Abstract
The 35S rRNA genes at the RDN1 locus in Saccharomyces cerevisiae can be transcribed by RNA polymerase (Pol) II in addition to Pol I, but Pol II transcription is usually silenced. The deletion of RRN9 encoding an essential subunit of the Pol I transcription factor, upstream activation factor, is known to abolish Pol I transcription and derepress Pol II transcription of rRNA genes, giving rise to polymerase switched (PSW) variants. We found that deletion of histone deacetylase gene RPD3 inhibits the appearance of PSW variants in rrn9 deletion mutants. This inhibition can be explained by the observed specific inhibition of Pol II transcription of rRNA genes by the rpd3Δ mutation. We propose that Rpd3 plays a role in the maintenance of an rRNA gene chromatin structure(s) that allows Pol II transcription of rRNA genes, which may explain the apparently paradoxical previous observation that rpd3 mutations increase, rather than decrease, silencing of reporter Pol II genes inserted in rRNA genes. We have additionally demonstrated that Rpd3 is not required for inhibition of Pol I transcription by rapamycin, supporting the model that Tor-dependent repression of the active form of rRNA genes during entry into stationary phase is Rpd3 independent.
Histone acetyltransferases and histone deacetylases play important roles in the regulation of gene expression and maintenance of chromatin structure. Extensive genome-wide and individual gene-specific studies have been carried out to define the distribution and functions of these enzymes in the yeast Saccharomyces cerevisiae (called yeast hereafter in this paper) (for a review, see, e.g., reference 27). However, there have been only limited studies on non-protein-coding genes, such as rRNA genes present at RDN1 on chromosome XII. Here we describe our studies related to the role of the histone deacetylase Rpd3 in regulation of transcription activities at rRNA genes and discuss some earlier published results on the basis of the new observations.
Yeast cells carry ∼150 copies of tandemly repeated rRNA genes but during exponential growth use only ∼50% of those genes (“active” or “open” copies); the remaining genes are kept completely inactive (“inactive” or “closed” copies) (9, 13, 39). It is well-known that the rRNA synthesis rate decreases during the transition from exponential phase to stationary phase in yeast cells (22) (defined as the “post-diauxic phase” [16]). This decrease in synthesis rate is achieved by two different mechanisms. First, the percentage of open rRNA gene copies decreases during the post-diauxic phase (9, 39). Second, the transcription of individual open genes is also decreased (39). It was discovered that an rpd3Δ mutation prevents the conversion of open rRNA gene copies to closed copies (an Rpd3-dependent mechanism) but allows decrease of rRNA transcription from individual open copies (an Rpd3-independent mechanism). In fact, this decrease is greater than that in a control RPD3 strain so that the overall rRNA transcription rate in the post-diauxic phase is the same in both rpd3Δ mutant and control RPD3 strains despite the presence of more open genes in the rpd3Δ mutant (39). Thus, the mechanisms involved in regulation of transcription of individual open rRNA genes appear to involve a system to adjust the overall rRNA synthesis rate to what is most suitable for cells entering into quiescent states.
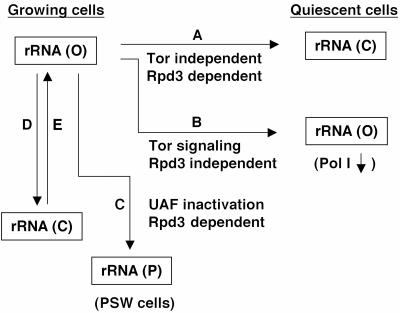
Entry into the post-diauxic/stationary phase is known to involve the Tor signaling pathway (reviewed in reference 21). Rapamycin, which inhibits the Tor signaling pathway, is known to inhibit the transcription of rRNA genes by RNA polymerase (Pol) I (34, 54). In a previous study (8), we asked whether the inhibition of rRNA synthesis by rapamycin is caused by decreasing the number of open genes (which is Rpd3 dependent) or by inhibiting the presumed Rrn3-dependent initiation step at the open rRNA genes (which is Rpd3 independent) or both. (Rrn3 is essential for Pol I transcription [30, 53] and binds Pol I in the absence of DNA template, forming an initiation-competent Pol I-Rrn3 complex [12, 24, 53]. The complex formation appears to be regulated by the phosphorylation state of Pol I or Rrn3 both in yeast and mammalian systems [6, 12, 28].) First, we found that rapamycin treatment caused a decrease in the amount of the Rrn3-Pol I complex. This result and previous observations made both in yeast (8, 29) and mammalian (28) cells strongly suggest that the Rpd3-independent mechanism may involve decreasing the amount of the initiation-competent form of RNA polymerase I, the Pol I-Rrn3 complex (Pol I-TIF-1A complex in the mouse). Second, electron microscopy (EM) Miller chromatin spread analysis of rapamycin-treated cells showed the predicted decrease in Pol I density on rRNA genes but failed to show any significant decrease in the number of active (open) rRNA genes relative to inactive (closed) genes, thus demonstrating that the Tor system does not participate in the conversion of rRNA genes from the open to closed state observed for cells in the post-diauxic phase (8). In Fig. 1, which summarizes our understanding of different rRNA gene chromatin states, the two pathways involved in down regulation of transcription as cells leave exponential growth are shown as pathways A and B.
FIG. 1.
Summary of information on several rRNA gene chromatin states. The open or active form and the closed or inactive form of rRNA gene copies are shown as rRNA gene (O) and rRNA gene (C), respectively, both for exponentially growing cells and cells in the post-diauxic/stationary phase (quiescent cells). The state of rRNA gene copies in PSW cells is shown as rRNA gene (P). Pathways A, B, C, D, and E are shown with information regarding Rpd3 dependency, participation of rapamycin-sensitive Tor system and UAF alteration, which is discussed in the present paper.
In contrast to these conclusions, a paper was published independently, proposing a direct requirement of Rpd3 for regulation of Pol I transcription of rRNA genes by the Tor signaling system (48). When yeast cells were treated with rapamycin, it was reported that Pol I delocalized as a result of structural alterations of the nucleolus and that an rpd3 deletion abolished rapamycin-dependent inhibition of Pol I transcription by preventing this structural alteration. Thus, the work by Tsang et al. (48) implies that rapamycin causes a decrease in the number of active (open) rRNA genes, and not an Rpd3-independent decrease in the activity of individual open rRNA genes as observed in rpd3Δ mutants in the post-diauxic phase (39). In this paper, we describe various experiments to reexamine this question and demonstrate that Rpd3 is not required for Tor-dependent regulation of Pol I transcription, confirming our previous conclusions on the roles of Rpd3 and Tor signaling pathways (Fig. 1, pathways A and B).
Another question regarding the role of the Rpd3 histone deacetylase in transcription activities at rRNA genes is related to Sir2-dependent silencing of reporter Pol II genes integrated into rRNA gene repeats (3, 14, 44). Sir2 is an NAD-dependent histone deacetylase, responsible mainly for deacetylation of acetylated lysine at position 16 (K16) of histone H4 and of K9 and K14 of H3 (20). The importance of histone deacetylation by Sir2 in transcriptional silencing of reporter genes has been supported by an observed correlation between hypoacetylation of histone tails and silent chromatin (1, 2, 4, 18, 38, 46). Paradoxically, however, deletion of another histone deacetylase, the NAD-independent histone deacetylase Rpd3, was found to increase, rather than to decrease, silencing of reporter genes at rRNA genes, mating type loci, and telomeres (45, 47). This deacetylase is predicted to function at all acetylation sites on histones H4, H3, H2A, and H2B, with the possible exception of K16 of H4 (37, 46). Since histone acetylation at silenced loci in an rpd3 deletion mutant did not change significantly and expression of many other genes is affected by the rpd3 mutation, it was suggested that this increase in silencing may be an indirect effect, i.e., a consequence of altered expression of some other gene(s) (35).
As mentioned previously, exponentially growing yeast cells use only ∼50% of ∼150 copies of rRNA genes. By following a single tagged rRNA gene copy integrated into chromosomal rRNA gene repeats and using psoralen cross-linking to separate active from inactive copies of rRNA genes, Dammann et al. (10) demonstrated that individual rRNA gene copies frequently alternate between open and closed states (these are shown as pathways D and E in Fig. 1; it is not known whether pathway E is a simple reversal of pathway D). In our previous study on silencing at rRNA genes (7), we found that silencing of a reporter Pol II gene in an rRNA gene copy takes place when the rRNA gene copy is in the active (open) state and that no significant silencing takes place when the reporter gene is in inactive (closed) copies. The results suggest that the rRNA gene chromatin structure in the open form allows Pol I but not Pol II transcription and that the closed form allows Pol II but not Pol I transcription; that is, expression of Pol I and expression of Pol II in rRNA genes are in a reciprocal relationship (7). This conclusion is supported by the finding that Pol II reporter genes integrated into rRNA gene repeats in strains carrying a mutation in upstream activation factor (UAF) and growing by Pol II transcription of the 35S rRNA genes are not subject to silencing (4, 7).
Upstream activation factor is a multiprotein complex, which binds tightly to the upstream element of the yeast Pol I promoter and is essential for a high level of rRNA transcription. It consists of six protein subunits, three proteins (Rrn5, Rrn9, and Rrn10) encoded by “nearly essential” genes (RRN5, RRN9, and RRN10), Uaf30p encoded by nonessential gene UAF30, and histones H3 and H4 (23, 25, 42). It was previously observed that deletion of any of the three genes, RRN5, RRN9, and RRN10, abolishes Pol I transcription and derepresses Pol II transcription at several start sites upstream of the Pol I start site (33, 51). The majority of cells carrying these mutations fail to form colonies (called N-PSW for polymerase switched but no growth) (33, 51), almost certainly due to a very low level of 35S rRNA transcription by Pol II, but give rise to variants (called PSW for polymerase switched) able to grow as a result of an expansion of chromosomal rRNA gene repeats (33) (Fig. 1, pathway C). Deletion of UAF30, which decreases the growth rate, was found to allow the use of both Pol I and Pol II for transcription of the 35S rRNA genes (42). Thus, UAF has dual functions; one function is to act as a positive transcription factor for Pol I, and the other is to act as a silencer for Pol II transcription of rRNA genes, consistent with the role of UAF in the reciprocal relationship between Pol I and Pol II transcription in rRNA genes (7). As discussed previously, deletion of any one of the subunit protein genes encoding essential nonhistone proteins causes derepression of Pol II transcription of the 35S rRNA genes and, as a consequence, abolishes silencing of Pol II reporter genes integrated at rRNA genes (7). In the course of silencing studies of Pol II reporter genes, we discovered that an rpd3Δ mutation inhibits the appearance of PSW cells from N-PSW cells and growth of PSW cells and that this inhibition is a result of specific inhibition of Pol II transcription of rRNA genes (pathway C in Fig. 1). In this paper, we first describe these new observations and then discuss them in connection with previously known effects of rpd3Δ mutations on silencing of Pol II reporter genes at rRNA genes and on conversion of rRNA genes from open to closed states during the post-diauxic phase.
MATERIALS AND METHODS
Media and strains.
Yeast extract-peptone-galactose (YEPG), yeast extract-peptone-glucose (YEPD), synthetic galactose (SG), and synthetic glucose (SD) complete media, were described previously (8, 40). For [methyl-3H]methionine incorporation experiments, methionine was omitted from SD complete medium. In all the experiments, cells were grown at 30°C unless otherwise stated.
The yeast strains and plasmids used are described in Table 1. Disruption of SIR2 and RPD3 was carried out using pNOY718 and pNOY725 (or pM1061), respectively.
TABLE 1.
Yeast strains and plasmids used
| Strain or plasmid | Description |
|---|---|
| Strains | |
| NOY388 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 |
| NOY680 | MATα ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 rrn5Δ::LEU2, PSW |
| NOY684 | Same as NOY680, but rrn9Δ::HIS3, PSW |
| NOY703 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 rrn9Δ::HIS3, N-PSW; pNOY103 |
| NOY852 | MATα ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 rrn9Δ::HIS3; PSW (51) |
| NOY902 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 rrn9Δ::HIS3; PSW |
| NOY1022 | MATaade2-1 ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 uaf30Δ::HIS3 |
| NOY1045 | Same as NOY388, but sir2Δ::LEU2 |
| NOY1075 | Same as NOY388, but rrn3(S213P) encodes a protein with seven-hemagglutinin and six-histidine tags on the N terminus (8) |
| NOY2015 | Same as NOY388, but rpd3Δ::LEU2 |
| NOY2107 | Same as NOY1022, but rpd3Δ::URA3 |
| NOY2108 | MAT? ade2-1 ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 rrn9Δ::HIS3 sir2Δ::LEU2; PSW |
| NOY2109 | Same as NOY2015, but carrying pMV34 |
| NOY2110 | MATaade2-1 ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 rpd3Δ::LEU2 rrn9Δ::HIS3, pNOY199, pMV34; PSW |
| NOY2111 | MAT? ade2-1 ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 rrn9Δ::HIS3, pNOY199; N-PSW |
| NOY2156 | Same as NOY388, but rpd3Δ::His3MX6 |
| NOY2168 | Same as NOY703, but rpd3Δ::LEU2 |
| JS772 | MATahis3Δ leu2Δ ura3Δ (39) |
| JS777 | Same as JS772, but rpd3Δ::kanMX4 (39) |
| Plasmids | |
| pM1061 (pNOY726) | rpd3Δ::URA3 disruption plasmid, linearized with XbaI prior to transformation (47) |
| pMV34 (pNOY671) | A 3-kb EcoRI-SphI genomic fragment carrying RPD3 in a URA3CEN plasmid (49) |
| pNOY103 | High-copy-number plasmid carrying GAL7-35S rRNA gene, URA3, ADE3, 2μm, Amp (30) |
| pNOY199 | High-copy-number plasmid carrying Gal7-35S rRNA gene, TRP1, 2μm, Amp (33) |
| pNOY718 | sir2Δ::LEU2 disruption plasmid, linearized with SalI prior to transformation |
| pNOY725 | rpd3Δ::LEU2 disruption plasmid, linearized with XbaI prior to transformation |
Biochemical analyses.
Analyses of rRNA synthesis by labeling RNA with [14C]uracil or [methyl-3H]methionine were carried out as previously described (8, 52). Analysis of 5′ ends of precursor rRNA by primer extension was carried out as described previously, using a primer which hybridizes to 35S rRNA 130 nucleotides downstream of the Pol I start site (33). Quantification was done with a phosphorimager (Bio-Rad, Hercules, California).
EM thin-section, IFM, and EM Miller chromatin spread analyses.
EM analysis was carried out as described previously using a JEOL 100CX electron microscope (31). Immunofluorescence microscopy (IFM) was done with a Zeiss Axioskop (Carl Zeiss Inc., Oberkochen, Germany), using anti-A190 and anti-Nop1 antibodies (31).
EM Miller chromatin spread analysis was performed as described previously (8, 13). For quantitative analysis, entire EM grids were scanned, and all rRNA genes were photographed and analyzed. The polymerase number per gene was determined by counting the number of RNA polymerases (or nascent rRNA transcripts) on all rRNA genes that could be unambiguously followed from the 5′ end to the 3′ end.
RESULTS
RPD3 is required for the appearance of PSW variants from rrn9 or rrn5 deletion mutant cells.
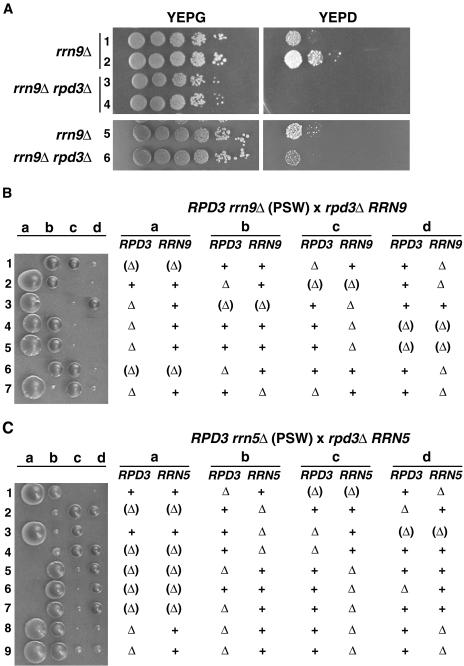
Cells carrying mutations of RRN5, RRN9, and RRN10 encoding the three essential nonhistone protein subunits of UAF were originally isolated from a standard yeast strain carrying a helper plasmid, which contains the 35S rRNA coding region fused to the GAL7 promoter (GAL7-35S rRNA gene fusion gene), as mutants that can grow on galactose but not on glucose (30). It was subsequently discovered that a small fraction of these mutant cells growing in galactose-containing cultures give rise to PSW variants able to grow on glucose-containing medium (33, 51) (see Fig. 2A for an rrn9Δ mutant). PSW cells grow by transcribing the chromosomal rRNA genes by Pol II and hence, can grow in the absence of helper plasmid. We discovered that deletion of RPD3 in the rrn9Δ strain growing on galactose by the use of a helper plasmid inhibited appearance of PSW variants on glucose medium without inhibiting growth on galactose (Fig. 2A). However, the degree of inhibition varied depending on clones (compare the left gel with the right gel in Fig. 2A) similar to the known variation in the frequency of appearance of PSW cells (compare clones 1 and 2 in Fig. 2A). As explained in the introduction, the switch to the PSW state, i.e., growth by transcribing rRNA genes by Pol II involves two key steps; the first is the derepression of Pol II transcription of rRNA genes by mutational inactivation of one of the essential subunits of UAF, and the second is an expansion of rRNA gene copy numbers (33). We considered the possibility that transcription of rRNA genes by Pol II not only requires inactivation of UAF as a repressor but also requires intact Rpd3 for a proper chromatin structure at the promoter. A possible explanation for the variability in the degree of inhibition of the appearance of PSW cells invokes the heterogeneity in rRNA gene repeat number in these cells. A given N-PSW rrn9Δ culture (or other UAF mutants carrying the GAL7-35S rRNA gene fusion gene on a helper plasmid) growing on galactose gradually accumulates cells with higher repeat numbers presumably due to some small growth advantages, such that even a strong (but partial) decrease in Pol II transcription of individual rRNA genes by the rpd3Δ mutation may be compensated by a higher gene dosage at the time of disruption of RPD3. Thus, rpd3Δ rrn9Δ N-PSW clones derived from cells with higher rRNA gene repeat numbers may produce PSW variants with a higher efficiency approaching that observed for control RPD3 rrn9Δ N-PSW clones, whereas those derived from cells with lower rRNA gene repeat numbers may produce PSW variants with much reduced efficiency.
FIG. 2.
rpd3Δ mutation inhibits appearance of PSW variants from N-PSW cells carrying rrn9Δ or rrn5Δ mutations. (A) RPD3 was disrupted in strain NOY2111 (rrn9Δ::HIS3; carrying helper pNOY199 and N-PSW). In one experiment, two colonies (colonies 3 and 4) of the resultant rrn9Δ rpd3Δ strain and two colonies (colonies 1 and 2) of its parent, NOY2111 (rrn9Δ), were analyzed for their ability to switch to the PSW state. In another experiment, disruption of RPD3 was repeated, and the rpd3Δ mutant isolated (rrn9Δ rpd3Δ colony 6) was analyzed together with a control rrn9Δ colony (rrn9Δ colony 5). An approximately equal number of cells (corresponding to a 2-mm-diameter colony) were picked from plates and suspended in H2O, and 10-fold serial dilutions of the indicated strains were spotted on YEPG and YEPD complete media. Plates were incubated for 7 days at 30°C. (B) Tetrad analysis showing rrn9Δ rpd3Δ spores without helper plasmid are inviable. Diploid cells (His+ Leu+) formed by crossing strain NOY684 (rrn9Δ::HIS3) with strain NOY2015 (rpd3Δ::LEU2) were sporulated, and tetrads were dissected and incubated for 9 days at 30°C. After a photograph was taken (shown), the colonies were analyzed for the presence of rrn9Δ (His+) and rpd3Δ (Leu+). The genotype of spores that failed to form colonies was deduced from the genotypes of colonies formed from the three other spores and concluded to be rrn9Δ rpd3Δ (shown in parentheses). We note that the colonies formed at the left-hand side of the photograph (e.g., spores a) were generally larger in size than those at the right-hand side (e.g., spores d) even if they had the same genotype, perhaps because they were farther away from the streak of zymolase-treated cells containing tetrad spores (the streak is not shown but is present to the right of spores d). (C) Tetrad analysis showing rrn5Δ rpd3Δ spores without helper plasmid are inviable. Diploid cells (Leu+ His+) formed by crossing NOY680 (rrn5Δ::LEU2) with NOY2156 (rpd3Δ::His3MX6) were sporulated, and tetrads were dissected and incubated for 9 days at 30°C. Colonies were analyzed as described above for panel B, and genotypes of haploid segregants are indicated. The genotype of the spores that failed to form colonies was concluded to be rrn5Δ rpd3Δ.
In order to overcome the variability observed in the effects of rpd3Δ mutation on production of PSW cells, we introduced an rpd3Δ mutation into an rrn9Δ strain by mating and examined its effect on PSW variant formation by tetrad analysis. A diploid strain was first constructed by crossing an rpd3Δ strain with an rrn9Δ (PSW) strain which does not carry any helper plasmid. This strain, which is RPD3/rpd3Δ and RRN9/rrn9Δ, was grown for many generations by repeated streaking on the synthetic medium used for selection of the original diploid and then subjected to sporulation followed by tetrad analysis. As shown in Fig. 2B, RPD3 rrn9Δ haploid spores formed small colonies after 9 days of incubation at 30°C (e.g., spores 1d) as observed in the original study (25, 51), but none of the spores expected to carry the rpd3Δ rrn9Δ genotype formed colonies (see spores 1a, 2c, 3b, 4d, 5d, and 6a in Fig. 2B).
Similar tetrad analysis was also carried out using an rrn5Δ (PSW) strain instead of the rrn9Δ (PSW) strain. As shown in Fig. 2C, RPD3 rrn5Δ haploid spores formed small colonies after 9 days of incubation, as observed in the earlier study (25), but none of the spores that must have carried the rpd3Δ rrn5Δ genotype formed colonies (see spores 1c, 2a, 3d, 4a, 5a, 6a, and 7a in Fig. 2C). The results of these experiments demonstrate that the rpd3Δ mutation decreases the ability of mutants with defects in UAF subunit genes RRN9 and RRN5 (and presumably RRN10 which we have not examined) to grow by transcribing rRNA genes with Pol II, that is, to grow as PSW variant cells.
RPD3 is required for efficient Pol II transcription, but not Pol I transcription, of 35S rRNA genes.
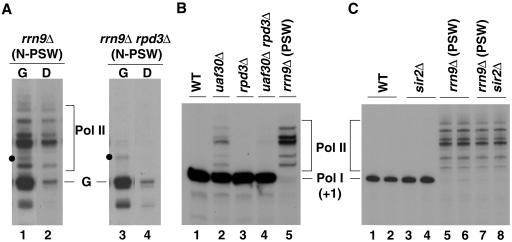
We have previously shown that there is (weak) Pol II transcription of chromosomal 35S rRNA genes in rrn9Δ cells even in the N-PSW state, and this can be clearly recognized after shifting galactose-grown N-PSW cells to glucose which represses Pol II transcription from the GAL7-35S rRNA gene fusion gene (33). As shown in Fig. 3A, rrn9Δ N-PSW cells grown in galactose and shifted to glucose for 1 h showed Pol II transcripts from several start sites (Pol II in Fig. 3A, lanes 1 and 2), which could be distinguished from the main rRNA transcript from the GAL7 promoter (band labeled G in Fig. 3A). Shifting to glucose caused a very large (≥90%) reduction in transcription of the GAL7-35S rRNA gene fusion gene on the helper plasmid but only a partial (∼50% in Fig. 3A) apparent reduction in Pol II transcription of chromosomal rRNA genes (Fig. 3A, compare lane 2 to lane 1). (Shifting of rrn9Δ N-PSW cultures from galactose to glucose causes a large decrease in the growth rate, leading to an eventual cessation of cell mass increase. The degree of decrease in Pol II transcription of chromosomal rRNA genes was variable, depending on the experiment, but was usually less than ∼50%. This decrease might result from growth defects caused by the virtual cessation of rRNA synthesis in glucose.) In contrast to the rrn9Δ N-PSW control strain, rrn9Δ rpd3Δ N-PSW cells did not show significant Pol II transcription of chromosomal rRNA genes either in galactose or after the shift to glucose (Fig. 3A, compare lanes 3 and 4 with lanes 1 and 2, respectively; see the legend for quantification). These results support the conclusion that the rpd3Δ deletion inhibits Pol II transcription of chromosomal rRNA genes and hence, largely abolishes the ability of rrn9Δ or rrn5Δ spores to grow as PSW variants and form colonies.
FIG. 3.
rpd3Δ mutation inhibits Pol II transcription of rRNA genes, but not Pol I transcription of rRNA genes or Pol II transcription of GAL7-35S rRNA gene fusion gene. (A) The two strains, NOY2111 (rrn9Δ, N-PSW) and its rpd3Δ derivative (Fig. 2A), were grown in YEPG complete medium to a cell density of A600 of ∼0.2. Cells from half of each culture were collected by centrifugation, resuspended in YEPD complete medium, and incubated for 1 h (D lanes). The remaining half of each culture was kept in YEPG complete medium and incubated as controls (G lanes). Total cellular RNA was then isolated from each culture. RNA transcribed from the GAL7-35S rRNA gene fusion gene (the main band labeled G) and RNA derived from chromosomal rRNA genes by Pol II transcription (bands labeled Pol II) were analyzed by primer extension using 2.5 μg RNA. Relevant lanes from an autoradiogram of a single gel are shown. It should be noted that there are some bands (e.g., those indicated with a dot) above the main transcription start site (G) of the GAL7-35S rRNA gene fusion gene that, like band G, were strongly reduced upon transfer to glucose and therefore, almost certainly represent transcripts from the GAL7 promoter. Quantification of radioactive bands indicated that rRNA synthesized from the GAL7 promoter in galactose was comparable in the two strains (the amount of band G in lane 3/amount of band G in lane 1 = 0.78), whereas Pol II transcription of chromosomal rRNA genes in the rrn9Δ rpd3Δ strain was negligibly small relative to the control rrn9Δ strain (amount of Pol II bands in lane 4/amount of Pol II bands in lane 2 = 0.017). We also ascertained chromosomal rRNA gene repeat numbers per genome and found that the rrn9Δ strain used had ∼50% more than the rrn9Δ rpd3Δ strain. (N-PSW cells tend to have increased rRNA gene copy numbers even on galactose due to a slight growth advantage [see text].) This difference is small and does not affect the conclusion that the rpd3Δ mutation inhibits Pol II transcription of chromosomal rRNA genes. (B) RNA was prepared from strains NOY388 (WT), NOY1022 (uaf30Δ), NOY2015 (rpd3Δ), NOY2107 (uaf30Δ rpd3Δ), and NOY902 (rrn9Δ, PSW), which were grown in YEPD complete medium to an A600 of ∼0.5. Primer extension was carried out using 2.5 μg of RNA. (C) RNA was prepared from strain NOY388 (WT; lanes 1 and 2), NOY902 (rrn9Δ, PSW; lanes 5 and 6), and the sir2Δ derivatives of these strains (NOY1045 and NOY2108, respectively; lanes 3 and 4 and lanes 7 and 8, respectively), as described above for panel B. Primer extension was carried out in duplicate using 1.5 μg total RNA for NOY388 and NOY1045, and 5 μg total RNA for NOY902 and NOY2108. Autoradiograms are shown. The Pol I start site (+1) and Pol II start sites are indicated. In panel B, phosphorimager analysis showed the Pol II/Pol I ratios to be 0.17 for lane 2 and ∼0.05 for lane 4. We note that the rrn9Δ (PSW) strain (lane 5 in panel B and lanes 5 and 6 in panel C) was derived from a rrn9Δ (N-PSW) strain carrying a helper plasmid (similar to the one shown in lane 1 in panel A), but the helper plasmid was lost. Primer extension using RNA from cells grown in YEPG complete medium also showed the pattern identical to those shown here: that is, no RNA corresponding to band G (lanes 1 and 3 in panel A) was present.
As mentioned above, rRNA gene repeat numbers in rrn9Δ (and rrn5Δ or rrn10Δ) N-PSW cell populations tend to increase when they are maintained on galactose. Therefore, comparison of the rates of total rRNA synthesis by Pol II between two N-PSW cultures by biochemical analyses such as that shown in Fig. 3A has to be corrected for differences in rRNA gene copy numbers. For this reason, we also carried out experiments using strains deleted for UAF30, which encodes the nonessential UAF subunit Uaf30. The uaf30Δ mutation allows Pol II transcription of rRNA genes without completely inhibiting Pol I transcription (42), and therefore, the effects of the rpd3Δ mutation on Pol II transcription of rRNA genes relative to Pol I transcription can be analyzed independently of variations of rRNA gene copy numbers. We constructed an rpd3Δ derivative of the uaf30Δ mutant (in the absence of any helper plasmid) and found no obvious growth defects. We then examined the effects of the rpd3Δ mutation on transcription of rRNA genes by primer extension. As shown in Fig. 3B, a large decrease (≥70%) in Pol II transcription was observed relative to the parent RPD3 uaf30Δ strain, while Pol I transcription was not significantly affected (compare lanes 4 and 2). The absence of a significant effect of the rpd3Δ mutation on Pol I transcription was also confirmed by comparison of the control rpd3Δ strain with the RPD3 (wild-type [WT]) strain (compare lanes 3 and 1). In addition, the growth rates of these two control strains were approximately the same (doubling time, ∼110 min). The growth rates of the uaf30Δ and uaf30Δ rpd3Δ strains were also approximately the same (doubling time, ∼190 min). Since the contribution of Pol II transcription to the synthesis of total rRNA is small in the uaf30Δ strain (15% in the experiment shown in Fig. 3B, lane 2), even the large inhibition of Pol II transcription of rRNA genes is not expected to decrease the growth rate significantly.
Because deletion of SIR2 is known to weaken silencing of reporter genes inserted into rRNA genes, whereas deletion of RPD3 strengthens silencing, we examined the effects of a sir2Δ mutation on transcription of rRNA genes by Pol I and Pol II. In contrast to RPD3 deletion, SIR2 deletion did not affect the growth of rrn9Δ PSW strains (data not shown) or Pol II transcription of the rRNA genes (Fig. 3C, lanes 5 to 8). The same sir2Δ mutation also had no effect on Pol I transcription (Fig. 3C, lanes 1 to 4), consistent with the fact that sir2Δ mutants and control SIR2 strains generally have equal growth rates. These results demonstrate a striking difference between the two histone deacetylases; Rpd3, but not Sir2, is required for efficient transcription of rRNA genes by Pol II in rrn9Δ cells (and, almost certainly, in rrn5Δ and rrn10Δ cells also).
Reexamination of inhibitory effects of rapamycin on nucleolar structures and Pol I transcription of rRNA genes in rpd3Δ and RPD3 control strains.
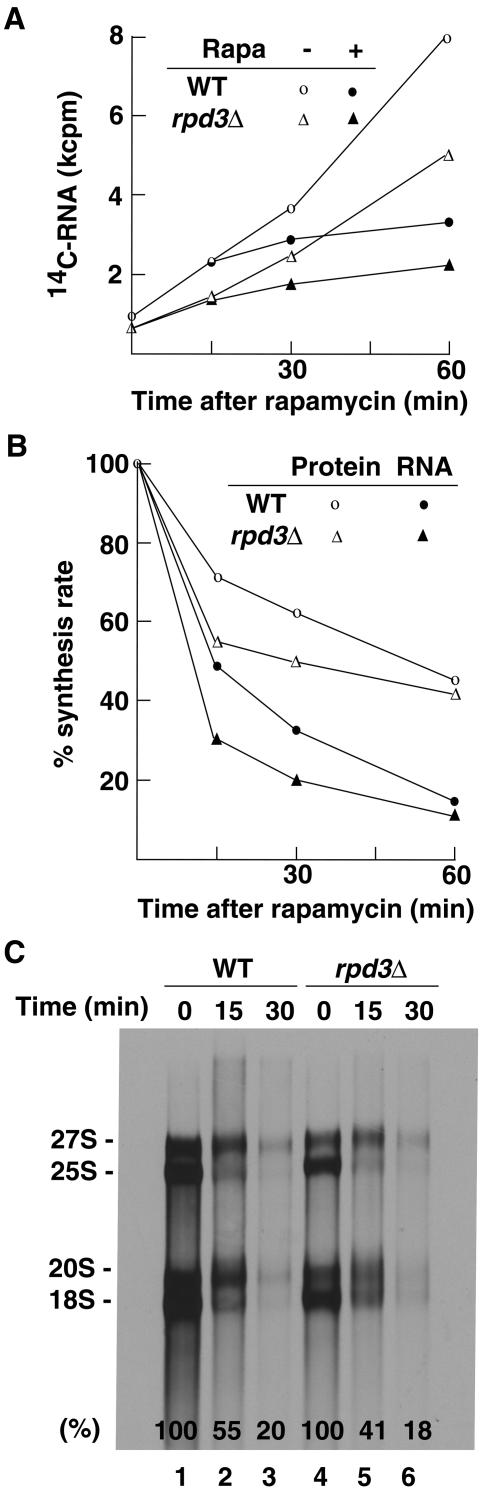
To clarify the discrepancy between the observations of Tsang et al. (48) that an rpd3Δ mutation prevents inhibition of Pol I transcription by rapamycin and those in our previous work (8) (see introduction), we carried out a series of experiments to compare the effects of rapamycin in rpd3Δ and control RPD3 strains. First, we measured the accumulation of total radioactive RNA in the presence of [14C]uracil. As shown in Fig. 4A, rapamycin inhibited stable RNA accumulation equally well in both the rpd3Δ and control WT RPD3 strains. Since rRNA represents ∼85% of total RNA under growth conditions used (our unpublished experiments), we conclude that there is little, if any, difference in the effect of rapamycin on rRNA accumulation.
FIG. 4.
Pol I transcription in rpd3Δ mutant cells is as sensitive to rapamycin as Pol I transcription in control WT cells is. (A) Accumulation of total RNA. Both rpd3Δ cells (NOY2015) and control RPD3 (NOY388; WT) cells were grown in SD complete medium supplemented with uracil (5 μg/ml). Each culture was diluted to a cell density of A600 of ∼0.2 and divided into two. [14C]uracil (0.5 μCi/ml) was added, and 15 min later, rapamycin (Rapa) (0.2 μg/ml) or vehicle was added to one of the duplicate cultures, respectively (time zero). Aliquots of the cultures were taken at the indicated times, and the amounts of 14C label (counts per minute in thousands [kcpm]) incorporated into the trichloroacetic acid (TCA)-insoluble fraction (total RNA) were determined. The degrees of inhibition of accumulation between 0 and 30 min and between 30 and 60 min by rapamycin were 64% and 85%, respectively, for the WT, and 58% and 85%, respectively, for the rpd3Δ strains. (B) The rpd3Δ and WT strains were grown in SD complete medium without methionine and were treated with rapamycin (0.2 μg/ml; time zero). Aliquots were taken at indicated times, mixed with [methyl-3H]methionine, and incubated for 5 min. Incorporation of 3H label into the TCA-insoluble fraction (“Protein”; mostly protein together with small amounts of RNA) and the RNA fraction (obtained after phenol extraction) was measured. The values normalized for those at the time of rapamycin addition are shown. (C) In a separate experiment carried out as described above for panel B, RNA samples were prepared after 5 min of 3H pulse-labeling at 15 and 30 min after rapamycin addition, and portions derived from an equal volume of the original culture were subjected to polyacrylamide/agarose composite gel electrophoresis followed by autoradiography. The amounts of 3H in radioactive rRNA bands (precursor 27S and 20S and mature 25S and 18S) were quantified, and the sum of these values normalized for those at time zero were calculated and are indicated near the bottom of each lane.
We also pulse-labeled cells with [methyl-3H]methionine to measure the rRNA synthesis rate as recommended for the analysis of the rRNA synthesis rate in yeast cells (52). Both total RNA fractions (Fig. 4B) and individual large rRNAs separated by gel electrophoresis (Fig. 4C) were analyzed. The rpd3Δ strain was at least as sensitive as the control RPD3 strain was to rapamycin.
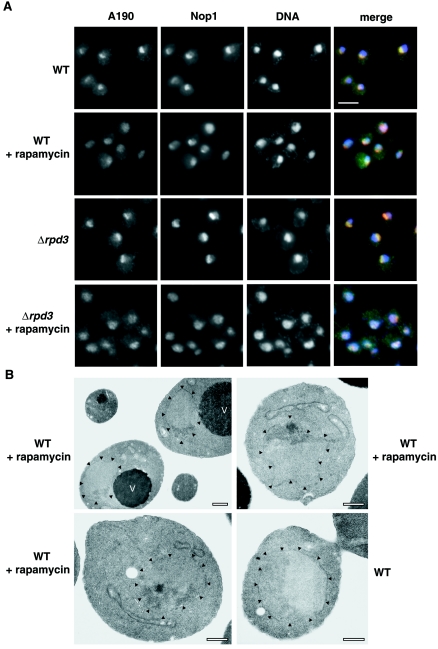
Next, we examined alterations of nucleolar structures after rapamycin treatment by IFM using antibodies against the A190 subunit of Pol I and against nucleolar protein Nop1 (Fig. 5A). Treatment of cells with rapamycin (0.2 μg/ml) for 1 h led to changes in nucleolar morphology. In some cells, Nop1 appeared to condense into a small spot with A190 spread through the nucleus, as described by Tsang et al. (48). In other cells, both A190 and Nop1 appeared to be spread through the nucleus. Examination of thin sections of rapamycin-treated (control WT RPD3) cells by EM also showed variable nucleolar morphology, with most nucleoli smaller, irregularly shaped, and somewhat denser than nucleoli in untreated control WT cells (Fig. 5B, top left). Nucleoli with a small dense spot (Fig. 5B, top right and bottom left), similar to those reported previously (48), were found but only in ∼9% of cells examined (n = 54). Using IFM, we also observed significant alteration of nucleolar structures after rapamycin treatment in rpd3Δ mutant cells (Fig. 5A). The pattern of alterations was generally similar, though not identical, to that observed for control RPD3 cells. Some rpd3Δ cells showed smaller Nop1 spots, and A190 either colocalized with it or spread through the nucleus. Other cells showed that both Nop1 and A190 spread through the nucleus. It is clear that both the rpd3Δ mutant cells and the control RPD3 cells undergo similar, though perhaps not identical, nucleolar alterations upon rapamycin treatment. It should be noted that the rpd3Δ mutation we used increased the degree of silencing of Pol II reporter genes inserted into rRNA genes (data not shown), a mutant phenotype that is well established by previous workers (45, 47).
FIG. 5.
IFM analysis of nucleolar structures in WT and rpd3Δ strains and EM analysis of WT strain treated with rapamycin. (A) Double-label indirect IFM of WT and rpd3Δ strains. Images of WT (NOY388) cells and rpd3Δ (NOY2015) cells are shown. Cells growing exponentially in YEPD complete medium at 30°C were treated with rapamycin (0.2 μg/ml for 1 h) and compared to control cells without rapamycin treatment. Images across each row represent the same field of cells. The first two images in the row depict the localization of two nucleolar proteins, A190 and Nop1, using anti-A190 and anti-Nop1 antibodies, respectively. The third image shows DNA stained with 4′,6′-diamidino-2-phenylindole (DAPI). The first three images are merged in the last panel with A190 in green, Nop1 in red, and DNA in blue. Bar, 5 μm. (B) EM analysis of WT cells grown as described above for panel A with or without rapamycin. The top left image represents the most common nucleolar morphology after treatment with rapamycin. The top right and bottom left images are representative of ∼9% of nucleolar cross sections observed in the EM. The bottom right panel is a WT cell without rapamycin treatment. Arrowheads point to the nuclear envelope. Vacuoles(V) are indicated. Bars, 0.5 μm.
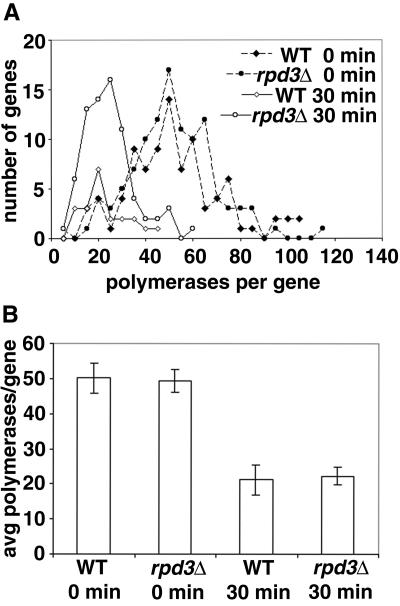
Finally, we examined the sensitivity of Pol I transcription to rapamycin using the EM Miller chromatin spread method. We compared the same rpd3Δ mutant and control RPD3 strains that were used to study the effect of rpd3Δ on conversion of open rRNA gene copies to the closed state during entry into stationary phase (39). Exponentially growing cells were treated with rapamycin (0.2 μg/ml) for 30 min, and RNA polymerase density for active genes was measured. As shown in Fig. 6, rapamycin treatment caused a 55% decrease in polymerase density in the rpd3Δ cells, similar to that (57% decrease) observed in control cells. There was no significant difference in the pattern of polymerase distribution between rpd3Δ and control RPD3 cells treated with rapamycin. These results strongly support the conclusion that the rpd3Δ mutation does not confer any rapamycin resistance to Pol I-transcribed rRNA genes.
FIG. 6.
Decrease in polymerase density per gene after rapamycin treatment in rpd3Δ and WT strains as analyzed by the EM Miller chromatin spread method. (A) WT RPD3 (JS772) and rpd3Δ (JS777) cells without rapamycin treatment (0 min) or treated with rapamycin (0.2 μg/ml) for 30 min were analyzed for the number of polymerases per gene. (B) The average (avg) number of polymerases that transcribe each active 35S rRNA gene was calculated from the data shown in panel A and is presented as a bar graph with 95% confidence intervals indicated.
Mutational inactivation of Rrn3 causes structural alterations of the nucleolus.
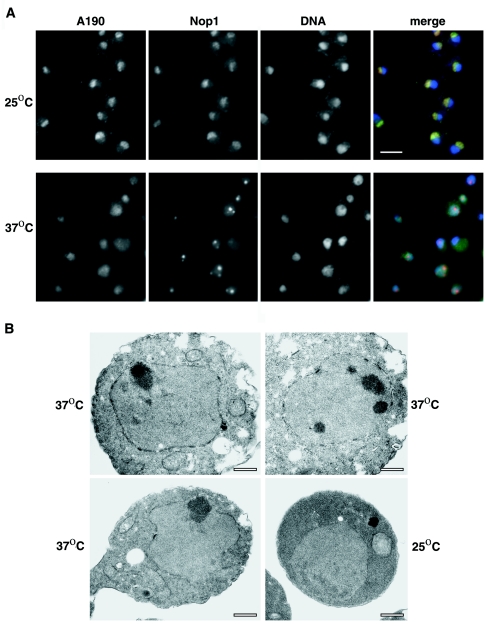
In the work proposing that rapamycin inhibits Pol I by recruiting Rpd3 to rRNA genes, it was also reported that temperature shift-up of an rrn3 temperature-sensitive (TS) mutant strain (rrn3-1/syc1-8) (5) did not cause morphological changes in nucleoli (48). On the basis of this observation, the authors concluded that morphological changes following rapamycin treatment are not a secondary consequence of inhibition of Pol I transcription. However, they did not show whether the conditions used (temperature shift-up to 37°C for 1 h), were sufficient to inhibit Pol I transcription in that particular rrn3 mutant. We used the rrn3 mutant [rrn3(S213P)] isolated and characterized in this laboratory (8) and reexamined this question. In rrn3(S213P) cells, a shift from 25°C to 37°C followed by incubation for 3 h was shown to cause 95 to 98% inhibition of Pol I activity relative to the same strain kept at 25°C (or relative to RRN3 cells shifted to 37°C) (8). Clear morphological alterations (Fig. 7) were observed in the rrn3(S213P) cells after temperature shift from 25° to 37°C for 3 h. Condensation of the nucleolus into several smaller and denser particles was evident by IFM using anti-Nop1 antibodies (Fig. 7A) or by EM examination of thin sections (Fig. 7B). Simultaneous examination of the mutant cells by IFM using anti-A190 antibodies revealed that Pol I did not colocalize with Nop1 and that Pol I was distributed throughout the nucleoplasm under such conditions. We also constructed a strain carrying both rrn3(S213P) and rpd3Δ and examined morphological changes at 3 h after shift to 37°C. Changes in nucleolar morphology observed by IFM were very similar to those in rrn3(S213P) cells (data not shown). Clearly, changes were induced as a consequence of inactivation of Rrn3, a Pol I-specific transcription factor, and were independent of Rpd3.
FIG. 7.
IFM and EM analyses of nucleolar structures in an rrn3 TS strain grown at a permissive temperature and after a shift to the nonpermissive temperature. (A) Double-label indirect IFM of rrn3 TS strain NOY1075. Mutant TS cells grown at 25°C in YEPD complete medium are shown in the top row; cells in the bottom row are shown after temperature shift to 37°C for 3 h. Images across each row are from the same field of cells. In the first three images, the localization of A190, Nop1, and DNA is observed, respectively, as described in the legend to Fig. 5A. The last image in each row is a merged image as in Fig. 5. Bar, 5 μm. (B) EM analysis of the same rrn3 TS strain grown at 25°C (bottom right image) and after temperature shift to 37°C for 3 h (remaining images). Bars, 0.5 μm.
The morphological features observed after the inactivation of Rrn3 were similar, but not identical, to those observed after rapamycin treatment. This is not unexpected, since Tor signaling affects various cellular activities in addition to Pol I activities, whereas inactivation of Rrn3 leads to specific inhibition of initiation of Pol I transcription. The morphological change of the nucleolus in rapamycin-treated cells seen by Tsang et al. (48) resembles that observed after the inactivation of Rrn3 presented here. In summary, our results contradict the conclusions reached by Tsang et al. (48) that inhibition of Pol I transcription and morphological alterations of the nucleolus are caused mainly by recruitment of Rpd3 to rRNA genes and that deletion of RPD3 results in resistance to the inhibitory effects of rapamycin on Pol I transcription and nucleolar structures.
DISCUSSION
In this paper, we first describe the new observation that deletion of histone deacetylase gene RPD3 inhibits the ability of rrn9Δ or rrn5Δ mutant cells to grow by transcribing rRNA genes by Pol II, i.e., to attain the PSW state. By studying the effects of rpd3Δ using an rrn9Δ strain carrying a GAL7-35S rRNA gene helper plasmid and a viable uaf30Δ strain which uses both Pol I and Pol II for rRNA transcription, we have demonstrated that the main cause of growth defects is a strong inhibition of rRNA gene transcription by Pol II, but not by Pol I, caused by the absence of histone deacetylase Rpd3 in these UAF mutant cells. These data support a specific role for Rpd3 in allowing transcription of 35S rRNA genes by Pol II in these mutant cells. We also describe a series of experiments to reexamine the effects of rapamycin on Pol I transcription in rpd3Δ strains. Although we cannot exclude participation of Rpd3 in the inhibition of Pol I by rapamycin in wild-type RPD3 cells, it is clear that Rpd3 is not necessary for such inhibition. Various states of rRNA gene chromatin defined by experimental observations together with known transitions between the states that require Rpd3 function(s) are summarized in Fig. 1 and discussed below. It should be noted that we omit discussion of Pol III transcription of 5S RNA genes and limit discussion of silencing to reporter genes inserted in the 35S rRNA coding region.
Inactivation of Rpd3 inhibits Pol II transcription of rRNA genes and reporter genes inserted in rRNA genes.
In growing yeast cells, about half of ∼150 tandemly repeated rRNA genes are actively transcribed, i.e., in the open state (9, 13, 39), and each of the rRNA gene copies is assumed to alternate rapidly between the open and closed states (10). Evidence strongly suggests that a Pol II reporter gene inserted in rRNA genes is silenced when the rRNA gene copy takes the open form, but that it is transcribed when the rRNA gene copy is in the closed form (4, 7). Reporter genes inserted in rRNA gene repeats are not silenced in PSW strains or when Pol I transcription is inactivated (4, 7). We have now found that inactivation of Rpd3 in rrn9Δ N-PSW strains or uaf30Δ strains leads to a large decrease in Pol II (but not Pol I) transcription of rRNA genes and leads to inhibition of production of PSW variants from rrn9Δ N-PSW cells or rrn5Δ N-PSW cells. We note that, in the presence of a helper plasmid, rpd3Δ rrn9Δ N-PSW cells grow on galactose at about the same growth rate as control RPD3 rrn9Δ N-PSW cells by synthesizing rRNA from the GAL7-35S rRNA gene fusion gene on a helper plasmid. Thus, contribution of Rpd3-dependent Pol II transcription of chromosomal rRNA genes to the total synthesis of 35S rRNA is small in the latter control cells. Therefore, it appears that inhibition of Pol II transcription of rRNA genes by the rpd3Δ mutation may be specific and is not a consequence of a general decrease in Pol II transcription caused, e.g., by a (hypothetical) decrease in the amount of Pol II in the rpd3Δ mutant strain. In addition, we also note that inhibition of Pol II transcription of rRNA genes is likely at the initiation step, because the transcription (i.e., elongation) of the GAL7-35S rRNA gene fusion genes on the helper plasmid by Pol II is not affected by the rpd3Δ mutation. Of course, the inhibition of Pol II transcription of rRNA genes could be a consequence of altered expression of some other genes caused by the rpd3 mutation, e.g., decreasing synthesis of a (hypothetical) positive factor required for Pol II (but not for Pol I) transcription of rRNA genes (nor for Pol II transcription of the GAL7-35S rRNA gene fusion gene). However, it is possible and perhaps more likely that the chromatin state of a specific rRNA gene promoter region is altered, either directly or indirectly as a result of the rpd3Δ mutation, so that Pol II transcription becomes unfavorable. It was previously suggested that UAF binding to the upstream element of the promoter forms a chromatin structure that activates Pol I and silences Pol II initiation of transcription of rRNA genes and that this structure forms a nucleation site for spreading of this chromatin state (7). According to this model, rpd3Δ mutation alters chromatin structures at the nucleation site, making the structure more repressive for Pol II transcription of both rRNA genes and Pol II reporter genes. Similarly, the observation that sir2Δ mutation increases transcription of Pol II reporter genes, i.e., abolishes silencing (3, 14, 44) but does not increase or inhibit Pol II transcription of rRNA genes (this paper) indicates that Sir2 may be required for spreading, but not initial nucleation, of the proposed Pol II repressive chromatin structure. We note that previous analysis of histone acetylation did not find any significant difference in the degree of acetylation of histones H3 and H4 in several regions of rRNA genes analyzed between rpd3Δ and control RPD3 strains (39). Thus, if the chromatin alteration caused by the rpd3 mutation at the proposed nucleation site is a direct consequence of the mutation, one might expect a difference(s) in the degree of acetylation of histones H3 and/or H4 associated with UAF or nearby histones or possibly in the (hypothetical) acetylation of nonhistone UAF subunit proteins between the rpd3 mutant and control RPD3 strains. The search for such possible difference(s) will be a subject of future studies.
The interpretation of the various experimental observations suggests that the chromatin structure of closed rRNA gene copies in growing cells [rRNA gene (C) in growing cells (Fig. 1)] may have a feature similar to that of rRNA gene copies in PSW cells [rRNA gene (P) (Fig. 1)]. We note that even WT strains reveal Pol II transcription of rRNA genes under some conditions, e.g., after incubation at 37°C (7), and this may likely take place in the closed state of rRNA gene, which allows Pol II transcription of reporter Pol II genes but not Pol I transcription of rRNA genes. However, the physiological significance of the observed (weak) Pol II transcription of rRNA genes in WT strains is presently unclear.
Rpd3-dependent change from the open to closed state of rRNA genes during the post-diauxic phase.
The change from the open to closed state during entry into stationary phase (pathway A in Fig. 1) requires Rpd3 (39), but its significance and other factors involved in this conversion are unknown. The new observations described here suggest that the closed state in stationary phase shares common features with the state in PSW cells. Besides the absence or the great reduction of Pol I transcription of rRNA genes, both states can be generated from the open state in growing cells only in the presence of active Rpd3; in the rpd3 mutant, pathway A does not take place, and pathway C, leading to the PSW cells in the absence of functional UAF, is greatly reduced. Since the chromatin state rRNA gene (P) in PSW cells can be formed by mutations in UAF components, it may be interesting to examine possible (reversible) modifications of these components (including histones H3 and H4 of UAF) in rRNA gene chromatin in post-diauxic-phase or stationary-phase cells as well as in closed rRNA gene copies in growing cells. It should be noted that Rpd3 is required for conversion of the open state to the closed state during the post-diauxic phase but is not required for the maintenance of the closed state in the post-diauxic and stationary phases. Or simply stated, in rpd3Δ mutant cells going from exponential to stationary phase, the open genes fail to close normally but the closed genes apparently remain closed (39). This known feature is somewhat difficult to explain in terms of simple acetylated states of rRNA gene chromatin-associated histones. On the basis of the observed inhibition of Pol II transcription of rRNA genes by the rpd3Δ mutation, one could formulate some models to explain this feature; for example, one can hypothesize that, during the post-diauxic phase, Rpd3-dependent Pol II transcription of rRNA genes similar to that observed in UAF mutant cells might be induced via some signaling system(s) and that the products of such transcription reaction are required for the conversion of the open to closed state of rRNA genes. Such a scenario may explain why Rpd3 is required for the formation, but not the maintenance, of the closed state of rRNA gene copies in the post-diauxic phase. Testing the validity of such a scenario is a subject for future studies.
Inhibition of Pol I transcription of rRNA genes by rapamycin does not require Rpd3.
We have not observed any significant difference in the sensitivity of Pol I transcription to rapamycin between rpd3Δ strains and control RPD3 strains as judged by measuring the rate of accumulation of total RNA, the rate of synthesis of rRNA using [methyl-3H]methionine pulse-labeling, and by measuring polymerase density in individual genes visualized in Miller chromatin spreads. Tsang et al. (48) concluded that Pol I transcription was resistant to rapamycin in an rpd3 mutant on the basis of measurements of the amounts of unstable 35S pre-rRNA by Northern blot analysis. We also analyzed the amount of 5′ ends of 35S rRNA in rpd3Δ and control RPD3 strains after rapamycin treatment by primer extension. Although the decrease in rpd3Δ cells was somewhat smaller than that in WT cells, even the decrease in WT cells was much smaller than the decrease in rRNA synthesis rate measured by the other methods described in this paper (our unpublished experiments). Rapamycin causes an inhibition of rRNA processing in addition to inhibiting Pol I transcription, leading to an increased appearance of 35S pre-rRNA relative to the control in pulse-labeling experiments (34). Therefore, the use of 5′-end analysis or Northern analysis of 35S pre-rRNA may not be appropriate for measurements of Pol I transcription rate after rapamycin treatment.
Regarding morphological changes observed after rapamycin treatment, we did not observe any significant difference between the rpd3Δ mutant and control RPD3 strain, whereas Tsang et al. (48) reported morphological changes in RPD3, but not rpd3Δ, cells. The reason for the discrepancy is unknown.
The discrepancy between our observations on morphological changes in nucleoli of rrn3 mutant cells and the results of Tsang et al. (48) may be due to the use of different mutations. The degree of Pol I inhibition in their rrn3 mutant under the conditions used in their studies was not shown. It is difficult therefore to directly compare observations, since different rrn3 TS mutations respond to temperature shift with different rapidities. Our results clearly demonstrate that morphological alterations of the nucleolus, including dispersion of Pol I through the nucleoplasm, take place as a result of inactivation of Rrn3 by temperature shift up in the absence of rapamycin and in both RPD3 and rpd3Δ cells. Clearly, inactivation of Rrn3 itself (or the resultant inhibition of Pol I transcription) disrupts nucleolar organization and disperses Pol I into the nucleoplasm separate from the fragmented nucleolar particles containing Nop1. Thus, although we cannot exclude the possibility that rapamycin treatment can cause morphological changes of the nucleolus independently of its inhibition of Pol I transcription, such changes can take place in the absence of Rpd3. In addition, it is possible that disruption of the nucleolus observed in rapamycin-treated cells is a consequence of rapamycin effects on the Pol I transcription machinery (including Rrn3) and/or the resultant inhibition of rRNA transcription. By comparing the effects of Pol I subunit mutations with the effects of several inhibitors of rRNA synthesis, it was suggested that Pol I may play a structural role in the maintenance of nucleolar structures in addition to its functional role in rRNA synthesis (32). It is possible that Rrn3, which is known to interact with Pol I (12, 24, 53), plays a similar role.
In summary, the results of reexamination of the effects of rapamycin on Pol I transcription and nucleolar morphology presented in this paper combined with those reported previously (8, 39) support signaling pathways A and B shown in Fig. 1; that is, during the transition from exponential phase to post-diauxic/stationary phase, yeast cells decrease Pol I transcription rate by two mechanisms: (i) in pathway A, the number of active genes is decreased by an unknown mechanism, which is Rpd3 dependent and is independent of the rapamycin-sensitive Tor pathway, and (ii) in pathway B, the transcription activity of individual “open” rRNA genes is decreased by inhibiting Tor signaling, which resembles rapamycin inhibition and is Rpd3 independent. We note that inhibition of ribosomal protein gene expression by rapamycin appears to be achieved at least in part by a recruitment of Rpd3 to the promoters of these genes (19, 36) and that a similar model invoking recruitment of Rpd3 to the rRNA gene promoters (48) might be attractive. However, our results do not support such a model. Inactivation of Tor by rapamycin is known to cause inactivation of some components of the Pol I transcription machinery, such as Rrn3 (or its mouse homolog, TIF-IA) and/or Pol I, as shown in both mammalian and yeast systems (8, 28; our unpublished results), and we now conclude that such alterations must almost certainly take place without participation of the Rpd3 histone deacetylase.
It is evident that regulation of rRNA transcription is complex and that rRNA gene chromatin structures participate in this regulation. Chromatin structures of rRNA genes also play important roles in various functions unrelated to Pol I transcription of rRNA genes, such as regulation of cell cycle progression by sequestration of and controlled release of Cdc14 (11, 41, 50), regulation of recombination between rRNA gene repeats (15, 26), and regulation of cell aging (17, 43). Molecular characterization of various rRNA gene chromatin states and pathways depicted in Fig. 1 and discussed in this paper represent an effort toward achieving the challenging goal of understanding the complex structures of the nucleolus that organize and regulate these various essential cellular functions.
Acknowledgments
We thank M. Grunstein and M. Hampsey for gifts of plasmid pMV34 and pM1061, respectively. We also thank Kristilyn Eliason and P. Tongaonkar for participation in earlier stages of this work and D. A. Schneider for critical reading of the manuscript.
The work was supported by Public Health Service grants GM-35949 (to M.N.), GM-63952 (to A.L.B.) and AG-23719 (to J.P.A.).
REFERENCES
- 1.Braunstein, M., A. B. Rose, S. G. Holmes, C. D. Allis, and J. R. Broach. 1993. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 7:592-604. [DOI] [PubMed] [Google Scholar]
- 2.Braunstein, M., R. E. Sobel, C. D. Allis, B. M. Turner, and J. R. Broach. 1996. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol. Cell. Biol. 16:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryk, M., M. Banerjee, M. Murphy, K. E. Knudsen, D. J. Garfinkel, and M. J. Curcio. 1997. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 11:255-269. [DOI] [PubMed] [Google Scholar]
- 4.Buck, S. W., J. J. Sandmeier, and J. S. Smith. 2002. RNA polymerase I propagates unidirectional spreading of rDNA silent chromatin. Cell 111:1003-1014. [DOI] [PubMed] [Google Scholar]
- 5.Cadwell, C., H. J. Yoon, Y. Zebarjadian, and J. Carbon. 1997. The yeast nucleolar protein Cbf5p is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3. Mol. Cell. Biol. 17:6175-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanaugh, A. H., I. Hirschler-Laszkiewicz, Q. Hu, M. Dundr, T. Smink, T. Misteli, and L. I. Rothblum. 2002. Rrn3 phosphorylation is a regulatory checkpoint for ribosome biogenesis. J. Biol. Chem. 277:27423-27432. [DOI] [PubMed] [Google Scholar]
- 7.Cioci, F., L. Vu, K. Eliason, M. Oakes, I. Siddiqi, and M. Nomura. 2003. Silencing in yeast rDNA chromatin: reciprocal relationship in gene expression between RNA polymerase I and II. Mol. Cell 12:135-145. [DOI] [PubMed] [Google Scholar]
- 8.Claypool, J. A., S. L. French, K. Johzuka, K. Eliason, L. Vu, J. A. Dodd, A. L. Beyer, and M. Nomura. 2004. Tor pathway regulates Rrn3p-dependent recruitment of yeast RNA polymerase I to the promoter but does not participate in alteration of the number of active genes. Mol. Biol. Cell 15:946-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dammann, R., R. Lucchini, T. Koller, and J. M. Sogo. 1993. Chromatin structures and transcription of rDNA in yeast Saccharomyces cerevisiae. Nucleic Acids Res. 21:2331-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dammann, R., R. Lucchini, T. Koller, and J. M. Sogo. 1995. Transcription in the yeast rRNA gene locus: distribution of the active gene copies and chromatin structure of their flanking regulatory sequences. Mol. Cell. Biol. 15:5294-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Amours, D., and A. Amon. 2004. At the interface between signaling and executing anaphase—Cdc14 and the FEAR network. Genes Dev. 18:2581-2595. [DOI] [PubMed] [Google Scholar]
- 12.Fath, S., P. Milkereit, G. Peyroche, M. Riva, C. Carles, and H. Tschochner. 2001. Differential roles of phosphorylation in the formation of transcriptional active RNA polymerase I. Proc. Natl. Acad. Sci. USA 98:14334-14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French, S. L., Y. N. Osheim, F. Cioci, M. Nomura, and A. L. Beyer. 2003. In exponentially growing Saccharomyces cerevisiae cells, rRNA synthesis is determined by the summed RNA polymerase I loading rate rather than by the number of active genes. Mol. Cell. Biol. 23:1558-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritze, C. E., K. Verschueren, R. Strich, and R. E. Esposito. 1997. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 16:6495-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb, S., and R. E. Esposito. 1989. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56:771-776. [DOI] [PubMed] [Google Scholar]
- 16.Gray, J. V., G. A. Petsko, G. C. Johnston, D. Ringe, R. A. Singer, and M. Werner-Washburne. 2004. “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 68:187-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarente, L. 2000. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 14:1021-1026. [PubMed] [Google Scholar]
- 18.Hoppe, G. J., J. C. Tanny, A. D. Rudner, S. A. Gerber, S. Danaie, S. P. Gygi, and D. Moazed. 2002. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol. Cell. Biol. 22:4167-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey, E. L., A. F. Shamji, B. E. Bernstein, and S. L. Schreiber. 2004. Rpd3p relocation mediates a transcriptional response to rapamycin in yeast. Chem. Biol. 11:295-299. [DOI] [PubMed] [Google Scholar]
- 20.Imai, S., C. M. Armstrong, M. Kaeberlein, and L. Guarente. 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795-800. [DOI] [PubMed] [Google Scholar]
- 21.Jacinto, E., and M. N. Hall. 2003. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell Biol. 4:117-126. [DOI] [PubMed] [Google Scholar]
- 22.Ju, Q., and J. R. Warner. 1994. Ribosome synthesis during the growth cycle of Saccharomyces cerevisiae. Yeast 10:151-157. [DOI] [PubMed] [Google Scholar]
- 23.Keener, J., J. A. Dodd, D. Lalo, and M. Nomura. 1997. Histones H3 and H4 are components of upstream activation factor required for the high-level transcription of yeast rDNA by RNA polymerase I. Proc. Natl. Acad. Sci. USA 94:13458-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keener, J., C. A. Josaitis, J. A. Dodd, and M. Nomura. 1998. Reconstitution of yeast RNA polymerase I transcription in vitro from purified components. TATA-binding protein is not required for basal transcription. J. Biol. Chem. 273:33795-33802. [DOI] [PubMed] [Google Scholar]
- 25.Keys, D. A., B. S. Lee, J. A. Dodd, T. T. Nguyen, L. Vu, E. Fantino, L. M. Burson, Y. Nogi, and M. Nomura. 1996. Multiprotein transcription factor UAF interacts with the upstream element of the yeast RNA polymerase I promoter and forms a stable preinitiation complex. Genes Dev. 10:887-903. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, T., T. Horiuchi, P. Tongaonkar, L. Vu, and M. Nomura. 2004. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell 117:441-453. [DOI] [PubMed] [Google Scholar]
- 27.Kurdistani, S. K., and M. Grunstein. 2003. Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell Biol. 4:276-284. [DOI] [PubMed] [Google Scholar]
- 28.Mayer, C., J. Zhao, X. Yuan, and I. Grummt. 2004. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 18:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milkereit, P., and H. Tschochner. 1998. A specialized form of RNA polymerase I, essential for initiation and growth-dependent regulation of rRNA synthesis, is disrupted during transcription. EMBO J. 17:3692-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogi, Y., L. Vu, and M. Nomura. 1991. An approach for isolation of mutants defective in 35S ribosomal RNA synthesis in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 88:7026-7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakes, M., J. P. Aris, J. S. Brockenbrough, H. Wai, L. Vu, and M. Nomura. 1998. Mutational analysis of the structure and localization of the nucleolus in the yeast Saccharomyces cerevisiae. J. Cell Biol. 143:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oakes, M., Y. Nogi, M. W. Clark, and M. Nomura. 1993. Structural alterations of the nucleolus in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Mol. Cell. Biol. 13:2441-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oakes, M., I. Siddiqi, L. Vu, J. Aris, and M. Nomura. 1999. Transcription factor UAF, expansion and contraction of ribosomal DNA (rDNA) repeats, and RNA polymerase switch in transcription of yeast rDNA. Mol. Cell. Biol. 19:8559-8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers, T., and P. Walter. 1999. Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae. Mol. Biol. Cell 10:987-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robyr, D., Y. Suka, I. Xenarios, S. K. Kurdistani, A. Wang, N. Suka, and M. Grunstein. 2002. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109:437-446. [DOI] [PubMed] [Google Scholar]
- 36.Rohde, J., and M. E. Cardenas. 2003. The Tor pathway regulates gene expression by linking nutrient sensing to histone acetylation. Mol. Cell. Biol. 23:629-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rundlett, S. E., A. A. Carmen, R. Kobayashi, S. Bavykin, B. M. Turner, and M. Grunstein. 1996. HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc. Natl. Acad. Sci. USA 93:14503-14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2002. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol. Biol. Cell 13:2207-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandmeier, J. J., S. French, Y. Osheim, W. L. Cheung, C. M. Gallo, A. L. Beyer, and J. S. Smith. 2002. RPD3 is required for the inactivation of yeast ribosomal DNA genes in stationary phase. EMBO J. 21:4959-4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman, F., G. R. Fink, and J. B. Hicks. 1986. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Shou, W., J. H. Seol, A. Shevchenko, C. Baskerville, D. Moazed, Z. W. Chen, J. Jang, A. Shevchenko, H. Charbonneau, and R. J. Deshaies. 1999. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97:233-244. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqi, I. N., J. A. Dodd, L. Vu, K. Eliason, M. L. Oakes, J. Keener, R. Moore, M. K. Young, and M. Nomura. 2001. Transcription of chromosomal rRNA genes by both RNA polymerase I and II in yeast uaf30 mutants lacking the 30 kDa subunit of transcription factor UAF. EMBO J. 20:4512-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinclair, D. A., and L. Guarente. 1997. Extrachromosomal rDNA circles—a cause of aging in yeast. Cell 91:1033-1042. [DOI] [PubMed] [Google Scholar]
- 44.Smith, J. S., and J. D. Boeke. 1997. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 11:241-254. [DOI] [PubMed] [Google Scholar]
- 45.Smith, J. S., E. Caputo, and J. D. Boeke. 1999. A genetic screen for ribosomal DNA silencing defects identifies multiple DNA replication and chromatin-modulating factors. Mol. Cell. Biol. 19:3184-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suka, N., Y. Suka, A. A. Carmen, J. Wu, and M. Grunstein. 2001. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 8:473-479. [DOI] [PubMed] [Google Scholar]
- 47.Sun, Z. W., and M. Hampsey. 1999. A general requirement for the Sin3-Rpd3 histone deacetylase complex in regulating silencing in Saccharomyces cerevisiae. Genetics 152:921-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsang, C. K., P. G. Bertram, W. Ai, R. Drenan, and X. F. Zheng. 2003. Chromatin-mediated regulation of nucleolar structure and RNA Pol I localization by TOR. EMBO J. 22:6045-6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidal, M., and R. F. Gaber. 1991. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:6317-6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visintin, R., E. S. Hwang, and A. Amon. 1999. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398:818-823. [DOI] [PubMed] [Google Scholar]
- 51.Vu, L., I. Siddiqi, B. S. Lee, C. A. Josaitis, and M. Nomura. 1999. RNA polymerase switch in transcription of yeast rDNA: role of transcription factor UAF (upstream activation factor) in silencing rDNA transcription by RNA polymerase II. Proc. Natl. Acad. Sci. USA 96:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner, J. R. 1991. Labeling of RNA and phosphoproteins in Saccharomyces cerevisiae. Methods Enzymol. 194:423-428. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto, R. T., Y. Nogi, J. A. Dodd, and M. Nomura. 1996. RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. EMBO J. 15:3964-3973. [PMC free article] [PubMed] [Google Scholar]
- 54.Zaragoza, D., A. Ghavidel, J. Heitman, and M. C. Schultz. 1998. Rapamycin induces the G0 program of transcriptional repression in yeast by interfering with the TOR signaling pathway. Mol. Cell. Biol. 18:4463-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]