Abstract
Nucleophosmin (NPM/B23) is a key regulator in the regulation of a number of processes including centrosome duplication, maintenance of genomic integrity, and ribosome biogenesis. While the mechanisms underlying NPM function are largely uncharacterized, NPM loss results in severe dysregulation of developmental and growth-related events. We show that NPM utilizes a conserved CRM1-dependent nuclear export sequence in its amino terminus to enable its shuttling between the nucleolus/nucleus and cytoplasm. In search of NPM trafficking targets, we biochemically purified NPM-bound protein complexes from HeLa cell lysates. Consistent with NPM's proposed role in ribosome biogenesis, we isolated ribosomal protein L5 (rpL5), a known chaperone for the 5S rRNA. Direct interaction of NPM with rpL5 mediated the colocalization of NPM with maturing nuclear 60S ribosomal subunits, as well as newly exported and assembled 80S ribosomes and polysomes. Inhibition of NPM shuttling or loss of NPM blocked the nuclear export of rpL5 and 5S rRNA, resulting in cell cycle arrest and demonstrating that NPM and its nuclear export provide a unique and necessary chaperoning activity to rpL5/5S.
As the most prominent of subnuclear structures, the nucleolus has long been recognized as the site of active transcription of rRNA and ribosome assembly (8). Various nucleolar proteins, RNAs, and other factors have been implicated in the complex process of ribosome production and maturation (18). Recently, several groups reported the successful isolation and mapping of the mammalian nucleolar proteome (1, 2, 44). While these studies clearly identified proteins and ribonucleoproteins with purported roles in ribosome biogenesis, a surprising number of proteins within the nucleolar proteome (>100) have no known function. In previous decades, it was assumed that all nucleolar proteins must somehow contribute to static ribosome biogenesis simply by virtue of their localization. However, more-recent findings have demonstrated that the nucleolus is a dynamic subnuclear organelle which regulates numerous cellular processes, prompting a broadened view of the potential functions of nucleolar proteins (28).
Nucleophosmin (NPM/B23) is an abundant phosphoprotein that resides within the granular regions of the nucleolus (46). Proliferating cells express NPM at high levels (9, 13), and NPM has been associated with a variety of cellular events, including ribosomal biogenesis, protein chaperoning, and centrosome duplication (13, 23, 35, 36). Structurally, NPM is present in both monomeric and multimeric states, although NPM multimers appear predominant in the nucleolus and may be crucial for the assembly of maturing ribosomes (33, 34, 53). Furthermore, NPM, along with other nucleolar proteins, is believed (or has been shown) to actively mobilize into distinct subcellular pools, supporting the notion that NPM trafficking may be essential for its (proper) function (6). Indeed, NPM's transit from the nucleolus/nucleus is an essential event in S phase progression; when NPM export was inhibited by the nucleolar tumor suppressor ARF, cells arrested in G1 (7). Moreover, loss of NPM expression results in severe attenuation of cellular proliferation and increased apoptosis (5, 7, 16, 19), underscoring NPM's indispensable role within the cell.
Given that nuclear export of NPM promotes cell growth, we aimed to further elucidate the crucial roles of NPM's trafficking. While numerous proteins, such as Mdm2, cdc14p, and telomerase reverse transcriptase, are topologically restrained in the nucleolus following receipt of defined cellular cues, newly synthesized ribosomal subunits must be exported from the nucleolus to promote proper protein translation in the cytosol (45). Recent work with Xenopus laevis and Saccharomyces cerevisiae has shown that nuclear export of ribosomes utilizes the CRM1-RanGTP export receptor pathway (20) as well as the conserved nuclear adaptor protein NMD3 (51). While investigating the critical nature of NPM trafficking, we noted that NPM's exit from the nucleus also involved the classical CRM1-dependent nuclear export pathway. In search of proteins that are targeted for NPM-mediated nuclear export, we observed that nuclear and cytosolic NPM proteins directly bound to the ribosomal L5 protein (rpL5), a 60S subunit protein that chaperones the 5S rRNA into the nucleolus and out into the cytosol (31). Here we report that NPM mediates rpL5/5S nuclear export through a CRM1-dependent mechanism, allowing NPM to directly access the maturing ribosome and potentially regulate the protein translational machinery.
MATERIALS AND METHODS
Cell culture.
HeLa and NIH 3T3 cells and wild-type (WT) mouse embryonic fibroblasts (MEFs) (ArtisOptimus, Carlsbad, CA) were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 2 mM glutamine, 0.1 mM nonessential amino acids, and 100 U penicillin and streptomycin.
Plasmid constructs.
Vectors encoding full-length His-tagged murine NPM are described elsewhere (7). The His epitope-tagged NPM coding sequence was subcloned into pcDNA3.1 (Invitrogen) and pEGFP (Clontech) vectors. His-NPMΔ42-61, His-NPMΔ62-83, or His-NPMdL mutants were generated using the primers 5′-GAAAATGAGCACCAGGCAGAAGCAATGAAC-3′ (sense) and 5′-GTTCATTGCTTCTGCCTGGTGCTCATTTTC-3′ (antisense), 5′-GTTACACATCGTAGAGCAACCAACAGTTTCC-3′ (sense) and 5′-GGAAACTGTTGGTTGCTCTACGATGTGTAAC-3′ (antisense), or 5′-GAAAATGAGCACCAGGCGTCAGCAAGAACGGTC-3′ (sense) and 5′-CTAAACTGACCGTTCTTGCTGACGCCTGGTGCTCATTTTC-3′ (antisense), respectively, by QuikChange mutagenesis (Stratagene). A myc-tagged NPC-M9 (40) in pcDNA3 and a green fluorescent protein (GFP)-tagged rpL5 plasmid (41) were generous gifts from Alan Diehl (University of Pennsylvania) and Joachim Hauber (Universitat Erlangen-Nurnberg).
Heterokaryon assay.
HeLa cells (2 × 105) were seeded onto glass coverslips and transfected with plasmids. NIH 3T3 cells (6 × 105) were seeded onto the HeLa cells 24 h posttransfection. Cocultures were then incubated for 30 min with cycloheximide (100 μg/ml), followed by incubation with 50% polyethylene glycol in phosphate-buffered saline for 105 s. Cocultures were incubated with Dulbecco's modified Eagle's medium containing cycloheximide (100 μg/ml) for an additional 4 h. Heterokaryons were fixed and stained with a rabbit anti-His antibody (Santa Cruz) or mouse anti-myc antibody (Zymed), followed by either fluorescein isothiocyanate-conjugated or rhodamine X-conjugated anti-rabbit or anti-mouse immunoglobulin (Pierce) as described previously (7). Nuclei were stained with Hoechst (Sigma). Fluorescent signals were detected using a Nikon epifluorescence compound microscope (×100) fitted with a Nikon FDX-35 camera.
Immunoprecipitation and Western blot analysis.
Cells were transduced as recommended by the manufacturer (Amaxa) with vectors encoding His-NPM, His-NPMdL, and GFP-rpL5 and lysed in binding buffer (25 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40) 48 h after the Nucleofector process. Primary antibody to the NPM N terminus (custom rabbit; Sigma Genosys), GFP (Santa Cruz), His (Santa Cruz), rpL5 (12), or nonimmune rabbit serum (NRS) was added to the binding reaction mixtures. Immune complexes were precipitated with protein A-Sepharose (Amersham). The precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. NPM, His-tagged proteins, and GFP-tagged proteins were visualized by direct immunoblotting with NPM (Zymed), His (Santa Cruz), and rpL5 and GFP (Santa Cruz) antibodies, respectively.
Fluid phase liquid chromatography.
For affinity chromatography, a rabbit polyclonal antibody recognizing the N terminus of NPM (Sigma) was coupled to N-hydroxysuccinimide-activated Sepharose (Amersham). HeLa cells were lysed in 20 mM Tris, pH 7.4, and 0.1% Tween 20 and sonicated. Lysates (600 μg) were injected onto the NPM affinity column, washed with 20 mM Tris, and eluted with an increasing NaCl gradient (0.1 to 1 M) using BioLogic fluid phase liquid affinity chromatography and HR software (Bio-Rad). Fractions were precipitated with trichloroacetic acid (TCA). Proteins were resuspended in 1 M Tris-HCl (pH 7.4), separated by SDS-PAGE, and visualized with Coomassie blue stain (Sigma).
Proteomic analysis.
Proteins from fluid phase liquid affinity chromatography fractions were precipitated with TCA and resuspended in Laemmli buffer. SDS-PAGE-separated proteins were stained with SYPRO-Ruby (Bio-Rad). Bands of interest were excised and processed for trypsin digestion. Tryptic peptides were calibrated with Sequazyme peptide mass standard kit (PE Biosystem) and analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (Voyager DE Pro; Applied Biosystems). Identification of proteins was performed using MS-Fit software (http://prospector.ucsf.edu/ucsfhtml4.0/msfit.htm). MALDI-TOF spectra and sequences were verified using a 4700 Proteomics tandem mass spectrometry system (Applied Biosystems). Identified proteins were additionally verified by direct Western blot analysis.
Bacterial protein purification.
BL21 cells were transformed with pET28a vectors encoding NPM, NPMdL, rpL5, and p27kip1 proteins. Protein production was induced for 3 h with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Harvested cells were lysed in phosphate-buffered saline containing protease inhibitors and 1% Triton X-100 with sonication. Cleared lysates were subjected to affinity purification using Ni-nitrilotriacetic acid columns as described by the manufacturer (Sigma). Purified proteins were separated by SDS-PAGE and visualized for purity using Coomassie blue stain.
Subcellular fractionation.
HeLa cells were subjected to the Nucleofector process with scrambled or small interfering NPM RNAs or control vector, His-NPM, and His-NPMdL and harvested. Pellets containing equal cell numbers were resuspended in HEPES buffer (10 mM HEPES, pH 7.4, with 4 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μg/ml pepstatin) and lysed with a syringe. Lysates were pelleted, and the supernatant was saved as the cytoplasmic fraction. The pellet was resuspended in fractionation buffer (10 mM Tris, pH 7.5, 10 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 4 mM MgCl2, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μg/ml pepstatin), subjected to Dounce homogenization, layered over a cushion of sucrose (45%, wt/vol, in fractionation buffer), and centrifuged. The pellet was washed and resuspended in EBC buffer (50 mM Tris-HCl, pH 7.4, 120 mM NaCl, 1 mM EDTA, 0.5% NP-40, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μg/ml pepstatin, 1 mM NaF, 10 mM NaVO4, β-glycerophosphate). Nuclear or cytoplasmic protein was subjected to SDS-PAGE. Superoxide dismutase (SOD; Cu/Zn-specific form), lamin A/C, and rpL5 proteins were visualized by direct immunoblotting with anti-SOD (Calbiochem), anti-lamin A/C (Santa Cruz), and anti-rpL5 antibodies (12), respectively. Similarly, total RNA was isolated from the fractions obtained above and separated on formaldehyde-agarose gels. Separated RNA from each nuclear and cytoplasmic fraction was analyzed by Northern blotting using a probe specific for the 5S rRNA. The 5S rRNA probe was obtained by PCR using HeLa cell genomic DNA as the template and the following primers: sense, 5′-CCTTCAGCGTCTACGGCCATACC-3′; antisense, 5′-GCCAAGAAAAAGCCTACAGCAGG-3′. The PCR product was cloned and confirmed by sequencing.
RNA FISH.
HeLa cells were subjected to the Nucleofector process with pcDNA3.1 His, His-tagged NPM, or His-NPMdL and plated on coverslips. Cells were subjected to RNA fluorescence in situ hybridization (FISH) as described previously (3) using a tetramethyl rhodamine isocyanate (TRITC)-labeled 5S rRNA probe (Genedetect). DNA was counterstained with DAPI (4′,6′-diamidino-2-phenylindole).
Ribosome fractionation.
Cells were subjected to cytosolic and nuclear ribosome fractionation, and lysates were separated on sucrose gradients as previously described (48). RNA was continuously monitored over the gradient by measuring UV absorbance at 254 nm. Fractions were collected, and proteins were precipitated with TCA. Proteins were separated by SDS-PAGE and immunoblotted with antibodies recognizing NPM (Zymed) and rpL5.
RESULTS
NPM nuclear export requires a CRM1-dependent nuclear export signal involving leucines 42 and 44.
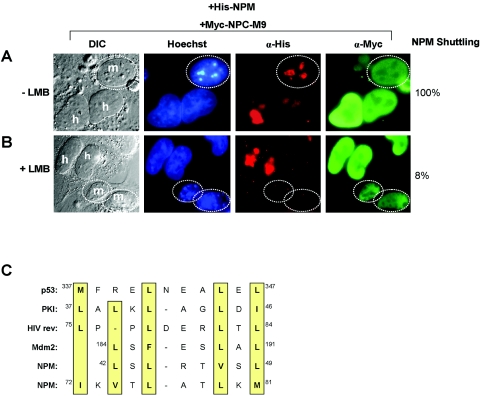
NPM is a ubiquitously expressed nucleolar phosphoprotein capable of regulated nuclear import (6). When NPM is transiently expressed in mammalian cells, it localizes predominantly to the nucleolus. Moreover, using in vivo heterokaryon shuttling assays (50), we have previously shown that NPM readily shuttles between the nucleolus/nucleus and cytoplasm (7). NPC-M9, a nuclear hnRNP protein that readily mobilizes to the cytoplasm, serves as a shuttling control (40). To distinguish between human donor and murine acceptor nuclei, chromosomal DNA was stained with Hoechst, clearly demarcating greater heterochromatin foci of NIH 3T3 mouse cells (speckled pattern, Fig. 1, Hoechst). As shown in Fig. 1A, NPM readily shuttles out of the human nucleolus, into the fused cytoplasm, and back into the mouse acceptor nucleus/nucleolus.
FIG. 1.
Nuclear export of NPM is CRM1 dependent. NIH 3T3 cells were seeded onto HeLa cells that had been transfected with His-NPM in combination with Myc-NPC-M9 (shuttling control) in the (A) absence or (B) presence of LMB. Heterokaryons were incubated in media containing cycloheximide for an additional 4 h before fixation. Heterokaryon formation was verified by phase-contrast microscopy, while His-NPM and Myc-NPC-M9 proteins were visualized with antibodies against His (red) and Myc (green), respectively. DNA was stained with Hoechst. Mouse nuclei are demarcated with dotted circles. Human and mouse nuclei are labeled h and m, respectively. These data are representative of at least five independent heterokaryons formed for each transfection condition in three independent experiments. The percentages of His-NPM shuttling in heterokaryons are given. DIC, differential interference contrast; α, anti. (C) Sequence alignment of putative NPM NESs with known NESs of CRM1-dependent nuclear export proteins (p53, protein kinase inhibitor [PKI], rev, and Mdm2). Critical hydrophobic residues are indicated in yellow.
Given that a wide range of shuttling proteins utilize the CRM1 transport protein for their nuclear export, we further investigated the underlying export mechanism of NPM both in the presence and absence of leptomycin B (LMB), a potent inhibitor of CRM1-mediated nuclear export (24). In the absence of LMB, NPM readily migrated from human nucleoli to mouse nucleoli (Fig. 1A). However, in the presence of LMB, NPM failed to shuttle and was restricted to human nucleoli within heterokaryons (92% inhibition; Fig. 1B). The addition of LMB did not hinder the nucleocytoplasmic trafficking of Myc-NPC-M9, an hnRNP that readily shuttles in a CRM1-independent nuclear export pathway (38).
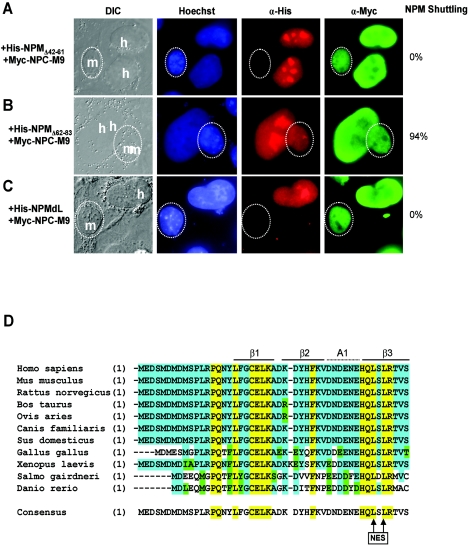
A sequence alignment of NPM residues with known CRM1-dependent shuttling proteins revealed two motifs containing short leucine-rich hydrophobic stretches of amino acids characteristic of CRM1-dependent nuclear export sequences (NESs) (Fig. 1C) (14, 15). In order to identify which region(s) of NPM contains its NES, we generated deletion mutants of NPM lacking either of the two potential NESs (NPMΔ42-61 and NPMΔ62-83). Using these NPM constructs, we again conducted interspecies heterokaryon assays. As shown in Fig. 2A, deletion of amino acids 42 to 61 of NPM (His-NPMΔ42-61) prevented its shuttling (100% inhibition) to mouse nucleoli. Importantly, a myc-tagged NPC-M9 shuttling control readily shuttled in the same human-mouse heterokaryon, indicating that these heterokaryons were not impaired for nucleocytoplasmic shuttling in general. In contrast, deletion of amino acids 62 to 83 of NPM (His-NPMΔ62-83) did not prevent NPM from shuttling between human and mouse nucleoli (6% inhibition; Fig. 2B), revealing that the putative NES resides within amino acids 42 to 61 of the NPM protein.
FIG. 2.
Leucine 42 and leucine 44 are identified as critical nuclear export residues. NIH 3T3 cells were seeded onto HeLa cells that had been transfected with (A) His-NPMΔ42-61, (B) His-NPMΔ62-83, or (C) NPMdL in combination with Myc-NPC-M9. Ectopic NPM proteins and Myc-NPC-M9 proteins were visualized with antibodies against His (red) and Myc (green), respectively. DNA was stained with Hoechst. Mouse nuclei are demarcated with dotted circles. Human and mouse nuclei are labeled h and m, respectively. These data are representative of at least five independent heterokaryons formed for each transfection condition in three independent experiments. The percentages of His-NPM shuttling in heterokaryons are given. DIC, differential interference contrast; α, anti. (D) Sequence alignment of NPM homologues throughout evolution. Identical residues in all species are marked yellow, identical residues in at least seven species are highlighted blue, and conserved residues are marked green. Crystal structure features are identified above the sequences. The consensus NPM sequence for all 11 identified homologues is given, with conserved nuclear export leucines 42 and 44 marked with arrows (NES).
Since the type of NES recognized and bound by the CRM1 export receptor is dependent on closely spaced hydrophobic amino acids (particularly leucines) (14, 15), we introduced point mutations into the corresponding leucine residues within the NES of NPM (Leu-42 and Leu-44 to Ala-42 and Ala-44). First, we tested this NPM mutant (designated NPMdL for double-leucine mutant) with Myc-NPC-M9 as a shuttling control. As expected, NPMdL was unable to transit from a human nucleus to the cytoplasm and into a murine nucleus (100% inhibition), indicating that these two leucine residues are critical for nuclear export of the NPM protein (Fig. 2C). Sequence alignment of numerous nucleophosmin homologues underscores the evolutionary importance of this amino-terminal export motif as it is nearly identical from zebra fish to humans (Fig. 2D).
Heterogeneous complexes containing NPM NES mutants and wild-type NPM fail to shuttle.
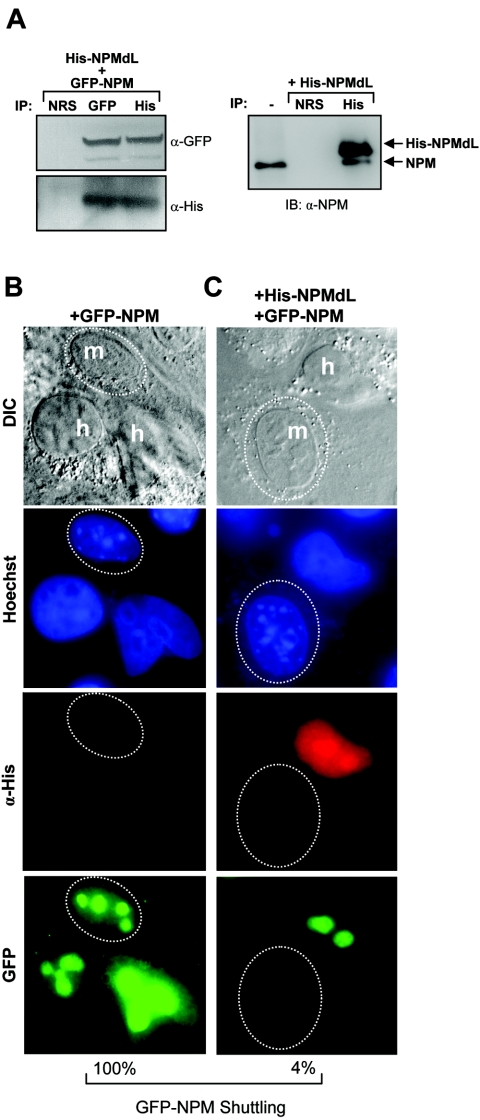
Because NPM readily self-oligomerizes (32, 33, 34, 53), we considered the possibility that mutant NPM molecules could form hetero-oligomers with wild-type NPM proteins. To test this hypothesis, HeLa cells were transduced with His-tagged NPMdL expression vectors. Immunoprecipitation of His-NPMdL proteins revealed the coprecipitation of wild-type endogenous NPM proteins, demonstrating the formation of mutant-wild-type hetero-oligomers in cells (Fig. 3A, right). Additionally, cells transduced with His-NPMdL and GFP-NPM displayed formation of hetero-oligomers, as observed by coprecipitation of both proteins using antibodies directed at either epitope tag (His or GFP; Fig. 3A, left). Given our finding that mutant NPM forms oligomers with wild-type NPM, we next examined whether the NPM shuttling mutant NPMdL could also block wild-type NPM from shuttling. In the absence of the shuttling mutant, GFP-tagged NPM readily shuttled from human to mouse nucleoli (Fig. 3C). However, in the presence of His-tagged NPMdL, GFP-NPM was retained in human nuclei (Fig. 3D; 96% inhibition). Although we were unable to determine the exact stoichiometry between mutant proteins and wild-type proteins in the NPM oligomer, it is clear that overexpression of NPMdL severely impaired the shuttling activity of nearly all NPM oligomers.
FIG. 3.
NPM shuttling mutants act as dominant negative inhibitors of NPM nuclear export. (A, left) HeLa cells transduced with His-NPMdL and GFP-NPM were lysed, and the whole-cell lysate was subjected to immunoprecipitation (IP) with NRS or antibodies recognizing His and GFP epitopes. Precipitated protein complexes were separated by SDS-PAGE, and ectopic NPM proteins were visualized with antibodies against GFP and His epitopes. (A, right) HeLa cells transfected with His-NPMdL were lysed, and the whole-cell lysate was subjected to immunoprecipitation with NRS or antibodies recognizing His epitopes. Precipitated protein complexes were separated by SDS-PAGE, and ectopic mutant and endogenous wild-type NPM proteins were visualized with antibodies against NPM. Untransfected HeLa whole-cell lysate was loaded as a marker for endogenous NPM expression (lane 1). IB, immunoblot; α, anti; DIC, differential interference contrast. NIH 3T3 cells were seeded onto HeLa cells that had been transfected with GFP-NPM (B) alone or (C) in combination with His-NPMdL. Heterokaryon assays were performed, and His-NPMdL and GFP-NPM proteins were visualized with antibodies against His (red) or naturally emitting GFP spectra (green). DNA was stained with Hoechst. Mouse nuclei are demarcated with dotted circles. Human and mouse nuclei are labeled h and m, respectively. These data are representative of at least five independent heterokaryons formed for each transfection condition in three independent experiments. The percentages of GFP-NPM shuttling in heterokaryons are given.
NPM associates with cytoplasmic and nuclear rpL5 ribosome complexes.
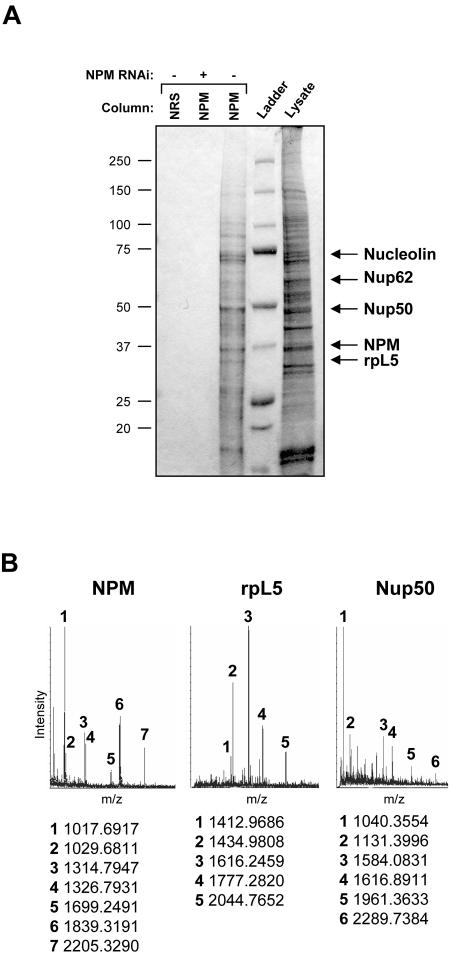
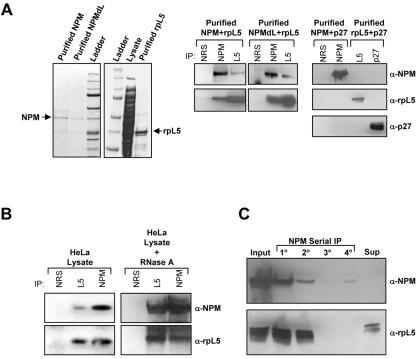
Previous studies have indicated that NPM might function as an integral component of ribosome maturation through its RNA binding activities (36). However, most hypotheses in this regard are largely based on the fact that NPM is nucleolar and, thus, most likely to be involved in the major process in the nucleolus: ribosome biogenesis. To formally test the nucleolar function of NPM, we examined the composition of in vivo NPM protein complexes in HeLa cell lysates. We generated a custom NPM polyclonal antibody affinity column and used a control nonimmune immunoglobulin column to preclear protein lysates. NPM complexes were eluted with increasing salt concentrations and visualized following SDS-PAGE and SYPRO-Ruby staining. As seen in Fig. 4A, we observed very little protein bound to our nonimmune rabbit serum column (lane 1). However, some proteins (∼18) were specifically eluted from the NPM antibody column (lane 3), including NPM and the previously known binding protein nucleolin. To determine whether the eluted proteins were in fact bound to the column through their interaction with NPM, we depleted NPM from HeLa cells using NPM-targeted RNA interference. Knockdown of NPM resulted in a loss of specific proteins bound and eluted from the NPM antibody column, demonstrating that our identified NPM protein complex is specific for NPM (lane 2). Protein bands were excised and identified using MALDI-TOF and tandem mass spectrometry analyses. Among those proteins bound to NPM, a cluster of proteins associated with ribosome biogenesis, including rpL5 and nucleolin, as well as the nuclear pore complex proteins Nup50 and Nup62, were identified (Fig. 4A and B), with nucleolin (C23) being the only known NPM binding protein (26, 27). Western blot analysis of NPM protein complexes verified the presence of these proteins in salt-eluted fractions (data not shown).
FIG. 4.
Isolation of endogenous NPM protein complexes. (A) HeLa cell lysates (600 μg) transduced with control (−) or NPM-directed RNA interference (RNAi) constructs (+) were injected onto either an NRS column or custom NPM polyclonal antibody affinity columns and eluted with an increasing NaCl gradient (0.1 to 1.0 M). Eluted proteins were separated by SDS-PAGE and visualized with Coomassie blue stain. Identified bands are labeled. (B) Representative MALDI-TOF spectra of labeled protein bands from panel A are shown with labeled matching peptide masses.
Given the novelty and potentially significant ribosome biology of finding rpL5 in the NPM complex, we focused on verifying the NPM-rpL5 interaction. Purified recombinant NPM, NPMdL, and rpL5 proteins (Fig. 5A, left panels) mixed overnight were coprecipitated (Fig. 5A, middle panels), demonstrating that the NPM-rpL5 interaction is direct and independent of the NPM nuclear export signal. To show that the interaction of recombinant proteins was specific, NPM and rpL5 were mixed overnight with recombinant p27kip1 proteins (equally charged proteins not bound to the NPM antibody column). Precipitated proteins exhibited no complex formation between NPM and p27kip1 or rpL5 and p27kip1 (Fig. 5A, right panels). Both NPM and rpL5 readily interact with RNAs through conserved nucleic acid binding domains. To determine whether RNA binding is required for the NPM-rpL5 interaction, HeLa lysates were subjected to RNase A treatment prior to coprecipitation of NPM-rpL5 complexes. Even in the presence of RNase A, NPM and rpL5 visibly formed in vivo protein complexes (Fig. 5B) indistinguishable from those from untreated cells and consistent with our earlier finding that the interaction can be recapitulated with purified recombinant proteins (Fig. 5A). While NPM and rpL5 formed complexes in vivo, serial immunoprecipitation of NPM proteins from HeLa lysates showed that NPM and rpL5 are not exclusive partners. We failed to detect rpL5 in some NPM complexes (Fig. 5C, 3o and 4o), and we also noted that there was a significant amount of rpL5 free from NPM complexes in the remaining supernatant (Fig. 5C, Sup), indicating that both NPM and rpL5 can exist in complexes independent of one another.
FIG. 5.
NPM interacts directly with rpL5. (A, left) Recombinant NPM, NPMdL, and rpL5 were purified from bacterial lysates using Ni-nitrilotriacetic acid affinity chromatography. Purified proteins were separated by SDS-PAGE and detected with Coomassie blue stain. (A, middle) Purified NPM or NPMdL proteins were incubated overnight with rpL5 and immunoprecipitated (IP) with NRS or antibodies recognizing NPM or rpL5. Precipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with NPM and rpL5 antibodies. (A, left) Purified NPM or rpL5 proteins were incubated overnight with recombinant p27 and immunoprecipitated with NRS or antibodies recognizing NPM, rpL5, or p27. Precipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with NPM, rpL5, and p27 antibodies. α, anti. (B) Proteins from HeLa cell lysates were immunoprecipitated with NRS, rpL5 antibody, or NPM antibodies. Precipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with NPM and rpL5 antibodies. Alternatively, HeLa lysates were pretreated for 1 h with RNase A prior to immunoprecipitation as described above. (C) HeLa lysates were subjected to serial immunoprecipitation with NPM antibodies (lanes 1o to 4o). Precipitated proteins and proteins in the final supernatant (unbound) were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies recognizing NPM and rpL5.
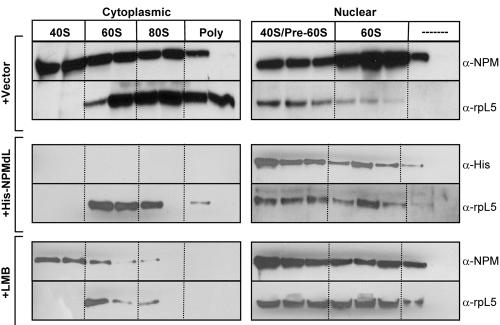
Having identified a critical member of the 60S ribosomal subunit, namely, rpL5, in NPM complexes, we wanted to evaluate the colocalization of NPM with ribosomes in vivo. In order to follow the spatial control of NPM-rpL5 complexes in vivo, we utilized the UV absorbance of the ribosome. Ribosomal protein L5 is known to supply the maturing 60S ribosomal subunit with 5S rRNA prior to nucleolar/nuclear export of the 60S subunit (47), providing NPM an ideal time to form nucleolar complexes with rpL5. Cytoplasmic and nuclear extracts of HeLa cells were subjected to sucrose gradient centrifugation, and the gradients were fractionated with continuous UV monitoring. As shown in Fig. 6, NPM associates with the 40S, 60S, 80S, and polysome fractions in the cytoplasm while nuclear pools of NPM associate with the 40S/pre-60S and 60S fractions in the nucleus. Consistent with previous reports (30), we found rpL5 associated with the 60S, 80S, and polysome fractions in the cytoplasm and the 40S/pre-60S and 60S fractions in the nucleus (Fig. 6). These data demonstrate that NPM and rpL5 are localized with the maturing 60S ribosomal subunits in the nucleus and are maintained in the mature ribosome once it reaches the cytosol. They also indicate that NPM also associates with the 40S subunit, which is devoid of rpL5 (Fig. 6) (30).
FIG. 6.
NPM and rpL5 colocalize with nuclear and cytosolic ribosome subunits. HeLa cells transduced with vector (top panels) or His-NPMdL (middle panels) or treated with LMB (bottom panels) were divided into cytoplasmic and nuclear fractions and subjected to sucrose gradient centrifugation. Absorbance was monitored at 254 nm, and fractions containing 40S, 60S, 80S, and polysome units were collected. Proteins from each fraction were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies recognizing NPM, the His epitope, and rpL5. α, anti.
Transduction of HeLa cells with His-NPMdL resulted in a dramatic redistribution of rpL5 in cytosolic ribosomes; rpL5 was maintained in the 60S subunits but severely reduced in 80S ribosomes (Fig. 6, middle left panels). Given these findings, we cannot rule out the possibility that rpL5 proteins are still capable of some NPM-independent shuttling. However, it is more likely that rpL5 association with cytosolic ribosomes in the presence of NPMdL is a result of preexisting, stable cytosolic rpL5 complexes. This notion is further substantiated by treatment of HeLa cells with LMB. LMB treatment yielded results that were consistent with NPMdL overexpression (Fig. 6, lower panel). Both NPM and rpL5 proteins were found in the cytosol of LMB-treated cells (at reduced levels), even though the nuclear export of both proteins is LMB sensitive. This finding suggests that some preexisting cytosolic NPM and rpL5 ribosome complexes are fairly stable (∼24 h) and that, if either protein utilizes CRM1-independent export, it is minimal.
NPM is required for rpL5 nuclear export.
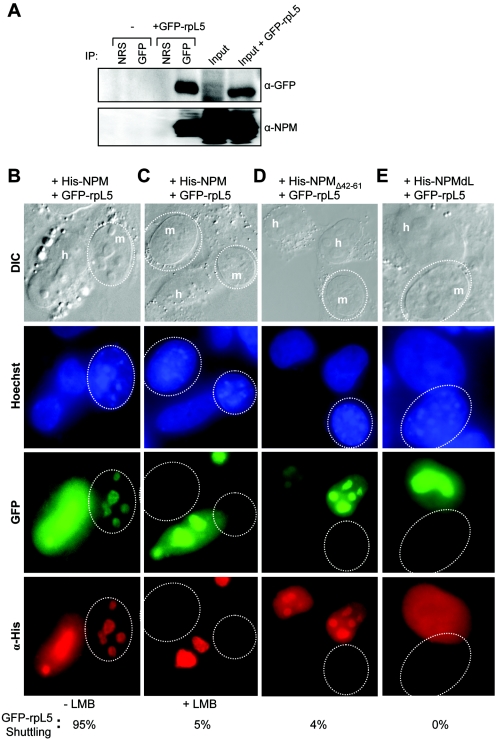
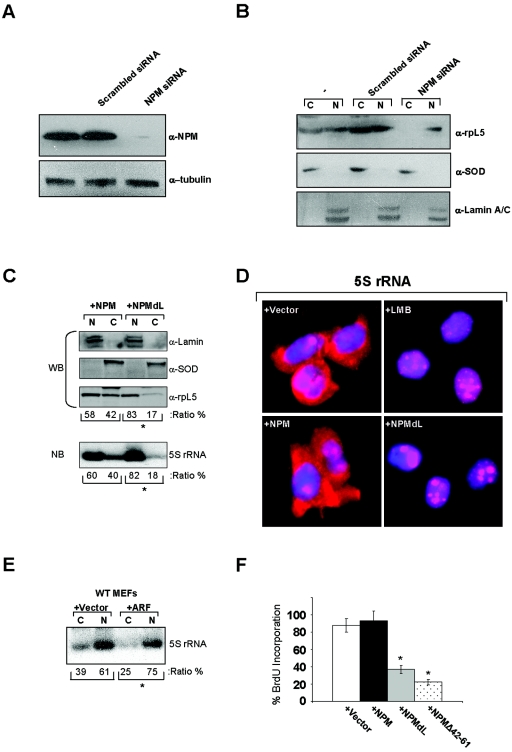
Having demonstrated a reduction of rpL5 associated with cytosolic ribosome subunits in the absence of NPM nuclear export signals, we next examined the direct influence of NPM shuttling mutants on rpL5 nuclear export using a previously characterized GFP-tagged rpL5 protein (41). To confirm that GFP-rpL5 retained the NPM-binding properties of the endogenous rpL5 protein, we transiently overexpressed GFP-rpL5 in HeLa cells and performed Western blot analysis of GFP-immunoprecipitated complexes. As shown in Fig. 7A, precipitated GFP-rpL5 complexes contained endogenous NPM, confirming that the GFP moiety does not adversely affect the formation of NPM-rpL5 complexes in vivo. GFP-rpL5 and His-NPM readily migrated from human nucleoli to mouse nucleoli, as visualized in interspecies heterokaryons (Fig. 7B). However, in the presence of LMB, both GFP-rpL5 and His-NPM failed to shuttle (95% inhibition; Fig. 7C). Introduction of NPM shuttling mutant NPMΔ42-61 or NPMdL inhibited GFP-rpL5 shuttling into mouse nucleoli, restricting its expression to human nuclei (Fig. 7D and E; 96% and 100% inhibition, respectively), establishing that NPM nuclear export is required for the export of rpL5. To more definitively show that NPM is required for rpL5 nuclear export, we knocked down NPM expression in HeLa cells (Fig. 8A). Cells lacking NPM protein expression failed to accumulate rpL5 in the cytoplasm, while cells transduced with scrambled small interfering RNA (siRNA) as a control exhibited an equal distribution of rpL5 between the nucleus and cytoplasm (Fig. 8B). These data underscore the necessity of NPM proteins for the efficient transport of rpL5 out of the nucleus and into the cytoplasm.
FIG. 7.
NPM nuclear export signals are required for the efficient export of GFP-rpL5. (A) HeLa cells either untransfected or transfected with GFP-tagged L5 for 48 h were harvested and lysed. Proteins were immunoprecipitated (IP) with NRS or a rabbit GFP antibody. Precipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with GFP and NPM antibodies. α, anti; DIC, differential interference contrast. Loading inputs are indicated. (B to E) NIH 3T3 cells were seeded onto HeLa cells that had been transfected with GFP-rpL5 in combination with (B and C) His-NPM, (D) His-NPMΔ42-61, and (E) His-NPMdL. Additionally, HeLa cells in panel C were treated with LMB for 18 h prior to fusion. Heterokaryon assays were performed with NPM and GFP-rpL5 proteins being visualized with antibodies against His (red) and naturally emitting GFP spectra (green), respectively. DNA was stained with Hoechst. Mouse nuclei are demarcated with dotted circles. Human and mouse nuclei are labeled h and m, respectively. These data are representative of at least five independent heterokaryons formed in three independent experiments. The percentages of heterokaryons exhibiting GFP-rpL5 shuttling are given.
FIG. 8.
NPM is essential for rpL5 nuclear export. (A) HeLa cells (−) or cells transduced with siRNAs encoding either scrambled control or NPM-specific sequences were harvested 72 h posttransduction for Western blot analysis. Proteins separated by SDS-PAGE were transferred to PVDF membranes and immunoblotted with antibodies recognizing NPM and γ-tubulin. α, anti. (B) HeLa cells (−) or cells transduced with siRNAs encoding either scrambled control or NPM-specific sequences were harvested 72 h posttransduction for cellular fractionation. Proteins from nuclear (N) and cytosolic (C) fractions were analyzed by SDS-PAGE and immunoblotted with antibodies recognizing rpL5, SOD (cytoplasm control), and lamin A/C (nuclear control). (C) HeLa cells were transfected with His-NPM or His-NPMdL, and 24 h later equal numbers of cells were subjected to fractionation into cytoplasmic (C) and nuclear (N) extracts. L5 protein was detected by Western blot analysis (top panels; WB). Lamin A/C and SOD are shown as nuclear and cytoplasmic fractionation controls, respectively (top panels; WB). 5S rRNA was detected by Northern blot analysis of total RNA extracted from the nuclear and cytosolic fractions (bottom panel; NB). The ratios of nuclear to cytoplasmic accumulation of rpL5 and 5S rRNA are given as percentages of totals (*, P > 0.001). (D) HeLa cells were transfected with vector, His-NPM, or His-NPMdL, and cells were plated on glass coverslips. Twenty-four hours later, cells were subjected to RNA FISH with a TRITC-labeled 5S rRNA probe. Nuclei were stained with DAPI. Untransfected HeLa cells were treated with LMB for 18 h prior to RNA FISH analysis. Results of 5S rRNA localization are each representative of three independent experiments. (E) Wild-type (WT) MEFs were infected with control retroviruses or those encoding ARF, and 48 h later equal numbers of cells were subjected to fractionation into cytoplasmic (C) and nuclear (N) extracts. 5S rRNA was detected by Northern blot analysis of total RNA extracted from the nuclear and cytosolic fractions (*, P > 0.005). (F) HeLa cells were transfected with vector, His-NPM, His-NPMdL, or His-NPMΔ42-61 and plated on glass coverslips. Cells were incubated with BrdU 72 h posttransfection and fixed 20 h later. Fixed cells were stained with antibodies recognizing BrdU and His epitopes and visualized by immunofluorescence using fluorescein isothiocyanate- and TRITC-labeled secondary antibodies, respectively. Cells (100) were counted for each condition in triplicate. Standard deviations are reported as error bars (*, P > 0.005).
Ribosomal protein L5 is known to bind specifically to the mature 5S rRNA and aid in its nucleocytoplasmic transport (31, 37, 47). We hypothesized that NPM export, through its influence on rpL5, is the critical determinant for 5S rRNA nuclear export. To test this hypothesis, we performed Northern blot analysis of similar cellular fractions in the presence and absence of NPM shuttling. Indeed, in the presence of dominant negative NPM shuttling mutants, 5S rRNA failed to accumulate in the cytosol and instead was retained in the nucleus in a ratio similar to that for rpL5 (Fig. 8C). In addition to our fractionation studies, we performed RNA FISH to visualize the localization of steady-state levels of 5S rRNA. As shown in Fig. 8D, 5S rRNA was distributed throughout the nucleoli/nuclei and cytoplasm of HeLa cells transduced with empty vector as well as with wild-type NPM. Consistent with our fractionation data, inhibition of NPM and rpL5 nuclear export with LMB or NPMdL resulted in a severe attenuation of 5S rRNA export to the cytosol (Fig. 8D).
To further expand on this theme, we transduced wild-type MEFs with ARF, a known inhibitor of NPM nuclear export (7). Again, in the presence of the ARF tumor suppressor, 5S rRNA failed to transit to the cytosol and instead was retained the nucleoplasm (Fig. 8E). These data imply that ARF and NPM mutants defective in shuttling act similarly to prevent rpL5-5S rRNA nuclear export. To determine whether NPM shuttling mutants also prevent cell cycle progression, HeLa cells transduced with NPM expression constructs were labeled with 5-bromodeoxyuridine (BrdU) to measure active DNA synthesis. Similar to ARF's known cell cycle arrest properties (7), cells expressing NPMdL or NPMΔ42-61 failed to enter S phase (Fig. 8F). Thus, NPM shuttling activity is not only required for the nuclear export of the rpL5-5S rRNA complex but also necessary for continued cell proliferation.
DISCUSSION
The nucleolus, a highly specialized and structured organelle, has been described as the cell's control center for ribosomal synthesis, maturation, and assembly, with a host of proteins, RNAs, and other factors being implicated in these processes (8). Recently, numerous proteins (cdc14, NPM, cyclin E, Mybbp1a, telomerase reverse transcriptase, and others) have been shown to continuously shuttle from the nucleolus to various subcellular compartments in a regulated manner, providing evidence that the nucleolus is a dynamic site of multiple cellular events (4, 7, 21, 22, 52).
One such protein, NPM/B23, has been linked to a variety of important cellular processes, both in and out of the nucleolus, including ribosome processing, molecular chaperoning, maintenance of genomic integrity, centrosome duplication, and transcriptional regulation (9, 10, 13, 16, 23, 35). Initially, NPM which was imported into the nucleolus from the cytoplasm was presumed to move about the various compartments of the nucleus (6), a feature shared by many critical cell cycle regulators. This shuttling of proteins between the nucleus and cytoplasm is now recognized as a key mechanism for ensuring proper cell cycle progression (39, 43). In previous reports, we and others identified NPM as a novel p53-independent target of the ARF tumor suppressor protein (5, 7, 19). We have since shown that, in response to hyperproliferative signals, nucleolar ARF directly binds NPM, effectively inhibiting NPM's nucleocytoplasmic shuttling. Here, we have further explored the mechanism and significance of NPM intracellular trafficking. First, we have described the CRM1-dependent nuclear export of NPM, identifying the two leucine residues (42 and 44) that are critical to this process. In addition, we have shown that alteration of the NPM NES resulted in the failure of wild-type NPM to be exported out of the nucleolus, providing evidence that these mutations function in a dominant-negative fashion, through the formation of NPM-NPMdL heteromultimers. Thus, NPMdL mimics the effects of ARF induction by directly impeding the nucleocytoplasmic shuttling of NPM through direct interaction, further demonstrating that NPM must exit the nucleolus/nucleus to maintain and promote cell growth.
We have previously proposed that targets of nucleolar sequestration might in fact “ride the ribosome” from the nucleolus to the cytoplasm to engage in growth-promoting events (45). In agreement with this hypothesis, our findings reveal a direct interaction between NPM and rpL5, providing the first physical link between NPM and ribosomal subunits. Much of the field's focus has been on the putative role of rpL5 in delivering 5S rRNA to the nucleolus, following the initial transcription of 5S rRNA by RNA polymerase III in the nucleoplasm (31, 37, 47). However, it is also possible that rpL5 is a critical player in the export of the large ribosomal subunit (60S), containing 5S rRNA, from the nucleolus/nucleus to the cytoplasm after its assembly. Clearly, the latter events would render themselves sensitive to NPM regulation, given that NPM provides the necessary export signals and chaperoning capabilities (via rpL5) required to transport components of the ribosome to the cytosol. Indeed, inhibition of NPM nuclear export via deletion or mutation of its NES prevented the trafficking of rpL5, an integral component of the 60S ribosomal subunit. Moreover, reduction of NPM expression through RNA interference completely abolished the cytosolic stores of rpL5, underscoring the absolute requirement for NPM in rpL5 nuclear export. Thus, our initial hypothesis of “riding the ribosome” should be revised to “taking the ribosome for a ride.”
While many components of the ribosome, including rpL5, encode their own NESs, it is clear that a single NES forms a relatively weak interaction with CRM1 (25), suggesting a requirement for additional NESs in the efficient export of complexes. Consequently, proteins like NPM and NMD3 may have evolved to serve this purpose. Additionally, NPM and rpL5 were found, in reduced amounts, in cytosolic 40S and 60S complexes, respectively, after LMB treatment, implying either that these particular protein-ribosome complexes are fairly stable or that minor fractions of NPM and rpL5 utilize CRM1-independent modes of transport from the nucleus. Considering that the predominant function of rpL5 is to bind and mobilize 5S rRNA molecules, it was not surprising that 5S transport was also NPM sensitive, and thus NPM contributes to the efficient nuclear export of rpL5-5S rRNA complexes. However, NPM was present in 40S, 60S, 80S, and polysomes in the cytoplasm, implying that NPM, free (within the 40S subunit) or bound to rpL5, remains associated with the mature ribosome as it assembles and forms actively translating polysomes in the cytosol. Taken together, these findings open up the possibility that NPM might transmit additional cues (beyond nuclear export) to cytosolic ribosomes during translation, consistent with nucleolus's proposed role in dictating translation rates (28).
While it has been appreciated for several decades that changes in nucleolar structure are reliable markers of cellular transformation, experiments that provide a direct link between nucleolar dysfunction and tumorigenesis remain to be conducted. In fact, the nucleolus has largely been dismissed as a static organelle, having little to no impact on the overall well-being of the cell. However, this “nucleolar stigma” recently has been challenged with the discovery that tumor suppressors, such as p53 and ARF, play a direct role in regulating nucleolar processes (5, 7, 42, 49). Interestingly, rpL5 is also a binding partner of Mdm2 and p53 (12, 17, 29), suggesting that rpL5 may provide an intriguing mechanistic link between ARF and ARF-binding partners. Clearly, through its interaction with NPM, ARF is capable of inhibiting nuclear export of rpL5-5S rRNA complexes. Inhibition of NPM-directed rpL5-5S nuclear export by ARF or NPM mutants defective in shuttling results in cell cycle arrest, demonstrating the importance of rpL5-5S export in maintaining cell proliferation. Moreover, NPM itself is a unique player in both the p53 and ARF responses (10, 11), providing us with a glimpse of how this network of protein interactions may inevitably become sensitive to oncogenic and tumor-suppressive signals in determining tumorigenic cell fates.
Acknowledgments
We thank J. Alan Diehl and Joachim Hauber for plasmid constructs as well as Loryn Rikimaru, Yijun Yi, and Myla Ashfaq for their excellent technical assistance. We are extremely grateful to Alan Diehl, Chuck Sherr, Greg Longmore, Helen Piwnica-Worms, David Gutmann, and Joseph Baldassare for insightful discussions.
Y.Y. and A.J.A. are recipients of a grant-in-aid from the Department of Defense Breast Cancer Research Program (BC030793). L.B.M. is supported by the Department of Defense Prostate Cancer Research Program under award number PC040009. H.L. is supported by a grant from the National Cancer Institute (CA-095441). J.D.W. thanks the Pew Charitable Trusts and is a recipient of grant-in-aid from the National Institutes of Health (GM-066032).
Views and opinions of, and endorsements by, the author(s) do not reflect those of the U.S. Army or the Department of Defense.
REFERENCES
- 1.Andersen, J. S., Y. W. Lam, A. K. Leung, S. E. Ong, C. E. Lyon, A. I. Lamond, and M. Mann. 2005. Nucleolar proteome dynamics. Nature 433:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J. S., C. E. Lyon, A. H. Fox, A. K. Leung, Y. W. Lam, H. Steen, M. Mann, and A. I. Lamond. 2002. Directed proteomic analysis of the human nucleolus. Curr. Biol. 12:1-11. [DOI] [PubMed] [Google Scholar]
- 3.Ashe, H. L., J. Monks, M. Wijgerde, P. Fraser, and N. J. Proudfoot. 1997. Intergenic transcription and transinduction of the human beta-globin locus. Genes Dev. 11:2494-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzam, R., S. L. Chen, W. Shou, A. S. Mah, G. Alexandru, K. Nasmyth, R. S. Annan, S. A. Carr, and R. J. Deshaies. 2004. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 305:516-519. [DOI] [PubMed] [Google Scholar]
- 5.Bertwistle, D., M. Sugimoto, and C. J. Sherr. 2004. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol. Cell. Biol. 24:985-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borer, R. A., C. F. Lehner, H. M. Eppenberger, and E. A. Nigg. 1989. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 56:379-390. [DOI] [PubMed] [Google Scholar]
- 7.Brady, S. N., Y. Yu, L. B. Maggi, Jr., and J. D. Weber. 2004. ARF impedes NPM/B23 shuttling in an Mdm2-sensitive tumor suppressor pathway. Mol. Cell. Biol. 24:9327-9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busch, H., and K. Smetana. 1970. The nucleolus. Academic Press, New York, N.Y.
- 9.Chan, W. Y., Q. R. Liu, J. Borjigin, H. Busch, O. M. Rennert, L. A. Tease, and P. K. Chan. 1989. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry 28:1033-1039. [DOI] [PubMed] [Google Scholar]
- 10.Colombo, E., P. Bonetti, E. Lazzerini Denchi, P. Martinelli, R. Zamponi, J. C. Marine, K. Helin, B. Falini, and P. G. Pelicci. 2005. Nucleophosmin is required for DNA integrity and p19Arf protein stability. Mol. Cell. Biol. 25:8874-8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo, E., J. C. Marine, D. Danovi, B. Falini, and P. G. Pelicci. 2002. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat. Cell Biol. 4:529-533. [DOI] [PubMed] [Google Scholar]
- 12.Dai, M. S., and H. Lu. 2004. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J. Biol. Chem. 279:44475-44482. [DOI] [PubMed] [Google Scholar]
- 13.Feuerstein, N., S. Spiegel, and J. J. Mond. 1988. The nuclear matrix protein, numatrin (B23), is associated with growth factor-induced mitogenesis in Swiss 3T3 fibroblasts and with T lymphocyte proliferation stimulated by lectins and anti-T cell antigen receptor antibody. J. Cell Biol. 107:1629-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda, M., S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, and E. Nishida. 1997. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308-311. [DOI] [PubMed] [Google Scholar]
- 16.Grisendi, S., R. Bernardi, M. Rossi, K. Cheng, L. Khandker, K. Manova, and P. P. Pandolfi. 2005. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 437:147-153. [DOI] [PubMed] [Google Scholar]
- 17.Guerra, B., and O. G. Issinger. 1998. p53 and the ribosomal protein L5 participate in high molecular mass complex formation with protein kinase CK2 in murine teratocarcinoma cell line F9 after serum stimulation and cisplatin treatment. FEBS Lett. 434:115-120. [DOI] [PubMed] [Google Scholar]
- 18.Hadjiolov, A. A. 1984. The nucleolus and ribosome biogenesis. Springer-Verlag, New York, N.Y.
- 19.Itahana, K., K. P. Bhat, A. Jin, Y. Itahana, D. Hawke, R. Kobayashi, and Y. Zhang. 2003. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol. Cell 12:1151-1164. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, A. W., E. Lund, and J. Dahlberg. 2002. Nuclear export of ribosomal subunits. Trends Biochem. Sci. 27:580-585. [DOI] [PubMed] [Google Scholar]
- 21.Juan, G., and C. Cordon-Cardo. 2001. Intranuclear compartmentalization of cyclin E during the cell cycle: disruption of the nucleoplasm-nucleolar shuttling of cyclin E in bladder cancer. Cancer Res. 61:1220-1226. [PubMed] [Google Scholar]
- 22.Keough, R. A., E. M. Macmillan, J. K. Lutwyche, J. M. Gardner, F. J. Tavner, D. A. Jans, B. R. Henderson, and T. J. Gonda. 2003. Myb-binding protein 1a is a nucleocytoplasmic shuttling protein that utilizes CRM1-dependent and independent nuclear export pathways. Exp. Cell Res. 289:108-123. [DOI] [PubMed] [Google Scholar]
- 23.Kondo, T., N. Minamino, T. Nagamura-Inoue, M. Matsumoto, T. Taniguchi, and N. Tanaka. 1997. Identification and characterization of nucleophosmin/B23/numatrin which binds the anti-oncogenic transcription factor IRF-1 and manifests oncogenic activity. Oncogene 15:1275-1281. [DOI] [PubMed] [Google Scholar]
- 24.Kudo, N., B. Wolff, T. Sekimoto, E. P. Schreiner, Y. Yoneda, M. Yanagida, S. Horinouchi, and M. Yoshida. 1998. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242:540-547. [DOI] [PubMed] [Google Scholar]
- 25.Kutay, U., and S. Guttinger. 2005. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 15:121-124. [DOI] [PubMed] [Google Scholar]
- 26.Li, Y. P., R. K. Busch, B. C. Valdez, and H. Busch. 1996. C23 interacts with B23, a putative nucleolar-localization-signal-binding protein. Eur. J. Biochem. 237:153-158. [DOI] [PubMed] [Google Scholar]
- 27.Liu, H. T., and B. Y. Yung. 1999. In vivo interaction of nucleophosmin/B23 and protein C23 during cell cycle progression in HeLa cells. Cancer Lett. 144:45-54. [DOI] [PubMed] [Google Scholar]
- 28.Maggi, L. B., Jr., and J. D. Weber. 2005. Nucleolar adaptation in human cancer. Cancer Investig. 23:599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marechal, V., B. Elenbaas, J. Piette, J. C. Nicolas, and A. J. Levine. 1994. The ribosomal L5 protein is associated with mdm-2 and mdm-2-p53 complexes. Mol. Cell. Biol. 14:7414-7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marion, M. J., and C. Marion. 1987. Localization of ribosomal proteins on the surface of mammalian 60S ribosomal subunits by means of immobilized enzymes. Correlation with chemical cross-linking data. Biochem. Biophys. Res. Commun. 149:1077-1083. [DOI] [PubMed] [Google Scholar]
- 31.Michael, W. M., and G. Dreyfuss. 1996. Distinct domains in ribosomal protein L5 mediate 5 S rRNA binding and nucleolar localization. J. Biol. Chem. 271:11571-11574. [DOI] [PubMed] [Google Scholar]
- 32.Namboodiri, V. M., I. V. Akey, M. S. Schmidt-Zachmann, J. F. Head, and C. W. Akey. 2004. The structure and function of Xenopus NO38-core, a histone chaperone in the nucleolus. Structure 12:2149-2160. [DOI] [PubMed] [Google Scholar]
- 33.Namboodiri, V. M., S. Dutta, I. V. Akey, J. F. Head, and C. W. Akey. 2003. The crystal structure of Drosophila NLP-core provides insight into pentamer formation and histone binding. Structure (Cambridge) 11:175-186. [DOI] [PubMed] [Google Scholar]
- 34.Namboodiri, V. M., M. S. Schmidt-Zachmann, J. F. Head, and C. W. Akey. 2004. Purification, crystallization and preliminary X-ray analysis of the N-terminal domain of NO38, a nucleolar protein from Xenopus laevis. Acta Crystallogr. Sect. D Biol. Crystallogr. 60:2325-2327. [DOI] [PubMed] [Google Scholar]
- 35.Okuda, M., H. F. Horn, P. Tarapore, Y. Tokuyama, A. G. Smulian, P. K. Chan, E. S. Knudsen, I. A. Hofmann, J. D. Snyder, K. E. Bove, and K. Fukasawa. 2000. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 103:127-140. [DOI] [PubMed] [Google Scholar]
- 36.Okuwaki, M., K. Matsumoto, M. Tsujimoto, and K. Nagata. 2001. Function of nucleophosmin/B23, a nucleolar acidic protein, as a histone chaperone. FEBS Lett. 506:272-276. [DOI] [PubMed] [Google Scholar]
- 37.Pieler, T., and F. Rudt. 1997. Nucleocytoplasmic transport of 5S ribosomal RNA. Semin. Cell Dev. Biol. 8:79-82. [DOI] [PubMed] [Google Scholar]
- 38.Pinol-Roma, S. 1997. HnRNP proteins and the nuclear export of mRNA. Semin. Cell Dev. Biol. 8:57-63. [DOI] [PubMed] [Google Scholar]
- 39.Piwnica-Worms, H. 1999. Cell cycle. Fools rush in. Nature 401:535, 537. [DOI] [PubMed] [Google Scholar]
- 40.Pollard, V. W., W. M. Michael, S. Nakielny, M. C. Siomi, F. Wang, and G. Dreyfuss. 1996. A novel receptor-mediated nuclear protein import pathway. Cell 86:985-994. [DOI] [PubMed] [Google Scholar]
- 41.Rosorius, O., B. Fries, R. H. Stauber, N. Hirschmann, D. Bevec, and J. Hauber. 2000. Human ribosomal protein L5 contains defined nuclear localization and export signals. J. Biol. Chem. 275:12061-12068. [DOI] [PubMed] [Google Scholar]
- 42.Rubbi, C. P., and J. Milner. 2003. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 22:6068-6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan, K. M., A. C. Phillips, and K. H. Vousden. 2001. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13:332-337. [DOI] [PubMed] [Google Scholar]
- 44.Scherl, A., Y. Coute, C. Deon, A. Calle, K. Kindbeiter, J. C. Sanchez, A. Greco, D. Hochstrasser, and J. J. Diaz. 2002. Functional proteomic analysis of human nucleolus. Mol. Biol. Cell 13:4100-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherr, C. J., and J. D. Weber. 2000. The ARF/p53 pathway. Curr. Opin. Genet. Dev. 10:94-99. [DOI] [PubMed] [Google Scholar]
- 46.Spector, D. L., R. L. Ochs, and H. Busch. 1984. Silver staining, immunofluorescence, and immunoelectron microscopic localization of nucleolar phosphoproteins B23 and C23. Chromosoma 90:139-148. [DOI] [PubMed] [Google Scholar]
- 47.Steitz, J. A., C. Berg, J. P. Hendrick, H. La Branche-Chabot, A. Metspalu, J. Rinke, and T. Yario. 1988. A 5S rRNA/L5 complex is a precursor to ribosome assembly in mammalian cells. J. Cell Biol. 106:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strezoska, Z., D. G. Pestov, and L. F. Lau. 2000. Bop1 is a mouse WD40 repeat nucleolar protein involved in 28S and 5.8S rRNA processing and 60S ribosome biogenesis. Mol. Cell. Biol. 20:5516-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugimoto, M., M. L. Kuo, M. F. Roussel, and C. J. Sherr. 2003. Nucleolar Arf tumor suppressor inhibits ribosomal RNA processing. Mol. Cell 11:415-424. [DOI] [PubMed] [Google Scholar]
- 50.Tao, W., and A. J. Levine. 1999. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc. Natl. Acad. Sci. USA 96:3077-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trotta, C. R., E. Lund, L. Kahan, A. W. Johnson, and J. E. Dahlberg. 2003. Coordinated nuclear export of 60S ribosomal subunits and NMD3 in vertebrates. EMBO J. 22:2841-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong, J. M., L. Kusdra, and K. Collins. 2002. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat. Cell Biol. 4:731-736. [DOI] [PubMed] [Google Scholar]
- 53.Yung, B. Y., and P. K. Chan. 1987. Identification and characterization of a hexameric form of nucleolar phosphoprotein B23. Biochim. Biophys. Acta 925:74-82. [DOI] [PubMed] [Google Scholar]