Abstract
The symbiosome of nitrogen fixing root nodules mediates metabolite exchange between endosymbiotic rhizobia bacteria and the legume host. In the present study, the ion currents of the symbiosome membrane of the model legume Lotus japonicus were analyzed by patch-clamp recording. Both excised and symbiosome-attached patches exhibited a large inward (toward the cytosolic side of the membrane) current that is activated in a time-dependent manner by negative (on the cytosolic side) potentials. Based on reversal potential determinations and recordings with the impermeant cation N-methyl-glucamine, this current shows a high permeability for monovalent cations with no apparent permeability for anions. The current also showed a finite Ca2+ permeability. However, the currents were predominantly carried by univalent cations with a slightly greater selectivity for NH4+ over K+. Increased Ca2+ concentration inhibited the current with a K0.5 for inhibition of 0.317 mm. The current showed strong rectification that is mediated by divalent cations (either Mg2+ or Ca2+). The influence of divalent cations is symmetrical in nature, because rectification can be exerted in either direction depending upon which side of the membrane has the highest concentration of divalent cations. However, based on observations with symbiosome-attached patches, the direction of the current in vivo is proposed to be toward the cytosol with cytosolic Mg2+ acting as the putative gating regulator. The findings suggest that L. japonicus possesses a voltage-dependent cation efflux channel that is capable of exporting fixed NH4+, and may also play an additional role in Ca2+ transport.
The formation of nitrogen fixing symbioses between legumes and Rhizobiaceae bacteria represents a developmental program that leads to the formation of a novel organ, the nodule, on the roots of the plant host. During the formation of the nodule, the bacteria infect the cells of the host and become enclosed within a specialized organelle, termed the “symbiosome” (Roth et al., 1988). The endosymbiotic bacteroids are separated from the infected plant cell cytosol by a membrane of plant origin, the symbiosome membrane. This membrane performs several functions, including protection of the endosymbionts from plant defense responses and serving as a selectively permeable membrane that controls metabolic flux and exchange between the host and symbiont (for review, see Udvardi and Day, 1997). The principal metabolic exchange that is mediated by this membrane is the efflux of fixed nitrogen (NH3 or NH4+) and the uptake of reduced carbon (e.g. dicarboxylates) from the plant cytosol to serve as an energy source to support the nitrogen fixation process (for review, see Udvardi and Day, 1997).
The mechanism of nitrogen efflux from the symbiosome to the plant cytosol has been the subject of much debate (for review, see Day et al., 2001). Initially, it was proposed that because a large concentration gradient of ammonia exists between the bacteroid and plant cytosol, the simple diffusion of uncharged NH3 across both the bacteroid and symbiosome membrane was adequate to account for the observed rates of nitrogen assimilation (Streeter, 1989; Udvardi and Day, 1990). More recently, this view of NH3 diffusion across the bilayer was revised, and a facilitated diffusion pathway was observed which was inhibited by mercurials (Niemietz and Tyerman, 2000). This observation, along with the previous finding that the symbiosome membrane major intrinsic protein nodulin 26 forms a mercurial-sensitive water and solute channel (Rivers et al., 1997; Dean et al., 1999), suggests that major intrinsic protein-mediated NH3 efflux may occur (Niemietz and Tyerman, 2000).
Besides these proposed pathways for NH3 diffusion, pathways for NH4+ transport have been detected on soybean symbiosome membranes by patch-clamp recording (Tyerman et al., 1995; Whitehead et al., 1998). These analyses show that soybean symbiosomes possess a voltage-activated cation channel that is capable of transporting NH4+. Because the concentration of NH4+ is proposed to be higher than that of NH3 within the acidic symbiosome space, this cation current may be important for the efflux of this charged species to the cytosol for assimilation (Tyerman et al., 1995; Whitehead et al., 1998).
Thus, there are multiple potential pathways for NH4+/NH3 efflux across the symbiosome membrane. To investigate the participation of these various pathways in the symbiosis, it would be advantageous to identify the proteins/activities involved and to study them in a genetically tractable organism in which their expression could be altered. For legumes such as soybean that form determinant nodules, Lotus japonicus has emerged as a model organism (Handberg and Stougaard, 1992). In the present work we have used patch-clamp recording to investigate the transport properties of the L. japonicus symbiosome membrane.
RESULTS
Voltage-Activated, Time-Dependent Current on L. japonicus Symbiosome Membranes
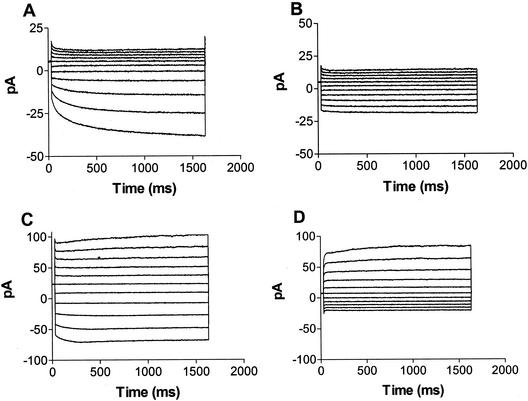
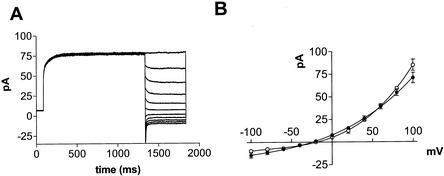
Reprentative records from inside-out, excised patches of L. japonicus symbiosomes are shown in Figure 1. Standard recording conditions used to characterize the patch currents included 20 mm NH4Cl in the bath, which faces the symbiosome lumen side of the membrane, and 150 mm KCl in the pipette, which faces the cytoplasmic side of the membrane. Under initial recording conditions (pipette 10 mm Ca2+, and bath 0.25 μm Ca2+) rectified, time-dependent inward currents (toward the pipette) are readily observed at negative applied potentials (Fig. 1A). Elevation of Ca2+ in the bath to 1 mm resulted in a reduction of the inward current (Fig. 1B), suggesting that Ca2+ within the pipette was responsible for the rectification observed under the standard recording conditions. This was verified by perfusion of the pipette with a solution containing low Ca2+ (Fig. 1, C and D). In recordings performed under conditions in which both the pipette and bath solutions possessed a low (0.25 μm) free Ca2+ concentration, rectification was lost and the time dependence of the current was substantially reduced (Fig. 1C). It is interesting that the direction of the rectification can be reversed by elevating the bath Ca2+ (Fig. 1D). These observations suggest that Ca2+ can exert its inhibitory influence in a symmetrical manner on either side of the patch.
Figure 1.
Time-dependent currents of excised inside-out patches of L. japonicus symbiosome membranes. Shown are current versus time traces of a representative inside-out symbiosome membrane patch of L. japonicus. The records represent currents recorded during 1.6-s voltage pulses at 20-mV intervals from +80 (top trace) to −120 mV (bottom trace). Under all conditions, the pipette contained 150 mm KCl and the bath contained 20 mm NH4Cl and the following differences in calcium concentration. A, Pipette solution containing 10 mm CaCl2 and the bath solution containing 0.5 mm CaCl2 and 1 mm EGTA (free Ca2+ = 0.25 μm); B, pipette solution containing 10 mm CaCl2 and the bath containing 2 mm CaCl2 and 1 mm EGTA (free Ca2+ = 1 mm); C, pipette perfused with pipette solution with 1 mm EGTA and bath solution containing 0.5 mm CaCl2 and 1 mm EGTA; and D, pipette perfused with pipette solution with 1 mm EGTA and bath solution containing 5 mm CaCl2 and 1 mm EGTA (free Ca2+ = 4 mm).
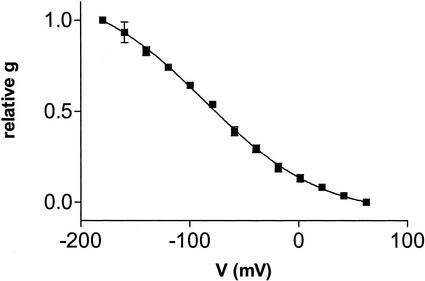
Voltage-activation profiles of inwardly rectified currents obtained with excised inside-out patches are shown in Figure 2. Stepwise decreases in potential from a holding potential of +60 mV resulted in an initial activation of the current at 0 mV with increased opening apparent as the potential becomes more negative, reaching saturation at −180 mV. Voltage-dependent activation is well fit with a Boltzmann relation, yielding a V0.5 of −86 mV (se = 2.7, n = 3). From the slope factor (−53.5; se = 3.15), the gating charge of the channel (zδ) was calculated to be 0.475.
Figure 2.
Voltage dependence of activation of time-dependent cation current. Recording conditions were 40 mm KCl and 10 mm CaCl2 in the pipette and 60 mm NH4Cl, 0.5 mm CaCl2, and 1 mm EGTA in the bath. Relative conductance was determined and was plotted against potential as described in “Materials and Methods.” The data represent the average ± se of data from three separate patches.
Rectification by Divalent Cations
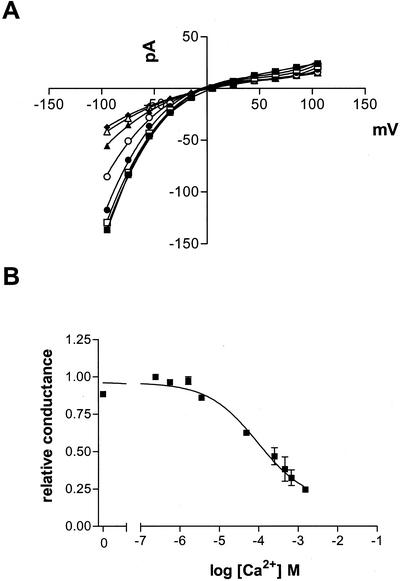
By elevating the calcium concentration in the bath under the standard recording conditions, the relative sensitivity of the inward current to calcium concentration was determined (Fig. 3). By plotting the relative conductance of the inward current versus the concentration of Ca2+, a K0.5 for inhibition of 0.317 mm (se = 0.17, n = 4) with a Hill coefficient of 0.7 was calculated. Most of the inward current is blocked by high Ca2+ concentrations and it could not be determined if the residual current was carried by monovalent cation or by Ca2+ because the currents approached that for the patch seal. Overall, the data suggest that current is gated by Ca2+, which can exert its influence from either side of the membrane.
Figure 3.
Calcium-dependent rectification of steady-state inward currents of L japonicus symbiosome membrane patches. A, Current-voltage plots were generated from steady-state currents obtained with a standard voltage pulse protocol similar to that shown in Figure 1. All recordings were performed with a pipette solution containing 40 mm KCl and 10 mm CaCl2 and a bath solution containing 20 mm NH4Cl and 1 mm EGTA in which CaCl2 was added to generate free Ca2+ concentrations of 0.25 μm (▪), 1.7 μm (□), 3.5 μm (●), 49 μm (○), 250 μm (▴), 460 μm (▵), and 670 μm (♦). B, Relative conductance at −100 mV as a function of free calcium concentration. The data were fit to a Hill equation.
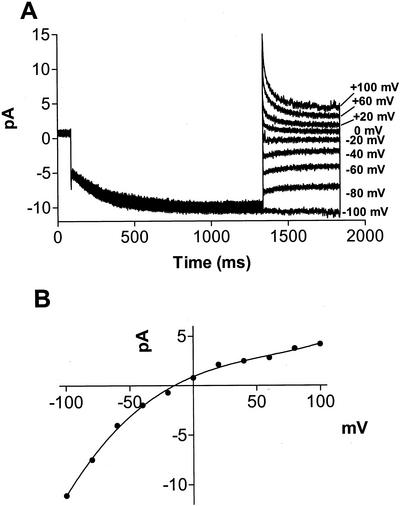
Because of the symmetrical nature of the rectification, it was uncertain whether this current flows in an inward (cations toward the cytosolic compartment) or an outward (cations toward the symbiosome space) manner under physiological conditions. To test this, the time-dependent current was evaluated in intact symbiosome-attached patches (Fig. 4). Symbiosome-attached membranes show voltage-activated, time-dependent openings at negative potentials. Current-voltage plots show that the current measured in symbiosome-attached patches exhibits a rectification similar to that observed in excised patches containing the same pipette solution. These findings suggest that the flow of current in intact symbiosomes is predominantly in the inward direction, and that the internal Ca2+ activity in the symbiosome space is low.
Figure 4.
Rectified time-dependent inward currents are observed in symbiosome-attached patches. Recording of intact symbiosome-attached patches were done with pipette solution containing 150 mm KCl and 10 mm CaCl2 and a bath solution containing 10 mm KCl, 0.5 mm CaCl2, and 1 mm EGTA (free Ca2+ = 0.25 μm). A, Tail currents were obtained by activating the time-dependent current with a 1.25 s pulse to −100 mV from a holding potential of 0 mV, followed by a step to the indicated potentials. B, Representative current-voltage plot of steady-state currents under the same recording conditions.
Based on previous studies with other divalent gated ion channels (Pei et al., 1999; Oliver et al., 2000), including the voltage-dependent cation channel previously characterized in soybean symbiosome membranes (Tyerman et al., 1995; Whitehead et al., 1998), it is clear that other cations besides Ca2+ can regulate gating. Because high Ca2+ concentrations are required to achieve high resistance seals with the symbiosome membrane (Whitehead et al., 1998), most recordings are done with high Ca2+ concentrations on the pipette side of the membrane. As shown in Figure 1, after obtaining a high resistance seal in excised patches, the pipette solution can be perfused with a low calcium containing solution and the effects of different cations could be evaluated by varying the bath solution. The substitution of Mg2+ for Ca2+ in the bath solution resulted in a channel with nearly identical properties with respect to voltage activation and rectification properties (Fig. 5), suggesting that either Mg2+ or Ca2+ can serve as the agent responsible for the rectification of the current. Similar to Ca2+, Mg2+ was able to exert rectification from the pipette side as well as the bath side of the membrane patch (data not shown).
Figure 5.
Magnesium and calcium show a similar affect on time-dependent currents of L. japonicus symbiosome membrane patches. Shown are the currents recorded with inside-out symbiosome membrane patches in which the standard pipette solution was perfused with 150 mm KCl, 1 mm EGTA, 10 mm HEPES-Tris, pH 7.0. A, The bath solution contained 20 mm NH4Cl, 1 mm EGTA, 2 mm MgCl2, and 10 mm HEPES-Tris, pH 7.0. Tail currents were recorded by activating the time-dependent current with a 1.25-s pulse to +100 mV from a holding potential of 0 mV, followed by a step to various potentials ranging from −100 to +100 mV (in 20-mV increments). B, Current-voltage plots of steady-state currents obtained with a bath solution of 20 mm NH4Cl, 1 mm EGTA, 2 mm MgCl2, and 10 mm HEPES-Tris, pH 7.0 (○) and 20 mm NH4Cl, 1 mm EGTA, 5 mm CaCl2, 10 mm HEPES-Tris, pH 7.0 (●).
Relative Permeabilities
To determine relative permeabilities, the concentration of NH4Cl or KCl in the bath was varied and the reversal potential was measured by using a tail current protocol as described in “Materials and Methods.” As the concentration of either NH4Cl or KCl was elevated in the bath, the reversal potential shifted to more positive potentials (Fig. 6, A–C) suggesting that the current is carried by the flux of cations from the bath (lumen) toward the pipette (cytosol). To test whether anion (i.e. Cl−) was transported, recordings were done under standard conditions (20 mm NH4Cl) supplemented with various concentrations of N-methyl-d-glucamine chloride. Because N-methyl-d-glucamine is a large, impermeant cation, this approach allowed us to evaluate the transport of Cl− independent of the cation concentration. The results showed that increasing the Cl− concentration did not affect the reversal potential of the voltage-activated current. Similar results were observed for other anions including nitrate and malate (data not shown).
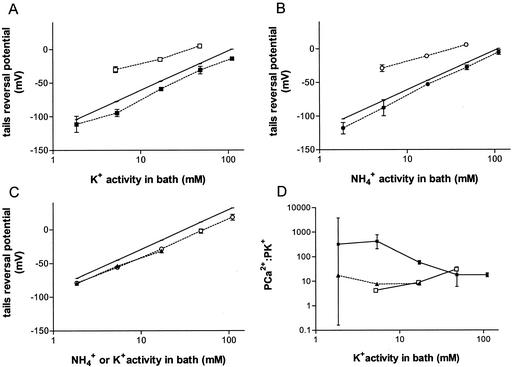
Figure 6.
Cation permeability of the time-dependent inward current of L. japonicus symbiosome membrane. Reversal potentials were calculated from tail currents of inside-out excised patches by using the tails recording protocol outlined in Figure 4. The error bars show the se of between two and six patches. A, Recordings with 150 mm KCl and 10 mm HEPES-Tris, pH 7.0 in the pipette, and a base bath solution of 10 mm HEPES-Tris, pH 7.0 in which the KCl concentration was altered. █, Outwardly directed Ca2+ gradient (pipette Ca2+ = 4.1 mm; bath Ca2+ = 126 nm); □, inwardly directed Ca2+ gradient (pipette Ca2+ = 340 nm; bath Ca2+ = 4 mm). The solid line drawn represents the Nernst potential for K+. B, Identical recording conditions, buffer, and Ca2+ activities as in A but with NH4Cl replacing KCl in the bath. C, Recordings as in A except with 40 mm KCl, 10 mm HEPES-Tris, pH 7.0, in the pipette with an outwardly directed Ca2+ gradient (pipette Ca2+ = 4.1 mm; bath Ca2+ = 126 nm). Plots are shown with KCl (▴) or NH4Cl (○) in the bath solution. D, The PCa2+ to PK+ ratios as a function of K+ activity of the bath: 150 mm KCl in pipette and outward Ca2+ gradient (█), 150 mm KCl in pipette and inward Ca2+ gradient (□), 40 mm KCl in pipette and outward Ca2+ gradient (▴).
The change in reversal potential as a function of the conductant in the bath paralleled the predicted Nernst potential for monovalent cations (Fig. 6, A–C). However, under conditions of an outward calcium gradient (4.1 mm Ca2+ in the pipette; 126 nm Ca2+ in the bath), the reversal potentials were consistently more negative than the predicted Nernst potential for monovalent cations (solid symbols, Fig. 6, A–C). The polarity of this discrepancy and the fact that there is an outward-directed gradient for Ca2+ suggests that there was a finite Ca2+ permeability in the time-dependent current. By using the Goldman–Hodgkin–Katz equation modified for Ca2+ permeability, we calculated a PCa2+ to PK+ ratio that was consistently greater than unity over the tested range of external monovalent K+ activities (Fig. 6D), ranging from a PCa2+:PK+ of 8.6 (40 mm KCl in the pipette) to >18 (150 mm KCl in the pipette). An enhanced PCa2+ to PNH4+ permeability ratio was also apparent when NH4+ was substituted for K+ in the bath (Fig. 6B).
Reversing the Ca2+ gradient across the patch and with 150 mm KCl in the pipette, the reversal potential became more positive than the Nernst potential for K+ (Fig. 6, A and B, white symbols), consistent with the symmetrical nature of the channel and the fact that calcium can interact from either side of the membrane. In this case a PCa2+:PK+ between 4 and 30 was calculated (Fig. 6D, white squares). The PNH4+ to PK+ ratio was calculated with the assumption that the calcium permeability ratio was independent of the monovalent cation present in the bath (i.e. PCa2+:PNH4+ = PCa2+:PK+). In all cases PNH4+:PK+ was slightly greater than one (1.1–1.3).
Relative Selectivity
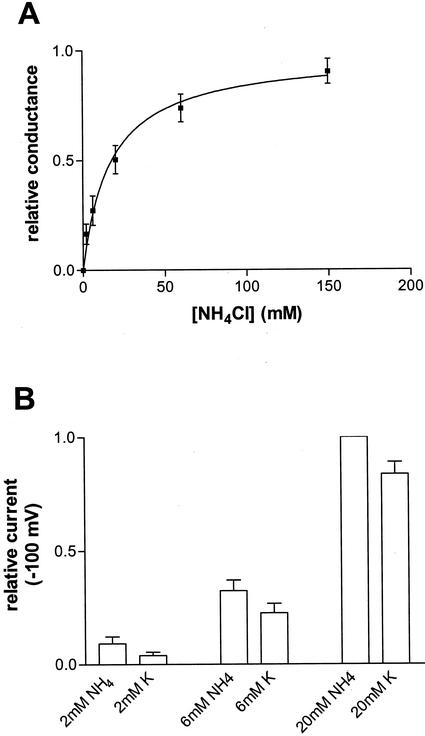
The magnitude of the currents was strongly dependent on the univalent cation concentration, emphasizing that it is predominantly carried by either K+ or NH4+. Consistent with the calculated PNH4+ to PK+ ratio discussed above, measurements of the relative conductance obtained for K+ and NH4+ show that slightly higher currents are obtained with NH4+ in the bath solution, particularly at lower concentrations of conductant (Fig. 7). A plot of relative conductance as a function of NH4Cl concentration (Fig. 7) yields an apparent Km of 17 mm (se = 2.9, n = 4).
Figure 7.
Comparison of the steady-state currents of NH4+ and K+. Recordings were done with excised inside-out patches of L. japonicus symbiosome membranes and a pipette solution containing 150 mm KCl and 10 mm CaCl2 and a bath solution containing 0.5 mm CaCl2, 1 mm EGTA (free Ca2+ = 0.25 μm), and the indicated concentrations of either NH4Cl or KCl. A, The relative conductance of NH4Cl at −100 mV of data for four separate patches fit to the Michalis-Menten equation: grel = [NH4]/Km + [NH4]. B, Comparisons of currents carried at −100 mV by K+ and NH4+ at 2, 6, and 20 mm.
DISCUSSION
Patch-clamp recording of excised patches of L. japonicus symbiosome membranes show a voltage-activated current that exclusively carries cations. The current is carried predominantly by monovalent cations (NH4+ and K+) although evidence for Ca2+ permeability was also observed. The monovalent cation current was strongly inhibited by Ca2+ and Mg2+ and these agents were responsible for the observed voltage dependence and rectification of the current. Rectification was observed both in the inward and outward direction, depending on which side of the membrane possessed the highest concentration of divalent cations. Despite the bidirectional nature of the current, measurements with symbiosome-attached patches suggest that the current would be inwardly rectifying in vivo. Based on previous observations (Tyerman et al., 1995) for a similar current observed in soybean symbiosome membrane, as well as preliminary nonstationary noise analysis of the L. japonicus current (data not shown), the data suggest that this symbiosome membrane current represents a subpicoSiemen cation channel that is voltage-gated by divalent cations. However, in contrast to soybean (Whitehead et al., 1998) the L. japonicus channel differs in its interaction with Ca2+ and also exhibits permeability to Ca2+.
Voltage-Dependent Gating and Rectification
Inwardly rectifying K+ channels can be divided into two classes: those that possess an intrinsic gate that is part of the integral structure of the channel, and those that are regulated by the association of small gating charged particles, such as divalent cations or polyamines (for review, see Schroeder et al., 1994; Reimann and Ashcroft, 1999; Schachtman, 2000). Most higher plant inward-rectifying cation channels (e.g. KAT1, AKT1) belong to the former category (Schachtman, 2000). In contrast, the voltage-dependent properties and rectification of the symbiosome-membrane cation channel requires the presence of a divalent cation, similar to animal inwardly rectifying K channels (e.g. Kir), which also lack an intrinsic gate (Reimann and Ashcroft, 1999; Oliver et al., 2000).
The finding that gating of the symbiosome membrane cation channel occurs in either direction suggests that divalent cations can bind from either end of the channel and that the flow of ions could theoretically proceed in either direction. However, measurements with symbiosome-attached patches show inward rectification (i.e. flow of cations toward the cytosol). This is somewhat surprising since the concentration of Ca2+ within the symbiosome space has been reported to be high (Udvardi and Day, 1997). Since significant inward current is observed with intact symbiosomes, it argues that the concentration of “free” Ca2+ is likely low, presumably by buffering by Ca2+ binding proteins or binding to other sites within the symbiosome space. This observation also suggests that although the gating is symmetrical in nature, the direction of transport favored is toward the cytosol.
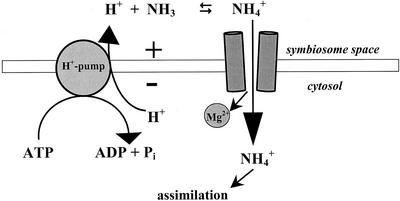
The divalent cation-dependent properties of voltage activation and inward rectification are similar to those previously described for the soybean symbiosome membrane cation channel (Tyerman et al., 1995; Whitehead et al., 1998). A model for the regulation of this channel (Whitehead et al., 1998) has been proposed in which cytosolic Mg2+ (likely to be present at mm concentrations in excess of the Kd of the channel for divalent cations), or perhaps other cytosolic cations such as a polyamines (Whitehead et al., 2001), serve as gating particles that diffuse into the channel pore and block current in an outward direction at positive potentials. Upon hyperpolarization of the symbiosome membrane, the gating particle would be displaced in a time-dependent manner resulting in channel opening and cation influx (Fig. 8). The low Hill coefficient for divalent cation inhibition and the calculated gating charge are consistent with one divalent cation binding site, similar to the animal Kir channel (for review, see Oliver et al., 2000).
Figure 8.
Model for ammonium/cation transport through the symbiosome membrane inward rectifying channel. Shown diagrammatically is the electrogenic H+-ATPase pump that hyperpolarizes the symbiosome membrane, resulting in the opening of the time-dependent ammonium permeable channel that is inwardly rectified by cytosolic magnesium block.
Transport Selectivity and Physiological Function
The low selectivity between NH4+ and K+ and the relatively high Km for cation transport suggests that the symbiosome cation channel activity is distinct from the high affinity ammonium (e.g. the AMT family) and potassium (e.g. HKT) uptake channels that show higher specificity and operate at micromolar concentrations of cations (Howitt and Udvardi, 2000; Schachtman, 2000). Although the symbiosome membrane channel could theoretically transport several monovalent cations, a strong case can be made for its role in the transport of fixed nitrogen in the form of NH4+ (for discussion, see Whitehead et al., 1995). First, the Km for ammonium (17 mm) is within the range of concentrations estimated within the symbiosome space (12 mm; Streeter, 1989). Further, a large gradient of NH4+ is proposed to exist between the symbiosome space and cytosol due to: 1) The acidity of the symbiosome space, which is estimated to be 1.0 to 1.5 units lower than the cytosol (Udvardi et al., 1991) favoring the predominance of the NH4+ species; and 2) the rapid assimilation of free ammonia/ammonium by Gln synthetase that maintains a low steady-state concentration of cytosolic ammonium (Streeter, 1989). Second, as discussed above, the channel is inwardly rectified. Thus, even though the concentration of cytosolic K+ is high (60–100 mm), the direction of current flow through the channel will be toward the cytosol at negative potentials (Fig. 8).
Besides a metabolic role in the transport of fixed NH4+ for nitrogen assimilation, the NH4+ permeable symbiosome channel can also have other nonmetabolic functions. Similar to the plasma membrane of plant cells, hyperpolarization of the symbiosome membrane is proposed to occur via the action of an electrogenic ATPase activity (Udvardi and Day, 1989; Udvardi et al., 1991). If not controlled, this activity could lead to severe hyperpolarization and membrane damage. Similar to the roles proposed for the inwardly rectified K channels of animal and plant plasma membranes (for review, see Maathuis et al., 1997; Reimann and Ashcroft, 1999), the symbiosome membrane cation channel could aid in regulation of the symbiosome membrane potential. At low membrane potentials the channel would remain closed because of the action of cytosolic magnesium. However, upon hyperpolarization of the membrane mediated by the H+-ATPase (Fig. 8) the channel would open, facilitating the flux of cations from the symbiosome space to the cytosol. The voltage threshold for channel opening might be further regulated by the electrochemical gradient of permeant monovalent cations across the symbiosome membrane (Whitehead et al., 1998). This coordinate regulation of the pump and the inwardly rectified channel would allow the maintenance of the membrane potential and electrochemical gradient that would serve as a driving force for efflux of fixed ammonium as well as the uptake of malate and possibly other anions (Ou Yang et al., 1990; Udvardi et al., 1991). Further, efflux of cations such as fixed NH4+, not only would serve a function in voltage regulation, but would also aid in symbiosome pH homeostasis, since the flux of this ion would carry a proton out of the symbiosome space (Fig. 8). Whether the voltage-dependent cation channel is involved in the compartmentation or release of other monovalent cations besides ammonium also remains to be addressed.
Another feature of the L. japonicus channel that needs to be considered is its permeability to Ca2+ ions. Although Ca2+ blocks the monovalent cation current of the L. japonicus channel, the actual affinity of the channel for divalent cations is somewhat lower than that observed for the previously characterized soybean symbiosome channel (apparent Kd = 0.32 mm versus 8 μm, Whitehead et al., 1998), and the L. japonicus channel exhibits a finite Ca2+ permeability. These observations suggest that Ca2+ is less tightly bound at a site in the pore and therefore may be able to permeate the channel.
The calcium permeability exhibited by the L. japonicus channel appears to be unique to this activity since a similar permeability is lacking in the analogous channel from the soybean symbiosome membrane (Tyerman et al., 1995; Whitehead et al., 1998). Because symbiosomes have also been reported to contain high amounts of Ca2+, similar to vacuoles (Udvardi and Day, 1997), calcium release through the L. japonicus channel may therefore play a role in cell signaling. In this respect, the Ca2+ permeability of the L. japonicus channel is similar to that of the SV channel in the tonoplast, which also shows slow activation kinetics, is cation selective and Ca2+-permeable (Ward and Schroeder, 1994; Allen and Sanders, 1996) and has been proposed to be involved in Ca2+-induced Ca2+-release in vacuoles (Ward and Schroeder, 1994; Pottosin et al., 1997; Allen et al., 1998). The presence of a distinct calcium permeability on the L. japonicus symbiosome membrane is intriguing and merits further investigation as a potential target of calcium signaling and homeostasis in this particular legume.
The finding of similar voltage-dependent cation channels that are permeable to ammonium ions in the symbiosomes of soybean and L. japonicus argues for a fundamental conserved role of this activity in nitrogen-fixing symbioses. Further, the demonstration that the channel is permeable to Ca2+ raises the possibility of alternative roles for this voltage-dependent channel, at least in the L. japonicus system. Given the ease of genetic manipulation of L. japonicus, this organism has emerged as a candidate as a model legume for the study of the molecular genetics of nitrogen fixation (Handberg and Stougaard, 1992; Stiller et al., 1997; Szczyglowski et al., 1997; Stougaard, 2000). Further analysis of the L. japonicus genes encoding the proteins responsible for these symbiosome membrane activities, and the use of transgenic plant technologies to alter their expression, may allow an evaluation of their functional role in the symbiosis.
MATERIALS AND METHODS
Lotus japonicus B-129-56 cv Gifu seeds were scarified and germinated in sterile water for a week. Seedlings were planted in a 1:1 mix of sand:vermiculite and were grown under greenhouse conditions. On the day of planting, seedlings were watered with a 3-d-old culture of Mesorhizobium loti NZP 2235 (Stiller et al.1997), diluted 1:1 with Herridges nutrient solution as previously described (Guenther and Roberts, 2000). The plants were re-inoculated with M. loti 7 d after planting and were watered on alternative weeks with water or Herridges solution.
Nodules were harvested from L. japonicus plants between 8 and 11 weeks after planting and were washed briefly in 20 mm MOPS-NaOH, pH 7.0, 350 mm mannitol, and 3 mm MgSO4. Single nodules were crushed on ice in 20 mm MES-NaOH, pH 7.0, 20 mm sodium ascorbate, 10 mm MgSO4, 10 mm EGTA, 350 mm mannitol, and 1 mm dithiothreitol. After 5 min, 5 μL of the extract was spotted onto a microscope slide bath (1 mL) containing 100 mm K-Glu, 2 mm MgCl2, 2.3 mm CaCl2, 10 mm EGTA, and 5 mm HEPES-Tris, pH 7.0, (standard bath solution) and the symbiosomes were allowed to adsorb to the base of the chamber as previously described (Whitehead et al., 1998). The bath was perfused with 5 mL of standard bath reagent before patch-clamp analysis.
Unless otherwise noted, all recordings were done in solutions buffered with 10 mm HEPES-Tris, pH 7.0, and adjusted to an osmolarity of 400 mOsm/kg with mannitol. Patch pipettes were made from GC150-10 borosilicate glass capillaries (Clark Electronic Instruments, Reading, UK) by using a PP-83 electrode puller (Narishige, Tokyo) and a two-step protocol. The tips were polished to a diameter of approximately 0.5 μm and were coated with Sylgard (Dow Corning, Corning, NY) to reduce the capacitance. Unless otherwise noted, the standard pipette filling solution was 150 mm KCl, 10 mm CaCl2, and 10 mm HEPES-Tris, pH 7.0. High resistance (10–50 GΩ) inside-out, excised patches of the symbiosome membrane were obtained essentially as previously described (Whitehead et al., 1998). The reference AgCl electrode was connected to the bath by an agar bridge filled with 100 mm KCl. Current measurements were made with an Axopatch 200B patch amplifier (Axon Instruments, Burlingame, CA), and were filtered at 200 or 500 Hz with a low pass Bessel filter. Data acquisition was done using the P-Clamp6 analysis acquisition system (Axon Instruments). Data fitting was done by using pClamp 6.0, Clampfit 8.0 (Axon Instruments), and Graphpad Prism (Graphpad Software, San Diego). As discussed previously (Whitehead et al., 1998), the sign convention for voltage was relative to the cytosolic side of the membrane, and the sign for current was such that positive current represents the flux of cations from the cytosol into the symbiosome space.
The relative permeability of time-dependent currents was determined by activating the current by a prepulse to −100 mV followed by measuring the tail currents upon a rapid step to various holding potentials ranging from +100 to −100 mV. The potential that no longer showed current deactivation, was taken as the reversal potential. The relative permeability between Ca2+ and K+ (PCa2+:PK+) was determined by measuring reversal potentials with a constant KCl concentration in the pipette and varied KCl concentrations in the bath. Calcium activity was kept constant with a gradient directed either inwards or outwards across the patch in different experiments. The relative permeability between NH4+ and K+ (PNH4+:PK+) was determined by measuring reversal potentials with constant KCl concentration in the pipette and varied NH4Cl concentration in the bath and assuming that the PCa2+:PNH4+ was equal to PCa2+:PK+ under the same univalent cation activities and Ca2+ activity gradient. The modified Goldman–Hodgkin–Katz voltage equation (Johannes and Sanders, 1995) was used to calculate relative permeabilities.
For Ca2+ titrations, an EGTA buffered system (1 mm) was used, and CaCl2 was varied to yield free Ca2+ concentrations ranging from 10−7 to 10−3 m as determined by the GEOCHEM program (Parker et al., 1987). IV plots were fit with low order polynomials and chord conductances measured at −100 mV were determined. This relative conductance was plotted against free Ca2+ and was fit with a Hill equation:
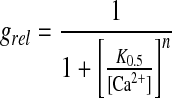 |
1 |
The voltage dependence of activation was performed essentially as described by Whitehead et al. (1998). Briefly, excised, inside-out patches were held at +60 mV, and 1.6-s pulses were done in 20-mV increments from +60 mV to −180 mV. Relative conductance was determined from the tail currents measured upon return to the holding potential. The relationship of relative conductance to potential was fitted to a simple Boltzmann function.
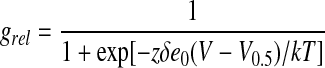 |
2 |
where grel is relative conductance, z is the gating charge, δ is the distance the gating charge moves across the bilayer, e0 is the elementary charge, T is absolute temperature, and k is the Boltzmann constant.
ACKNOWLEDGMENTS
The authors would like to thank Wendy Sullivan for expert technical assistance and Drs. David A. Day and C. David Weaver for critical comments regarding the manuscript. D.M.R. would like to acknowledge the long time support and encouragement of David L. Roberts.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (award no. 9703548) and by the National Science Foundation (grant no. MCB–9904978 to D.M.R.), by the Australian Research Council (to S.D.T.), and by a Career Development award to D.M.R. from the University of Tennessee Office of Research Administration.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010568.
LITERATURE CITED
- Allen GJ, Sanders D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996;10:1055–1069. doi: 10.1046/j.1365-313x.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Sanders D, Gradmann D. Calcium-potassium selectivity: kinetic analysis of current-voltage relationships of the open, slowly activating channel in the vacuolar membrane of Vicia fabaguard-cells. Planta. 1998;204:528–541. [Google Scholar]
- Day DA, Poole PS, Tyerman SD, Rosendahl L. Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell Mol Life Sci. 2001;58:61–71. doi: 10.1007/PL00000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RM, Rivers RL, Zeidel ML, Roberts DM. Purification and functional reconstitution of soybean nodulin 26: an aquaporin with water and glycerol transport properties. Biochemistry. 1999;38:347–353. doi: 10.1021/bi982110c. [DOI] [PubMed] [Google Scholar]
- Guenther JF, Roberts DM. Water selective and multifunctional aquaporins from nodules of Lotus japonicus. Planta. 2000;210:741–748. doi: 10.1007/s004250050675. [DOI] [PubMed] [Google Scholar]
- Handberg K, Stougaard J. Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J. 1992;2:487–496. [Google Scholar]
- Howitt SM, Udvardi MK. Structure, function and regulation of ammonium transporters in plants. Biochim Biophys Acta. 2000;1465:152–170. doi: 10.1016/s0005-2736(00)00136-x. [DOI] [PubMed] [Google Scholar]
- Johannes E, Sanders D. Lumenal calcium modulates unitary conductance and gating of a plant vacuolar calcium release channel. J Membr Biol. 1995;146:211–224. doi: 10.1007/BF00238010. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Ichida AM, Sanders D, Schroeder JI. Roles of higher plant K+channels. Plant Physiol. 1997;114:1141–1149. doi: 10.1104/pp.114.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett. 2000;465:110–114. doi: 10.1016/s0014-5793(99)01729-9. [DOI] [PubMed] [Google Scholar]
- Oliver D, Baukrowitz T, Fakler B. Polyamines as gating molecules of inward-rectifier K+channels. Eur J Biochem. 2000;267:5824–5829. doi: 10.1046/j.1432-1327.2000.01669.x. [DOI] [PubMed] [Google Scholar]
- Ou Yang L-J, Udvardi MK, Day DA. Specificity and regulation of the dicarboxylate carrier on the peribacteroid membrane of soybean nodules. Planta. 1990;182:437–444. doi: 10.1007/BF02411397. [DOI] [PubMed] [Google Scholar]
- Parker DR, Zelazny LW, Kinraide TB. Improvements to the program GEOCHEM. Soil Sci Soc Am J. 1987;51:488–491. [Google Scholar]
- Pei Z-M, Ward JM, Schroeder JI. Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in fava bean guard cells. Plant Physiol. 1999;121:977–986. doi: 10.1104/pp.121.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin II, Tikhonova I, Hedrich R, Schönknecht G. Slowly activating vacuolar channels can not mediate Ca2+-induced Ca2+release. Plant J. 1997;12:1387–1398. [Google Scholar]
- Reimann F, Ashcroft FM. Inwardly rectifying potassium channels. Curr Opin Cell Biol. 1999;11:503–508. doi: 10.1016/S0955-0674(99)80073-8. [DOI] [PubMed] [Google Scholar]
- Rivers RL, Dean RM, Chandy G, Hall JE, Roberts DM, Zeidel ML. Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J Biol Chem. 1997;272:16256–16261. doi: 10.1074/jbc.272.26.16256. [DOI] [PubMed] [Google Scholar]
- Roth E, Jeon K, Stacey G. Homology in endosymbiotic systems: the term “symbiosome.”. In: Palcios R, Verma DPS, editors. Molecular Genetics of Plant Microbe Interactions. ADS Press, St. Paul. 1988. pp. 220–225. [Google Scholar]
- Schachtman DP. Molecular insight into the structure and function of plant K+transport mechanisms. Biochim Biophys Acta. 2000;1465:127–139. doi: 10.1016/s0005-2736(00)00134-6. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+channels in higher plants. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Stiller J, Martirani L, Tuppale S, Chian R, Chiurazzi M, Gresshoff PM. High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus. J Exp Bot. 1997;48:1357–1365. [Google Scholar]
- Stougaard J. Regulators and regulation of legume root nodule development. Plant Physiol. 2000;124:531–540. doi: 10.1104/pp.124.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter JG. Estimation of ammonium concentration in the cytosol of soybean nodules. Plant Physiol. 1989;90:779–782. doi: 10.1104/pp.90.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczyglowski K, Hamburger D, Kapranov P, de Bruijn FJ. Construction of a Lotus japonicuslate nodulin EST library and identification of novel nodule-specific genes. Plant Physiol. 1997;144:1335–1346. doi: 10.1104/pp.114.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Whitehead LF, Day DA. A channel-like transporter for NH4+ on the symbiotic interface of N2-fixing plants. Nature. 1995;378:629–632. [Google Scholar]
- Udvardi MK, Day DA. Electrogenic ATPase activity on the peribacteroid membrane of soybean (Glycine maxL.) root nodules. Plant Physiol. 1989;90:982–987. doi: 10.1104/pp.90.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Day DA. Ammonia (14C-methylamine) transport across the bacteroid and peribacteroid membranes of soybean root nodules. Plant Physiol. 1990;94:71–76. doi: 10.1104/pp.94.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Day DA. Metabolite transport across symbiotic membranes of legume nodules. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:493–523. doi: 10.1146/annurev.arplant.48.1.493. [DOI] [PubMed] [Google Scholar]
- Udvardi MK, Lister DL, Day DA. ATPase activity and anion transport across the peribacteroid membrane of isolated soybean symbiosomes. Arch Microbiol. 1991;156:362–366. [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead LF, Day DA, Tyerman SD. Divalent cation gating of an ammonium permeable channel in the symbiotic membrane of soybean nodules. Plant J. 1998;16:313–324. [Google Scholar]
- Whitehead LF, Tyerman SD, Salom CL, Day DA. Transport of fixed nitrogen across symbiotic membranes of legume nodules. Symbiosis. 1995;19:141–154. [Google Scholar]
- Whitehead LF, Tyerman SD, Day DA. Polyamines as potential regulators of nutrient exchange across the peribacteroid membrane in soybean root nodules. Aust J Plant Physiol. 2001;28:675–681. [Google Scholar]