Abstract
Mouse models of orthopoxvirus disease provide great promise for probing basic questions regarding host responses to this group of pathogens, which includes the causative agents of monkeypox and smallpox. However, some essential tools for their study that are taken for granted with other mouse models are not available for these viruses. Here we map and characterize the initial CD8+ T-cell determinants for poxviruses in H-2d-haplotype mice. CD8+ T cells recognizing these three determinants make up around 40% of the total responses to vaccinia virus during and after resolution of infection. We then use these determinants to test if predicted conservation across orthopoxvirus species matches experimental observation and find an unexpectedly cross-reactive variant peptide encoded by ectromelia (mousepox) virus.
Vaccinia virus (VACV) is the live virus vaccine that was used to eradicate smallpox in the late 1970s (9). Since that time, VACV has been developed as a vector for recombinant vaccines targeting infectious diseases and cancer (7, 19, 25). Despite its unparalleled success against smallpox, ongoing use in experimental medicine, and ease of use with mouse models, VACV lags behind lymphocytic choriomeningitis and influenza A viruses as a model pathogen for understanding antiviral immunity. Due to its relaxed packaging constraints, ease of genetic manipulation, and broad ability to infect cells of various lineages from a variety of vertebrates (and even invertebrates), VACV shines as a vector to express foreign antigens and cytokines. But what of immunity to VACV itself, and how might immune responses to the vector compare to (or compromise) responses to foreign antigens in VACV-vectored vaccines? These issues are of increasing importance given renewed calls to vaccinate against smallpox in the face of biodefense concerns (16) and evidence that VACV vectors require improvement for better translation into human medicine (6, 24).
Although CD8+ T cells (TCD8+) are important antiviral effectors in poxvirus immunity (13), studies of TCD8+ responses to poxviruses have been hampered by the absence of information regarding the peptide determinants they recognize. The initial TCD8+ determinants recognized in the context of Kb and Db were described recently (18, 26). Given the influence of major histocompatibility complex (MHC) and non-MHC genes on pathogenicity of poxviruses (3), it is important to study immunity to poxviruses in a variety of mouse strains. BALB/c mice have been widely used as a model for studying VACV immunology (1, 10, 30, 32) and pathogenesis, especially by the intranasal route (27, 28, 31).
Here we define the initial TCD8+ determinants for VACV in H-2d-haplotype mice and explore cross-reactivity of TCD8+ specific for these peptides with natural variants found in other orthopoxviruses, including cowpox virus (CPXV) and ectromelia virus (ECTV), the causative agent of mousepox.
MATERIALS AND METHODS
Viruses and cell lines.
VACV strains WR and MVA were grown and titrated using standard methods, as were CPXV strain Brighton (a kind gift from Bernard Moss, NIAID, NIH, Bethesda, MD) and a thymidine kinase-negative mutant of ECTV, ECTV-TK− (12). 293A cells were maintained in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (D10). P815 was maintained in RPMI 1640 medium (QIMR and JCSMR medium services) with 10% fetal bovine serum (R10). For use as stimulators in TCD8+ assays, 1 × 106 to 5 × 106 P815 cells were infected with VACV at 5 to 10 PFU/cell in less than 200 μl of phosphate-buffered saline (PBS) at 37°C for 30 to 60 min with regular shaking, after which 9 ml R10 was added and the incubation continued for at least 3 h.
Synthetic peptides.
Peptides were synthesized by A & A Labs (San Diego, CA) at the John Curtin School of Medical Research Biomolecular Resources Facility or purchased as crude material from Mimotopes (Minneapolis, MN) or Pepscan Systems B.V. (Lelystad, The Netherlands). Peptides were resuspended at 4 to 20 mg/ml in 100% dimethyl sulfoxide and then diluted to required concentrations in PBS, PBS with 0.05% NP-40, or RPMI 1640. Peptides for use as probes were radiolabeled using the chloramine T method (22) after purification to >95% by reverse-phase high-pressure liquid chromatography.
Mice, infections, and immunizations.
Specific-pathogen-free BALB/c mice were obtained from Taconic (Gaithersburg, MD), Animal Resource Centre (Perth, Australia), or Animal Services Division, John Curtin School of Medical Research. Mice were housed and experiments done in accordance with relevant ethics requirements. In all VACV experiments, mice were infected intraperitoneally (i.p.) and doses ranged from 5 × 104 to 1 × 106 PFU/mouse for WR and 5 × 107 to 1 × 108 PFU/mouse for MVA. Mice were also infected with CPXV i.p., using a dose of 1 × 105 PFU. ECTV-TK− infections were done subcutaneously (s.c.) in the shank, using 2 × 107 PFU. A TK− mutant was chosen for these experiments, because fully virulent ECTV is lethal in BALB/c mice at any infective dose. The s.c. route was used, since ECTV is thought to be transmitted by a peripheral route and pathogenesis after i.p. infection is thought to be less true to the natural infection (8, 17).
Generation of 293 cells expressing mouse MHC genes.
The complete coding sequences for H-2Kd (Kd), H-2Dd (Dd), and H-2Ld (Ld) were amplified by PCR from recombinant VACV encoding these products and cloned into pcDNA3.1D/V5-His-TOPO (Invitrogen). Primers used were as follows: Kd, CACCATGGCACCCTGCACGC and TCACGCTAGAGAATGAGGGTC; Dd, CACCATGGGGGCGATGGCTC and TCACACTTTACAATCTGGGAGAGAC; Ld, CACCATGGGGGCGATGGCTC and TCACGCTTTACAATCTCGGA. All clones were verified by sequencing and comparison to GenBank entries: Kd, U47329; Dd, U47326; Ld, M33151. To make stable cell lines, clones were cleaved with SmaI and transfected into 293A cells using Lipofectamine 2000 reagent (Invitrogen). After overnight incubation (37°C, 9% CO2), cells were harvested and six serial 1:5 dilutions made in a fresh six-well plate in medium supplemented with 0.5 mg/ml G418 (Biofluids, MD). Transfectants were cloned by limiting dilution or fluorescence-activated cell sorting (FACS) (based on surface staining for the relevant H-2 product). followed by single-cell deposition (FACStar Plus, BD Biosciences, San Jose, CA) following several weeks of growth in selective medium. Potential H-2-expressing clones were selected by staining with monoclonal antibodies (Pharmingen, BD Biosciences) and analysis on a FACSCalibur instrument (BD biosciences). Clones were maintained in 0.5 mg/ml G418 (but expression of H-2 antigens was found to be stable for several passages in nonselecting medium) and are referred to collectively as 293H-2 cells or individually as 293Kd, 293DdC5, and 293LdA3.
Stimulations and intracellular cytokine staining (ICS).
Whole splenocytes (0.2 × 106 to 1 × 106) or TCD8+ (1 × 105) (CD8a+ T-Cell Isolation kit; Miltenyi Biotec, Aubern, CA) were incubated with (i) transfected cells (26), (ii) peptides, (iii) P815 cells pulsed with peptides, or (iv) P815 cells infected with VACV at 37°C and 5% CO2. Where P815 cells were used as stimulators, they were included at a P815 cell:splenocyte ratio of 1:5. Brefeldin A (10 μg/ml) was added after 1 h, and the incubation was continued for another 3 to 4 h. Cells were then stained with anti-CD8-phycoerythrin (PE) (clone 53-6.7; Pharmingen) (some experiments used fluorescein isothiocyanate or PE-Cy5), washed, fixed with 1% paraformaldehyde, washed, and finally stained with anti-gamma interferon (IFN-γ)-allophycocyanin (clone XMG1.2; Pharmingen) (some experiments used fluorescein isothiocyanate or PE) in the presence of 0.5% saponin. A FACSCalibur or FACSCanto instrument (BD Biosciences) was used for acquisition of data, and analysis was done using Flowjo software (Tree Star, Inc., Ashland, OR). Events were gated for live lymphocytes on a forward- and side-scatter gate followed by gating for CD8+ cells using CD8 and side scatter and displayed as CD8 by IFN-γ. Backgrounds determined using irrelevant (or no) peptide or uninfected cells were subtracted from test values.
MHC binding assays.
Binding of peptides to H-2 allomorphs was determined using quantitative assays based on the inhibition of binding of a radiolabeled standard peptide (22, 26). Briefly, 1 to 10 nM radiolabeled peptide was coincubated at room temperature with 1 μM to 1 nM purified H-2 molecules in the presence of 1 μM human β2-microglubulin (Scripps Laboratories, San Diego, CA). After a two-day incubation, MHC-peptide complexes were captured on microplates (Greiner Bio-one, Longwood, FL) coated with monoclonal antibody 28-14-8S, SF1-1.1.1, or 34-5-8S for Ld, Kd, or Dd, respectively, and bound radioactivity was measured using a TopCount (Packard Instrument Co.). The concentration of peptide yielding 50% inhibition of the binding of the radiolabeled probe peptide was calculated (IC50).
Sequence comparison.
Open reading frame (ORF) and determinant conservation between poxviruses was done with the aid of poxvirus orthologous clusters (29) maintained at the University of Victoria, Victoria, British Columbia, Canada, and accessed through the Poxvirus Bioinformatics Resource (www.poxvirus.org).
RESULTS AND DISCUSSION
Identification of proteins with H-2d-restricted VACV TCD8+ determinants.
Immunogenic VACV gene products were identified using a screening approach based on a library of 258 predicted VACV ORF expression clones (26) transiently transfected into 293 cells stably expressing H-2Kd, Ld, or Dd. Transfectants, each expressing a single VACV ORF, were tested for their ability to activate ex vivo splenic TCD8+ obtained from VACV-infected BALB/c mice as measured by ICS for IFN-γ (26).
Three immunogenic VACV ORFs were identified, one restricted by each of the three H-2d class I allomorphs (Table 1). Two of the three genes identified encode known VACV immunomodulators, a finding that echoes data from our screen for immunogenic ORFs in C57BL/6 mice (26). A52R encodes an inhibitor of signaling via Toll-like receptors and the interleukin 1 receptor (2), and a VACV lacking this gene is attenuated in mouse models (11). The product of E3L is an inhibitor of the antiviral state induced by interferons (5), and several regions in the E3 protein contribute independently to VACV virulence (14).
TABLE 1.
Immunogenic proteins, TCD8+ determinants, and binding to MHC
| ORF | H-2 | Function | Peptides testeda | IC50 (nM)b | Determinant | Namec |
|---|---|---|---|---|---|---|
| A52R | Kd | Intracellular inhibitor of TLR and IL-1 signaling | KYGRLFNEI | <0.5 | KYGRLFNEI | A5275-83 |
| DYIKVQKQDI | 80 | |||||
| KYCLRAIKL | 187 | |||||
| EYFMYRGLL | 1,524 | |||||
| LFSRWKYCL | 5,002 | |||||
| F2L | Ld | DUTPase | SPYAAGYDL | <0.5 | SPYAAGYDL | F226-34 |
| SPYAAGYDLY | 230 | |||||
| MPKFCYGRI | 256 | |||||
| IPPGERQLI | 543 | |||||
| SPTRQSPYA | 1,922 | |||||
| E3L | Dd | IFN resistance, inhibition of PKR, and Z-DNA binding | VGPSNSPTF | <0.5 | VGPSNSPTF | E3140-148 |
| DAMADVII | 267 | |||||
| CEAIKTIGI | 543 | |||||
| DDVSREKSM | 651 | |||||
| CQITRRDWSF | 1,083 |
Top five peptides (by H-2 binding) predicted for each gene. Total no. of peptides tested: A52R, 25; F2L 8; E3L, 13.
Peptide binding to relevant H-2 molecule.
Name derived from protein (rather than ORF) name and position of determinant within protein sequence.
For two mouse strains, then, our expression screening approach has found that immunomodulators are overrepresented as targets for TCD8+. This may reflect the tendency of this class of genes to be expressed abundantly at early times of infection. All things being equal, abundant expression will increase the chances of numbers of a given determinant class I complex reaching the threshold to activate naive TCD8+. Early expression may be an important factor in VACV immunogenicity due to multiple factors including the inability of VACV to express late viral genes in dendritic cells (4) and the ability of “first-responder” TCD8+ clones to suppress other clones in the phenomenon of immunodomination (21).
Mapping and characterization of H-2d-restricted VACV TCD8+ determinants.
Immunogenic ORFs were examined for MHC-binding motifs and peptides synthesized for testing in MHC binding and T-cell assays. In each case, the peptide that bound MHC with highest measured affinity was the immunogenic peptide (Table 1). Based on the frequency of responding splenic TCD8+ to primary VACV infection, F226-34 tops the immunodominance hierarchy, followed by A5275-83 and then E3140-148. At ∼10% of splenic TCD8+ in some mice, the size of responses to F226-34 is among the largest seen for individual determinants in virus infections of mice and comparable to the dominant VACV B820-27 determinant in C57Bl/6 mice (26).
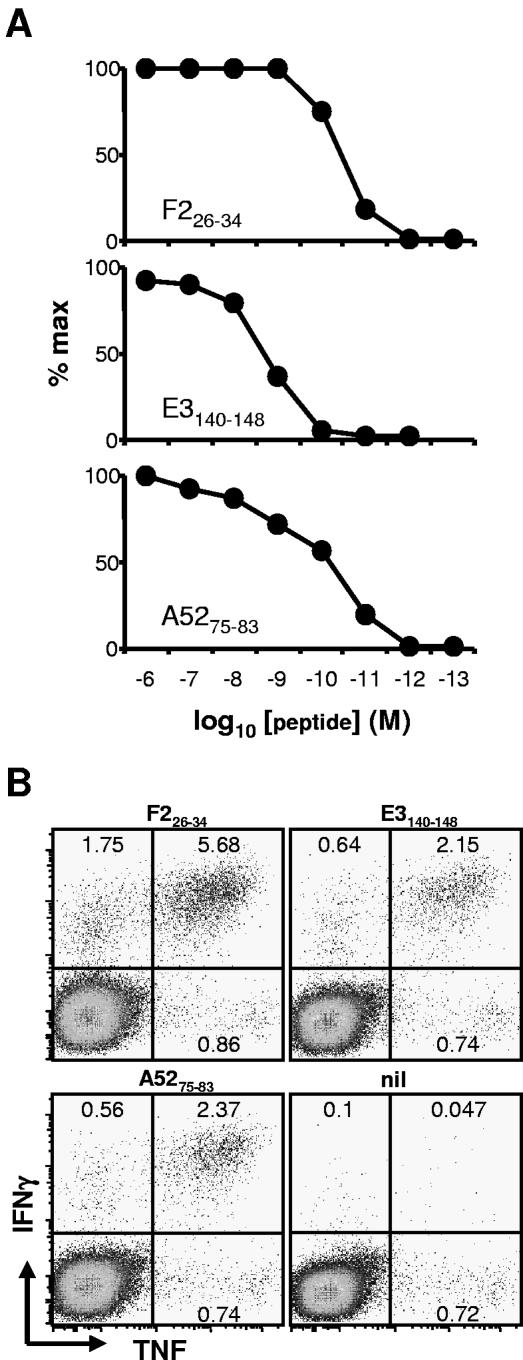
We characterized the potency of synthetic peptides for activating splenic TCD8+ cells from mice infected 7 days previously with VACV (Fig. 1A). All peptides stimulated CD8+ T cells at physiologically relevant peptide concentrations, with half-maximal stimulation being <10−8 M for each. As an additional test of the validity of the determinants, we determined that peptide-specific TCD8+ lines derived by repeated in vitro stimulation of VACV-immune splenocytes recognized the expected (but not control) VACV gene transfected into 293H-2 cells expressing the appropriate (but not mismatched) restriction element (not shown). These TCD8+ lines also recognized VACV-infected P815 cells (not shown).
FIG. 1.
Characterization of H-2d-restricted VACV TCD8+ determinants. Mice were infected with 106 PFU VACV-WR i.p., and 7 days later, splenocytes were used in ICS assays. (A) Antigenic potency of synthetic peptides. Peptides at the indicated concentrations were used as stimulators for ICS (IFN-γ) assays. Data are expressed as percentages of maximum stimulation and are representative of two or more experiments. (B) Anti-TNF and anti-IFN-γ expression in VACV determinant-specific TCD8+. TNF was used in addition to IFN-γ in ICS. Plots show CD8+ gated events and are representative of several experiments. Note that TNF+, IFN-γ− events are similar in negative controls and for all VACV peptides.
To further characterize the T cells responding to these peptides, the ICS protocol was expanded to include tumor necrosis factor (TNF) (Fig. 1B). Approximately 70% of TCD8+ making IFN-γ in response to each determinant also made TNF; these cells were those that made the largest amounts of IFN-γ. The proportion of IFN-γ+ cells that also make TNF in responses to VACV-infected P815 cells was similar (not shown). In all cases, the TNF+ population of peptide-specific TCD8+ was entirely contained within the IFN-γ+ population, as has been shown previously with mice by use of VACV (using infected cells as stimulators) and other viruses, such as lymphocytic choriomeningitis and influenza A viruses (10, 15, 23).
TCD8+ recognizing mapped determinants are a significant proportion of the response to VACV.
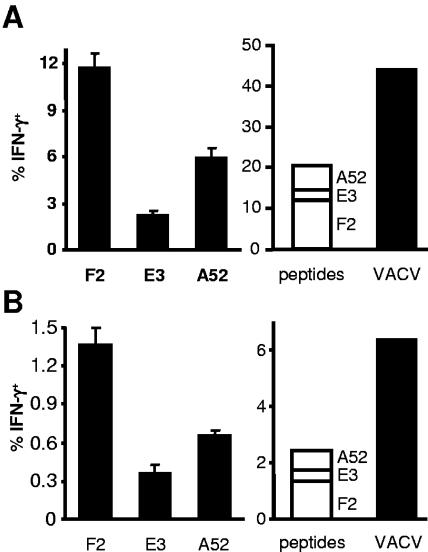
Next, we determined the fraction of the total TCD8+ response to VACV that is attributable to clones responding to the three determinants. BALB/c mice were infected with VACV, and responses to peptides and VACV-infected P815 cells were measured by ICS (Fig. 2A). Added together, cells recognizing the three determinants account for around 20% of TCD8+ in spleen. In the same experiment, VACV-infected cells were able to stimulate around 45% of splenic TCD8+. This indicates that the mapped determinants account for the specificity of around 40% of the TCD8+ response to acute VACV infection. Similar infections in DBA/2 mice (also H-2d haplotype) gave comparable results (not shown). Determinant-specific TCD8+ were also measured for BALB/c mice infected 12 weeks previously (Fig. 2B), and these data show that for memory responses, while the total size of each response is around eightfold lower, each response contracts proportionally.
FIG. 2.
TCD8+ responses to VACV determinants in the context of the total anti-VACV response. Mice were immunized i.p. with 106 PFU of VACV-WR, and TCD8+ responses of splenocytes were measured by ICS 7 days (A) and 12 weeks (B) later. (Left) Graphs show the percentages of TCD8+ that produce IFN-γ in ex vivo stimulations with peptides indicated (name of gene shown). (Right) Graphs compare a summation of the peptide data with similar data from the same splenocytes stimulated with VACV-infected P815 cells. Data are means and standard errors of the means from groups of four mice.
Predicted conservation of TCD8+ determinants across orthopoxvirus species.
We determined the conservation of the three determinants in the genomes of several orthopoxviruses, including VACV strain MVA, CPXV, ECTV (mousepox), and variola (VARV) (smallpox) viruses using POCs software (29). We used the Bimas algorithm (20) (http://bimas.dcrt.nih.gov/molbio/hla_bind) to predict relevant class I allomorph binding affinity for each variant peptide (Table 2). None of the determinants is conserved in all these viruses.
TABLE 2.
Variant TCD8+ determinants across poxvirus species and strains
| WR gene | Sequences | Virusa | Abbrev.b | Bimas result
|
IC50 (nM)c | |
|---|---|---|---|---|---|---|
| Rank | Score | |||||
| F2L | SPYAAGYDL | WR, VARV | F2 | 1 | 150.0 | <0.5 |
| SPGAAGYDL | MVA, CPXV | F2(G) | 1 | 150.0 | 3.7 | |
| SNHAAGYDL | ECTV | F2(NH) | 15 | 5.0 | 5.3 | |
| E3L | VGPSNSPTF | WR, MVA, CPXV, VARV | E3 | 1 | 120.0 | <0.5 |
| VGPSNSPIF | ECTV | E3(I) | 1 | 120.0 | 12 | |
| A52R | KYGRLFNEI | WR, CPXV, ECTV, VARV | A52 | 2 | 4,147.2 | <0.5 |
| ORF absent | MVA | |||||
F226-34 exists in three variant sequences in all 27 orthopoxviruses for which the full genome sequence is available. The most common variants are SPYAAGYDL, as in VACV-WR, and VARV and SPGAAGYDL, as in VACV-MVA and CPXV. Both are ranked as the highest-H-2Ld-binding peptides within their respective proteins and would be predicted to be immunogenic. ECTV strains have a third variant, namely, SNHAAGYDL, which is not predicted to bind well to H-2Ld.
E3140-148 is highly conserved, being present in all sequenced orthopoxviruses, with the determinant in nearly all cases being identical to that in VACV-WR. The exception is ECTV, in which the penultimate residue of the peptide is changed from Thr to Ile. This variant (VGPSNSPIF) is ranked as the highest H-2Dd-binding peptide in the ECTV homologue of E3 by Bimas and is predicted to be immunogenic.
Finally, the A52R ORF is absent in MVA and several other orthopoxviruses, but where present, the A5275-83 determinant is identical to that found in VACV-WR.
Observed conservation of TCD8+ determinants across orthopoxvirus species.
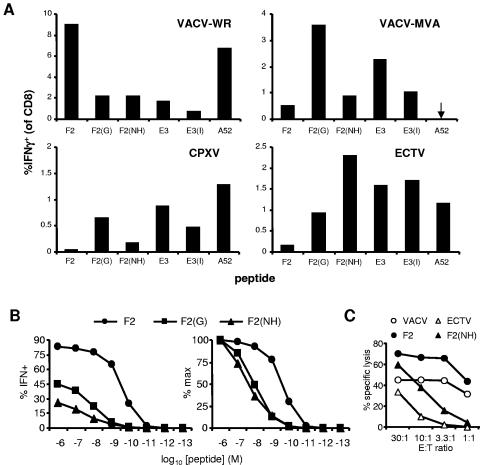
The difficulties associated with working with VARV means that much smallpox vaccine development will go forward on the basis of conservation of determinant sequences among poxviruses and predictions of antigenicity. The use of poxviruses that infect mice and mapping of determinants allow us to test predictions of antigenic cross-reactivity across orthopoxvirus species. To this end, we determined TCD8+ responses of mice infected with VACV-WR, VACV-MVA, CPXV, or ECTV to peptides corresponding to all variants of the three VACV-WR determinants (Fig. 3). In each case, all variants were recognized by splenic TCD8+ from infected mice, but the largest response was always to the peptide encoded by the infecting virus (e.g., SPGAAGYDL for MVA- or CPXV-infected mice). This result was not surprising for SPGAAGYDL and VGPSNSPIF, both predicted to bind well to their MHC restriction element. For SNHAAGYDL, which is not predicted to bind H-2Ld, we were surprised to find that not only did TCD8+ from VACV-infected (WR and MVA) and CPXV-infected mice respond to this variant of F226-34, but ECTV was able to prime responses to this peptide in vivo.
FIG. 3.
Cross-reactivity between TCD8+ elicited by VACV-WR and variant peptides from other orthopoxviruses. In all panels, VACV-WR peptides are indicated by protein name; variants are F2(G) (SPGAAGYDL), F2(NH) (SNHAAGYDL), and E3(I) (VGPSNSPIF). (A) Mice were infected with the orthopoxvirus shown in the top left of each graph: VACV-WR and MVA, 106 and 108 PFU, respectively, i.p.; CPXV, 105 PFU i.p.; and ECTV-TK−, 2 × 107 PFU s.c. in the rear leg shank. Splenic TCD8+ responses to peptides were measured by ICS after 7 days (VACV strains) and 8 days (CPXV and ECTV). Data are percentages of TCD8+ that produce IFN-γ in ex vivo stimulations with peptides. Means and standard errors of the means for groups of four or five mice are plotted and are representative of repeated experiments. (B and C) A TCD8+ line was derived from VACV-WR immune splenocytes by five restimulations with SPYAAGYDL in vitro and tested for cross-reactivity with variant peptides at various concentrations by ICS (B) and to peptides and virus-infected cells by cytotoxicity assay (C). (B, left) Percent TCD8+ that produce IFN-γ after a short stimulation with the peptide shown (circle, SPYAAGYDL; square, SPGAAGYDL; triangle, SNHAAGYDL); (B, right) the same data presented as percentages of the maximum response. Panel C shows percent specific lysis at the E:T ratios indicated for this TCD8+ line against ECTV-infected (ECTV, unfilled triangle) or VACV-WR-infected (VACV, unfilled circle) P815 cells or peptide-pulsed P815 as indicated above the graph (symbols as in panel B).
As an aside, in these experiments ECTV-TK− was used by a peripheral route (s.c.) to better replicate the natural route of infection (8, 17), and we noted that the hierarchy of dominance for the three antigens was F2 followed by E3 followed by A52, which is different from that found using VACV-WR by the i.p. route (F2 followed by A52 followed by E3). To see if this might be in part a result of the different route, as was found for VACV infection in C57BL/6 mice (26), we infected a group of mice with VACV via the s.c. route. In these mice the order of dominance was F2 followed by E3, whose dominance was greater than or equal to that of A52 (not shown), more closely resembling that in mice infected s.c. with ECTV-TK− rather than VACV-WR by i.p. This result was repeated in two other experiments and together with results in our previous study suggests that the effect of the infection route on the immunodominance hierarchy is a general feature of CD8+ T-cell responses to poxviruses.
We then returned to the issue of cross-reactivity between variant peptides, and to investigate this further, we measured the actual affinities of all peptide variants for their restricting MHC (Table 2) and generated a TCD8+-reactive line to F226-34 (SPYAAGYDL) and estimated the size and avidity of cross-reactive TCD8+ populations using peptide titrations in ICS (Fig. 3B). The affinity measurements showed that despite a prediction to the contrary in the case of SNHAAGYDL, all peptides bound their restriction element with very high (<20 nM) affinity. In the TCD8+ line experiments, nearly 85% of the cells in the F226-34 line responded to SPYAAGYDL at 1 μM, but only half of these cells were cross-reactive with SPGAAGYDL and around a quarter with SNHAAGYDL. These data were also plotted as percentages of maximum stimulation (left graph in Fig. 3B) to give a better comparison of the relative sensitivities of the TCD8+ populations that cross-reacted to the peptides. Comparison of these two plots indicates that while roughly twice as many SPYAAGYDL cells in the culture recognized SPGAAGYDL compared with recognition of SNHAAGYDL, the overall sensitivity of the cross-reactive TCD8+ for each case was similar. Given that these peptides have similar affinities for H-2Ld (Table 2), these data suggest that the cross-reactive T-cell populations recognizing these variants have similar avidity. Finally, to demonstrate that the cross-reactivity between SPYAAGYDL and SNHAAGYDL can lead to antiviral killing, we tested the ability of the same TCD8+ line to lyse VACV- or ECTV-infected cells (Fig. 3c). TCD8+ specific for F226-34 from VACV were able to lyse cells pulsed with both forms of F226-34 as well as those infected with VACV and with ECTV. These data, combined with those from work with H-2b-restricted peptides (26), show that current bioinformatic methods are not always able to predict cross-reactivities of TCD8+ responses across orthopoxvirus species and highlight the need for refined predictive algorithms supported by more experimental data.
Concluding remarks.
We have mapped the first TCD8+ determinants for VACV in BALB/c (and other H-2d haplotype) mice, and these three determinants were responsible for a significant proportion of the total TCD8+ response to VACV. Synthetic peptides based on each determinant were recognized at physiologically relevant concentrations in in vitro assays, and approximately 70% of the TCD8+ that responded to each made TNF as well as IFN-γ. Finally, a comparison of predicted and actual cross-reactivities of these determinants across four different orthopoxviruses revealed an unexpectedly cross-reactive variant peptide encoded by ECTV.
Acknowledgments
We thank B. Moss for providing PCR products for the VACV ORF library, CPXV stocks, and helpful discussions; L. Wyatt for titrated stocks of MVA; Calvin Eigsti and the NIAID flow cytometry section for single-cell sorting; and L. Morrison, A. Asgari, and D. Tokarchick for technical assistance.
This work was funded by the NIAID-NIH intramural program and grants from the NHMRC (Australia) to D.C.T. (Howard Florey Centenary Fellowship no. 224273) and G.K. (no. 153836), the Howard Hughes Medical Institute (G.K.), and NIAID-NIH to A.S. and J.S. (R01 grant AI56268 and contract N01-AI-40023). I.G.S. is the recipient of an ANU Ph.D. scholarship.
REFERENCES
- 1.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briody, B. 1959. Response of mice to ectromelia and vaccinia viruses. Bacteriol. Rev. 23:61-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronte, V., M. W. Carroll, T. J. Goletz, M. Wang, W. W. Overwijk, F. Marincola, S. A. Rosenberg, B. Moss, and N. P. Restifo. 1997. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc. Natl. Acad. Sci. USA 94:3183-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooney, E. L., A. C. Collier, P. D. Greenberg, R. W. Coombs, J. Zarling, D. E. Arditti, M. C. Hoffman, S. L. Hu, and L. Corey. 1991. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet 337:567-572. [DOI] [PubMed] [Google Scholar]
- 7.Drexler, I., C. Staib, and G. Sutter. 2004. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr. Opin. Biotechnol. 15:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenner, F. 1949. Mouse-pox (infectious ectromelia of mice): a review. J. Immunol. 63:341-373. [PubMed] [Google Scholar]
- 9.Fenner, F., D. Henderson, I. Arita, Z. Jezek, and I. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 10.Harrington, L. E., R. van der Most, J. L. Whitton, and R. Ahmed. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 76:3329-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. J. O'Neill. 2003. The poxvirus protein A52R targets toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson, R. J., D. J. Maguire, L. A. Hinds, and I. A. Ramshaw. 1998. Infertility in mice induced by a recombinant ectromelia virus expressing mouse zona pellucida glycoprotein 3. Biol. Reprod. 58:152-159. [DOI] [PubMed] [Google Scholar]
- 13.Karupiah, G., R. M. Buller, N. Van Rooijen, C. J. Duarte, and J. Chen. 1996. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J. Virol. 70:8301-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, Y. G., M. Muralinath, T. Brandt, M. Pearcy, K. Hauns, K. Lowenhaupt, B. L. Jacobs, and A. Rich. 2003. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl. Acad. Sci. USA 100:6974-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Gruta, N. L., S. J. Turner, and P. C. Doherty. 2004. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 172:5553-5560. [DOI] [PubMed] [Google Scholar]
- 16.Lane, H. C., J. L. Montagne, and A. S. Fauci. 2001. Bioterrorism: a clear and present danger. Nat. Med. 7:1271-1273. [DOI] [PubMed] [Google Scholar]
- 17.Marchal, J. 1930. Infectious ectromelia. A hitherto undescribed virus disease of mice. J. Pathol. Bacteriol. 33:713-728. [Google Scholar]
- 18.Mathew, A., M. Terajima, K. West, S. Green, A. L. Rothman, F. A. Ennis, and J. S. Kennedy. 2005. Identification of murine poxvirus-specific CD8+ CTL epitopes with distinct functional profiles. J. Immunol. 174:2212-2219. [DOI] [PubMed] [Google Scholar]
- 19.Panicali, D., S. W. Davis, R. L. Weinberg, and E. Paoletti. 1983. Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 80:5364-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker, K. C., M. A. Bednarek, and J. E. Coligan. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163-175. [PubMed] [Google Scholar]
- 21.Roy-Proulx, G., C. Baron, and C. Perreault. 2005. CD8 T-cell ability to exert immunodomination correlates with T-cell receptor: epitope association rate. Biol. Blood Marrow Transplant. 11:260-271. [DOI] [PubMed] [Google Scholar]
- 22.Sidney, J., S. Southwood, C. Oseroff, M. F. Del Guercio, A. Sette, and H. Grey. 1998. Measurement of MHC/peptide interactions by gel filtration, p. 18.13.11-18.13.19. In Current protocols in immunology. John Wiley & Sons, Inc., Hoboken, N.J. [DOI] [PubMed]
- 23.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164:208-216. [DOI] [PubMed] [Google Scholar]
- 24.Smith, C. L., F. Mirza, V. Pasquetto, D. C. Tscharke, M. J. Palmowski, P. R. Dunbar, A. Sette, A. L. Harris, and V. Cerundolo. 2005. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J. Immunol. 175:8431-8437. [DOI] [PubMed] [Google Scholar]
- 25.Smith, G. L., M. Mackett, and B. Moss. 1983. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 302:490-495. [DOI] [PubMed] [Google Scholar]
- 26.Tscharke, D. C., G. Karupiah, J. Zhou, T. Palmore, K. R. Irvine, S. M. M. Haeryfar, S. Williams, J. Sidney, A. Sette, J. R. Bennink, and J. W. Yewdell. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tscharke, D. C., P. C. Reading, and G. L. Smith. 2002. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. J. Gen. Virol. 83:1977-1986. [DOI] [PubMed] [Google Scholar]
- 28.Turner, G. S. 1967. Respiratory infection of mice with vaccinia virus. J. Gen. Virol. 1:399-402. [DOI] [PubMed] [Google Scholar]
- 29.Upton, C., S. Slack, A. L. Hunter, A. Ehlers, and R. L. Roper. 2003. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 77:7590-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weltzin, R., J. Liu, K. V. Pugachev, G. A. Myers, B. Coughlin, P. S. Blum, R. Nichols, C. Johnson, J. Cruz, J. S. Kennedy, F. A. Ennis, and T. P. Monath. 2003. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 9:1125-1130. [DOI] [PubMed] [Google Scholar]
- 31.Williamson, J. D., R. W. Reith, L. J. Jeffrey, J. R. Arrand, and M. Mackett. 1990. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J. Gen. Virol. 71:2761-2767. [DOI] [PubMed] [Google Scholar]
- 32.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]