Abstract
Rotavirus circulates extraintestinally in animals used as models for rotavirus infection and in children. Rotavirus infection in mice was used to define host or viral factors that affect rotavirus viremia. Antigenemia was observed with homologous and heterologous rotaviruses, and neither age nor mouse strain genetics altered the occurrence of rotavirus antigenemia or viremia. Rotavirus RNA and infectious virus were present in sera and associated with the plasma fraction of blood in all infected mice. These findings indicate that antigenemia/viremia occurs routinely in rotavirus infections and imply that infectious rotavirus has access to any extraintestinal cell within contact of blood.
Rotavirus infection and disease are worldwide health concerns resulting in 111 million episodes of diarrhea in children <5 years of age (25). Initially, it was thought that rotavirus infection was restricted to the gastrointestinal tract. However, the detection of rotavirus proteins or RNA outside the intestine (7, 9, 16, 18-20, 23) suggested that rotavirus infection is not limited to the intestine. Extraintestinal rotavirus has been attributed to infections with specific rotavirus strains or in children with immunologic defects (14). However, we and others have demonstrated that proteins and RNA of rotavirus can be commonly detected in the sera of children infected with rotavirus (2, 7, 13).
The mouse model has been widely utilized to define the pathogenesis of rotavirus (5, 12, 15, 27, 31). Both homologous and heterologous rotaviruses have been shown to cause viremia in both infant and adult mice (2, 17, 21). Rotavirus has also been associated with two migrating cell populations isolated from lymph nodes of infected mice, B cells and macrophages (4), suggesting that rotavirus viremia can be both plasma and cell associated. However, rotavirus antigenemia was found to be plasma associated rather than cell associated in piglets (1). In the studies described here, we further investigated the properties of rotavirus viremia in mice and demonstrate the predominant association of the virus with the plasma fraction of blood.
Rotavirus antigenemia does not depend on dose of viral inoculum or on genetic strain or age of mouse.
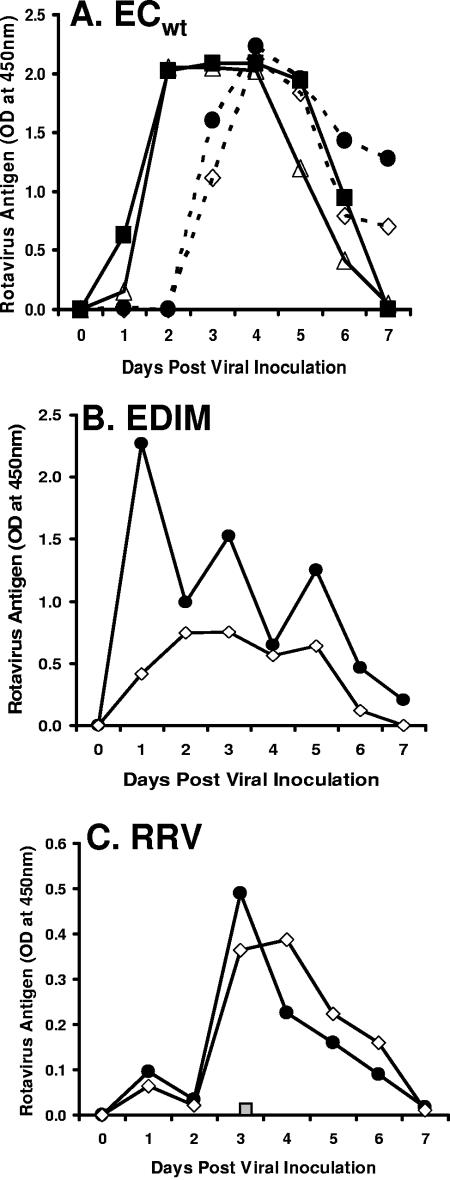
Six- to eight-week-old female outbred CD-1 mice (Charles River Laboratories, Wilmington, MA) were inoculated with 10 or 105 50% infectious doses (ID50) of the murine rotavirus strain ECwt (G3P[17]) (12), 104 ID50 of the murine rotavirus strain EDIM (G3P[17]) (31), 10 ID50 (∼109 PFU) of the rhesus rotavirus strain RRV (G3P5[3]) (28), or an equivalent amount of inactivated RRV (6). To detect antigenemia, fecal and serum samples collected from individual mice were analyzed by enzyme-linked immunosorbent assay (ELISA) (24). Samples with an optical density at 450 nm of >0.100 were considered positive for virus. Antigenemia was detected concurrently with fecal rotavirus excretion at both low and high ID50 inocula (Fig. 1). Antigen was not detected in fecal or serum samples collected prior to 24 h post-viral inoculation. Antigenemia required replication because inactivated RRV did not result in antigenemia (Fig. 1C). This conclusion is consistent with the report that in piglets, nonreplicating virus-like particles do not cause antigenemia (1).
FIG. 1.
Rotavirus antigenemia is not rotavirus strain dependent. Rotavirus naïve adult CD-1 mice were orally inoculated on day 0 with the indicated strains of rotavirus. Mice were sacrificed at the indicated time points and 10% (wt/vol) fecal suspensions (squares and circles) and undiluted sera (open diamonds and open triangles) analyzed for rotavirus antigens by ELISA. Points represent average values for each time point (n = 3 to 5). A. Mice were inoculated with either 10 ID50 (dashed lines) or 105 ID50 (solid lines) ECwt. B. Mice were inoculated with 104 ID50 EDIM. C. Mice were inoculated with 10 ID50 RRV. The gray box indicates mice inoculated with inactivated RRV (n = 3).
We recently reported that susceptibility to rotavirus infection is genetically determined in mice (3). To determine whether genetic background influenced antigenemia, several mouse strains (CD-1, CF-1, BALB/c, C57BL/6, and 129) were orally inoculated with a dose of ECwt equivalent to 105 ID50 in CD-1 mice. Antigenemia was present and approximately equivalent in all mouse strains examined (data not shown), indicating that rotavirus antigenemia does not depend on host genetics.
To determine whether rotavirus antigenemia was dependent on age, three litters of five-day-old CD-1 pups (Charles River Laboratories) were orally inoculated with 105 ID50 ECwt. Four days after inoculations, sera and intestines from each litter were pooled and tested for rotavirus antigenemia. All sera and intestinal homogenate pools from ECwt inoculated mice were antigen positive (Fig. 2A), indicating that rotavirus antigenemia also occurs in infant mice.
FIG. 2.
Detection of rotavirus antigens. A. Six litters of 5-day-old naïve CD-1 pups were randomized (6 to 8 mice/litter) and administered either phosphate-buffered saline (PBS) (white bars, n = 3 litters) or 105 ID50 ECwt (black bars, n = 3 litters). Four days after inoculation, the intestines and the blood from each litter were pooled, gut homogenates or sera recovered, respectively, and samples analyzed for rotavirus antigens by ELISA. Bars represent average values of samples pooled from each of three litters ± the standard deviation. B. Naïve adult CD-1 mice were orally inoculated with 105 ID50 ECwt (black bars) or PBS (white bars). Three days after inoculation, whole blood was collected in EDTA-containing collection tubes for each mouse to prevent clotting. Cells were recovered by low-speed centrifugation after a washing and cell lysates made by addition of 150 μl PBS to each tube followed by three freeze-thaw cycles. Plasma and cell fractions were analyzed by ELISA for the presence of rotavirus antigens. Each bar represents the average value from three mice ± the standard deviation.
Rotavirus antigenemia and viremia are associated with the plasma fraction of blood.
Whole blood collected using lithium heparin or potassium EDTA 3 to 4 days after inoculation of CD-1 mice with 105 ID50 ECwt was separated into plasma and cell fractions. Each fraction was analyzed for rotavirus antigenemia by ELISA or for infectious virus by testing the ability of the sample obtained from the donor mouse to cause rotavirus fecal excretion in a naïve mouse (recipient). Rotavirus antigen was detected in the plasma but not the cell fraction (Fig. 2B). Both sera and plasma, but not cell lysates, collected from ECwt inoculated infant or adult donor mice resulted in rotavirus fecal shedding in recipient mice (Table 1). Neither plasma collected using EDTA (resulting in the generation of noninfectious double-layered particles) nor sera, plasma, or cell lysates isolated from uninfected donor animals initiated infection in recipient mice (Table 1). Infection of infant mice with homologous rotavirus resulted in antigenemia (Fig. 2A), viremia (Table 1), and disease at 4 days postinoculation (data not shown); but heterologous rotavirus viremia was observed only at 24 to 48 h after inoculation (22), suggesting that the kinetics of homologous and heterologous rotavirus viremia may differ in pups or that viremia is dependent on virulence of the infecting strain. Another explanation may involve the difference in PFU/ID50 ratio which has been reported to be 104 to 105 times higher for RRV than ECwt (12). Our findings indicate that active plasma-associated viremia is a prominent feature in rotavirus infection. Rotavirus has been detected in mouse lymph node macrophages, dendritic cells, and B cells (4, 11), as well as infectious virus isolated from blood cells from immunocompromised mice inoculated with the live Rotashield vaccine (26), suggesting that rotavirus viremia is also cell associated. Our lack of detection of rotavirus in blood cells suggests that cell-associated viremia occurs at low levels or in small numbers of circulating cells. Further work is needed to determine the possible role of cell associated viremia in rotavirus pathogenesis and whether it is a common feature of infections in humans and other animals.
TABLE 1.
Infectious virus is present in the plasma fraction of sera from ECwt-infected micea
| Donor sample | Donor antigenemia | Donor age | Recipient mice infected/totalb |
|---|---|---|---|
| Serum | − | Adult | 0/6 |
| + | Adult | 6/6 | |
| Diluted serumc | + | Adult | 1/6 |
| Serum pool | − | Pup | 0/3 |
| + | Pup | 3/3 | |
| Plasma | − | Adult | 0/3 |
| + | Adult | 6/6 | |
| Cells | − | Adult | 0/3 |
| + | Adult | 0/6 | |
| Plasma/EDTAd | + | Adult | 0/3 |
To detect infectious virus, sample was collected at 3 to 4 days postinfection from an ECwt-inoculated mouse (donor) and administered to a naïve mouse (recipient).
Number of naïve recipient mice excreting rotavirus antigen after oral inoculation with a sample from donor mouse/total number of mice inoculated.
Serum samples diluted 1:10.
Plasma obtained after treatment of whole blood with 25 mM EDTA, which prevents clotting by Ca2+ chelation and also removes the outer capsid proteins from infectious virus (to yield double-layered particles which are not infectious).
Quantification of infectious virus present in sera from mice excreting rotavirus.
Two approaches were utilized to estimate the amount of infectious virus present in sera. First, donor sera from ECwt infected mice were diluted 1:10 and administered to naïve recipient mice. Only one of six naïve recipient mice became infected with rotavirus, suggesting the infectious virus titer in the sera is low (Table 1). Second, the number of double-stranded RNA copies/μl was quantitated at the peak of antigenemia by quantitative reverse transcriptase PCR (QRT-PCR), as described previously (11). For QRT-PCR, a primer pair specific to the ECwt NSP3 gene was used. Sera from infected mice contained double-stranded RNA (Table 2), but the amount varied greatly (0 to 1,236 copies/μl). Similar variation in copy number was observed in feces (2.4 × 105 to 2.4 × 108 copies/μl), but the level in feces greatly exceeded that in sera (Table 2). The low RNA copy number was not consistent with the high amounts of protein detectable in both sera and feces by ELISA. The apparent difference in rotavirus RNA and protein levels in sera and feces suggests the production of excess amounts of free viral proteins, release of noninfectious rotavirus particles that do not contain RNA, or disruption of virus and degradation of viral RNA in sera. Fischer et al. (13) reported discrepancies in which human serum samples were rotavirus positive by ELISA and rotavirus negative by RT-PCR and vice versa, supporting the idea that there is a discordance in the results between the two methods in the detection of rotavirus in the blood. Similar discrepancies were also observed between ELISA and QRT-PCR results for rotavirus-infected neonatal mice (11). However, studies in rat pups have shown a correlation between antigenemia and infectious virus (10). Further studies are necessary to determine whether rotavirus protein, RNA, and infectious virus in the blood do not always agree due to methodology issues or biologic differences.
TABLE 2.
QRT-PCR determination of total viral RNA levels in sera and fecal suspensions from ECwt-infected micea
| Mouse | Value for indicated sample typeb:
|
|||
|---|---|---|---|---|
| Serum samples
|
Fecal samples
|
|||
| RNAc | Proteind | RNAc | Proteind | |
| 1 | 1,668 | 2.316 | 192,000,000 | 2.996 |
| 2 | 0 | 0.236 | 381,000 | 2.122 |
| 3 | 1,236 | 2.433 | 240,000,000 | 3.020 |
| 4 | 0 | 2.781 | 28,380,000 | 3.030 |
| 5 | 1,452 | 2.532 | 240,000 | 2.908 |
Samples were collected 3 days postinfection.
Serum samples were analyzed undiluted, and fecal samples were analyzed as 10% (wt/vol) solutions.
Numbers indicate average copies of double-stranded RNA/μl.
Numbers indicate optical density at 450 nm.
The identification of rotavirus viremia raises important questions as to whether viremia occurs solely as a result of intestinal replication or whether replication of virus at extraintestinal sites also contributes to viremia. Our work demonstrates that rotavirus replication is necessary for viremia to be established in mice, and the kinetics of antigen detection in feces compared to that in sera indicates that antigenemia lags behind intestinal replication. Although it is experimentally difficult to prove unequivocally, the suggestion that intestinal replication precedes the presence of antigen or infectious virus in the blood is supported by kinetic studies with mouse pups (11, 22), rats (10), and pigs (1). The presence of infectious rotavirus within the circulatory system provides one explanation for the findings of rotavirus at extraintestinal locations (30). Determination of whether the virus in the circulatory system represents virus produced in the intestine, at extraintestinal sites, or both in the intestine and at extraintestinal sites will require development of a model in which viremia and intestinal replication are discordant or methods that can differentiate the origin of viral replication, neither of which are currently available.
One additional consequence of viremia, beyond infection of extraintestinal tissues, is the enhancement of intestinal infection. Three previous findings support the idea that rotavirus viremia could result in enhanced intestinal infection: (i) in vitro results demonstrating that Caco-2 cells can be infected by rotavirus at the basolateral surface (8, 29), (ii) intravenous inoculation of gnotobiotic piglets with rotavirus results in intestinal virus shedding (1), and (iii) subcutaneous and intraperitoneal administration of RRV to neonatal mice results in intestinal infection (21). Clearly, infectious virus circulating in the blood may gain retrograde access to the intestine, as well as most tissues. However, the lack of technical approaches to separate the circulatory system from the intestine and extraintestinal organs limits our current ability to address the source of rotavirus viremia and its impact on intestinal and extraintestinal infection. New approaches are needed to gain more information as to the source of rotavirus viremia and its contribution to rotavirus pathogenesis.
Acknowledgments
The authors would like to thank Sue Crawford and Mary Estes for helpful discussions and David Keeland, Erin Sargent, Jillian Pennington, and Fred Basile for excellent technical assistance.
This work was supported by NIH AI10604, NIH AI24998, NIH AI21362, the Gulf Coast Digestive Disease Center (NIH DK56338), and by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
REFERENCES
- 1.Azevedo, M. S., L. Yuan, K. I. Jeong, A. Gonzalez, T. V. Nguyen, S. Pouly, M. Gochnauer, W. Zhang, A. Azevedo, and L. J. Saif. 2005. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J. Virol. 79:5428-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 3.Blutt, S. E., K. L. Warfield, C. M. O'Neal, M. K. Estes, and M. E. Conner. 2006. Host, viral, and vaccine factors that determine protective efficacy induced by rotavirus and virus-like particles (VLPs). Vaccine 24:1170-1179. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. A., and P. A. Offit. 1998. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb. Pathog. 24:327-331. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. W., A. A. Krishnaney, P. T. Vo, R. V. Rouse, L. J. Anderson, and H. B. Greenberg. 1995. Analyses of homologous rotavirus infection in the mouse model. Virology 207:143-153. [DOI] [PubMed] [Google Scholar]
- 6.Casola, A., M. K. Estes, S. E. Crawford, P. L. Ogra, P. B. Ernst, R. P. Garofalo, and S. E. Crowe. 1998. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology 114:947-955. [DOI] [PubMed] [Google Scholar]
- 7.Chiappini, E., C. Azzari, M. Moriondo, L. Galli, and M. de Martino. 2005. Viraemia is a common finding in immunocompetent children with rotavirus infection. J. Med. Virol. 76:265-267. [DOI] [PubMed] [Google Scholar]
- 8.Ciarlet, M., S. E. Crawford, and M. K. Estes. 2001. Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J. Virol. 75:11834-11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cioc, A. M., and G. J. Nuovo. 2002. Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod. Pathol. 15:914-922. [DOI] [PubMed] [Google Scholar]
- 10.Crawford, S. E., D. G. Patel, E. Cheng, Z. Berkova, J. M. Hyser, M. Ciarlet, M. J. Finegold, M. E. Conner, and M. K. Estes. 2006. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J. Virol. 80:4820-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenaux, M., M. A. Cuadras, N. Feng, M. C. Jaimes, and H. B. Greenberg. 2006. Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J. Virol. 80:5219-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, N., J. W. Burns, L. Bracy, and H. B. Greenberg. 1994. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J. Virol. 68:7766-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer, T. K., D. Ashley, T. Kerin, E. Reynolds-Hedmann, J. Gentsch, M. A. Widdowson, L. Westerman, N. Puhr, R. M. Turcios, and R. I. Glass. 2005. Rotavirus antigenemia in patients with acute gastroenteritis. J. Infect. Dis. 192:913-919. [DOI] [PubMed] [Google Scholar]
- 14.Gilger, M. A., D. O. Matson, M. E. Conner, H. M. Rosenblatt, M. J. Finegold, and M. K. Estes. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120:912-917. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg, H. B., P. T. Vo, and R. Jones. 1986. Cultivation and characterization of three strains of murine rotavirus. J. Virol. 57:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iturriza-Gomara, M., I. A. Auchterlonie, W. Zaw, P. Molyneaux, U. Desselberger, and J. Gray. 2002. Rotavirus gastroenteritis and central nervous system (CNS) infection: characterization of the VP7 and VP4 genes of rotavirus strains isolated from paired fecal and cerebrospinal fluid samples from a child with CNS disease. J. Clin. Microbiol. 40:4797-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraft, L. M. 1958. Observations on the control and natural history of epidemic diarrhea of infant mice (EDIM). Yale J. Biol. Med. 31:122-136. [PMC free article] [PubMed] [Google Scholar]
- 18.Li, N., and Z. Y. Wang. 2003. Viremia and extraintestinal infections in infants with rotavirus diarrhea. Di Yi Jun Yi Da. Xue Xue Bao 23:643-648. [PubMed] [Google Scholar]
- 19.Lynch, M., W. J. Shieh, K. Tatti, J. R. Gentsch, T. Ferebee-Harris, B. Jiang, J. Guarner, J. S. Bresee, M. Greenwald, S. Cullen, H. D. Davies, C. Trevenen, S. R. Zaki, and R. I. Glass. 2003. The pathology of rotavirus-associated deaths, using new molecular diagnostics. Clin. Infect. Dis. 37:1327-1333. [DOI] [PubMed] [Google Scholar]
- 20.Morrison, C., T. Gilson, and G. J. Nuovo. 2001. Histologic distribution of fatal rotaviral infection: an immunohistochemical and reverse transcriptase in situ polymerase chain reaction analysis. Hum. Pathol. 32:216-221. [DOI] [PubMed] [Google Scholar]
- 21.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossel, E. C., and R. F. Ramig. 2003. A lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouse. J. Virol. 77:12352-12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuovo, G. J., G. Owor, T. Andrew, and C. Magro. 2002. Histologic distribution of fatal rotaviral pneumonitis: an immunohistochemical and RT in situ PCR analysis. Diagn. Mol. Pathol. 11:140-145. [DOI] [PubMed] [Google Scholar]
- 24.O'Neal, C. M., S. E. Crawford, M. K. Estes, and M. E. Conner. 1997. Rotavirus VLPs administered mucosally induce protective immunity. J. Virol. 71:8707-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parashar, U. D., E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. I. Glass. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao, H., H. F. Clark, M. DiVietro, and M. Riepenhoff-Talty. 2004. A comparison of the effects of oral inoculation with Rotashield and pentavalent reassortant rotavirus vaccine (WC3-PV) on suckling CB17scid mice. J. Gen. Virol. 85:2245-2253. [DOI] [PubMed] [Google Scholar]
- 27.Ramig, R. F. 1988. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb. Pathog. 4:189-202. [DOI] [PubMed] [Google Scholar]
- 28.Stuker, G., L. S. Oshiro, and N. J. Schmidt. 1980. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J. Clin. Microbiol. 11:202-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svensson, L., B. B. Finlay, D. Bass, C. H. von Bonsdorff, and H. B. Greenberg. 1991. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J. Virol. 65:4190-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhnoo, I., M. Riepenhoff-Talty, T. Dharakul, P. Chegas, J. E. Fisher, H. B. Greenberg, and P. L. Ogra. 1990. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J. Virol. 64:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward, R. L., M. M. McNeal, and J. F. Sheridan. 1990. Development of an adult mouse model for studies on protection against rotavirus. J. Virol. 64:5070-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]