Abstract
The productive program of human papillomaviruses (HPVs) in epithelia is tightly linked to squamous differentiation. The E7 proteins of high-risk HPV genotypes efficiently inactivate the pRB family of proteins that control the cell cycle, triggering S phase in suprabasal keratinocytes. This ability has until now not been demonstrated for the low-risk HPV-6 or HPV-11 E7 proteins. An inducible system in which HPV-16 E7 is fused to the ligand binding domain of the human estrogen receptor (ER) was described by Smith-McCune et al. (K. Smith-McCune, D. Kalman, C. Robbins, S. Shivakumar, L. Yuschenkoff, and J. M. Bishop, Proc. Natl. Acad. Sci. USA 96:6999-7004, 1999). In the absence of hormone, E7ER is cytoplasmic, and upon addition of 17β-estradiol, it translocates to the nucleus. Using organotypic epithelial raft cultures developed from primary human keratinocytes, we show that 16E7ER promotes either S-phase reentry or p21cip1 accumulation in differentiated keratinocytes in a stochastic manner as early as 6 h postinduction with 17β-estradiol. A vector expressing the ER moiety alone had no effect. These observations prove unequivocally that the E7 protein drives S-phase reentry in postmitotic, differentiated keratinocytes rather than preventing S-phase exit while the cells ascend through the epithelium. HPV-11 E7ER and, much less efficiently, HPV-6 E7ER also promoted S-phase reentry by differentiated cells upon exposure to 17β-estradiol. S-phase induction required the consensus pRB binding motif. We propose that the elevated nuclear levels of the low-risk HPV E7 protein afforded by the inducible system account for the positive results. These observations are entirely consistent with the fact that low-risk HPV genotypes replicate in the differentiated strata in patient specimens, as do the high-risk HPVs.
Human papillomaviruses (HPVs) are small DNA viruses that infect cutaneous or mucosal epithelia. The closely related HPV type 6 (HPV-6) and HPV-11 are low-risk (LR) genotypes and cause benign warts in the anogenital tract and laryngeal papillomas in the upper respiratory mucosal epithelium; these infections rarely progress to cancers. In contrast, HPV-16, HPV-18, and related types are high-risk (HR) viruses, and their infections can lead to high-grade squamous intraepithelial neoplasias that pose a risk of further progression to carcinomas in situ and invasive carcinomas (47). HPV infection is thought to initiate in the undifferentiated basal stratum, presumably gaining access through a wound. The double-stranded circular DNA genome is maintained in the undifferentiated basal stratum as extrachromosomal nuclear plasmids at approximately 20 to 50 copies per cell. Viral-DNA amplification and progeny virion production are only observed in the more superficial, differentiated strata of the squamous epithelium (9, 22, 36; see reference 6 for a review). Because viral-DNA replication depends on the host DNA replication machinery to complement the viral origin binding protein E2 and the viral DNA helicase E1 (7), the virus must reestablish an S-phase milieu in the differentiated strata to achieve productive infections.
The high-risk HPV E7 proteins efficiently target the key tumor suppressor retinoblastoma susceptibility protein (pRB) and related pocket proteins p107 and p130 (25) that control the cell cycle and differentiation (8, 34). In particular, the HPV-18 E7 gene expressed under the control of the homologous viral promoter and enhancer elements contained in the upstream regulatory region (URR) was able to induce S-phase reentry in suprabasal cells in organotypic raft cultures of primary human keratinocytes (PHKs) (3, 5). HPV-16 and HPV-18 E7 mutants unable to bind the pocket proteins do not support viral-DNA amplification in such cultures (11, 21). The most plausible interpretation is that E7 promotes S-phase reentry in postmitotic, differentiated cells when it is expressed from the differentiation-dependent promoter (29, 44-46). However, there has been a lingering uncertainty as to whether E7 protein keeps the daughters of basal/parabasal cells from exiting the S phase while being pushed upward into the spinous strata and undergoing differentiation.
A second issue not yet resolved is the fact that the LR HPV-11 E7 gene or E6/E7 genes driven by the HPV-11 URR or the highly homologous HPV-6 E7 gene under the control of the retroviral LTR have so far been unable to induce S phase in suprabasal cells in PHK raft cultures (3, 15). The E7 protein of the LR HPV binds to pRB with much-reduced affinity relative to the HR HPV E7 (16, 26, 32, 43). Thus, it is possible that, relative to the HR HPV E7, a higher level of the LR HPV E7 protein would be required to bind to and inactivate the pocket proteins. However, such high concentrations have not been attainable in the experimental raft cultures due to the low copy number of the E7 gene transduced into PHKs via retrovirus-mediated gene transfer. Moreover, HPV-11 genomic DNA transduced into PHKs has not been demonstrated to amplify in raft cultures (37), contrary to what has been shown for many high-risk HPVs (11-13, 23, 28). Thus, it is not clear whether the LR HPV E7 alone is capable of inducing S phase in the differentiated keratinocytes or what conditions must be met for the LR HPV E7 to do so.
In this study, we addressed both issues by taking advantage of a previously reported inducible system. Smith-McCune et al. (35) described a constitutively expressed but conditionally activated HPV-16 E7 protein. HPV-16 E7 was fused at its carboxyl terminus to the ligand binding domain (amino acids 287 to 595) of the human estrogen receptor α (ER) (20). The fusion protein was expressed from the retroviral long terminal repeat (LTR) promoter, but its functional activation occurred only upon addition of 17β-estradiol (E2), whereby E7ER was transported from the cytoplasm into the nucleus and gained access to the pocket proteins, enabling human fibroblasts arrested by serum starvation to reenter S phase. We have now demonstrated the functionality of this inducible HPV-16 E7ER in PHK raft cultures in that it induces S phase in postmitotic, differentiated human keratinocytes. We have further shown that the LR HPV-11 E7 also induces S-phase reentry efficiently when it is expressed constitutively as a fusion to this ER ligand binding domain and when nuclear import is induced by E2. In contrast, the highly homologous HPV-6 E7ER triggered S phase rather ineffectively.
MATERIALS AND METHODS
Retroviral vectors.
The retroviral constructs pLNCXER (Fig. 1A) and pMXE7ER (Fig. 1B) were kind gifts from Karen Smith-McCune (35). pMXE7ER (hereafter called pMX16E7ER to distinguish it from the other E7ER expression vectors constructed for this study) contains HPV-16 E7 fused to the ligand binding domain of the human ER, whereas pLNCXER contains ER alone. Both genes are under the control of the retroviral LTR promoter, and both vectors express the neomycin resistance gene from the simian virus 40 promoter. We additionally constructed retroviral vectors in pBabe Puro (24). To prepare pBabe Puro-ER (Fig. 1C), 6E7ER (Fig. 1D), or 11E7ER (Fig. 1E), ER alone or in combination with HPV-6 E7 or HPV-11 E7 were amplified by PCR and inserted downstream of the LTR promoter. A linker peptide, GGSDP, separates the two functional domains in both constructs. To generate pBabe Puro-11E7(ΔGLHC)ER (Fig. 1F), the wild-type 11E7 gene was swapped with a PCR-amplified sequence with the core of the consensus pRB binding sequence (LxCxE) deleted.
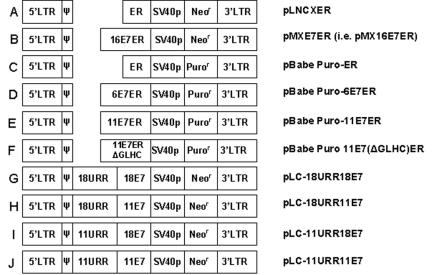
FIG. 1.
Schematic representation of retroviral vectors used in this study. (A) pLNCXER and (B) pMX16E7ER (35). (C) pBabe Puro-ER expresses the ligand binding domain of human estrogen receptor α from the LTR. (D) pBabe Puro-6E7ER expresses the HPV-6 E7ER fusion from the LTR. (E) pBabe Puro-11E7ER expresses the HPV-11 E7ER fusion from the LTR. (F) pBabe Puro-11E7(ΔGLHC)ER expresses a fusion of ER with HPV-11E7 with critical residues in the pocket protein binding domain from the LTR deleted. (G) pLC-18URR18E7 expresses HPV-18 E7 from the HPV-18 URR. (H) pLC-18URR11E7 expresses HPV-11 E7 from the HPV-18 URR. (I) pLC-11URR18E7 expresses HPV-18 E7 from the HPV-11 URR. (J) pLC-11URR11E7 expresses HPV-11E7 under the control of the HPV-11URR.
pLC-18URR18E7 (previously termed pLC-18URRE7) (Fig. 1G), which expresses HPV-18 E7 from the HPV-18 URR and the neomycin resistance gene from the simian virus 40 early promoter, has been described (5). During construction of this plasmid, a HindIII site was created immediately upstream of the E7 ATG, which is located in an NsiI site. HPV-11 E7 between NsiI and SalI sites was recovered from pLJd-11URRE6E7 (3) and swapped in place of the HPV-18E7 in pBSSK + 18E7 to construct pBSSK + 11E7. HPV-11E7 was then recovered as a HindIII-SalI fragment and used to replace HPV-18 E7 in pLC-18URRE7, generating pLC-18URR11E7 (Fig. 1H). To assemble pLC-11URR18E7 (Fig. 1I) and pLC-11URR11E7 (Fig. 1J), the HPV-11 URR was PCR amplified to incorporate 5′ BamHI and 3′ HindIII sites and was used to replace the HPV-18 URR in pLC-18URR18E7 and pLC-18URR11E7. All clones were verified by sequence determination.
Retroviruses and infection of PHKs.
Retrovirus stocks for each vector construct were prepared and used to infect PHKs isolated from neonatal foreskins (40) as previously described (reference 2 and references therein). Briefly, the retroviral vector plasmids were separately electroporated into the ecotropic producer cell line Bosc23 for transient production of retroviruses. The NIH 3T3-derived amphotropic packaging cell line GP+envAM12 (ATCC CRL-9641; ATCC, Manassas, VA) was infected with culture media containing the ecotropic retroviruses. Stably transduced GP+envAM12 producer cells were selected with the appropriate antibiotics. PHKs were then infected with amphotropic retroviruses and selected with the appropriate antibiotics (1 μg/ml of puromycin or 250 μg/ml of G418) for 2 days to eliminate all untransduced cells.
Organotypic raft cultures of PHKs.
Normal PHKs or retrovirus-transduced PHKs were seeded onto a dermal equivalent consisting of collagen with embedded Swiss 3T3 J2 fibroblasts (initially provided by Elaine Fuchs) within 24 h after drug selection, lifted onto a stainless steel stand, and cultured at the medium-air interface as described previously (3, 29) with the following modification. After 5 days at the medium-air interface, the cultures were fed for the next 4 days (for harvest on day 9) or 5 days (for harvest on day 10) with raft culture medium prepared from Dulbecco's modified Eagle's medium lacking phenol red, Ham's F12, and bovine fetal serum, which was treated with activated charcoal to deplete estrogenic compounds (35). Other additives in the media were unmodified. As specified in each experiment, 17β-estradiol (E2) (Sigma-Aldrich, St Louis, MO) was added to various final concentrations to the stripped medium for different durations prior to harvest on day 9 (for induction of up to 2 days) or day 10 (for induction of 3 days). Bromodeoxyuridine (BrdU) (50 μg/ml) was added at 6 or 12 h before harvest to mark cells in S phase. This two-stage protocol was necessary, as PHKs failed to establish a properly stratified and differentiated raft culture if depleted medium was used on day 1 upon lifting the cultures to the medium-air interface. The cultures were harvested, fixed in 10% buffered formalin, and embedded in paraffin.
To track basal cell migration, raft cultures of PHKs or PHKs transduced with the empty retrovirus (pBabe Puro) or HPV 11E7ER were prepared as described above with stripped medium, pulsed with BrdU (50 μg/ml) for 6 h, washed three times with phosphate-buffered saline to remove BrdU-containing media, and cultured in fresh stripped medium. E2 was added to 5 μM final concentration for 24 h or 48 h before harvest on day 9. A second set was harvested on day 9 without E2 addition.
Antigen detection by in situ methods.
Thin sections (4 μm) were cut and analyzed by in situ methods. Chromogenic detection of BrdU was conducted with anti-BrdU monoclonal antibody (clone ZBU 30 at 1:50 dilution; Zymed Laboratories, Invitrogen, Carlsbad, CA) (5). Images were captured using an Olympus BH-2 microscope with a SPOT digital camera at ×20 magnification (Diagnostic Instruments, Sterling Heights, Mich.). Simultaneous detection of BrdU and p21cip1 by indirect immunofluorescence was conducted with anti-p21cip1 mouse monoclonal antibody (OP64; Calbiochem, EMD Biosciences, La Jolla, CA), biotin-conjugated horse anti-mouse immunoglobulin G (Vector Laboratories, Inc., Burlingame, CA), streptavidin-tagged Texas red (Vector Laboratories), and fluorescein-conjugated anti-BrdU antibody (clone BMC9318; Roche Applied Science, Indianapolis, IN) as described before (27). For dual detection of ER and BrdU, the sections were reacted overnight with anti-ER rabbit monoclonal antibody (1:50 dilution; Clone SP1; Lab Vision Corp., Fremont, CA) at 4°C. Bound anti-ER antibody was detected with sheep anti-rabbit immunoglobulin G and F(ab′)2 fragment-Cy3 conjugate (1:200 dilution; C2306; Sigma-Aldrich). BrdU was then detected by fluorescein-conjugated anti-BrdU antibody. DAPI (4′,6′-diamidino-2-phenylindole) staining was carried out on sections in all immunofluorescent assays. All sections were analyzed at ×20 magnification. Images were recorded with an Olympus Provis AX70 microscope equipped with an AxioCam camera and AxioVision image capture software (Carl Zeiss MicroImaging Inc., Thornwood, NY). All the images were digitally processed using Photoshop 6.0 (Adobe Systems, San Jose, CA).
Immunoblot experiments.
To determine the relative steady-state expression levels of ER (alone), PHKs were transduced separately with HPV-6, HPV-11, and HPV-16 E7ER retroviruses, selected with antibiotics, grown in submerged cultures to 50% confluence in serum-free media, and treated for 24 h with 5 μM Ε2. The cells were then lysed in buffer containing 50 mM Tris (pH 8.0), 5 mM EDTA, 100 mM NaCl, 10 mM NaF, 0.5% NP-40, 2 mM dithiothreitol, and protease inhibitor cocktail (Sigma). Thirty micrograms of total protein from each lysate was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 10% to 20% gradient acrylamide gel and transferred to a polyvinylidene difluoride membrane. The membrane was probed with anti-ER rabbit monoclonal antibody (Lab Vision; clone SP1; 1:250 dilution) overnight at 4°C and developed via chemiluminescence by using an ECL kit (GE-Amersham). Actin was probed with horseradish peroxidase-conjugated anti-actin antibody (catalog no. sc-1616 HRP; Santa Cruz Biotechnology, Santa Cruz, CA) at 1:5,000 dilution and detected similarly.
RESULTS AND DISCUSSION
HPV-16 E7ER induces S-phase reentry in differentiated PHKs only in the presence of 17β-estradiol.
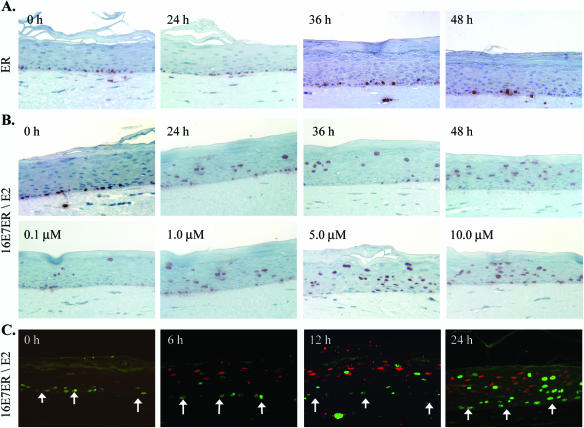
To establish the validity of the inducible HPV-16 E7ER system (35) in PHK raft cultures, we probed 4-μm sections of raft cultures transduced by pMX16E7ER or pLNCXER by immunohistochemical staining for BrdU, which was added to the cultures 12 h prior to harvest. Western blots confirmed the synthesis of ER and HPV-16 E7ER (see Fig. 4B, lanes 1 and 4). Cultures expressing ER moiety alone had S-phase cells only in the basal stratum in the absence of E2 or in the presence of 1 μM E2 over 48 h (Fig. 2A), as in cultures of untransduced PHKs (data not shown). In contrast, cultures transduced with the pMX16E7ER retrovirus had S-phase cells in the differentiated strata in the presence of 1 μM E2. Because of the stochastic nature of S-phase reentry induced by E7 (27), the numbers of BrdU-positive S-phase cells in the differentiated strata varied in different experiments. For the experiments shown in Fig. 2B (upper row), BrdU-positive cells in the differentiated strata increased from 284 (± 73) at 24 h to 393 (± 44) at 48 h postinduction when counted along the entire length of four tissue sections. In the absence of E2 (zero hour), there were no S-phase cells in the differentiated strata. A dose-dependent increase in the number of S-phase cells was observed between 0.1 μM and 5 μM of E2. There was no significant difference between 5 μM and 10 μM of E2 (Fig. 2B, lower row). Thus, we selected 5 μM E2 for all subsequent studies. Collectively, these results establish that the activity of E7ER is tightly controlled by the addition of E2 and that, in the presence or absence of E2, ER cannot promote S-phase reentry in differentiated cells.
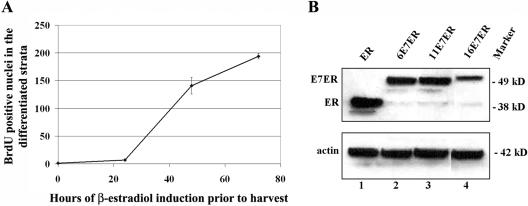
FIG. 4.
(A) Graphic representation of time-dependent increase in the number of BrdU-positive cells in the differentiated strata following E2-induced nuclear entry of HPV-11 E7ER. The error bars indicate standard deviations. (B) Western blot showing retroviral LTR-driven expression of ER, HPV 6E7ER, HPV-11E7ER, and HPV-16 E7ER after 24 h of E2 induction in submerged cultures of transduced PHK. Equal loading in the same gel was verified by probing for actin.
FIG. 2.
Functional activation of HPV-16 E7ER in PHK raft cultures by E2. The raft cultures were left untreated (0 h) or treated with E2 for the specified durations prior to harvest on day 9. BrdU was added 12 h (A and B) or 6 h (C) prior to harvest. Immunohistochemistry to detect BrdU incorporation (in red) (A and B) was performed on 4-μm sections to reveal S-phase cells. (A) Raft cultures expressing ER after induction with 1 μM E2 for 0 h, 24 h, 36 h, or 48 h. (B) Raft cultures expressing 16E7ER in the absence of E2 or in the presence of 1 μM E2 for 24 h, 36 h, or 48 h (upper images) or after induction for 24 h with 0.1 μM, 1.0 μM, 5.0 μM, or 10 μM E2 (lower images). (C) Double immunofluorescence revealing time-dependent induction of p21cip1 protein stabilization (red) or S-phase reentry, as indicated by BrdU incorporation (green), in separate populations of differentiated cells in the presence of 5 μM of E2 for 0 h, 6 h, 12 h, or 24 h. The arrows point to the basal stratum.
S-phase reentry or p21cip1 stabilization in postmitotic, differentiated strata by HPV-16 E7ER upon activation by 17β-estradiol.
Previously, we demonstrated that HR HPV-18 E7 expression from the HPV-18 URR induces S-phase reentry or costabilization of p21cip1 and cyclin E proteins in separate cell populations in the differentiated strata of raft cultures (4, 5, 17, 18, 27, 33). We have also shown that, in suprabasal strata of neonatal foreskin samples and normal PHK raft cultures derived from them, the p21cip1 RNA is constitutively transcribed, but the protein is below detection level due to rapid degradation by proteasomes. In contrast, variable levels of p27kip1 protein were stably expressed in the differentiated strata in the presence or in the absence of E7, and its abundance tends to increase in the upper, more differentiated cells. p21cip1 and p27kip1 are both potent inhibitors of cyclin E/cdk2 and cyclin A/cdk2 (34). Thus, in cells containing high levels of the p27kip1 protein, the E7-induced cyclin E/cdk2 kinase was inhibited after forming a stable complex with p27kip1, thereby preventing S-phase reentry. In turn, the high levels of cyclin E stabilize the p21cip1 protein in a separate kinase-inactive complex with cdk2. In contrast, in cells with low levels of the p27kip1 protein, the E7-induced cyclin E/cdk2 complex phosphorylates p27kip1, facilitating its degradation and promoting S-phase reentry. Thus, the cells that reenter S phase do not accumulate p27kip1, p21cip1, or cyclin E. These novel virus-host interactions were also observed in clinical biopsies of benign laryngeal papillomas caused by HPV-6 or HPV-11.
Taking advantage of the tightly controlled inducible HPV E7ER, we examined the time course of BrdU incorporation and p21cip1 stabilization. Raft cultures of HPV-16 E7ER-transduced PHKs were either uninduced (the control) or induced with 5 μM E2 for 6 h, 12 h, and 24 h before harvest; in each case, BrdU was added for the last 6 h to label S-phase cells. Using indirect immunofluorescence, we did not observe S-phase reentry or p21cip1 stabilization in cells in the differentiated strata in the absence of E2 (Fig. 2C, 0h). Weak BrdU incorporation or p21cip1 protein stabilization was first observed in separate populations of differentiated cells at 6 h postinduction with E2 (Fig. 2C). Both populations increased progressively over time through 24 h of induction, as did the signal intensities (Fig. 2C). At the 24-h time point, we observed a few cells with both BrdU and p21cip1 signals. This observation will be discussed below in conjunction with experiments involving HPV-11 E7ER raft cultures.
We have previously shown that basal cells migrate upward by no more than two or three cell layers within a span of 1 to 2 days in the presence of HPV-18 URR-E7 (4). In those experiments, raft cultures were pulse-labeled with [3H]thymidine (3H-TdR) for 6 h and then chased for 0 to 48 h in 6-h increments before being pulsed again for 6 h with BrdU prior to harvest on day 10. Without a chase, 30% of the 3H-TdR-positive cells in the differentiated strata were also positive for BrdU. Similarly, some basal cells were positive for 3H-TdR, BrdU, or both. Conversely, most of the BrdU-positive basal or spinous cells were not positive for 3H-TdR. Thus, the cells positive for both 3H-TdR and BrdU were in the same S phase when they were exposed to the two reagents back to back without a chase. The doubly positive cells in the differentiated strata decreased with the duration of the chase to 2% (with an 18-h chase), and they rebounded after a 30-h chase, when 11.8% of the 3H-TdR-positive spinous cells were also positive for BrdU. This percentage again decreased with longer chases. These results demonstrate that E7 does not promote the migration of basal S-phase cells into the spinous strata, where they continue to synthesize host DNA. Furthermore, once the spinous cells reenter S phase, E7 does not keep them in continuous S phase. Had either been the case, all 3H-TdR-positive cells would have been positive for BrdU, regardless of the duration of the chase. Rather, E7 stochastically induces a fraction of the differentiated cells to reenter S phase, and a small fraction of these cells can enter another round of S phase after a long, presumably G2 arrest. The fact that these 3H-TdR- or BrdU-positive spinous cells endoreduplicated accounts for their enlarged nuclei or for having multiple nuclei and their higher ploidy (4). The E7ER effects in the form of S-phase reentry or p21cip1 stabilization as early as 6 h after induction with β-estradiol are entirely consistent with the above-mentioned published results and demonstrate conclusively that E7 induces S-phase reentry de novo in postdifferentiated cells. As described below, we reached the same conclusion after following the migration of basal cells in the raft cultures.
URR swapping has little influence on the properties of HPV-11 or HPV-18 E7.
The HPV-18 URR has been reported to provide a stronger enhancer-promoter than the HPV-16 URR, accounting for higher immortalization efficiency (31, 38). To determine whether the lack of activity exhibited by HPV-11 E7 driven by the HPV-11 URR might be attributable in part to a weaker promoter strength relative to the HPV-18 URR, we constructed retroviruses in which the expression of HPV-11 E7 was under the control of the HPV-18 URR (pLC-18URR11E7) (Fig. 1H) or the HPV-11URR (pLC-11URR11E7) (Fig. 1J). For comparison, we also prepared retroviruses expressing HPV-18 E7 from the HPV-18URR (pLC-18URR18E7) (Fig. 1G) or HPV-11 URR (pLC-11URR18E7) (Fig. 1I). Immunohistochemical staining for BrdU, which was added for 12 h prior to harvest, revealed that expression of HPV-11 E7 under either the HPV-11 URR or HPV-18 URR (Fig. 3A, left two images) did not induce any BrdU incorporation in the differentiated strata; all BrdU-positive cells were found in the basal stratum. In contrast, HPV-18 E7 induced S-phase reentry in differentiated PHKs when under the transcriptional regulation of either the HPV-11 URR or the HPV-18 URR (Fig. 3A, right two images). Thus, we conclude that the relative strengths of the URR enhancer-promoter regions of these two HPVs play no determining role in the distinct activities of the HR versus the LR HPV E7-containing retroviruses. Rather, the difference primarily reflects the intrinsic properties of the E7 proteins.
FIG. 3.
Ability of the low-risk HPV E7 or E7ER protein to induce unscheduled DNA synthesis in differentiated cells. (A, B, and D) BrdU was added to the raft culture medium 12 h prior to harvest. Four-micrometer sections of raft cultures were probed for BrdU incorporation by immunohistochemistry (in red) to reveal S-phase cells (A) Raft cultures expressing HPV-11 E7 or HPV-18 E7 from either the HPV-11 URR or the HPV-18 URR. (B) Raft cultures expressing HPV-6 E7ER (upper row), HPV-11 E7ER (middle row), or HPV-11 E7(ΔGLHC)ER (lower row) in the absence (0 h) or in the presence of 5 μM of E2 for 24 h, 48 h, or 72 h. (C) Immunofluorescence detection of BrdU (green) in the basal and parabasal layers after a 6-h BrdU pulse followed by 24-h (a and c) or 48-h (b and d) E2 induction. (a and b) Raft cultures of pBabe-Puro vector-transduced PHKs. (c and d) Raft cultures of HPV-11 E7ER-transduced PHKs. Nuclei were labeled with DAPI. (D) S-phase reentry by differentiated cells coincides with nuclear import of E7ER. (a to g) Raft cultures were probed by double indirect immunofluorescence for ER (red) and BrdU (green) incorporation. (a to c) Cultures transduced with pBabe Puro-ER in the absence of E2 (a) or in the presence of 5 μM E2 for 24 h (b) or 48 h (c). (d) A normal untransduced PHK raft culture. (e to h) Cultures transduced with pBabe Puro-11E7ER in the absence of E2 (e) or in the presence of 5 μM E2 for 24 h (f) or 48 h (g). (h) Stabilization of p21cip1 protein (red) and BrdU (green) incorporation at 48 h after induction with 5 μM of E2. Colocalization of p21cip1 and BrdU (yellow) was observed in a small fraction of the differentiated cells. The arrows point to the basal stratum.
Retroviral LTR-driven HPV-6 and 11 E7ER induce S-phase reentry in postmitotic, differentiated keratinocytes.
We then tested whether the biological activities of the LR HPV E7 proteins might be revealed in raft cultures by using the constitutively expressed but functionally inducible E7ER system. We reasoned that, relative to the native E7, an elevated bolus of nuclear E7ER protein might be expected upon the addition of E2 to the medium. The ER moiety alone, HPV-6 E7ER, or HPV-11 E7ER was expressed from the retroviral vector pBabe Puro under the control of the LTR promoter (Fig. 1C, D, and E). Raft cultures of transduced PHKs were prepared. Five micromolar E2 was added at 24, 48, and 72 h before harvest, and BrdU was added for the last 12 h to monitor cells in S phase. Immunohistochemical staining for BrdU revealed basal proliferating cells and a very small number of S-phase cells in the differentiated keratinocytes in cultures expressing HPV-6 E7ER, and only when E2 was added to the medium (Fig. 3B, upper row). We believe that this inefficient induction is nevertheless significant, in agreement with the findings of Zhang et al. (43) who showed that HPV-6 E7 can degrade p130. In contrast, the native HPV-6 E7 driven by the LTR promoter did not elicit any S-phase cells in the differentiated strata (N. J. Genovese, T. R. Broker, and L. T. Chow, unpublished results), in agreement with its inability to induce PCNA, as reported by Halbert et al. (15).
Interestingly, S-phase induction in the differentiated strata was observed in raft cultures transduced with pBabe Puro-11 E7ER at 24 h, and the induction was robust at 48 h and 72 h postinduction with E2 (Fig. 3B, middle row, right 3 images). No S-phase reentry by the suprabasal cells was observed in the absence of E2 (Fig. 3B, middle row, left image). Figure 4A provides quantification of S-phase cells in the spinous strata during the time course of induction. To verify that the effect observed with 11E7ER is dependent on an interaction between E7 and the pRB family of pocket proteins, we constructed pBabe Puro-11E7(ΔGLHC)ER, which has critical residues within the pocket protein binding domain deleted (Fig. 1F). This mutation failed to promote S-phase reentry by cells in the differentiated strata in the presence or absence of E2 (Fig. 3B, lower row). These results demonstrate that the ability to bind the pRB family proteins is essential for S-phase reentry induced by the LR HPV-11 E7, as it is with the HR HPV-18 E7 (4). Western blots demonstrated a similar level of steady-state HPV-11 E7ER and HPV-6 E7ER proteins in infected PHKs after 24-h treatment with E2 (Fig. 4B, lanes 2 and 3). Surprisingly, both were higher than that of 16E7ER. Thus, the low biological activity of 6E7ER cannot be attributed to protein expression or stability. The low mobility of HR HPV-16 E7 relative to LR HPV E7 (1) can also be discerned in the context of fusions to ER (compare lanes 2, 3, and 4).
To verify that HPV-11 E7ER functions in a fashion similar to that of the native HPV-18 E7 or the HPV-16 E7ER and did not accelerate upward mobility of basal cells, we followed their migration with time. Raft cultures were prepared as described above. The cells were pulsed for 6 h with BrdU in stripped medium and then chased for 24 h or 48 h in the presence or in the absence of E2 prior to harvest on day 9. Many BrdU-positive cells remained basal, but a small number of BrdU-positive cells did ascend to the lower spinous strata after the chase over a period of 2 days. Importantly, extremely rarely were labeled cells observed in the upper spinous or granular strata. This pattern was similar in control raft cultures of untransduced PHKs (data not shown), PHKs transduced with pBabe-Puro (Fig. 3C, images a and b), or PHKs transduced with HPV-11 E7ER (Fig. 3C, images c and d). The same observation was made when E2 was not added during the chase (data not shown). This distribution of BrdU-positive cells is distinct from cultures that were induced with E2 first and then exposed to BrdU immediately prior to harvest. In that case, many of the BrdU-positive cells were located in the upper spinous or granular strata (Fig. 3B). Collectively, these experiments show that the LR HPV E7 protein induces de novo S-phase reentry in postmitotic, differentiated keratinocytes, as do the E7 proteins of HR HPVs.
S-phase reentry promoted by HPV-11 E7ER is accompanied by nuclear import of ER.
Antibody probing in sections of raft cultures transduced with pBabe Puro-ER revealed abundant signals of ER but, in the absence of E2, these signals remained mostly in the cytoplasm (Fig. 3D, image a). The addition of E2 to the media led to significant nuclear localization of the ER signals. However, BrdU-positive cells were observed only in the basal stratum, verifying that the ER moiety alone in the nucleus cannot promote S-phase reentry by the differentiated keratinocytes (Fig. 3D, images b and c). Indirect immunofluorescence and Western blot studies revealed little or no human estrogen receptor α in raft cultures of untransduced PHKs or PHKs transduced with an empty-vector-only retrovirus (Fig. 3D, image d, and data not shown). In contrast, in raft cultures of PHKs transduced with HPV-11 E7ER, S-phase reentry by cells in the differentiated strata was associated with E2-induced nuclear import of E7ER (Fig. 3D, compare image e to images f and g). However, only some of the nuclei were positive for both DNA synthesis and ER antigen, whereas others were positive only for ER. We suggest that this is because 11E7ER expression stabilizes the p21cip1 protein in some of the differentiated cells and prevents their S-phase reentry, as was observed with HPV-18 E7 or HPV-16 E7ER in raft cultures and in patient specimens infected by HPV-6 and HPV-11 (references 4, 17, 18, 27, and 33 and the present study). Indeed, indirect immunofluorescence probing for BrdU and p21cip1 proteins revealed two distinct, mutually exclusive populations of differentiated cells (Fig. 3D, image h).
Unlike the observation in laryngeal papillomas associated with HPV-6 or HPV-11 infections, a small number of BrdU-positive, differentiated cells were also positive for p21cip1 at 24 h postinduction, as was also observed with HPV-16 E7ER (Fig. 2C, 24h). We previously demonstrated with the native HPV-18 E7 that S-phase cells can subsequently accumulate cyclin E and hence the p21cip1 protein when they continue to differentiate and accumulate high levels of p27kip1 protein (4, 27). Thus, the colocalization of p21cip1 and BrdU could be a matter of timing of the S-phase induction by the bolus of E7ER relative to cell differentiation. However, it is also possible that the high levels of stable E7ER achieved in the inducible system might have inactivated p21cip or p27kip1, as previously observed in studies conducted with HPV-16 E7 or in productively infected HPV-16 lesions (14, 19, 41, 42). Despite this small difference between 11E7ER raft cultures and LR HPV benign patient lesions, our observations collectively demonstrate that HPV-11 E7 stochastically promotes the transition of postmitotic, differentiated keratinocytes into S phase when sufficient levels of nuclear E7 protein are achieved.
In conclusion, our results with the inducible system employing the ligand binding domain of the human estrogen receptor have conclusively demonstrated that both the high-risk and the low-risk HPV E7 can induce S-phase reentry in a fraction of postmitotic, differentiated cells and that this activity requires an interaction with the pRB family of proteins. Moreover, the three fusion proteins exhibited distinct abilities to induce S phase in differentiated cells and different kinetics of induction, possibly reflecting their respective threshold levels required for this induction. In contrast to URR-driven HPV-11 E7, LTR-driven HPV-11 E7 induced S-phase reentry in differentiated cells (N. J. Genovese, T. R. Broker, and L. T. Chow, unpublished observation), but the efficiency of S-phase induction was lower than that achieved by HPV-11 E7ER. This difference again suggests that the steady-state level of the low-risk HPV E7 is critical to its biological activity. Our observations that URR swapping between the HR and LR HPVs did not alter the abilities of the two viral E7 proteins to promote S-phase reentry in differentiated cells further highlight the distinct activities of the E7 proteins to inactivate pRB and related proteins. Finally, the HPV-16 and HPV-11 E7ER recapitulated the induction of p21cip1 protein in differentiated cells negative for BrdU incorporation when BrdU was added to the medium for 6 to 12 h immediately prior to harvest, as previously observed in patient specimens infected by the low-risk HPVs and in raft cultures transduced with pLC-18URR18E7. It is also clinically relevant to note that the higher efficiency of HPV-11 E7ER in inducing S-phase reentry in differentiated cells relative to HPV-6 E7ER might potentially explain the more aggressive regrowth of laryngeal papillomas caused by HPV-11 relative to those caused by HPV-6 after surgical excision (10, 30, 39).
Acknowledgments
This research was supported by US PHS grant CA36200 and CA83679. N.J. Genovese is the recipient of a Fellowship from the Basic Mechanisms in AIDS Pathogenesis Training Grant T32 AI07493.
We thank Karen Smith-McCune for the gift of pMXE7ER and pLNCX-ER and Christopher Fisher for discussions. We thank Ge Jin for paraffin embedding and sectioning of the raft cultures.
REFERENCES
- 1.Armstrong, D. J., and A. Roman. 1992. Mutagenesis of human papillomavirus types 6 and 16 E7 open reading frames alters the electrophoretic mobility of the expressed proteins. J. Gen. Virol. 73:1275-1279. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, N. S., L. T. Chow, and T. R. Broker. 2005. Retrovirus mediated gene transfer to analyze HPV gene regulation and protein functions in “organotypic” raft culture, p. 187-202. In C. Davy and J. Doorbar (ed.), Human papillomaviruses: methods and protocols, methods in molecular medicine. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 3.Cheng, S., D.-C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 9:2335-2349. [DOI] [PubMed] [Google Scholar]
- 4.Chien, W.-M., F. Noya, H. M. Benedict-Hamilton, T. R. Broker, and L. T. Chow. 2002. Alternative fates of keratinocytes transduced by human papillomavirus type 18 E7 during squamous differentiation. J. Virol. 76:2964-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien, W.-M., J. N. Parker, D.-C. Schmidt-Grimminger, T. R. Broker, and L. T. Chow. 2000. Casein kinase II phosphorylation of the human papillomavirus-18 E7 protein is critical for promoting S-phase entry. Cell Growth Differ. 11:425-435. [PubMed] [Google Scholar]
- 6.Chow, L. T., and T. R. Broker. Human papillomavirus RNA transcription. In D. DiMaio and R. Garcea (ed.), The papillomaviruses, in press. Plenum Publishers, New York, N.Y.
- 7.Chow, L. T., and T. R. Broker. 2006. Mechanisms and regulation of papillomavirus DNA replication, p. 53-71. In M. S. Campo (ed.), Papillomavirus research: from natural history to vaccines and beyond. Caister Academic Press, Norwich, United Kingdom.
- 8.Classon, M., and N. Dyson. 2001. p107 and p130: versatile proteins with interesting pockets. Exp. Cell Res. 264:135-147. [DOI] [PubMed] [Google Scholar]
- 9.Dollard, S. C., J. L. Wilson, L. M. Demeter, W. Bonnez, R. C. Reichman, T. R. Broker, and L. T. Chow. 1992. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. Genes Dev. 6:1131-1142. [DOI] [PubMed] [Google Scholar]
- 10.Draganov, P., S. Todorov, I. Todorov, T. Karchev, and Z. Kalvatchev. 2006. Identification of HPV DNA in patients with juvenile-onset recurrent respiratory papillomatosis using SYBR Green real-time PCR. Int. J. Pediatr. Otorhinolaryngol. 70:469-473. [DOI] [PubMed] [Google Scholar]
- 11.Flores, E. R., B. L. Allen-Hoffmann, D. Lee, and P. F. Lambert. 2000. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J. Virol. 74:6622-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frattini, M. G., H. B. Lim, J. Doorbar, and L. A. Laimins. 1997. Induction of human papillomavirus type 18 late gene expression and genomic amplification in organotypic cultures from transfected DNA templates. J. Virol. 71:7068-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frattini, M. G., H. B. Lim, and L. A. Laimins. 1996. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc. Natl. Acad. Sci. USA 93:3062-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1992. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J. Virol. 66:2125-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heck, D. V., C. L. Yee, P. M. Howley, and K. Münger. 1992. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl. Acad. Sci. USA 89:4442-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jian, Y., D.-C. Schmidt-Grimminger, W.-M. Chien, X. Wu, T. R. Broker, and L. T. Chow. 1998. Post-transcriptional induction of p21cip1 protein by human papillomavirus E7 inhibits unscheduled DNA synthesis reactivated in differentiated keratinocytes. Oncogene 17:2027-2038. [DOI] [PubMed] [Google Scholar]
- 18.Jian, Y., B. A. Van Tine, W.-M. Chien, G. M. Shaw, T. R. Broker, and L. T. Chow. 1999. Concordant induction of cyclin E and p21cip1 in differentiated keratinocytes by the human papillomavirus E7 protein inhibits cellular and viral DNA synthesis. Cell Growth Differ. 10:101-111. [PubMed] [Google Scholar]
- 19.Jones, D. L., R. M. Alani, and K. Münger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, V., S. Green, A. Staub, and P. Chambon. 1986. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 5:2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin-Drubin, M. E., J. L. Bromberg-White, and C. Meyers. 2005. The role of the human papillomavirus type 18 E7 oncoprotein during the complete viral life cycle. Virology 338:61-68. [DOI] [PubMed] [Google Scholar]
- 22.Meyers, C., M. G. Frattini, J. B. Hudson, and L. A. Laimins. 1992. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 257:971-973. [DOI] [PubMed] [Google Scholar]
- 23.Meyers, C., T. J. Mayer, and M. A. Ozbun. 1997. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J. Virol. 71:7381-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Münger, K., A. Baldwin, K. M. Edwards, H. Hayakawa, C. L. Nguyen, M. Owens, M. Grace, and K. Huh. 2004. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 78:11451-11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Münger, K., W. C. Phelps, V. Bubb, P. M. Howley, and R. Schlegel. 1989. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noya, F., W.-M. Chien, T. R. Broker, and L. T. Chow. 2001. p21cip1 degradation in differentiated keratinocytes is abrogated by costabilization with cyclin E induced by human papillomavirus E7. J. Virol. 75:6121-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozbun, M. A., and C. Meyers. 1997. Characterization of late gene transcripts expressed during vegetative replication of human papillomavirus type 31b. J. Virol. 71:5161-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker, J. N., W. Zhao, K. J. Askins, T. R. Broker, and L. T. Chow. 1997. Mutational analyses of differentiation-dependent human papillomavirus type 18 enhancer elements in epithelial raft cultures of neonatal foreskin keratinocytes. Cell Growth Differ. 8:751-762. [PubMed] [Google Scholar]
- 30.Rabah, R., W. D. Lancaster, R. Thomas, and L. Gregoire. 2001. Human papillomavirus-11-associated recurrent respiratory papillomatosis is more aggressive than human papillomavirus-6-associated disease. Pediatr. Dev. Pathol. 4:68-72. [DOI] [PubMed] [Google Scholar]
- 31.Romanczuk, H., L. L. Villa, R. Schlegel, and P. M. Howley. 1991. The viral transcriptional regulatory region upstream of the E6 and E7 genes is a major determinant of the differential immortalization activities of human papillomavirus types 16 and 18. J. Virol. 65:2739-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sang, B. C., and M. S. Barbosa. 1992. Single amino acid substitutions in “low-risk” human papillomavirus (HPV) type 6 E7 protein enhance features characteristic of the “high-risk” HPV E7 oncoproteins. Proc. Natl. Acad. Sci. USA 89:8063-8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Grimminger, D.-C., X. Wu, Y. Jian, T. R. Broker, and L. T. Chow. 1998. Post-transcriptional induction of p21cip1 protein in condylomata and dysplasias is inversely related to human papillomavirus activities. Am. J. Pathol. 152:1015-1024. [PMC free article] [PubMed] [Google Scholar]
- 34.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 35.Smith-McCune, K., D. Kalman, C. Robbins, S. Shivakumar, L. Yuschenkoff, and J. M. Bishop. 1999. Intranuclear localization of human papillomavirus 16 E7 during transformation and preferential binding of E7 to the Rb family member p130. Proc. Natl. Acad. Sci. USA 96:6999-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoler, M. H., S. M. Wolinsky, A. Whitbeck, T. R. Broker, and L. T. Chow. 1989. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology 172:331-340. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, J. T., S. T. Oh, S. S. Terhune, and L. A. Laimins. 2001. Cellular changes induced by low-risk human papillomavirus type 11 in keratinocytes that stably maintain viral episomes. J. Virol. 75:7564-7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa, L. L., and R. Schlegel. 1991. Differences in transformation activity between HPV-18 and HPV-16 map to the viral LCR-E6-E7 region. Virology 181:374-377. [DOI] [PubMed] [Google Scholar]
- 39.Wiatrak, B. J., D. W. Wiatrak, T. R. Broker, and L. Lewis. 2004. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope 114(Suppl. 104):1-23. [DOI] [PubMed] [Google Scholar]
- 40.Wilson, J. L., S. C. Dollard, L. T. Chow, and T. R. Broker. 1992. Epithelial-specific gene expression during differentiation of stratified primary human keratinocyte cultures. Cell Growth Differ. 3:471-483. [PubMed] [Google Scholar]
- 41.Zehbe, I., A. Ratsch, A., M. Alunni-Fabbroni, A. Burzlaff, E. Bakos, M. Dürst, E. Wilander, and M. Tommasino. 1999. Overriding of cyclin-dependent kinase inhibitors by high and low risk human papillomavirus types: evidence for an in vivo role in cervical lesions. Oncogene 18:2201-2211. [DOI] [PubMed] [Google Scholar]
- 42.Zerfass-Thome, K., W. Zwerschke, B. Mannhardt, R. Tindle, J. W. Botz, and P. Jansen-Dürr. 1996. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene 13:2323-2330. [PubMed] [Google Scholar]
- 43.Zhang, B., W. Chen, and A. Roman. 2005. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc. Natl. Acad. Sci. USA 103:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, W., L. T. Chow, and T. R. Broker. 1999. A distal element in the HPV-11 upstream regulatory region contributes to promoter repression in basal keratinocytes in squamous epithelium. Virology 253:219-229. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, W., L. T. Chow, and T. R. Broker. 1997. Transcription activities of human papillomavirus type 11 E6 promoter-proximal elements in raft and submerged cultures of foreskin keratinocytes. J. Virol. 71:8832-8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao, W., F. Noya, W. Y. Chen, T. M. Townes, L. T. Chow, and T. R. Broker. 1999. Trichostatin A up-regulates human papillomavirus type 11 upstream regulatory region-E6 promoter activity in undifferentiated primary human keratinocytes. J. Virol. 73:5026-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nature 2:342-350. [DOI] [PubMed] [Google Scholar]