. Gantelet, C. Egile, I. Lasa, H. Ohayon, V. Villiers, P. Gounon, P. J. Sansonetti, and P. Cossart. 1999. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J. Cell Sci. 112:1697-1708. [DOI] [PubMed] [Google Scholar]
- 12.Heinzen, R. A., S. S. Grieshaber, L. S. Van Kirk, and C. J. Devin. 1999. Dynamics of actin-based movement by Rickettsia rickettsii in Vero cells. Infect. Immun. 67:4201-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loisel, T. P., R. Boujemaa, D. Pantaloni, and M. F. Carlier. 1999. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401:613-616. [DOI] [PubMed] [Google Scholar]
- 15.Lommel, S., S. Benesch, K. Rottner, T. Franz, J. Wehland, and R. Kuhn. 2001. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2:850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machesky, L. M., and K. L. Gould. 1999. The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol. 11:117-121. [DOI] [PubMed] [Google Scholar]
- 17.Machesky, L. M., and R. H. Insall. 1998. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8:1347-1356. [DOI] [PubMed] [Google Scholar]
- 18.Machesky, L. M., R. D. Mullins, H. N. Higgs, D. A. Kaiser, L. Blanchoin, R. C. May, M. E. Hall, and T. D. Pollard. 1999. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA 96:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Quiles, N., R. Rohatgi, I. M. Anton, M. Medina, S. P. Saville, H. Miki, H. Yamaguchi, T. Takenawa, J. H. Hartwig, R. S. Geha, and N. Ramesh. 2001. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat. Cell Biol. 3:484-491. [DOI] [PubMed] [Google Scholar]
- 20.May, R. C., E. Caron, A. Hall, and L. M. Machesky. 2000. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2:246-248. [DOI] [PubMed] [Google Scholar]
- 21.May, R. C., M. E. Hall, H. N. Higgs, T. D. Pollard, T. Chakraborty, J. Wehland, L. M. Machesky, and A. S. Sechi. 1999. The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes. Curr. Biol. 9:759-762. [DOI] [PubMed] [Google Scholar]
- 22.McGee, K., M. Zettl, M. Way, and M. Fallman. 2001. A role for N-WASP in invasin-promoted internalisation. FEBS Lett. 509:59-65. [DOI] [PubMed] [Google Scholar]
- 23.Miki, H., K. Miura, and T. Takenawa. 1996. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 15:5326-5335. [PMC free article] [PubMed] [Google Scholar]
- 24.Miki, H., T. Sasaki, Y. Takai, and T. Takenawa. 1998. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391:93-96. [DOI] [PubMed] [Google Scholar]
- 25.Mimuro, H., T. Suzuki, S. Suetsugu, H. Miki, T. Takenawa, and C. Sasakawa. 2000. Profilin is required for sustaining efficient intra- and intercellular spreading of Shigella flexneri. J. Biol. Chem. 275:28893-28901. [DOI] [PubMed] [Google Scholar]
- 26.Moreau, V., F. Frischknecht, I. Reckmann, R. Vincentelli, G. Rabut, D. Stewart, and M. Way. 2000. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat. Cell Biol. 2:441-448. [DOI] [PubMed] [Google Scholar]
- 27.Mounier, J., V. Laurent, A. Hall, P. Fort, M. F. Carlier, P. J. Sansonetti, and C. Egile. 1999. Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J. Cell Sci. 112:2069-2080. [DOI] [PubMed] [Google Scholar]
- 28.Mullins, R. D. 2000. How WASP-family proteins and the Arp2/3 complex convert intracellular signals into cytoskeletal structures. Curr. Opin. Cell Biol. 12:91-96. [DOI] [PubMed] [Google Scholar]
- 29.Mullins, R. D., J. A. Heuser, and T. D. Pollard. 1998. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA 95:6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruyne, D., M. Evangelista, C. Yang, E. Bi, S. Zigmond, A. Bretscher, and C. Boone. 2002. Role of formins in actin assembly: nucleation and barbed-end association. Science 297:612-615. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh, N., I. M. Anton, J. H. Hartwig, and R. S. Geha. 1997. WIP, a protein associated with Wiskott-Aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl. Acad. Sci. USA 94:14671-14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohatgi, R., H. Y. Ho, and M. W. Kirschner. 2000. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 150:1299-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaplehorn, N., A. Holmstrom, V. Moreau, F. Frischknecht, I. Reckmann, and M. Way. 2002. Grb2 and nck act cooperatively to promote actin-based motility of vaccinia virus. Curr. Biol. 12:740-745. [DOI] [PubMed] [Google Scholar]
- 34.Shibata, T., F. Takeshima, F. Chen, F. W. Alt, and S. B. Snapper. 2002. Cdc42 facilitates invasion but not the actin-based motility of Shigella. Curr. Biol. 12:341-345. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, T., H. Miki, T. Takenawa, and C. Sasakawa. 1998. Neural Wiskott-Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 17:2767-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teyssiere, N., C. Chiche-Portiche, and D. Raoult. 1992. Intracellular movements of Rickettsia conorii and R. typhi based on actin polymerization. Res. Microbiol. 143:821-829. [DOI] [PubMed] [Google Scholar]
- 37.Van Kirk, L. S., S. F. Hayes, and R. A. Heinzen. 2000. Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins. Infect. Immun. 68:4706-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch, M. D., A. Iwamatsu, and T. J. Mitchison. 1997. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature 385:265-269. [DOI] [PubMed] [Google Scholar]
- 39.Welch, M. D., J. Rosenblatt, J. Skoble, D. A. Portnoy, and T. J. Mitchison. 1998. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281:105-108. [DOI] [PubMed] [Google Scholar]
- 40.Winkler, H. H. 1990. Rickettsia species (as organisms). Annu. Rev. Microbiol. 44:131-153. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi, H., H. Miki, S. Suetsugu, L. Ma, M. W. Kirschner, and T. Takenawa. 2000. Two tandem verprolin homology domains are necessary for a strong activation of Arp2/3 complex-induced actin polymerization and induction of microspike formation by N-WASP. Proc. Natl. Acad. Sci. USA 97:12631-12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeile, W. L., D. L. Purich, and F. S. Southwick. 1996. Recognition of two classes of oligoproline sequences in profilin-mediated acceleration of actin-based Shigella motility. J. Cell Biol. 133:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]