Summary
In this study, we examined the tissue-specific expression of two electroneutral Na/HCO3 cotransporter (NBCn1) variants that differ from each other by the presence of the N-terminal 123 amino acids (cassette II). A rat Northern blot with the probe to nucleotides encoding cassette II detected a 9 kb NBCn1 mRNA strongly in the heart and weakly in skeletal muscles, but absent from most of the tissues including kidney, brain, and pancreas. In the rat heart, PCR with primers flanking cassette II preferentially amplified a DNA fragment that lacked cassette II. However, in the human heart, PCR preferentially amplified a fragment that contained cassette II. This larger PCR product was found virtually in all regions of the human cardiovascular system with strong amplification in the apex, atrium, and atrioventricular nodes. These findings indicate that the variant containing cassette II is almost absent in tissues including brain, kidney, and pancreas, where NBCn1 has been extensively examined.
Keywords: acid-base regulation, bicarbonate transporter, sodium bicarbonate symporter
The proton concentration in the cardiovascular system is important for maintaining cardiac function (for review see Ref [1]). Intracellular acidification decreases contractility by affecting almost all steps in excitation-contraction coupling. Cardiac cells have precise regulatory mechanisms to extrude acids from the cytoplasm. More than 40% of acid extrusion in cardiac myocytes occur by Na/HCO3 transport [2]. The carrier protein is proposed as an Na/HCO3 cotransporter with the 1:1 stoichiometry of Na+ versus HCO3− [2]. The electroneutral Na/HCO3 cotransporter (NBCn1) was cloned from the heart and vascular smooth muscle 6–7 years ago [3, 4]. Many studies have subsequently characterized its cellular and physiological function in epithelial and non-epithelial tissues (for review see Ref. [5]). While these provide useful information on physiological properties of NBCn1, recent reports suggest that the transporters in many tissues appear different from the initially identified cardiovascular clone; NBCn1 in adult brain and kidney lacks, in the N-terminal domain, a region of 123 amino acids (cassette II) that comprise ~10% of the total amino acids in the protein [6, 7]. Identification of this deletion variant led us to reinvestigate the cardiac tissue specificity of NBCn1.
For experimental methods, we used Northern blot and PCR to distinguish the deletion variant from the non-deletion variant in rat and human tissues. A multiple-tissue Northern blot membrane of adult rats was purchased from BD Bioscience (Palo Alto, CA). The nucleotides (964–1,334 of rat NBCn1; GenBank accession number NM_058211) corresponding to cassette II were 32P-labeled and hybridized with the membrane at 68 °C for 2 h (probe concentration: 0.6 × 106 cpm/ml). The membrane was washed in 2 × SSC/0.1% SDS for 40 min, and then in 0.1 × SSC/0.1% SDS for 1 h at 55 °C. The autoradiograph was done 24 h later. For PCR, total RNA was extracted from adult rat (Sprague-Dawley) whole heart using Trizol (Life Technologies, Gaithersburg, MD) according to the manufacturer protocol. cDNA was synthesized using Superscript Reverse Transcriptase (Invitrogen, Carlsbad, CA) primed with random hexamers. Primers were designed from the flanking regions of cassette II; 819–846 of rat NBCn1 (forward primer) and 2,266–2,295 (reverse primer). In addition, cDNAs of human cardiovascular system (BD Bioscience) were purchased. Primers corresponded to 135–164 (forward) and 1,662–1,691 (reverse) of human NBCn1 sequence (NBC3; GenBank accession number NM_003615). PCR was done in 30 cycles of 94 °C for 30 s, 65 °C for 45 s, and 72 °C for 1 min. PCR was repeated minimum three times to verify the results.
Figure 1(A) shows the rat northern blot probed with cassette II. A 9 kb NBCn1 mRNA was predominantly detected in the heart. The signals in other tissues were very weak or negligible. In particular, the NBCn1 mRNA was almost absent in spleen and testis, kidney, and brain, where a probe to the transmembrane domains of NBCn1 detected moderate to strong signals [3]. The absence of signals in the brain and kidney is consistent with recent reports that the transporter in these tissues lacks cassette II [6, 7]. Our data are also similar to the data from human northern blot, except that, in human, NBCn1 signals are strongly detected in both heart and skeletal muscles [4]. Figure 1(B) shows the relative abundance of the NBCn1 variant containing cassette II in the rat heart. PCR with primers flanking cassette II preferably generated an 1.0 kb product, which corresponds to the deletion variant. The 1.5 kb product, which corresponds to the non-deletion variant, was very rare. These data indicate that only a small fraction of the total transporters in the rat heart has cassette II. In contrast, when PCR was repeated on the human heart with similar primers, an 1.5 kb product was preferentially amplified (Figure 1C). This larger PCR product was found virtually in all regions of the human cardiovascular system we examined. The smaller product was also amplified in most tissues though its abundance was minor in the apex of the heart, atrium, and atrioventricular nodes. Therefore, the relative expression of deletion and non-deletion variants varies between rat and human hearts and among different regions of the heart.
Figure 1.
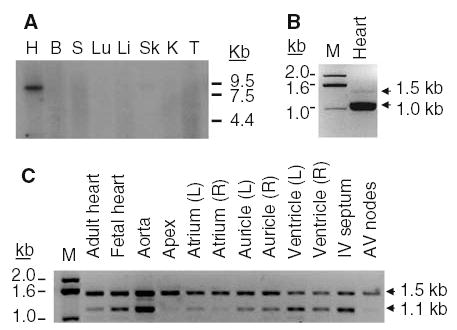
Cardiac expression of NBCn1 variants. (A) Northern blot of rat tissues probed to nucleotides 964–1,334 (encoding N-terminal cassette II) of NBCn1. H, heart; B, brain; S, spleen; Lu, lung; Li, liver; Sk, skeletal muscle; K, kidney; T, testis. (B) PCR of rat whole heart. Primers were designed from the conserved flanking regions of cassette II. The 1.0 kb PCR product represents NBCn1 without cassette II, and the 1.5 kb product represents the one with the cassette. (C) PCR of human cardiovascular system. Primers were designed to amplify a 1.1 kb product for NBCn1 without cassette II and a 1.5 kb product for the one with the cassette. M, molecular marker; IV, interventricular; AV, Atrioventricular. L and R in parenthesis are left and right.
The significance of our data is the almost exclusive presence of the cassette II-containing NBCn1 variant in the cardiovascular system. However, its relative abundance differs between rat and human hearts. Most NBCn1 variants in the rat heart lack cassette II, whereas most variants in the human heart contain the cassette. It is important to note that the transporter initially identified in the cardiovascular systems of rat and human is the non-deletion clone containing cassette II. Our northern blot data show that this clone is almost undetectable in tissues including brain, kidney, and pancreas, where NBCn1 has been extensively examined in vivo and in vitro. Therefore, caution is required when studying NBCn1 in these tissues. Nevertheless, we do not insist that the non-deletion variant is expressed only in the heart (and vascular smooth muscle). It may be possible that the non-deletion variant is also present in other tissues we did not examine in this study.
The function of cassette II is unclear. Cassette II does not directly alter the biophysical properties of the transporter [7], thus raising the possibility of transporter regulation. The cytoplasmic N- and C-terminal domains of other Na/HCO3 transporters have been proposed to affect protein expression and trafficking, cellular signaling, and interactions with other proteins [5, 8]. The non-deletion variant containing cassette II has a slow expression on Xenopus oocytes membranes compared to the one without the cassette (unpublished observation). Thus, the function of cassette II would likely relate to protein trafficking or retention. Alternatively, cassette II might involve interactions with other proteins. We note that the Cl/HCO3 exchanger AE1 has a binding motif for ankyrin in the region corresponding to cassette II of NBCn1. Thus, it is plausible that cassette II in NBCn1 may be a site for docking other cytosolic proteins.
In conclusion, the presence of two NBCn1 variants in the cardiovascular system is an interesting finding in that their dual expression could be advantageous to some cardiac cells, where pHi and ion gradients have to be carefully regulated. Nonetheless, understanding the mechanism of such regulation will require more complicated investigation.
Acknowledgments
We are grateful to Eun Jung Shin for PCR. This work was supported by NIDDK Grant DK-061418 (C.C. Yun) and the American Heart Association Southeast Affiliate and Emory URC grant (I. Choi).
References
- 1.Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990;258:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- 2.Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- 4.Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- 5.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- 6.Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J. Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol. 2004;555:205–218. doi: 10.1113/jphysiol.2003.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem. 2005;280:17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- 8.McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]


