Abstract
Objectives
To identify clinical indicators of immediate, early, and late mortality in children at admission to a sub-Saharan district hospital and to develop prognostic scores.
Design
Prospective cohort study.
Setting
One district hospital in Kenya.
Participants
Children aged over 90 days admitted to hospital from 1 July 1998 to 30 June 2001.
Main outcome measures
Prognostic indicators of mortality.
Results
Of 8091 children admitted up to 1 June 2000, 436 (5%) died. Sixty (14%) died within four hours after admission (immediate), 193 (44%) after 4-48 hours (early), and 183 (42%) after 48 hours (late). There were marked differences in the clinical features associated with immediate, early, and late death. Seven indicators (neurological status, respiratory distress (subcostal indrawing or deep breathing), nutritional status (wasting or kwashiorkor), severe anaemia, jaundice, axillary temperature, and length of history) were included in simplified prognostic scores. Data from 4802 children admitted from 1 July 2000 to 30 June 2001 were used to validate the scores. For simplified prognostic scores the areas under the receiver operating characteristic curves were 0.93 (95% confidence interval 0.92 to 0.94), 0.82 (0.80 to 0.83), and 0.82 (0.81 to 0.84) for immediate, early, and late death, respectively.
Conclusion
In children admitted to a sub-Saharan hospital, the prognostic indicators of early and late deaths differ but a small number of simple clinical signs predict outcome well.
What is already known on this topic
Prognostic indicators have been described for individual diseases—including malaria, lower respiratory tract infection, diarrhoea, and malnutrition—in African children
Making a single diagnosis is often difficult or inappropriate as many children are admitted with several coexisting problems, the clinical features of common illnesses may be indistinguishable, and laboratory facilities are often inadequate
What this study adds
The signs that are prognostic of immediate, early, and late deaths in children admitted to hospital in Africa differ
A small number of simple clinical signs have good sensitivity and specificity for predicting outcome and could be used for risk assessment in individuals or groups
Introduction
One in six African children dies before the age of 5 years.1 District hospitals have an important role in reducing mortality2 but resources are often limited and care may be of limited quality.3 The implementation of simple guidelines4,5 for common childhood illnesses may lead to improvements, but tools are needed to evaluate the quality of care between hospitals and over time.3,6
Scores designed to predict mortality enable adjustment for severity of illness.7 For example, the PRISM score has recently been used to standardise and evaluate outcome in paediatric meningococcal disease in the United Kingdom.8,9 An appropriately developed score might be useful in audit, research, and clinical practice in sub-Saharan Africa.
Although prognostic indicators have been described for specific diseases—including malaria,10,11 lower respiratory tract infection,12,13 gastroenteritis,14,15 and malnutrition16,17—children commonly present with multiple problems. These typically include malnutrition, anaemia, malaria parasitaemia, or HIV infection. Malnutrition increases the risk of death in children admitted to hospital, and presents with almost every illness.18 Additionally, without adequate laboratory facilities clinical features of malaria may be indistinguishable from lower respiratory tract infection19 or meningitis.20
We identified prognostic indicators in children admitted to a rural district hospital in Kenya, irrespective of diagnosis, to produce practical prognostic scores.
Methods
Location
Kilifi district hospital is located on the Kenyan coast. It serves a mainly rural population of about 200 000 people. A Kenya Medical Research Institute (KEMRI) research centre is situated within the hospital. The outpatient department is staffed by government employed clinical officers who refer admissions to the paediatric ward (35 beds) or the high dependency ward (six beds). Research clinicians provide inpatient paediatric clinical cover.
The KEMRI Scientific Steering Committee and National Ethical Review Committee approved the study.
Participants and clinical methods
Data were prospectively collected on admission and at death or discharge for all 8477 admissions of children age over 90 days who were admitted from 1 July 1998 to 30 June 2001. We excluded children who were admitted for trauma or elective procedures. Trained clinical assistants collected demographic data, and research clinical officers and doctors from Kenya and Europe with 2-12 years' paediatric experience collected clinical data. Training was given in the recognition of clinical signs, including videos for respiratory signs.21 Data were recorded on standardised proforma that also served as the clinical notes. Table 1 gives definitions of clinical signs.
Table 1.
Definitions of clinical signs
| Definition (age to which it applies)
|
|
|---|---|
| Prostrate | Inability to sit unassisted (⩾1 year); inability to drink or breast feed (<1 year) |
| Impaired consciousness | Inability to localise a painful stimulus (>8 months); non-directed eye movements (⩽8 months) |
| Raised respiratory rate | >50/min (<12 months); >40/min (⩾1 year) |
| Subcostal recession | Indrawing of lower chest wall* |
| Severe anaemia | Haemoglobin concentration <50 g/l |
| History of diarrhoea | ⩾3 episodes in 24 hours |
| Hyperthermia | Axillary temperature >39°C |
| Hypothermia | Axillary temperature <36°C |
| Wasting | WAZ <−3† |
Differentiated from deep breathing (Kussmaul's respiration).
Weight for age (WAZ) and height for age (HAZ) z scores calculated with reference data from American National Center for Health Statistics (NCHS). Scores <−3 indicate severe wasting.
Sampling and laboratory methods
After we obtained consent from the carers in their own languages a blood sample was taken from all children admitted. For diagnosis of malaria thick and thin blood smears were examined at ×1000. A full blood count was performed with a Coulter MDII-18 counter (Beckman-Coulter, USA). Results were available immediately to clinicians. We investigated the association between HIV infection and mortality in a nested case-control study using stored plasma samples from all children who died during a six month period. We used a random number table to select four times as many controls as cases, stratified by age (<18 months and ⩾18 months). Anonymous HIV testing was performed using enzyme linked immunosorbent assay (ELISA, Vironostika, Organon Teknika, USA) and by an IgG antibody capture particle agglutination test (Central Public Health Laboratory, London). Samples from children age <18 months in which results were positive were confirmed by polymerase chain reaction (PCR) for proviral DNA.22 All laboratory procedures were monitored through internal and external quality assessment protocols.
Immediate, early, and late deaths
We had observed differences in presentation between children who died soon after admission and those who died later. Furthermore, there seemed to be a bimodal distribution of deaths. For example, most deaths in children with a clinical syndrome similar to acute severe malaria occurred within 48 hours after admission, while other deaths generally occurred later. We therefore hypothesised that the predictors of early and late mortality differed and developed these as separate models. We also wanted to distinguish children close to death on admission, in whom a model might be the diagnosis of the process of dying rather than prediction. Thus we divided deaths into immediate (within four hours after admission), early (4-48 hours), and late (>48 hours).
Statistical methods
We double entered and verified data using FoxPro for Windows. We used EpiInfo 6.04b and Stata 6.0 for statistical analysis.
We used data from children admitted in the first two years to investigate the prognostic ability of clinical features and to derive prognostic models. The positive and negative likelihood ratios were calculated for immediate, early, and late deaths. We selected variables that were univariately predictive, with a likelihood ratio ⩾2 or ⩽0.5, and adjusted them for potential confounding of related variables in a multivariate analysis according to the method of Spiegelhalter and Knill-Jones.23,24 The final indicators were chosen by further excluding any variables with an adjusted likelihood of ⩽1.5 and ⩾0.67, suggesting they were of little independent predictive value. Simplified prognostic scores were constructed by assigning points approximating to the natural logarithm of the adjusted likelihood ratio for each indicator. When potential for a negative score existed we added a constant.
We prospectively validated the prognostic scores using data collected from children in the third year of the study. We used receiver operating curve characteristic analysis and calculated the area under the curve.
Results
Patient characteristics
From 1 July 1998 to 30 June 2000, 8477 children (47% girls) aged 90 days to 13 years were admitted. Of these, we excluded 386 who were admitted electively or after trauma. Of the 8091 included children, 4314 (53%) were <2 years of age, 2687 (33%) were 2-5 years, and 1090 (14%) were >5 years old. The mean z scores for weight for age and height for age were –1.95 (SD 1.41) and –1.32 (SD 1.21), respectively. Carers of 2031 (25%) children reported that the child had had convulsions, 974 (12%) children were prostrate, 572 (7%) had impaired consciousness, and 1853 (23%) had respiratory distress (deep breathing or subcostal indrawing). In 4537 (56%) children test results for malaria were positive, and 1264 (16%) were severely anaemic.
Four hundred and thirty six children (5%) died while in hospital. Sixty (14%) deaths were classified as immediate, 193 (44%) as early, and 183 (42%) as late. HIV infection was detected in 17/79 (22%) children who died between 1 October 1999 and 31 March 2000 and in 13/311 (4%) children who were discharged alive (odds ratio 6.29, 2.71 to 14.7). The proportion was similar in children who died before and after 48 hours after admission.
Clinical prognostic indicators
Table 2 shows the crude likelihood ratios for clinical features in predicting immediate, early, and late deaths. Table 3 shows the adjusted likelihood ratios.
Table 2.
Clinical predictors of immediate, early, and late death in children admitted to hospital in Kenya. Figures are numbers of children* and likelihood ratios
| Alive (n=7655)
|
Immediate (n=60)
|
Early (n=193)
|
Late (n=183)
|
Crude likelihood ratio (95% CI)
|
||||
|---|---|---|---|---|---|---|---|---|
| Immediate
|
Early
|
Late
|
||||||
| Length of history (days): | ||||||||
| ⩽7 | 6729 | 54 | 152 | 92 | 0.96 (0.87 to 1.07) | 0.89 (0.83 to 0.96) | 0.57 (0.49 to 0.66) | |
| >7 | 898 | 9 | 41 | 91 | 1.27 (0.70 to 2.33) | 1.80 (1.37 to 2.38) | 4.22 (3.61 to 4.95) | |
| History of fever: | ||||||||
| No | 1002 | 13 | 24 | 50 | 1.66 (1.02 to 2.69) | 0.95 (0.65 to 1.39) | 2.09 (1.64 to 2.66) | |
| Yes | 6653 | 47 | 168 | 133 | 0.90 (0.79 to 1.03) | 1.01 (0.95 to 1.06) | 0.84 (0.76 to 0.91) | |
| History of diarrhoea: | ||||||||
| No | 5928 | 47 | 139 | 96 | 1.01 (0.89 to 1.16) | 0.93 (0.86 to 1.02) | 0.68 (0.59 to 0.78) | |
| Yes | 1727 | 13 | 53 | 87 | 0.96 (0.59 to 1.56) | 1.22 (0.97 to 1.54) | 2.11 (1.80 to 2.47) | |
| History of cough: | ||||||||
| No | 4028 | 36 | 95 | 71 | 1.14 (0.92 to 1.40) | 0.94 (0.81 to 1.09) | 0.74 (0.61 to 0.89) | |
| Yes | 3626 | 24 | 97 | 112 | 0.84 (0.62 to 1.15) | 1.07 (0.93 to 1.23) | 1.29 (1.15 to 1.45) | |
| History of seizures: | ||||||||
| No | 5722 | 41 | 130 | 163 | 0.91 (0.77 to 1.09) | 0.91 (0.82 to 1.00) | 1.19 (1.13 to 1.26) | |
| Yes | 1930 | 19 | 62 | 20 | 1.26 (0.86 to 1.82) | 1.27 (1.03 to 1.57) | 0.43 (0.29 to 0.66) | |
| Jaundice: | ||||||||
| No | 7502 | 55 | 184 | 179 | 0.95 (0.89 to 1.02) | 0.97 (0.94 to 0.99) | 1.00 (0.98 to 1.02) | |
| Yes | 152 | 4 | 9 | 4 | 3.41 (1.31 to 8.91) | 2.36 (1.22 to 4.55) | 1.10 (0.41 to 2.94) | |
| Severe anaemia (haemoglobin <50 g/l): | ||||||||
| No | 3436 | 30 | 148 | 149 | 0.62 (0.49 to 0.79) | 0.91 (0.85 to 0.99) | 0.96 (0.90 to 1.03) | |
| Yes | 1160 | 27 | 43 | 34 | 3.10 (2.15 to 4.10) | 1.47 (1.13 to 1.93) | 1.22 (0.89 to 1.66) | |
| Wasting: | ||||||||
| No | 6241 | 39 | 162 | 65 | 0.85 (0.72 to 1.01) | 0.75 (0.67 to 0.84) | 0.44 (0.36 to 0.53) | |
| Yes | 1405 | 17 | 74 | 118 | 1.65 (1.11 to 2.46) | 2.12 (1.76 to 2.55) | 3.51 (3.12 to 3.95) | |
| Kwashiorkor: | ||||||||
| No | 6844 | 50 | 144 | 84 | 0.93 (0.83 to 1.04) | 0.84 (0.78 to 0.91) | 0.51 (0.44 to 0.60) | |
| Yes | 806 | 10 | 47 | 99 | 1.58 (0.89 to 2.80) | 2.34 (1.81 to 3.02) | 5.13 (4.43 to 5.96) | |
| Temperature: | ||||||||
| <36°C | 203 | 7 | 13 | 22 | 4.70 (2.32 to 9.52) | 2.61 (1.52 to 4.49) | 4.54 (3.00 to 6.88) | |
| 36-39°C | 4934 | 41 | 128 | 143 | 1.13 (0.97 to 1.33) | 1.06 (0.96 to 1.17) | 1.21 (1.12 to 1.31) | |
| >39°C | 2490 | 8 | 46 | 17 | 0.44 (0.23 to 0.83) | 0.75 (0.57 to 0.97) | 0.29 (0.18 to 0.45) | |
| Raised respiratory rate: | ||||||||
| No | 5373 | 26 | 98 | 119 | 0.75 (0.57 to 0.97) | 0.73 (0.64 to 0.85) | 0.94 (0.85 to 1.05) | |
| Yes | 2176 | 23 | 90 | 58 | 1.63 (1.21 to 2.20) | 1.66 (1.42 to 1.94) | 1.14 (0.92 to 1.41) | |
| Subcostal indrawing: | ||||||||
| No | 6213 | 24 | 107 | 135 | 0.49 (0.36 to 0.47) | 0.68 (0.60 to 0.77) | 0.91 (0.83 to 0.99) | |
| Yes | 1432 | 36 | 86 | 48 | 3.20 (2.59 to 3.96) | 2.38 (2.02 to 2.80) | 1.40 (1.09 to 1.79) | |
| Deep breathing: | ||||||||
| No | 7182 | 26 | 132 | 166 | 0.46 (0.35 to 0.62) | 0.73 (0.66 to 0.80) | 0.97 (0.92 to 1.04) | |
| Yes | 468 | 34 | 61 | 17 | 9.26 (7.30 to 11.8) | 5.17 (4.12 to 6.47) | 1.52 (0.96 to 2.41) | |
| Neurological status: | ||||||||
| Not prostrated | 6851 | 13 | 91 | 162 | 0.24 (0.15 to 0.39) | 0.53 (0.45 to 0.61) | 0.99 (0.94 to 1.04) | |
| Prostrated with seizures | 206 | 1 | 11 | 1 | 0.62 (0.09 to 4.35) | 2.12 (1.17 to 3.82) | 0.20 (0.03 to 1.44) | |
| Prostrated without seizures | 364 | 17 | 43 | 9 | 5.94 (3.93 to 9.00) | 4.67 (3.53 to 6.20) | 1.03 (0.54 to 1.97) | |
| Impaired consciousness with seizures | 147 | 12 | 17 | 6 | 10.4 (6.13 to 17.7) | 4.59 (2.83 to 7.42) | 1.71 (0.76 to 3.81) | |
| Impaired consciousness without seizures | 67 | 17 | 30 | 5 | 32.4 (20.3 to 51.7) | 17.8 (11.8 to 26.7) | 3.12 (1.27 to 7.65) | |
Data are missing where numbers are less than the totals given in each column.
Table 3.
Adjusted likelihood ratios and simplified prognostic scores for death in children admitted to hospital in Kenya
| Indicators
|
Adjusted likelihood ratios
|
Simplified scores
|
|||
|---|---|---|---|---|---|
| Indicator present
|
Indicator absent
|
Indicator present
|
Indicator absent
|
||
| Immediate deaths | |||||
| Severe anaemia (haemoglobin <50 g/l) | 1.76 | 0.79 | +1 | +0 | |
| Jaundice | 1.89 | 0.97 | +1 | +0 | |
| Subcostal indrawing | 2.51 | 0.57 | +1 | −1 | |
| Deep breathing | 2.08 | 0.77 | +1 | +0 | |
| Prostrated with seizures | 2.03 | 0.99 | +1 | +0 | |
| Prostrated without seizures | 14.1 | 0.80 | +3 | +0 | |
| Impaired consciousness with seizures | 9.27 | 0.70 | +2 | +0 | |
| Impaired consciousness without seizures | 32.2 | 0.72 | +3 | +0 | |
| Axillary temperature <36 °C | 1.75 | 0.96 | +1 | +0 | |
| Axillary temperature >39 °C | 0.59 | 1.17 | −1 | +0 | |
| Constant | +2 | ||||
| Early deaths | |||||
| Jaundice | 1.99 | 0.98 | +1 | +0 | |
| Subcostal indrawing | 2.18 | 0.71 | +1 | +0 | |
| Prostrated with seizures | 4.70 | 0.94 | +2 | +0 | |
| Prostrated without seizures | 5.11 | 0.93 | +2 | +0 | |
| Impaired consciousness with seizures | 6.97 | 0.78 | +2 | +0 | |
| Impaired consciousness without seizures | 21.2 | 0.84 | +3 | +0 | |
| Wasting | 1.58 | 0.84 | +1 | +0 | |
| Kwashiorkor | 2.55 | 0.83 | +1 | +0 | |
| Constant | +0 | ||||
| Late deaths | |||||
| History >7 days | 1.88 | 0.78 | +1 | +0 | |
| Prostrated with seizures | 0.54 | 1.01 | −1 | +0 | |
| Prostrated without seizures | 1.53 | 0.99 | +0 | +0 | |
| Impaired consciousness with seizures | 2.24 | 1.03 | +1 | +0 | |
| Impaired consciousness without seizures | 3.16 | 0.98 | +1 | +0 | |
| Axillary temperature <36 °C | 2.30 | 0.94 | +1 | +0 | |
| Axillary temperature >39 °C | 0.57 | 1.15 | −1 | +0 | |
| Wasting | 2.05 | 0.63 | +1 | +0 | |
| Kwashiorkor | 2.09 | 0.74 | +1 | +0 | |
| Constant | +2 | ||||
For immediate deaths, severe anaemia, jaundice, subcostal indrawing, deep breathing, neurological status, and axillary temperature were univariately predictive (table 2). When we included these features in a multivariate analysis they all remained predictive (table 3).
Jaundice, subcostal indrawing, deep breathing, neurological status, axillary temperature, wasting, and kwashiorkor were univariately predictive for early deaths (table 2). When we included these features in the multivariate model, the likelihood ratios for deep breathing and axillary temperature were poorly predictive and were excluded (table 3).
For late deaths, neurological status, axillary temperature, wasting, kwashiorkor, history >7 days, seizures, fever, and diarrhoea were univariately predictive and were included in the multivariate analysis (table 2). The adjusted likelihood ratios for a history of fever, seizures, and diarrhoea had reduced prognostic ability so we excluded these variables from the late score (table 3). The resulting prognostic scores ranged from 0 to 10 for immediate deaths and from 0 to 7 for early and late deaths.
Validation of prognostic scores
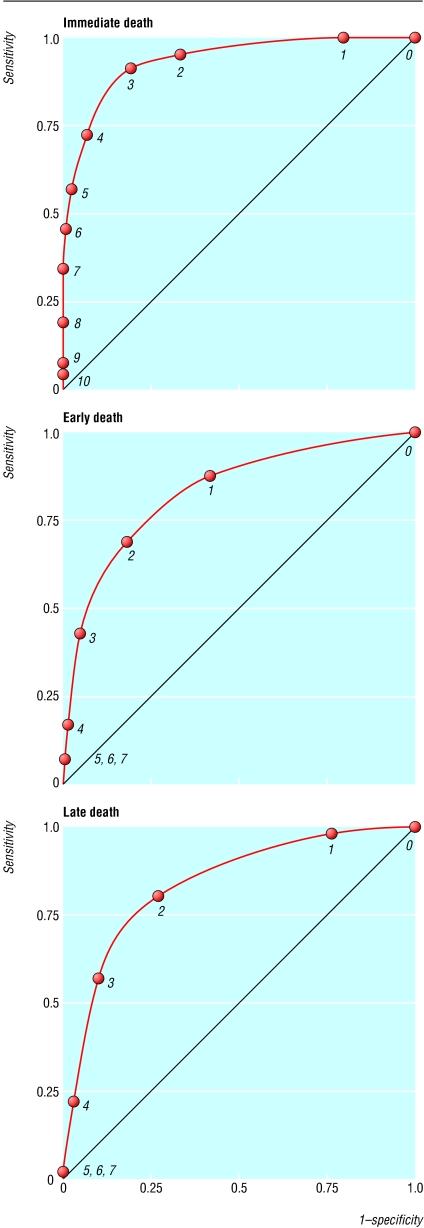
Between 1 July 2000 and 30 June 2001, 4995 children age >90 days were admitted. We excluded 193 who were admitted for trauma or elective admissions. This left 4802 children, of whom 222 (4.6%) died: 26 (12%) immediate, 88 (40%) early, and 108 (48%) late. Table 4 shows the distributions of immediate, early, and late prognostic scores. The areas under the receiver operating characteristic curves for the scores were 0.93 (95% confidence interval 0.92 to 0.94) for immediate, 0.82 (0.80 to 0.83) for early, and 0.82 (0.81 to 0.84) for late deaths (fig).
Table 4.
Distribution of prognostic scores and outcome in 4802 children admitted to hospital in Kenya
| Score
|
Immediate
|
Early
|
Late
|
|||||
|---|---|---|---|---|---|---|---|---|
| No admitted
|
No (%) who died
|
No admitted
|
No (%) who died
|
No admitted
|
No (%) who died
|
|||
| 0 | 698 | 0 | 2779 | 11 (0.4) | 15 | 0 | ||
| 1 | 2196 | 1 (0.1) | 1101 | 16 (1.5) | 1081 | 2 (0.2) | ||
| 2 | 657 | 1 (0.2) | 668 | 23 (3.4) | 2310 | 18 (0.8) | ||
| 3 | 612 | 5 (0.8) | 183 | 23 (13) | 869 | 26 (3.0) | ||
| 4 | 217 | 4 (1.8) | 55 | 10 (18) | 347 | 38 (11) | ||
| 5 | 72 | 3 (4.2) | 15 | 5 (33) | 170 | 22 (13) | ||
| 6 | 34 | 3 (8.8) | 1 | 0 | 10 | 2 (20) | ||
| 7 | 31 | 4 (13) | 0 | 0 | 0 | 0 | ||
| 8 | 12 | 3 (25) | — | — | — | — | ||
| 9 | 2 | 1 (50) | — | — | — | — | ||
| 10 | 1 | 1 (100) | — | — | — | — | ||
Discussion
Our prognostic scores showed good predictive ability despite being based on simple clinical signs. None of the indicators was specific for aetiology, which suggests that they could be applicable throughout the region. Our results need to be externally validated, ideally, in areas of differing HIV prevalence and malaria transmission. About half of the children admitted and a third of those who died had a malaria parasitaemia, and the overall rate of HIV infection was about 6%. However, nearly a quarter of children who died and were tested were infected with HIV.
Clinical indicators
Our findings overlap with those of some studies of disease specific prognostic indicators. The principal indicators of immediate and early death are similar to those for malaria10,11 and severe lower respiratory tract infection,12 particularly with respect to the increased risk in afebrile, malnourished children. Importantly, diarrhoea or seizures (other than in prostrate children or those with impaired consciousness) were not significant independent predictors of death. Among prostrate children or those with impaired consciousness, a history of seizures was associated with a better outcome, suggesting a postictal state in many of these children. We did not include biochemical or microbiological variables in our models as laboratory facilities are not widely available.
Our treatment practices did not differ significantly from those recommended by WHO4 or in other well established guidelines for common illnesses. In common with other African hospitals, the paediatric ward was often overcrowded. Close monitoring was possible only in the high dependency unit. Basic drugs were available throughout the study period but affordable antibiotics—for example, such as penicillin, chloramphenicol, and gentamicin—were normally used. While laboratory services were good, radiography was not always available. The presence of the research unit may have reduced inpatient mortality, though the level of care was not beyond that possible at a district hospital.2
Prognostic scoring systems are usually developed from patients given treatment, which may influence their outcome. “False positives” are therefore expected as many children are admitted in extremis but receive prompt lifesaving treatments such as fluid resuscitation. In practice, although some patients inevitably die despite treatment, prognostic models derived from treated cohorts remain valuable in identifying areas for improvement and in identifying individuals at high risk.7 “False negatives” may also occur when the condition of a child deteriorates after admission. This was seen in the early score, where 11 children in the validation year died early despite having had an early score of zero at admission. The use of negative likelihood ratios allowed the possibility of the inclusion of the prognostic value of the absence of a clinical sign.
Current strategies
The WHO/Unicef integrated management of childhood illness (IMCI) strategy is aimed at improving community based care and referral. Current efforts to improve health care at the first referral level include an algorithm for emergency assessment and triage (ETAT). It is aimed at prioritising children who need admission or urgent resuscitation. Our prognostic scores differ in that the main outcome is inpatient mortality. Mortality varies considerably between hospitals, with patterns of use depending on location and availability of other healthcare options. By adjusting for risk of death, outcomes could be compared either between centres or over time.
We have shown that a small number of important clinical indicators are prognostic for death in children admitted to a rural Kenyan district hospital, irrespective of clinical diagnosis. If these scores can be suitably validated elsewhere in the region, we believe they could be valuable in assessing the clinical care at district hospitals, standardising clinical studies, and identifying children “at risk.”
Figure.
Receiver operating characteristic curves for immediate, early, and late death scores in 4802 children. Numbers on curves refer to scores
Acknowledgments
We thank the medical officer of health, the medical superintendent, and the staff of Kilifi District Hospital for their support, and N Peshu, director of the centre. This paper is published with the permission of the director of KEMRI (Kenyan Medical Research Institute).
Footnotes
Conflict of interest: None declared.
Funding: This study was supported by KEMRI and the Wellcome Trust. JAB is a Wellcome Trust research training fellow in clinical tropical medicine (053439) and KM (031342) and CRJCN (050533) are Wellcome Trust senior clinical fellows.
References
- 1.WHO. World health report 1999: making a difference. Geneva: WHO; 1999. [Google Scholar]
- 2.Snow RW, Howard SC, Mung'Ala-Odera V, English M, Molyneux CS, Waruiru C, et al. Paediatric survival and re-admission risks following hospitalization on the Kenyan Coast. Trop Med Int Health. 2000;5:377–383. doi: 10.1046/j.1365-3156.2000.00568.x. [DOI] [PubMed] [Google Scholar]
- 3.Nolan T, Angos P, Cunha AJ, Muhe L, Qazi S, Simoes EA, et al. Quality of hospital care for seriously ill children in less-developed countries. Lancet. 2001;357:106–110. doi: 10.1016/S0140-6736(00)03542-X. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Management of the child with a serious infection or severe malnutrition. Guidelines at the first referral level in developing countries. Geneva: WHO; 2000. [Google Scholar]
- 5.Gove S, Tamburlini G, Molyneux E, Whitesell P, Campbell H. Development and technical basis of simplified guidelines for emergency triage assessment and treatment in developing countries. WHO integrated management of childhood illness (IMCI) referral care project. Arch Dis Child. 1999;81:473–477. doi: 10.1136/adc.81.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molyneux E. Paediatric emergency care in developing countries. Lancet. 2001;357:86–87. doi: 10.1016/S0140-6736(00)03536-4. [DOI] [PubMed] [Google Scholar]
- 7.Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323:224–228. doi: 10.1136/bmj.323.7306.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booy R, Habibi P, Nadel S, de Munter C, Britto J, Morrison A, et al. Reduction in case fatality rate from meningococcal disease associated with improved healthcare delivery. Arch Dis Child. 2001;85:386–390. doi: 10.1136/adc.85.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorburn K, Baines P, Thomson A, Hart CA. Mortality in severe meningococcal disease. Arch Dis Child. 2001;85:382–385. doi: 10.1136/adc.85.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 11.Schellenberg D, Menendez C, Kahigwa E, Font F, Galindo C, Acosta C, et al. African children with malaria in an area of intense Plasmodium falciparum transmission: features on admission to the hospital and risk factors for death. Am J Trop Med Hyg. 1999;61:431–438. doi: 10.4269/ajtmh.1999.61.431. [DOI] [PubMed] [Google Scholar]
- 12.Shann F, Barker J, Poore P. Clinical signs that predict death in children with severe pneumonia. Pediatr Infect Dis J. 1989;8:852–855. doi: 10.1097/00006454-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal V, Sethi GR, Sachdev HP, Satyanarayana L. Predictors of mortality in subjects hospitalized with acute lower respiratory tract infections. Indian Pediatr. 1997;34:213–219. [PubMed] [Google Scholar]
- 14.Bhutta ZA, Nizami SQ, Thobani S, Issani Z. Risk factors for mortality among hospitalized children with persistent diarrhoea in Pakistan. J Trop Pediatr. 1997;43:330–336. doi: 10.1093/tropej/43.6.330. [DOI] [PubMed] [Google Scholar]
- 15.Sachdev HP, Kumar S, Singh KK, Satyanarayana L, Puri RK. Risk factors for fatal diarrhea in hospitalized children in India. J Pediatr Gastroenterol Nutr. 1991;12:76–81. doi: 10.1097/00005176-199101000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Collins S, Myatt M. Short-term prognosis in severe adult and adolescent malnutrition during famine: use of a simple prognostic model based on counting clinical signs. JAMA. 2000;284:621–626. doi: 10.1001/jama.284.5.621. [DOI] [PubMed] [Google Scholar]
- 17.Dramaix M, Brasseur D, Donnen P, Bawhere P, Porignon D, Tonglet R, et al. Prognostic indices for mortality in hospitalized children in central Africa. Am J Epidemiol. 1996;143:1235–1243. doi: 10.1093/oxfordjournals.aje.a008711. [DOI] [PubMed] [Google Scholar]
- 18.Man WD, Weber M, Palmer A, Schneider G, Wadda R, Jaffar S, et al. Nutritional status of children admitted to hospital with different diseases and its relationship to outcome in The Gambia, West Africa. Trop Med Int Health. 1998;3:678–686. doi: 10.1046/j.1365-3156.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–665. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 20.Berkley JA, Mwangi I, Mellington F, Mwarumba S, Marsh K. Cerebral malaria versus bacterial meningitis in children with impaired consciousness. Q J Med. 1999;92:151–157. doi: 10.1093/qjmed/92.3.151. [DOI] [PubMed] [Google Scholar]
- 21.English M, Murphy S, Mwangi I, Crawley J, Peshu N, Marsh K. Interobserver variation in respiratory signs of severe malaria. Arch Dis Child. 1995;72:334–336. doi: 10.1136/adc.72.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham SM, Mtitimila EI, Kamanga HS, Walsh AL, Hart CA, Molyneux ME. Clinical presentation and outcome of Pneumocystis carinii pneumonia in Malawian children. Lancet. 2000;355:369–373. doi: 10.1016/S0140-6736(98)11074-7. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelhalter DJ, Knill-Jones RP. Statistical and knowledge-based approaches to clinical decision-support systems, with an application in gastroenterology. J R Stat Soc. 1984;147:35–77. [Google Scholar]
- 24.Straus SE, McAlister FA, Sackett DL, Deeks JJ. The accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. CARE-COAD1 Group. Clinical assessment of the reliability of the examination-chronic obstructive airways disease. JAMA. 2000;283:1853–1857. doi: 10.1001/jama.283.14.1853. [DOI] [PubMed] [Google Scholar]