Abstract
Canine X-linked severe combined immunodeficiency (XSCID) is due to mutations in the common gamma chain (γc) gene and is identical clinically and immunologically to human XSCID, making it a true homologue of the human disease. Bone marrow-transplanted (BMT) XSCID dogs not only engraft donor T cells and reconstitute normal T-cell function but, in contrast to the majority of transplanted human XSCID patients, also engraft donor B cells and reconstitute normal humoral immune function. Shortly after our initial report of successful BMT of XSCID dogs, it soon became evident that transplanted XSCID dogs developed late-onset severe chronic cutaneous infections containing a newly described canine papillomavirus. This is analogous to the late-onset cutaneous papillomavirus infection recently described for human XSCID patients following BMT. Of 24 transplanted XSCID dogs followed for at least 1 year post-BMT, 71% developed chronic canine papillomavirus infection. Six of the transplanted dogs that developed cutaneous papillomas were maintained for >3 1/2 years post-BMT for use as breeders. Four of these six dogs (67%) developed invasive squamous cell carcinoma (SCC), with three of the dogs (75%) eventually developing metastatic SCC, an extremely rare consequence of SCC in the dog. This finding raises the question of whether SCC will develop in transplanted human XSCID patients later in life. Canine XSCID therefore provides an ideal animal model with which to study the role of the γc-dependent signaling pathway in the response to papillomavirus infections and the progression of these viral infections to metastatic SCC.
Severe combined immunodeficiency (SCID) is a heterogeneous group of diseases characterized by the inability to mount humoral and cell-mediated immune responses, and it is invariably fatal within the first 2 years of life (9, 46). X-linked SCID (XSCID) is the most common form of the disease, representing approximately 50% of all human SCID cases (9, 18). XSCID is caused by mutations in the common gamma (γc) subunit of the receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21 (reviewed in references 4 and 34). Thus, the XSCID phenotype is the complex result of multiple cytokine defects. The shared usage of the γc subunit by receptors for growth factors that are critical for normal B-, NK-, and T-cell development and function explains the profound immunologic abnormalities and clinical severity of the disease.
Since the first successful HLA-identical bone marrow transplant in a boy with SCID in 1968 (19), bone marrow transplantation (BMT) has become the treatment of choice for all forms of SCID (10, 18, 22). SCID patients receiving a histocompatible (HLA-identical) BMT have >90% long-term survival rates (10, 18, 22). However, the majority of patients do not have a histocompatible donor. Haploidentical (half-matched) BMT with T-cell depletion to prevent fatal graft-versus-host disease has become the standard therapy for SCID patients who lack a histocompatible donor (10, 18, 22). Although T-cell depletion makes BMT possible for virtually all SCID patients, long-term immune reconstitution and survival are less favorable than those after histocompatible BMT, with 60 to 78% of patients surviving for 3 to 6 months posttransplantation (10, 22). The most common immunologic problems in human XSCID patients following both histocompatible and haploidentical BMT are a failure to engraft donor B cells and poor humoral immune reconstitution that can be managed by intravenous immunoglobulin therapy (18, 23, 53, 55). A recent retrospective study of 41 SCID patients who have survived for more than 10 years following BMT reported that 50% of XSCID and Jak3-deficient SCID patients (9/18) developed chronic severe papillomavirus infections, with a median age of onset of 8 years post-BMT, while patients with other forms of SCID did not develop cutaneous papillomas (30). The importance of this finding is that the γc subunit interacts with Jak3, and therefore only SCID patients with defects in the γc/Jak3 signaling pathway are susceptible to chronic severe cutaneous papillomavirus disease.
Our laboratory has identified and characterized an X-linked severe combined immunodeficiency in dogs that has a clinical and immunologic phenotype virtually identical to that of human XSCID (15, 16). We have shown that XSCID dogs can be transplanted successfully with histocompatible and haploidentical unfractionated bone marrow and highly purified bone marrow CD34+ cells from genotypically normal neonatal donors, resulting in full immunologic reconstitution and engraftment of both donor B and T cells without the need for pretransplant conditioning (17, 25, 26). Following our initial report of successful BMT, it soon became clear that transplanted dogs followed past the initial evaluation period (6 to 8 months) developed cutaneous papillomas between 8 and 14 months post-BMT. This study describes the occurrence of papillomavirus infections in transplanted XSCID dogs with unusual site predilection and histologic characteristics that progress to metastatic squamous cell carcinomas (SCCs), which may be predictive of possible long-term sequelae of the papillomavirus infections observed in transplanted human XSCID patients.
MATERIALS AND METHODS
Dogs.
The XSCID dogs used in this study were derived from a breeding colony established from a single carrier female (15, 16). All affected dogs have the same γc mutation, a 4-bp deletion in exon 1, and were diagnosed shortly after birth by a PCR-based mutation detection assay using DNA isolated from whole blood (17, 27). DLA-identical or DLA-haploidentical donors for transplantation were determined by a PCR assay for highly polymorphic major histocompatibility complex class I and class II microsatellite marker polymorphisms (54).
Bone marrow preparation.
Bone marrow cells were collected from donors following euthanasia by removing a segment of the femur, flushing the marrow into a sterile petri dish containing Hanks' buffered salt solution without calcium and magnesium (Mediatech, Fisher Scientific, Philadelphia, PA), and filtering it through a fine mesh filter (17, 26). The cells were centrifuged and resuspended in ammonium chloride lysing buffer (Sigma Chemical, St. Louis, MO) to remove red blood cells, washed twice in Hanks' buffered salt solution, and resuspended in sterile saline for use in unfractionated BMT or in phosphate-buffered saline (PBS) for isolation of CD34+ cells. Isolation of bone marrow CD34+ cells was performed as previously described (26). Briefly, unfractionated marrow cells were resuspended at a final concentration of 1 × 108 cells/ml in a PBS solution containing 2% horse serum and incubated with 40 μg/ml of anti-canine CD34 antibody 1H6 (36). Cells were incubated with anti-mouse immunoglobulin G (IgG) MACS magnetic microbeads, followed by selection on varioMACS columns according to the manufacturer's protocol (Miltenyi, Auburn, CA). Aliquots of positively selected cells were labeled with phycoerythrin-labeled streptavidin (Jackson Immunoresearch, West Grove, PA) and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) to determine the purity of the eluted cells. The purity of the cells was >96%.
Flow cytometry.
Peripheral blood mononuclear cells were isolated from heparinized whole blood by centrifugation over a discontinuous density gradient of Hypaque-Ficoll and stained for flow cytometric analysis as previously described (17, 51). Analysis gates were adjusted to 2% positive staining with isotype controls. For each sample, 10,000 cells were analyzed by using a Becton Dickinson FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The murine monoclonal antibodies used in this study were CA17.3G9 (canine CD3), CA13.1E4 (canine CD4), CA9.JD3 (canine CD8), and CA4.1D3 (canine CD45RA) (13, 39). Fluorescein isothiocyanate-conjugated F(ab′)2 goat anti-dog IgG (heavy and light chain specific) was purchased from Jackson Immunoresearch, West Grove, PA. Fluorescein isothiocyanate- and phycoerythrin-labeled secondary antibodies were purchased from Fisher Scientific (Pittsburgh, PA).
Proliferation assays.
The response of peripheral blood lymphocytes to in vitro mitogenic stimulation with phytohemagglutinin P (5 μg/ml; Sigma, St. Louis, MO) was determined as previously described, using the incorporation of tritiated thymidine (17). The results are expressed in counts per minute.
Quantitation of serum IgG.
Serum IgG concentrations were measured by using a commercial radial immunodiffusion kit for quantitating canine IgG (Bethyl Laboratories, Montgomery, TX).
Assessment of specific antibody production.
The transplanted dogs were immunized with one of two T-cell-dependent antigens, either bacteriophage ΦX174 or tetanus toxoid, when their serum IgG concentrations reached normal levels. Bacteriophage ΦX174 was administered intravenously in a dose of ∼3 × 109 PFU/kg of body weight. A secondary immunization was given 6 weeks after the primary immunization. Phage clearance and specific phage-neutralizing antibody activity, expressed as the rate of phage inactivation (K value, or Kv), were determined as previously described (17). Dogs immunized with tetanus toxoid (Lederle, Pearl River, NY) were administered a dose of 0.5 ml intramuscularly. Animals were reimmunized with tetanus toxoid 2 weeks after the initial immunization. Serum samples were obtained from the immunized animals at weekly intervals for 4 weeks after the initial immunization. The amount of IgG-specific, tetanus toxoid-specific antibody was determined by using an enzyme-linked immunosorbent assay (26).
Immunohistochemistry.
Immunohistochemistry was performed on frozen sections, using a DakoCytomation automatic autostainer (DakoCytomatix, Carpinteria, CA). After the sections were washed with PBS, they were incubated with rabbit anti-sera (anti-canine oral papillomavirus [anti-COPV] or anti-CaPV2 capsids) or the negative control (preimmune serum) at a 1:250 dilution with antibody diluent (DakoCytomatix) for 30 min at room temperature. After washing of the sections with PBS, the secondary antibody Envision Plus polymer (anti-rabbit polymer conjugated to horseradish peroxidase [DakoCytomatix]) was applied for 30 min. Slides were washed, incubated for 5 min with DAB Plus substrate (DakoCytomatix), washed again, and counterstained in Mayer's hematoxylin for 10 min.
RESULTS
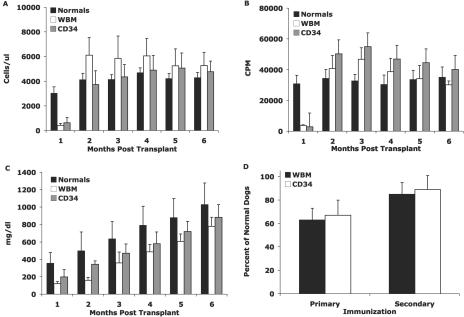
Since our first report of successful immune reconstitution of XSCID dogs following BMT in 1997, 24 transplanted XSCID dogs have been maintained for at least 1 year post-BMT, primarily for use as potential breeders. All dogs were transplanted within the first 3 weeks of life with bone marrow cells from DLA-identical healthy littermates. Eleven dogs were transplanted with unfractionated bone marrow containing >20 × 106 CD34+ cells/kg, and 13 dogs were transplanted with highly purified bone marrow CD34+ cells at doses ranging from 10 × 106 to 30 × 106 cells/kg. None of the dogs received pretransplant conditioning. At 8 to 15 months post-BMT, 17 of the dogs (71%) developed severe chronic cutaneous papillomavirus infection. In the approximately 15-year history of our XSCID colony, papillomas have never been observed in healthy males or female carriers. Eight of the dogs received unfractionated bone marrow transplants, and the remaining nine dogs received purified bone marrow CD34+ transplants. All of the transplanted dogs had normal levels of CD45RA+ (naïve) T cells (Fig. 1A) that responded to mitogenic stimulation (Fig. 1B) at 2 to 3 months post-BMT. Normal serum IgG concentrations were attained between 5 and 6 months post-BMT (Fig. 1C), with the dogs developing normal levels of antigen-specific IgG antibody following immunization with either bacteriophage ΦX174 or tetanus toxoid (Fig. 1D). Therefore, all dogs had reconstituted normal systemic immune function prior to the development of papillomas.
FIG. 1.
Immune reconstitution of bone marrow-transplanted XSCID dogs that have developed papillomas. Results are divided into those for dogs transplanted with whole bone marrow and those for dogs transplanted with purified CD34+ bone marrow cells. (A) Absolute numbers of CD45RA+ T cells; (B) in vitro proliferative responses following stimulation with phytohemagglutinin; (C) serum IgG concentrations. (D) IgG-specific antibody titers following bacteriophage ΦX174 or tetanus toxoid immunization, expressed as percentages of those in age-matched healthy dogs.
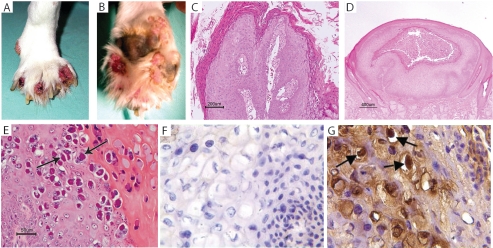
In contrast to the typical oral mucosa predilection for canine papillomas, the papillomas observed in the transplanted XSCID dogs had an unusual cutaneous site predilection (Fig. 2A to C). The majority of the papillomas involved the feet, either as interdigital papillomas (Fig. 2A) or, more typically, on the footpads (Fig. 2B).
FIG. 2.
Papillomas in transplanted XSCID dogs. Typical interdigital (A) and footpad (B) papillomas in 1-year-old BMT XSCID dogs. Histologic characteristics of papillomas in bone marrow-transplanted XSCID dogs included exophytic papilloma (C), endophytic (infundibular) papilloma (D), and endophytic papilloma with an unusual histologic phenotype consisting of orthokeratosis and parakaratosis with cytoplasmic accumulation of keratin tonofilaments (denoted by arrows) (E). (F and G) Immunohistochemical demonstration of canine papillomavirus type 2 antigen in a typical papilloma (arrow) from a transplanted XSCID dog (F, anti-COPV; G, anti-CfPV-2). Magnification, ×400.
The papillomas in the transplanted dogs also had a very unusual histologic appearance. Although a few of the papillomas were exophytic papillomas typically seen in dogs (Fig. 2C), the majority of the papillomas in the transplanted dogs were endophytic (infundibular) (Fig. 2D). Several of the dogs had an unusual type of endophytic papilloma that was characterized by orthokeratosis and parakaratosis with cytoplasmic accumulation of keratin tonofilaments (Fig. 2E). The papillomas were positive for a newly described canine papillomavirus, CfPV-2 (H. Yuan et al., unpublished data) (Fig. 2F and G).
The clinical course of the papillomas in the transplanted dogs also differed from that normally observed in dogs, in which both oral and cutaneous papillomas usually spontaneously regress within 6 to 8 weeks. Nine dogs developed persistent papillomas on the footpads and had to be euthanized within 4 to 8 months of papilloma development. Because of their location, these papillomas are very painful, resulting in varying degrees of lameness in the affected dogs. Two dogs developed cutaneous papillomas at approximately 12 months posttransplantation that became generalized, essentially covering the entire body, resulting in their euthanasia between 2 and 2 1/2 years posttransplantation. The remaining six dogs with isolated interdigital papillomas had remarkably similar clinical courses. The papillomas appeared between 9 and 14 months posttransplantation and persisted for 11 to 18 months. In three of the dogs, the papillomas spontaneously regressed in between 12 and 15 months. Three dogs were treated with interferon, but their papillomas persisted for 11 to 16 months, at which time they regressed. Two of the six dogs remained papilloma-free for 2 1/2 to 6 years. However, papillomas reappeared in the same areas in the other four dogs between 1 and 3 years following regression of the original papillomas. The courses of papillomavirus infection and clinical outcomes are summarized in Table 1.
TABLE 1.
Course of papillomavirus infection in bone marrow-transplanted XSCID dogs surviving past 3 1/2 years posttransplantation
| Dog | Date of BMT (mo/day/yr) | Dates of papillomas (mo/yr) | Date of SCC (mo/yr) | Date of metastatic SCC (mo/yr) | Status |
|---|---|---|---|---|---|
| R350 | 10/13/94 | 1/96-12/96 | Euthanized (7/99) | ||
| R468 | 10/12/95 | 6/96-7/97 | Alive | ||
| R619 | 8/7/97 | 4/98-8/99,a 6/01b | 10/01 | 12/01 | Euthanized (12/01) |
| R743 | 8/13/98 | 8/99-3/00,a 4/03b | 8/03 | Alive | |
| R868 | 8/27/99 | 6/00-7/01,a 1/03b | 4/03 | 10/03 | Euthanized (12/03) |
| R1163 | 3/24/01 | 4/02-8/03, 9/04b | 11/04 | 11/04 | Euthanized (11/04) |
Treated with interferon.
Recurrence at original site.
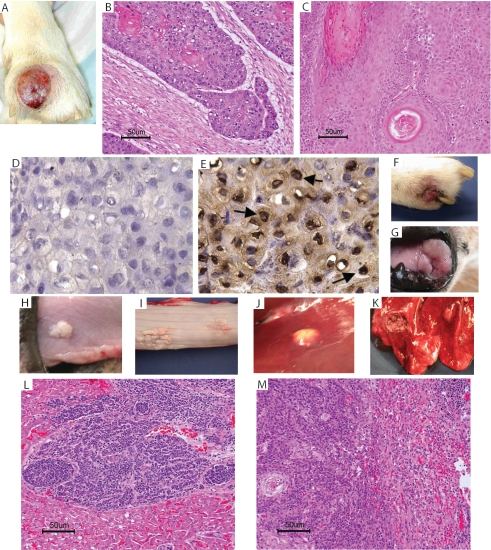
A mass developed between the digits of the right front foot in one of the dogs, R619, 3 months after recurrence of the interdigital papilloma (Fig. 3A; Table 1). A biopsy of the mass was diagnosed as invasive SCC (Fig. 3B), with the SCC emanating from the papilloma (Fig. 3C) and the presence of CfPV-2 antigen within the cells of the SCC (Fig. 3E). The two digits involved in the SCC in R619 were amputated; however, within 2 months, another interdigital SCC developed (Fig. 3F), in addition to histologically confirmed SCC lesions at various mucosal sites, including the nares, tongue, and esophagus (Fig. 3G to I). At necropsy, metastatic lesions were also observed in the bone, liver, and lung (Fig. 3J and K). Histologic confirmation of metastatic SCC in the liver and lung is shown in Fig. 3L and M. Within 3 to 4 months following the recurrence of interdigital papillomas in the other three dogs, interdigital masses developed that were histologically diagnosed as invasive SCC. When the interdigital mass from dog R1163 was biopsied, a 3-cm mass was observed in the soft palate, and a decision was made to euthanize the dog. At necropsy, lesions were observed along the esophagus and in the lung that were histologically diagnosed as SCC in situ and metastatic SCC, respectively. The other two dogs were routinely monitored for radiographic evidence of metastatic disease. Four months after the original diagnosis of SCC, one of the two dogs developed radiographic evidence of metastatic lung disease that was confirmed histologically at necropsy. The last dog has remained free of metastatic disease for 8 months. At the time of diagnosis of SCC, the only immunologic abnormality was an inverted CD4:CD8 ratio in two of the dogs (Table 2).
FIG. 3.
Squamous cell carcinoma in dog R619. The primary SCC lesion is shown in panels A to C. (A) Gross SCC lesion; (B) histopathology illustrating locally invasive SCC; (C) histopathology illustrating SCC emanating from a papilloma (*). (D and E) Immunohistochemistry of the primary SCC lesion, using antibodies to COPV (D) and CfPV-2 (E), demonstrating the presence of CfPV-2 antigen within the SCC cells (arrow). Magnification, ×400. Secondary and metastatic SCC lesions are shown in panels F to M. Gross lesions (F, digit; G, nares; H, tongue; I, esophagus; J, liver; K, lung) and the histopathology of metastatic SCC (L, liver; M, lung) are shown.
TABLE 2.
Immunologic findings at the time of SCC diagnosis in transplanted XSCID dogs
| Parameter | Value for indicated dog
|
|||
|---|---|---|---|---|
| R619 | R743 | R868 | R1163 | |
| Age post-BMT (yr) | 4.5 | 5 | 4 | 3.5 |
| Lymphocytes (cells/μl) | 3,500 | 3,210 | 3,980 | 1,840 |
| Cell phenotype (%) | ||||
| B cells | 7.6 | 12.5 | 4.3 | 5.8 |
| CD3 | 69.2 | 78.5 | 79.1 | 77.2 |
| CD4 | 39.1 | 28.9 | 34.4 | 36.6 |
| CD8 | 29.4 | 53.2 | 52.6 | 28.1 |
| CD45RA+ T cells | 82.3 | 71.2 | 80.6 | 86.7 |
| Proliferation (cpm) | 28,052 | 25,867 | 31,065 | 24,181 |
DISCUSSION
Oral papillomas are the most common clinical manifestation of papillomavirus infection in the dog and are caused by COPV. Canine oral papillomas normally occur in young dogs, have a typical exophytic histologic phenotype, and usually spontaneously regress within 4 to 8 weeks (41). Cutaneous papillomas occur uncommonly in the dog and are caused by a papillomavirus that differs from COPV, suggesting at least a second type of canine papillomavirus (12, 33, 49, 52). Reports describing cutaneous papillomas have primarily been for dogs receiving various forms of immununosuppressive therapy, with the papillomas spontaneously regressing shortly after cessation of the immunosuppressive drug (8, 33, 49, 52). One study described cutaneous papillomas in a healthy dog that spontaneously regressed 3 weeks after the diagnosis (50). The majority of cutaneous papillomas are exophytic, but some cutaneous papillomas have an endophytic (inverted) histologic phenotype (12, 33, 50). A rare form of cutaneous papilloma has been reported for the dog that histologically has an endophytic morphology with intracytoplasmic accumulation of keratin tonofilaments (33). A second canine papillomavirus, designated CfPV-2, has recently been cloned (Hang et al., unpublished data). CfPV-2 was isolated from a cutaneous papilloma and differs significantly from COPV with respect to its epithelial tropism and genomic structure. The genomes of COPV and CfPV-2 are highly divergent, and CfPV-2 contains an open reading frame for the early (E) protein E5, which is lacking in COPV. Phylogenetic analysis indicates that CfPV-2 is distantly related to all other papillomaviruses and represents a new genus among the papillomaviruses.
The cutaneous papillomas, and their biologic behavior, in the transplanted XSCID dogs in this study differed significantly from those previously reported. In contrast to the majority of reported cases of cutaneous papillomas in the dog, there was no evidence of systemic immunosuppression in the transplanted dogs prior to or at the time that papillomas developed. Immunologically, the transplanted dogs did not differ from carrier females or healthy males in our colony, in which papillomas have never been observed in over 15 years. The interdigital and footpad site predilection and endophytic morphology also differ from the majority of reported cutaneous papillomas in the dog. Lastly, the chronic nature and nonresponse to immune response modifiers are in contrast to what has been previously reported.
Although SCC has been associated with COPV, the biologic behavior of the SCCs in the transplanted dogs differed significantly from that in previously reported dogs and SCCs in general. In a series of 12 beagle dogs immunized at 3 months of age with a live COPV vaccine administered intramuscularly, 10 of the dogs developed a single squamous cell carcinoma localized to the site of immunization 2 to 3 years following immunization (6). Interestingly, five of the lesions contained small numbers of cells positive for structural antigens by immunohistochemistry using an antibody specific for group-specific papillomavirus antigen. However, electron microscopy of these same five lesions failed to detect any virions, suggesting the absence of a productive infection. None of the dogs had evidence of metastatic SCC. This contrasts with the aggressive biologic behavior of the SCCs that developed in the transplanted XSCID dogs, in which SCCs developed at multiple sites, with metastasis occurring in 75% of the dogs. Although SCC is the most common form of epidermal cancer in the dog, metastatic SCC is extremely rare (21).
The development of severe chronic cutaneous papillomavirus infections in the transplanted XSCID dogs shares many similarities with a recent report documenting a similar finding for human XSCID patients following bone marrow transplantation (30). The development of papillomas in the transplanted human patients and XSCID dogs had a late onset rather than occurring in the immediate posttransplantation period, when the dogs or boys were immunocompromised. Immunologically, there was no difference between transplanted XSCID dogs or XSCID boys who developed papillomas and those that did not. Only one of the nine transplanted patients responded to treatment, including immune response modifiers, which is similar to the lack of response in all three transplanted XSCID dogs treated with interferon. The major difference between the transplanted human patients and the transplanted XSCID dogs is that none of the human patients had evidence of neoplastic disease, whereas 67% of the transplanted XSCID dogs surviving past 3 1/2 years posttransplantation developed invasive squamous cell carcinoma, with 75% of these dogs developing metastastic SCC. One possible explanation for this discrepancy is that the oldest patient in the human study was 16 years posttransplantation. A 3 1/2-year-old dog is approximately equivalent to a 30-year-old human based upon comparison of biologic aging between humans and dogs (20). This raises the question of whether SCC will develop in the transplanted human XSCID patients later in life.
It is clear from the findings for transplanted human XSCID/Jak3 patients and XSCID dogs that the γc/Jak3 signaling pathway is somehow involved in the pathogenesis of papillomavirus infections. Further evidence for a role of the γc/Jak3 signaling pathway in papillomavirus infection is that severe chronic cutaneous papillomas have also been reported to be a common problem for a large kindred of human XSCID patients who have a missense mutation in the cytoplasmic domain of the γc subunit that permits partial Jak3 signaling and who can survive into late childhood or early adulthood without treatment (7, 48).
A cell-mediated immune response with a predominant CD4+ T-cell infiltrate has been associated with regression of both canine and human papillomas (35, 40). Although not reported for human XSCID patients, a common feature of the papillomas in the transplanted XSCID dogs was the lack of any evidence of an immune response to the papillomas. This is in contrast to the mononuclear cell infiltrate normally seen in biopsies of papillomas from healthy dogs. It is generally accepted that induction of a cell-mediated immune response to papillomavirus occurs by cross-presentation of papillomavirus antigen by Langerhans cells (LCs), professional antigen-presenting cells (APCs) within the epidermis (44). LCs and dermal dendritic cells, the other professional APCs in the skin, are both derived from the myeloid lineage and express a functional γc subunit (31, 32). A recent report has suggested that Toll-like receptor signaling through APCs may be an important component in the immune response to papillomavirus infection (56). Persistent or nonresponsive papillomas have been associated with either an absence of LCs or defective LC function (1, 3, 44, 47). Commonly used immune response modifiers, including imiquimod, which was used in the transplanted XSCID boys, act through normally functioning LCs and dermal dendritic cells (3, 11, 47). Although XSCID boys have been shown to possess LCs histologically (14, 24), their function has not been evaluated. Transplanted XSCID boys and dogs do not engraft donor cells of the myeloid lineage (17, 23, 26), and therefore, LCs remain of host origin.
Keratinocytes, the target cells of papillomavirus infection, are also part of the innate immune system. They can function as nonprofessional APCs, but it has been suggested that they may provide a tolerogenic response in the absence of normally functioning professional APCs (35, 42). Keratinocytes produce similar cytokines to those produced by APCs, including the γc-dependent cytokines IL-7 and IL-15, which have been shown to be involved in the differentiation of LCs (5, 28, 37, 38). They also express similar Toll-like receptors to those produced by professional APCs (45). Keratinocytes express various γc-dependent cytokine receptors, respond to γc-dependent cytokines, and express Jak3, suggesting that the γc/Jak3 signaling pathway may be important in keratinocyte function (2, 29, 43, 57). As with cells of the myeloid lineage, keratinocytes are not reconstituted following BMT.
Therefore, one possible explanation for the extensive papillomavirus infections observed in this study might relate to defective functioning of LCs and/or keratinocytes. Canine XSCID provides an ideal animal model with which to explore this possiblilty and to further evaluate the role of the γc-dependent signaling pathway in the response to papillomavirus infection and the progression to SCC.
Acknowledgments
This study was supported by NIH grants AI43745, AI41562, CA53371, and RR02512.
We thank Patty O'Donnell for excellent supervision of the XSCID dog colony.
REFERENCES
- 1.Arany, I., and S. K. Tyring. 1996. Status of local cellular immunity in interferon-responsive and -nonresponsive human papillomavirus-associated lesions. Sex. Transm. Dis. 23:475-480. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, R., M. Seifert, K. Asadullah, and H. D. Volk. 1999. Crosstalk between keratinocytes and T lymphocytes via Fas/Fas ligand interaction: modulation by cytokines. J. Immunol. 162:7140-7147. [PubMed] [Google Scholar]
- 3.Arrese, J., P. Paquet, N. Claessens, C. Pierard-Franchimont, and G. Pierard. 2001. Dermal dendritic cells in anogenital warty lesions unresponsive to an immune-response modifier. J. Cutan. Pathol. 28:131-134. [DOI] [PubMed] [Google Scholar]
- 4.Asao, H., C. Okuyama, S. Kumaki, N. Ishii, S. Tsuchiya, D. Foster, and K. Sugamura. 2001. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167:1-5. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt, A., H. Asada, V. Klaus-Kovtun, D. J. Altman, D. R. Lucey, and S. I. Katz. 1996. Interleukin-15 mRNA is expressed by human keratinocytes, Langerhans cells, and blood-derived dendritic cells and is downregulated by ultraviolet B radiation. J. Investig. Dermatol. 106:1047-1052. [DOI] [PubMed] [Google Scholar]
- 6.Bregman, C. L., R. S. Hirth, J. P. Sundberg, and E. F. Christensen. 1987. Cutaneous neoplasms in dogs associated with canine oral papillomavirus vaccine. Vet. Pathol. 24:477-487. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, E. G., F. C. Schmalstieg, D. P. Wirt, H. M. Rosenblatt, L. T. Adkins, D. P. Lookingbill, H. E. Rudloff, T. A. Rakusan, and A. S. Goldman. 1990. A novel X-linked combined immunodeficiency disease. J. Clin. Investig. 86:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks, M. A., and K. L. Campbell. 1992. Concurrent hypothyroidism, IgM deficiency, impaired T cell mitogenic response, and multifocal cutaneous squamous papillomas in a dog. Canine Pract. 17:15-21. [Google Scholar]
- 9.Buckley, R. H., R. I. Schiff, S. E. Schiff, M. L. Markert, L. W. Williams, T. O. Harville, J. L. Roberts, and J. M. Puck. 1997. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J. Pediatr. 130:378-387. [DOI] [PubMed] [Google Scholar]
- 10.Buckley, R. H., S. E. Schiff, R. I. Schiff, L. Markert, L. W. Williams, J. L. Roberts, L. A. Myers, and F. E. Ward. 1999. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N. Engl. J. Med. 340:508-516. [DOI] [PubMed] [Google Scholar]
- 11.Burns, R. P., Jr., B. Ferbel, M. Tomai, R. Miller, and A. A. Gaspari. 2000. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans' cells. Clin. Immunol. 94:13-23. [DOI] [PubMed] [Google Scholar]
- 12.Campbell, K. L., J. P. Sundberg, M. H. Goldschmidt, C. Knupp, and M. E. Reichmann. 1988. Cutaneous inverted papillomas in dogs. Vet. Pathol. 25:67-71. [DOI] [PubMed] [Google Scholar]
- 13.Cobbold, S., and S. Metcalfe. 1994. Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW). Tissue Antigens 43:137-154. [DOI] [PubMed] [Google Scholar]
- 14.Emile, J. F., A. Durandy, F. Le Deist, A. Fischer, and N. Brousse. 1997. Epidermal Langerhans' cells in children with primary T-cell immune deficiencies. J. Pathol. 183:70-74. [DOI] [PubMed] [Google Scholar]
- 15.Felsburg, P. J., B. J. Hartnett, P. S. Henthorn, P. F. Moore, S. Krakowka, and H. D. Ochs. 1999. Canine X-linked severe combined immunodeficiency. Vet. Immunol. Immunopathol. 69:127-135. [DOI] [PubMed] [Google Scholar]
- 16.Felsburg, P. J., R. L. Somberg, B. J. Hartnett, P. S. Henthorn, and S. R. Carding. 1998. Canine X-linked severe combined immunodeficiency. A model for investigating the requirement for the common gamma chain (gamma c) in human lymphocyte development and function. Immunol. Res. 17:63-73. [DOI] [PubMed] [Google Scholar]
- 17.Felsburg, P. J., R. L. Somberg, B. J. Hartnett, S. F. Suter, P. S. Henthorn, P. F. Moore, K. I. Weinberg, and H. D. Ochs. 1997. Full immunologic reconstitution following nonconditioned bone marrow transplantation for canine X-linked severe combined immunodeficiency. Blood 90:3214-3221. [PubMed] [Google Scholar]
- 18.Fischer, A., E. Haddad, N. Jabado, J. L. Casanova, S. Blanche, F. Le Deist, and M. Cavazzana-Calvo. 1998. Stem cell transplantation for immunodeficiency. Springer Semin. Immunopathol. 19:479-492. [DOI] [PubMed] [Google Scholar]
- 19.Gatti, R. A., H. J. Meuwissen, H. D. Allen, R. Hong, and R. A. Good. 1968. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet ii:1366-1369. [DOI] [PubMed] [Google Scholar]
- 20.Glickman, L. T., and L. M. Domanski. 1986. An alternative to laboratory animal experimentation for human health risk assessment: epidemiological studies of pet animals. ALTA 13:267-285. [Google Scholar]
- 21.Goldschmidt, M. H., and F. S. Shofer. 1998. Skin tumors of the dog and cat. Butterworth-Heinemann, Woburn, Mass.
- 22.Haddad, E., P. Landais, W. Friedrich, B. Gerritsen, M. Cavazzana-Calvo, G. Morgan, Y. Bertrand, A. Fasth, F. Porta, A. Cant, T. Espanol, S. Muller, P. Veys, J. Vossen, and A. Fischer. 1998. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood 91:3646-3653. [PubMed] [Google Scholar]
- 23.Haddad, E., F. Le Deist, P. Aucouturier, M. Cavazzana-Calvo, S. Blanche, G. De Saint Basile, and A. Fischer. 1999. Long-term chimerism and B-cell function after bone marrow transplantation in patients with severe combined immunodeficiency with B cells: a single-center study of 22 patients. Blood 94:2923-2930. [PubMed] [Google Scholar]
- 24.Hale, L. P., R. H. Buckley, J. M. Puck, and D. D. Patel. 2004. Abnormal development of thymic dendritic and epithelial cells in human X-linked severe combined immunodeficiency. Clin. Immunol. 110:63-70. [DOI] [PubMed] [Google Scholar]
- 25.Hartnett, B. J., P. S. Henthorn, P. F. Moore, K. I. Weinberg, H. D. Ochs, and P. J. Felsburg. 1999. Bone marrow transplantation for canine X-linked severe combined immunodeficiency. Vet. Immunol. Immunopathol. 69:137-144. [DOI] [PubMed] [Google Scholar]
- 26.Hartnett, B. J., D. P. Yao, S. E. Suter, N. M. Ellinwood, P. S. Henthorn, P. F. Moore, P. A. McSweeney, R. A. Nash, K. I. Weinberg, and P. J. Felsburg. 2002. Transplantation of X-linked severe combined immunodeficient dogs with CD34+ bone marrow cells. Biol. Blood Marrow Transpl. 8:188-197. [DOI] [PubMed] [Google Scholar]
- 27.Henthorn, P. S., R. L. Somberg, V. M. Fimiani, J. M. Puck, D. F. Patterson, and P. J. Felsburg. 1994. IL-2R gamma gene microdeletion demonstrates that canine X-linked severe combined immunodeficiency is a homologue of the human disease. Genomics 23:69-74. [DOI] [PubMed] [Google Scholar]
- 28.Heufler, C., G. Topar, A. Grasseger, U. Stanzl, F. Koch, N. Romani, A. E. Namen, and G. Schuler. 1993. Interleukin 7 is produced by murine and human keratinocytes. J. Exp. Med. 178:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junghans, V., T. Jung, and C. Neumann. 1996. Human keratinocytes constitutively express IL-4 receptor molecules and respond to IL-4 with an increase in B7/BB1 expression. Exp. Dermatol. 5:316-324. [DOI] [PubMed] [Google Scholar]
- 30.Laffort, C., F. Le Deist, M. Favre, S. Caillat-Zucman, I. Radford-Weiss, M. Debre, S. Fraitag, S. Blanche, M. Cavazzana-Calvo, G. de Saint Basile, J. P. de Villartay, S. Giliani, G. Orth, J. L. Casanova, C. Bodemer, and A. Fischer. 2004. Severe cutaneous papillomavirus disease after haemopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common gammac cytokine receptor subunit or JAK-3 deficiency. Lancet 363:2051-2054. [DOI] [PubMed] [Google Scholar]
- 31.Larregina, A., A. Morelli, E. Kolkowski, and L. Fainboim. 1996. Flow cytometric analysis of cytokine receptors on human Langerhans' cells. Changes observed after short-term culture. Immunology 87:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larregina, A. T., A. E. Morelli, E. Kolkowski, N. Sanjuan, M. E. Barboza, and L. Fainboim. 1997. Pattern of cytokine receptors expressed by human dendritic cells migrated from dermal explants. Immunology 91:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Net, J. L., G. Orth, J. P. Sundberg, P. Cassonnet, L. Poisson, M. T. Masson, C. George, and L. Longeart. 1997. Multiple pigmented cutaneous papules associated with a novel canine papillomavirus in an immunosuppressed dog. Vet. Pathol. 34:8-14. [DOI] [PubMed] [Google Scholar]
- 34.Leonard, W. J. 1996. The molecular basis of X-linked severe combined immunodeficiency: defective cytokine receptor signaling. Annu. Rev. Med. 47:229-239. [DOI] [PubMed] [Google Scholar]
- 35.Majewski, S., and S. Jablonska. 1998. Immunology of HPV infection and HPV-associated tumors. Int. J. Dermatol. 37:81-95. [DOI] [PubMed] [Google Scholar]
- 36.McSweeney, P. A., K. A. Rouleau, P. M. Wallace, B. Bruno, R. G. Andrews, L. Krizanac-Bengez, B. M. Sandmaier, R. Storb, E. Wayner, and R. A. Nash. 1998. Characterization of monoclonal antibodies that recognize canine CD34. Blood 91:1977-1986. [PubMed] [Google Scholar]
- 37.Mohamadzadeh, M., F. Berard, G. Essert, C. Chalouni, B. Pulendran, J. Davoust, G. Bridges, A. K. Palucka, and J. Banchereau. 2001. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J. Exp. Med. 194:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamadzadeh, M., A. Takashima, I. Dougherty, J. Knop, P. R. Bergstresser, and P. D. Cruz, Jr. 1995. Ultraviolet B radiation up-regulates the expression of IL-15 in human skin. J. Immunol. 155:4492-4496. [PubMed] [Google Scholar]
- 39.Moore, P. F., P. V. Rossitto, and T. Olivry. 1994. Development of monoclonal antibodies to canine T cell receptor-γδ (TCR-γδ) and their utilization in the diagnosis of epidermotropic cutaneous T cell lymphoma. Vet. Pathol. 31:597. [Google Scholar]
- 40.Nicholls, P. K., P. F. Moore, D. M. Anderson, R. A. Moore, N. R. Parry, G. W. Gough, and M. A. Stanley. 2001. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology 283:31-39. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls, P. K., and M. A. Stanley. 1999. Canine papillomavirus—a centenary review. J. Comp. Pathol. 120:219-233. [DOI] [PubMed] [Google Scholar]
- 42.Nickoloff, B. J., L. A. Turka, R. S. Mitra, and F. O. Nestle. 1995. Direct and indirect control of T-cell activation by keratinocytes. J. Investig. Dermatol. 105:25S-29S. [DOI] [PubMed] [Google Scholar]
- 43.Nishio, H., K. Matsui, H. Tsuji, A. Tamura, and K. Suzuki. 2001. Immunolocalisation of the Janus kinases (JAK)-signal transducers and activators of transcription (STAT) pathway in human epidermis. J. Anat. 198:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Offringa, R., A. de Jong, R. E. Toes, S. H. van der Burg, and C. J. Melief. 2003. Interplay between human papillomaviruses and dendritic cells. Curr. Top. Microbiol. Immunol. 276:215-240. [DOI] [PubMed] [Google Scholar]
- 45.Pivarcsi, A., L. Bodai, B. Rethi, A. Kenderessy-Szabo, A. Koreck, M. Szell, Z. Beer, Z. Bata-Csorgoo, M. Magocsi, E. Rajnavolgyi, A. Dobozy, and L. Kemeny. 2003. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 15:721-730. [DOI] [PubMed] [Google Scholar]
- 46.Rosen, F. S., M. D. Cooper, and R. J. Wedgwood. 1995. The primary immunodeficiencies. N. Engl. J. Med. 333:431-440. [DOI] [PubMed] [Google Scholar]
- 47.Sauder, D. N. 2003. Imiquimod: modes of action. Br. J. Dermatol. 149(Suppl. 66):5-8. [DOI] [PubMed] [Google Scholar]
- 48.Schmalstieg, F. C., W. J. Leonard, M. Noguchi, M. Berg, H. E. Rudloff, R. M. Denney, S. K. Dave, E. G. Brooks, and A. S. Goldman. 1995. Missense mutation in exon 7 of the common gamma chain gene causes a moderate form of X-linked combined immunodeficiency. J. Clin. Investig. 95:1169-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seibel, W., J. P. Sundberg, L. J. Lesko, J. J. Sauk, L. B. McCleary, and T. M. Hassell. 1989. Cutaneous papillomatous hyperplasia in cyclosporine-A treated beagles. J. Investig. Dermatol. 93:224-230. [DOI] [PubMed] [Google Scholar]
- 50.Shimada, A., K. Shinya, T. Awakura, I. Narama, H. Maeda, and T. Umemura. 1993. Cutaneous papillomatosis associated with papillomavirus infection in a dog. J. Comp. Pathol. 108:103-107. [DOI] [PubMed] [Google Scholar]
- 51.Somberg, R. L., J. P. Robinson, and P. J. Felsburg. 1994. T lymphocyte development and function in dogs with X-linked severe combined immunodeficiency. J. Immunol. 153:4006-4015. [PubMed] [Google Scholar]
- 52.Sundberg, J. P., E. K. Smith, A. J. Herron, A. B. Jenson, R. D. Burk, and M. Van Ranst. 1994. Involvement of canine oral papillomavirus in generalized oral and cutaneous verrucosis in a Chinese Shar Pei dog. Vet. Pathol. 31:183-187. [DOI] [PubMed] [Google Scholar]
- 53.Ting, S. S., S. G. Tangye, J. Wood, R. A. French, and J. B. Ziegler. 2001. Reduced memory B-cell populations in boys with B-cell dysfunction after bone marrow transplantation for X-linked severe combined immunodeficiency. Br. J. Haematol. 112:1004-1011. [DOI] [PubMed] [Google Scholar]
- 54.Wagner, J. L., R. C. Burnett, S. A. DeRose, L. V. Francisco, R. Storb, and E. A. Ostrander. 1996. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation 62:876-877. [DOI] [PubMed] [Google Scholar]
- 55.White, H., A. Thrasher, P. Veys, C. Kinnon, and H. B. Gaspar. 2000. Intrinsic defects of B cell function in X-linked severe combined immunodeficiency. Eur. J. Immunol. 30:732-737. [DOI] [PubMed] [Google Scholar]
- 56.Yang, R., F. M. Murillo, H. Cui, R. Blosser, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2004. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J. Virol. 78:11152-11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yasumura, S., W. C. Lin, E. Weidmann, P. Hebda, and T. L. Whiteside. 1994. Expression of interleukin 2 receptors on human carcinoma cell lines and tumor growth inhibition by interleukin 2. Int. J. Cancer 59:225-234. [DOI] [PubMed] [Google Scholar]