Abstract
Objective
Some patients with bipolar disorder experience mood episodes following emotional life events, whereas others do not. There is evidence that orbitofrontal hypoactivity may be related to this, because the orbitofrontal cortex is involved in the regulation of emotional and behavioural responses to external events. The close anatomical and functional connection between the orbitofrontal cortex and olfactory processing suggests that patients with bipolar disorder and heightened emotional reactivity may exhibit altered olfactory function compared with patients with bipolar disorder who do not exhibit this sensitivity.
Methods
In this pilot study, olfactory function was assessed in patients with bipolar disorder and a history of event-triggered episodes (n = 7) and in patients with bipolar disorder without such a history (n = 9) at the Department of Psychiatry and the Taste and Smell Clinic of the University of Dresden, Germany. Each patient's bipolar disorder was in remission at study entry, and they were on monotherapy with mood stabilizers. Assessment included olfactory event-related potentials (ERP) and psychophysical tests for odour threshold, odour identification and olfactory quality discrimination.
Results
Odour thresholds were lower in patients with bipolar disorder and event-triggered episodes compared with the other patient group. In addition, patients with event-triggered episodes exhibited shorter N1 peak latencies of the olfactory ERP.
Conclusions
Our findings indicate disinhibition of orbitofrontal areas involved in the processing of emotional events in a subset of patients with bipolar illness.
Medical subject headings: bipolar disorder, evoked potentials, orbitofrontal cortex, smell
Abstract
Objectif
Certains patients atteints de trouble bipolaire ont des épisodes thymiques à la suite d'événements émotionnels de la vie, tandis que d'autres n'en ont pas. Les données indiquent qu'une hypoactivité orbitofrontale peut être reliée à ce phénomène, parce que le cortex orbitofrontal joue un rôle dans la régulation des réactions émotionnelles et comportementales aux événements externes. Le lien anatomique et fonctionnel étroit entre le cortex orbitofrontal et le traitement olfactif indique que les patients atteints de trouble bipolaire et qui ont une réactivité émotionnelle accentuée peuvent présenter une altération de la fonction olfactive comparativement aux patients atteints de trouble bipolaire qui n'ont pas cette sensibilité.
Méthodes
Au cours de cette étude pilote, on a évalué, au Département de psychiatrie et à la Clinique du goût et de l'odorat de l'Université de Dresde, en Allemagne, la fonction olfactive de patients atteints de trouble bipolaire et ayant des antécédents de crises déclenchées par des événements (n = 7) et de patients atteints de trouble bipolaire qui ne présentent pas ces antécédents (n = 9). Le trouble bipolaire de chaque patient était en rémission au début de l'étude et les patients suivaient une monothérapie aux thymorégulateurs. L'évaluation a inclus les potentiels évoqués cognitifs (PEC) olfactifs et des tests psychophysiques visant à déterminer le seuil olfactif, l'identification des odeurs et la discrimination de la qualité olfactive.
Résultats
Les seuils olfactifs étaient moins élevés chez les patients atteints de trouble bipolaire et qui avaient des crises déclenchées par des événements comparativement à ceux de l'autre groupe. Les patients qui avaient des crises déclenchées par des événements montraient en outre une période de latence de pointe N1 du PEC olfactif de plus courte durée.
Conclusions
Nos constatations indiquent une désinhibition des régions orbitofrontales qui interviennent dans le traitement des événements émotionnels chez un sous-ensemble de patients atteints de trouble bipolaire.
Introduction
Bipolar disorder (BD) is characterized by episodes of fluctuating moods of opposite polarity separated by periods of remission.1 Clinical remission from an acute episode is generally seen as a “symptom-free period.” However, there is growing evidence that in many patients this state is frequently accompanied by increased emotional reactivity, as well as mood lability.2–4 These impairments are reported to be more pronounced in patients with BD2,4 than in recovered patients with major depressive disorder.5 Although often undetected clinically, these phenomena suggest that, despite remission of symptoms, patients remain in an unstable affective state. This state is thought to contribute significantly to the vulnerability of some patients with BD to external stressors such as life events or biological factors that may trigger new episodes.6
In 2 recent studies using positron emission tomography (PET), euthymic patients with BD exhibited a decreased regional cerebral blood flow in the orbitofrontal cortex at rest and an even stronger decrease after provocation with a sad mood–induction paradigm compared with healthy volunteers.7,8 This finding was interpreted as a trait effect and was clinically associated with a higher level of emotional arousal in these patients. The following questions arose, however: Why do some, but not all, patients with bipolar disorder exhibit this vulnerability to external stressors? How could those patients be identified?
Because the heightened emotional responsiveness in patients whose BD is in remission seems, at least in part, to be linked to orbitofrontal hypoperfusion, and because the orbitofrontal cortex is strongly involved in odour processing,9,10 we hypothesized that euthymic patients with BD have alterations in olfactory function in the form of either an increased or a decreased olfactory sensitivity. This ambiguity is also the reason why 2-tailed testing was performed. To this effect, olfactory function in clinically euthymic patients with BD was assessed on both psychophysical (phenyl ethyl alcohol odour thresholds, olfactory quality discrimination and odour identification) and electrophysiological levels (olfactory event-related potentials [ERP]).
Methods
The study was conducted at the Departments of Psychiatry and Otorhinolaryngology of the University of Dresden Medical School, Dresden, Germany. Sixteen patients with BD type I, whose disease was in remission after an acute episode of depression or mania/mixed mania, were included in the study. All participants were outpatients of the Department of Psychiatry. The BD had previously been diagnosed by the treating physician according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10),11 and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).1 Exclusion criteria were other Axis I or II diagnoses, smoking, history of head trauma or of substance abuse, serious medical and neurological comorbidity, and taking medications other than mood stabilizers (lithium and anticonvulsants), because there is no evidence that these drugs have a major impact on olfaction, although there are reports indicating possible effects on gustatory function.12 Testing was only performed in patients without signs of an acute upper respiratory tract infection. The study was performed in accordance with the Declaration of Helsinki concerning biomedical studies in human subjects. All patients gave informed consent.
Remission (euthymia) was defined as the absence of symptoms of depression, mania or mixed mania for a minimum of 3 months without recurrence. This was verified by both review of clinical records and interview with one of the authors (S.K.). During this evaluation, the Hamilton Rating Scale for Depression13 was administered to quantify current depressive symptoms and the Self-Report Manic Inventory (SRMI)14 to assess a possible manic/hypomanic episode. Only patients with a HAM-D score of 6 or less and a SRMI score of 2 or less were enrolled. Although self-reporting of manic symptoms may be limited by lack of insight, all our subjects acknowledged their bipolar illness, thus justifying the use of the SRMI instead of an observer-rated instrument.
Patients were then divided into 2 groups: group A (n = 7) comprised patients whose recent episode had been triggered by an external event that the patients had experienced as emotionally stressful. These events included break-up of a relationship (n = 2), birthday preparations (n = 2), marriage (n = 1), occupational stress due to working longer hours (n = 1) and overseas travel (n = 1). In the absence of a systematic instrument to assess event-triggered episodes, events considered to have triggered the last episode had been identified in the psychoeducational program for BD conducted at the hospital; they were documented in the patients' charts. Group B (n = 9) comprised patients whose episodes occurred without a trigger. Many patients in group B reported that they had experienced similar events to those experienced by group A patients but that they had not been affected by them.
Demographic and illness-related parameters at baseline are summarized in Table 1. Patients differed with respect to their ages: group A, mean 33.9 (standard deviation [SD] 10.7) years versus group B, mean 46.1 (SD 11.6) years. To exclude effects of age on the results, age was introduced as a covariate in the analysis (see below). Despite fewer years of illness, patients with event-triggered episodes had as many full-blown episodes as patients without event-triggered episodes. In addition, in the patient group with event-triggered episodes, there were significantly fewer women compared with the other group (χ2 = 5.13, p = 0.024).
Table 1
Stimulation procedures
Testing sessions took place during summer/fall 2003 in an air-conditioned laboratory (21°C, about 40% relative humidity). To record olfactory ERP, chemosensory nasal stimulation was performed using an apparatus (Olfactometer OM2s; Burghart Instruments, Wedel, Germany) that allows application of chemical stimuli without causing concomitant stimulation of mechanoreceptors or thermoreceptors.15 This was achieved by embedding chemical stimuli of 200-ms duration in a constantly flowing airstream (7.2 L/min) applied to the nasal mucosa by a cannula, with an inner diameter of 4 mm, inserted about 1 cm into the nostril beyond the nasal valve area. The temperature and humidity of the airstream were kept constant (36.5°C, 80% relative humidity). Stimulus onset occurred within less than 20 ms. Both hydrogen sulfide ([H2S] 4 parts per million, smell of rotten eggs) and phenyl ethyl alcohol ([PEA] 40% volume [of solute] per volume [of solvent], smell of roses) were used for olfactory stimulation; they are known to specifically activate the olfactory system with little or no simultaneous trigeminal activation.16,17 Twenty stimuli of each odorant were alternately applied to the left or right nostril with an interstimulus interval of 40 s to avoid habituation.18 Patients were seated in a room that was darkened and acoustically shielded to minimize concomitant sensory stimulation; during measurements, patients also received white noise through headphones. Further, the patient's movements were monitored through a video camera system.
Olfactory ERP
Electroencephalography (EEG) recordings were obtained from 3 positions of the International 10–20 EEG System (Cz, Fz and Pz) referenced to linked earlobes (A1+A2) (see inset in Fig. 1). Blink artifacts were monitored from an additional site (Fp2). Stimulus-linked EEG segments of 2048 ms were digitally recorded at a frequency of 250 Hz (bandpass filter of 0.2–30 Hz; off-line filtering with 15-Hz low-pass). Olfactory ERP were obtained through off-line averaging of the digitized EEG segments. Records contaminated by eye blinks (> 50 μV in the Fp2 lead) or other disturbances (e.g., high-frequency motor artifacts) were discarded during off-line, visual inspection of single trials.
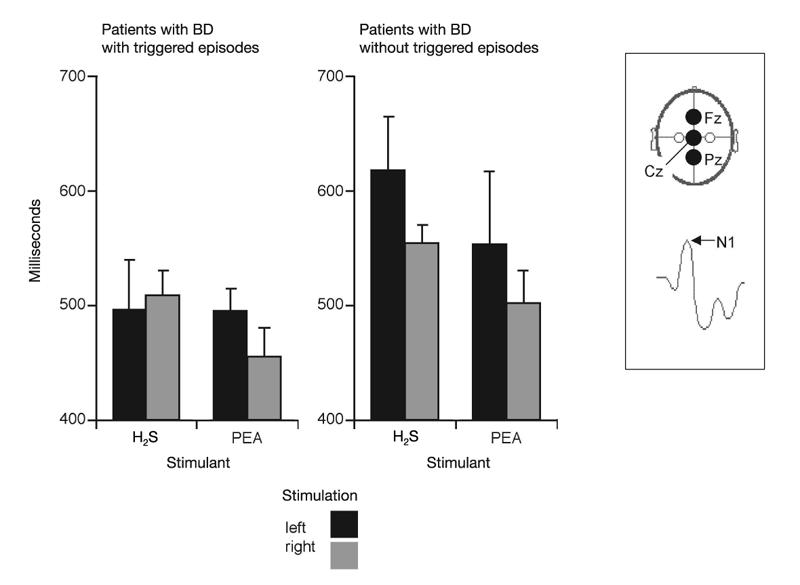
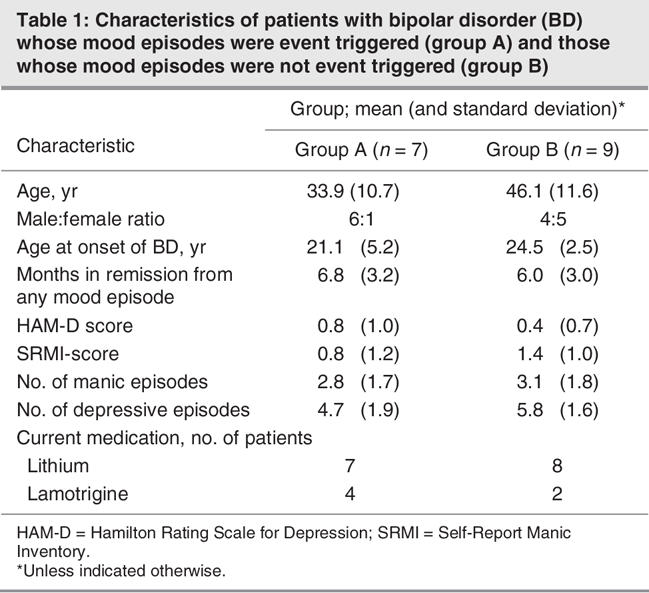
Fig. 1: Electrophysiological measures of olfactory function: peak latencies N1 of olfactory event-related potentials (ERP) obtained at recording position Cz (means and standard error of the mean). The results are presented separately for groups A (left side) and B (right side), responses to olfactory stimulation with H2S and phenyl ethyl alcohol (PEA), and for left-sided (black bars) or right-sided stimulation (grey bars). Inset: schematic drawings of the 3 recording sites and of the ERP peaks are presented. Compared with patients without event-triggered episodes, patients with event-triggered episodes had significantly shorter latencies N1.
In this study, olfactory ERP peaks were named N1 and P2, according to the widely accepted nomenclature of Evans et al.19 On average, the first negative peak (N1) of olfactory ERP in response to right-sided stimulation with H2S in this study occurred at position Pz at a latency of 532 ms, followed by a major positive peak (P2) at 709 ms (see inset in Fig. 1). ERP peak latencies N1 and P2 (in relation to stimulus onset) and peak-to-peak amplitudes N1P2 were evaluated by an experienced observer (T.H.), who was blinded to the patients' diagnoses.
Psychophysical testing of olfactory function
Psychophysical testing was performed separately for the left and right nostrils; the sequence of testing was randomized across all patients. Using the “Sniffin' Sticks” test kit,20,21 odorants were presented in dispensers similar to felt-tip pens. The pens are about 14 cm long and have an inner diameter of 1.3 cm. Instead of liquid dye, the pen is filled with 4 mL of liquid odorants or odorants dissolved in propylene glycol. For odour presentation, the cap is removed by the experimenter for about 3 s, and the pen's tip is placed about 2 cm in front of each nostril. Testing involved tests for odour threshold, olfactory quality discrimination and odour identification.
Odour threshold
Odour thresholds for PEA were assessed using a single-staircase, triple-forced-choice procedure. Sixteen dilutions were prepared in a geometric series starting from a 4% PEA solution (dilution ratio 1:2; diluent:propylene glycol). Three pens were presented in a randomized order, with 2 containing the solvent and the third the odorant at a certain dilution. The patient's task was to identify the odour-containing pen. Triplets were presented at intervals of 20 s. Reversal of the staircase was triggered when the odour was correctly identified in 2 successive trials. The threshold was defined as the mean of the last 4 of 7 staircase reversals.
Olfactory quality discrimination
In the olfactory quality discrimination task, triplets of pens were presented in a randomized order, with 2 containing the same odorant and the third, a different odorant. Patients had to determine which of 3 odour-containing pens smelled different. Presentation of triplets was separated by 20–30 s. The interval between presentation of individual pens of a triplet was about 3 s. Sixteen of the following combinations were tested: butanol — 2-phenyl ethanol; isoamyl acetate — anethole; anethole — eugenol; limonene — fenchone; (-)carvone — (+)carvone; eugenol — cinnamon aldehyde; dihydro rosenoxide — menthol; acetaldehyde — isoamylacetate; citronellal — linalool; pyridine — limonene; limonene — citronellal; eucalyptol — dipyridyl; dipyridyl — cyclopentadecanoate; butanol — fenchone; octyl acetate — cinnamon aldehyde; and carvone — acetaldehyde (for a more detailed description, see Hummel et al20). When measuring odour thresholds and olfactory quality discrimination, patients were blindfolded to prevent visual identification of some of the odorant-containing pens.
Odour identification
Odour identification was assessed by means of 16 common odours, namely, orange, peppermint, turpentine, cloves, leather, banana, garlic, rose, fish, lemon, coffee, anise, cinnamon, licorice, apple and pineapple. Using a multiple-choice task, identification of individual odorants was performed from a list of 4 descriptors (for a more detailed description, see Hummel et al20). The interval between odour presentations was 20–30 s. All measurements were performed in a quiet, air-conditioned room.
Statistical analysis
The results were submitted to analyses of variance, adopting “side of stimulation” (left/right) and, in the case of olfactory ERP recordings, “recording site” (positions Fz, Cz and Pz) and “odour” (H2S, PEA) as within-subjects factors. The factor “group” (groups of patients with BD with/without event-triggered episodes) was used as a between-subjects factor, and the subjects' age was introduced as covariate. Degrees of freedom were adjusted according to the Greenhouse–Geisser method. Only significant main effects or 2-way interactions will be reported. t tests for independent samples were used for additional comparisons between groups. The level of significance was set at 0.05.
Results
Electrophysiological investigations
Descriptive statistics for electrophysiological investigations are presented in Table 2. Because of significant contamination with artifacts (blink artifacts, muscular artifacts, etc.), some of the recordings could not be analyzed, which reduced the sample size in group B to n = 4.
Table 2
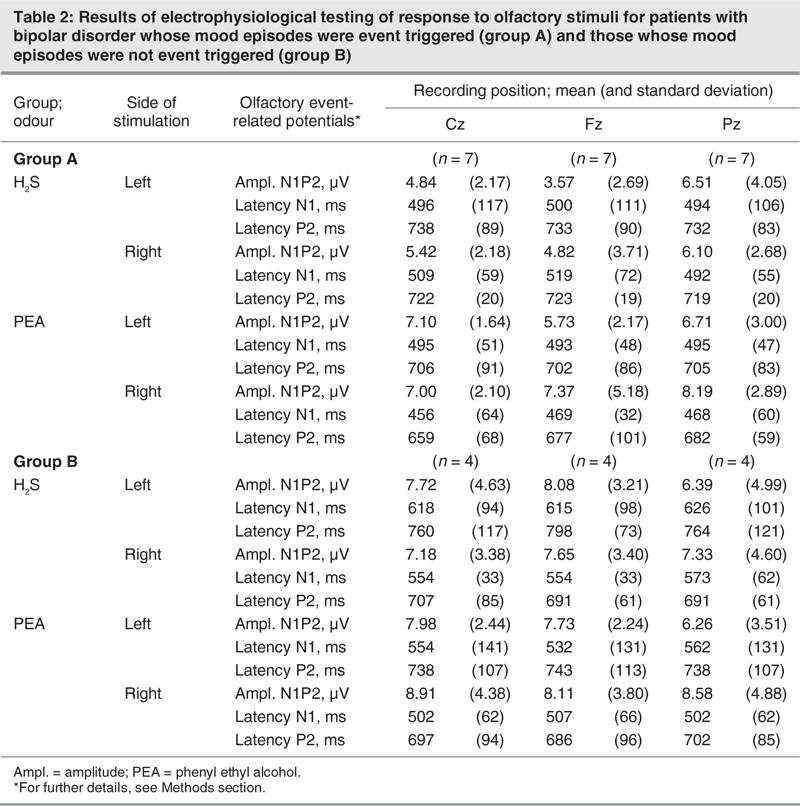
No significant main effect of the factor “group” was observed for amplitudes (F1,8 = 1.64, p = 0.24). For latencies N1, there was a significant main effect of the factor “group” (F1,8 = 10.6, p = 0.012, η2 = 0.57), indicating that group A patients exhibited shorter N1 latencies compared with group B patients (Fig. 1). For latencies P2, a significant interaction between the factors “side of stimulation” and “group” (F1,8 = 6.97, p = 0.03, η2 = 0.47) emphasized the fact that group A patients showed a pronounced difference in response to left-sided or right-sided stimulation, whereas on average there was little difference for group B patients. However, post hoc comparisons for individual parameters using t tests did not exhibit significant group differences.
Psychophysical testing
Descriptive statistics for psychophysical testing are presented in Table 3. The results of psychophysical tests of olfactory function performed separately for the left and right nostril did not differ between the 2 groups (F1,13 < 0.84, p > 0.38). There was neither a significant effect of the factor “side of testing,” nor a significant interaction between the factors “side of testing” and “group.” However, when comparing results for the better nostril, group A patients were found to exhibit lower PEA thresholds (F1,14 = 5.27, p = 0.038, η2 = 0.27), whereas no significant difference was observed for olfactory quality discrimination (F1,14 = 0.083, p = 0.78) or odour identification (F1,14 = 1.51, p = 0.24) (Fig. 2). There was no seasonal clustering of the measurements of the 2 groups of patients.
Table 3
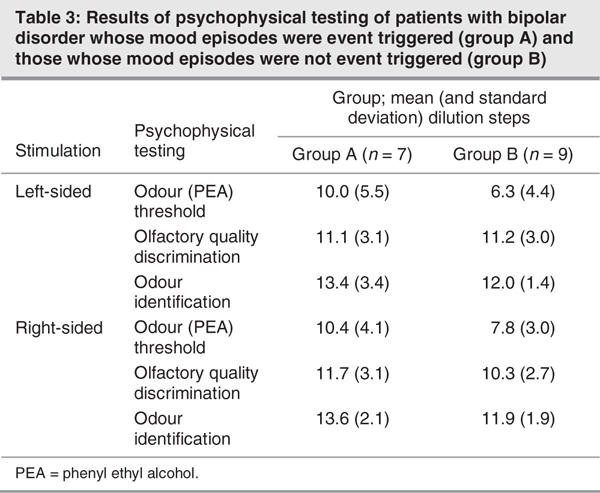
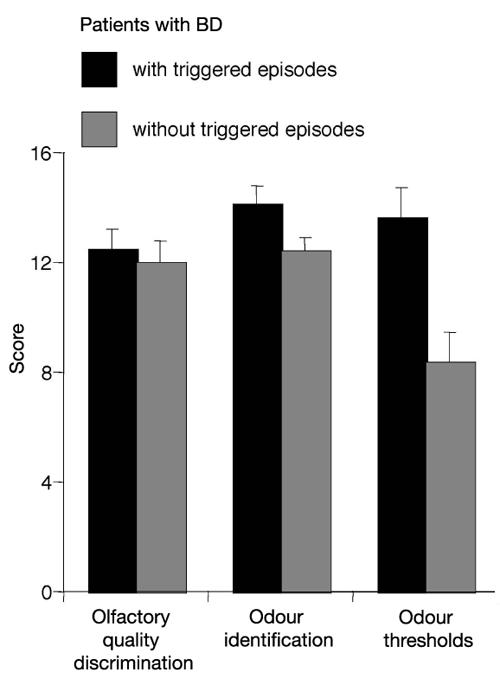
Fig. 2: Psychophysical olfactory testing: results obtained for the better nostril (means and standard error of the mean) separately for groups A (black bars) and B (grey bars), for olfactory quality discrimination, odour identification and phenyl ethyl alcohol (PEA) odour thresholds. Higher scores indicate higher sensitivity. Compared with patients without event-triggered episodes, patients with event-triggered episodes were significantly better at detecting PEA at lower concentrations (higher dilution steps).
Discussion
The results from the current study in a relatively small group of subjects indicate a difference in odour thresholds between euthymic patients with BD whose mood episodes were event triggered (group A) and those whose episodes were not event related (group B). Furthermore, with respect to olfactory ERP, group A patients showed significantly shorter peak latencies than group B patients, indicating a relative increase of processing speed of olfactory information. In addition, group A patients exhibited larger differences for the latency of P2 in response to left or right-sided stimulation.
To our knowledge, there are no published studies of olfactory function in BD. However, in patients with “first-episode psychosis,” one study has reported an increased olfactory sensitivity and another has reported normal thresholds.22,23 The diagnosis of first-episode psychosis comprises nosologically heterogeneous patients, all of whom present with prominent delusions and hallucinations. It is likely that at least some of the patients in these studies may have had bipolar illness, whereas others may have had schizophrenia. That said, the contradictory findings in these 2 studies may be the result of heterogeneous patient samples subsumed under the diagnostic term first-episode psychosis.
Studies of olfactory function in affective disorders diagnosed according to DSM-IV criteria have focused on olfactory function during acute episodes or during periods shortly after antidepressant treatment in recurrent depression and seasonal affective disorder.24–30 These studies have yielded controversial results in that 2 studies24,26 found a correlation between odour identification scores and severity of depression (increased scores when depressed, normalization after treatment), whereas 2 other studies did not.25,29 Specifically, Postolache et al27 found that patients with seasonal affective disorder exhibited a more acute sense of smell than healthy controls, independent of the season during which they were studied. Gross-Isseroff et al25 reported a greater rather than weaker odour detection ability in patients with major depression than in healthy controls. Olfactory acuity was highest after treatment with antidepressants. Lower odour thresholds in depression that normalized after successful treatment have been identified in 3 studies.27–29
One recent study investigated the similarities and differences in the olfactory and visual processing of emotional stimuli in healthy subjects and in patients with major depressive disorder before and after treatment. Before treatment, visual stimulus processing was attenuated in depressive subjects at a relatively late processing level, whereas olfactory stimulus processing had already been affected at an early level. After successful medical treatment, ERP had normalized. The authors suggested that functional deviations within the primary olfactory cortex may be responsible for the lower olfactory sensitivity, as well as for the altered emotional stimulus processing in depressed patients.30
Our finding of a relative increase of olfactory sensitivity in patients with BD with a known vulnerability to emotional stress may be related to orbitofrontal and cingulate function in these patients. The left medial region of the rostral orbitofrontal cortex and the more lateral orbitofrontal cortex are known to be involved in olfactory processing in humans.31 The anterior cingulate cortex is also part of the olfactory network and is activated when pleasant and intense odours are detected.31–34
Although our study results do not allow definitive conclusions concerning the association between emotional vulnerability, orbitofrontal cortex function and olfactory processing, there are functional neuroimaging data in mood disorders using PET suggesting7,8,33–35 that orbitofrontal hypoactivity and dorsal anterior cingulate hyperactivity may be trait markers of emotional vulnerability in patients with BD, which may put these patients at risk for new episodes.
Clinically, orbitofrontal hypoactivity seems to be related to disinhibition of emotional modulation. The orbitofrontal cortex has extensive connections to the dorsal anterior cingulate and other structures of the limbic system known to process emotion. The interplay between these brain regions is known to serve as a substrate to integrate viscerosensory information with affective signals;36 to guide behaviour, regulate mood and reappraise spontaneous emotional responses;8,37 and to modulate the reward system.38 One might speculate that because of their close anatomical proximity, orbitofrontal cortex dysfunction contributing to emotional disinhibition may affect olfactory processing areas by increasing sensitivity in a subset of patients with BD. This does not explain, however, why only sensitivity and not other aspects of olfactory function are altered. Future studies are required to further define the association of orbitofrontal cortex function, emotional vulnerability and olfactory sensitivity in patients with BD.
The available literature does not allow for conclusive explanations with respect to the finding of lateralization of P2 latencies in group A but not in group B.39,40 Furthermore, because the presently observed interactions were not confirmed by individual group comparisons, the effect appears to be weak. Future studies are needed to determine whether the present observations might be related to anatomical or functional peculiarities in patients with BD with event-triggered episodes.
Limitations of the present pilot study include the small number of patients investigated. In addition, we did not adjust for menstrual cycle in our female participants. Although menstrual cycle has been shown to be a modulator of olfactory function, and endocrine, cardiovascular and psychological correlates of olfactory sensitivity change across the menstrual cycle, these effects are subtle.41 In fact, numerous carefully conducted studies have failed to demonstrate such effects (see Hummel et al42). Accordingly, it can be assumed that changes of olfactory sensitivity in relation to the menstrual cycle may not have been a major confounding factor.
Although the groups were not balanced with regard to the sex of the participants, it is important to note that group A was largely composed of men. Considering that numerous studies indicate a higher olfactory sensitivity/responsiveness in women compared with men,43 the present findings are not readily explained by sex differences, with group A subjects being relatively more sensitive than group B subjects. However, future studies will have to take sex-related differences into account.
Conclusion
This is to our knowledge the first study to investigate olfactory function in 2 subsets of euthymic patients with BD. Based on psychophysical and electrophysiological measures, our results point to a heightened olfactory acuity in those patients with BD whose mood episodes were triggered by emotional events as opposed to those patients whose episodes occurred without such triggers, possibly linking olfactory function to a labile and disinhibited emotional modulation system in these patients. In addition, the present findings point to a close relation between olfactory function and mood regulation. Replication studies with larger groups of patients are required to validate the results of this study.
Acknowledgments
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft to Dr. Hummel (DFG HU441/2-1). We would like to thank Matthias Fülle for his help in scheduling the olfactory tests.
Footnotes
Contributors: Drs. Krüger, Bräunig and Hummel designed the study and wrote the article. Drs. Krüger, Frasnelli and Hummel acquired the data. Drs. Krüger and Hummel analyzed the data. All authors reviewed the written article and gave final consent for its publication.
Competing interests: None declared.
Correspondence to: Dr. Thomas Hummel, Smell & Taste Clinic, Department of Otorhinolaryngology, University of Dresden Medical School, Fetscherstrasse 74, 01307 Dresden, Germany; fax 49-351-458-4326; thummel@rcs.urz.tu-dresden.de
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th ed. Washington: American Psychiatric Press; 1996.
- 2.Fukuda K, Etoh T, Iwadate T, et al. The course and prognosis of manic-depressive psychosis: a quantitative analysis of episodes and intervals. Tohoku J Exp Med 1983;139:299-307. [DOI] [PubMed]
- 3.Keller MB, Lavori PW, Coryell W, et al. Differential outcome of pure manic, mixed/cycling and pure depressive episodes in patients with bipolar illness. JAMA 1986;255:3138-42. [PubMed]
- 4.Tsuang MT, Woolson RF, Fleming JA. Long-term outcome of major psychoses: I. schizophrenia and affective disorders compared with psychiatrically symptom-free surgical conditions. Arch Gen Psychiatry 1979;36:1295-301. [DOI] [PubMed]
- 5.Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv 2001;52:51-5. [DOI] [PubMed]
- 6.Lauer CJ, Schreiber W, Modell S, et al. The Munich Vulnerability Study of Affective Disorders. Overview of the results at index study. Nervenarzt 1998;69:574-85. [DOI] [PubMed]
- 7.Krüger S, Seminowicz D, Goldapple K, et al. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry 2003;54:1274-83. [DOI] [PubMed]
- 8.Krüger S, Alda M, Young T, et al. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry 2006;163:257-64. [DOI] [PubMed]
- 9.Zatorre RJ, Jones-Gotman M, Evans AC, et al. Functional localization and lateralization of human olfactory cortex. Nature 1992;360:339-40. [DOI] [PubMed]
- 10.Fulbright RK, Skudlarski P, Lacadie CM, et al. Functional MR imaging of regional brain responses to pleasant and unpleasant odors. AJNR Am J Neuroradiol 1998;19:1721-6. [PMC free article] [PubMed]
- 11.World Health Organization. International statistical classification of diseases and related health problems. 10th rev. Geneva: WHO; 1992.
- 12.Henkin RI. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf 1994;11:318-77. [DOI] [PubMed]
- 13.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed]
- 14.Shugar G, Schertzer S, Toner BB, et al. Development, use and factor analysis of a self-report inventory for mania. Compr Psychiatry 1992;33:325-31. [DOI] [PubMed]
- 15.Kobal G, Hummel C. Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr Clin Neurophysiol 1988;71:241-50. [DOI] [PubMed]
- 16.Doty RL, Brugger WE, Jurs PC, et al. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav 1978;20:175-85. [DOI] [PubMed]
- 17.Kobal G, Hummel T. Olfactory and intranasal trigeminal event-related potentials in anosmic patients. Laryngoscope 1998;108:1033-5. [DOI] [PubMed]
- 18.Hummel T, Kobal G. Olfactory event-related potentials. In: Simon SA, Nicolelis MAL (eds). Methods and frontiers in chemosensory research. Boca Raton (Fla.): CRC Press; 2001. p. 429-64.
- 19.Evans WJ, Kobal G, Lorig TS, et al. Suggestions for collection and reporting of chemosensory (olfactory) event-related potentials. Chem Senses 1993;18:751-6.
- 20.Hummel T, Sekinger B, Wolf SR, et al. “Sniffin' Sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997;22:39-52. [DOI] [PubMed]
- 21.Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol 2000;257:205-11. [DOI] [PubMed]
- 22.Sirota P, Davidson B, Mosheva T, et al. Increased olfactory sensitivity in first episode psychosis and the effect of neuroleptic treatment on olfactory sensitivity in schizophrenia. Psychiatry Res 1999;86:143-53. [DOI] [PubMed]
- 23.Kopala LC, Clark C, Hurwitz T. Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res 1993;8:245-50. [DOI] [PubMed]
- 24.Warner MD, Peabody CA, Csernansky JG. Olfactory functioning in schizophrenia and depression. Biol Psychiatry 1990;27:457-8. [DOI] [PubMed]
- 25.Gross-Isseroff R, Luca-Haimovici K, Sasson Y, et al. Olfactory sensitivity in major depressive disorder and obsessive compulsive disorder. Biol Psychiatry 1994;35:798-802. [DOI] [PubMed]
- 26.Postolache TT, Doty RL, Wehr TA, et al. Monorhinal odor identification and depression scores in patients with seasonal affective disorder. J Affect Disord 1999;56:27-35. [DOI] [PubMed]
- 27.Postolache TT, Wehr TA, Doty RL, et al. Patients with seasonal affective disorder have lower odor detection thresholds than control subjects. Arch Gen Psychiatry 2002;59:1119-22. [DOI] [PubMed]
- 28.Pause BM, Miranda A, Goder R, et al. Reduced olfactory performance in patients with major depression. J Psychiatr Res 2001;35:271-7. [DOI] [PubMed]
- 29.Thomas HJ, Fries W, Distel H. Evaluation of olfactory stimuli by depressed patients. Nervenarzt 2002;73:71-7. [DOI] [PubMed]
- 30.Pause BM, Raack N, Sojka B, et al. Convergent and divergent effects of odors and emotions in depression. Psychophysiology 2003;40:209-25. [DOI] [PubMed]
- 31.Shiino A, Morita Y, Ito R, et al. Functional MRI of the human brain: responses to olfactory stimulation. No Shinkei Geka 1999;27:1105-10. [PubMed]
- 32.Weismann M, Yousri I, Heuberger E, et al. Functional magnetic resonance imaging of human olfaction. Neuroimaging Clin N Am 2001;11:237-50. [PubMed]
- 33.Liotti M, Mayberg SH, McGinnis S, et al. Mood challenge in remitted unipolar depression unmasks disease-specific cerebral blood flow abnormalities. Am J Psychiatry 2002;159:1830-40. [DOI] [PubMed]
- 34.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999;156:675-82. [DOI] [PubMed]
- 35.Liotti M, Mayberg HS, Brannan SK, et al. Mood challenge in remitted depression: a 15O-Water PET study. Neuroimage 1997;5:S114.
- 36.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000;10:206-19. [DOI] [PubMed]
- 37.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 1999;877:383-96. [DOI] [PubMed]
- 38.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn 2004;55:11-29. [DOI] [PubMed]
- 39.Hummel T, Mohammadian P, Kobal G. Handedness is a determining factor in lateralized olfactory discrimination. Chem Senses 1998;23:541-4. [DOI] [PubMed]
- 40.Fulbright RK, Skudlarski P, Lacadie CM, et al. Functional MR imaging of regional brain responses to pleasant and unpleasant odors. AJNR Am J Neuroradiol 1998;19:1721-6. [PMC free article] [PubMed]
- 41.Doty RL, Snyder PJ, Huggins GR, et al. Endocrine, cardiovascular and psychological correlates of olfactory sensitivity changes during the human menstrual cycle. J Comp Physiol Psychol 1981;95:45-60. [DOI] [PubMed]
- 42.Hummel T, Gollisch R, Wildt G, et al. Changes in olfactory perception during the menstrual cycle. Experientia 1991;47:712-5. [DOI] [PubMed]
- 43.Doty RL. Gender and endocrine-related influences upon olfactory sensitivity. In: Meiselman H, Rivlin RS, editors. Clinical measurement of taste and smell. New York: MacMillan; 1986. p. 377-413.


