Abstract
Infection of human dermal microvascular endothelial (HMVEC-d) cells and human foreskin fibroblast (HFF) cells in vitro by Kaposi's sarcoma-associated herpesvirus (KSHV) provides an excellent in vitro model system to study viral latency. KSHV infection is characterized by the induction of preexisting host signal cascades; sustained expression of the latency-associated open reading frame 73 (ORF73) (LANA-1), ORF72, and K13 genes; transient expression of a limited number of lytic genes, including the lytic cycle switch ORF50 (replication and transcription activator) gene; and reprogramming of host transcriptional machinery regulating a variety of cellular processes, including several proinflammatory responses. The cyclooxygenase 2 (COX-2) gene was one of the host cell genes that was highly up-regulated at 2 and 4 h postinfection (p.i.) of HMVEC-d and HFF cells (P. P. Naranatt, H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran, Cancer Res. 64:72-84, 2004). Since COX-2 is an important mediator of inflammatory and angiogenic responses, here, using real-time PCR, Western blot, and immunofluorescence assays, we characterized the COX-2 stimulation and its role in KSHV infection. KSHV induced a robust COX-2 expression, which reached a maximum at 2 h p.i. in HMVEC-d cells and at 8 h p.i. in HFF cells, and significantly higher levels were continuously detected for up to 72 h p.i. Constitutive COX-1 protein levels were not modulated by KSHV infection. Moderate levels of COX-2 were also induced by UV-irradiated KSHV and by envelope glycoproteins gB and gpK8.1A; however, viral gene expression appears to be essential for the increased COX-2 induction. High levels of prostaglandin E2 (PGE2), a COX-2 product, were released in the culture supernatant medium of infected cells. PGE2 synthase, catalyzing the biosynthesis of PGE2, also increased upon infection and inhibition of COX-2 by NS-398, and indomethacin drastically reduced the levels of PGE2 and PGE2 synthase. COX-2 inhibition did not affect KSHV binding, internalization of virus, or the trafficking to the infected cell nuclei. However, latent ORF73 gene expression and ORF73 promoter activity were significantly reduced by COX-2 inhibitors, and this inhibition was relieved by exogenous supplementation with PGE2. In contrast, lytic ORF50 gene expression and ORF50 promoter activity were unaffected. These studies demonstrate that COX-2 and PGE2 play roles in facilitating latent viral gene expression and the establishment and maintenance of latency and suggest that KSHV has evolved to utilize the inflammatory responses induced during infection of endothelial cells for the maintenance of viral latent gene expression.
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is etiologically associated with Kaposi's sarcoma (KS) (12) and two lymphoproliferative disorders, namely, body cavity-based B-cell lymphoma (BCBL) (also called primary effusion lymphoma) (11) and some forms of multicentric Castleman's disease, a polyclonal B-cell angiolymphoproliferative disorder (60). In vivo, viral DNA and transcripts have been detected in human B cells, macrophages, keratinocytes, endothelial cells, and epithelial cells (18, 21, 40, 85). Similarly to other herpesviruses, KSHV displays latent and lytic cycles in the infected cells. In KS tissues, KSHV DNA is present in a latent form in the vascular endothelial and spindle cells, and a lytic cycle is detected in a low percentage of infiltrating inflammatory monocytes of KS lesions (18, 21, 41, 59, 60, 65, 85). BCBL cell lines such as BCBL-1 and BC-3 carry KSHV in a latent form, and a lytic cycle can be induced by 12-O-tetradecanoyl-phorbol-13-acetate (60). KSHV carries more than 80 complete open reading frames (ORFs), which are designated as ORF4 to ORF75 by their homology to ORFs of herpesvirus saimiri (50, 57). Divergent regions between the conserved gene blocks contain more than 20 KSHV unique genes, which are designated with the prefix K. Several KSHV-encoded proteins are homologs of host proteins. These genes include K2 (encoding viral interleukin-6 [vIL-6]), K4 (encoding viral macrophage-inhibitory protein II [vMIP-II]), K3 and K5 (encoding immunomodulatory proteins MIR-1 and MIR-2), K6 (encoding vMIP-I), K7 (encoding an antiapoptotic protein), K9 (encoding viral interferon-regulatory factor [vIRF]), K11.1 (encoding vIRF2), ORF16 (encoding vBcl-2), K13 (encoding v-FLICE-inhibitory protein), K14 (encoding vOX-2), ORF72 (encoding viral cyclin D), and ORF74 (encoding viral G protein-coupled receptor) (50, 57).
Infection by members of the herpesvirus family is characterized by the establishment of latent infection in a variety of host target cells and the expression of a limited number of viral genes. Several aspects of latent infection are not well defined, including the mechanisms of establishment and maintenance of latent infection, how the limited viral genes manipulate the host cell functions, and how the virus evades the host intrinsic, innate, and immune responses during latent infection. A lack of well-defined in vitro models to analyze the latent infection for alphaherpesviruses such as herpes simplex virus type 1 (HSV-1) and HSV-2 and for betaherpesviruses such as cytomegalovirus (CMV) hampers these types of investigations. Moreover, in vitro infection by alpha- and betaherpesviruses results in the rapid initiation of the lytic cycle, virus progeny formation, and cell death. This is not unexpected, since the majority of cells used in these studies do not represent the in vivo target cells in which these viruses enter latency. In contrast, in vitro infection of human primary B cells by gamma-1 Epstein-Barr virus (EBV) results in the establishment of latent infection, transformation, and the establishment of B-lymphoblastoid cell lines (30).
Although KSHV infection of primary B cells does not result in transformation, unlike EBV, KSHV infects a variety of in vitro target cells such as human B, endothelial, epithelial, and fibroblast cells (1-4, 7, 17, 22, 33, 44, 54, 73, 77) as well as animal cells, such as owl monkey kidney cells, baby hamster kidney fibroblast (BHK-21) cells, Chinese hamster ovary (CHO) cells, and primary embryonic mouse fibroblast cells (3, 4, 18, 32, 48). In contrast to the case for alpha- and betaherpesviruses, in vitro infection by KSHV does not result in a productive lytic cycle. Instead, KSHV establishes a latent infection (55), and it thus provides a good in vitro model for studying viral and host factors involved in the establishment and maintenance of latent infection.
KSHV infection of primary human adult dermal microvascular endothelial (HMVEC-d) cells and human foreskin fibroblast (HFF) cells is characterized by the sustained expression of latency-associated ORF73 (LANA-1 or latency-associated nuclear antigen), ORF72 (v-cyclin), and K13 (v-FLIP) genes. However, an unique aspect of this in vitro infection is our demonstration of concurrent transient expression of a limited number of lytic KSHV genes, such as the lytic cycle switch gene ORF50 (replication and transcription activator [RTA]) and the immediate-early lytic K5, K8, and v-IRF2 genes (32). Similar results were also seen during KSHV primary infection of human 293 cells (34). Among these lytic proteins, the K5 gene product, involved in the down-regulation of major histocompatibility complex class I A and C, ICAM-1, CD31/PECAM, and B7-2 molecules, could be detected for up to 5 days in the infected HMVEC-d cells (32). How KSHV establishes in vitro latency and the roles played by viral and host genes in the establishment and maintenance of infection are not well understood. Several overlapping dynamic events occur during the early stages of KSHV infection, and current evidence suggests that these events play active roles in infection (46). KSHV binds to the cell surface heparan sulfate molecules via its envelope glycoproteins gB and gpK8.1A, to integrins via gB, and possibly to other yet-to-be identified molecules (61). This is followed by virus entry into the target cells, probably overlapping with the induction of host cell signal pathways that facilitate the entry. KSHV envelope glycoprotein gB possesses the integrin binding RGD motif, and virus binding and entry into the target cells induce extensive cytoskeletal rearrangement in the target cells and signal cascades such as focal adhesion kinase (FAK), Src, phosphatidylinositol 3-kinase (PI-3K), Rho-GTPases, protein kinase C ζ (PKC-ζ), MEK, and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathways (62). We have demonstrated the role of FAK and PI-3K in virus entry, the role of RhoA in microtubule modulation and transport of the capsid in the cytoplasm, and the role of ERK1/2 in the initiation of lytic (ORF50) and latent (ORF73) gene expression (61).
As an initial step toward understanding how KSHV establishes in vitro latent infection, we have previously examined the modulation of host cell gene expression at 2 and 4 h postinfection (p.i.) of primary HMVEC-d and HFF cells by using oligonucleotide arrays (47). We observed the reprogramming of host transcriptional machinery regulating a variety of cellular processes, including apoptosis, transcription, cell cycle regulation, signaling, the inflammatory response, and angiogenesis (47). Notable among the KSHV-induced proinflammatory genes is the angiogeneic stress response cyclooxygenase 2 (COX-2) gene, whose expression was the most strongly up-regulated in both HMVEC-d and HFF cells.
Three isoforms of COX, namely, COX-1, COX-2, and COX-3, have been identified to date. COX is a key rate-limiting cellular enzyme involved in the synthetic pathway of prostaglandins and thromboxane from arachidonic acid (76). The COX-1 gene is constitutively expressed and displays the characteristics of a housekeeping gene in most tissues, and it is involved in mediating physiological responses. COX-2 is an inducible immediate-early gene product whose synthesis in cells can be up-regulated by either mitogenic or inflammatory stimuli (64, 76). COX-2 has also emerged as the mediator critically involved in cancer progression (64). COX-2 levels are barely detected in normal tissues (19), but the gene has been shown to be overexpressed in a wide range of tumors and possesses proangiogenic and antiapoptotic properties (83). COX-2 is widely regarded as a potential pharmacological target for preventing and treating malignancies (16). Pharmacological agents such as nonsteroidal anti-inflammatory drugs target COX-1 and COX-2 (64, 75). Moreover, nonsteroidal anti-inflammatory drugs blocking COX and prostaglandin production have been recognized as potentially effective antiviral compounds (63, 78, 86). COX-2 produces an important inflammatory prostaglandin, prostaglandin E2 (PGE2), which is a potent immunoregulatory lipid mediator that plays key roles in the regulation of a number of cellular processes, including the acute and chronic inflammatory responses as well as innate immune responses which are generally produced in response to cytokines, mitogens, bacterial lipopolysaccharide (LPS), and viral infections (53, 66, 74).
Since COX-2 and PGE2 play important roles in host inflammatory and stress responses, including viral infections, we designed studies to decipher the kinetics of KSHV-induced COX-2 and PGE2 induction and their role in infection. We present several lines of evidence to show that KSHV-induced COX-2 and PGE2 play roles in the continued latent ORF73 gene expression, suggesting that KSHV orchestrates the host cell inflammatory responses for its advantage.
MATERIALS AND METHODS
Cells.
Primary HFF cells (Clonetics, Walkersville, Md.) and CV-1 cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, Utah), 2 mM l-glutamine, and antibiotics. Primary HMVEC-d cells (CC-2543; Clonetics) were grown in endothelial basal medium 2 with growth factors (Clonetics). Recombinant green fluorescent protein-KSHV (GFP-KSHV-γKSHV.152)-carrying BCBL-1 cells (77) were cultured in RPMI 1640 (Gibco BRL) medium (48). All cells were cultured in LPS-free medium.
Virus.
Induction of the KSHV lytic cycle in GFP-BCBL-1 cells, supernatant collection, and virus purification procedures were described previously (48), and purity was assessed according to general guidelines established in our laboratory (3, 32, 48). The virus preparations used in our studies represent mostly enveloped KSHV virion particles (32, 48), and only enveloped virus particles were seen by electron microscopy, indicating the purity of virus preparations (1, 2, 4). Since every viral genome contains a single copy of the ORF73 gene, the number of viral DNA molecules could be calculated from the corresponding copy numbers of the ORF73 gene. Hence, we extracted the KSHV DNA from the purified virus, and copy numbers were quantitated by real-time DNA PCR using primers amplifying the KSHV ORF73 gene (32, 62). To prepare replication-defective virus, KSHV was irradiated with UV light (365 nm) for 20 min at a 10-cm distance. KSHV DNA was extracted from the UV-irradiated virus, and the copy numbers were quantitated by real-time DNA PCR as described previously (32).
Preparation of DNA and RNA.
Total DNAs from the viral stocks and cells were prepared using a DNeasy tissue kit (QIAGEN, Inc., Valencia, Calif.), and total RNA was isolated from infected or uninfected cells using an RNeasy kit as described previously (32, 61).
KSHV gB and gpK8.1A.
The generation and purification of baculovirus-expressed purified ΔTMgB and ΔTMgpK8.1A have been described in detail previously (80, 81, 87). Recombinant protein purifications were carried out in buffers prepared with LPS-free water, and stock preparations of proteins were monitored for endotoxin contamination by standard Limulus assay (Limulus amebocyte lysate endochrome; Charles River Endosafe, Charleston, S.C.) as recommended by the manufacturer.
Antibodies and reagents.
Monoclonal antibodies against human COX-2 and PGE2 and rabbit polyclonal antibody against human membrane-associated PGE2 synthase were purchased from Cayman Chemical, Ann Arbor, Mich. Heparin, sodium orthovanadate, benzamidine, leupeptin, aprotinin, and monoclonal antibody against human β-actin (clone AC-40) were obtained from Sigma, St. Louis, Mo. Rhodamine-labeled phalloidin, Alexa 488 (excitation, 495 nm; emission, 591 nm)-coupled anti-mouse antibody and antifade with DAPI (4′,6′-diamidino-2-phenylindole) were from Molecular Probes, Eugene, OR. Conjugates of anti-mouse-alkaline phosphatase and -horseradish peroxidase were obtained from Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD. The COX-1 and COX-2 inhibitor indomethacin and the COX-2-specific inhibitor NS-398 [N-(2-cyclohexyloxy-4-nitrophenyl)-methanesulfonamide] were purchased from Calbiochem, La Jolla, Calif. NS-398 was reconstituted in dimethyl sulfoxide, indomethacin was reconstituted in ethanol, and PGE2 was reconstituted in phosphate-buffered saline (PBS).
Plasmids.
A human COX-2 cDNA construct (1,815 bp) cloned in the eukaryotic expression vector pcDNA (Invitrogen) (24) was a kind gift from Timothy Hla, University of Connecticut. The 774-bp full-length KSHV ORF73 promoter (58) was cloned in the pGL3 vector (Promega) to create the pGL3.6-luciferase reporter construct. The p2500Luc construct, a 2,500-bp ORF50 promoter-luciferase reporter cloned in the pcDNA3.1 vector (Invitrogen) (84), was a kind gift from George Miller, Yale University School of Medicine, New Haven, Connecticut.
Cytotoxicity assay.
DMEM containing different concentrations of various inhibitors was incubated with HFF cells or HMVEC-d cells for 4 h or 4 days. At different time points, supernatants were collected and assessed for cellular toxicity by using a cytotoxicity assay kit (Promega).
Western blotting.
Total cell lysates prepared from HFF as well as HMVEC-d cells were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels, and the resolved proteins were transferred onto nitrocellulose paper and immunoblotted with primary antibodies (anti-COX-2 and anti-COX-1). To confirm equal protein loading, blots were also reacted with antibodies against human β-actin. Secondary antibodies conjugated either to horseradish peroxidase or to alkaline phosphatase were used for detection. Immunoreactive bands were developed by either enhanced chemiluminescence reaction (NEN Life Sciences Products, Boston, Mass.) or CDP-Star (Roche Diagnostics Corp., Indianapolis, Ind.) and quantified by following standard protocols (62).
Immunofluorescence assay (IFA).
Confluent HMVEC-d cells in eight-well chamber slides (Nalge Nunc International, Naperville, Ill.) were either uninfected or infected with KSHV at a multiplicity of infection (MOI) of 10 at 37°C for 2 h. For COX-2 immunostaining, cells were fixed with 4% paraformaldehyde, permeabilized with 0.4% Triton X-100, and stained with anti-COX-2 monoclonal antibody (Cayman Chemical) overnight at 4°C. HMVEC-d cells were washed and incubated with a 1:200 dilution of Alexa 488 (excitation, 495 nm; emission, 591 nm)-coupled anti-mouse antibodies (Molecular Probes, Eugene, OR) for 1 h at room temperature. Nuclei were visualized by using DAPI (excitation, 358 nm; emission, 461 nm; Molecular Probes, Eugene, OR) as a counterstain. Stained cells were washed and viewed with appropriate filters under a fluorescence microscope with the Nikon Metamorph digital imaging system.
Inhibition of GFP-KSHV infectivity.
The ability of the inhibitors indomethacin and NS-398 to inhibit GFP-KSHV infectivity was tested by use of procedures described before (49). Briefly, cells were incubated with different concentrations of specific inhibitors at 37°C for 1 h with DMEM and then infected with GFP-KSHV at 37°C for 2 h in the presence and absence of inhibitors. Cells were washed and incubated at 37°C with growth medium for 3 days. After 3 days, green fluorescent cells were examined under a fluorescence microscope and were counted (49). After being examined for GFP expression, cells were fixed with acetone and examined for KSHV ORF73 protein by IFA (3).
Real-time RT-PCR.
The ORF50 and ORF73 transcripts were detected by real-time reverse transcription-PCR (RT-PCR) using gene-specific real-time primers and specific TaqMan probes (Applied Biosystems, Foster City, Calif.) according to procedures described previously (32). To monitor the induction of COX-2 by KSHV, a real time RT-PCR assay was designed and standardized. Total RNA was isolated from uninfected and KSHV-infected HFF cells as well as HMVEC-d cells by using the RNeasy kit (QIAGEN Inc., Valencia, Calif.). RNA was normalized to contain 250 ng/5 μl, and COX-2 mRNA was amplified in the presence of TaqMan probe (5′-6carboxyfluorescein-TCCTACCACCAGCAACCCTGCCA-6-carboxytetramethylrhodamine-3′) and COX-2 specific primers (forward, 5′-GAATCATTCACCAGGCAAATTG-3′; reverse, 5′-TCTGTACTGCGGGTGGAACA-3′). The product length was 67 bp.
To ascertain that contamination with host DNA would not pose a problem, the COX-2 probe was designed to span the splice junction between COX-2 exons 8 and 9. Different dilutions of known amounts (106, 105, 104, 103, 102, and 101 copies) of in vitro-transcribed human COX-2 transcripts were used for copy estimation. The human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene was used as the internal housekeeping gene control. The reaction conditions used for the ORF50 and ORF73 gene amplification consisted of four stages: 50°C for 2 min, 60°C for 30 min, and 95°C for 10 min, followed by 44 cycles of 95°C for 15 s and 60°C for 30 s. COX-2 gene amplifications consisted of four stages: 50°C for 2 min, 60°C for 30 min, and 95°C for 10 min, followed by 44 cycles of 95°C for 30 s and 55°C for 1 min. GAPDH conditions varied slightly in the amplification stage, with denaturation of the strands at 95°C for 20 s and 62°C for 1 min, which was repeated for 40 cycles. Known concentrations of DNase I-treated in vitro-transcribed COX-2 transcripts were used in a real-time RT-PCR to construct a standard graph from which the relative copy numbers of KSHV-induced COX-2 transcripts were calculated and normalized, with GAPDH used as the internal control.
KSHV binding to target cells.
[3H]thymidine-labeled, density gradient-purified KSHV (5,000 cpm) (1, 2) was incubated with medium alone or medium with 10 μg/ml of heparin for 90 min at 4°C. These mixtures were added to HFF cells and incubated for 90 min at 4°C. To test whether indomethacin prevents the binding of virus or entry into target cells, HFF cells were preincubated with a nontoxic dose of indomethacin before addition of the radiolabeled KSHV. After incubation, the cells were washed five times and lysed with 1% SDS and 1% Triton X-100, and the radioactivity was precipitated with trichloroacetic acid (TCA) and counted in a scintillation counter.
Internalization and nuclear trafficking of KSHV DNA.
The effects of COX-2 inhibitors on KSHV internalization and nuclear trafficking were assessed by real-time PCR detecting the internalized KSHV DNA by procedures described before (46). Briefly, untreated HFF cells or HFF cells incubated with 500 μM of indomethacin for 1 h at 37°C were infected with GFP-KSHV at an MOI of 5 per cell. After 2 h of incubation with virus, cells were washed twice with PBS to remove the unbound virus. Cells were treated with trypsin-EDTA for 5 min at 37°C to remove the bound noninternalized virus. Detached cells were washed twice to remove the virus and trypsin-EDTA. The effect of COX-2 inhibitors on KSHV trafficking was monitored by isolating pure nuclear fractions as described earlier (46). Total DNA isolated from cells or from nuclear fractions was tested by real-time PCR for ORF73, as described in detail elsewhere (46).
ELISA for PGE2 detection.
Levels of PGE2 in the culture supernatants of uninfected and KSHV-infected HFF and HMVEC-d cells were measured with a commercially available enzyme immunoassay (Cayman Chemical). Supernatants were centrifuged to remove cell debris and were stored at −80°C until assayed.
Luciferase reporter assays.
The effect of the COX-1 and COX-2 inhibitor indomethacin on the ORF73 promoter (pORF73-luc) and ORF50 promoter (p2500Luc) was measured using the dual luciferase kit according to the manufacturer's protocol (Promega, Madison, WI). Briefly, CV-1 cells (1 × 105) seeded in 24-well tissue culture plate were fed with antibiotic-free, low-serum (0.5% FBS) Dulbecco's modified Eagle's medium 12 to 18 h before transfection. Low-serum conditions were maintained throughout the experiment. Transfection of CV-1 cells was performed with 0.4 μg of ORF73 or ORF50 promoter luciferase constructs and with 4 ng of p-Renilla luciferase as a transfection efficiency control, using Lipofectamine 2000 (Invitrogen). After 24 h, transfected cells were untreated or pretreated with 500 μM indomethacin for 1 h at 37οC and then infected with KSHV at an MOI of 10 for 2 h, 8 h, and 24 h. Cells were harvested at the indicated time points after KSHV infection in 1× passive lysis buffer, and luciferase assays were carried out in triplicates. Briefly, the aliquot of cell extract and luciferase assay reagents were gently mixed (1:1), and firefly luciferase activity was measured for 10 s using a luminometer. The addition of Stop and Glo reagent terminated the reaction and allowed the measurement of the constitutive Renilla luciferase activity. Firefly luciferase activities for each time point were normalized to those of parallel controls for each time point. Alterations in promoter activities of ORF73 or ORF50 as a result of KSHV infection were determined after normalization, using the Renilla luciferase activity as control.
RESULTS
Induction and persistence of COX-2 gene expression during in vitro infection of target cells by KSHV.
As an initial step toward understanding how KSHV establishes the in vitro latent infection and to decipher the role of host cell genes, we have previously analyzed the transcriptional reprogramming of 22,283 host genes from KSHV-infected HMVEC-d and HFF cells at 2 and 4 h p.i. (47). With a criterion of >2-fold gene induction as significant, transcriptional modulation of several genes was detected, and among these genes, we observed a high level of COX-2 gene induction in both HMVEC-d and HFF cells. In HMVEC-d cells, we observed 85-fold and 45-fold up-regulation at 2 h and 4 h p.i., respectively, and 11-fold and 10-fold inductions of the COX-2 gene were observed in HFF cells at 2 h and 4 h p.i., respectively (47). Since COX-2 plays important roles in inflammatory responses, angiogenesis, and cancer, we examined here in detail the induction of COX-2 by KSHV and its role in infection.
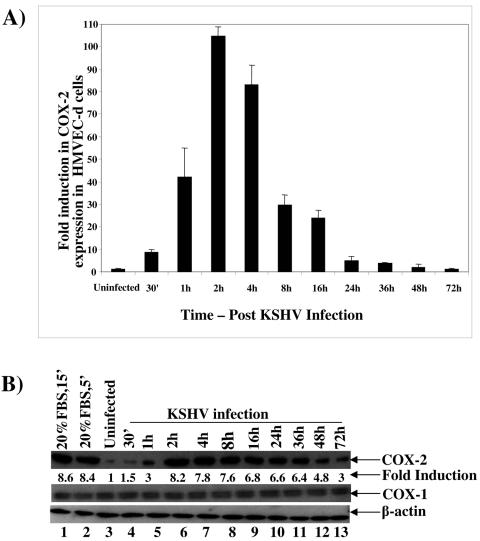
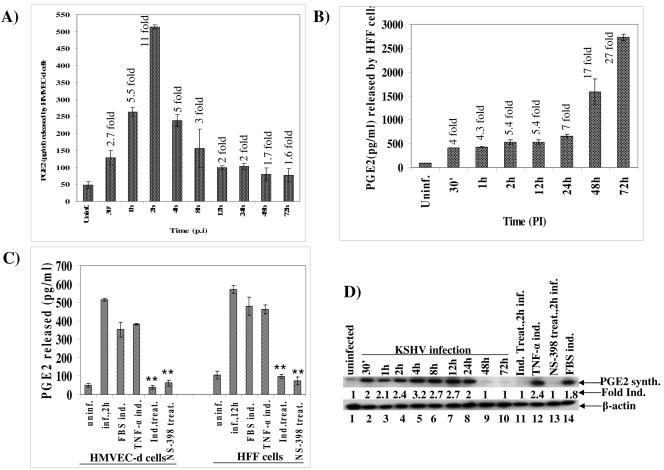
Initially, using real-time RT-PCR with COX-2-specific primers, we examined the time kinetics of COX-2 mRNA induction in cells infected with KSHV at an MOI of 10 (Fig. 1A). In HMVEC-d cells, increased levels of COX-2 mRNA were detected as early as 30 min p.i., and these increased steadily. We observed about 9-, 43-, 104-, 83-, 30-, and 24-fold COX-2 gene induction at 30 min, 1 h, 2 h, 4 h, 8 h, and 16 h p.i., respectively (Fig. 1A). This induction decreased to four- and threefold at 24 h and 36 h p.i. (Fig. 1A), respectively, and reached closer to the basal levels of expression by 48 h and 72 h p.i. (Fig. 1A). We next examined the induction of COX-2 at the protein level by Western blotting using equal amount of proteins from uninfected and infected cells (Fig. 1B). Compared to COX-2 levels in uninfected HMVEC-d cells (Fig. 1B, top panel, lane 3), COX-2 protein levels increased by about 1.5-, 3-, 8.2-, 7.8-, 7.6-, 6.8-, 6.6-, 6.4-, 4.8-, and 3-fold at 30 min, 1 h, 2 h, 4 h, 8 h, 16 h, 24 h, 36 h, 48 h, and 72 h p.i., respectively (Fig. 1B, top panel, lanes 4 to 13). Treatment of serum-starved HMVEC-d cells with 20% FBS for 5 min or 15 min induced COX-2 protein levels about 8.6- and 8.4-fold, respectively (Fig. 1B, top panel, lanes 2 and 1). In contrast, we did not observe any significant changes in COX-1 protein levels (Fig. 1B, middle panel, lanes 1 to 13) or in β-actin protein levels (Fig. 1B, bottom panel, lanes 1 to 13), thus demonstrating the specificity of COX-2 induction in HMVEC-d cells by KSHV.
FIG. 1.
Detection of COX-2 mRNA and protein in KSHV-infected HMVEC-d cells. HMVEC-d cells grown to 80% confluence were serum starved for 10 h and infected with KSHV at an MOI of 10, and expression of COX-2 mRNA and protein was monitored at the indicated time points. (A) Infected and uninfected cells were washed and lysed, and total RNA was prepared. DNase I-treated RNA (250 ng) was subjected to real-time RT-PCR with COX-2 gene-specific primers. Known concentrations of DNase I-treated, in vitro-transcribed COX-2 transcripts were used in a real-time RT-PCR to construct a standard graph from which the relative copy numbers of viral transcripts were calculated and normalized, with GAPDH used as the internal control. Each reaction was done in duplicate, and each bar represents the average ± standard deviation from three independent experiments. The COX-2 level normalized to GAPDH in the uninfected cells was considered 1 for comparison. (B) Lysates prepared from uninfected or KSHV-infected HMVEC-d cells were adjusted to contain equal amount of protein, resolved by SDS-10% polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. Membranes were immunoblotted with monoclonal antibodies against COX-2 protein (upper panel), COX-1 protein (middle panel), or cytoplasmic β-actin (bottom panel). Each blot is representative of at least three independent experiments. The COX-2 level in the uninfected cells was considered 1 for comparison.
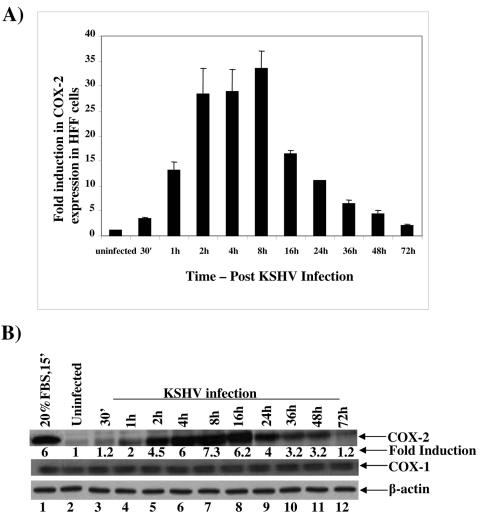
As in HMVEC-d cells, we also observed significant levels of COX-2 mRNA induction in HFF cells as early as 30 min p.i. (3.5-fold), which increased by about 13-, 28-, and 29-fold at 1 h, 2 h, and 4 h p.i., respectively (Fig. 2A). In contrast to the case in HMVEC-d cells, the maximum (33-fold) induction in COX-2 mRNA level was observed at 8 h p.i. in HFF cells (Fig. 2A), which decreased thereafter, with about 16-, 11-, 6-, 4-, and 2-fold induction by 16 h, 24 h, 36 h, 48 h, and 72 h p.i., respectively. Similarly, COX-2 protein levels increased by about 1.2-, 2-, 4.5-, 6-, 7.3-, 6.2-, 4-, 3.2-, 3.2-, and 1.2-fold at 30 min, 1 h, 2 h, 4 h, 8 h, 16 h, 24 h, 36 h, 48 h, and 72 h p.i., respectively (Fig. 2B, top panel, lanes 3 to 12). No significant changes in the levels of COX-1 protein (Fig. 2B, middle panel, lanes 1 to 12) or β-actin protein (Fig. 2B, bottom panel, lanes1 to 12) were observed in the KSHV-infected HFF cells. Incubation of KSHV with 100 μg/ml of heparin reduced the COX-2 induction by >75% (data not shown). Further, all of the reagents used in the preparation of virus stocks were free of bacterial LPS, as tested by Limulus amebocyte lysate assay (data not shown). These results demonstrated that COX-2 was specifically induced by KSHV infection and not by contaminating host cell factors and/or LPS.
FIG. 2.
Detection of COX-2 mRNA and protein in KSHV-infected HFF cells. HFF cells grown to 80% confluence were serum starved for 24 h before being infected with KSHV at an MOI of 10. COX-2 mRNA expression (A) and protein levels (B) in infected cells were monitored over a 72-h period as described in the Fig. 1 legend. Error bars indicate standard deviations.
These results suggested that KSHV strongly up-regulates the COX-2 mRNA expression early during infection of target cells, thus supporting and extending our gene array analyses (47). The differences in the kinetics of COX-2 expression in HMVEC-d cells and HFF cells demonstrated the specificity of induction and cell type differences in the induction by KSHV.
Detection of KSHV-induced COX-2 by IFA.
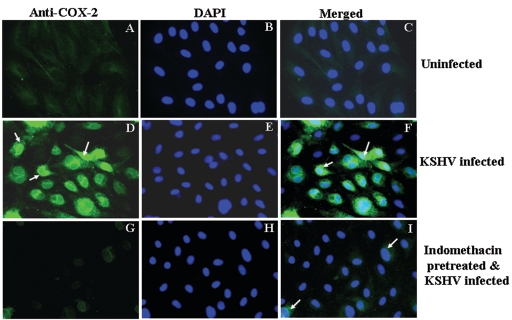
COX-1 and COX-2 are integral membrane proteins and localize to the luminal membrane of the endoplasmic reticulum and the inner and outer membranes of the nucleus (68). COX-2 localization in the nuclear envelope and perinuclear region has been demonstrated in LPS-stimulated brain endothelial cells during lipopolysaccharide-induced fever (38). When we examined the induction of COX-2 by IFA, uninfected HMVEC-d cells did not show any significant staining with anti-COX-2 antibodies (Fig. 3A). In contrast, we observed a strong, well-defined perinuclear staining characteristic of COX-2 expression in KSHV-infected HMVEC-d cells at 2 h p.i., and about 70 to 80% of the cells were positive for COX-2 (Fig. 3D and F). This strong COX-2 detection was abolished by treating HMVEC-d cells with a 500 μM concentration of the COX-2 inhibitor indomethacin prior to infection, and about a 90% reduction in COX-2 staining was observed (Fig. 3G). These results not only demonstrated the specificity of COX-2 induction but also further supported the data showing that KSHV induces strong up-regulation of COX-2 early during infection of target cells.
FIG. 3.
Immunofluorescence observation of KSHV infection-induced COX-2 protein expression in HMVEC-d cells. Uninfected HMVEC-d cells (A, B, and C), cells infected with KSHV (MOI = 10) for 2 h (D, E, and F), or cells pretreated with 500 μM indomethacin for 1 h at 37οC and then infected with KSHV at an MOI of 10 for 2 h (G, H, and I) were permeabilized and stained with anti-COX-2 monoclonal antibody. COX-2 was detected with Alexa 488-coupled anti-mouse antibody. Nuclei were counterstained with DAPI (B, E, and H). Perinuclear staining characteristic of COX-2 expression is indicated by arrows. Magnification, ×40.
COX-2 induction depends upon KSHV binding and entry into the target cells, and increased levels require viral transcription.
To determine whether KSHV gene expression is essential for COX-2 up-regulation, replication-incompetent KSHV was prepared by UV irradiation. We have previously demonstrated that KSHV exposed to UV for 20 min binds and enters HMVEC-d and HFF cells as efficiently as the untreated virus (61). In addition, our studies have shown that UV-inactivated KSHV did not induce any KSHV gene expression, whereas an equal MOI of live virus induced a quantitative increase in the latency-associated ORF73 gene expression as well as lytic cycle-associated ORF50, K8, and K5 gene expression (61). However, similar to the case for live KSHV, a robust ERK1/2 activation was observed in target cells incubated with UV-irradiated KSHV early during infection, and this ERK1/2 activation returned to the basal level at 60 to 120 min p.i. (61). These results suggested that ERK activation of KSHV was not dependent on KSHV gene expression but probably depended upon the induction of preexisting signal cascades by a KSHV envelope glycoprotein(s) (61) during the binding and entry stages of infection.
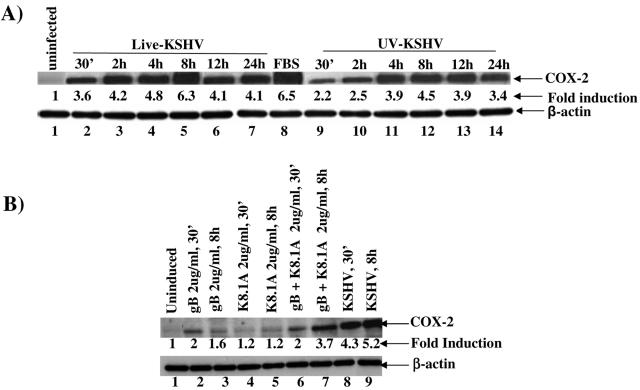
To determine whether COX-2 induction requires KSHV-induced signal cascade and/or viral gene expression, we examined the protein levels of COX-2 in HFF cells infected with either live or UV-inactivated KSHV at an MOI of 10. Incubation with 20% FBS for 15 min induced an increase in COX-2 levels of about 6.5-fold over uninfected cells (Fig. 4A, top panel, lanes 8 and 1). Live KSHV induced about 3.6-, 4.2-, 4.8-, 6.3-, 4.1-, and 4.1-fold increases in COX-2 at 30 min, 2 h, 4 h, 8 h, 12 h, and 24 h p.i., respectively (Fig. 4A, top panel, lanes 2 to 7). We also detected about 2.2-, 2.5-, 3.9-, 4.5-, 3.9-, and 3.4-fold increases in COX-2 in cells infected with UV-irradiated KSHV at 30 min, 2 h, 4 h, 8 h, 12 h, and 24 h p.i., respectively (Fig. 4A, top panel, lanes 9 to 14). In contrast, there was no change in the β-actin protein levels (Fig. 4A, bottom panel, lanes 1 to 14). The absence of LPS contamination in our virus preparations clearly demonstrated the specificity of COX-2 induction by UV-irradiated KSHV. Although COX-2 induction with UV-irradiated KSHV was significantly higher than that in uninfected cells and was sustained, this induction was comparatively lower than the induction observed with live KSHV at all parallel time points. This suggested that, similar to ERK1/2 induction, COX-2 induction was partially dependent upon virus binding and entry stages. However, in contrast to ERK1/2 induction, KSHV viral gene expression appears to be required for the augmented induction of COX-2.
FIG. 4.
Induction of COX-2 by UV-inactivated KSHV and viral glycoproteins gB and gpK8.1A. (A) HFF cells grown to 80% confluence were serum starved for 24 h before being infected with either live KSHV(top panel, lanes 2 to 7) or UV-irradiated KSHV (top panel, lanes 9 to 14) for the indicated times. Cell extracts were harvested at 30 min, 2 h, 4 h, 8 h, 12 h, and 24 h p.i. and analyzed by Western blotting using a COX-2 polyclonal antibody (top panel). As a positive control, serum-starved HFF cells were treated with 20% FBS (top panel, lane 8) for 30 min. Part of the lysates were also probed with anti-β-actin antibody as a loading control (bottom panel). Each lane contained 10 μg of total protein. Each blot is representative of at least three independent experiments. The COX-2 level in the uninfected HFF cells was considered 1 for comparison. (B) Serum-starved HFF cells were either uninduced or induced with KSHV glycoproteins gB (lanes 2 and 3) and gpK8.1A (lanes 4 and 5) alone or together (lanes 6 and 7) for the indicated times. Cell lysates from KSHV-infected cells at 30 min (lane 8) and 8 h (lane 9) were used for comparison. Cell extracts were harvested and Western blotted, and COX-2 (top panel) or β-actin (bottom panel) was detected. Each lane contained 10 μg of total protein, and each blot is representative of at least three independent experiments. The COX-2 level in the uninfected HFF cells was considered 1 for comparison.
Since COX-2 induction by UV-irradiated KSHV suggested stimulation during the binding and entry stages of infection, we next examined the ability of KSHV envelope glycoproteins gB and gpK8.1A to induce COX-2. Incubation of HFF cells with 2 μg/ml of gB for 30 min or 8 h induced the COX-2 levels by 2- or 1.6-fold, respectively (Fig. 4B, top panel, lanes 2 and 3). Incubation with 2 μg/ml of gpK8.1A for 30 min as well as 8 h also induced the COX-2 protein levels by 1.2-fold (Fig. 4B, top panel, lanes 4 and 5). However, when cells were treated with 2 μg/ml of a mixture of gB and gpK8.1A proteins, higher levels of COX-2 induction (2-fold and 3.7-fold at 30 min and 8 h of incubation, respectively) were observed (Fig. 4 B, top panel, lanes 6 and 7). The absence of LPS contamination in our purified preparations (61, 62) and inhibition of COX-2 induction by preincubation of virus with heparin (data not shown) clearly demonstrated the specificity of COX-2 induction by UV-irradiated KSHV. These data further verified the conclusion that COX-2 induction in KSHV-infected cells was initiated by the binding and entry of virions into the target cells and demonstrated the role of KSHV envelope glycoproteins gB and gpK8.1A in this induction. The higher levels of COX-2 detected in live-virus-infected cells (Fig. 4 B, top panel, lanes 8 and 9) suggested that viral gene expression early during infection, and possibly together with viral gene-induced host cell genes, is probably essential for the increased and sustained induction of COX-2 levels.
KSHV infection of HMVEC-d and HFF cells induces the release of PGE2.
Viral infections stimulate a variety of host inflammatory responses which are often successful in controlling the extent of infection. Part of the host cell inflammatory responses includes the release of soluble factors such as PGE2, which is the most abundant lipid mediator produced in response to cytokines and mitogens and mediates many symptoms of inflammation (53, 74). Previous studies have shown that infection by several viruses leads to PGE2 release by the infected cells (6, 23, 27, 43, 86). PGE2 biosynthesis has been shown to be dysregulated in a variety of neoplasms, and KS is classified as a chronic-inflammation-associated malignancy. Since COX-1 and -2 are the key rate-limiting cellular enzymes involved in the synthetic pathway of prostaglandins from arachidonic acid (76), we next examined the release of PGE2 from the infected cell culture supernatants.
A significant level of PGE2 (128 pg/ml), about 2.7-fold over that in uninfected cells, was detected as early as 30 min p.i. in HMVEC-d cells, which peaked to about 11-fold (513 pg/ml) by about 2 h p.i. (Fig. 5A). KSHV-induced PGE2 decreased slowly thereafter to 238, 156, 99, 102, 79, and 77 pg/ml at 4 h, 8 h,12 h, 24 h, 48 h, and 72 h p.i., respectively (Fig. 5A). Similarly, PGE2 levels of 409, 428, 529, 530, 655, 1,590, and 2,730 pg/ml were detected in infected HFF cell supernatants at 30 min, 1 h, 2 h, 12 h, 24 h, 48 h, and 72 h p.i., respectively (Fig. 5B). These results demonstrated the ability of KSHV-infected cells to produce a high and sustained level of PGE2. Since COX-2 inhibitors can abrogate the COX-2-mediated induction of PGE2 accumulation, to determine the role of KSHV-induced COX-2 in the observed release of PGE2 and establish the specificity of PGE2 release, we next pretreated the target cells with nontoxic doses of either 500 μΜ indomethacin or 50 μΜ NS-398 at 37°C for 1 h and then infected the cells with KSHV at an MOI of 10 for 2 h (Fig. 5C). Significant release of PGE2 was observed in cells treated with either 20% FBS for 30 min or 20 ng/ml tumor necrosis factor alpha (TNF-α) for 30 min (used as a positive control) (Fig. 5C). Pretreatment of HMVEC-d cells with 500 μΜ indomethacin or 50 μΜ NS-398 significantly reduced the release of PGE2, by 93% and 88%, respectively (Fig. 5C). Similarly, compared to cells infected with KSHV at an MOI of 10 for 12 h in the absence of drugs, pretreatment of HFF cells with 500 μΜ indomethacin and 50 μΜ NS-398 significantly reduced the release of PGE2, by 83% and 87%, respectively (Fig. 5C). Since we used nontoxic doses of these drugs and the cell viability was not altered in these experiments (data not shown), the data suggested that the observed responses were specific responses and not due to nonspecific toxicity of drugs.
FIG. 5.
Induction of PGE2 release from KSHV-infected HMVEC-d and HFF cells. Serum-starved HMVEC-d cells (A) and HFF cells (B) were either uninfected or infected with KSHV at an MOI of 10 for the indicated times, and the levels of PGE2 released in the cell-free culture supernatants were measured by enzyme-linked immunosorbent assay. Each reaction was done in duplicate, and each point represents the average ± standard deviation from three independent experiments. PGE2 released from uninfected cells was considered 1 for comparison, and fold inductions in infected cells are indicated. (C) Culture supernatants of uninfected HMVEC-d and HFF cells and cells infected with KSHV (MOI of 10) for 2 h and 12 h, respectively, with and without pretreatment with either 50 μM NS-398 or 500 μM indomethacin for 1 h at 37°C, were measured for released PGE2. Cells were also induced with 20% FBS for 30 min or with 20 ng/ml of TNF-α for 30 min for positive controls. Each reaction was done in duplicate, and each point represents the average ± standard deviation from three independent experiments. PGE2 released from uninfected cells was considered 1 for comparison, and fold inductions in infected cells are indicated. **, statistically significant (P < 0.001). (D) Lysates from uninfected HMVEC-d cells and cells infected with KSHV at an MOI of 10 for various times, with and without pretreatment with either 500 μM indomethacin or 50 μM NS-398 at 37οC for 1 h, were Western blotted and quantitated for PGE2 synthase (top panel) or β-actin (bottom panel). Cells were also induced with TNF-α for 30 min or with 20% FBS for 30 min for positive controls. Each lane contained 10 μg of total protein, and each blot is representative of at least three independent experiments. The PGE2 synthase level in the uninfected cells was considered 1 for comparison.
Cyclooxygenase catalyzes a two-step reaction. In the first step, it converts arachidonic acid to prostaglandin G2, an unstable product, which is rapidly converted to prostaglandin H2 (PGH2) by the peroxide activity of COX-2 in the second step. PGH2 is then converted by microsomal prostaglandin E synthase to PGE2. Since HFF as well as HMVEC-d cells infected with KSHV had an augmented release of PGE2 in the culture supernatants, we next examined the induction of PGE2 synthase, which is known to catalyze the biosynthesis of PGE2. Similar to the increase in the PGE2 release, compared to that in uninfected cells, we observed increases of about 2-, 2.1-, 2.4-, 3.2-, 2.7-, 2.7-, and 2-fold in the PGE2 synthase levels at 30 min,1 h, 2 h, 4 h, 8 h,12 h, and 24 h p.i. of infected HMVEC-d cells (Fig. 5D, top panel, lanes 1 to 8). At 48 h and 72 h p.i., we did not observe any PGE2 synthase (Fig. 5D, top panel, lanes 9 to 10), suggesting that the observed decreased PGE2 release in HMVEC-d cells at later time points in infection (Fig. 5A) is probably due to the lower levels of PGE2 synthase. Pretreatment of HMVEC-d cells with either 500 μM indomethacin or 50 μM NS-398 significantly decreased the PGE2 synthase levels, to the basal level (Fig. 5D, top panel, lanes 11 and 13), as observed in the uninfected cells (Fig. 5D, top panel, lane 1). Significant levels of PGE2 synthase were observed in cells treated with either 20 ng/ml TNF-α for 30 min or 20% FBS for 30 min (used as a positive control) (Fig. 5D, top panel, lanes 12 and 14). In contrast, we did not observe any change in the protein level of β-actin (Fig. 5D, bottom panel, lanes 1 to 14), demonstrating the specificity of the observed changes in PGE2 synthase levels in the infected cells. These results clearly demonstrated that KSHV-induced COX-2 is responsible for the release of PGE2 from the infected target cells.
Inhibition of COX-2 negatively regulates KSHV infection.
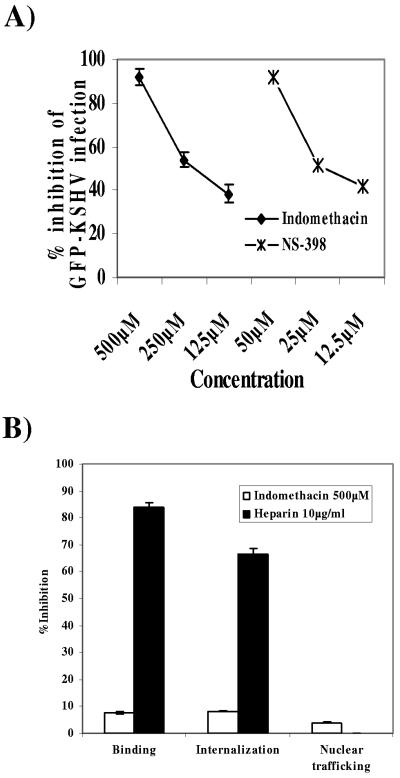
To determine the role of the stress response COX-2 protein in KSHV biology, we examined KSHV infection in the presence of COX-2 inhibitors. HMVEC-d cells pretreated with different concentrations of indomethacin and NS-398 at 37°C for 1 h were infected with GFP-KSHV at an MOI of 10 for 2 h, washed, incubated with growth medium containing inhibitors at 37°C for 3 days, and then examined for green fluorescent cells. We observed a dose-dependent reduction in GFP-KSHV infection by both inhibitors (Fig. 6A). At 125 μΜ, 250 μΜ, and 500 μΜ, indomethacin reduced the GFP-KSHV infection by about 38%, 52%, and 92%, respectively (Fig. 6A). Similarly, pretreatment of HMVEC-d cells with 12.5 μΜ, 25 μΜ, and 50 μΜ concentrations of the COX-2 selective inhibitor NS-398 inhibited KSHV infection by 42%, 51%, and 92%, respectively (Fig. 6A). These results were highly reproducible, and similar results were also observed with HFF cells (data not shown). These data clearly suggested that COX-2 is critical for a successful in vitro KSHV infection.
FIG. 6.
Effect of COX-2 inhibition on KSHV infection. (A) Neutralization of GFP-KSHV infectivity by COX inhibitors. HMVEC-d cell monolayers in eight-well chamber slides were incubated with DMEM containing different concentrations of nontoxic doses of indomethacin (nonselective COX-1/2 inhibitor) or NS-398 (COX-2 specific inhibitor) at 37°C for 1 h before infection with GFP-KSHV at an MOI of 10. After incubation for 2 h at 37°C with virus in the presence of inhibitors, cells were washed and further incubated with growth medium containing the same concentration of inhibitors for 3 days at 37°C. Green fluorescent cells indicative of GFP-KSHV entry and infection were counted. In control wells approximately 100 green fluorescent HMVEC-d cells per well were detected. Each reaction was done in triplicate, and each point represents the average ± standard deviation from three experiments. (B) Effect of COX-2 inhibition on KSHV binding, internalization, and virus trafficking to the cell nucleus. [3H]thymidine-labeled, purified KSHV (5,000 cpm) was added to untreated HFF cells or cells pretreated with indomethacin. As a control, labeled KSHV mixed with 10 μg/ml of heparin for 90 min at 4°C was added to cells. After incubation for 90 min at 4°C with the virus, cells were washed, lysed, and precipitated with TCA, and the cell-associated virus radioactivity (counts per minute) was counted. Each reaction was done in triplicate, and each point represents the average ± standard deviation from three independent experiments. To demonstrate the effect of COX-2 inhibition on KSHV internalization, HFF cells were either untreated or treated with 500 μM of indomethacin at 37°C for 1 h and infected with KSHV at an MOI of 10 for 2 h. Unbound KSHV was removed by washing the cells twice with PBS. Bound, noninternalized virus was also removed by treating the cells with trypsin-EDTA for 5 min. Cells were washed, the internalized viral DNA was isolated, and ORF73 DNA levels were checked by real-time DNA PCR (61). The effect of indomethacin treatment on virus nuclear trafficking was studied by isolating pure nuclear fractions at 3 h p.i. in either the presence or absence of the inhibitor. Total DNA isolated from either cells or purified nuclear fractions was normalized to contain 100 ng/5 μl and subjected to real-time DNA PCR for KSHV ORF73. Copy standards (106 to 101) and nontemplate controls were run in parallel along with samples for absolute copy number in test samples. Data are presented as percent inhibition of KSHV binding, internalization, and nuclear trafficking relative to cells infected with virus alone. Each reaction was done in duplicate, and each bar represents the average ± standard deviation from three independent experiments.
Inhibition of COX-2 does not affect KSHV binding, internalization, and trafficking to the infected cell nucleus.
Since the GFP readout in the experiment described above indicated the expression of KSHV genomes that have entered the infected cell nuclei, inhibition of GFP expression with the COX-2 inhibitor did not identify the stage at which this inhibition takes place. Reduced infection as measured by GFP readout could be due to a block in virus binding, a block in virus entry into these cells, a block in the movement of virus in the cytoplasm, a block in the delivery of viral DNA into the infected cell nucleus, or a block in viral gene expression in the nucleus. To determine the stage of KSHV infection at which COX-2 plays the key role, virus binding, entry, and nuclear trafficking in the infected cells were assessed (Fig. 6B). To determine whether COX-2 induction has any role in virus binding, [3H]thymidine-labeled purified KSHV was added to HFF cells pretreated with 500 μM of indomethacin for 1 h. After incubation with the virus for 90 min at 4°C, cells were washed, lysed, and precipitated with TCA and the cell-associated virus counts per minute were counted. In the presence of heparin, virus binding was inhibited by 84%, whereas only negligible (8%) inhibition of virus binding was observed in the presence of indomethacin (Fig. 6B). This suggested that the COX-2 inhibitor does not affect virus binding to the target cells.
To determine whether COX-2 induction has any role in virus internalization, we carried out a quantitative real-time DNA PCR assay (32). By this method, we have previously shown that internalized viral DNA could be detected in HFF cells as early as 5 min p.i., increasing rapidly during the first 60 min to 90 min of infection and reaching a plateau at around 90 min to 120 min p.i. (32). Preincubation of KSHV with 10 μg of heparin per ml blocked the viral DNA entry by 66% (Fig. 6B), and in contrast, no significant reduction of KSHV DNA internalization was observed in HFF cells pretreated with 500 μM of indomethacin (Fig. 6B). This suggested that COX-2 does not play any role in the virus internalization stage of KSHV infection.
To assess the role of indomethacin treatment in KSHV trafficking to the nucleus, HFF cells pretreated with indomethacin were infected with KSHV for 2 h and unbound KSHV was removed by washing the cells. Bound, noninternalized virus was removed by treating the cells with trypsin-EDTA for 5 min. Infected cell nuclei were isolated and checked for purity, and viral DNA copy numbers were quantified by real-time PCR. The purity of nuclei was confirmed by immunoblotting with anti-lamin B antibodies, and cytoskeletal contamination was assessed by the use of anti-β-tubulin antibodies for the detection of α-tubulin protein (46). Indomethacin treatment did not affect the nuclear delivery of viral DNA in HFF cells (Fig. 6B), thus demonstrating that COX-2 induction does not play any role in the nuclear delivery of viral DNA. Taken together, these results suggested that inhibition of GFP-KSHV infectivity by COX-2 inhibitor must be due to a block in viral gene expression.
Inhibition of COX-2 blocks the expression of KSHV latent ORF73 gene expression.
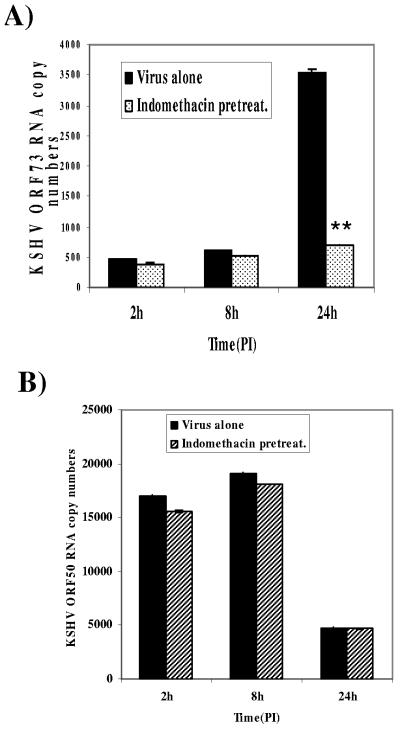
To analyze the potential role of COX-2 induction in KSHV latent gene expression, HFF cells were preincubated with 500 μM indomethacin at 37°C for 1 h, infected with KSHV for 2 h, and analyzed for viral mRNAs at different times p.i. by quantitative real-time RT-PCR. Similar to our earlier observations (32), we observed lower levels of ORF73 gene expression at earlier time p.i. and a steady increase at later time points in KSHV infection (Fig. 7A). Treatment with 500 μΜ indomethacin appeared to have only a moderate effect on the expression of the ORF73 gene at 2 h and 8 h p.i. (Fig. 7A). However, the expression of ORF73 was significantly reduced, by 80%, at 24 h p.i. (Fig. 7A) by indomethacin pretreatment. The specificity of these reactions was demonstrated by the absence of contaminating DNA in any of the DNase-treated RNA samples. Furthermore, treatment of HFF cells with COX-2 inhibitors did not affect the RNA levels of two housekeeping genes β-actin and GAPDH (data not shown). Even though we observed slight differences in the copy numbers of viral transcripts in different experiments and batches of viruses, the patterns of gene expression were closely similar and highly reproducible. The KSHV latency ORF71, ORF72, and ORF73 genes are located immediately adjacent to each other, and mRNAs for these three latent proteins originate from the same promoter (28, 34, 58). ORF73 protein is expressed from a latency-associated large 5.32-kb transcript which also encodes the ORF71 and ORF72 proteins. This 5.32-kb latent transcript is spliced to form a 1.7-kb transcript from which the ORF71 and ORF72 protein are translated. Since the real-time PCR primers used here measure the 5.32-kb transcript that is common to all three latency-associated KSHV proteins, these results suggested that COX-2 definitely plays an important role in modulating the KSHV latent (ORF73) gene expression.
FIG. 7.
Effect of COX-2 inhibition on KSHV viral gene expression. Serum-starved HFF cells were either untreated or indomethacin treated prior to infection with KSHV for 2 h, 8 h, and 24 h. At the indicated times p.i., RNA was isolated and treated with DNase I, and 250 ng of DNase-treated RNA was subjected to real-time RT-PCR with KSHV ORF73 (A) and ORF50 (B) gene-specific primers and TaqMan probes (32). The relative copy numbers of viral transcripts were calculated using a standard graph generated by using known concentrations of DNase-treated, in vitro-transcribed ORF73 and ORF50 transcripts in real-time RT-PCR and normalized with GAPDH. Each reaction was done in duplicate, and each point represents the average ± standard deviation from three independent experiments. **, statistically significant (P < 0.001).
Inhibition of COX-2 has no effect on KSHV lytic gene ORF50 expression.
The 110-kDa KSHV immediate-early ORF50 (RTA) protein is a transcriptional activator and acts as a molecular switch for KSHV reactivation (18, 21, 60, 69, 82). We have previously shown that immediately after infection of HMVEC-d and HFF cells, KSHV expresses the latent genes as well as the lytic cycle switch ORF50 gene (32). To analyze the potential role of COX-2 induction in KSHV lytic gene expression, HFF cells were preincubated with 500 μM indomethacin at 37°C for 1 h and infected with KSHV for 2 h, and messages at different times p.i. were quantitated by real-time RT-PCR for ORF50. HFF cells infected with KSHV showed similar kinetics for ORF50 expression as described earlier (32), and the peak level of ORF50 gene expression was observed at 8 h p.i., followed by a sharp decline by 24 h p.i. (Fig. 7B). The data in Fig. 7A and B clearly show that ORF73 gene expression was inhibited drastically, in contrast to ORF50 gene expression, in the presence of indomethacin. Pretreatment of cells with indomethacin reduced the ORF73 expression by about 17%, 10%, and 80% at 2 h, 8 h, and 24 h p.i., respectively, whereas ORF50 expression was reduced by only 8%, 6%, and 5% at 2 h, 8 h, and 24 h, respectively. These results suggested that KSHV-induced COX-2 has no effect on KSHV lytic gene expression (Fig. 7B) and further indicated the specificity of inhibition of ORF73 gene expression by the COX-2 inhibitor.
COX-2 inhibitors decrease ORF73 protein expression.
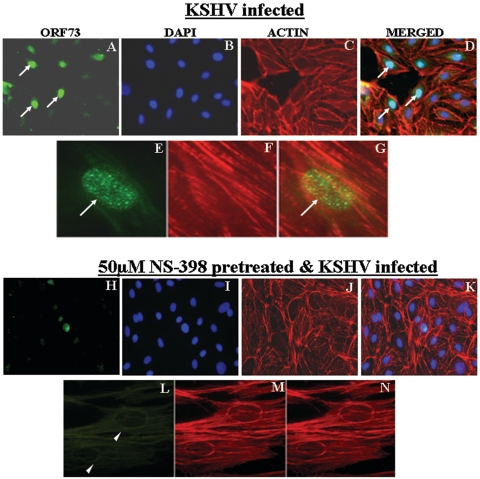
The effect of indomethacin on ORF73 protein levels was examined by immunostaining of KSHV-infected HMVEC-d cells with ORF73-specific antibody, DAPI staining for the nuclei, and rhodamine-labeled phalloidin staining for F-actin (Fig. 8A to C). At 72 h p.i., more than 50% of cells showed nuclear staining for ORF73 (Fig. 8A to D) and exhibited the characteristic punctate nuclear staining of ORF73 protein (Fig. 8E to G). In contrast, when cells were pretreated with 50 μΜ of NS-398 prior to infection, ORF73 was not detected in about 90% of infected HMVEC-d cells (Fig. 8H), which is more clearly shown by the nonstaining nuclei (Fig. 8L to N). Similar inhibition of ORF73 expression was also observed in cells pretreated with indomethacin for 1 h prior to KSHV infection (data not shown). These results further verified the role of KSHV-induced COX-2 in the maintenance of ORF73 expression in the infected cells.
FIG. 8.
Immunofluorescence observation of ORF73 expression in the presence of COX-2 inhibitors. HMVEC-d cell monolayers were infected with KSHV at an MOI of 10 for 72 h (A, B, C, D, E, F, and G) or treated with the COX-2 inhibitor indomethacin (H, I, J, K, L, M, and N) for 1 h before being infected with KSHV at an MOI of 10 for 72 h. Monolayers were fixed, permeabilized, and stained for ORF73 protein (A, E, H, and L) and for cytoplasmic β-actin (C, F, J, and M) using rabbit polyclonal antibodies against ORF73 and rhodamine-labeled phalloidin, respectively. Nuclei were counterstained with DAPI (B and I). Arrows indicate nuclei demonstrating positive staining for ORF73, and arrowheads indicate the lack of nuclear staining for ORF73, representing the absence of ORF73 expression. The merged signals are shown in panels D, G, K, and N. Magnifications, ×40 (panels A, B, C, D, H, I, J, and K) and ×100 (panels E, F, G, L, M, and N).
PGE2 relieves the indomethacin inhibition of ORF73 expression.
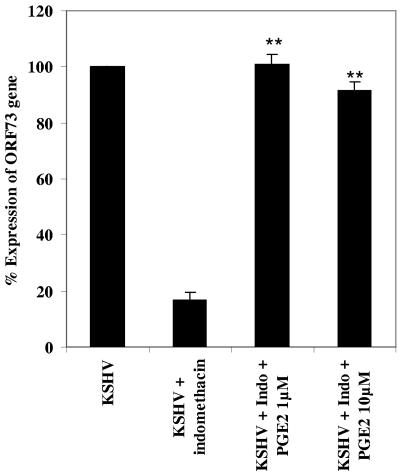
Because PGE2 is the product of COX-2 action, we reasoned that PGE2 should reverse the drug-induced block of KSHV ORF73 expression. To determine whether the release of PGE2 was a host cell antiviral response or a virus-induced phenomenon that is required for the successful establishment of latency, HFF cells were untreated or pretreated with 500 μM of indomethacin for 1 h at 37οC before infection with KSHV for 24 h (Fig. 9). In a parallel experiment, cells were pretreated with 500 μM of indomethacin for 1 h at 37οC and then infected with KSHV and simultaneously supplemented with either 1 μM or 10 μM of PGE2 (Fig. 9). As demonstrated in Fig. 7, treatment of HFF cells with COX-2 inhibitor indomethacin decreased the expression of viral latent ORF73 gene expression by 83% at 24 p.i. (Fig. 9). Exogenously added PGE2 substantially relieved the inhibitory effect of the drug, and supplementation with 1 or 10 μM PGE2 relieved the inhibition of ORF73 gene expression; close to 100% of ORF73 gene expression was restored (Fig. 9). These results suggested that COX-2 induced PGE2 released by host cells in response to KSHV infection plays an important role in the maintenance of viral latent gene expression.
FIG. 9.
Effect of PGE2 on indomethacin inhibition of ORF73 expression. HFF cells were untreated or pretreated with indomethacin before infection with KSHV. In a parallel experiment, cells were pretreated with indomethacin, infected with KSHV for 2 h, and then supplemented with 1 μM or 10 μM PGE2. Total RNA was harvested at 24 h p.i. and subjected to real time RT-PCR for ORF73 gene expression. Expression of the ORF73 gene in untreated infected cells is considered 100% for calculations. Data represent the averages ± standard deviations from three independent experiments. **, statistically significant (P < 0.001).
KSHV-induced COX-2 modulates the latent ORF73 promoter but not the lytic ORF50 promoter.
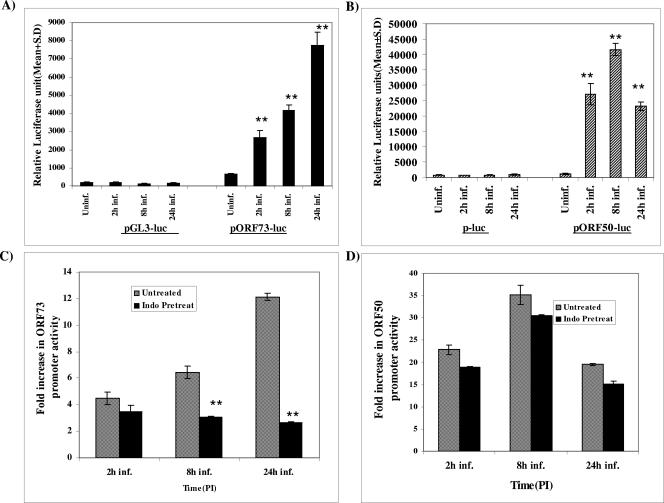
Since the results described above demonstrated the role of COX-2 in ORF73 gene expression, we next determined the effect of COX-2 inhibitor on KSHV latent and lytic promoters. CV-1 cells were transfected with a luciferase vector control (pGL3) or ORF73 promoter-luciferase construct (pORF73-luc), and transfected cells were either uninfected or infected with KSHV at an MOI of 10 for different time points. The data in Fig. 10A represents the mean relative luciferase activities of the pGL3-luc and pORF73-luc constructs at all time points, from triplicate experiments, normalized by cotransfected Renilla activity. The luciferase activity from the basic reporter pGL3-luc construct was not affected by KSHV infection at any time during infection (Fig. 10A). In contrast, ORF73 promoter activity increased by about 4-, 6-, and 12-fold at 2 h, 8 h, and 24 h, respectively, after KSHV infection compared to the activities in the uninfected cells transfected with pORF73-luc (Fig. 10A). The lower fold ORF73 promoter activation at early time points and the subsequent steady increase at later time points of infection are probably due to a combination of the following reasons: (i) the ability of ORF50 protein synthesized early during infection to positively regulate the ORF73 promoter (39), (ii) increased levels of ORF73 protein at later time points and the ability of ORF73 protein to positively regulates its own promoter (28), and (iii) increased COX-2 and PGE2 levels at later time points, as demonstrated in Fig. 1, 2, and 7, leading to increased ORF73 promoter activity.
FIG. 10.
Effect of indomethacin on KSHV ORF50 and ORF73 promoter activities. (A and B) Effect of KSHV infection on ORF73 and ORF50 promoter activities, respectively. CV-1 cells were transfected with control pGL3-luc, ORF73 promoter-luciferase, control p-Luc, or ORF50 promoter-luciferase constructs. After 24 h, cells were either uninfected or infected with KSHV at an MOI of 10 for 2 h, 8 h, and 24 h. At the indicated times p.i., cells were harvested, lysed, and assayed for luciferase activity. The data represents the mean relative luciferase units after normalizing with the cotransfected Renilla activity. Each reaction was done in triplicate, and each point represents the average ± standard deviation from three experiments. **, statistically significant (P < 0.001). (C and D) Effect of COX-2 inhibitor on the ORF50 and ORF73 promoter activities, respectively. CV-1 cells transfected with control constructs or KSHV ORF73 or ORF50 promoter-luciferase constructs were untreated or pretreated with indomethacin and infected with KSHV for different times. Uninfected and infected cells were harvested, lysed, and assayed for luciferase activity. The fold luciferase activity was calculated for each time point of infection by taking the activity of each promoter in the uninfected cells as 1. Each reaction was done in triplicate, and each point represents the average ± standard deviation from three experiments. **, statistically significant (P < 0.001).
CV-1 cells were also transfected with pluc or ORF50 promoter constructs (p2500-luc) (Fig. 10B), and transfected cells were either uninfected or infected with KSHV at an MOI of 10 for different times (Fig. 10B). KSHV infection induced the ORF50 promoter activity by about 23-fold, 35-fold, and 20-fold at 2 h, 8 h, and 24 h p.i., respectively (Fig. 10B). The steady increase of ORF50 promoter activity at earlier later time points and the comparative lower level at 24 h p.i. are probably due to a combination of the following reasons: (i) the ability of the ORF50 protein to positively regulate its promoter (37, 70), (ii) increased ORF50 protein synthesis at earlier time points and its subsequent decline by 24 h p.i. (32), and (iii) the ability of ORF73 protein to negatively regulate the ORF50 promoter (34) coupled with the increased ORF73 gene expression at later times p.i. (Fig. 7).
To assess the effect of COX-2 inhibitor on the ORF73 and ORF50 promoter activities, transfected cells were untreated or pretreated with 500 μM of indomethacin for 1 h at 37°C and then infected with KSHV at an MOI of 10 for different times (Fig. 10C and D). The fold inductions of the ORF73 and ORF50 promoter activities were calculated by considering the respective promoter activities in the uninfected cells as 1. Indomethacin pretreatment reduced the ORF50 promoter activity moderately, with about 19-, 30-, and 16-fold inductions at 2 h, 8 h, and 24 h p.i., respectively (Fig. 10D), in contrast to 23-, 35-, and 20-fold observed in at 2 h, 8 h, and 24 h p.i., respectively, in untreated infected cells.
Indomethacin pretreatment significantly reduced ORF73 promoter activity, to about 3-, 3.15-, and 2.7-fold at 2 h, 8 h, and 24 h p.i., respectively (Fig. 10C), compared to about 5-, 7-, and 12-fold at 2 h, 8 h, and 24 h p.i., respectively, in untreated infected cells. These data clearly suggested that COX-2 inhibitor pretreatment has a profound impact on the promoter activity of ORF73 compared to promoter activity of the lytic gene ORF50 (Fig. 10C and D), especially at later times postinfection. Taken together, the results presented here suggested that KSHV infection-induced COX-2 is critical for the maintenance of KSHV latency in infected cells.
DISCUSSION
Latent infection in the infected host is the hallmark of herpesviruses. For the successful establishment and maintenance of latent infection, herpesviruses need to overcome several obstacles, such as apoptosis, transcriptional restriction, and host intrinsic, innate, and adaptive immune responses. Several of these host obstacles need to be effectively neutralized very early during infection prior to de novo viral gene transcription, as well as after latent viral gene expression. As KSHV infection of in vitro target cells leads to latent infection, this provides unique opportunities to decipher the mechanism of herpesvirus latency.
Our previous studies have shown that one of the best strategies to overcome these obstacles is the manipulation of the host cell preexisting signal pathways by KSHV via the interactions with cell surface receptors. KSHV interacts with cell surface heparan sulfate, α3β1 integrin, and probably other, yet-to-be-identified receptors in the endothelial and fibroblast cells, leading to the activation of FAK, Src, PI-3K, Rho-GTPases, PKC-ζ, MEK, and ERK1/2 signaling pathways within minutes of infection (Fig. 11) (62). PI-3K is a well-known upstream effector for the host antiapoptotic Akt pathway. FAK and PI-3K play roles in virus entry (48, 62), RhoA plays roles in microtubule modulation and transport of capsids in the cytoplasm (46), and ERK1/2 play roles in the initiation of lytic ORF50 and latent ORF73 gene expression, as well as host cell genes (61). KSHV K5 protein, which is detected for up to 5 days in infected HMVEC-d cells, has been shown to down-regulate cell surface major histocompatibility complex class I A and C, ICAM-1, and B7-2 molecules, which are essential for the recognition and effective elimination of infected target cells by the host cytotoxic lymphocytes (32). The other transiently expressed lytic KSHV genes, such as those for vIRF2, vIL-6, and vMIP I, II, and III, are also known to mediate antiapoptotic and immune evasion functions (32), thus providing ample survival time for the virus to express latent genes. Once sufficient levels of KSHV latent LANA-1 (ORF73), v-cyclin (ORF72), v-FLIP (ORFK13), and Kaposin (ORFK12) proteins are synthesized, these proteins probably mediate antiapoptotic and other functions to establish latent infection.
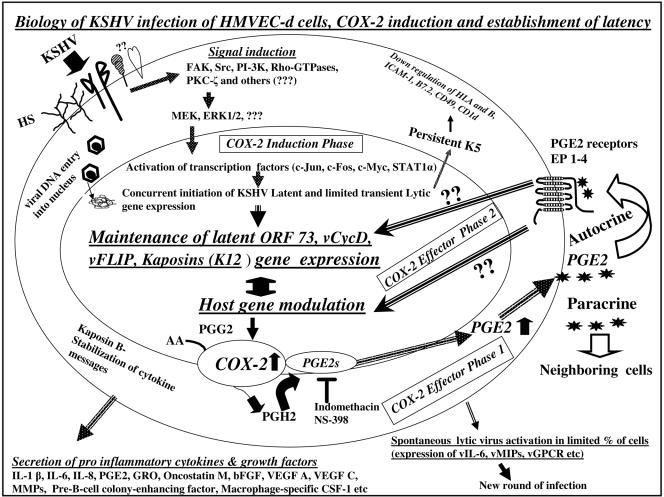
FIG. 11.
Schematic diagram depicting the overlapping induction and effector phases of COX-2 during in vitro KSHV infection of human HMVEC-d cells and their potential roles in the establishment and maintenance of latent infection. During the early stages of KSHV infection of HMVEC-d or fibroblast target cells, KSHV binds to the cell surface heparan sulfate (HS) molecules via its envelope glycoproteins gpK8.1A and gB (1, 3, 80, 81), followed by interaction with α3β1 integrin via gB (3), and possibly to other, yet-to-be-identified molecules. Interactions with receptors trigger the host cell preexisting signal cascades, such as FAK, Src, PI-3K, Rho-GTPases, PKC-ζ, MEK1/2, ERK1/2, and others, and KSHV enters the target cells via endocytosis (3, 48, 61). KSHV infection via the induction of signal pathways also reprograms and modulates the host cell genes (47). KSHV-induced MEK and ERK1/2 are essential for the initial KSHV ORF73 and ORF50 gene expression and for some of the KSHV-induced host gene expression (47, 61). Viral DNA entry into the nuclei is followed by concurrent KSHV latent and lytic cycle gene expression influenced by the KSHV-induced signal pathways and expressed host cell genes (47). Initial higher levels of ORF50 expression is followed by a rapid decline. While the expression of latent ORF72, ORF73, and K13 genes continues, that of nearly all of the lytic genes declines or is undetectable by 24 h p.i. The lytic K2, K4, K5, K6, and vIRF2 genes, with immune modulation functions, and the K7 gene, with antiapoptotic function, are expressed, which may provide sufficient survival time for the infected cells from the host immune surveillance apparatus and apoptosis and the advantage necessary for the establishment of latent infection during the initial time of infection. The K5 gene expression persists, and several proinflammatory cytokines and growth factors are released to the extracellular environment of infected cells. The results in this paper suggest that in the COX-2 induction phase, KSHV-activated signal pathways induce COX-2 gene expression and COX-2 protein synthesis significantly. In COX-2 effector phase 1, COX-2 catalyzes the synthesis of PGH2 from arachidonic acid (AA) through an unstable intermediate PGG2 (64, 76). PGH2 is converted by microsomal prostaglandin E synthase (mPGES) to PGE2, which is released to the infected cell supernatant. The COX-2 inhibitors indomethacin and NS-398 significantly reduce the release of KSHV-induced PGE2. In the COX-2 effector phase 2, PGE2, the proinflammatory bioactive lipid, probably acts in an autocrine fashion by binding to one of its four cognate EP receptors in the infected cells or in a paracrine fashion by binding to the neighboring cells. The EP receptors belong to the family of seven transmembrane G-protein-coupled rhodopsin-type receptors, and PGE2 binding to these receptors can lead to a variety of signal cascades, such as PI-3K, Ras, Raf MEK, and ERK, as well as the induction of VEGF, bFGF, IL-10, Bcl-2, etc. (79). The results presented here suggest that COX-2 effector phase 2 is critical for KSHV latent gene expression and maintenance, since latent ORF73 gene expression was significantly reduced by COX-2 inhibitors and this inhibition was relieved by exogenous supplementation with PGE2. These studies suggest that during in vitro infection of target cells, KSHV utilizes the proinflammatory COX-2 and PGE2 for viral latent gene expression and maintenance, thus facilitating the establishment of latency.
Another facet of KSHV in vitro infection is the ability of the virus to reprogram the host transcriptional machinery regulating a variety of cellular processes, including apoptosis, transcription, cell cycle regulation, signaling, the inflammatory response, and angiogenesis (47). In the present study, we provide several lines of evidence to suggest that KSHV infection-induced proinflammatory COX-2 molecules play roles in the maintenance of latent gene expression. During KSHV infection, COX-2 modulation and its effect probably occur in overlapping dynamic phases, and for descriptive purpose, we have arbitrarily divided these into COX-2 induction and effector phases (Fig. 11). The initial expression of COX-2 genes and proteins is considered the induction phase, induction of PGE2 is considered effector phase 1, and the effect of PGE2 is considered effector phase 2.
The induction of significant amounts of COX-2 early during infection (Fig. 1 and 2) and the ability of UV-irradiated KSHV and envelope glycoproteins to induce a moderate level of COX-2 suggest that the initial binding and entry stages of KSHV infection initiate the COX-2 induction phase (Fig. 11). This is similar to COX-2 up-regulation by UV-inactivated human CMV (HCMV) (86). In contrast, COX-2 was induced by live but not by UV-inactivated murine gammaherpesvirus 68 (MHV-68) infection, suggesting that MHV-68 induces COX-2 via mechanisms that depend on viral gene expression (71). Similar to our observation of the ability of KSHV gB and gPK8.1A to induce COX-2, incubation of fibroblast cells with HCMV gB induced the accumulation of a wide variety of cellular mRNAs, including COX-2 mRNA (86). KSHV gene expression early during infection and subsequent modulation of host genes are probably essential for the increased induction of COX-2 levels. Sustained induction of COX-2 by UV-irradiated KSHV could be due to the combination of induced host genes, with induction of COX-2 leading to the synthesis and release of PGE2, which in turn induces COX-2. Investigations to decipher the roles of KSHV-induced signal cascades and host cell genes involved in the initial induction of COX-2 genes are under way.
In our earlier studies we have demonstrated that KSHV-induced ERK1/2 plays a role in the expression of the ORF50 and ORF73 genes, probably in the initiation of their expression (61). The moderate effect on the expression of the ORF73 gene at 2 h and 8 h p.i. by COX-2 inhibitor (Fig. 7A) could be due to the overall lower level of ORF73 gene expression early during infection as well as possible role of ERK1/2 in the early ORF73 expression (61). Studies show that a variety of transcription factors, including activator protein-1 (AP-1) can stimulate COX-2 expression (27, 67). There are several binding sites for a variety of transcription factors, including NF-κB, NF-IL-6/C/EBP, AP-1, and the cyclic AMP response element in the 5′ region of the COX-2 gene (26). We have shown that KSHV infection-induced ERK phosphorylation activated a variety of transcription factors, including c-Fos, c-Jun, c-Myc, and STAT1α (61). AP-1 is composed of a mixture of heterodimeric complexes of proteins derived from the Fos and Jun families, including c-Fos, FosB, Fra-1, Fra-2, c-Jun, JunB, and JunD. At present it is not clear whether these transcription factors are involved in the initial stimulation of COX-2 by KSHV. Expression of GFP from the GFP-KSHV genome is under the control of promiscuous elongation factor 1-α promoter (GenBank accession number E02627) which possesses several important transcription binding motifs, including AP-1, SP1, CREB, CRE-BP, and NF-κB, that are activated by multiple signaling pathways. Inhibition of GFP expression in addition to ORF73 expression by pretreatment of cells with COX-2 inhibitor (Fig. 6) could be due to the involvement of COX-2 in the activation and regulation of the above-mentioned transcription factors.
Induction of COX-2 in the target cells is not unique for KSHV, since infection by several viruses has been shown to up-regulate COX-2 expression. These include herpesviruses such as HSV (9), HCMV (86), pseudorabies virus (52), HHV-6 (27), EBV (45), and MHV-68 (71), as well as other viruses such as hepatitis B virus (15, 35) and human T-cell leukemia virus type 1 (HTLV-1) (42, 43, 45). In addition, rhesus cytomegalovirus even encodes a COX-2 homolog in its genome, thus emphasizing the importance of this enzyme in viral infections.
In the COX-2 effector phase 1, we observed a strong stimulation of PGE2 in the infected cell supernatant. KSHV-induced COX-2 catalyzes the oxidation of arachidonic acid to PGH2, which is the precursor of several biologically active prostanoids, such as PGE2, PGD2, PGF2α, and thromboxane A2. Among these, KSHV-induced PGE2 is one of the most abundant lipid mediators produced during viral infections and inflammatory reactions (66). Increased PGE2 production has been observed in infection by a variety of viruses, including HCMV (86), HHV-6 (27), coxsackie B3 virus (23), bovine leukemia virus (51), and HTLV-1 (43). Human immunodeficiency virus type 1 (HIV-1) infection also up-regulates PGE2, as demonstrated in monocytes as well as sera of infected individuals (20). Evidence suggests a role for PGE2 in the regulation of viral replication and modulation of inflammatory responses to viral infections (14, 23, 27, 29, 86), and these actions appear to be dependent upon both the virus type and the host cells. A role for PGE2 in virus replication and infectivity has been shown for CMV (86), HSV (29), HHV-6 (27), vesicular stomatitis virus (14), HIV-1 (20), bovine leukemia virus (51), HTLV-1 (43), hepatitis B virus (25), and coxsackie B3 virus (23). As in pseudorabies virus and HCMV infection of target cells (52, 86), constitutive COX-1 protein levels were not modulated by KSHV infection, thus demonstrating that COX-2 is the major isoform involved in the PGE2 synthesis observed in KSHV-infected target cells.
Pharmacological agents blocking COX and prostaglandin production have been recognized as potentially effective antiviral compounds (78, 86). In vitro infection by HCMV (86), HSV-1 (9, 29), HSV-2, vesicular stomatitis virus, (14), and Japanese encephalitis virus (13) has been shown to be sensitive to COX inhibitors. Although COX-2 inhibitors also affected KSHV infection, whereas COX-2 appears to have a role in lytic gene expression in the above-mentioned viral infections, our studies demonstrated that COX-2 plays a role in latent KSHV gene expression. Our data clearly demonstrated that COX-2 inhibitor pretreatment has a profound impact on the promoter activity of ORF73 compared to that of lytic gene ORF50, suggesting that KSHV infection-induced COX-2 is critical for the maintenance of KSHV latency in infected cells in the COX-2 effector phase 2 (Fig. 11). Whether COX-2 plays a direct role in reducing the ORF50 expression and thus blocking the full-fledged lytic cycle cascade is not known. Since studies suggest that ORF73 protein has the ability to suppress lytic reactivation and maintain latency through the repression of the transcriptional activity of the RTA promoter (34), it is possible that the COX-2 effect on the lytic cycle could be an indirect one as it facilitates latent gene expression and thus leads to repression of lytic cascade.
PGE2 has been shown to prevent overactivation of cellular immunity and to exert antiviral activities. Treatment of monocyte-derived macrophages with PGE2 decreased HIV-1 virion penetration by suppressing the expression of HIV-1 coreceptor CCR5 (72). Prostaglandins have very short lives, and hence they act in either a paracrine or autocrine fashion in order to stimulate transforming pathways through G protein-coupled receptors. PGE2 has been shown to act as a signal molecule, as it has been reported to bind to one of four E-prostanoid (EP) receptors. Among these, EP receptor 2 and EP receptor 4 activate the G protein-coupled adenylate cyclase, causing a transient rise in cyclic AMP, protein kinase A and Rac activation, and accelerating endothelial cell adhesion, spreading, and migration (56), whereas EP receptor 1 activates phospholipase C to induce the protein kinase C pathway. Our studies implicate PGE2 in controlling viral latent gene expression. PGE2 has been shown to activate several viral promoters, including the HTLV-1 long terminal repeat promoter (43) and the HCMV major immediate-early promoter (31). Our studies suggested that KSHV modulates the COX-2 gene to induce PGE2 for the maintenance of viral latent gene expression. Further studies are in progress to define the molecular mechanism of PGE2-modulated KSHV latent gene expression.
Besides providing clues to how KSHV establishes and maintains latent infection, our findings also have some implications for KSHV biology and KS pathogenesis. KS is an enigma among the known human tumors, due to its multifocal nature and the variety of cell types, such as spindle-shaped cells and inflammatory cells, in the lesions. KSHV ORF73, ORF72, K13, and K12 were detected in vascular endothelial and spindle cells of KS lesions, and the lytic cycle proteins were detected in about 1 to 10% of infiltrating monocytic cells of KS lesions (18, 21, 41, 59, 60, 65, 85). Several lines of evidence suggest that KSHV lytic reactivation under immuosuppressed conditions precedes KS development (8). KS tumorigenesis appears to require an ongoing lytic infection, since interruption of lytic replication by drugs such as ganciclovir appears to prevent KS development (10). Reactivation leading to increased progeny virus probably leads to multiple foci of infected endothelial cells throughout the body.
KS is classified as a chronic inflammation-associated malignancy and is characterized by the presence of abundant proinflammatory cytokines and growth factors in the KS lesions (8). COX-2 activity increases the expression of growth factors such as platelet-derived growth factor, basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs) (66). Since early during in vitro infection of endothelial cells KSHV also induced VEGF, bFGF, MMP, and other cytokine and growth factor mRNAs (47), it is possible that COX-2 and PGE2 induction during in vivo infection could be responsible for the growth factors and cytokines seen in the microenvironment of KS lesions. The detection of higher levels of PGE2 in KS tissues (5) supports this hypothesis.
KSHV probably utilizes COX-2 and other host cell genes to its advantage in the establishment of latent infection and immune modulation (Fig. 11). However, the combination of factors such as (i) the absence of immune regulation, (ii) an unchecked KSHV lytic cycle and increased virus load resulting in widespread KSHV infection, and (iii) induction of inflammatory cytokines and growth factors may contribute to the pathogenesis of KS lesions. A recent study demonstrated the occurrence of COX-2-related multicentric Castleman's disease, a disease associated with the lytic cycle of KSHV (36). COX-2 is a target for anticancer therapy, as COX-2 inhibitors have been shown to possess anticancer effects. The detection of higher levels of PGE2 in KS tissue compared to surrounding normal tissue (5), together with our studies demonstrating the importance of COX-2 in KSHV latent infection, suggests that effective inhibition of COX-2 could lead to reduced latent KSHV infection of endothelial cells, which may lead in turn to a reduction in the accompanying inflammation and KS lesions.
Acknowledgments
This study was supported in part by Public Health Service grants CA 75911 and CA 099925 and the Rosalind Franklin University of Medicine and Science-H.M. Bligh Cancer Research Fund to B.C.
We thank Timothy Hla, University of Connecticut, for the kind gift of the human COX-2 cDNA construct. We thank Yuan Chang, University of Pittsburgh, for the generous gift of the ORF73 promoter-luciferase reporter construct. We thank George Miller, Yale University School of Medicine, for the kind gift of p2500Luc, an ORF50 promoter-luciferase reporter construct.
REFERENCES
- 1.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. [DOI] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., N. P. Pramod, F.-Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD49c/28) is a cellular receptor for Kaposi's sarcoma associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 4.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrus, J. L., H. L. Stoll, E. A. Klein, C. P. Karakousis, and S. Stadler. 1992. Increased prostaglandin E2 and cAMP phosphodiesterase levels in Kaposi's sarcoma—a virus against host defense mechanism. Res. Commun. Chem. Pathol. Pharmacol. 78:249-252. [PubMed] [Google Scholar]
- 6.Bartz, H., F. Buning-Pfaue, O. Turkel, and U. Schauer. 2002. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin. Exp. Immunol. 129:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackbourn, D. J., E. Lennette, B. Klencke, A. Moses, B. Chandran, M. Weinstein, R. G. Glogau, M. H. Witte, D. L. Way, T. Kutzkey, B. Herndier, and J. A. Levy. 2000. The restricted cellular host range of human herpesvirus 8. AIDS 14:1123-1133. [DOI] [PubMed] [Google Scholar]
- 8.Boshoff, C., and R. Weiss. 2002. AIDS-related malignancies. Nat. Rev. Cancer 2:373-382. [DOI] [PubMed] [Google Scholar]
- 9.Bratcher, D. F., C. J. Harrison, N. Bourne, L. R. Stanberry, and D. I. Bernstein. 1993. Effect of indomethacin on ultraviolet radiation-induced recurrent herpes simplex virus disease in guinea-pigs. J. Gen. Virol. 74:1951-1954. [DOI] [PubMed] [Google Scholar]
- 10.Casper, C., W. G. Nichols, M. L. Huang, L. Corey, and A. Wald. 2004. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood 103:1632-1634. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 13.Chen, C. J., S. L. Raung, M. D. Kuo, and Y. M. Wang. 2002. Suppression of Japanese encephalitis virus infection by non-steroidal anti-inflammatory drugs. J. Gen. Virol. 83:1897-1905. [DOI] [PubMed] [Google Scholar]
- 14.Chen, N., J. L. Warner, and C. S. Reiss. 2000. NSAID treatment suppresses VSV propagation in mouse CNS. Virology 276:44-51. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, A. S., H. L. Chan, N. W. Leung, C. T. Liew, K. F. To, P. B. Lai, and J. J. Sung. 2002. Expression of cyclooxygenase-2 in chronic hepatitis B and the effects of anti-viral therapy. Aliment. Pharmacol. Ther. 16:251-260. [DOI] [PubMed] [Google Scholar]
- 16.Dannenberg, A. J., and K. Subbaramaiah. 2003. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 4:431-436. [DOI] [PubMed] [Google Scholar]
- 17.Dezube, B. J., M. Zambela, D. R. Sage, J.-F. Wang, and J. D. Fingeroth. 2002. Characterization of Kaposi sarcoma-associated herpesvirus/human herpesvirus-8 infection of human vascular endothelial cells: early events. Blood 100:888-896. [DOI] [PubMed] [Google Scholar]
- 18.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67:175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois, R. N., S. B. Abramson, L. Crofford, R. A. Gupta, L. S. Simon, L. B. Van De Putte, and P. E. Lipsky. 1998. Cyclooxygenase in biology and disease. FASEB J. 12:1063. [PubMed] [Google Scholar]
- 20.Dumais, N., B. Barbeau, M. Olivier, and M. J. Tremblay. 1998. Prostaglandin E2 up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-kappaB-dependent and -independent signaling pathways. J. Biol. Chem. 273:27306-27314. [DOI] [PubMed] [Google Scholar]
- 21.Ganem, D. 1997. KSHV and Kaposi's sarcoma: the end of the beginning? Cell 91:157-160. [DOI] [PubMed] [Google Scholar]
- 22.Gao, S. J., J. H. Deng, and F. C. Zhou. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 77:9738-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke, A., H. P. Spengler, A. Stelzner, M. Nain, and D. Gemsa. 1992. Lipopolysaccharide suppresses cytokine release from coxsackie virus-infected human monocytes. Res. Immunol. 143:65-70. [DOI] [PubMed] [Google Scholar]
- 24.Hla, T., and K. Neilson. 1992. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. USA 89:7384-7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyman, A., C. Yim, M. Krajden, S. Read, A. S. Basinski, I. Wanless, G. Levy, and J. Heathcote. 1999. Oral prostaglandin (PGE2) therapy for chronic viral hepatitis B and C. J. Viral Hepat. 6:329-336. [DOI] [PubMed] [Google Scholar]
- 26.Inoue, H., S. Yokoyama, S. Hara, Y. Tone, and T. Tanabe. 1995. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. J. Biol. Chem. 270:24965-24971. [DOI] [PubMed] [Google Scholar]
- 27.Janelle, M. E., A. Gravel, J. Gosselin, M. J. Tremblay, and L. Flamand. 2002. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 277:30665-30674. [DOI] [PubMed] [Google Scholar]
- 28.Jeong, J., J. Papin, and D. Dittmer. 2001. Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters. J. Virol. 75:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khyatti, M., and J. Menezes. 1990. The effect of indomethacin, prostaglandin E2 and interferon on the multiplication of herpes simplex virus type 1 in human lymphoid cells. Antivir. Res. 14:161-172. [DOI] [PubMed] [Google Scholar]
- 30.Kieff, E., and A. B. Rickinson. 2002. Epstein-Barr virus and its replication, p. 251.1-2573. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 31.Kline, J. N., G. M. Hunninghake, B. He, M. M. Monick, and G. W. Hunninghake. 1998. Synergistic activation of the human cytomegalovirus major immediate early promoter by prostaglandin E2 and cytokines. Exp. Lung Res. 24:3-14. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan, K., D. A. Kuppers, S. C. Verma, N. Sharma, M. Murakami, and E. S. Robertson. 2005. Induction of Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency. J. Virol. 79:7453-7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lara-Pezzi, E., M. V. Gomez-Gaviro, B. G. Galvez, E. Mira, M. A. Iniguez, M. Fresno, A. C. Martinez, A. G. Arroyo, and M. Lopez-Cabrera. 2002. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclo-oxygenase-2 expression. J. Clin. Investig. 110:1831-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, J., S. Han, J. Ding, K. Wu, J. Miao, and D. Fan. 2005. COX2-related multicentric mixed-type Castleman's disease in a young man. Nat. Clin. Pract. Oncol. 2:370-375. [DOI] [PubMed] [Google Scholar]
- 37.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumura, K., C. Cao, M. Ozaki, H. Mori, K. Nakadate, and Y. Watanbe. 1998. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J. Neurosci. 18:6279-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumura, S., Y. Fujita, E. Gomez, N. Tanese., and A. C. Wilson. 2005. Activation of the Kaposi's sarcoma-associated herpesvirus major latency locus by the lytic switch protein RTA (ORF50). J. Virol. 79:8493-8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mocarski, E. S., and C. M. Courcell. 2002. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 41.Moore, P. S., and Y. Chang. 2002. Kaposi's sarcoma associated herpesvirus, p. 2803-2833. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 42.Mori, N., H. Inoue, T. Yoshida, T. Tanabe, and N. Yamamoto. 2001. Constitutive expression of the cyclooxygenase-2 gene in T-cell lines infected with human T cell leukemia virus type I. Int. J. Cancer 94:813-819. [DOI] [PubMed] [Google Scholar]
- 43.Moriuchi, M., H. Inoue, and H. Moriuchi. 2001. Reciprocal interactions between human T-lymphotropic virus type 1 and prostaglandins: implications for viral transmission. J. Virol. 75:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murono, S., I. Hiroyasu, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naranatt, P. P., H. H. Krishnan, M. S. Smith, and B. Chandran. 2005. Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J. Virol. 79:1191-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/KSHV) infected endothelial, fibroblast and B cells: insights into modulation events early during infection. Cancer Res. 64:72-84. [DOI] [PubMed] [Google Scholar]
- 48.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naranatt, P. P., Akula, S. M., and B. Chandran. 2002. Characterization of gamma2-human herpesvirus-8 glycoproteins gH and gL. Arch. Virol. 147:1349-1370. [DOI] [PubMed] [Google Scholar]
- 50.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pyeon, D., J. F. Diaz, and G. A. Splitter. 2000. Prostaglandin E2 increases bovine leukemia virus tax and pol mRNA levels via cyclooxygenase 2: regulation by interleukin-2, interleukin-10, and bovine leukemia virus. J. Virol. 74:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ray, N., and L. W. Enquist. 2004. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J. Virol. 78:3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy, R. C., G. H. Chen, K. Tateda, W. C. Tsai, S. M. Phare, P. Mancuso, M. Peters-Golden, and T. J. Standiford. 2001. Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L537-L543. [DOI] [PubMed] [Google Scholar]
- 54.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roizman, B., and P. E. Pellett. 2002. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 56.Ruegg, C., O. Dormond, and A. Mariotti. 2004. Endothelial cell integrins and COX-2: mediators and therapeutic targets of tumor angiogenesis. Biochim. Biophys. Acta 1654:51-67. [DOI] [PubMed] [Google Scholar]
- 57.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelmen, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi's sarcoma-associated herpesvirus (HHV-8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarid, R., J. S. Wiezorek, P. S. Moore, and Y. Chang. 1999. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 73:1438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz, T. F., J. Sheldon, and J. Greensill. 2002. Kaposi's sarcoma associated herpesvirus (KSHV) or human herpesvirus 8 (HHV-8). Virus Res. 82:115-126. [DOI] [PubMed] [Google Scholar]
- 60.Schulz, T. F., Y. Chang, and P. S. Moore. 1998. Kaposi's sarcoma associated herpesvirus (human herpesvirus 8), p. 87-134. In D. J. McCance (ed.), Human tumor viruses. American Society for Microbiology, Washington, D.C.
- 61.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 79:10308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 78:4207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shelby, B. D., A. Nelson, and C. Morris. 2005. γ-Herpesvirus neoplasia: a growing role for COX-2. Microsc. Res. Tech. 68:120-129. [DOI] [PubMed] [Google Scholar]
- 64.Smith, W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69:145-182. [DOI] [PubMed] [Google Scholar]
- 65.Staskus, K. A., A. W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. D. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steer, S. A., and J. A. Corbett. 2003. The role and regulation of COX-2 during viral infection. Viral Immunol. 16:447-460. [DOI] [PubMed] [Google Scholar]
- 67.Subbaramaiah, K., P. A. Cole, and A. J. Dannenberg. 2002. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Res. 62:2522-2530. [PubMed] [Google Scholar]
- 68.Subbaryan, V., A. L. Sabichi, N. Llansa, S. M. Lippman, and D. G. Menter. 2001. Differential expression of cyclooxygenase-2 and its regulation by tumor necrosis factor-α in normal and malignant prostate cells. Cancer Res. 61:2720-2726. [PubMed] [Google Scholar]
- 69.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Symensma, T. L., D. Martinez-Guzman, Q. Jia, E. Bortz, T. T. Wu, N. Rudra-Ganguly, S. Cole, H. Herschman, and R. Sun. 2003. COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J. Virol. 77:12753-12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thivierge, M., C. L. Gouill, M. J. Tremblay, J. Stanková, and M. Rola-Pleszczynski. 1998. Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood 92:40-45. [PubMed] [Google Scholar]
- 73.Tomescu, C., W. K. Law, and D. H. Kedes. 2003. Surface downregulation of major histocompatibility complex class I, PE-CAM, and ICAM-1 following de novo infection of endothelial cells with Kaposi's sarcoma-associated herpesvirus. J. Virol. 77:9669-9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ushikubi, F., E. Segi, Y. Sugimoto, T. Murata, T. Matsuoka, T. Kobayashi, H. Hizaki, K. Tuboi, M. Katsuyama, A. Ichikawa, T. Tanaka, N. Yoshida, and S. Narumiya. 1998. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395:281-284. [DOI] [PubMed] [Google Scholar]
- 75.Vane, J. R., and R. M. Botting. 1998. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 47(Suppl. 2):S78-S87. [DOI] [PubMed] [Google Scholar]
- 76.Vane, J. R., Y. S. Bakhle, and R. M. Botting. 1998. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 38:97-120. [DOI] [PubMed] [Google Scholar]
- 77.Vieira, J., O. Hearn, L. E. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus lytic replication by human cytomegalovirus. J. Virol. 75:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wachsman, M., L. Aurelian, and J. W. Burnett. 1990. The prophylactic use of cyclooxygenase inhibitors in recurrent herpes simplex infections. Br. J. Dermatol. 123:375-380. [DOI] [PubMed] [Google Scholar]
- 79.Wang, D., and R. N. DuBois. 2006. Prostaglandin and Cancer Gut 55:115-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, F.-Z., S. M. Akula, N. S. Walia, L. Zeng, and B. Chandran. 2003. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J. Virol. 77:3131-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparin sulfate. J. Virol. 75:7517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.West, J. T., and C. Wood. 2003. The role of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression. Oncogene 22:5150-5163. [DOI] [PubMed] [Google Scholar]
- 83.Williams, C. S., M. Mann, and R. N. DuBois. 1999. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18:7908-7916. [DOI] [PubMed] [Google Scholar]
- 84.Ye, J., D. Shedd, and G. Miller. 2005. An Sp1 response element in the Kaposi's sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J. Virol. 79:1397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu, L., V. Puri, and B. Chandran. 1999. Characterization of human herpesvirus-8 K8.1 A/B glycoproteins by monoclonal antibodies. Virology 262:237-249. [DOI] [PubMed] [Google Scholar]