Abstract
The aim of the present work was to characterize Na+ currents through nonselective cation channels (NSCCs) in protoplasts derived from root cells of Arabidopsis. The procedure of the protoplast isolation was modified to increase the stability of Arabidopsis root protoplasts in low external Ca2+ by digesting tissue in elevated Ca2+. Experiments in whole-cell and outside-out modes were carried out. We found that Na+ currents in Arabidopsis root protoplasts were mediated by cation channels that were insensitive to externally applied tetraethylammonium+ and verapamil, had no time-dependent activation (permanently opened or completely activated within 1–2 ms), were voltage independent, and were weakly selective for monovalent cations. The selectivity sequence was as follows: K+ (1.49) > NH4+ (1.24) > Rb+ (1.15) ≈ Cs+ (1.10) ≈ Na+ (1.00) > Li+ (0.73) > tetraethylammonium+ (0.47). Arabidopsis root NSCCs were blocked by H+ (pK ≈ 6.0), Ca2+ (K1/2 ≈ 0.1 mm), Ba2+, Zn2+, La3+, Gd3+, quinine, and the His modifier diethylpyrocarbonate. They were insensitive to most organic blockers (nifedipine, verapamil, flufenamate, and amiloride) and to the SH-group modifier p-chloromercuriphenyl sulfonic acid. Voltage-insensitive, Ca2+-sensitive single channels were also resolved. Properties of Arabidopsis root NSCCs are discussed and compared with characteristics of similar conductances studied previously in plants and animals. It is suggested that NSCCs present a distinct group of plant ion channels, mediating toxic Na+ influx to the cell and probably having other important roles in physiological processes of plants.
Excess salt in soil is an important environmental factor limiting plant growth and the yield of crops. About 6% of total global land area and one third of the world's irrigated land are significantly affected by soil salinity, and one of the major components limiting plant growth in such soils is high Na+ (Bergmann, 1992; Flowers and Yeo, 1995). Although numerous studies have been carried out on plant responses to high Na+, mechanisms of Na+ transport, toxicity, and tolerance in plants are far from understood. Increase of Na+ content occurs when passive influx of Na+ through cation channels prevails over processes that actively remove Na+ from the cytoplasm to the extracellular space. Several classes of cation channels seem to be involved in mediating toxic Na+ influx, including outward and inward-rectifying K+-selective channels and nonselective cation channels (NSCCs; see reviews by Amtmann and Sanders, 1999; Tyerman and Skerrett, 1999). Evidence is now accumulating that suggests that NSCCs are the major pathway for Na+ influx into root cells (White and Lemtiri-Chlieh, 1995; Roberts and Tester, 1997; Tyerman et al., 1997; Buschmann et al., 2000; Davenport and Tester, 2000). This is mainly because these channels do not select strongly against Na+ (contrary to outward and inward-rectifying K+-selective channels) and are time and voltage independent (Amtmann and Sanders, 1999). Furthermore, the partial sensitivity of NSCCs to Ca2+ and Mg2+ is directly reflected in the partial inhibition by these cations of Na+ influx into intact roots (Davenport and Tester, 2000).
Although NSCCs and NSCC-like conductances in roots have been characterized in several cereals (barley [Hordeum vulgare], wheat [Triticum aestivum], corn [Zea mays], and rye [Secale cereale]), they have not been described in roots of the important model plant, Arabidopsis. Recent sequencing of the Arabidopsis genome creates unique opportunities for the molecular identification of Na+ entry pathways. Therefore, the electrophysiological characterization of NSCCs in Arabidopsis roots is clearly essential. The aim of the present work was to characterize NSCC-mediated Na+ fluxes in protoplasts derived from Arabidopsis root cells. Experiments in whole-cell and outside-out modes were carried out.
RESULTS
Na+ Influx through NSCCs
Inward and outward Na+ currents could be measured in all protoplasts, with currents increasing with increasing external Na+ (Fig. 1). In 69.5% of total protoplasts studied (n = 380), an inward Na+ current could be measured that had no visible time dependence or that only slightly decreased (by up to 30% of the initial current amplitude during voltage pulses of 2.5 s from −70 mV to −160 mV). Eleven and one-half percent of protoplasts revealed a stronger time-dependent decrease of this current (with up to 40%–60% decrease of the initial current amplitude over 2.5-s pulses). The remaining 19% of protoplasts revealed a time-dependent increase of the inward Na+ current, from 10% to 2- to 3-fold. Only protoplasts having no time-dependent component or showing a slight decrease in the current were used for studying the NSCCs; this was the most abundant group of protoplasts measured.
Figure 1.
Instantaneous currents through the plasma membrane of Arabidopsis root protoplasts in response to voltage-clamp steps (ΔE = 15 mV) from −160 to 80 mV (holding potential = −70 mV). Solutions contained 10, 20, or 100 mm NaCl. Data were obtained from the same protoplast (dp = 21 μm). Before recording, the cell was exposed to each NaCl concentration for 15 min. Concentrations are given in mm.
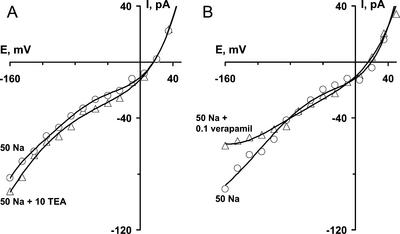
To distinguish Na+ influx catalyzed by NSCCs from that catalyzed by K+-selective and Ca2+-selective channels, experiments with the K+ and Ca2+ channel blockers tetraethylammonium (TEA+) and verapamil were carried out (Fig. 2). The addition of 10 mm TEA+ to a background of 50 mm NaCl did not decrease currents; in fact, it usually slightly increased the inward current, probably because TEA+ permeates the NSCCs (see below). In the same conditions, 100 μm verapamil slightly decreased the inward currents (by up to 30% of the current amplitude) at voltages negative of −90 mV, linearizing the current-voltage (I/V) curve. It is notable that some protoplasts revealed linear I/V curves in 10 to 100 mm NaCl solutions before the addition of verapamil. Inward Na+ currents in such cells were insensitive to verapamil. It is proposed that the nonlinear increase in inward Na+ currents at hyperpolarized potentials is due to activation of verapamil-sensitive hyperpolarization-activated Ca2+ channels, which are, in the relatively low Ca2+ concentration used in the present study, permeable to Na+ (Fairley-Grenot and Assmann, 1992). Most Na+ currents measured were unrelated to activities of K+ and Ca2+ channels and were evidently mediated by NSCCs.
Figure 2.
Changes in NSCC I/V relations caused by extracellular application of TEA+ (A) (dp = 19.5 μm) or verapamil (B) (dp = 20.5 μm). Concentrations are indicated in mm. Na+ was present as the chloride salt. Data were obtained after 10-min exposure to TEACl or verapamil.
In addition to these relatively stable NSCC currents, in 42% of cells, large “spiky” inward currents (nS range) were seen at voltages negative of −100 mV and NaCl concentrations above 20 mm. These currents were due to the Na+ influx, because they were not present when Na+ was removed from the external solution (data not shown). “Spiky” inward Na+ currents, reminiscent of those seen in wheat by Tyerman et al. (1997), generally increased with increasing time in NaCl-containing solutions, although, in some cases, “spiky” currents were only seen in some individual recordings and were not repeatable later on the same cell. However, in 58% of protoplasts, the whole-cell Na+ influx was dominated quantitatively by the stable conductance due to the activity of the NSCCs. The “spiky” conductances superimposed on this stable conductance were related to the activity of another transport system and were not considered further in this study.
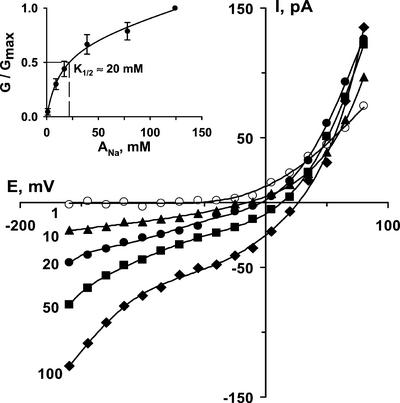
Inward Na+ current increased with increasing external Na+, tending to saturate by about 50 mm and with a K1/2 of approximately 20 mm (Fig. 3). However, at NaCl concentrations over 100 mm, conductance again increased, although we obtained only a few successful measurements at 150 mm NaCl because of instability of patches at high NaCl.
Figure 3.
The dependence of instantaneous Na+ currents of Arabidopsis root NSCCs on external NaCl concentration (indicated in mm) (dp = 22 μm). Inset shows dose-response relation (dp = 21.5 ± 0.7 μm; n = 5; ±se). G/Gmax is the ratio between conductance recorded at tested Na+ activities (G) and conductance at 124 mm NaCl (Gmax). Conductance values are conductances for inward current immediately negative of the reversal potential. Data were fitted by hyperbolic function (double rectangular, five parameters) by Sigma Plot software. K1/2 was estimated graphically.
An increase in external NaCl concentration also shifted the reversal potential positive, consistent with currents being dominated by the movement of monovalent cations (Fig. 3). However, in all protoplasts used, there was also a Cl− influx component, which contributed to the outward current (see also Tyerman et al., 1997). Due to the variable effects of pharmacological agents on reversal potentials (see below), it is likely that the relative contributions of Na+ and Cl− conductances to total protoplast conductance varied between cells. Therefore, we did not use reversal potentials to assess the selectivity of NSCCs. Instead, we measured conductance from the inward Na+ current, averaged over the 30 mV immediately negative of the reversal potential (Piñeros and Tester, 1995; Table I). This was, in any case, arguably more physiologically relevant to consideration of toxic Na+ influx. Protoplasts having only a linear I/V curve (recorded with 25 mm external NaCl) or verapamil-treated protoplasts (2-min exposure to 100 μm verapamil) were used for selectivity measurements. In all selectivity measurements, a background of 25 mm NaCl was used to prevent permeation of the test ion through the time-dependent inward-rectifying K+-selective channels (and a control background current measured in simply 25 mm NaCl was then subtracted from all currents). NSCCs from Arabidopsis roots were found to be slightly more permeable to K+ than to Rb+, Cs+, and Na+ (Table I) but had a 2- to 3-fold lower permeability to Li+ and TEA+.
Table I.
Selectivity ratios for nonselective cation channels measured by patch clamping protoplasts from root cortex of Arabidopsis
| Cation Tested | Permeability Relative to Na+ |
|---|---|
| K+ | 1.49 ± 0.15 |
NH
|
1.24 ± 0.11 |
| Rb+ | 1.15 ± 0.10 |
| Cs+ | 1.1 ± 0.08 |
| Li+ | 0.73 ± 0.07 |
| TEA+ | 0.47 ± 0.04 |
Data were obtained on the basis of conductances over the 30 mV immediately negative of the reversal potential (Piñeros and Tester, 1995). Solutions contained 25 mm NaCl + 25 mm Cl− salt of the test cation, all on a background of 0.5 mm CaCl2. Values of currents for conductance calculations were obtained by subtraction of background currents caused by 25 mm NaCl. Cells used were insensitive to verapamil. Values are the average of three to four measurements ± se.
Pharmacology
A wide range of inorganic cations inhibited the activity of NSCCs (Table II). Gd3+, La3+, and Zn2+ were the most potent blockers, causing a 10-fold decrease of NSCC conductance in 72% of protoplasts. In 37% of cells, 100 μm Gd3+ or La3+ completely inhibited the inward current with 50 mm NaCl in the bathing solution. Ba2+ or Zn2+ induced the same effect in 26% of protoplasts when applied at 0.3 to 1 mm.
Table II.
Effects of cation channel blockers and modifiers on nonselective cation channels measured by patch clamping protoplasts from root cortex of Arabidopsis
| Blockers, Modifiers | Range Tested | Effecta | Description of Block |
|---|---|---|---|
| m | |||
| Ca2+ | 5 × 10−5 to 5 × 10−2 | + | Partial; Ki ≈ 0.1 mm |
| H+ | 10−9 to 10−4 (pH 9.5–3.8) | + | Partial; pK ≈ 6 |
| Zn2+ | 10−7 to 10−3 | + | Complete by 0.3–1 mm |
| Ba2+ | 10−7 to 10−3 | + | Complete by 0.3–1 mm |
| La3+, Gd3+ | 10−7 to 10−3 | + | Complete by 0.1 mm |
| Quinine | 10−5 to 10−3 | + | Partial; Ki ≈ 0.5 mm |
| Verapamil | 10−5 to 10−3 | − | Linearized I/V curve |
| Nifedipine, flufenamate, amiloride | 10−6 to 10−3 | − | |
| DEPC | 1-h pretreatment in 10−5–10−4 | + | Partial (4–6-fold) |
| p-Chloromercuriphenyl sulfonic acid | 1-h pretreatment in 10−4–3 × 10−4 | − |
+, Addition of blocker inhibited currents; −, addition of blocker had no effect.
The sensitivity of NSCCs to H+ and Ca2+ differed from cell to cell. In most cells (77%), the minimal NSCC current was found at pH 3.8 (usually 10–20 pA at 100 mm NaCl, 0.5 mm CaCl2; Fig. 4), although lower pHs were not tested as they caused membrane breakdown. The current at pH 9.5 (the highest tested pH value) was 10 to 20 times greater than that at pH 3.8. The pK of NSCC conductance (Fig. 4, inset) was approximately 6.
Figure 4.
Inhibition of inward Na+ currents through NSCCs by external acidification. pH values are indicated (dp = 23.5 mm). Inset shows dose-response relation (dp = 22.5 ± 0.8 μm; n = 4; ±se). G/Gmax is the ratio between conductance recorded at the tested pH (G) and maximal conductance registered at pH 9.5 (Gmax). Conductance values are conductances for inward current immediately negative of the reversal potential. Solutions contained 100 mm NaCl. Data were fitted by sigmoidal function (Hill, four parameters) by Sigma Plot software. PK was determined graphically.
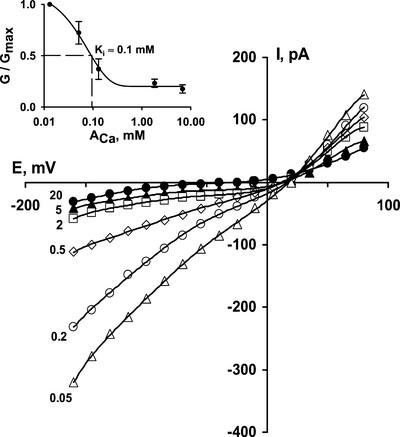
The effect of Ca2+ on NSCCs was studied at Ca2+ concentrations from 0.05 to 20 mm (Fig. 5). Lower or higher concentrations were not used because they caused, respectively, destabilization of the seal or an increase in conductance due to movement of Ca2+ (probably through NSCCs and/or Ca2+ channels). When Ca2+ in the bathing solution was decreased from 20 to 0.05 mm, the NSCC conductance increased by 5- to 6-fold. Half-maximal block of Ca2+ was found at an activity of 0.1 mm (Fig. 5). The change in reversal potential seen upon blockade of inward currents is presumably due to the increasing influence of anion currents on the whole-cell currents (ECl being −67 to −120 mV in these experiments, moving more negative upon addition of CaCl2). As Erev always remains well positive of ECl, it is likely that there always remains a significant component of Na+ conductance—as reported in other systems, blockade of Na+ currents by Ca2+ is incomplete.
Figure 5.
Inhibition of Na+ currents through NSCCs by external Ca2+ (dp = 23.5 μm). Added CaCl2 concentrations (in mm) are indicated on the figure. Inset shows dose-response relation (average dp = 19.5 ± 0.6 μm; n = 4; ±se). G/Gmax is the ratio between conductance recorded at tested Ca2+ activity (G) and maximal conductance registered at 0.05 mm Ca2+ (Gmax). Conductance values are conductances for inward current immediately negative of the reversal potential. Solutions contained 100 mm NaCl. Data were fitted by exponential decay function (double, five parameters) by Sigma Plot software. Ki was determined graphically.
It should be noted that the sensitivity of inward Na+ current to H+ and Ca2+ varied between protoplasts. NSCCs were less sensitive to protons and divalent cations in 23% of cells (data not shown). In such protoplasts, only 50% to 70% of inward Na+ current was inhibited upon addition of high Ca2+ or low pH. However, La3+ and Gd3+ reduced currents by 90% in all protoplasts.
At concentrations of 1 to 1,000 μm, externally added flufenamate, amiloride, and nifedipine did not inhibit NSCCs (data not shown). Among the organic blockers tested, only quinine inhibited NSCCs (Fig. 6). Quinine blockade developed over a few minutes and was reversible. Because quinine is poorly soluble in water, the highest concentration used was 1 mm. The 0.5 mm quinine blocked half of inward Na+ current (bathing solution: 0.5 mm CaCl2, 100 mm NaCl; see Fig. 6). More than a 10- to 20-min exposure to quinine concentrations over 0.5 mm was toxic, causing membrane breakdown and cell death. It is also notable that in 23% of protoplasts, 1 mm quinine caused only a 35% decrease in current—these were the same protoplasts that were less sensitive to H+ and Ca2+ (see above).
Figure 6.
Inhibition of Na+ currents through NSCCs by quinine and DEPC. A, 0.2 to 1 mm quinine (dp = 24 μm); B, 50 μm DEPC (dp = 18 μm). Solutions contained 100 mm NaCl.
Amino Acid Modifiers
The states of external His residues and sulfhydryl groups are crucially important for the functioning of the cation channel protein. We have examined the effects of two amino acid modifiers on NSCCs. Pretreatment for 0.5 to 1 h in 100 to 300 μm p-chloromercuriphenyl sulfonic acid, a sulfhydryl group reagent, did not modify the nonselective cation currents (conditions: 100 mm NaCl, 0.5 mm CaCl2, 50 μm verapamil). Conversely, a 1-h pretreatment in 10 to 100 μm diethylpyrocarbonate (DEPC), a His residue modifier, decreased currents 4- to 6-fold in 56% of the cells used (Fig. 6). This inhibition was mostly irreversible. In some cases, even traces of DEPC in the working chamber strongly affected Na+ currents.
Single Channel Characteristics
Single channel recordings performed in outside-out mode are shown in Figure 7. It was difficult to maintain stable seals in outside-out patches when high external Na+ was applied with low Ca2+, with the total proportion of successful patches (where background current level was stable during the recording) being less than 10% in conditions of 50 mm NaCl and 0.5 mm CaCl2.
Figure 7.
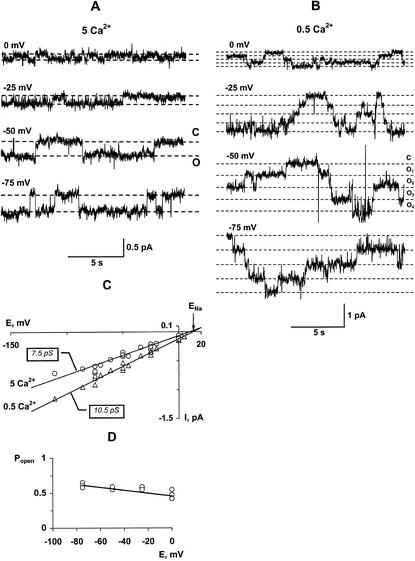
A and B, Single-channel recordings of NSCCs in outside-out configuration at voltages indicated adjacent to traces. External solution contained 50 mm NaCl and either 5 mm CaCl2 (A) or 0.5 mm CaCl2 (B). C, I/V relations of unitary NSCCs (outside: 50 mm NaCl) at 0.5 (triangles) and 5 (circles) mm CaCl2 with unitary conductances of 10.5 and 7.5 pS, respectively. Data were collected from five independent recordings. D, Dependence of open probability on membrane voltage (5 mm CaCl2, 100 mm NaCl); data were obtained from three independent recordings.
Reducing external Ca2+ significantly increased the number of open channels (Fig. 7, A and B), whereas the amplitude of unitary current only slightly increased (Fig. 7C). Decreasing Ca2+ concentration from 5 to 0.5 mm on a background of 50 mm NaCl increased the number of simultaneously open channels from 1 through 2 to 4 through 8. The open-state probability increased 10 to 15 times with a decrease in Ca2+ concentration but was unaffected by voltage (Fig. 7D). I/V relations obtained on the basis of unitary channel currents show that the reversal potential coincides with the reversal potential for Na+ (Fig. 7C). Changes in Ca2+ concentration did not modify values of the reversal potential.
DISCUSSION
In the present work, we have identified in protoplasts isolated from roots of Arabidopsis cation channels that are weakly selective for monovalent cations; they have no potential dependence and no time-dependent activation (or are, at least, completely activated within 1–2 ms). These channels are inhibited by external H+, divalent and trivalent cations (such as Ca2+, Zn2+, and Gd3+), quinine, and the His modifier DEPC. They are insensitive to most organic blockers, which are known to inhibit different classes of cation channels, such as K+- and Ca2+-selective channels.
The NSCCs studied here are distinct from Arabidopsis root inward K+ and Ca2+ rectifiers that were studied in earlier reports. According to Maathuis and Sanders (1995), hyperpolarization-activated inward-rectifying K+ channels from Arabidopsis roots were much more selective to K+ (PNa/PK = 0.17) than to NSCCs (PNa/PK = 0.67), were not blocked by 1 mm quinidine, and were significantly inhibited by 5 to 10 mm Cs+. Currents mediated by Arabidopsis inward-rectifying K+ channels revealed no time-independent component and were voltage dependent. Hyperpolarization-activated Ca2+ channels in protoplasts derived from Arabidopsis root hairs and elongation zone cortex were highly permeable for Ca2+ (PCa/PK = 15) and revealed steep voltage and time dependence (Kiegle et al., 2000; Véry and Davies, 2000). These facts, as well as the insensitivity of Arabidopsis root NSCCs to K+ and Ca2+ channel blockers (TEA+ and verapamil) and to other cation channel inhibitors (flufenamate, amiloride, and nifedipine), make the NSCCs described in this work distinct from K+ and Ca2+ channels, as well as from mechanosensitive cation channels.
At the same time, the sensitivity to quinine suggests similarities to channels that are selective for monovalent cations (Chen et al., 1993). The sensitivity of NSCCs to quinine (K1/2 ≈ 0.5 mm) resembles that of NSCC-like conductances described in the plasma membrane of cells of maize (Zea mays) suspension culture (0.5 mm quinine induced about 3-fold decrease of currents; Ketchum and Poole, 1990) and slowly activating NSCCs (SV channels) in the vacuolar membrane isolated from suspension cells of Chenopodium rubrum (K1/2 ≈ 0.35 mm; Weiser and Bentrup, 1993), although NSCC-like conductances of rye root protoplasts were less sensitive to quinine (1 mm caused 20–30% block; White and Lemtiri-Chlieh, 1995). Wheat and rye root plasma membrane NSCCs incorporated into PLBs were strongly inhibited by millimolar concentrations of quinine (White and Tester, 1992; Davenport and Tester, 2000). In animal preparations, 0.05 to 1 mm quinine and quinidine blocked NSCCs in the plasma membrane of human T lymphocytes (Schlichter, 1992), guinea pig (Cavia porcellus) gastric myocytes (Kim et al., 1995), in the basolateral membrane of isolated strial marginal cells (Takeuchi et al., 1995), and acetylcholine-activated NSCCs in guinea pig ileal smooth muscle (Chen et al., 1993).
Besides finding NSCCs that were highly sensitive to quinine, H+, and divalent and trivalent cations, we found NSCCs in 23% of protoplasts that were weakly sensitive to these blockers. This shows that probably two different classes of NSCCs occurred in Arabidopsis protoplasts. However, in this study, the more abundant channel was investigated.
The selectivity sequence of NSCCs from Arabidopsis roots reported here (Table I) is similar to that measured for rye root NSCCs characterized in planar lipid bilayers, namely K+ (1.36) = Rb+ (1.36) > Cs+ (1.17) > Na+ (1.0) ≈ Li+ (0.97) > TEA+ (0.41) (White and Tester, 1992). The same preparation made with wheat gave a slightly different NSCC selectivity sequence of NH4+ (2.06) > Rb+ (1.38) > K+ (1.23) ≈ Cs+ (1.18) > Na+ (1.00) > Li+ (0.83) > TEA+ (0.20) (Davenport and Tester, 2000). The lower NH4+ conductance measured in Arabidopsis is the only notable difference with wheat and may reflect differences in the physiological role of these channels between the two species. In a number of whole-cell studies on NSCC and NSCC-like conductances in higher and lower plants, the selectivity to cations varied only slightly, with channels almost equally permeable for K+, Rb+, Na+, NH4+, and Cs+, whereas TEA+, divalent cations, and in some cases Li+, were less permeable (Sokolik, 1990, 1999; Tyerman et al., 1997; Amtmann et al., 1997; Demidchik et al., 1997, 2001; Roberts and Tester, 1997; Véry et al., 1998; Buschmann et al., 2000).
Voltage and time independence of Arabidopsis root NSCCs resembles K+-permeable channels in maize cell culture (Ketchum et al., 1989), rye root protoplasts (White and Lemtiri-Chlieh, 1995), barley suspension cells (Amtmann et al., 1997), Na+-permeable channels in leaves of Aster tripolium and Aster amellus (Véry et al., 1998), and leakage currents of intact Nitella flexilis cells (Demidchik et al., 1997, 2001; Sokolik, 1999). Slight voltage dependence together with instantaneous activation was found in rye and wheat root NSCC-like channels incorporated into planar lipid bilayers (White and Tester, 1992; Davenport and Tester, 2000) and Na+ currents in wheat and maize root protoplasts (Roberts and Tester, 1997; Tyerman et al., 1997; Buschmann et al., 2000). The instantaneous character of cationic currents found in our study and in other investigations has two possible explanations. The first is that NSCCs are open at any voltages; the second is that they activate very rapidly (within 1 ms), as described by Zhang et al. (2000) for outward cation currents in developing seeds of bean (Phaseolus vulgaris). In animal preparations, classical Na+ channels of axons also activate very rapidly in response to changes in voltage (0.1–1 ms; Hille, 1992), as do ligand-gated channels (for review, see Lerma et al., 1998; Dingledine et al., 1999).
In this research, we have reported the inhibition of Arabidopsis NSCCs by the His modifying agent, DEPC, suggesting the existence of a histidyl group (or groups) on the external surface of the channel, which is important for NSCC functioning. This provides direct evidence for the protein nature of the nonselective conductances. High sensitivity to DEPC indicates that the Arabidopsis NSCC does not have a similar surface structure as classical animal excitatory Na+ channel, which is only slightly sensitive to DEPC (Spires and Begenisich, 1990).
Unitary conductance of Arabidopsis root NSCC (10.5 pS measured with 50 mm Na+) is similar to unitary conductances of weakly selective K+-permeable channels of rye roots (49 pS at 280 mm Na+) and wheat root NSCC (44 pS channel at 280 mm Na+) (White and Tester, 1992; Davenport and Tester, 2000). It is also similar to the unitary conductance of a Na+-permeable channel in maize root protoplasts (15 pS at 100 mm NaCl; Roberts and Tester, 1997). It appears that NSCCs are a group of ion channels having similar single channel properties in different plant species and, interestingly, animal NSCCs tend to have unitary conductances in the same order.
The high sensitivity of single NSCC to Ca2+ reported here also resembles that of single cation channels from maize root protoplasts (Roberts and Tester, 1997). Unitary NSCC conductance was only slightly inhibited by elevated Ca2+ (Fig. 7), but a decrease in external Ca2+ concentration (from 5 mm to 0.5 mm) appeared to cause opening of new channels in the outside-out patches or appearance of additional substates of NSCC (Fig. 7, A and B). Open-state probability of Na+-dependent currents of maize root protoplasts (Roberts and Tester, 1997) and leaf protoplasts from A. tripolium and A. amellus (Véry et al., 1998) also increased with decreasing external Ca2+.
In saline conditions, NSCCs are probably responsible for much of the toxic influx of Na+ into the cytoplasm of root cells. The pharmacological properties of 22Na+ influx into wheat root segments resembled properties of NSCCs from both wheat roots (Davenport and Tester, 2000) and Arabidopsis roots. The ameliorative effect of Ca2+ and some other divalent cations (Mg2+, Zn2+) on Na+ toxicity (Bergmann, 1992) can be explained by the blockade of the toxic Na+ influx through NSCCs.
The molecular identity of these channels remains obscure. Twenty genes encoding ionotropic glutamate receptors have been recently discovered and analyzed in Arabidopsis (Lacombe et al., 2001). Although they have yet to be functionally characterized, they may encode amino acid-gated NSCCs in plants. Another candidate for NSCCs are cyclic nucleotide-gated channels, 20 genes for which can also be found in the Arabidopsis genome. These genes encode proteins that are similar in overall structure to mammalian cyclic nucleotide-gated NSCCs. However, plant AtCNGC2 (expressed in Xenopus laevis oocytes) revealed properties of inward-rectifying K+ channels (strong time and voltage dependence of currents; Leng et al., 1999), showing a difference to the NSCCs reported here.
In conclusion, NSCCs were identified and characterized in Arabidopsis roots. The properties of these channels are close to other nonselective cation conductances described before in roots of monocotyledons and leaves of dicotyledons. Electrophysiological and pharmacological analyses show that plant root NSCCs are probably a distinct group of ion channels, rather than a class of K+ or Ca2+ channels. NSCCs can probably mediate toxic Na+ influx to root cells. These channels also potentially have other important roles for plant cell ion balance, mineral nutrition, and ecophysiology. Future studies should provide a more detailed understanding of NSCC involvement in Na+ stress mechanisms and other physiological processes in plants.
MATERIALS AND METHODS
Plant Material
Seedlings of Arabidopsis ecotype C24 were grown at 22°C in 16-h daylight (100 μmol m−2 s−1 irradiance) in sterile, vertical plates on standard medium containing 0.25% Phytagel (Sigma) with full-strength Murashige and Skoog (Duchefa, Haarlem, The Netherlands) and 1% (w/v) Suc. Approximately 50 surface sterilized seeds were planted along one edge of each 10-cm wide square sterile plate and vernalized for 2 d in the dark at 4°C. Seven- to 14-d-old plants were selected as giving the highest quality and quantity of protoplasts.
Roots from about 50 seedlings were chopped into pieces 0.5 to 1 mm long in 3 mL of enzyme solution (1.5% Cellulase Onozuka RS [Yakult Honsha, Tokyo], 1% cellulysin [CalBiochem, Nottingham, UK], 0.1% pectolyase Y-23 [Yakult Honsha, Tokyo], 0.1% bovine serum albumin [Sigma], 10 mm KCl, 10 mm CaCl2, 2 mm MgCl2, 2 mm MES [2-(N-morpholino)-ethanesulfonic acid], pH 5.6 adjusted with Tris, π0 = 290 to 300 mOsM adjusted with 330 mm sorbitol or mannitol). The high Ca2+ concentration in this solution was found to increase up to 1,000-fold the yield of protoplasts that were suitable for patch clamping, and it significantly increased seal frequency and quality. Roots were gently shaken (at 60 rpm) in the enzyme solution at 28°C for 30 to 50 min: Longer exposure to the enzyme solution decreased the quality of seal contacts without greatly increasing protoplast yield. The rigidity of the plasma membrane and the visible density of the cytoplasm were significantly less in such protoplasts, which also tended to have a rougher surface texture.
Protoplasts were filtered through a nylon mesh with 30-μm diameter pores, then undigested tissues were squeezed and rinsed with “holding solution” (5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm Suc, 10 mm Glc, 2 mm MES, pH 5.7 adjusted with Tris, π0 = 290–300 mOsM adjusted with sorbitol or mannitol). Protoplasts were collected on the bottom of a 15-mL plastic tube by 5-min centrifugation at 200 g. Approximately 0.3 to 0.5 mL of the solution containing protoplasts remained in the tube after removal of supernatant. This volume was diluted with 8 to 10 mL of the fresh holding solution. About 0.5 to 1 mL of this stock was used for one patch clamp experiment. Isolated protoplasts were stored on ice for up to 24 h.
Electrophysiology
Protoplasts of 15- to 25-μm diameter were patch clamped using standard techniques (Roberts and Tester, 1997). High-resistance seals were formed over a period of 1 to 2 min in sealing solution, which contained 10 or 20 mm CaCl2, 2 mm MES, pH 5.7 adjusted with Tris, π0 = 290 to 300 mOsM adjusted with sorbitol or mannitol. After gigaseal formation, Ca2+ concentrations were reduced to 0.5 mm CaCl2, essential to be able to measure the (Ca2+-sensitive) Na+ currents described in the present study. Stable seals could be maintained with this low Ca2+ concentration due to the modified protoplast isolation procedures used (i.e. high Ca2+ and short exposure to enzymes).
Patch clamp pipettes were pulled on a vertical electrode puller (model PE-2, Narishige, Tokyo) by the usual two-step approach from 1.5 to 1.8 ×100 mm glass capillaries (Kimax 51, Kimble Products, Vineland, NJ) and were filled with “pipette solution” (PS: 25 mm Na-gluconate, 5 mm NaCl, 10 mm EGTA, 5 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.2 adjusted with NaOH). Final pipette resistances were 20 to 25 MΩ. In several trial experiments, we added 0.2 to 2 mm Mg-ATP to the PS. We found no effect of ATP addition on inward Na+ currents, but steady-state Cl−-selective inward currents increased (data not shown). To avoid possible uncertainties arising from this Cl−-selective inward current, we omitted ATP from the PS. Steady-state Cl−-selective inward current was monitored in each protoplast using an external solution containing only 0.5 mm CaCl2. If the Cl−-selective inward current exceeded 5 to 7 pA at −150 mV, we corrected values of inward Na+ currents. (This only occurred in about 10% of protoplasts.)
Currents were recorded and processed using a standard patch clamp amplifier (IM/PCA, List, Darmstadt, Germany), Digidata 1200 digitiser and pClamp software, version 6.0.2 (Axon Instruments, Foster City, CA). Data were low pass filtered at 0.5 to 1 kHz with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA) and sampled at 3 to 10 kHz. Liquid junction potentials (which were not more than 10 mV) were measured and corrected as described elsewhere (Ward and Schroeder, 1994). Holding potentials were maintained at −70 to −100 mV throughout experiments, as specified in figure legends. Ion activities were calculated using GEOCHEM (Parker et al., 1995). Statistical analysis and curve fitting was done using standard software packages (Statistica version 6.0, StatSoft, Tulsa, OK and SigmaPlot for Windows Version 4.01, SPSS Science, Chicago). All solutions (except the enzyme solution) were filtered (0.22-μm pore size, Millipore, Watford, UK). Experiments were carried out at room temperature (20°C–22°C) with continuous bath perfusion (1 mL min−1). The osmotic pressure of solutions was measured using a vapor pressure osmometer (model 5520, Wescor, Logan, UT).
ACKNOWLEDGMENTS
We thank John Banfield, Dr Romola Davenport, Pauline Essah, Matt Gilliham, and Dr Fouad Lemtiri-Chlieh for helpful discussions and technical advice. We also gratefully acknowledge the support of Dr. Julia Davies.
Footnotes
This work was supported by the NATO/Royal Society Fellowship (grants to M.T. and V.D.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010524.
LITERATURE CITED
- Amtmann A, Laurie S, Leigh R, Sanders D. Multiple inward channels provide flexibility in Na+/K+discrimination at the plasma membrane of barley suspension culture cells. J Exp Bot. 1997;48:481–497. doi: 10.1093/jxb/48.Special_Issue.481. [DOI] [PubMed] [Google Scholar]
- Amtmann A, Sanders D. Mechanisms of Na+uptake by plant cell. Adv Bot Res. 1999;29:75–112. [Google Scholar]
- Bergmann W. In: Nutritional Disorders of Plants—Development, Visual and Analytical Diagnosis. Fisher G, editor. Stuttgart, Germany: Jena; 1992. [Google Scholar]
- Buschmann PH, Vaidynathan R, Gassmann W, Schroeder JI. Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+starvation in wheat root cells. Plant Physiol. 2000;122:1387–1397. doi: 10.1104/pp.122.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Inoue R, Ito Y. Pharmacological characterization of muscarinic receptor-activated channels in guinea-pig ileum. Brt J Pharmacol. 1993;109:793–801. doi: 10.1111/j.1476-5381.1993.tb13644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Tester M. A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol. 2000;122:823–834. doi: 10.1104/pp.122.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Sokolik A, Yurin V. The effect of Cu2+on ion transport systems of the plant cell plasmalemma. Plant Physiol. 1997;114:1313–1325. doi: 10.1104/pp.114.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Sokolik A, Yurin V. Characteristics of non-specific permeability and H+-ATPase inhibition induced in the plasma membrane of Nitella flexilis by excessive Cu2+ Planta. 2001;212:583–590. doi: 10.1007/s004250000422. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharm Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fairley-Grenot KA, Assmann SM. Permeation of Ca2+ through K+ channels in the plasma membrane of Vicia fabaguard cells. J Membrane Biol. 1992;128:103–113. doi: 10.1007/BF00231883. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants: where next? Aust J Plant Physiol. 1995;22:875–884. [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Ketchum KA, Poole RJ. Pharmacology of the Ca2+-dependent K+channel in corn protoplasts. FEBS Lett. 1990;274:115–118. doi: 10.1016/0014-5793(90)81343-m. [DOI] [PubMed] [Google Scholar]
- Ketchum KA, Shrier A, Poole RJ. Characterization of potassium-dependent currents in protoplasts of corn suspension cells. Plant Physiol. 1989;89:1184–1192. doi: 10.1104/pp.89.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Gilliham M, Haseloff J, Tester M. Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thalianaroots. Plant J. 2000;21:225–229. doi: 10.1046/j.1365-313x.2000.00659.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Ahn SC, So I. Quinidine blockade of the carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Br J Pharmacol. 1995;115:1407–1414. doi: 10.1111/j.1476-5381.1995.tb16631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Becker D, Hedrich R, Chiu J, DeSalle R, Heinemann S, Hollmann M, Kwak J, Le Novere N, Nam HG et al. On the identity of plant glutamate receptors. Science. 2001;292:1486–1487. doi: 10.1126/science.292.5521.1486b. [DOI] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Yao W, Berkowitz GA. Cloning and first functional characterization of a plant cyclic nucleotide-gated channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Salvador N, Somohano F, Morales M, Casado M. Excitatory amino acid-activated channels. In: Soria B, Cena V, editors. Ion Channel Pharmacology. Oxford, England: Oxford University Press; 1998. pp. 399–421. [Google Scholar]
- Maathuis FJM, Sanders D. Contrasting roles in ion transport of two K+-channel types in root cells of Arabidopsis thaliana. Planta. 1995;197:456–464. doi: 10.1007/BF00196667. [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL. GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In: Loeppert RH, Schwab AP, Goldberg S, editors. Chemical Equilibrium and Reaction Models, Special Publication 42. Madison, WI: Soil Science Society of America; 1995. pp. 253–269. [Google Scholar]
- Piñeros M, Tester M. Characterization of a voltage-dependent Ca2+-selective channel from wheat roots. Planta. 1995;195:478–488. [Google Scholar]
- Roberts SK, Tester MA. Patch clamp study of Na+transport in maize roots. J Exp Bot. 1997;48:431–440. doi: 10.1093/jxb/48.Special_Issue.431. [DOI] [PubMed] [Google Scholar]
- Schlichter LC. Nonselective cation channels in intact human lymphocytes-T. Can J Physiol Pharm. 1992;70:247–258. doi: 10.1139/y92-031. [DOI] [PubMed] [Google Scholar]
- Sokolik AI. Leakage conductance of the plant cell plasmalemma. Cytologia. 1990;55:S-949. (in Russian) [Google Scholar]
- Sokolik AI. Nonselective ion conductivity of plasmalemma as an important component of the system of membrane transport of ions in plants. Dokl Acad Nauk Belarus. 1999;43:77–80. (in Russian) [Google Scholar]
- Spires S, Begenisich T. Modification of potassium channel kinetics by histidine-specific reagents. J Gen Physiol. 1990;96:757–775. doi: 10.1085/jgp.96.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Ando M, Kozakura K, Saito H, Irimajiri A. Ion channels in basolateral membrane of marginal cells dissociated from Gerbilstria vascularis. Hearing Res. 1995;83:89–100. doi: 10.1016/0378-5955(94)00191-r. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Skerrett M. Root ion channels and salinity. Sci Hortic. 1999;78:175–235. [Google Scholar]
- Tyerman SD, Skerrett M, Garrill A, Findlay GP, Leigh RA. Pathways for the permeation of Na+ and Cl−into protoplasts derived from the cortex of wheat roots. J Exp Bot. 1997;48:459–480. doi: 10.1093/jxb/48.Special_Issue.459. [DOI] [PubMed] [Google Scholar]
- Véry A, Davies JM. Hyperpolarization-activated calcium channels at the tip of Arabidopsisroot hairs. Proc Natl Acad Sci USA. 2000;97:9801–9806. doi: 10.1073/pnas.160250397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A, Robinson MF, Mansfield TA, Sanders D. Guard cell cation channels are involved in Na+-induced stomatal closure in a halophyte. Plant J. 1998;14:509–521. [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+channels and calcium-induced calcium release by slow vacuolar ion channels in guard cells. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser T, Bentrup FW. Pharmacology of the SV channel in the vacuolar membrane of Chenopodium rubrumsuspension cells. J Membr Biol. 1993;136:43–54. doi: 10.1007/BF00241488. [DOI] [PubMed] [Google Scholar]
- White PJ, Lemtiri-Chlieh F. Potassium currents across the plasma membrane derived from rye roots: a patch-clamp study. J Exp Bot. 1995;46:497–511. [Google Scholar]
- White PJ, Tester M. Potassium channels from the plasma membrane of rye roots characterized following incorporation into planar lipid bilayers. Planta. 1992;186:188–202. doi: 10.1007/BF00196248. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Walker NA, Tyerman SD, Patrick JW. Fast activation of a time-dependent outward current in protoplasts derived from coats of developing Phaseolus vulgarisseeds. Planta. 2000;211:894–898. doi: 10.1007/s004250000391. [DOI] [PubMed] [Google Scholar]