Abstract
Muscle satellite cells are quiescent precursors interposed between myofibers and a sheath of external lamina. Although their activation and recruitment to cycle enable muscle repair and adaptation, the activation signal is not known. Evidence is presented that nitric oxide (NO) mediates satellite cell activation, including morphological hypertrophy and decreased adhesion in the fiber-lamina complex. Activation in vivo occurred within 1 min after injury. Cell isolation and histology showed that pharmacological inhibition of nitric oxide synthase (NOS) activity prevented the immediate injury-induced myogenic cell release and delayed the hypertrophy of satellite cells in that muscle. Transient activation of satellite cells in contralateral muscles 10 min later suggested that a circulating factor may interact with NO-mediated signaling. Interestingly, satellite cell activation in muscles of mdx dystrophic mice and NOS-I knockout mice quantitatively resembled NOS-inhibited release of normal cells, in agreement with reports of displaced and reduced NOS expression in dystrophin-deficient mdx muscle and the complete loss of NOS-I expression in knockout mice. Brief NOS inhibition in normal and mdx mice during injury produced subtle alterations in subsequent repair, including apoptosis in myotube nuclei and myotube formation inside laminar sheaths. Longer NOS inhibition delayed and restricted the extent of repair and resulted in fiber branching. A model proposes the hypothesis that NO release mediates satellite cell activation, possibly via shear-induced rapid increases in NOS activity that produce “NO transients.”
INTRODUCTION
After muscle injury, satellite cells are activated and recruited to cycle as precursors for new muscle formation. Between injury and proliferation in vivo, satellite cells express immediate early genes after 3–6 h (Weiss, 1994; Kami et al., 1995) and muscle regulatory genes after 6 h (Grounds et al., 1992) in concert with proliferating cell nuclear antigen (Chambers and McDermott, 1996). The expression of these genes, release of growth factors such as bFGF, and DNA synthesis 24–30 h later are used to characterize muscle regeneration in injured and dystrophic muscle (Grounds and McGeachie, 1989; Anderson et al., 1995; Floss et al., 1997, 1998). The timing and sequence of events are specific to those of repair (Megeney et al., 1996; Li et al., 1997; McIntosh et al., 1998) but similar to those of development (Rudnicki and Jaenisch, 1995; Yun and Wold, 1996).
The fine structure of satellite cells, positioned intimately between the fiber sarcolemma and the external lamina (Mauro, 1961; Ishikawa, 1966), changes during their transition from quiescence to activation. Nuclei enlarge and become euchromatic. The typical attenuated organelle-poor cytoplasm expands, and organelles such as mitochondria and rough endoplasmic reticulum hypertrophy (Schultz, 1976; Snow, 1977; Schultz et al., 1978, 1985). However, although activation is recognized as essential to repair and defined as precursor stimulation and recruitment to cycle (Bischoff, 1990a), the initial signal, timing, and character of activation are not known (Schultz and McCormick, 1994).
To date, the earliest indicator of satellite cell transformation during activation is the colocalization of hepatocyte growth factor (also called scatter factor; HGF/SF) with its receptor c-met shortly after injury in normal rat muscle (Tatsumi et al., 1998). In normal and regenerating muscle, satellite cells express c-met (Cornelison and Wold, 1997; Tatsumi et al., 1998) and m-cadherin (Moore and Walsh, 1993; Irintchev et al., 1994; Rose et al., 1994). Although HGF/SF also plays a role in differentiation (Gal-Levi et al., 1998), it is the activating agent in extracts from crushed muscle (Tatsumi et al., 1998). Thus, the shift of HGF/SF from the periphery of the intact fiber to satellite cells means that activation follows soon after muscle damage.
Other observations indicate that the activation signal is transmitted along fibers from the site of direct injury. After segmental damage, satellite cells proliferate and fuse to form new myotubes both adjacent to the injury (Grounds and McGeachie, 1987) and also at some distance from the injury near the ends of fibers (Klein-Ogus and Harris, 1983; Schultz et al., 1985; Bischoff, 1990b; Grounds et al., 1992; McIntosh et al., 1994; McIntosh and Anderson, 1995). Satellite cells are also activated without trauma and make DNA after exercise, training, stretching, cold, compression, hypertrophy, suspension, and denervation (Bischoff, 1986a,b; Darr and Schultz, 1987; Appell et al., 1988, 1989; White and Esser, 1989, 1990b; Snow, 1990; Winchester et al., 1991; Buonanno et al., 1992; Alway, 1997). Therefore, multiple signals initiate or mediate activation. Nonetheless, it is clear that DNA synthesis some 24–30 h after injury is a delayed index of previous and completed satellite cell activation.
A novel insight linking biophysical shear, muscle structure, fiber hypercontraction in injury, and the rapid shift of HGF/SF to its receptor suggested the idea that nitric oxide (NO) release from fibers may mediate satellite cell activation. NO is a very small, freely diffusible, and ubiquitous molecule produced constitutively at high levels in muscle by neuronal nitric oxide synthase (NOS-Iμ) (Nakane et al., 1993; Kobzik et al., 1994; Silvagno et al., 1996). NOS-Iμ is complexed at its N terminus to α1-syntrophin, which, in turn, is linked to the dystrophin cytoskeleton, especially in fast-twitch fibers; in dystrophic muscles without dystrophin, NOS-Iμ is reduced and displaced to the cytoplasm (Brenman et al., 1995, 1996; Chang et al., 1996; Chao et al., 1996; Grozdanovic et al., 1996; Wakayama et al., 1997; Hemler, 1999). NO is also made constitutively by NOS-III and by inducible NOS-II activity and transduces signals in vascular endothelium and smooth muscle, brain, and liver. NO is the subject of exciting new ideas of pathophysiology (Kanner et al., 1991; Lowenstein and Snyder, 1992; Palmer, 1993; Lowenstein et al., 1994; Schmidt and Walter, 1994; Garthwaite and Boulton, 1995; Wang et al., 1995; Kröncke et al., 1997; Gossrau, 1998; Reid, 1998). Critical controls on NO action are imposed by the biophysical properties of a tissue. Importantly, NO release is also regulated by mechanical forces such as shear, which is produced by pressure in a structure when its layers shift laterally across one another (Rubanyi et al., 1986; Nathan and Xie, 1994; Busse and Fleming, 1998; Chien et al., 1998). Gradients and contours of NO concentration signal to nearby cells (Lancaster, 1994, 1997), whereas hemoglobin heme acts as a huge sink to neutralize NO (Beckman and Koppenol, 1996).
Because satellite cells are intimately contoured to fibers and often stay attached to the external lamina as the sarcolemma buckles after injury (Schultz and McCormick, 1994), they are ideally positioned to be “first responders” to a shear-induced release of NO from the subjacent NOS-Iμ. In the present experiments, the release of myogenic cells from single crush-injured muscles was used as an index of the collective processes in muscle. It was reasoned that activation would increase the harvest of myogenic cells from a single muscle by reducing their adhesion to fibers and lamina and would also affect subsequent muscle repair. Experiments were carried out in normal mice pretreated to inhibit or augment NOS activity, and one tibialis anterior (TA) muscle was subjected to crush injury 30 min later. Cell yields from injured and undamaged muscles were determined for 30 min immediately after injury, and longer-term repair was also examined. Muscle in mdx mice lacks subsarcolemmal NOS-Iμ and shows rapid repair and precursor cycling (McIntosh et al., 1994; McIntosh and Anderson, 1995; Pernitsky and Anderson, 1996), whereas NOS-I knockout mice have complete loss of NOS-I expression (Huang et al., 1993). Therefore, the effects of low or absent NOS expression (similar in outcome to NOS inhibition) on cell yield in mdx and NOS-I knockout mice, and on mdx satellite cells and muscle repair, were examined. The rapid activation of satellite cells by injury, shown by increased myogenic cell release and morphological changes, was delayed by NOS inhibition induced pharmacologically by Nω-nitro-l-arginine methyl ester and by primary and secondary defects in NOS-I gene expression. Activation was transiently observed with a slower time course in intact contralateral muscles, and NOS inhibition negatively affected muscle regeneration.
MATERIALS AND METHODS
In all experiments, 6- to 8-wk-old male normal mice (C57BL/6 and B6129SF; Jackson Laboratories, Bar Harbor, ME), mdx mutant mice (C57BL/10 ScSn; Central Animal Care Services, University of Manitoba), and NOS-I knockout mice (B6129S-Nos1tm1Plh; Jackson Laboratories) were treated double blind and in accord with the guidelines of the Canadian Council on Animal Care (reference No. R-99-003). Studies were designed to determine the effects of manipulating NOS activity on the number and myogenic nature of cells isolated from muscles with and without injury and to examine the longer-term effects of NOS inhibition.
NOS activity was influenced by an intraperitoneal injection (80–100 μl by Hamilton syringe) exactly 30 min before crush injury to the right TA muscle of mice rested for at least 1 week after transport from the breeding facility. Mice were injected with saline or saline containing one of three drug treatments as follows: the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 7.5, 10, or 15 mg/kg), the NO donor l-arginine (l-Arg; 225 mg/kg), or combined l-NAME (7.5 mg/kg) plus l-Arg. Fifteen minutes later, animals were anesthetized (intraperitoneal ketamine:xylazine). The crush injury was delivered to the right TA muscle (RTA) with the use of a hemostat clamp closed for 3 s (McIntosh et al., 1994). Skin was held closed or sutured for longer recovery (see below). The time-course study from 0 to 30 min after injury was completed in 1 d for each treatment group, treatments were coded, and each set of experiments was carried out by the same individual(s).
The time course of treatment effects was determined at two intervals: during the early response 0, 5, 10, and 30 min after injury, and over the longer term after 6 d of recovery. Short-term experiments were repeated at least twice. The animals used in longer-term experiments were maintained on either plain drinking water or water containing fresh l-NAME at 12.5 mg/100 ml (30 mg·kg−1·d−1), based on an intake of 6–7 ml/d per mouse (McIntosh et al., 1994). In the longer-term-repair studies, there were four normal mice in each saline- and l-NAME–treated group. An additional two normal and two mdx mice were injected once before injury with l-NAME and given plain water for 6 d.
Tissues were harvested rapidly within 1–2 min after cervical dislocation under anesthesia. Whole muscles were carefully dissected from animals in the following order: RTA, left TA (LTA), left extensor digitorum longus (LEDL), left soleus (LSOL); and right soleus (RSOL); TAs and RSOL were then weighed. Muscles were used to determine cell yield or embedded for cryosectioning (7 μm thick) to examine morphology.
Cell yield was determined immediately after tissue collection. Satellite cells from RTA, LTA (representative fast-twitch muscles), and RSOL (a representative slow-twitch muscle) were isolated by standard procedures (Allen et al., 1998) modified for brevity and to collect only the cells available for harvest after a short digestion. Briefly, connective tissue was removed, and muscles were minced to a slurry in PBS and digested for 1 h (37°C) in 1 ml of 0.125% protease XIV (Sigma, St. Louis, MO) with inversion every 15 min. Samples were triturated for 1 min, and enzyme action was stopped by adding 10 ml of growth medium (DMEM containing 15% FBS, 1% antimycotic, 0.5% gentamicin, and 2% chick embryo extract; Life Technologies/BRL, Grand Island, NY). Cells were pelleted by centrifugation (1500 × g for 4 min), and the supernatant was discarded. Cells were resuspended in 15 ml of warm PBS, filtered through Nitex gauze, and centrifuged (1500 × g for 4 min). The pellet was resuspended in 500 μl of sterile PBS. A 100-μl aliquot of cell suspension was diluted in 10 ml of isotone for Coulter counting. The number of cells isolated per muscle (cell yield) was calculated and plotted over time. In three preliminary experiments, cells were counted with the use of a hemocytometer to ensure that they were nucleated cells and not isolated myonuclei or red blood cells.
To characterize the cell yield from each muscle, remaining cells were plated on 35-mm Petri dishes precoated with polylysine and fibronectin and cultured in growth medium for 1–5 d under 95%:5% CO2:O2 at 37°C. Some cultures were incubated for the final 30 min with bromodeoxyuridine (BrdU; 1 mg in 2 ml of medium) to label DNA synthesis. After washing in PBS, cells were fixed (10 min) in 1% paraformaldehyde in PBS and blocked (10% horse serum plus 1% BSA in PBS) before routine immunostaining (Tatsumi et al., 1998) with the use of antibodies against BrdU (diluted 1:1000; Sigma) or c-met receptor protein (diluted 1:400; Santa Cruz Laboratories, Santa Cruz, CA). Negative control slides were incubated in blocking solution without primary antibody. Appropriate peroxidase-conjugated secondary antibodies (diluted 1:250–1:400) and 3,3′-diaminobenzidine tetrahydrochloride/nickel chloride visualization (Dimension Laboratories, Mississauga, Ontario, Canada) were used to determine the relative myogenicity (c-met+ staining) and level of proliferation (BrdU+ staining).
In the same experiments (n = 8 animals, repeated twice), the LSOL and LEDL were embedded for cryosectioning to monitor the effects of treatment or remote injury on tissue histology, as visualized by fresh hematoxylin and eosin (H&E) staining and immunostaining for c-met and m-cadherin (see below).
In the longer-term study of NOS inhibition during repair, saline- and l-NAME–treated normal mice recovered for 6 d. Two mice per group were injected 2 h before being killed with BrdU (1.6 mg intraperitoneal in 0.4 ml of saline), and sections were immunostained with the use of anti-BrdU antibodies as described above.
A separate experiment was conducted to study the immediate effects of injury on muscle and satellite cell histology. LTA and RTA were collected immediately at 0 and 10 min after crush from normal mice after saline or l-NAME pretreatment (total n = 4). Muscles were bisected longitudinally, and half of each muscle was frozen in Tissue Tek optimal cutting temperature (Miles Scientific, Elkhart, IN) for cryosectioning. Sections were stained with H&E or immunostained with the use of primary antibodies to c-met (1:400), HGF/SF (1:1000; R&D Systems, Minneapolis, MN), or developmental myosin heavy chain (devMHC) (1:250; Novocastra Laboratories, Newcastle-upon-Tyne, United Kingdom) as reported (Pernitsky et al., 1996; Tatsumi et al., 1998) or against m-cadherin (1:50; Santa Cruz Laboratories). The other half of each muscle was fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.35, postfixed in osmium tetroxide, and embedded in methacrylate resin. Sections (0.5 μm thick) were collected on glass slides and stained with toluidine blue. The inhibition of NOS enzyme activity by l-NAME treatment 30 min before crush was confirmed with the use of NADPH-diaphorase enzyme histochemistry with jejunal epithelium as the positive control, according to Beesley (1995).
Sections and cultures were viewed on an Olympus (Tokyo, Japan) microscope equipped with epifluorescence and phase-contrast optics. Observations were based on systematic viewing of two to four longitudinal sections per muscle (separated by >100 μm). In the case of muscle regenerating from crush injury, observations (without knowledge of treatment group) were made in preset fields of muscle from the central crush region, the adjacent regenerating region, and the surviving region, as reported (McIntosh et al., 1994). Representative photographs of muscle fibers and satellite cells were taken on >700 frames of ASA 400 Fuji (Tokyo, Japan) Sensia slide film. Where stated, the number of satellite cells observed in each category, group, or condition was estimated from photographed slides rather than from direct counts made during observations under oil immersion. Selected slides were scanned (Olympus ES-10 film scanner), formatted into plates with little or no enlargement, and printed (Freehand 8.0, Macromedia, San Francisco, CA).
RESULTS
Effects of NOS Manipulation in Normal Muscle
The myogenic nature of cells isolated from muscles in the 0- to 30-min time course was confirmed by counting the proportion of c-met+ cells 12–24 h after plating. Myogenic cells formed the large majority of cells isolated from the normal LTA (83–94%) and RTA (86–92%) muscles (n = 997 cells). After 24 h in culture, cells were typically round or elongated, and 10–25% had nuclei that were intensely positive for BrdU incorporation. After 4–5 d in culture, dark c-met staining was present in single cells and in small multinucleated myotubes. Cultured cells from different treatments, recovery times, and muscles were identical in appearance despite differences in cell yield (see below).
Muscle weight as a proportion of body weight (Figure 1, A–D) was used to monitor edema secondary to tissue damage. The weight of muscles dissected from saline-treated normal mice showed a 10–15% increase in RTA over LTA that began immediately after injury. During l-NAME treatment, RTA weight increased only at 10 min relative to LTA weight, whereas l-Arg and combined l-NAME plus l-Arg treatment produced little or no change in muscle weight profile. Because the profile of RTA weight differed over time and among the four normal treatment groups, cell yield was expressed as cells per muscle, based on the assumption that LTA and RTA in one normal animal have similar-sized populations of myogenic precursors. Other observations made during tissue collection suggested that RTA hemorrhage at the crush site in the l-NAME–treated animals appeared later and at 30 min was subjectively more pronounced than in the other three groups.
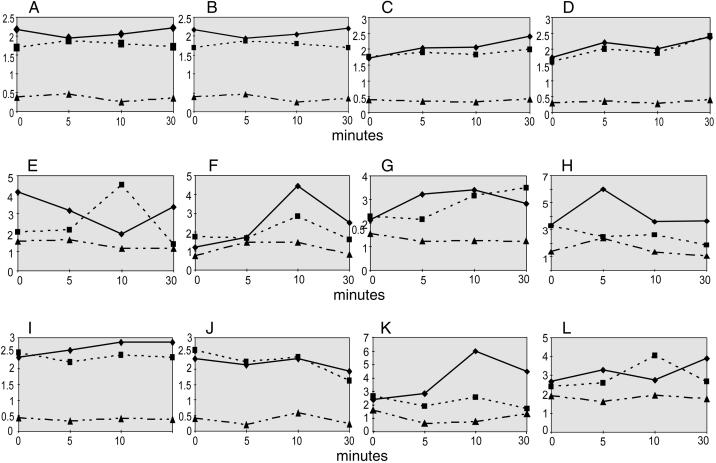
Figure 1.
Representative graphs from one experiment each on normal control (C57BL/6) (A–H) and mdx mice (I–L). Panels show the time course of changes in muscle weight to body weight (mg/g) (A–D, I, and J) and cell yield (cells/muscle × 105) (E–H, K, and L) in three muscles (RTA [♦], LTA [▪], and RSOL [▴]) for groups of mice treated 30 min before injury with saline (A, E, I, and K), l-NAME (B, F, J, and L), l-Arg (C and G), or l-NAME plus l-Arg (D and H). Data from the same animals are represented for muscle weight and cell yield. In normal mice, the immediate increase in RTA yield in saline-treated animals was absent after l-NAME treatment, and the transient increase in LTA yield at 10 min was reduced by l-NAME treatment. In mdx mice, there was no immediate increase in RTA yield above the LTA basal level after injury, whereas at 10 min RTA yield was increased. l-NAME treatment in mdx mice prevented the increased RTA yield at 10 min.
Cell yield in the time course 0–30 min after injury in normal mice changed dramatically with treatment and differed between LTA and RTA (and RSOL) muscles (Figure 1, E–H). After saline treatment, the LTA released 2.0 × 105 cells at 0 min (herein referred to as basal LTA level). In marked contrast, the crushed RTA from the same mouse yielded twofold more cells at 0 min. The RTA cell yield decreased briefly from 5–10 min and then increased again. Surprisingly, at 10 min the LTA yield doubled over the basal yield (LTA at 0 min) and then declined below the basal yield by 30 min. The yield from RSOL (an uninjured slow-twitch muscle ipsilateral to RTA and included for comparison with fast-twitch TA) was lower than the yield from LTA on a per muscle basis (although it was twofold to sevenfold higher when expressed as cells per milligram) and did not change during the 30-min time course. The data compiled from three repeat experiments on normal mice treated with saline (including C57BL/6 and B6129SF mice) are presented as the ratio of cell yield in RTA/LTA (mean ± SEM) in Figure 2 and demonstrate the consistent large immediate increase in cell yield at 0 min.
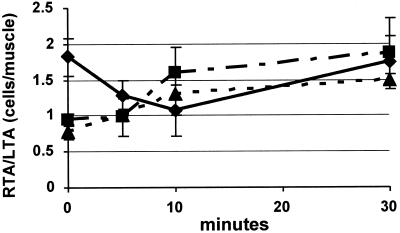
Figure 2.
Time course of cell yield (cells/muscle) expressed as the ratio of RTA to LTA (mean ± SEM) for normal mice (C57BL/6 and B6129SF, three experiments, ♦), normal mice treated with l-NAME (C57BL/6, three experiments, ▴) and “NOS mutant” mice including mdx and B6129S-Nos1tm1Plh (NOS-I knockout mice, three experiments, ▪). Satellite cell activation (cell yield ratio of RTA to LTA) in normal mice begins at 0 min and is significantly greater than in mice with NOS inhibition as a result of pharmacological treatment (by l-NAME), a primary gene defect (NOS-I knockout mice), or secondary to dystrophin deficiency (mdx mice).
l-NAME treatment (7.5 mg/kg) substantially changed the time course of cell yield, preventing the initial injury-induced increase in RTA yield (Figure 1F) and delaying the increased cell yield until 10 min after injury. The yields from LTA and RSOL were lower at 0 min than in the saline-treated mice (15 and 50%, respectively). Notably, 30% fewer cells were isolated at 0 min from RTA than LTA. By 10 min, yields from RTA and LTA were higher (3.5 and 2-fold, respectively) than at 0 min. By 30 min, cell yield from both RTA and LTA had decreased once again. This time course was consistent, as shown by a plot of relative cell yield (RTA/LTA ratio, mean ± SEM) from three experiments on normal muscle (Figure 2), demonstrating the prevention of immediate cell release from RTA relative to LTA at 0 min. l-NAME treatment at flanking doses indicated that the delayed peak RTA yield could be shorter (3 mg/kg) or longer (10 or 12.5 mg/kg) than 10 min after injury (one experiment at each dose) without the high RTA yield at 0 min. Interestingly, although peak cell yield in RTA was delayed (not reduced) by l-NAME, the peak cell yield in LTA (at 10 min) was reduced but not delayed after l-NAME treatment.
In the time course of cells isolated from mice treated with l-Arg, the basal yield in LTA was similar to that after saline, then increased and stayed high until 30 min. The RTA yield increased sharply at 5 min and also stayed high. RSOL counts were unchanged.
Combined treatment with l-NAME and l-Arg increased the yield in LTA and RTA by 50% at 0 min compared with saline-treated mice. Although LTA yield gradually decreased over 30 min, RTA yield at 5 min was the highest yield observed in a normal TA (6.0 × 105 cells/muscle) and decreased again by 10 min. The RSOL yield after combined treatment showed the only changes of any group of normal RSOL muscles. A sharp 80% increase between 0 and 5 min after RTA injury was followed by a decrease to the level at 0 min.
Effects of NOS Inhibition in mdx Dystrophic Muscle versus NOS-I Knockout Muscle
The myogenic proportions of cells isolated from mdx muscles were very high in LTA and RTA (95 and 96% respectively, 295 cells counted) and likely included both satellite cells from fibers and myoblasts from the interstitium of dystrophic muscles. Muscle weight as a proportion of body weight had a different profile in mdx and normal mice (Figure 1I). RTA weight increased later (after 5 min) and was maintained for 30 min in saline-treated mdx mice, whereas l-NAME abolished the increase in RTA weight for 30 min (Figure 1J). During tissue collection, mdx RTA muscles were subjectively less hemorrhagic after l-NAME than after saline treatment.
The time course of cell yield from saline-treated mdx mice (Figure 1K) showed five major distinctions from that in saline-treated normal mice and more closely resembled the profile of the normal muscle yield after NOS inhibition. First, the basal level of LTA yield was ∼30% more in mdx mice than in normal mice. In mdx mice, RTA yield did not show an immediate increase at time 0. Instead, counts for LTA and RTA were similar. Over 10 min, the RTA yield doubled and then leveled off somewhat. The cell yield in LTA did not change over time, whereas RSOL yields decreased by half from 0 to 10 min.
The cell yields in LTA and RTA from l-NAME–treated mdx mice at 0 min were similar to the yields from saline-treated mdx mice and again higher than yields from normal mice (Figure 1L). LTA yield increased by 50% at 10 min and returned to basal yield by 30 min (as in normal mice after l-NAME). RTA yield increased slowly during the 30-min time course. After l-NAME, the cell yield from mdx RSOL did not change over time after l-NAME. Thus, NOS inhibition in mdx mice increased cell yield in uninjured LTA. NOS inhibition also decreased and further delayed the peak of myogenic cells isolated from the injured mdx RTA muscle compared with saline treatment in mdx mice.
NOS-I knockout mice showed a time course of cell yield from LTA, RTA, and RSOL that was very similar to that in mdx mice (summarized in Figure 2; three experiments, pooled data from mdx and NOS-I knockout mice). The time course of cell yield in mdx and NOS knockout mice, expressed as the ratio of RTA/LTA yields, showed no difference from the profile of cell yield in l-NAME–treated normal muscle. The immediate increase in cell yield in RTA of normal mice was absent in RTA muscle of both mdx and NOS-I knockout mice.
Effects of NOS Inhibition on Early Muscle and Satellite Cell Responses to Injury
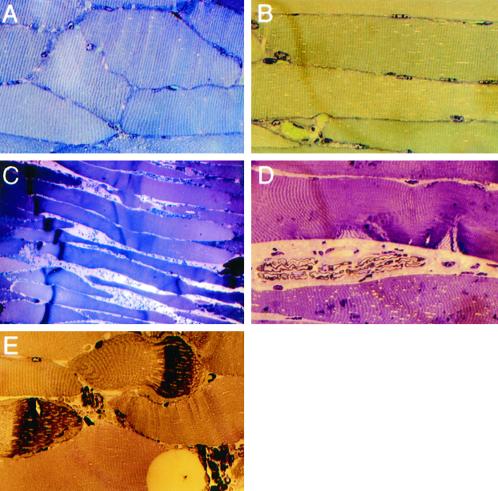
All the RTAs collected immediately after injury showed a crush site at 0 min that was very similar to uncrushed muscle (Figure 3; n = 4). After only 10 min, however, overt microscopic damage was present in the crushed region of all RTA sections of both saline- and l-NAME–treated mice, including transverse bands of fiber hypercontraction, delta lesions, and empty or disrupted external lamina directly at the crush site.
Figure 3.
Representative effects of crush injury in normal muscle at 0 min (A and B) and 10 min (C–E) after injury and after saline (A, B, and E) or l-NAME (C and D) pretreatment. (A) LTA section shows normal undamaged muscle. (B) RTA section at 0 min after crush injury. (C) At low magnification, a dark band of hypercontraction in fiber segments (to the left) and extravasated blood cells between fibers appear across the muscle belly at 10 min in a saline-treated animal. Fibers are thin and retracted to the right of the hypercontracted region. (D) Two delta lesions in a fiber after l-NAME treatment and 10 min after crush. (E) Higher-magnification view of muscle 10 min after injury showing extravasated blood cells between hypercontracted and retracted fiber segments and segments with early sarcomere disruption. Original magnification in A, B, D, and E ×132; in C, ×33.
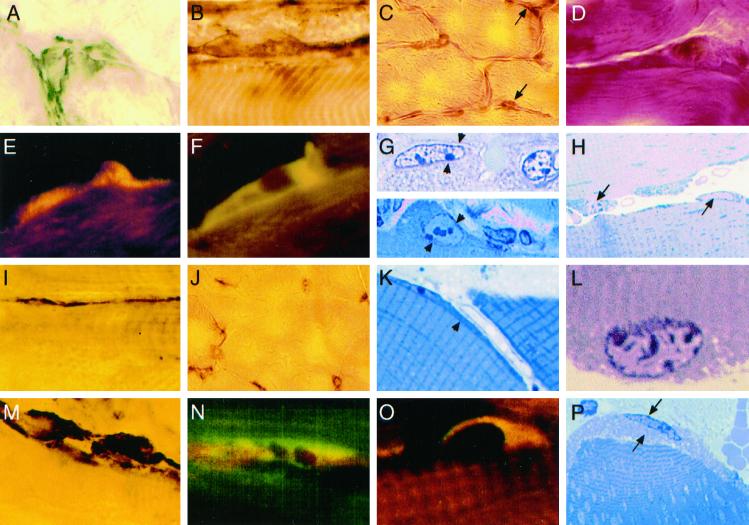
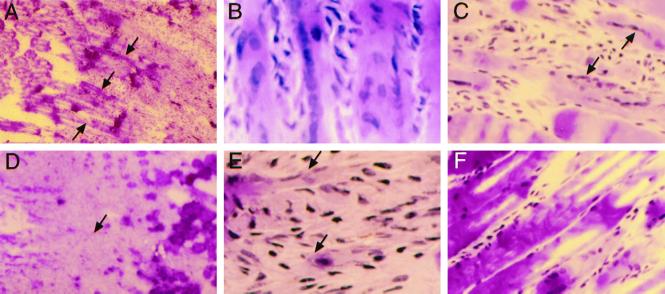
Histology and immunostaining showed that normal rapid changes in satellite cell size and position were consistently delayed and restricted after l-NAME treatment (Figure 4; n = 10–12, except n = 2 per group for resin sections). NADPH-diaphorase staining experiments on sections from the same mice confirmed that pretreatment with l-NAME inhibited NOS activity, detected as a thin outline located just inside the sarcolemma of all muscle fibers from saline-injected animals. The identity, position, and configuration of ∼300 satellite cells observed on fibers were confirmed by m-cadherin and c-met staining. M-cadherin was interposed between fibers and all satellite cells observed in undamaged muscle, and typically surrounded large satellite cells on fibers in saline-treated RTA at 0 and 10 min (Figure 4A). At 10 min, large m-cadherin+ cells were very often observed on the empty external lamina sheaths present after fiber retraction (Figure 4B). Interestingly, satellite cells were easily visible on nearly every fiber by H&E staining at 0 min at the RTA fiber periphery (Figure 4C) and were often prominent in the RSOL, LEDL, LTA, and RTA at 10 min. They contained large vesicular nuclei and many crimson cytoplasmic granules, likely mitochondria (Figure 4D). After saline treatment, c-met staining of LTA at time 0 consistently showed typical attenuated satellite cells very close to fibers. However, RTA at 0 min showed many large satellite cells (∼50 of 65 satellite cells, and a higher proportion in the crushed regions) that were c-met+ and HGF/SF+ (Figure 4E) and that had a higher ratio of cytoplasm to nucleus than satellite cells in the contralateral LTA. Those enlarged satellite cells often bulged from the fiber contour even at a distance from the crush region in RTAs. The same features were more pronounced at 10 min after crush in RTAs (Figure 4F), although 15–20% of satellite cells were small and attenuated and positive for m-cadherin or c-met in their location on some undamaged fibers at the edge of the crushed region.
Figure 4.
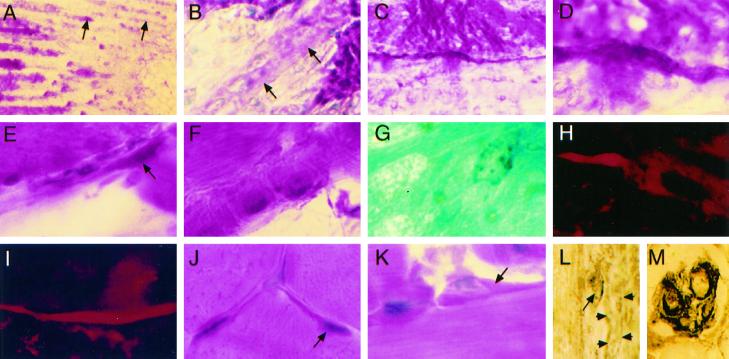
Satellite cell changes in vivo are delayed by NOS inhibition in normal mice treated with saline (A–H) or l-NAME (I–P). (A) M-cadherin outlines a large satellite cell at 0 min after injury. (B) Large m-cadherin+ satellite cell on the external lamina 10 min after injury. (C) H&E-stained satellite cells (arrows) in low-magnification RTA fibers at 0 min. (D) At high magnification, hypertrophic satellite cells on fibers in RSOL at 10 min. (E and F) Large satellite cell shows colocalized (yellow) staining for HGF/SF (Texas Red) and c-met (FITC) at 0 min (E) and 10 min (F). (G) Two resin sections (stained with toluidine blue) show large satellite cells (between arrowheads) at 0 min in RTA. (H) At 10 min in RTA, satellite cells (arrows) with granulated cytoplasm and euchromatic nuclei are partially lifting off adjacent fibers. (I) After l-NAME treatment, m-cadherin stains an attenuated satellite cell at 0 min in RTA. (J) Satellite cells are not prominent by H&E staining of RTA at 0 min. (K) Thin strips of cytoplasm and a contoured nucleus are probable satellite cells (at arrowhead) at the fiber periphery in resin sections. (L) At high magnification, a myonucleus in a resin section from RTA 10 min after injury shows a folded upper membrane near the contracted fibrils. (M) Ten minutes after injury, large m-cadherin+ satellite cells are adjacent to an unstained fiber. (N) At 0 min after injury, c-met (FITC) in satellite cells is not colocalized with HGF/SF (red). (O) A large satellite cell at 10 min after injury shows colocalization (yellow) of c-met (FITC) and HGF/SF (red) fluorescence. (P) A hypertrophic satellite cell (between arrows) is partly separated from an RTA fiber 10 min after injury. Original magnification, ×330 except in C and J (×132).
In contrast, in l-NAME–treated mice, the large majority of satellite cells (85% of >70 satellite cells identified with either m-cadherin+ or c-met+ staining) were thin and attenuated in both the RTA and LTA at 0 min (Figure 4I), were not prominent by H&E staining (Figure 4J), or were c-met+ but did not stain for HGF/SF (Figure 4N). However, by 10 min there were typically large m-cadherin+ satellite cells on many fibers in every section (Figure 4M), and c-met and HGF/SF were colocalized in at least 70% of satellite cells (25–30 were clearly observed per longitudinal section) bulging from fibers (Figure 4O) or at the external lamina. These features of cell enlargement and c-met/HGF colocalization were also less frequent after l-NAME treatment than saline treatment in observations made at the surviving ends of the RTA fibers not directly injured by the crush.
Resin sections showed details of more than 50 cells in the satellite position on fibers, later confirmed by electron microscopy as satellite cells and containing nuclei. Large satellite cells were present only at time 0 in the injured region of RTAs from saline-treated mice (Figure 4G). They were demarcated from the subjacent fibers, contained large vesicular nuclei with prominent nucleoli, and had many dark cytoplasmic granules identical to typical mitochondria between fibrils and at the fiber periphery (Figure 4G). By 10 min in RTA from a saline-treated mouse, the large satellite cells were often present and were observed lifting from fibers (Figure 4H). In comparison, nuclei and cells in the satellite position of LTA and in the RTA from an l-NAME–treated mouse at time 0 were typically thin and nearly agranular and their nuclei could seldom be distinguished from internal myonuclei because the cells were in tight apposition to fibers (Figure 4K). At 10 min after injury, many myonuclei inside fibers had a folded nuclear membrane on the aspect adjacent to hypercontracted fibrils (Figure 4L) and were easily distinguished from the smoothly contoured nuclei of enlarged satellite cells at the fiber periphery (Figure 4P).
Effects of NOS Inhibition on Longer-Term Repair in Normal Muscle
After 6 d of recovery from injury, normal mice treated with saline injection and plain drinking water (n = 4) had RTAs with small characteristic central necrotic crush sites flanked by small myotubes (Figure 5, A–C). In systematic observations of adjacent and surviving regions (McIntosh et al., 1994) of four different sections per muscle, adjacent regions contained many mononuclear cells and capillaries between the long myotubes. Surviving tissue at the ends of RTA contained fibers interspersed or continuous with new myotubes. Many mononuclear cells (more than half of 20–30 satellite cells clearly identified per section) stained for both c-met and HGF/SF, whereas myotubes did not stain for either protein.
Figure 5.
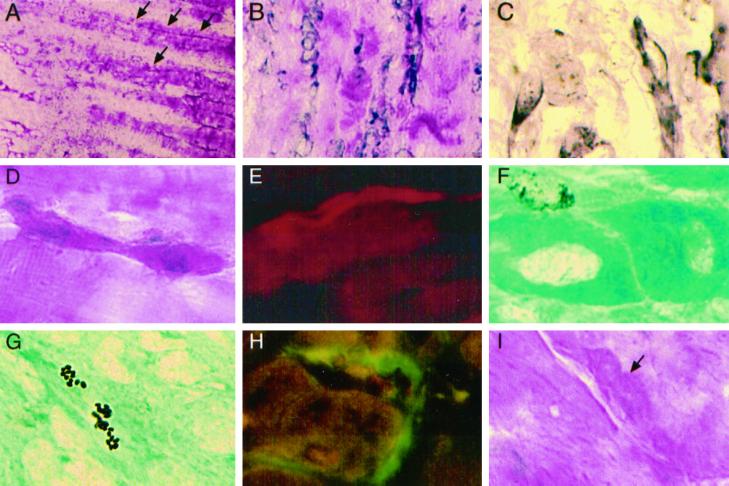
l-NAME treatment for 6 d reduces normal muscle regeneration. (A) At low magnification (H&E), normal muscle repair after saline pretreatment includes a small necrotic crushed region (to the right), a region of adjacent mononuclear cells and myotubes (arrows), and surviving fiber segments (to the left). (B) New myotubes in the adjacent region contain many central nuclei and eosinophilic sarcoplasm after 6 d of regeneration. (C) New myotubes (arrows) are also present among surviving fibers. (D) After continuous l-NAME treatment for 6 d, the necrotic area (to the left) and the adjacent region of mononuclear cells are enlarged, and a few myotubes (arrow) are present. (E) Among mononuclear cells in the adjacent region, new myotubes (arrows) are thin and contain immature, basophilic cytoplasm. (F) Very few myotubes are found between surviving fiber segments at the ends of the RTA. Original magnification in A and D, ×13; in B, C, E, and F, ×132.
In contrast, muscle regeneration was reduced by exposure to l-NAME during the 6 d of repair (n = 4) (Figure 5, D–F). Outside a large central crush site, persistent necrotic fiber segments contained macrophages and some calcified fiber segments that were infrequent in RTAs of saline-injected mice. Many mononuclear cells surrounded the thin basophilic (immature) myotubes, which, in addition, were seen at much lower density per field compared with myotubes in similar RTA fields from saline-treated mice. New myotubes were also infrequent among surviving fibers at the ends of RTA after l-NAME treatment. The prevalence and size of new myotubes were confirmed by devMHC-positive immunostaining.
Interestingly, a single injection of l-NAME before injury had also produced subtle effects on muscle regeneration after 6 d (n = 2) (Figure 6). RTAs had small remnant crush lesions, mononuclear cells in the adjacent regions, and numbers and size of myotubes similar to normal regenerating muscle, and many mononuclear cell nuclei were BrdU+ (Figure 6G). However, in regions of surviving segments, large cells in the satellite cell position (m-cadherin+) had granular cytoplasm and were connected directly with long, thin myotubes while still resident on fibers within the external lamina. This feature was observed at least once in every ×40 field containing surviving fiber segments in the region adjacent to the crush site. A small number (estimated at 5–10%) of myotubes appeared to be incompletely fused blocks of eosinophilic (Figure 6F) or devMHC+ cells, especially notable with phase-contrast optics. New, small devMHC+ myotubes were continuous with larger myotubes formed since the injury (Figure 6H) or were located among mononuclear cells adjacent to the injury site. Four m-cadherin+ satellite cells were seen on new myotubes (Figure 6L). Satellite cells were always very intensely stained in the spindle fiber complexes (Figure 6M), and in undamaged EDL or SOL from the same mice, satellite cells were very prominent and intensely eosinophilic on many fibers (Figure 6, J and K).
Figure 6.
A single l-NAME injection before injury affects myogenic repair in normal muscle. (A) At low magnification (H&E), the RTA 6 d after injury shows a large necrotic region (to the right), an adjacent area of mononuclear cells and small new myotubes (arrows), and surviving fiber segments (to the left). (B) At high magnification, a myotube (arrows) extends between mononuclear cells and a fiber segment. (C) A very thin intensely eosinophilic myotube originates immediately beside a surviving fiber segment. (D) At higher magnification, the same myotube has formed from the satellite cell position, apparently inside the external lamina. (E) An eosinophilic satellite cell (arrow) is elongated into a thin myotube. (F) A column of apparently unfused centrally nucleated cells with granular cytoplasm makes up a myotube. (G) A BrdU+ nucleus adjacent to a new myotube. (H and I) Thin new myotube segments are positive for devMHC (Texas Red fluorescence) whether they extend from a larger myotube (H) or are located among mononuclear cells near the crush (I). (J) A crimson satellite cell (arrow) on an EDL fiber. (K) A large satellite cell (arrow) with crimson cytoplasm on a SOL fiber. (L) M-cadherin is present between a satellite cell (arrow) and a small new myotube (arrowheads). (M) M-cadherin staining is intense on satellite cells located on the four intrafusal muscle fibers in a spindle complex. Original magnification in A, ×13; in C, ×33; in B, E, and M, ×132; in D and F–L, ×330.
Effects of NOS Inhibition on Longer-Term Repair in Dystrophic Muscle
A single l-NAME injection also produced subtle changes in regenerating mdx muscles during 6 d (n = 2) (Figure 7). In regenerating muscles of l-NAME–treated mdx mice, many large new myotubes extended from a small necrotic crush site through the adjacent region and between the fiber segments that survived the injury (Figure 7A). More large myotubes were present compared with normal regenerating muscle, as reported previously for mdx mice (McIntosh et al., 1994; McIntosh and Anderson, 1995), and many satellite cells, elongated mononuclear cells, and new myotubes were m-cadherin+ (Figure 7C). In one field, a binucleate satellite cell was lifted off the fiber sarcolemma (Figure 7D). DevMHC was expressed by new myotubes (Figure 7E), and BrdU+ nuclei were found in nearby mononuclear cells and in some muscle precursor cells close to surviving fiber segments and new myotubes (Figure 7F). Three fields of regenerating muscle (in the two animals) also contained small collections of intensely BrdU+ nuclear fragments in myotubes (Figure 7G). As in normal mice treated once with l-NAME, satellite cells (outlined by m-cadherin) were observed in continuity with the new myotubes that were anchored inside external lamina sheaths on remnant fiber segments (roughly 5% of new myotubes). Satellite cells in mdx LTA were very large and c-met+ (Figure 7H), as were satellite cells in NOS-I knockout LTAs, although their extensive cytoplasm was not as granulated or as distinct from fiber sarcoplasm as in normal undamaged muscles after l-NAME treatment (Figure 7I; compare with Figure 6, J and K).
Figure 7.
A single treatment with l-NAME 30 min before injury affects dystrophic muscle regeneration. (A) Low magnification (H&E) shows a large crush region (just to the left), an adjacent region of new myotubes (arrows), and surviving fiber segments (to the right). (B) Many large new myotubes adjacent to the crush. (C) Elongated mononuclear cells and myotubes are m-cadherin+. (D) An elongated crimson cell is binucleate and located in the satellite position on a surviving fiber segment. (E) A new myotube extends from a surviving segment and contains devMHC (Texas Red fluorescence). (F) A BrdU+ nucleus next to new myotubes with unstained central nuclei. (G) A new myotube contains apoptotic BrdU+ nuclear fragments. (H) Large c-met+ satellite cells (FITC) on HGF/SF+ fibers (Texas Red) in LTA. (I) A large satellite cell (arrow) with granular cytoplasm (H&E) on an LEDL fiber has less prominent margins than in undamaged normal muscles (see Figure 5, J and K). Original magnification in A, ×13; in B, ×130; in C–I, ×330.
DISCUSSION
The present results show that satellite cell activation occurs immediately upon muscle injury, is mediated by NO release, is briefly transmitted to distant muscles, and is prevented under pharmacological and genetic conditions that reduce the activity or expression of NOS-I. Time-course studies of myogenic cell yield and morphology showed two aspects of activation, namely altered adhesion and morphological changes. Before identifying HGF/SF as an activator of satellite cells, the nature of activation was elusive because it was studied with later markers, such as regulatory gene expression or DNA synthesis. The present demonstration in satellite cells of a rapid shift by HGF/SF to its “mitogenic and motogenic” receptor (Rong et al., 1994) upon activation confirms a previous report (Tatsumi et al., 1998). The disposition of satellite cells positive for both c-met and its ligand between fiber and laminar sheath suggested that the physical signal of injury was rapidly transduced from a fiber to activate its satellite cells. In vivo studies on myogenesis after injury demonstrated that pharmacological inhibition of NOS activity was detrimental to the outcome of muscle regeneration. Interestingly, two mutants with decreased or absent NOS-I expression showed enhanced activation in situations in which normal muscle is quiescent and showed very effective repair after an imposed injury. Together, these acute and chronic experiments strongly indicate a pivotal role for NO in transducing activation, satellite cell adhesion, and subsequent repair processes.
For the first time, the nature and possible impact of injury-induced activation were expressly addressed. Data showed that reduced NOS activity, by means of inhibition with l-NAME in normal muscle, from complete genetic loss of NOS-I expression (in NOS-I knockout mice), or secondary to dystrophin deficiency in mdx muscle, prevented the immediate increase in myogenic cells isolated from injured muscle. Rapid changes in the nuclear profile, cytoplasmic granularity, and ratio of cytoplasm to nucleus were consistent with the known hypertrophic alterations of satellite cells as they become activated and were also inhibited by l-NAME. That NOS inhibition thereby delayed and restricted injury-induced satellite cell activation, defined by changes in adhesion, cell yield, morphology, and expression in cells of two satellite cell markers, c-met and m-cadherin. Studies of noninjured normal muscles showed a surprising, albeit short-lived, increase in LTA cell yield after 10 min, coincident with hypertrophy of a large proportion of those satellite cells, and suggest that a circulating factor can at least transiently activate satellite cells in intact distant muscles. In regenerating muscle, longer-term NOS inhibition (by l-NAME in drinking water) delayed removal of debris, decreased the formation of new myotubes, and confined them closer than usual to the site of injury. Although more subtle changes in repair resulted from a single event of NOS inhibition at the time of injury, the appearance of fiber duplication inside the persistent external lamina on damaged fibers appeared to divert repair toward fiber branching. The apoptotic nuclear fragmentation (Blandino and Strano, 1997; Evan and Littlewood, 1998) that was present in regenerating muscles after briefly perturbing activation could reduce the number of nuclei in new fibers and potentially affect myotube domains (J. Kong and J.E. Anderson, unpublished observations) and the stability of repair. These data can now stimulate more focused examination of activation and the potential applications of NO manipulation.
A model is presented for the hypothesis that NO release, which is exquisitely responsive to shear in other systems (Traub and Berk, 1998; Dimmeler et al., 1999), mediates satellite cell activation by a similar mechanism (Figure 8). This working hypothesis broadens the field of NO signaling in muscle (reviewed by Grozdanovic and Baumgarten, 1999). The time course of satellite cell release and the onset of organelle hypertrophy were very rapid, occurring by 35–45 s after injury (the time from injury and death to collection and freezing of tissue was <2 min). To date, there are no other reports showing such rapid transduction of morphological or adhesion changes after injury or in repair. Normal cyclic loading of muscle produces pulsatile NO release (Tidball et al., 1998) by the rapid diffusion of NO down its concentration gradient and may maintain satellite cell quiescence. Thus, a large release of NO would move as a wave front across the narrow clefts between a fiber and its satellite cells. The subsequent lapse in pulsatile or bolus NO release would constitute the second phase of a powerful signal, a “nitric oxide transient” in physiological terms. Teleologically, the external lamina wrapping fibers may provide the potential for satellite cells to respond to shear between the sarcolemma and the lamina. Satellite cells hug fibers across an even 15 nm cleft without obvious junctional complexes, and they associate closely with external lamina (Bischoff, 1990a; Schultz and McCormick, 1994). Thus, satellite cells have the ideal topography to detect a rapid peak of NO release from underlying fibers after shear and also to be kept quiescent by normally continuous small pulses of NO from the fiber. The speed of the NO-mediated signal for activation suggests that the signal, such as mechanical shear forces, acts on constitutive NOS-I, because the response time is too short to induce expression or increase activity (McCall et al., 1991; Rubinstein et al., 1998). The effects of l-NAME on edema (and hemorrhage) were congruent with the known effects of NO on vascular tone (Busse and Fleming, 1998). Therefore, the time course of cell yield after injury and its change by NOS inhibition suggest that a large NO release mediates or directly signals activation. The transient decline in RTA yield at 10 min in saline-treated normal mice suggests that other signals are then needed to maintain or complete activation. The nature of those additional signals was suggested by the brief, delayed increase in LTA yield at 10 min in both saline- and l-NAME–treated normal mice. Because HGF/SF is released from crushed muscle and activates muscle precursors in vivo and in vitro (Tatsumi et al., 1998), HGF/SF or other factors may become activated themselves and circulate from RTA to initiate activation of satellite cells located outside the damaged muscle. Without injury or reinforcement in LTA, normal fibers would repress activation and their satellite cells would return to quiescence, whereas satellite cells in RTA would receive the secondary circulating signal on top of the damage-induced local changes and would complete the activation sequence. Combined treatment with a NO donor and a NOS inhibitor partly reversed the effects of NOS inhibition on RTA yield and prevented the temporary increase in LTA yield. Therefore, additional signals involved in fully activating precursor cells likely include both NO-mediated and NO-independent mechanisms.
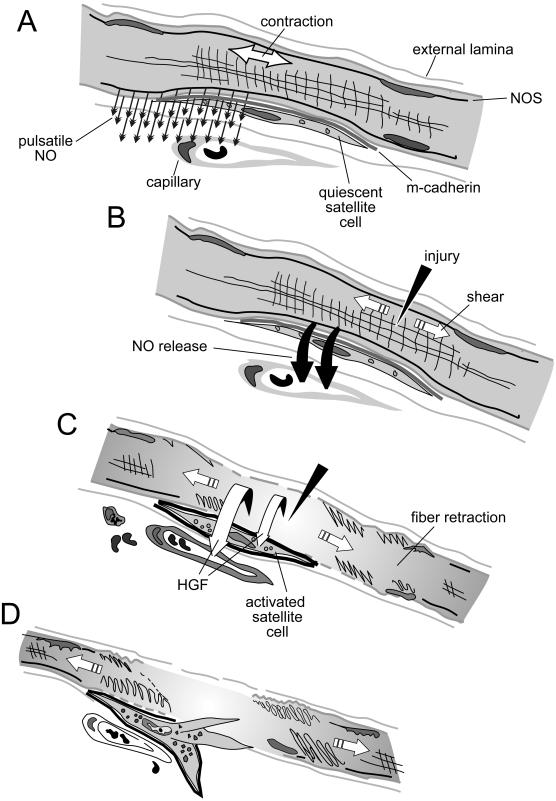
Figure 8.
A model for the process of shear-induced, NO-mediated events that activate satellite cells after skeletal muscle injury. (A) In undamaged muscle with normal contraction and relaxation, thin quiescent satellite cells are demarcated by m-cadherin and contain few organelles. They are interposed between the overlying external lamina and the sarcolemma of a subjacent fiber and are subject to pulsatile NO released from NOS-Iμ that is anchored to syntrophin. Normally, NO diffuses cylindrically out from the fiber to act on cells and enzymes in the interstitium or is neutralized by red cell hemoglobin in the vessels that wrap each fiber. (B) After sarcolemmal injury, depolarization is not followed by repolarization. A single large contraction produces intense shear between the fiber membrane and the external lamina. Shear induces a bolus release of NO that diffuses down its concentration gradient through the satellite cells hugging the fiber. (C) Satellite cells become activated and begin to enlarge as organelles such as mitochondria hypertrophy. HGF/SF from the damaged fiber is activated and shifts to the c-met receptor on satellite cells. Fibrils hypercontract and damaged segments retract within the external lamina, maintaining shear and NO release and activating satellite cells along the fiber length. The adhesiveness of m-cadherin decreases, and the damaged fiber releases proteins, including HGF/SF, to the interstitium. A released factor, such as HGF/SF, enters the circulation and can transiently activate distant satellite cells on undamaged muscles, although normal pulsatile NO release will mostly attenuate that response. Capillaries dilate and blood cells extravasate into the interstitium. (D) Fiber segments fully retract and satellite cells become motile precursors as HGF/SF binds to c-met. The external lamina remains as a scaffold for the satellite cells, now surrounded by less adhesive m-cadherin. The precursors may leave the fiber as the sequential expression of early immediate genes, muscle regulatory genes, proliferating cell nuclear antigen, and later DNA synthesis begin before proliferation.
NO-mediated satellite cell activation may account for recent findings by L. Hall-Martin, J. Morgan, and T.A. Partridge (personal communication). An isolated single intact normal fiber and attached satellite cells (∼10) was injected into mdx muscle. Results showed a 5000-fold more efficient production of dystrophin-positive fiber segments in mdx mice than myoblast transfer with the use of 5 × 106 cells, with equally wide-ranging dispersal. Although shear, produced by layers that shift laterally against each other, would be strong during segmental retraction within the external lamina, it would be very intense during fiber injection. Compared with myoblast transfer and according to the model, injection could maximize shear-induced satellite cell activation and supply crushed muscle extract containing HGF directly to the site of implantation. This hypothesis, therefore, can integrate diverse topics of NO physiology, mechanical force transduction, cell signaling, dystrophy, and repair. In that context, the gain of three magnitudes in repair outcome with the use of fiber injection reveals the huge potential for NO manipulation of satellite cell activation to dramatically improve muscle repair in health and disease. Transient precursor proliferation in denervation and persistent proliferation after trauma or segmental disease can be explained by applying the idea of NO-mediated, shear-induced satellite cell activation upon total synchronized nerve and fiber depolarization and then loss of membrane potential. Interestingly, intense m-cadherin+ satellite cells in muscle spindles suggest that high shear responsiveness may accompany the spindle function as a length-tension receptor. There is also a potential for NO interaction with m-cadherin in mediating loss of adhesion and normal quiescence during activation. The ratio of RTA/LTA of <1 at 0 min during NOS inhibition or decreased NOS-I expression (Figure 2) suggests that reduced NO after injury may mediate an increase in satellite cell adhesion to the fiber-lamina complex.
Until now, satellite cell activation was defined structurally as cytoplasmic and organelle hypertrophy and dynamically as recruitment to cycle. The close adherence of satellite cells to parent fibers must decrease during activation for satellite cells to move through the external lamina to form new fibers. Therefore the loss-of-adhesion feature was tested as a simple index of activation. The ability to isolate myogenic cells after brief standard digestion was a conservative estimate of available satellite cells and not an estimate of total myogenic cells. (Additional myogenic cells are found in the material collected on the Nitex filter during cell isolations.) NO is known to modulate leukocyte and platelet adhesion (Kubes et al., 1991; de Graaf et al., 1992), and m-cadherin mediates muscle precursor adhesion to fibers. So it is also possible that changes in adhesion during activation, and the m-cadherin molecule itself in repair, may be affected by NO. The present data also suggest that specifically manipulating satellite cell activation via changes in NOS-Iμ activity or shear, rather than giving systemic alkali dietary supplements to stimulate bone formation and indirectly stimulate muscle fibers (Landauer and Burke, 1998), could directly prevent muscle atrophy in microgravity.
The marked difference in activation time course between normal and mdx muscle is entirely congruent with the different locations of NOS-Iμ in the two types of muscle, as is the similarity between RTA/LTA yield ratios in muscles from mdx, NOS-I knockout, and l-NAME–treated normal mice. NOS-Iμ is subsarcolemmal and in mdx muscle is reduced and displaced to the cytosol as a result of the absence of dystrophin. Mdx muscle pathology was recently reported to be independent of NOS-I perturbation (Chao et al., 1998; Crosbie et al., 1998). The authors hypothesized that displaced NOS-I contributed free radical NO damage to the sarcoplasm of fibers and would exacerbate dystrophy. However, this idea was rejected, because total removal of NO by NOS-I knockout in mdx mice did not reduce dystrophy. An alternative explanation derives from the present data. Cytoplasmic NOS-I in mdx muscle would act as a diffuse areal source of NO rather than the nearby linear source, typically subjacent and parallel to satellite cells found in normal muscle fibers. The normally steep NO gradient across the cleft between fiber and satellite cell, therefore, would become more shallow and diffuse more slowly, and the small NO transient would be manifest as an attenuated responsiveness to shear forces. If normal pulsatile NO acts to maintain quiescence, a smaller gradient in dystrophy from the pulsatile NO of cytoplasmic origin could release mdx satellite cells from what is normally full quiescence and account for the greater proliferative activity and larger satellite cells in mdx muscle and primary cultures (McIntosh and Anderson, 1995; Pernitsky and Anderson, 1996; Moor et al., 2000). Rapid repair by mdx muscle is consistent with the notion that mdx satellite cells are partly activated or on “standby.” Likewise, it would follow that acute injury would not necessarily augment immediate activation, as reported here in cell yield studies for mdx and NOS-I knockout mice. By that reasoning, repair after imposed injury in the NOS-I × mdx double mutant should be less effective and/or delayed compared with mdx muscle repair. Dystrophy in that double mutant may be more severe than in mdx mice if it were assessed in younger mice (<12 mo) before the index of repair (central nucleation) has reached its theoretical plateau. In addition, because human fibers are larger than mdx fibers, cytoplasmic NOS-I in human fibers would serve as an even smaller nonlinear NO source than in mdx muscle. The resulting very shallow gradient or physiological NO transient across satellite cells could partly account for the severity of Duchenne dystrophy, almost as if the standby activation (like a “hair trigger”) contributes to overly enthusiastic successive repair events and early senescence (Decary et al., 1996, 1997). It is now clear that satellite cell activation needs to be considered separately from dystrophy.
Three observations are consistent with the cytosolic location of NOS-Iμ and the standing activation of mdx satellite cells. Hypertrophic c-met+ satellite cells are typical in mdx muscles without injury (Anderson, 1998; this study). Adult mdx muscle yields high-density myoblast cultures that rapidly begin to proliferate (Pernitsky and Anderson, 1996; Moor et al., 2000), and mdx muscle is more effective than normal in myogenic repair (Zacharias and Anderson 1991; McIntosh et al., 1994; McIntosh and Anderson, 1995; Pernitsky et al., 1996). The normal quiescence of mdx satellite cells should be restored along with subsarcolemmal NOS-I after mini-dystrophin gene transfer (Decrouy et al., 1998). The general inhibition of the delayed activation seen in injured and intact mdx muscles after l-NAME suggests that activation could still be modulated pharmacologically via NO, possibly in combination with deflazacort (glucocorticoid) treatment to improve repair (Anderson et al., 1996, 2000). Other studies of longer-term l-NAME exposure of regenerating mdx and normal muscle in vivo showed a high prevalence of branched myotubes (J.E. Anderson, unpublished data), emphasizing the distinct role of NO in fusion (Lee et al., 1994) separate from activation. Indeed, deflazacort itself may affect activation, because another glucocorticoid, dexamethasone, is a specific inhibitor of inducible NOS-II (McCall et al., 1991). Experiments are in progress to determine whether l-Arg can further augment mdx muscle satellite cell activation in vivo, as suggested by preliminary experiments on single-fiber cultures in vitro (O. Pilipowicz and J.E. Anderson, unpublished data). Other data demonstrated that regenerating muscle in NOS-I knockout mice (n = 2) had extensive myotube formation during 6 d after injury, similar to mdx mice (McIntosh et al., 1994; McIntosh and Anderson, 1995). Interestingly, NOS-I knockout mice also had modest focal myopathy (segmental muscle fiber damage and inflammation) in TA and diaphragm. That myopathy was not present in the control strain (B6129SF) and may relate to the absence of NOS-I expression in the nervous system or a constitutive heightening of satellite cell activation. Together, the mdx and NOS-I knockout experiments suggest that increased satellite cell activation from reduced or absent NOS expression may benefit myogenesis in the short term (and through a few cycles) by facilitating standing activation and precursor recruitment to cycle. However, in the longer term, that standby activation may be detrimental, such that mdx dystrophy may be reduced by increasing local (not systemic) pulsatile NO release in intact muscle fibers and the bolus of NO that activates satellite cells after fiber injury. We can now test whether human muscular dystrophy is more severe than mdx dystrophy as a result of even greater attenuation of the typical NO gradient through the larger fibers, overrecruitment of satellite cells, and accelerated precursor senescence.
NO has a broad impact on glucose uptake, insulin resistance, exercise, blood flow, and contractility (Balon and Nadler, 1994; Shen et al., 1995, 1997; Joyner and Dietz, 1997; Kapur et al., 1997; Chen et al., 1998; Young and Leighton, 1998). NO also mediates denervation responses, inflammatory myopathy, aging, and neuromuscular transmission (Tews et al., 1997a,b; Capanni et al., 1998; Ribera et al., 1998; Tews and Goebel, 1998). Therefore, the collective effects of NO have a significant impact on muscle pathophysiology. Although one study of rat muscle after crush injury showed that l-NAME could prevent traumatic shock by inhibiting NOS-II and NOS-III, no change in NOS-I was reported, and satellite cells and repair were not examined (Rubinstein et al., 1998).
A surprise from the present experiments was the observation of short-lived satellite cell activation in normal undamaged fast-twitch muscle, according to the dual criteria of hypertrophy in vivo and loss of adherence in cell yield studies. The consistency of the findings was emphasized by comparison with cell yield studies in the slow SOL muscle that expresses less NOS-Iμ (Kobzik et al., 1994). Although the idea that circulating HGF/SF can activate satellite cells in intact muscle needs to be tested, it recalls a report that serum collected after partial hepatectomy-induced shear will stimulate proliferation of liver cells (Wang and Lautt, 1999), which stain intensely for c-met (J.E. Anderson, unpublished observations). Distant activation may also involve NO interactions, possibly with a converting enzyme that activates HGF or other factors (Lowenstein et al., 1994; Miyazawa et al., 1996). The complex pharmacology of NOS (Nathan and Xie, 1994; Reid, 1998) suggests that careful trials to sustain the activation in undamaged normal muscle and attenuate it in dystrophic muscle are needed. However, the data for uninjured muscles suggest that manipulation of NO (through combinations of increased and decreased NO) holds a tantalizing potential to prevent or treat muscle atrophy (as from disuse, age, or zero gravity) and to promote hypertrophy and new fiber growth (as in meat production, athletic training, and animal racing) in otherwise healthy muscle. They also suggest that activation may require an initiating step (e.g., injury-induced NO release) and then a second step to be fully maintained or completed. That second phase of activation could involve factors activated by NO or released by damaged muscle (such as HGF/SF in crushed muscle extract) to act directly on injured muscle fibers and indirectly and transiently on uninjured muscles. These ideas are being tested in the isolated fiber culture model with the use of DNA synthesis as the marker of completed activation. Therapies that apply such findings by effectively and specifically manipulating NO levels will be extremely complex, given tissue interactions, NOS isoforms, NO diffusion and inactivation, and the variable cause and progression of atrophy and muscle diseases. Interestingly, in situ hybridization experiments show that satellite cells themselves express NOS-Iμ (J.E. Anderson, unpublished observations). This suggests that satellite cells may direct (in an autocrine manner) their own activation by shear or other stimuli in addition to receiving paracrine signals from fibers.
The present results address for the first time the initial steps of satellite cell activation. A single exposure to NOS inhibition had subtle effects on myotube formation that echo NO-stimulated myoblast fusion in vitro. Longer NOS inhibition reduced the effectiveness of repair and restricted its distribution, in agreement with the idea that shear-induced responses become attenuated longitudinally away from the injury. The significant negative effect of pharmacological NOS inhibition on myogenic repair reported here was further extended by the recent preliminary studies on repair in NOS-I knockout mice (n = 2) and during longer-term NOS inhibition in mdx mice (n = 22). Bearing in mind that l-NAME nonspecifically inhibits all NOS activity, including vascular smooth muscle and endothelial responsiveness, and will have a broad impact, 3 wk of systemic l-NAME treatment appeared to increase the severity of dystrophy in diaphragm, SOL, EDL, and TA in young mdx animals.
A model proposes the hypotheses that NO mediates rapid satellite cell activation, including hypertrophy and altered adhesion inside the fiber-lamina complex, and that distant muscle precursors may be transiently activated by circulating factors released from injured muscle. Although the model certainly needs exploration at many levels, perhaps the largest insights are the rapidity of activation and the notion that immediate satellite cell responses to muscle injury may be contributed by the physical character of the external lamina and mechanical shear. The signaling mechanism underlying NOS-I activity in response to shear can also be determined and may involve Akt/PKB-dependent phosphorylation of NOS-I, as reported recently for NOS-III (Dimmeler et al., 1999). With these signals better defined, new strategies to promote and regulate the action of satellite cells in disease and repair can be devised.
ACKNOWLEDGMENTS
The technical expertise of Cinthya Vargas and graphics by Jerry Kostur and Jay Anderson are gratefully acknowledged. The laboratory is supported by grants from the Muscular Dystrophy Association (USA) and the Heart and Stroke Foundation of Canada.
REFERENCES
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 1998;52:155–162. doi: 10.1016/s0091-679x(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Alway SE. Overload-induced C-Myc oncoprotein is reduced in aged skeletal muscle. J Gerontol A Biol Sci Med Sci. 1997;52:B203–B211. doi: 10.1093/gerona/52a.4.b203. [DOI] [PubMed] [Google Scholar]
- Anderson JE. Studies of the dynamics of skeletal muscle regeneration: the mouse came back! Biochem Cell Biol. 1998;76:13–26. [PubMed] [Google Scholar]
- Anderson JE, Garrett K, Moor A, McIntosh L, Penner K. Dystrophy and myogenesis in the mdx diaphragm. Muscle Nerve. 1998;21:1153–1165. doi: 10.1002/(sici)1097-4598(199809)21:9<1153::aid-mus6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Anderson JE, McIntosh LM, Poettcker R. Deflazacort but not prednisone improves muscle regeneration in mdx muscle. Muscle Nerve. 1996;19:1576–1585. doi: 10.1002/(SICI)1097-4598(199612)19:12<1576::AID-MUS7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Mitchell CM, McGeachie JK, Grounds MD. The time course of basic fibroblast growth factor expression in crush-injured skeletal muscles of SJL/J and BALB/c mice. Exp Cell Res. 1995;216:325–334. doi: 10.1006/excr.1995.1041. [DOI] [PubMed] [Google Scholar]
- Anderson, J.E., Weber, M., and Vargas, C. (2000). Deflazacort increases laminin expression and myogenic repair, and induces early persistent functional gain in mdx mouse muscular dystrophy. Cell Transplant. (in press). [DOI] [PubMed]
- Appell H-J, Forsberg S, Hollmann W. Satellite cell activation in human skeletal muscle after training: evidence for muscle fiber neoformation. Int J Sports Med. 1988;9:297–299. doi: 10.1055/s-2007-1025026. [DOI] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol. 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Beesley JE. Histochemical methods for detecting nitric oxide synthase. Histochem J. 1995;27:757–769. [PubMed] [Google Scholar]
- Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol. 1986a;115:140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986b;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Cell cycle commitment of rat muscle satellite cells. J Cell Biol. 1990a;111:201–207. doi: 10.1083/jcb.111.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990b;109:943–952. doi: 10.1242/dev.109.4.943. [DOI] [PubMed] [Google Scholar]
- Blandino G, Strano S. BCL-2: the pendulum of cell fate. J Exp Clin Cancer Res. 1997;16:3–10. [PubMed] [Google Scholar]
- Brenman JE, et al. Interaction of nitric oxide synthase with the synaptic density protein PSD-95 and α-1 syntrophin mediated by PDZ motifs. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Apone L, Morasso MI, Beers R, Brenner HR, Eftimie R. The MyoD family of myogenic factors is regulated by electrical activity: isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Res. 1992;20:539–544. doi: 10.1093/nar/20.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J Vasc Res. 1998;35:73–84. doi: 10.1159/000025568. [DOI] [PubMed] [Google Scholar]
- Capanni C, Squarzoni S, Petrini S, Millanova M, Muscari C, Maraldi NM, Guarnieri C, Caldarera CM. Increase of neuronal nitric oxide synthase in rat skeletal muscle during ageing. Biochem Biophys Res Commun. 1998;245:216–219. doi: 10.1006/bbrc.1998.8404. [DOI] [PubMed] [Google Scholar]
- Chambers RL, McDermott JC. Molecular basis of skeletal muscle regeneration. Can J Appl Physiol. 1996;21:155–184. doi: 10.1139/h96-014. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Iannoccone SR, Lau KS, Masters BSS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DS, Gorospe RM, Brenman JE, Rafael JA, Peters MF, Froehner SC, Hoffman EP, Chamberlain JS, Bredt DS. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J Exp Med. 1996;184:609–618. doi: 10.1084/jem.184.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DS, Silvagno F, Bredt DS. Muscular dystrophy in mdx mice despite lack of neuronal nitric oxide synthase. J Neurochem. 1998;71:784–789. doi: 10.1046/j.1471-4159.1998.71020784.x. [DOI] [PubMed] [Google Scholar]
- Chen L-E, Seaber AV, Nasser RM, Stamler JS, Urbaniak JR. Effects of S-nitroso-N-acetylcysteine on contractile function of reperfused skeletal muscle. Am J Physiol. 1998;274:R822–R829. doi: 10.1152/ajpregu.1998.274.3.R822. [DOI] [PubMed] [Google Scholar]
- Chien S, Li S, Shyy JY-J. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;19:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Crosbie RH, Straub V, Yun HY, Lee JC, Rafael JA, Chamberlain JS, Dawson VL, Dawson TM, Campbell KP. mdx muscle pathology is independent of nNOS perturbation. Hum Mol Genet. 1998;7:823–829. doi: 10.1093/hmg/7.5.823. [DOI] [PubMed] [Google Scholar]
- Darr KC, Schultz E. Exercise-induced satellite cell activation in mature and growing muscle. J Appl Physiol. 1987;63:1816–1821. doi: 10.1152/jappl.1987.63.5.1816. [DOI] [PubMed] [Google Scholar]
- Darr KC, Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol. 1989;67:1827–1834. doi: 10.1152/jappl.1989.67.5.1827. [DOI] [PubMed] [Google Scholar]
- Decary S, Mouly V, Ben Hamida C, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- Decary S, Mouly V, Butler-Browne GS. Telomere length: a biomarker to monitor satellite cell amplification for cell-mediated gene therapy. Hum Gene Ther. 1996;7:1347–1350. doi: 10.1089/hum.1996.7.11-1347. [DOI] [PubMed] [Google Scholar]
- Decrouy A, Renaud J-M, Lund JA, Dickson G, Jasmin BJ. Mini- and full-length dystrophin gene transfer induces the recovery of nitric oxide synthase at the sarcolemma of mdx4CV skeletal muscle fibers. Gene Ther. 1998;5:59–64. doi: 10.1038/sj.gt.3300553. [DOI] [PubMed] [Google Scholar]
- de Graaf JC, Banga JD, Moncada S, Palmer RMJ, de Groot PG, Sixma JJ. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation. 1992;85:2284–2290. doi: 10.1161/01.cir.85.6.2284. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- Floss T, Arnold H-H, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11:2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O. Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochim Biophys Acta. 1998;1402:39–51. doi: 10.1016/s0167-4889(97)00124-9. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Gossrau R. Caveolin-3 and nitric oxide synthase I in healthy and diseased skeletal muscle. Acta Histochem. 1998;100:99–112. doi: 10.1016/S0065-1281(98)80009-3. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Garrett KL, Lai MC, Wright WE, Bielharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- Grounds MD, McGeachie JK. A model of myogenesis in vivo, derived from detailed autoradiographic studies of regenerating skeletal muscle, challenges the concept of quantal mitosis. Cell Tissue Res. 1987;250:563–569. doi: 10.1007/BF00218947. [DOI] [PubMed] [Google Scholar]
- Grounds MD, McGeachie JK. A comparison of muscle precursor replication in crush-injured skeletal muscle of Swiss and BALBc mice. Cell Tissue Res. 1989;255:385–391. doi: 10.1007/BF00224122. [DOI] [PubMed] [Google Scholar]
- Grozdanovic Z, Goscztonyi G, Gossrau R. Nitric oxide synthase I (NOS-I) is deficient in the sarcolemma of striated muscle fibers in patients with Duchenne muscular dystrophy, suggesting an association with dystrophin. Acta Histochem. 1996;98:61–69. doi: 10.1016/S0065-1281(96)80051-1. [DOI] [PubMed] [Google Scholar]
- Grozdanovic Z, Baumgarten HG. Nitric oxide synthase in skeletal muscle fibers: a signaling component of the dystrophin-glycoprotein complex. Histol Histopathol. 1999;14:243–256. doi: 10.14670/HH-14.243. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Dystroglycan versatility. Cell. 1999;97:543–546. doi: 10.1016/s0092-8674(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dynamics. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Electron microscopic observations of satellite cells with special reference to the development of mammalian skeletal muscles. Z Anat Entwicklungsgesch. 1966;125:43–63. doi: 10.1007/BF00521974. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- Kami K, Noguchi K, Senba E. Localization of myogenin, c-fos, c-jun, and muscle-specific gene mRNAs in regenerating rat skeletal muscle. Cell Tissue Res. 1995;280:11–19. doi: 10.1007/BF00304506. [DOI] [PubMed] [Google Scholar]
- Kanner J, Harel S, Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991;289:130–136. doi: 10.1016/0003-9861(91)90452-o. [DOI] [PubMed] [Google Scholar]
- Kapur S, Bédard S, Marcotte B, Côté CH, Marette A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997;46:1691–1700. doi: 10.2337/diab.46.11.1691. [DOI] [PubMed] [Google Scholar]
- Klein-Ogus C, Harris JB. Preliminary observations of satellite cells in undamaged fibers of the rat soleus muscle assaulted by a snake-venom toxin. Cell Tissue Res. 1983;230:671–676. doi: 10.1007/BF00216210. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Kröncke K-D, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection. How, why, when, and where? Nitric Oxide Biol Chem. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JR. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci USA. 1994;9:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JR. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide Biol Chem. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- Landauer JA, Burke TJ. Proposed cause for and prevention of bone and muscle wasting in microgravity. Aviat Space Environ Med. 1998;69:699–702. [PubMed] [Google Scholar]
- Lee KH, Baek MY, Moon KY, Song WK, Chung CH, Ha DB, Kang M-S. Nitric oxide as a messenger molecule for myoblast fusion. J Biol Chem. 1994;269:14371–14374. [PubMed] [Google Scholar]
- Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell L-E, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for the myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol. 1997;139:129–144. doi: 10.1083/jcb.139.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Snyder SH. Nitric oxide, a novel biologic messenger. Cell. 1992;70:705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cells of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;87:225–251. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall TB, Palmer RMJ, Moncada S. Induction of nitric oxide synthase in rat peritoneal neutrophils and its inhibition by dexamethasone. Eur J Immunol. 1991;21:2523–2527. doi: 10.1002/eji.1830211032. [DOI] [PubMed] [Google Scholar]
- McIntosh LM, Anderson JE. Hypothyroidism prolongs and increases mdx muscle precursor proliferation and delays myotube formation in normal and dystrophic limb muscle. Biochem Cell Biol. 1995;73:181–190. doi: 10.1139/o95-022. [DOI] [PubMed] [Google Scholar]
- McIntosh LM, Garrett KL, Megeney L, Rudnicki MA, Anderson JE. Regeneration and myogenic cell proliferation correlate with taurine levels in dystrophin- and MyoD-deficient muscles. Anat Rec. 1998;252:311–324. doi: 10.1002/(SICI)1097-0185(199810)252:2<311::AID-AR17>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McIntosh LM, Pernitsky AN, Anderson JE. The effects of altered metabolism (hypothyroidism) on muscle repair in the mdx dystrophic mouse. Muscle Nerve. 1994;17:444–453. doi: 10.1002/mus.880170413. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Shimomura T, Naka D, Kitamura N. Proteolytic activation of hepatocyte growth factor in response to tissue injury. J Biol Chem. 1996;269:8966–8970. [PubMed] [Google Scholar]
- Moor AN, Rector ES, Anderson JE. Cell cycle behavior and MyoD expression in response to T3 differ in normal and mdx dystrophic primary muscle cell cultures. Microsc Res Tech. 2000;48:204–212. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<204::AID-JEMT8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Moore R, Walsh FS. The cell adhesion molecule M-cadherin is specifically expressed in developing and regenerating, but not denervated skeletal muscle. Development. 1993;110:1409–1420. doi: 10.1242/dev.117.4.1409. [DOI] [PubMed] [Google Scholar]
- Nakane M, Schmidt HHHW, Pollock JS, Förstermann U, Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993;316:175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie Q-W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Palmer RMJ. The discovery of nitric oxide in the vessel wall: a unifying concept in the pathogenesis of sepsis. Arch Surg. 1993;128:396–401. doi: 10.1001/archsurg.1993.01420160034004. [DOI] [PubMed] [Google Scholar]
- Pernitsky AN, Anderson JE. Differential effects of 3,5,3′-triiodothyronine on control and mdx myoblasts and fibroblasts: analysis by flow cytometry. Exp Cell Res. 1996;22:214–222. doi: 10.1006/excr.1996.0270. [DOI] [PubMed] [Google Scholar]
- Pernitsky AN, McIntosh LM, Anderson JE. Hyperthyroidism impairs early repair in normal but not dystrophic mdx mouse tibialis anterior muscle: an in vivo study. Biochem Cell Biol. 1996;74:315–324. doi: 10.1139/o96-034. [DOI] [PubMed] [Google Scholar]
- Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- Ribera J, Marsal J, Casanovas A, Hukkanen M, Tarabal O, Esquerda JE. Nitric oxide synthase in rat neuromuscular junctions and in nerve terminals of Torpedo electric organ: its role as regulator as acetylcholine release. J Neurosci Res. 1998;51:90–102. doi: 10.1002/(SICI)1097-4547(19980101)51:1<90::AID-JNR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Rong S, Segal S, Anver M, Resau JH, Vande Woude GF. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose O, Rohwedel J, Reinhardt S, Bachman M, Cramer M, Rotter M, Wobus A, Starzinski-Powitz A. Expression of M-cadherin protein in myogenic cells during prenatal mouse development and differentiation of embryonic stem cells in culture. Dev Dynamics. 1994;201:245–259. doi: 10.1002/aja.1002010308. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Abassi Z, Coleman R, Milman F, Winaver J, Better OS. Involvement of nitric oxide system in experimental muscle crush injury. J Clin Invest. 1998;101:1325–1333. doi: 10.1172/JCI810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. BioEssays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- Schmidt HHHW, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schultz E. Fine structure of satellite cells in growing skeletal muscle. Am J Anat. 1976;147:49–70. doi: 10.1002/aja.1001470105. [DOI] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8:217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Shen W, Zhang X, Zhao G, Wolin MS, Sessa W, Hintze TH. Nitric oxide production and NO synthase gene expression contribute to vascular regulation during exercise. Med Sci Sports Exercise. 1995;27:1125–1134. [PubMed] [Google Scholar]
- Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-μ, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem. 1996;271:11204–11208. doi: 10.1074/jbc.271.19.11204. [DOI] [PubMed] [Google Scholar]
- Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res. 1977;185:399–408. doi: 10.1007/BF00220299. [DOI] [PubMed] [Google Scholar]
- Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat Rec. 1990;227:437–446. doi: 10.1002/ar.1092270407. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH. Cell death and oxidative damage in inflammatory myopathies. Clin Immunol Immunopathol. 1998;87:240–247. doi: 10.1006/clin.1998.4527. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH, Schneider I, Gunkel A, Stennert E, Neiss WF. Expression of different isoforms of nitric oxide synthase in experimentally denervated and reinnervated skeletal muscle. J Neuropathol Exp Neurol. 1997a;56:1283–1289. doi: 10.1097/00005072-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH, Schneider I, Gunkel A, Stennert E, Neiss WF. Expression profile of stress proteins, intermediate filaments, and adhesion molecules in experimentally denervated and reinnervated rat facial muscle. Exp Neurol. 1997b;146:125–134. doi: 10.1006/exnr.1997.6512. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol. 1998;275:C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- Traub O, Berk BC. Laminar shear stress. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- Wakayama Y, Inoue M, Murahashi M, Shibuya S, Jimi T, Kojima H, Oniki H. Ultrastructural localization of α1-syntrophin and neuronal nitric oxide synthase in normal skeletal myofiber, and their relation to each other and to dystrophin. Acta Neuropathol. 1997;94:455–464. doi: 10.1007/s004010050733. [DOI] [PubMed] [Google Scholar]
- Wang HH, Lautt WW. Evidence of nitric oxide, a flow-dependent factor, being a trigger of liver regeneration in rats. Can J Physiol Pharmacol. 1999;76:1–8. doi: 10.1139/cjpp-76-12-1072. [DOI] [PubMed] [Google Scholar]
- Wang T, Xie Z, Lu B. Nitric oxide mediates activity-dependent synaptic suppression at developing neuromuscular synapses. Nature. 1995;374:262–265. doi: 10.1038/374262a0. [DOI] [PubMed] [Google Scholar]
- Weiss J. Jun, Fos, MyoD1, and Myogenin proteins are increased in skeletal muscle fiber nuclei after denervation. Acta Neuropathol. 1994;87:63–70. doi: 10.1007/BF00386255. [DOI] [PubMed] [Google Scholar]
- White T, Esser K. Satellite cell and growth factor involvement in skeletal muscle growth. Med Sci Sports Exercise. 1989;21:S158–S163. [PubMed] [Google Scholar]
- Winchester PK, Davis ME, Alway SE, Gonyea WJ. Satellite cell activation in the stretch-enlarged anterior latissimus dorsi muscle of the adult quail. Am J Physiol. 1991;260:C206–C212. doi: 10.1152/ajpcell.1991.260.2.C206. [DOI] [PubMed] [Google Scholar]
- Young ME, Leighton B. Evidence for altered sensitivity of the nitric oxide/cGMP signaling cascade in insulin-resistant skeletal muscle. Biochem J. 1998;329:73–79. doi: 10.1042/bj3290073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Wold B. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr Opin Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- Zacharias JM, Anderson JE. Muscle regeneration after imposed injury is better in younger than older mdx dystrophic mice. J Neurol Sci. 1991;104:190–194. doi: 10.1016/0022-510x(91)90309-u. [DOI] [PubMed] [Google Scholar]