Abstract
Paneth cells, highly secretory epithelial cells found at the bases of small intestinal crypts, release a variety of microbicidal molecules, including α-defensins and lysozyme. The secretion of antimicrobials by Paneth cells is thought to be important in mucosal host defense against invasion by enteric pathogens. We explored whether enteric pathogens can interfere with this arm of defense. We found that oral inoculation of mice with wild-type Salmonella enterica serovar Typhimurium decreases the expression of α-defensins (called cryptdins in mice) and lysozyme. Oral inoculation with Salmonella serovar Typhimurium strains that are heat killed, lack the PhoP regulon, and lack the SPI1 type III secretion system or with Listeria monocytogenes does not have this effect. Salmonella may gain a specific survival advantage in the intestinal lumen by decreasing the expression of microbicidal peptides in Paneth cells through direct interactions between Salmonella and the small intestinal epithelium.
The intestine possesses many arms of innate protection from microbial invasion, including peristalsis, mucus secretion, low pH, bile salts, digestive enzymes, and endogenous antimicrobials. Although vast numbers of bacteria colonize the cecum and large intestine, the small intestine has a significantly smaller bacterial burden, despite the presence of high concentrations of nutrients. Paneth cells, located at the base of the small intestinal crypts and rich in secretory granules containing microbicidal peptides and polypeptides, are thought to help maintain the relative paucity of bacteria within this environment (5). For enteric pathogens to be effective in an animal or a human, they must be able to survive in the gastrointestinal tract by evading the innate mucosal defenses, including the action of enteric antimicrobials.
In mice, Paneth cells constitutively produce large amounts of α-defensins (called cryptdins in mice) (30), lysozyme (8) and, in some strains, secretory phospholipase A2 (16, 21), suggesting that these cells have a role in mucosal host defense. Cryptdins are members of the α-defensin family that consists of cationic cysteine-rich antimicrobial peptides, which are widespread in nature, with broad-spectrum antibiotic activity, through their ability to disrupt bacterial membrane function (11, 23, 37). Cryptdin expression in the small intestine is specific to Paneth cells (32). Cryptdins are produced in the small intestine as prepropeptides and undergo posttranslational processing (1, 36) by the matrix metalloproteinase matrilysin. Neither cryptdins nor other α-defensins are found in murine neutrophils (7). To date, six different cryptdin peptides have been isolated (31). Recent work by Wilson et al. (36) has shown that the absence of active murine cryptdins increases the animal's susceptibility to Salmonella infection.
Salmonella enterica serovar Typhimurium is an important human pathogen in food-borne enteritis, causing significant morbidity and mortality. It is a facultative intracellular gram-negative bacterium that causes a systemic disease in mice that is analogous to typhoid fever in humans. Salmonella has evolved a complex variety of virulence mechanisms that make it an effective pathogen. Several pathogenicity islands have been identified in Salmonella. Two of the major pathogenicity islands being characterized are Salmonella pathogenicity island 1 (SPI1) and SPI2. SPI1 encodes for a type III secretion system that is required for Salmonella to invade enterocytes (10, 26), whereas SPI2 is involved in survival within the phagolysosome (6, 17, 27). Intracellular survival depends on the ability of Salmonella to resist the activity of cationic antimicrobial peptides within the phagolysosome (12, 13, 25). However, the mechanisms by which Salmonella survives in the small intestinal lumen prior to invasion is less clear. In the present study, to investigate the interplay between Salmonella and Paneth cell microbicidal peptides, we analyzed murine Paneth cell antimicrobial peptide expression at the mRNA and peptide levels after enteric infection with Salmonella serovar Typhimurium. We compare wild-type Salmonella serovar Typhimurium, heat-killed wild-type Salmonella, an isogenic Salmonella mutant lacking SPI1 that is attenuated when given orally, an avirulent isogenic PhoP-negative Salmonella mutant, and a wild-type gram-positive intracellular bacterium, Listeria monocytogenes, that is also known to invade through the intestine. This study identifies one virulence mechanism that Salmonella may use to survive in the antimicrobial-rich environment of the small intestine.
MATERIALS AND METHODS
Mice.
Female FvB mice were purchased from Taconic Laboratories (Germantown, N.Y.). The mice used were between 5 and 6 weeks of age.
Bacterial strains.
We used an L. monocytogenes wild-type strain 10403s, wild-type Salmonella serovar Typhimurium strain 14028s, and its isogenic mutant MS7953s (phoP::Tn10). The isogenic SPI1 mutant TK93, obtained from Samuel Miller (University of Washington, Seattle, Wash.), with the nonpolar deletion of prgK, does not secrete type III secreted proteins and shows a 100-fold invasion defect (20).
Bacterial culture.
For Salmonella, 10 ml of brain heart infusion (BHI) was inoculated with three to five colonies of Salmonella serovar Typhimurium. To select for motile bacteria, this culture was allowed to grow, standing, for 3.5 h at 37°C. The top 5 ml were removed, added to 45 ml of prewarmed BHI, and grown at 220 rpm for 1.5 h. Bacteria were quantified by counting by using a Petroff-Hauser chamber and then diluted as needed. For L. monocytogenes, bacteria were grown to mid-log phase to late log phase in BHI, quantified, and diluted as needed. An aliquot of inoculum was titered and plated on BHI agar to determine the actual doses administered for each experiment. Salmonella serovar Typhimurium 14028s and TK93 were given at one-quarter 50% lethal doses (LD50) unless otherwise indicated (2 × 106 CFU/mouse for 14028s and 1 × 107 CFU/mouse for TK93). L. monocytogenes was delivered at an LD50, which was 2 × 107 CFU/mouse. Salmonella serovar Typhimurium 7953s was given at 109 CFU/mouse (LD50 > 109CFU/mouse), and heat-killed Salmonella serovar Typhimurium 14028s was given at 109 bacteria/mouse. Bacteria were heat killed by incubating them for 1 h at 65°C.
Animal inoculations.
Animals were deprived of food overnight (18 h). They were inoculated per os (p.o.) with either sterile 0.2 M phosphate buffer (pH 8.0) alone or buffer containing one of the bacteria mentioned above. The total inoculum was 100 μl per mouse and was delivered orally by gavage needle. After inoculation, the mice were fed standard food. Mice were sacrificed postinoculation at the times indicated (ranging from 2 h to 7 days). The terminal ileum was examined by histology. Salmonella burden was determined by homogenization of each organ (spleen, liver, and mesenteric lymph node) in sterile phosphate-buffered saline (PBS) and plating in dilution on Salmonella-Shigella agar. Salmonella burden in the small intestine was determined by opening the lumen, washing the contents into sterile PBS, and plating in dilution on Salmonella-Shigella agar.
Northern analysis and quantification with a PhosphorImager.
Total RNA was isolated from the distal half of the small intestine of each mouse (Biotecx). First, 5 μg of RNA from each mouse was fractionated by formaldehyde-agarose gel electrophoresis and then blotted onto a nylon membrane (Hybond N+) by using a turboblot apparatus (Schleicher & Schuell). Northern blots were sequentially hybridized to GAPDH (AGCCCCAGCCTTCTCCATGGTGGTGAAGACGCCAGTAGACTCCACGAC), cryptdin 1 (CAGCCTGGACCTGGAAGGCCAGCAGGACAAGGGCAGAGAGGAGGACTA), lysozyme P (Paneth cell specific lysozyme) (CCTTGGCCTGGGCAGTGACAGAAAGCAGGAGGAGTCCCAGAGTCAGGA), and matrilysin (GGCCAGGTGGCCTGGCAGCAGACACACAAAGCAGAACAGGGTGAGCTG) 32P-labeled oligonucleotide probes. Hybridizations were done overnight at 37°C in 50% formamide and washed at room temperature for 1 h, followed by washing at 55°C in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS). The blots were exposed on film and then quantified by phosphorimager analysis (with a Storm PhosphorImager).
Isolation of small intestinal epithelium.
The distal small intestines of adult mice were opened and rinsed with sterile PBS. Segments were shaken in Ca2+-Mg2+-free PBS containing 30 mM EDTA at 4°C over a period of 45 min to strip the epithelium from its basement membrane. The epithelium was collected by centrifugation at 1,200 × g for 10 min.
Generation of anti-Paneth cell lysozyme (murine) antibodies.
According to standard procedures, total RNA from mouse small intestine was prepared and used to synthesize first-strand cDNA and used as a PCR template to amplify Lysozyme P (Paneth cell lysozyme) cDNA by using the lysozyme specific primers 5′-Ply (AGCCGGATCCTGCCCAGCCTCCAGTCACCATGAAGG) and 3′-Ly (AGAGAGAATTCGAGCTGCAGTAGAAGCACACCGCGG) and the following conditions: 30 cycles of 1.5 min at 94°C, 1.5 min at 70°C, and 2 min at 72°C. The PCR product was sequenced to verify the identity and then ligated into baculovirus vector pBacPAK9 for expression as described previously (35). Recombinant lysozyme was purified by by reversed-phase high-pressure liquid chromatography with a C8 column and an acetonitrile gradient by using 0.1% trifluoroacetic acid as pairing agent and used for generation of antiserum (Research Genetics, Inc., Huntsville, Ala.).
Isolation of cryptdin peptides, AU-PAGE, and Tricine-PAGE immunoblots.
Cryptdin peptides were isolated by the method of Ayabe et al. (2). Small intestinal epithelial cells were homogenized in 30% acetic acid and extracted by rotating overnight at 4°C. Extracts were diluted to 10% acetic acid and clarified by centrifugation at 100,000 × g for 2 h at 4°C. Supernatants were dialyzed against 5% acetic acid. Protein content of the samples was quantitated by using Bio-Rad protein assay (Bio-Rad). A number of 50-μg aliquots of dialysate were lyophilized, dissolved in 0.1% acetic acid, separated by Tricine-polyacrylamide gel electrophoresis (PAGE) in 16% gels (Bio-Rad, Hercules, Calif.) at 125 V for 90 min, and transferred to polyvinylidene difluoride membrane (Immobilon-P). The membrane was blocked with 5% nonfat milk and probed with sheep polyclonal anti-procryptdin antibody (1:1,000; generously provided by Andre Ouellette, University of California at Irvine, Irvine), followed by horseradish peroxidase-conjugated rabbit anti-sheep antibody (1:500,000; Pierce, Rockford, Ill.) and developed with chemiluminescent substrate (SuperSignal West Femto Substrate; Pierce). The blot was stripped (Restore Buffer; Pierce) and reprobed with rabbit polyclonal anti-lysozyme P antibody (1:500), followed by treatment with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:500,000; Pierce), and then developed as described. Then, 25-μg aliquots of dialysate were lyophilized, dissolved in 5% acetic acid, and separated by acid urea-PAGE (AU-PAGE) in 17% gels for 1 h at 130 V. The gel was Coomassie blue stained to document equal protein loading, destained, and then silver stained to reveal cryptdin peptides.
Immunoblotting for p38 and phospho-p38.
Small intestinal epithelial cells were solubilized with lysis buffer (7 mM K2HPO4, 3 mM KH2PO4, 1 mM EDTA, 5 mM EGTA, 10 mM MgCl2, 50 mM β-glycerophosphate, 0.5% NP-40, 0.1% Brij-35, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 0.5 μg of leupeptin/ml, 0.7 μg of pepstatin/ml; final pH 7.28), incubated on ice for 10 min, and spun in a microcentrifuge for 10 min at 4°C. An aliquot of supernatant was used for SDS-PAGE. The proteins were transferred to nitrocellulose membrane and probed with monoclonal antibody to p38 and phospho-p38 (1:1,000; New England Biolabs). Immunocomplexes were detected by using ECL. The p38 blot demonstrated equal loading of protein samples.
MAPKAPK2 immunoprecipitation and kinase assay.
Distal small intestinal epithelial cells were solubilized in lysis buffer as described above and MAPKAP kinase-2 (MAPKAPK2) was immunoprecipitated by using anti-MAPKAPK2 sheep polyclonal immunoglobulin G, and kinase assays were performed according to MAPKAPK2 kinase assay kit protocol (Upstate Biotechnology, Lake Placid, N.Y.). Assays were read by using a scintillation counter, and counts of experimental samples were compared to those of nonimmune controls.
RESULTS
Oral infection of mice with Salmonella serovar Typhimurium decreases levels of cryptdin and lysozyme mRNA in Paneth cells of the small intestine.
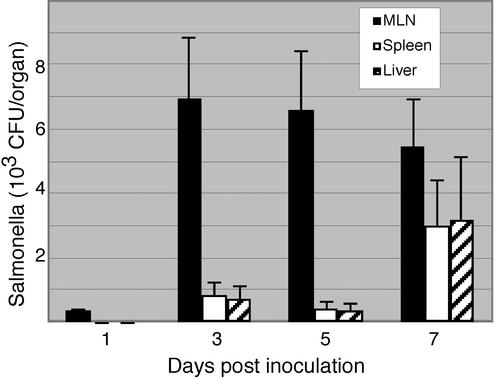
To study the role of enteric antimicrobials in response to Salmonella serovar Typhimurium infection, we evaluated mice infected with the 14028s wild-type strain over a 7-day time course. The mice demonstrated continued colonization of the small intestine but no external evidence of illness or sepsis (data not shown). After 1 day, Salmonella was present at low levels in mesenteric lymph nodes but not yet evident in the liver or spleen, suggesting that the bacteria have not spread systemically at this time point. Over the time course, bacterial levels in the mesenteric lymph nodes persisted, along with evidence of systemic spread (Fig. 1).
FIG. 1.
Progression of systemic infection after oral inoculation with Salmonella serovar Typhimurium. Mice were orally inoculated with wild-type Salmonella (14028s strain). The mice were sacrificed at 1, 3, 5, and 7 days postinoculation. Salmonella was cultured from mesenteric lymph nodes, liver, and spleen. Each time point represents an average of five mice.
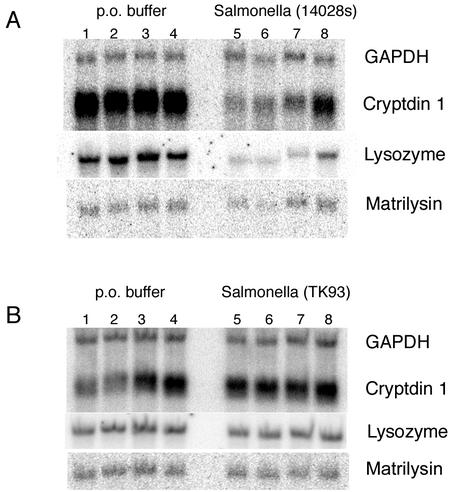
Analysis of antimicrobial mRNA expression revealed on average a threefold decrease in cryptdin and lysozyme mRNA expression (Fig. 2A and Table 1) in response to wild-type Salmonella infection. GAPDH expression was unchanged. We found that at 2 h the mRNA levels for cryptdins were already declining slightly (data not shown), achieving maximal decreases by 18 h and remaining at that level for as long as 7 days postinoculation.
FIG. 2.
Oral inoculation of FvB mice with Salmonella serovar Typhimurium evaluated by Northern blot analysis. Mice were orally inoculated with either buffer alone (lanes 1 to 4) or Salmonella in buffer (lanes 5 to 8). The mice were sacrificed after 24 h. Total RNA was isolated from distal small intestine of the above mice, fractionated by formaldehyde-agarose gel electrophoresis, blotted onto a nylon filter, and hybridized sequentially to the indicated probes. Each lane represents one mouse. (A) 14028s mouse virulent strain; (B) TK93 SPI1 type III secretion mutant, isogenic to 14028s strain.
TABLE 1.
mRNA expression of Paneth cell antimicrobial peptides in response to oral bacterial infection as quantified by Northern blot analysisa
| Inoculum | Mean mRNA level (cpm) ± SE or U/I ratio with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cryptdin
|
Lysozyme
|
Matrilysin
|
|||||||
| U | I | Ratio | U | I | Ratio | U | I | Ratio | |
| Salmonella serovar Typhimurium 14028s | 5.1 ± 0.6 | 1.9 ± 0.5 | 0.37b | 2.1 ± 0.3 | 0.62 ± 0.21 | 0.30b | 0.16 ± 0.02 | 0.16 ± 0.02 | 1.00 |
| Salmonella serovar Typhimurium 14028s heat- killed (109 bacteria) | 8.5 ± 0.4 | 10.0 ± 0.4 | 1.18 | 3.6 ± 0.3 | 3.8 ± 0.2 | 1.06 | 0.32 ± 0.03 | 0.34 ± 0.05 | 1.06 |
| Salmonella serovar Typhimurium MS7953s | 3.1 ± 0.2 | 3.0 ± 0.9 | 0.97 | 2.5 ± 0.4 | 2.6 ± 0.5 | 1.04 | 0.14 ± 0.02 | 0.17 ± 0.02 | 1.21 |
| Salmonella serovar Typhimurium TK93 | 2.2 ± 0.3 | 1.9 ± 0.2 | 0.86 | 6.8 ± 0.1 | 6.8 ± 0.1 | 1.00 | 0.71 ± 0.06 | 0.75 ± 0.05 | 1.06 |
| L. monocytogenes 10403s | 3.3 ± 0.6 | 3.6 ± 0.4 | 1.09 | 7.7 ± 0.4 | 7.5 ± 0.9 | 0.97 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.00 |
The radioactive oligonucleotide signals from the Northern blot analyses were quantitated by using a phosphorimager. The tabulated results show the normalized counts-per-minute (cpm) values for mRNA levels in infected mice (I) and uninfected mice (U) with the calculated standard errors. The tabulated results present individual experiments, each with four mice per infected group and four mice per uninfected group. The results for wild-type Salmonella are representative of six independent experiments. The results for the SPI1 mutant Salmonella are representative of three independent experiments.
P < 0.005 (Student t test) comparing uninfected and infected mice. Values for both mouse groups are normalized cpm values for mRNA levels (n = four mice per group).
To test whether the decrease in antimicrobial mRNA was due to a general dysfunction of Paneth cells in response to Salmonella infection, we examined the expression of matrilysin, a Paneth cell-specific enzyme involved in cryptdin processing (36). Matrilysin expression did not change in response to this dose of bacteria. In addition, histologic examination of the terminal ileum demonstrated mild focal acute inflammation with neutrophilic infiltrate, with normal-appearing Paneth cells (data not shown).
One possibility is that a decline in Paneth cell antimicrobial peptide expression is a universal response to enteric bacterial infection. We tested this possibility by using the 10403s wild-type strain of L. monocytogenes. L. monocytogenes is a gram-positive facultative intracellular bacterium that also invades through the small intestine in human and mice. Oral infection with an LD50 dose of L. monocytogenes, accompanied by translocation and lymphatic spread, did not result in any change in antimicrobial mRNA levels, suggesting that the modulation observed with Salmonella was not a stereotypic response to infection with enteric pathogens (Table 1).
Another feasible explanation is that the modulatory effect results from the general presence of gram-negative bacteria or their cell wall components within the small intestinal lumen. Mice were orally dosed with heat-killed wild-type 14028s Salmonella, at 107 or 109 bacteria/mouse. We found no alterations in antimicrobial mRNA levels (Table 1).
We then considered whether the change in antimicrobial peptide mRNA expression was due to specific interactions of Salmonella with the small intestinal epithelium. For these experiments we used TK93 Salmonella. TK93 is an SPI1 type III secretion mutant deleted of prgK and defective in the secretion of type III-secreted proteins and invasion (20). SPI1 mutants are attenuated in virulence when inoculated orally but are as virulent as the wild type when administered systemically (4).
When orally inoculated with this mutant, colonization was evident for 2 days (data not shown). To ensure that any measured differences were not due to the different abilities of each bacterial species to survive in the small intestine all experiments were done by sacrificing mice 1 day after inoculation. We determined the tititerd of inoculation doses to ensure that equivalent numbers of Salmonella species were present in the small intestine at the point of sacrificing the animals. Analysis of total small intestinal RNA from mice sacrificed 1 day postinoculation showed that oral infection with TK93 SPI1 mutant does not result in a decrease in Paneth cell antimicrobial mRNA levels (Fig. 2B and Table 1) in contrast to wild-type salmonella. These results suggest that the modulation of Paneth cell antimicrobial peptide mRNA expression by Salmonella may require direct interaction between the bacterium and the mucosal epithelium via the type III secretion system.
We also studied the mucosal response to avirulent MS7953s mutant Salmonella. MS7953s lacks the PhoP:PhoQ two-component regulatory system that controls the expression of many Salmonella virulence factors in response to its external environment. This mutant constitutively expresses the SPI1 type III secretion system and should be capable of invading the intestinal epithelium. It has been clearly demonstrated that MS7953s Salmonella cannot survive intracellularly (12, 24, 25). Since MS7953s is avirulent, we dosed the mice with higher numbers of bacteria (109 CFU/mouse). We were able to recover 10-fold-higher numbers of MS7953s (103) than the numbers of wild-type Salmonella (102 CFU) from the distal small intestine at this time point. We were not able to culture MS7953s Salmonella from Peyer's patches, mesenteric lymph nodes, liver, or spleen, at any point over a 7-day time course (data not shown). Despite the increased bacterial burden of MS7953s Salmonella in the lumen on day 1, there was no change in Paneth cell antimicrobial mRNA levels (Table 1).
Decreased levels of lysozyme and cryptdin mRNA are accompanied by lower levels of both lysozyme and cryptdin peptides in Paneth cells of the small intestine.
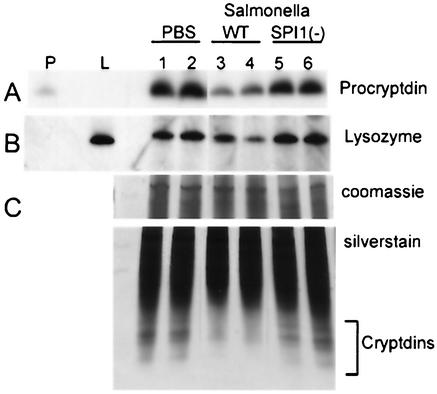
We then investigated whether the decrease in antimicrobial mRNA levels was reflected by a similar decrease in peptide. Western blot analysis of small intestinal extracts by Tricine-PAGE reveals significant decreases in procryptdin (Fig. 3A), the unprocessed precursor of the mature cryptdins, in response to wild-type Salmonella. The same Western blot also shows notable decreases in lysozyme protein expression (Fig. 3B). To compare the levels of mature cryptdin peptides, we used AU-PAGE. With this electrophoretic separation under acidic conditions, the cationic cryptdin peptides migrate the fastest of the intestinal peptides and demonstrate a characteristic multiband separation pattern (29, 33, 34). As shown in Fig. 3C, the upper panel showing a Coomassie blue-stained top portion of the gel demonstrates equal loading of protein extracts. The lower panel, showing the bottom portion of a silver-stained AU-PAGE gel, reveals variable but appreciable decreases in all mature cryptdin peptides in mice that received wild-type Salmonella compared to mice that received the TK93 mutant. There appears to be a greater loss of the most electrophoretically mobile cryptdin forms.
FIG. 3.
Regulation of antimicrobial peptide production in response to Salmonella infection evaluated by AU-PAGE and Western blot analysis. Mice were orally inoculated p.o. with buffer alone (lanes 1 and 2) or buffer containing wild-type Salmonella 14028s (lanes 3 and 4) or Salmonella TK93 (lanes 5 and 6). The mice were sacrificed after 24 h. Acetic acid extracts were made from the distal small intestinal epithelium. Then, 50 μg of protein was fractionated by Tricine-PAGE. (A) Western blot analysis of Tricine-PAGE with anti-procryptdin antibody (1:1,000; generously provided by Andre Ouellette, University of California at Irvine, Irvine). P, procryptdin control (0.5 μg). (B) Western blot analysis of Tricine-PAGE with anti-lysozyme antibody (1:500). L, human lysozyme control (1 μg). (C) A total of 25 μg of protein was fractionated by AU-PAGE. The top panel shows the top portion of the gel, which was Coomassie blue stained to demonstrate equal protein loading. The bottom panel shows the lower portion of the silver-stained gel. Cryptdins are the fastest-migrating proteins (bracket).
Oral infection of mice with Salmonella serovar Typhimurium but not with mutant TK93 that lacks the SPI1 type III secretion system activates the p38 MAPK pathway in the small intestinal epithelium.
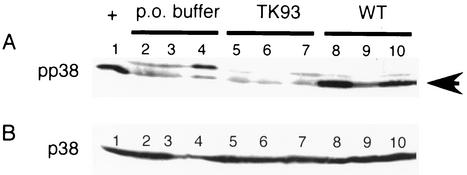
To investigate the cellular mechanisms involved in the modulation of enteric cryptdin and lysozyme expression, we investigated the signaling pathways activated by the SPI1 type III secretion system. One of the major signaling pathways activated by this system is the mitogen-activated protein kinase (MAPK) pathway. We investigated whether Salmonella was able to activate the p38 MAPK pathway in vivo. We evaluated the small-intestinal epithelium of mice for p38 MAPK activation by Western blot analysis with phospho-specific antibodies. A low basal level of p38 activation was seen in control mice (Fig. 4A). Administration of wild-type Salmonella induced a dramatic increase in p38 MAPK activation. In striking contrast, mice given TK93 mutant Salmonella showed no activation of p38 MAPK over control animals, whereas the levels of total p38 MAPK protein were equivalent in all samples as shown by immunoblot (Fig. 4B). Mice given MS7953s Salmonella or heat-killed 14028s Salmonella showed no increased activation of the p38 MAPK pathway over PBS controls (data not shown).
FIG. 4.
Activation of p38 MAPK pathway by Salmonella serovar Typhimurium evaluated by Western blot analysis. Mice were orally inoculated p.o. with buffer alone (lanes 2 to 4) or with buffer with Salmonella TK93 (lanes 5 to 7) or wild-type Salmonella 14028s (lanes 8 to 10). The mice were sacrificed after 24 h. Lysates were made from the distal small intestinal epithelium and fractionated by SDS-PAGE. Western blots were analyzed with phospho-p38 (A) and p38 (B) antibodies at 1:1,000. Each lane represents one mouse. Arrow, phosphorylated, active p38. The first lane of the phospho-p38 blot (+) contains lysate from NIH 3T3 cells that had been treated with sorbitol, which activates the p38 MAPK pathway.
Inhibition of the p38 MAPK pathway in vivo abrogates the ability of Salmonella to modulate cryptdin mRNA expression in Paneth cells.
We next investigated whether the p38 MAPK pathway was required for the Salmonella-associated inhibition of antimicrobial peptide expression by looking at the effect of SB 203580, a specific reversible p38 MAPK inhibitor (22). We found that the cryptdin mRNA levels of Salmonella-infected mice quickly returned to that of uninfected mice in the presence of p38 MAPK inhibitor (Table 2), whereas the levels of lysozyme mRNA are recovering but not as quickly. Matrilysin levels are not significantly changed by use of SB 203580 (Table 2).
TABLE 2.
Effects of p38 inhibition on modulation of antimicrobial peptide expression by Salmonella
| mRNA | Mean mRNA level (cpm) ± SE or I/U ratio with:
|
|||||
|---|---|---|---|---|---|---|
| No inhibitor
|
Inhibitor (SB 203580)
|
|||||
| U | I | Ratio | U | I | Ratio | |
| Cryptdin | 4.5 ± 0.6 | 2.5 ± 0.3 | 0.55b | 3.7 ± 0.4 | 3.4 ± 0.7 | 0.92 |
| Lysozyme | 6.5 ± 0.5 | 3.3 ± 0.7 | 0.51b | 7.1 ± 0.5 | 4.9 ± 1.4 | 0.69 |
| Matrilysin | 0.60 ± 0.03 | 0.58 ± 0.04 | 0.97 | 0.61 ± 0.02 | 0.73 ± 0.09 | 1.20 |
Mice were orally inoculated p.o. with either wild-type Salmonella in buffer or buffer alone. After 24 h, half of each group was given a 25-mg/kg oral dose of SB 203580 in acidified tragacanth. The other half was given vehicle alone. The mice were sacrificed after 2 h. Total RNA was isolated from distal small intestine, fractionated, blotted on to a nylon filter, and hybridized sequentially to G3PDH, cryptdin 1, lysozyme, and matrilysin oligonucleotide probes. The radioactive oligonucleotide signals from the Northern blot analyses were quantitated by using a phosphorimager. The tabulated results show the normalized cpm values for mRNA levels in Salmonella-infected mice (I) and uninfected mice (U) with the calculated standard errors. The tabulated results are representative of two individual experiments.
P ≤ 0.005 (Student t test) comparing uninfected and infected mice. Values for both groups are normalized cpm values for mRNA levels (n = four mice per group).
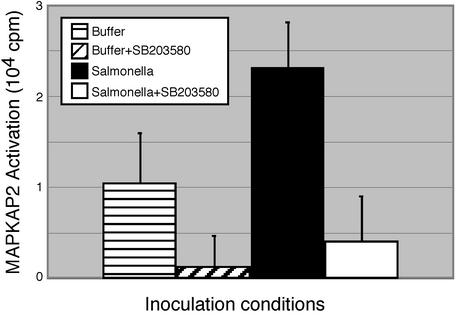
To demonstrate that the SB 203580 was effectively inhibiting p38 MAPK in vivo, we measured the activation of a kinase downstream of p38, MAPKAPK2 (Fig. 5). We observed basal MAPKAPK2 activity in mice treated with sterile buffer, just as was seen with p38 (first column). Activity was increased in response to Salmonella infection (comparison of column 1 to column 3). In specimens from mice treated with SB 203580, MAPKAPK2 activation is inhibited (comparison of column 1 to column 2 and of column 3 to column 4), confirming that the drug inactivated p38 MAPK in vivo (Fig. 5). These results confirm that inhibition of the p38 pathway reverses the decrease in cryptdin mRNA by Salmonella in vivo, suggesting that wild-type Salmonella-mediated reduction in antimicrobial production involves the p38 MAPK pathway.
FIG. 5.
Inhibition of p38 MAPK pathway by SB 203580 after Salmonella infection evaluated by MAPKAPK2 assay. Mice were orally inoculated p.o. with either wild-type Salmonella (12 mice) in buffer or with buffer alone (12 mice). At 24 h postinoculation, half of each group was given a 25-mg/kg oral dose of SB 203580 in acidified tragacanth (3). The other half was given vehicle alone. The mice were sacrificed 2 h thereafter. Lysates were prepared from distal small intestinal epithelium. MAPKAPK2 was immunoprecipitated from these lysates and subjected to in vitro kinase assays. Results show averages of six mice per group (Student t test: P < 0.005 compared p.o. buffer group to Salmonella group, P < 0.0005 comparing Salmonella plus SB203580 to Salmonella plus vehicle, and P < 0.05 comparing p.o. buffer plus SB203580 to p.o. buffer plus vehicle.).
DISCUSSION
In the results presented here, we find that an oral infection with wild-type Salmonella serovar Typhimurium results in a significant decrease in innate host defense effector molecules of the small intestine. The decreases in cryptdin and lysozyme expression are the first evidence that Paneth cell antimicrobial expression can be altered by bacterial infection with an intestinal pathogen in vivo. Salmonella-induced decrease of Paneth cell antimicrobial peptide mRNA and protein levels may be one of its survival mechanisms in the intestinal lumen and required for subsequent invasion.
The ability of intestinal pathogens to downregulate host antimicrobials is not restricted to Salmonella. Shigella flexneri infection is able to decrease the expression of human α-defensin-1 and LL-37 in colonic epithelial cell lines and human colonic biopsy specimens (19). These findings suggest that this regulation requires the Shigella virulence plasmid DNA alone, even in the absence of live bacteria (19). S. enterica serovar Typhi infection of human small intestinal xenografts, although inducing human β-defensin-2 in enterocytes, does not impact on Paneth cell antimicrobial expression (28), suggesting that distinct antimicrobial effector molecules of the intestinal mucosa are capable of responding differently, depending on the bacterial stimulus.
In Salmonella, the regulation of Paneth cell antimicrobial peptide expression appears to be linked to successful invasion via the SPI1 type III secretion, since neither the Salmonella strain lacking expression of SPI1 nor L. monocytogenes, which lacks a type III secretion system, alters the expression of these peptides. This finding suggests that specific interaction between the live bacterium and the mucosa and perhaps specific SPI1 secreted virulence factors are required for alterations in antimicrobial peptide expression. The avirulent Salmonella MS7953s, however, has no impact on antimicrobial peptide expression, although it should be capable of invading the mucosal epithelium. We have found that oral inoculation with high doses of MS7953s does not result in the activation of the p38 MAPK pathway in the small intestinal epithelium. One possibility is that this organism is not effectively interacting with the intestinal epithelial cells. It is also conceivable that this organism, which cannot survive within the phagolysosome, is killed so rapidly that it does not provoke the same inflammatory cascade by intestinal epithelial cells as does the wild-type bacterium. It appears, consequently, that direct interaction of the Salmonella with the epithelium is required but not sufficient for the alteration of Paneth cell antimicrobial peptide expression and that this phenomenon requires cellular epithelial responses that are activated by the process of invasion and persistence in the surrounding tissues. Additional work in this area will be needed to fully understand all of the factors involved in the Salmonella-Paneth cell interaction.
The decreased mRNA expression was accompanied by decreased peptide expression. Intracellular peptide levels are influenced by production and secretion rate. Recent work has shown that Paneth cells will release secretory granules containing cryptdins in response to bacterial challenge (both gram positive and negative), lipopolysaccharide, lipid A, and lipoteichoic acid (2). Thus, with comparable bacterial numbers of wild-type and SPI1-defective bacteria in the small-intestinal lumen, we favor the hypothesis that the decreases in peptide levels reflect differences in peptide production rather than variations in Paneth cell secretion rates.
Unlike cryptdins, which are exclusively found in Paneth cells, lysozyme can be found in Paneth cells, macrophages, and myeloid cells, and the antibody used reacts with both forms. Hence, this experiment is likely measuring the combination of Paneth cell and other cellular sources of lysozyme. We cannot distinguish whether the decrease in lysozyme mRNA and protein is specific to Paneth cells or whether Salmonella is also altering lysozyme mRNA expression in other hematopoietic cells.
There are a number of possible models for the mechanism by which Salmonella might impact Paneth cell antimicrobial peptide expression. First, it could be a result of a direct interaction between the Salmonella bacterium and the Paneth cell. Under normal circumstances bacteria are not found in the small intestinal crypt, but in the presence of active infection such contact could be conceivable. Another possibility is that the direct contact between Salmonella and the villous enterocytes causes the release of mediators from the enterocytes, which signal to Paneth cells. The interaction of Salmonella with enterocytes via the SPI1 type III secretion system involves the secretion of a number of effector molecules that are able to impact on several cell signaling pathways. Salmonella is able to activate p38 MAPK in intestinal epithelial cell lines (18). We have found that wild-type Salmonella is also able to activate p38 MAPK in small intestinal epithelium in vivo. In vivo inhibition of this pathway blocks the ability of Salmonella to modulate Paneth cell antimicrobial mRNA expression. This suggests that activation of the p38 MAPK pathway is involved in the negative modulation of enteric cryptdin expression by Salmonella either through regulation of transcription or decreased mRNA stability and message degradation.
For effective pathogenesis, Salmonella needs to survive in the small intestinal lumen. Salmonella-induced decreases in lysozyme and cryptdin peptides may be one of its survival mechanisms. This contrasts with Salmonella virulence in the phagolysosome, which depends in part on its ability to resist the action of cationic antimicrobial peptides (9, 12, 13) by altering its surface lipopolysaccharide (14, 15) in response to the phagolysosomal environment. The intestinal milieu differs from that of the phagolysosome and likely triggers different virulence mechanisms in Salmonella. Continued study of Salmonella and its earliest interactions with its host should provide further insight into this microbe's essential virulence mechanisms. At the same time, such studies will help us understand the regulation of the mucosal innate immune system and its significance in host protection from bacterial enteritis.
Acknowledgments
We thank Samuel I. Miller (University of Washington, Seattle) for TK93 serovar Typhimurium; Andre Ouellette (University of California at Irvine, Irvine) for anti-procryptdin antibody and technical advice on cryptdin isolation; Carole Wilson (Washington University School of Medicine, St. Louis, Mo.) for technical advice on cryptdin immunoblotting and recombinant procryptdin peptide; Koryn Carver (Medical College of Wisconsin, Milwaukee), Dayana Carcamo (California State University, Los Angeles), and Amie Chen (University of California at Los Angeles, Los Angeles [UCLA]) for technical assistance; and Tomas Ganz (UCLA), Charles Bevins (Cleveland Clinic Foundation, Cleveland, Ohio), and Martin Carroll (University of Pennsylvania School of Medicine, Philadelphia) for helpful discussions.
This study was supported by NIH grant K08-AI01525 (N.H.S.) and NIH grant AI36657 (Y.P.).
Editor: J. D. Clements
REFERENCES
- 1.Ayabe, T., D. P. Satchell, P. Pesendorfer, H. Tanabe, C. L. Wilson, S. J. Hagen, and A. J. Ouellette. 2002. Activation of Paneth cell α-defensins in mouse small intestine. J. Biol. Chem. 277:5219-5228. [DOI] [PubMed] [Google Scholar]
- 2.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 3.Badger, A. M., J. N. Bradbeer, B. Votta, J. C. Lee, J. L. Adams, and D. E. Griswold. 1996. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J. Pharmacol. Exp. Ther. 279:1453-1461. [PubMed] [Google Scholar]
- 4.Baumler, A. J., R. M. Tsolis, P. J. Valentine, T. A. Ficht, and F. Heffron. 1997. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella typhimurium to cause murine typhoid. Infect. Immun. 65:2254-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevins, C. L., E. Martin-Porter, and T. Ganz. 1999. Defensins and innate host defence of the gastrointestinal tract. Gut 45:911-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer, P. B., and R. I. Lehrer. 1992. Mouse neutrophils lack defensins. Infect. Immun. 60:3446-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erlandsen, S. L., J. A. Parsons, and T. D. Taylor. 1974. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J. Histochem. Cytochem. 22:401-413. [DOI] [PubMed] [Google Scholar]
- 9.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243(Pt. 1):1059-1062. [DOI] [PubMed] [Google Scholar]
- 10.Galan, J. E. 1996. Molecular genetic basis of Salmonella entry into host cells. Mol. Microbiol. 20:263-272. [DOI] [PubMed] [Google Scholar]
- 11.Ganz, T., M. E. Selsted, D. Szklarek, S. S. Harwig, K. Daher, D. F. Bainton, and R. I. Lehrer. 1985. Defensins: natural peptide antibiotics of human neutrophils. J. Clin. Investig. 76:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groisman, E. A., C. Parra-Lopez, M. Salcedo, and C. J. Lipps. 1992. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11939-11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo, L., B. L. Kheng, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 15.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 16.Harwig, S. S. L., L. Tan, X.-D. Qu, Y. Cho, P. B. Eisenhauer, and R. I. Lehrer. 1995. Bactericidal properties of murine intestinal phospholipase A2. J. Clin. Investig. 95:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Ganks, A. Vazques-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 18.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 19.Islam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. H. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune excape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7:180-185. [DOI] [PubMed] [Google Scholar]
- 20.Kimbrough, T. G., and S. I. Miller. 2000. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl. Acad. Sci. USA 97:11008-11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koopman, P., S. Povey, and R. H. Lovell-Badge. 1989. Widespread expression of human α1-antitrypsin in transgenic mice revealed by in situ hybridization. Genes Dev. 3:16-25. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, S., M. S. Jiang, J. L. Adams, and J. C. Lee. 1999. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 263:825-831. [DOI] [PubMed] [Google Scholar]
- 23.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli: mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, S. I., W. S. Pulkkinen, M. E. Selsted, and J. J. Mekalanos. 1990. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect. Immun. 58:3706-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills, D. M., V. Bajaj, and C. A. Lee. 1995. A 40-kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15:749-759. [DOI] [PubMed] [Google Scholar]
- 27.Ochman, H., F. C. Soncini, F. Solomon, and E. A. Groisman. 1996. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93:7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163:6718-6724. [PubMed] [Google Scholar]
- 29.Ouellette, A. J., M. M. Hsieh, M. T. Nosek, D. F. Cano-Gauci, K. M. Huttner, R. N. Buick, and M. E. Selsted. 1994. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect. Immun. 62:5040-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouellette, A. J., S. I. Miller, A. H. Henschen, and M. E. Selsted. 1992. Purification and primary structure of murine cryptdin-1, a Paneth cell defensin. FEBS Lett. 304:146-148. [DOI] [PubMed] [Google Scholar]
- 31.Ouellette, A. J., and M. E. Selsted. 1996. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 10:1280-1289. [DOI] [PubMed] [Google Scholar]
- 32.Porter, E., L. Liu, A. Oren, P. Anton, and T. Ganz. 1997. Localization of human intestinal defensin 5 in Paneth cell granules. Infect. Immun. 65:2389-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selsted, M. E., and H. W. Becker. 1986. Eosin Y: a reversible stain for detecting electrophoretically resolved proteins. Anal. Biochem. 155:270-274. [DOI] [PubMed] [Google Scholar]
- 34.Selsted, M. E., S. I. Miller, A. H. Henschen, and A. J. Ouellette. 1992. Enteric defensins: antibiotic peptide components of intestine host defense. J. Cell Biol. 118:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valore, E. V., and T. Ganz. 1997. Laboratory production of antimicrobial peptides in native conformation. Methods Mol. Biol. 78:115-131. [DOI] [PubMed] [Google Scholar]
- 36.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 37.Wimley, W. C., M. E. Selsted, and S. H. White. 1994. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 3:1362-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]