Abstract
The calcium-calmodulin-activated protein phosphatase calcineurin functions as a key mediator of diverse biologic processes, including differentiation, apoptosis, growth, and adaptive responses, in part through dephosphorylation and activation of nuclear factor of activated T-cell (NFAT) transcription factors. Apoptosis signal-regulating kinase 1 (ASK1) is an upstream component of the mitogen-activated protein kinases that serves as a pivotal regulator of cytokine-, oxidative-, and stress-induced cell death. Here, we performed a yeast two-hybrid screen with calcineurin B as bait, which identified ASK1 as a direct physical interacting partner. The C-terminal 218 amino acids of ASK1 were sufficient to mediate interaction with calcineurin B in yeast, as well as in mammalian cell lysates. Importantly, endogenous calcium binding B subunit (CnB) protein interacted with endogenous ASK1 protein in cardiomyocytes at baseline, suggesting that the interaction observed in yeast was of potential biologic relevance. Indeed, calcineurin directly dephosphorylated ASK1 at serine 967 using purified proteins or mammalian cell lysates. Dephosphorylation of ASK1 serine 967 by calcineurin promoted its disassociation from 14-3-3 proteins, resulting in ASK1 activation. Calcineurin and ASK1 cooperatively enhanced cardiomyocyte apoptosis, while expression of a dominant negative ASK1 blocked calcineurin-induced apoptosis. Mouse embryonic fibroblasts deficient in ask1 were also partially resistant to calcineurin- or ionomycin-induced apoptosis. Finally, ASK1 negatively regulated calcineurin-NFAT signaling indirectly through c-Jun NH2-terminal kinase (JNK)- and p38-mediated phosphorylation of NFAT, which blocked calcineurin- and agonist-dependent hypertrophic growth of cardiomyocytes. Thus, ASK1 and calcineurin-NFAT constitute a feedback regulatory circuit in which calcineurin positively regulates ASK1 through direct dephosphorylation, while ASK1 negatively regulates calcineurin-NFAT signaling through p38- and JNK-mediated NFAT phosphorylation.
Calcineurin (protein phosphatase 2B [PP2B]) is a calcium-calmodulin-activated, serine/threonine PP that is activated by sustained elevations in intracellular calcium (7, 8, 19). Calcineurin consists of a 59- to 62-kDa catalytic subunit (CnA), a 19-kDa calcium binding subunit (CnB), and calmodulin (7). Once activated, calcineurin directly dephosphorylates members of the nuclear factor of activated T-cell (NFAT) transcription factor family in the cytoplasm, promoting their translocation into the nucleus where they participate in the transcriptional induction of various genes with specific inducible functions (19). There are four calcineurin-regulated NFAT transcription factors, NFATc1 to -c4, each of which is widely expressed in vertebrate tissues. Calcineurin enzymatic activity is inhibited by the immunosuppressive drugs cyclosporine (CsA) and FK506 through complexes with immunophilin proteins (7). The calcineurin-NFAT signaling circuit has been shown to play a central role in regulating the hypertrophic growth response of cardiomyocytes (56). Indeed, mice expressing an activated mutant of calcineurin or NFATc4 in the heart by transgenesis demonstrated a profound hypertrophic response that was characterized by a two- to threefold increase in heart growth (32).
Calcineurin is also a critical effector of cell death, where it has been shown to either agonize or antagonize apoptosis, following stress stimulation in neurons, lymphocytes, and tumor cell lines (1, 2, 22, 39, 49, 50, 57, 61). The decision of cytoprotection versus apoptosis is likely regulated by coordinated signals from other costimulated signaling pathways, such as in coordination with p38 mitogen-activated protein kinase (MAPK) activation (29). With respect to the heart, we have previously shown that transgenic mice expressing a constitutively active mutant of calcineurin are protected from ischemia-reperfusion-induced DNA laddering (12). In cultured cardiomyocytes, adenovirus (Ad)-mediated gene transfer of activated calcineurin reduced 2-deoxyglucose-induced terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL), while calcineurin inhibition with a Cain-expressing Ad increased TUNEL (12). Moreover, endothelin-1-mediated cytoprotection from H2O2-induced apoptosis in cardiomyocytes was blocked by inhibition of calcineurin with CsA (23). Consistent with these observations, activation of NFAT functioned as an important mediator downstream of calcineurin in providing cellular protection (12, 35). Lastly, genetic disruption of the CnAβ gene in the mouse enhanced cardiac damage and myocyte apoptosis induced by ischemia-reperfusion injury (4). Collectively, these results suggest that calcineurin signaling imparts a degree of protection against cell death in the heart. In contrast, isoproterenol stimulation of neonatal cardiac myocytes promoted apoptosis, in part by stimulating calcineurin activity (36). Similarly, aldosterone-stimulated apoptosis in cardiomyocytes was inhibited by CsA and FK506, demonstrating a proapoptotic role for calcineurin (30). Thus, as in other cell types, calcineurin activation in cardiomyocytes can serve either a proapoptotic or prosurvival regulatory role, depending on the type and duration of the stimulation, as well as the milieu of other signaling pathways that are recruited.
Calcineurin-NFAT signaling is dynamically regulated by and in coordination with MAPK signaling (31). MAPK signaling is composed of a series of successively acting kinases that function as central regulators of cell growth, differentiation, transformation, and cell death (14, 54). Originally, MAPK signaling pathways consisted of three major branches named for their terminal effector kinases: the extracellular signal-regulated protein kinases (ERKs), the c-Jun NH2-terminal kinases (JNKs), and p38 (14, 54). More recently, additional MAPK signaling effector pathways have been identified that culminate in ERK3, ERK4, or ERK5 (big MAPK1) phosphorylation. ERK, p38, and JNK are each directly activated by a family of dual-specificity kinases referred to as MAPK kinases, which themselves are activated by specific upstream kinases referred to as MAPK kinase kinases. Apoptosis signal-regulating kinase 1 (ASK1) is a MAPK kinase kinase that is activated by oxidative stress stimulation, resulting in MKK3/6 and MKK4/7 activation, which in turn mediates p38 and JNK activation, respectively (45).
ASK1 is unique among the MAPK kinase kinases in that it appears to directly regulate cell death through p38, JNK, and other effectors (45). For example, expression of a constitutively active mutant of ASK1 in a wide variety of cell types induced apoptosis, while expression of dominant negative ASK1 blocked tumor necrosis factor alpha-, oxidative stress-, anticancer agent-, and growth factor withdrawal-induced cell death (5, 16, 20, 24, 37, 51). More importantly, mouse embryonic fibroblasts (MEFs) generated from ask1−/− mice were resistant to tumor necrosis factor alpha- and H2O2-induced cell death (47). In the heart, ASK1 has been implicated as a mediator of cellular remodeling and hypertrophy, as well as apoptotic and necrotic cell death. For example, ask1−/− mice showed reduced ventricular remodeling in response to angiotensin II infusion, myocardial infarction, and pressure overload stimulation (21, 58). Cardiomyocytes generated from ask1−/− mice were also resistant to H2O2-induced apoptosis (58). In vivo, ask1−/− mice showed less apoptotic and necrotic cell death, following ischemia-reperfusion injury to the heart (53). Thus, ASK1 has emerged as a necessary mediator of cardiac remodeling and cell death following a wide array of stimuli. Here, we demonstrate for the first time that ASK1 is directly regulated by calcineurin and that calcineurin-NFAT signaling is regulated by ASK1, which together function as a feedback loop to control cardiomyocyte death and hypertrophy.
MATERIALS AND METHODS
Identification of CnB-interacting proteins by yeast two-hybrid screening.
We selected the CnB subunit as the bait in our yeast two-hybrid screening strategy, since very little is understood of CnB binding targets outside of CnA and since multiple CnA binding proteins have already been elucidated. Yeast two-hybrid screening was performed using the Matchmaker Gal4 two-hybrid System 3 (Clontech, Palo Alto, CA). Plasmids, yeast strains, and culture conditions were used according to the manufacturer's instructions. The bait construct consisted of full-length murine CnB1 cloned in frame into the BamHI site of pGBKT7 using PCR-generated linkers. Bait integrity was confirmed by DNA sequencing and Western blot analysis. In addition, yeast strain AH109 (Clontech) cotransformed with the bait and a known interacting prey construct (pGAD10-CnAβ) grew blue colonies on highly selective complete synthetic medium lacking tryptophan, leucine, His, Ade, X-α-galactosidase plus 5 mM 3-amino-1,2,4-triazole, while AH109 cotransformed with either pGBKT7-CnB plus pGAD10, pGBKT7 plus pGAD10-CnAβ, or pGBKT7 plus pGAD10 did not produce growth on the same plates. After confirmation, the bait was used to screen a murine adult cardiac cDNA library constructed into the EcoRI site of pGAD10 (custom-made library; Clontech). Approximately 3.2 × 106 transformants were screened, covering the library at a 3× density. The initial screen was carried out on medium-stringency complete synthetic medium lacking tryptophan, leucine, and His plus 5 mM 3-amino-1,2,4-triazole, and putative interacting clones were identified by replica plating on high-stringency medium after 7 days of initial growth. Confirmation of the initial interaction was performed by cotransforming each unique bacterial prey plasmid into AH109 with the CnB bait construct and screening each combination on medium- and high-stringency media as previously described. Only prey clones identified more than once in the screen were considered valid CnB-interacting proteins.
Cell culture.
Primary neonatal rat cardiomyocytes were prepared from hearts of 1- to 2-day-old Sprague-Dawley rat pups as previously described (11). After separation from fibroblasts, enriched cardiomyocytes were plated on 1% gelatin-coated 12-well plates for luciferase assays or on 6-cm-diameter dishes for all other experiments. Cells were grown in M199 medium containing 100 U of penicillin-streptomycin/ml and 2 mM l-glutamine without serum for 24 h before infection. Adenoviral infections were performed as previously described at a multiplicity of infection of 10 to 50 PFU per ml (11). Cultures were harvested 24 h after infection, and luciferase assays were preformed as described previously (27). At harvest, cells were resuspended in 50 μl of lysis buffer, and 20 μl was used for luciferase activity determination.
Adenoviral constructs and expression vectors.
Adβgal, AdΔCnA, AdCnB, Adcain, AdNFAT-Luc, and AdNFATc1-GFP have been previously described (28, 38, 44, 55). AdASK1 and AdASK1-ΔN were a gift of Yong J. Lee (University of Pittsburgh). An ASK1-KM (dominant negative) cDNA was subcloned into the pAC-CMVpLpA vector to generate AdASK1-KM as described previously (11). ASK1-WT, -ΔN, and -KM in pcDNA3 have been previously described (37). ASK1-NT (amino acids 1 to 678), CT (amino acids 1158 to 1375), and ΔKD (amino acids 1 to 678 plus 936 to 1375) were amplified by PCR and subcloned into pcDNA3, pGEX-4T-1, or pGAD10 vectors.
GST pull-down assays.
To generate fusion proteins between glutathione S-transferase (GST) and CnB, CnA, ASK1, and ASK1-ΔN, select DNA sequences were amplified by PCR and subcloned into pGEX-4T-1 (Amersham Pharmacia Biotech, Piscataway, NJ). All fusion proteins were expressed in Escherichia coli BL21 cells, precipitated with glutathione-Sepharose beads, and eluted with 10 mM glutathione (in 50 mM Tris; pH 8.0). The purity and concentration of each fusion protein were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using bovine serum albumin standards. Binding assays were performed with labeled proteins synthesized in vitro using a transcription-translation-coupled reticulocyte lysate system (Promega, Madison, WI) in the presence of [35S]methionine (Amersham Pharmacia Biotech) as described previously (10). Equal amounts of immobilized GST fusion proteins were incubated for 2 h at 4°C with 10 μl of 35S-labeled proteins in GST binding buffer containing 40 mM HEPES (pH 7.2), 50 mM Na acetate (pH 7.0), 200 mM NaCl, 2 mM EDTA, 5 mM dithiothreitol, 0.5% NP-40, protease inhibitors, and 2 μg of bovine serum albumin/ml. Glutathione beads were washed three times in binding buffer and boiled in SDS sample buffer to elute complexed proteins, which were then resolved by SDS-PAGE and analyzed by autoradiography.
Western blot analysis.
Protein extracts were generated from cultured cardiomyocytes as described previously (44). Protein samples were subjected to SDS-PAGE (8% gels), transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech), blocked in 5% milk, and incubated with primary antibodies overnight in 5% milk at 4°C. Alkaline phosphatase-conjugated secondary antibodies in 5% milk were incubated for 1 h at room temperature. Chemifluorescent detection was performed with the Vistra ECF reagent (Amersham Pharmacia Biotech) and scanned using a Storm 860 (Amersham Pharmacia Biotech). Antibodies included ASK1, 14-3-3 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), phospho-ASK1 (Ser967), phospho-ASK1 (Thr845), phospho-ASK1 (Ser83) (Cell Signaling, Beverly, Mass.), CnB1 (Sigma, St. Louis, Mo.), and pan-CnA (Chemicon International, Inc., Temecula, CA).
Immunoprecipitation.
Neonatal rat cardiomyocytes were infected with or without specific adenoviruses for 24 h. Cells were lysed at 4°C in buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% Triton X-100) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1-μg/ml leupeptin, 1-μg/ml pepstatin, and 1-μg/ml aprotinin). Lysates were cleared by centrifugation at 18,000 × g for 10 min and then incubated with the indicated antibodies and protein A-Sepharose beads overnight at 4°C. The beads were washed extensively with binding buffer, and the proteins were resolved on a 7.5% SDS-PAGE for subsequent Western blotting.
In vitro phosphatase assay.
ASK1 was immunoprecipitated from cardiomyocytes infected with an ASK1-expressing adenovirus and then incubated with calcineurin purified from bovine brain (Sigma-Aldrich, St. Louis MO) in a reaction buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM CaCl2, 1 μM calmodulin, 0.1 μM calcineurin, 2 mM dithiothreitol, 20 mM β-glycerophosphate, 10 mg of leupeptin/ml, and 10 mg of aprotinin/ml) in a total volume of 30 μl for 15 min at 30°C. Dephosphorylation of ASK1 by calcineurin was also performed by using bacterium-purified GST-ASK1-ΔN. GST-ASK1-ΔN was first autophosphorylated in kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 50 μM ATP) for 15 min at 30°C. Phosphorylated GST-ASK1-ΔN was purified and then incubated with calcineurin as described above.
Immunoprecipitation kinase assay.
The kinase activity of ASK1 was determined essentially as described previously (20). At 24 h after transfection, cells (4 × 105) were lysed in buffer (20 mM Tris-Cl [pH 7.5], 10 mM glycerophosphate, 5 mM NaF, 1 mM sodium pyrophosphate, 150 mM NaCl, 1% NP-40, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 10-μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride). After clarification by centrifugation, ASK1 was immunoprecipitated with anti-ASK1 (Santa Cruz Biotechnology) and protein A/G-agarose. After washing the immunoprecipitates three times with lysis buffer and once with a kinase assay buffer (20 mM Tris-Cl p[H 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride), the immunoprecipitates were incubated with 1 μg of GST-MKK6 in 25 μl of kinase assay buffer with 0.3 μCi of [32P]ATP for 10 min at 30°C. Samples were subjected to SDS-PAGE (8%) and visualized by PhosphorImager analysis (Amersham Pharmacia Biotech).
TUNEL.
TUNEL staining was performed according to manufacturers' conditions (Roche). In brief, the cells in two chamber slides were fixed by 4% formaldehyde-phosphate-buffered saline at 4°C for 25 min and subjected to TUNEL. The sections were stained with Hoechst 33258 for 15 min at room temperature, washed in phosphate-buffered saline, and mounted for fluorescence microscopic quantitation.
Agarose gel electrophoresis for DNA fragmentation.
Cells (4 × 105) were lysed in 200 μl of lysis buffer (10 mM Tris-HCl [pH 7.4], 10 mM EDTA, 0.5% Triton X-100), followed by incubation with 40 μg of RNase (Roche) for 1 h at 37°C and 100 μg of proteinase K (Roche) for 1 h at 37°C, and DNA was extracted. The pellet was resuspended in TE buffer (10 mM Tris-HCl [pH 7.4], 1 mM EDTA) and treated with DNase-free RNase (Roche) for 1 h at 37°C. DNA was ethanol precipitated and resuspended in distilled water. The fragmented DNA was electrophoretically fractionated on a 2% agarose gel and stained with ethidium bromide.
Immunohistochemistry and hypertrophy analysis.
To assess cardiomyocyte hypertrophy, antibodies against α-actinin (EA-53; Sigma-Aldrich) and atrial natriuretic factor (ANF) (Peninsula Laboratories, San Carlos, CA) were used at a dilution of 1:800 and 1:300, respectively. Secondary antibodies included anti-mouse tetramethyl rhodamine isocyanate-conjugated antibody (Sigma-Aldrich) and anti-rabbit fluorescein isothiocyanate-conjugated antibody (Sigma-Aldrich) at a dilution of 1:400. Quantitation of cardiomyocyte cell surface area was performed on α-actinin-stained cardiomyocytes by confocal laser microscopy and NIH Image Analysis software, version 1.63. Cells from randomly selected fields in three independent experiments were examined, and the surface area was compared to that of control infected cells (200 cells each).
Statistical analysis.
Data were expressed as means ± standard error of the mean. Differences between experimental groups were evaluated for statistical significance using Student's t test for unpaired data or one-way analysis of variance for multiple groups, followed by Bonferroni's posttest. P values of <0.05 were considered to be statistically significant.
RESULTS
CnB directly interacts with ASK1.
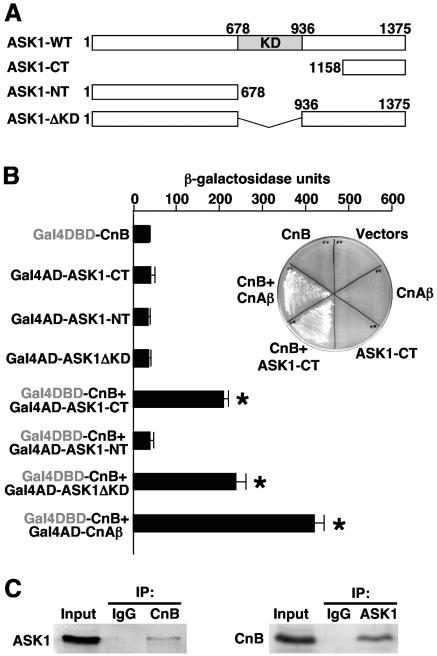
To investigate the downstream mechanisms whereby calcineurin regulates cardiomyocyte hypertrophy and apoptosis, a yeast two-hybrid screen was instituted with CnB. This screen of an adult heart library identified two independent clones containing a C-terminal (CT) fragment encoding amino acids 1158 to 1375 of mouse ASK1 as a CnB-specific interacting factor in yeast (Fig. 1A). Under growth-restricted conditions, no interaction was observed with CnB bait alone, ASK1 prey (amino acids 1158 to 1375) alone, CnAβ prey alone (control), or the empty bait and prey vectors alone (Fig. 1B, inset). However, CnB bait with either the CnAβ prey (control) or ASK1 prey gave a strong interaction that supported growth on selectable medium in yeast that contained interaction-dependent metabolic selection marker cassettes. These results were quantified by two-hybrid analysis using an interaction-dependent β-galactosidase reporter in yeast (Fig. 1B). Once again, CnB bait strongly interacted with CnAβ prey and the CT ASK1-prey (Fig. 1B). CnB bait also interacted with an ASK1 prey that was full-length except for deletion of the kinase domain (ΔKD), but not with the entire N terminus (designated NT) of ASK1 (Fig. 1B). While yeast two-hybrid screens uncover novel and direct interactions, it is still critical to demonstrate that the identified interaction occurs in mammalian cells at physiologic levels of each protein. Indeed, direct immunoprecipitation of either endogenous CnB or ASK1 from HEK293 cells identified the reciprocal protein as an interacting factor, yet immunoglobulin G (IgG) alone had no effect (Fig. 1C). Thus, CnB directly interacts with ASK1 in yeast, and this association was identified in a mammalian cell context at endogenous levels of protein.
FIG. 1.
ASK1 is a CnB binding protein. (A) Schematic representation of wild-type and mutant ASK1 proteins. The kinase domain (KD) is shown in the gray box. (B) Yeast two-hybrid β-galactosidase assay with strain AH109, which contains an interaction-dependent β-galactosidase reporter. Plasmids encoding full-length CnB fused to the Gal4 DNA binding domain (DBD) were cotransformed with plasmids encoding the indicated forms of ASK1 fused to the Gal4 activation domain (AD). The inset shows yeast that was streaked on a selectable medium plate to demonstrate interaction based on metabolic marker selection. (C) Endogenous CnB interacts with ASK1 in mammalian cells. Lysates from HEK293 cell were subjected to CnB immunoprecipitation, followed by Western blotting with anti-ASK1 antibody (left). ASK1 was immunoprecipitated from HEK293 cell lysates, followed by Western blotting with an anti-CnB antibody (right).
Molecular determinants of the ASK1-CnB interaction.
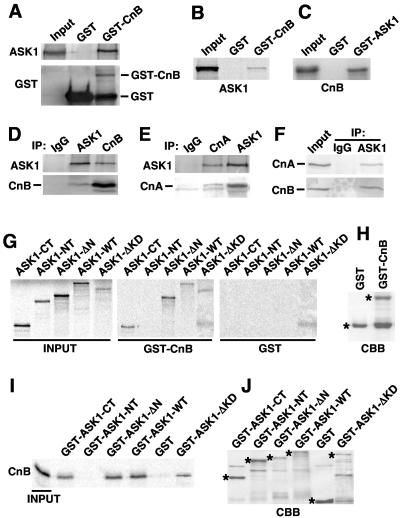
To further define the determinants of the interaction between CnB and ASK1, a series of in vitro biochemical assays were performed. First, neonatal cardiomyocyte cultures were infected with an adenovirus encoding full-length ASK1, and lysates from these cells were subjected to GST pull-down with GST-bound resin only or GST-CnB resin. The data showed that GST-CnB specifically interacted with full-length ASK1 from cardiomyocytes (Fig. 2A). A similar assay was performed with GST-CnB or GST-ASK1 resin in combination with [35S]methionine-labeled ASK1 or CnB, respectively, which also demonstrated a specific interaction in each direction but not with GST alone (Fig. 2B and C). ASK1 and CnB also coimmunoprecipitated from neonatal cardiomyocytes infected with AdASK1 and AdCnB but not with a nonspecific IgG antibody (Fig. 2D). Also of interest, immunoprecipitation of ASK1 resulted in the isolation of CnA, the obligate dimeric partner of CnB, while immunoprecipitation of CnA conversely identified ASK1 (Fig. 2E). However, CnA alone failed to bind ASK1 with [35S]methionine-labeled CnA and GST-ASK1 (data not shown). Thus, ASK1 associates with the dimeric CnA-CnB complex, consistent with the known instability of monomeric CnA (full-length) or CnB in mammalian cells (34). Finally, it was also of interest to determine if endogenous ASK1 could interact with endogenous CnB complexed with CnA in cardiomyocytes, without overexpression. Indeed, endogenous levels of ASK1 immunoprecipitated with CnA and CnB from cardiomyocytes (Fig. 2F). The relative strength of this interaction in cardiomyocytes was not altered by ionomycin (IONO), phenylephrine (PE), isoproterenol, or angiotensin II stimulation (data not shown).
FIG. 2.
Calcineurin physically interacts with ASK1. (A) Cardiomyocytes were infected with adenoviruses encoding ASK1, and cell extracts were subjected to GST pull-down with GST-CnB or GST alone, followed by Western blotting with the indicated antibodies. (B) GST pull-down was performed with GST alone or GST-CnB in conjunction with [35S]methionine-labeled ASK1 (full-length). (C) GST pull-down was performed with GST alone or GST-ASK1 in conjunction with [35S]methionine-labeled CnB. (D) Cardiomyocytes were infected with adenoviruses encoding ASK1 and CnB. Cell extracts were subjected to immunoprecipitation (IP) with anti-ASK1, anti-CnB, or preimmune IgG. The immunoprecipitates were Western blotted with the indicated antibodies. (E) Cardiomyocytes were infected with adenoviruses encoding CnA, CnB, and ASK1. Cell extracts were subjected to immunoprecipitation (IP) with anti-CnA, anti-ASK1, or preimmune IgG, followed by Western blotting with the indicated antibodies. (F) Immunoprecipitation with IgG or ASK1 from total cardiac extracts (no overexpression), followed by blotting for endogenous CnA and CnB. The input is extract alone without immunoprecipitation. (G) ASK1 truncations or deletions were [35S]methionine labeled and subjected to GST pull-down assay with GST-CnB or GST alone. (H) A CBB-stained gel shows GST fusion proteins used in panel G. (I) CnB was [35S]methionine labeled and subjected to a GST pull-down assay with GST-ASK1 truncations or deletions or GST alone. (J) Coomassie brilliant blue-stained gel of GST fusion proteins used in the results shown in panel I (asterisks indicate the fusion proteins).
Finally, the region of ASK1 that interacts with calcineurin was more carefully evaluated with deletion constructs of ASK1 by different pull-down assays. Full-length (wild-type [WT]) or ASK1 deletions fragments were [35S]methionine labeled in a coupled transcription-translation reaction and subjected to pull-down with GST alone or GST-CnB (Fig. 2G). This assay showed that ASK1-WT (amino acids 1 to 1375), ASK1-ΔN (amino acids 678 to 1375), ASK1-CT (amino acids 1158 to 1137), and ASK1-ΔKD (deletion of amino acids 678 to 936) each interacted with GST-CnB but not GST alone. The integrity of GST-CnB is also shown from a SDS-PAGE gel stained with Coomassie brilliant blue (CBB) (Fig. 2H). These results were confirmed by a reciprocal pull-down assay in which [35S]methionine-labeled CnB protein was subjected to pull down with the same series of ASK1 deletions fused to GST and generated in bacteria (Fig. 2I). The integrity of the GST-ASK1 fusion proteins was also shown from a CBB-stained SDS-PAGE gel (Fig. 2J). Thus, CnB specifically interacts with the C terminus of ASK1 (amino acids 1158 to 1375) in cells and in vitro.
Calcineurin dephosphorylates and activates ASK1.
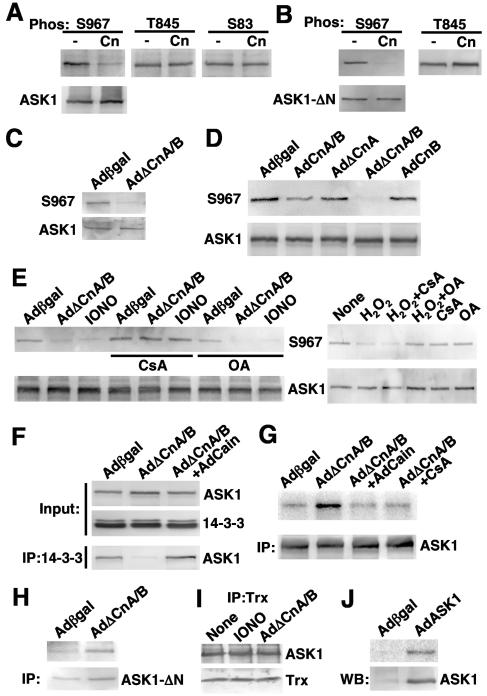
The formation of a stable complex between calcineurin and ASK1 suggested that one partner might directly regulate the other through a direct phosphorylation or dephosphorylation event, especially given the fact that ASK1 is phosphorylated at multiple sites that either activate or inhibit it (13, 15, 25, 60). Here, cardiomyocytes were infected with AdASK1 and immunoprecipitated to generate a source of in vivo-phosphorylated ASK1 for analysis with phospho-specific antibodies. The ASK1 immunoprecipitates were incubated with highly purified calcineurin in the presence of calmodulin and calcium for 15 min and then subjected to SDS-PAGE and Western blotting with ASK1 phospho-specific antibodies against serine 967, threonine 845, serine 83, or total ASK1 as a control (Fig. 3A). The data demonstrated that calcineurin treatment led to dephosphorylation of serine 967 but not threonine 845 or serine 83. This potential direct regulation was confirmed with purified calcineurin and a GST-ASK1-ΔN bacterial purified deletion construct (amino acids 678 to 1175) that were incubated together and subsequently Western blotted for serine 967 phosphorylation, showing dephosphorylation of serine 967 but not threonine 845 without a change in protein levels (Fig. 3B). An ASK1-ΔN deletion construct was utilized for this experiment, since this form of ASK1 undergoes autophosphorylation (activated mutant of ASK1) and since the endogenous kinase that phosphorylates this site is not known.
FIG. 3.
Calcineurin dephosphorylates ASK1 at serine 967 in vitro and in vivo. (A) ASK1 was immunoprecipitated from cardiomyocytes infected with ASK1 adenovirus. The ASK1 immunoprecipitates were incubated in the presence or absence of purified calcineurin in reaction buffer at 30°C for 15 min. Phosphorylation of ASK1 at serine 967, threonine 845, or serine 83 was determined by Western blotting with specific phospho-ASK1 antibodies. (B) GST-ASK1-ΔN was autophosphorylated in kinase buffer, purified, and then incubated with purified calcineurin; phosphorylation of ASK1 at S967 and T845 was determined by Western blotting. (C) Western blot for endogenous ASK1 S967 phosphorylation from HEK293 cells infected with the indicated adenoviruses. (D) Cardiomyocytes were infected with AdASK1 and the additional indicated adenoviruses for 24 h. Phosphorylation of ASK1 at serine 967 in cell lysates was determined by Western blotting. (E) Cardiomyocytes were infected with AdASK1, the additional indicated adenoviruses, and the calcium mobilizer IONO (1 μM) for 24 h or H2O2 (1 mM) for 15 min. Phosphorylation of ASK1 at serine 967 in cell lysates was determined by Western blotting. (F) Cardiomyocytes were infected with the indicated adenoviruses along with AdASK1. Endogenous 14-3-3 from cell lysates was immunoprecipitated, and the presence of ASK1 was detected by Western blotting. (G) Cardiomyocytes were infected with the indicated adenoviruses for 24 h. CsA (0.1 μM) was added to cells 1 h before infections. ASK1 enzymatic activity was determined by an immunoprecipitation kinase assay with GST-MKK6 as a substrate. Total ASK1 protein in each immunoprecipitation reaction was quantified by Western blotting. (H) ASK1 kinase assay against GST-MKK6 from cardiomyocytes infected with AdASK1-ΔN and the indicated additional adenoviruses. Total immunoprecipitated ASK1-ΔN is shown by Western blotting. (I) Western blot for ASK1 and thioredoxin (Trx) following immunoprecipitation of thioredoxin from cardiomyocytes infected with AdASK1 and AdΔCnA/B. (J) ASK1 kinase assay against GST-MKK6 from cardiomyocytes infected with AdASK1 or Adβgal.
The calcineurin-dependent regulation of ASK1 at serine 967 was further evaluated with cultured cardiomyocytes infected with combinations of adenoviruses and pharmacologic inhibitors, followed by Western blotting. Overexpression of activated calcineurin A (AdΔCnA) with CnB (AdCnB) led to a near-complete loss of endogenous serine 967 phosphorylation in HEK293 cells compared with Adβgal infection (Fig. 3C). This relationship was also examined with cultured cardiomyocytes infected with AdASK1 and various calcineurin-expressing subunits. It was also interesting that overexpression of activated CnA alone did not mediate dephosphorylation of ASK1 at serine 967 but that CnB was required in combination with activated CnA, consistent with the observation that CnB is required for mediating the direct interaction with ASK1 (Fig. 3D). Overexpression of full-length (WT) CnA with CnB had some effect on ASK1 serine 967 dephosphorylation, although it was milder than ΔCnA/B (Fig. 3D). To further evaluate the dependence on calcineurin or other phosphatases in regulating serine 967, cardiomyocytes were treated with the calcineurin activator IONO or with H2O2 in conjunction with pharmacologic inhibitors. Interestingly, AdΔCnA/B- or ionomycin-mediated dephosphorylation of serine 967 was blocked with the calcineurin inhibitor CsA, but not with the PP2A/PP1 inhibitor okadaic acid (Fig. 3E). However, H2O2-mediated dephosphorylation of serine 967 in ASK1 was not blocked with CsA but instead was reversed with okadaic acid (Fig. 3E). This later observation suggests that ASK1 can be activated at serine 967 by divergent upstream stimuli that are calcium or calcineurin dependent and independent. With respect to other cardiac-acting agonists, isoproterenol, PE, and angiotensin II treatment also promoted significant dephosphorylation of serine 967 within ASK1 in cultured cardiomyocytes, which was inhibited with CsA (data not shown).
The observed regulation of serine 967 in ASK1 through CnA/B was also associated with 14-3-3 protein binding. Indeed, serine 967 in ASK was previously shown to be tightly bound by 14-3-3 proteins when phosphorylated, leading to inactivation of ASK1 (60). In cardiomyocytes, 14-3-3 immunoprecipitated with ASK1, but expression of activated CnA/B, which promotes nearly complete dephosphorylation of ASK1, resulted in loss of 14-3-3 protein interaction with ASK1 (Fig. 3F). Cooverexpression of the calcineurin inhibitory protein Cain blocked this effect of calcineurin overexpression and maintained 14-3-3 protein interaction with ASK1 (Fig. 3F). An ASK1-specific kinase assay was also performed after ASK1 immunoprecipitation from cardiomyocytes infected with the indicated adenoviruses (Fig. 3G). The immunoprecipitates were incubated in kinase buffer with [32P]ATP and bacterium-generated MKK6 (substrate for ASK1). The data demonstrate that activated CnA/B, which dephosphorylates ASK1 and disassociates from 14-3-3, activates ASK1, leading to increased phosphorylation of MKK6 protein. Calcineurin-mediated activation of ASK1 was blocked with Adcain coinfection or by CsA treatment (Fig. 3G). Activated CnA/B also increased the kinase activity of ASK1-ΔN (Fig. 3H) but did not alter the interaction between ASK1 and thioredoxin (Trx) (Fig. 3I). As a final control, we observed that simple overexpression of ASK1 by adenovirus-mediated gene transfer in cultured cardiomyocytes led to an increase in total ASK1 kinase activity compared with Adβgal infection (Fig. 3J). In conclusion, these results indicate that calcineurin can regulate ASK1 activity through dephosphorylation of serine 967.
Calcineurin and ASK1 cooperatively regulate apoptosis.
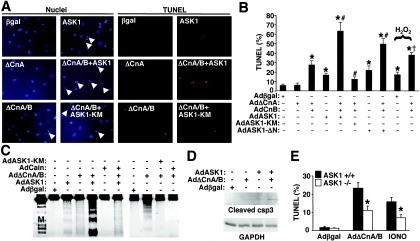
Both calcineurin and ASK1 have been shown to play critical regulatory roles in mediating cellular apoptosis, suggesting a physiologic function underlying the observed interaction between these enzymes. To evaluate this relationship, cultured neonatal cardiomyocytes were infected with different combinations of recombinant adenoviruses, followed by evaluation of cell death by nuclear fragmentation (as assessed with Hoechst-stained cells, demonstrated by blue nuclei), TUNEL, DNA laddering, or caspase 3 cleavage. Interestingly, AdΔCnA alone had no effect on nuclear fragmentation or TUNEL compared to Adβgal infection, yet coinfection with AdΔCnA plus AdCnB produced a significant increase, similar to that of AdASK1 infection (Fig. 4A to C). However, coinfection with AdASK1 together with AdΔCnA plus AdCnB induced a prominent increase in nuclear fragmentation, TUNEL, and DNA laddering (Fig. 4A to C). This cooperative increase in cell death was blocked by Adcain infection, indicating the requirement for calcineurin in augmenting ASK1-driven apoptosis (Fig. 4C). Moreover, expression of a dominant negative ASK1 (AdASK1-KM) inhibited the increase in cell death associated with AdΔCnA plus AdCnB (Fig. 4A to C). Thus, ASK1 was necessary in part for mediating calcineurin-dependent apoptosis in cardiomyocytes. Cleavage of caspase 3 in cardiomyocytes was also evaluated, showing increased levels after infection with AdASK1 or AdΔCnA plus AdCnB, which was dramatically increased when all three viruses were combined (Fig. 4D). AdΔCnA plus AdCnB overexpression also enhanced H2O2-induced TUNEL in cardiomyocytes (Fig. 4B). Finally, the requirement of ASK1 for mediating calcineurin- or ionomycin-induced cell death was evaluated in ask1+/+ or ask−/− MEFs. Consistent with the use of AdASK1-KM with cardiomyocytes, ask1−/− MEFs were partially protected from calcineurin- or ionomycin-induced TUNEL compared with wild-type MEFs, further suggesting that ASK1 is a necessary downstream mediator of calcineurin-regulated apoptosis (Fig. 4E).
FIG. 4.
Calcineurin activation promotes cardiomyocyte apoptosis through ASK1. (A) Microscopy of neonatal cardiomyocytes infected with the indicated adenoviruses for 48 h and subjected to TUNEL (red staining) and Hoechst staining of nuclei (blue staining). Arrowheads show condensing and fragmenting nuclei. (B) TUNEL-positive nuclei were quantified from 200 random selected fields of cardiomyocytes infected with the indicated combinations of adenoviruses, of which two conditions were subjected to H2O2 treatment to increase cell death. *, P < 0.001 versus Adβgal; #, P < 0.001 versus AdΔCnA/B; †, P < 0.01 versus H2O2 plus Adβgal. (C) Cardiomyocytes were infected with the indicated adenoviruses for 48 h, and total DNA was isolated and separated on a 2% agarose gel. (D) Western blot for cleaved caspase 3 (csp3) from cardiomyocytes infected with the indicated adenoviruses. (E) ask1+/+ or ask1−/− MEFs were infected with Adβgal or AdΔCnA/B or treated with 5 μM ionomycin. TUNEL-positive cells were determined as described in the legend to panel A. *, P < 0.001 versus ask1+/+.
ASK1 reciprocally modulates calcineurin-NFAT signaling.
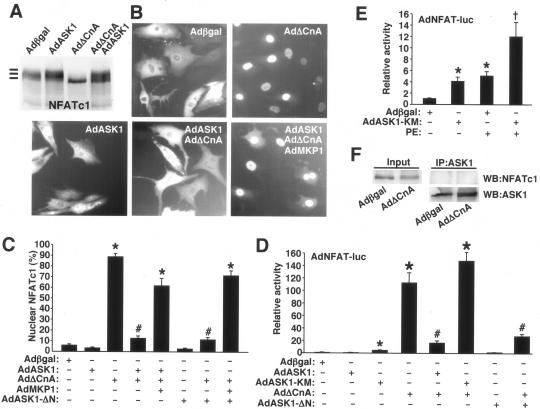
The data presented above demonstrate that calcineurin regulates ASK1 activity and promotion of cellular apoptosis. Here, we investigated the reciprocal regulatory relationship to determine if ASK1 regulated calcineurin-NFAT activation. While ASK1 did not directly phosphorylate calcineurin or alter its enzymatic activity (data not shown), it did affect NFAT activation. For example, NFATc1 dephosphorylation mediated by AdΔCnA, which dramatically increases NFATc1 migration on SDS-PAGE, was completely reversed by AdASK1 infection in cardiomyocytes (Fig. 5A). Consistent with the inhibition of NFATc1 dephosphorylation by ASK1 or ASK1-ΔN, nuclear translocation of NFATc1 was also blocked (Fig. 5B and C). Specifically, AdΔCnA infection promoted robust and sustained nuclear translocation of NFATc1-GFP in cardiomyocytes, which was inhibited by simultaneous infection with AdASK1 or AdASK1-ΔN (Fig. 5B and C). However, this effect appeared to be indirect through p38 and JNK activation, since coinfection with an adenovirus encoding MAPK phosphatase 1 (AdMKP1) reversed the inhibitory effect of ASK1 and ASK1-ΔN on NFATc1-GFP nuclear occupancy (Fig. 5B and C). JNK and p38 were each shown to function as NFAT kinases in diverse cell types, thus counterregulating NFAT nuclear translocation mediated by calcineurin (19, 31). MKP1 is also highly specific for p38 and JNK, which are essentially the dominant effector kinases downstream of ASK1 in this cellular system (45). A similar reversal of ASK1-mediated inhibition of NFATc1 nuclear translocation was also observed with SB203580 and coinfections with adenoviruses encoding dominant negative JNK1/2, together with dominant negative p38α (data not shown). Thus, while calcineurin and ASK1 physically interact, ASK1 does not appear to directly regulate NFAT but instead indirectly regulates it through JNK and p38 activation. Also, ASK1 did not physically interact with NFATc1, as assessed by immunoprecipitation in cardiomyocytes overexpressing NFATc1 and ASK1 (Fig. 5F).
FIG. 5.
ASK1 inhibits calcineurin-NFAT signaling in cardiomyocytes. (A) Cardiomyocytes were infected with adenoviruses as indicated, along with AdNFATc1, for 24 h. Whole-cell extracts were separated by 8% SDS-PAGE, followed by Western blotting for NFATc1. (B) Cardiomyocytes were infected with the indicated adenoviruses along with AdNFATc1-GFP for 24 h. Localization of NFATc1-GFP was determined by fluorescence microscopy. (C) Quantitation of nuclear NFATc1-GFP in cardiomyocytes as described in the legend to panel B. *, P < 0.001 versus Adβgal; #, P < 0.001 versus AdΔCnA. (D) Cardiomyocytes were infected with the indicated adenoviruses, along with an adenovirus containing an NFAT-luciferase reporter. Luciferase activity was determined 24 h after infection. *, P < 0.001 versus Adβgal; #, P < 0.01 versus AdΔCnA. (E) Cardiomyocytes were infected with the indicated adenoviruses, and some were stimulated with PE (50 μM). Luciferase activity was determined 24 h after infection. *, P < 0.001 versus Adβgal; †, P < 0.01 versus Adβgal plus PE. (F) Western blot for NFATc1 and ASK1, following immunoprecipitation of ASK1 from cardiomyocytes infected with AdNFATc1-GFP, AdASK1, and the other indicated adenoviruses. The input is cell lysate for NFATc1 without immunoprecipitation.
Consistent with the NFATc1 phosphorylation and translocation data presented above, ASK1 also inhibited NFAT transcriptional activity in cardiomyocytes. For example, AdΔCnA infection activated the AdNFAT-luciferase reporter by >100 fold (P < 0.05) in cardiomyocytes, an effect that was largely reversed by coinfection with AdASK1 or AdASK1-ΔN (Fig. 5D). Also of interest, infection with the dominant negative ASK1-KM-encoding adenovirus induced baseline NFAT-luciferase activity, suggesting that endogenous ASK1 plays a basal negative regulatory role with NFAT (Fig. 5D and E). AdASK1-KM also augmented PE-induced NFAT-luciferase activity, suggesting that hypertrophic stimuli partially induce ASK1 activity in cardiomyocytes.
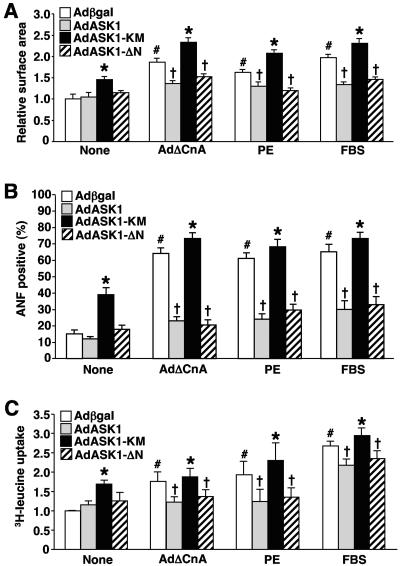
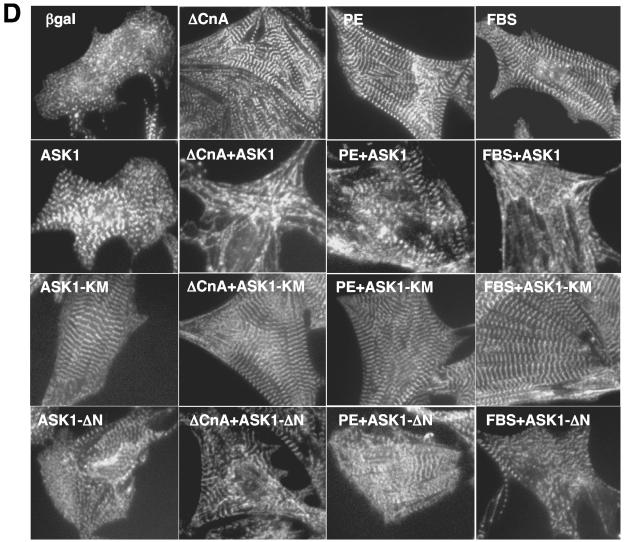
ASK1-regulated NFAT translocation is also predicted to influence the cardiac hypertrophic growth response, given the prominent role that calcineurin-NFAT signaling plays in this process (56). Indeed, AdASK1 or AdASK1-ΔN infection (24 h) antagonized AdΔCnA-PE- and fetal bovine serum (FBS)-induced cellular hypertrophy, ANF expression, and sarcomeric organization (Fig. 6). Cellular hypertrophy was assessed by direct measurement of myocyte surface area and by protein synthesis associated with [3H]leucine incorporation (Fig. 6A and C). Moreover, infection with AdASK1-KM (dominant negative) actually promoted mild hypertrophy and ANF expression at baseline, or enhanced AdΔCnA-, PE-, and FBS-induced growth, ANF expression, and sarcomeric organization (Fig. 6). The analysis of cardiac hypertrophy was performed in the presence of 50 μM zVADfmk (inhibitor of apoptosis through caspase blockade), so that propensity to cell death did not secondarily influence our investigation of cell growth. In summary, ASK1 indirectly regulates cardiomyocyte hypertrophic growth through its ability to alter calcineurin-NFAT activation (through p38 and JNK negatively regulating NFAT translocation and transcriptional activity).
FIG. 6.
ASK1 inhibits agonist-stimulated cardiomyocyte hypertrophy. (A) Cardiomyocytes were infected with the indicated adenoviruses and/or stimulated with PE (50 μM) or 1% FBS for 24 h. Cells were stained with α-actinin antibody, and cell surface areas were measured. The data are means ± standard error of the mean from 200 random cells measured in each group and were confirmed by two additional experiments. The analysis of cardiac hypertrophy was performed in the presence of 50 μM zVADfmk, so that propensity to cell death did not secondarily influence investigation of cell growth. (B) Cardiomyocytes were infected and treated as described in the legend to panel A. Cells were then fixed and stained with antibody against ANF. ANF-positive cells were quantified from 200 random cells. (C) Cardiomyocytes were infected and treated as described in the legend to panel A in the presence of [3H]leucine for assessment of protein synthesis (summation of four independent experiments). *, P < 0.001 versus Adβgal within each condition; #, P < 0.001 versus Adβgal none; †, P < 0.001 versus Adβgal-treated or AdASK1-KM-treated cells. (D) Representative pictures of α-actinin-stained neonatal cardiomyocytes for sarcomeric organization under serum-free conditions, following infection with the indicated adenoviruses and treatment with the indicated agonists.
DISCUSSION
The calcineurin-NFAT-ASK1 regulatory circuit.
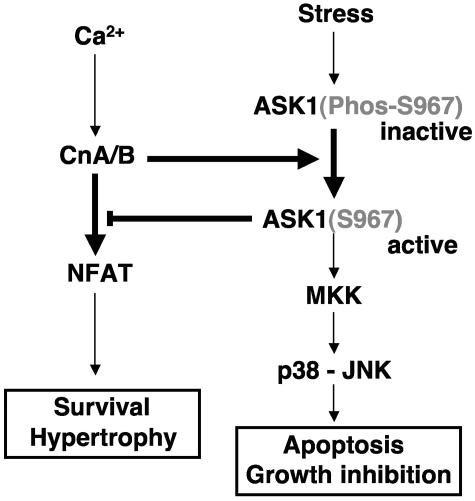
An interesting aspect of the current study is the identification of a physical interaction between a protein kinase and a protein phosphatase, each of which imparts some level of regulation upon the other. While the direct physical association between a protein kinase and a protein phosphatase is somewhat uncommon, ASK1 has been shown to interact with two other protein phosphatases, PP5 and CDC25A (33, 62). Similar to calcineurin, both PP5 and CDC25A interaction with ASK1 imparted some level of regulation, although only PP5 appears to function through dephosphorylation of ASK1. However, unlike calcineurin, PP5 and CDC25A negatively regulated ASK1, resulting in less activity and cell death (33, 62). PP5-dependent regulation of ASK1 likely includes the known activation-dependent phosphorylation site at threonine 845 (33, 48). In contrast to the effects of PP5 and CDC25A, calcineurin was shown to positively regulate ASK1 activity. Calcineurin directly dephosphorylated serine 967 in ASK1, a site that was previously shown to negatively regulate ASK1 when phosphorylated through association with 14-3-3 protein binding (15, 60). Thus, activation of calcineurin by overexpression or by ionomycin-mediated calcium mobilization activated ASK1 in association with dephosphorylation of serine 967 (Fig. 7).
FIG. 7.
Model of calcineurin and ASK1 signaling cross talk. Calcineurin dephosphorylates ASK1 at serine 967, which decreases the association of ASK1 with 14-3-3, leading to increased ASK1 activity. ASK1 then activates JNK and p38 in a unique manner leading to downstream coupling with apoptotic signaling effectors. ASK1 also inhibits NFAT nuclear translocation and transcriptional activity through JNK and p38, leading to inhibition of calcineurin-NFAT-mediated cell survival and hypertrophy.
Activation of ASK1 by calcineurin was shown to have two prominent biological effects in cardiomyocytes. First, ASK1 induced activation of p38 and JNK to promote the apoptotic program (Fig. 7). Second, ASK1 activation partially antagonized cardiomyocyte hypertrophic growth in association with JNK and p38 activation. Indeed, we have previously shown that p38 and JNK activation functions to inhibit calcineurin-NFAT-dependent cardiac hypertrophy (3, 26). JNK and p38 are known to serve as potent NFAT kinases, counteracting the ability of calcineurin to maintain NFAT in the nucleus, hence antagonizing myocyte growth to any stimulus that might utilize a calcineurin-dependent pathway (31). NFAT activation also provides an important prosurvival transcriptional signal in cardiomyocytes (12, 35). Therefore, ASK1-mediated activation of JNK and p38, which antagonizes NFAT activity, would further predispose cardiomyocytes to cell death by diminishing the NFAT prosurvival signal (Fig. 7). Thus, ASK1 and calcineurin-NFAT comprise a unique feedback signaling circuit. Stress-mediated signals that induce apoptosis or diminish cell growth through ASK1 function in part, through JNK and p38 activation, resulting in NFAT inhibition (Fig. 7). In contrast, calcium signals that would normally activate calcineurin and NFAT are subject to negative feedback through ASK1 activation, presumably limiting the extent of cardiac hypertrophy or cellular protection (Fig. 7). However, this later regulatory relationship may be slightly more complex, since calcineurin activation can have dichotomous effects on cellular protection (see below).
Calcineurin as a modulator of cellular apoptosis.
Calcineurin has been implicated as a modulator of cell death in countless studies, the majority of which relied on the inhibitory agents CsA and/or FK506. However, CsA can modulate apoptosis independently of calcineurin through direct effects on the mitochondrial permeability pore in association with cyclophilin D, potentially obscuring the interpretation of causation in many previous cell death studies (9). Despite this caveat, more directed and nonpharmacologic approaches have also failed to yield definitive data. Studies conducted with neurons, lymphocytes, and tumor cell lines have shown both pro- and antiapoptotic roles for calcineurin activation, and no unifying principle has yet emerged that reconciles these differing accounts (1, 2, 22, 39, 49, 50, 57, 61). In cardiomyocytes, calcineurin can have both pro- and antiapoptotic effects, depending on the nature of the stimulus. For example, overexpression of activated CnA in cultured cardiomyocytes (without CnB overexpression) reduced 2-deoxyglucose-induced TUNEL, while calcineurin inhibition with a Cain-expressing adenovirus increased TUNEL, suggesting a prosurvival role for calcineurin (12). Also in culture, endothelin-1-mediated cytoprotection from H2O2-induced apoptosis was blocked by inhibition of calcineurin, further supporting a prosurvival signaling role for calcineurin (23). In vivo, transgenic mice expressing activated CnA were protected from cell death following ischemia-reperfusion injury, while genetic disruption of the CnAβ gene enhanced myocyte death induced by ischemia-reperfusion injury (4, 12). In contrast to these studies, isoproterenol or aldosterone stimulation promoted apoptosis in association with calcineurin activation, suggesting that calcineurin could also promote cell death (30, 36).
While it is easy to simply attribute these conflicting accounts discussed above to differences in the type and duration of the stimulation employed, there may be another level of complexity that ultimately suggests a unifying hypothesis among the previous literature if ASK1 is involved. Overexpression of activated CnA in cultured cardiomyocytes or in the hearts of transgenic mice showed profound protection from apoptotic stimuli (12). However, the activated CnA subunit, which is truncated at the C terminus, does not effectively regulate ASK1 unless the CnB subunit is also included. Indeed, we have observed that the activated CnA subunit does not require CnB for functionality (data not shown). Calcineurin directly binds ASK1 through the CnB calcium regulatory subunit, although the CnA catalytic subunit is also a critical component of the complex and for regulation of ASK1. Thus, singular overexpression of ΔCnA (without CnB) could provide protection through NFAT activation, without recruiting significant ASK1 and its counterbalancing effect. This interpretation is also consistent with the known protective effect of NFAT activation alone (12, 35).
In addition to ASK1 activation, calcineurin might also regulate the survival versus apoptotic response of cells through other pathways or effectors. Calcineurin can localize to the mitochondria in fibroblasts in association with FKBP38, resulting in Bcl-2 and Bcl-xl redistribution and effects on cell death (40). Calcineurin has also been implicated as a direct inducer of apoptosis in primary hippocampal neurons by dephosphorylating the proapoptotic factor Bad, resulting in its induction of mitochondrion-dependent cell death (50). However, this observation has not been extended to cardiac myocytes, despite exhaustive efforts, suggesting that this regulatory paradigm might be specific to neurons or at least noncardiac myocytes (4). Another downstream mechanism whereby calcineurin might regulate apoptosis was suggested by the observation that Bcl-2 expression is modulated in association with calcineurin activity in neonatal cardiomyocytes (23).
ASK1 regulation in cardiomyocytes.
ASK1 is highly enriched in the heart, suggesting that it might have an important function there (20, 46). Indeed, ASK1 has been shown to function as both a regulator of cardiac remodeling and hypertrophy, as well as apoptotic and necrotic cell death. Mice lacking ask1 have been very important in elucidating these functional roles in the heart. For example, ask1−/− mice showed reduced ventricular remodeling, interstitial fibrosis, cardiac hypertrophy, and cardiomyocyte apoptosis in response to angiotensin II infusion, a neuroendocrine factor that is known to mediate heart disease (21). Mice lacking ask1 also showed significantly less left ventricular structural remodeling and TUNEL-positive myocytes following 4 weeks of pressure overload stimulation or 4 weeks after myocardial infarction (58). Cardiomyocytes generated from ask1−/− mice were also resistant to H2O2- and calcium overload-induced apoptosis (53, 58). Mice lacking ask1−/− also showed less apoptotic and necrotic cell death, following acute ischemia-reperfusion injury to the heart (53). Moreover, deletion of ask1 in the mouse rescued cardiomyopathy and the increase in cardiac apoptosis associated with a cardiac-specific deletion of the raf-1 gene (59), consistent with the ability of Raf-1 to directly interact with ASK1 and modulate cell death independently of the MAPK kinase 1-ERK signaling circuit (6). ASK1 was also shown to directly interact with cardiac troponin T, resulting in its phosphorylation and the promotion of contractile dysfunction, suggesting another mechanism whereby ASK1 might induce cardiomyopathy (17). Thus, previous data support a proapoptotic and procardiomyopathic role for ASK1 in the heart. Our results are entirely consistent with these data and further support the hypothesis that AK1 is a promyopathic signaling effector in the heart, although through different downstream pathways. Indeed, we proposed another mechanism here whereby ASK1 might facilitate cardiomyopathy and lead to increased cell death through inhibition of NFAT transcriptional activity.
Pressure overload, ischemia-reperfusion injury, and various neuroendocrine agonists activate ASK1 in cardiomyocytes (18, 53, 58). While these stimuli are known to induce cell death in association with ASK1 activation, the causal association between cardiac hypertrophy and ASK1 activation is less certain. Here, we observed increased cardiomyocyte hypertrophy by adenovirus infection with a dominant negative ASK1 mutant, while overexpression of wild-type ASK1 suppressed hypertrophy due to ΔCnA or phenylephrine and FBS stimulation. Hypertrophy was assessed by cell surface area measurements, total protein synthesis, ANF expression levels, and relative sarcomerogenesis. While these results are consistent with the mechanism discussed above whereby ASK1 negatively regulates NFAT transcriptional activity through JNK and p38, hence reducing hypertrophic growth due to calcineurin-NFAT signaling, it is in contrast to the results of two other studies. Overexpression of ASK1-ΔN was shown to induce cardiomyocyte hypertrophy in culture, while overexpression of dominant negative ASK1 attenuated hypertrophy (18). Similarly, ask1−/− mice showed reduced hypertrophy following angiotensin II infusion, suggesting that ASK1 could positively regulate cardiac growth (21). However, c-raf-1 null mice showed abundant ASK1 activity in the heart without corresponding hypertrophy, suggesting that activation of endogenous ASK1 need not lead to hypertrophy (59). Also, cardiac blood pressure overload induced by thoracic transverse aortic constriction did not result in less cellular hypertrophy in ask−/− mice than in wild-type mice, although assessment of hypertrophy was not a primary focus of the study (58). While the reasons underlying the disparity between our study and the previous two studies are uncertain, that disparity likely reflects differences in conditions employed or agonists selected (see below for a discussion of redox potential). Indeed, the use of cultured neonatal cardiomyocytes to address mechanisms of cardiac hypertrophy sometimes leads to differing results between groups, depending on culture cell density, the composition of the culturing media, the timing of adenoviral infection, the substrate used on the culturing plates for adherence, and the assays employed to detect hypertrophy itself. The observations that inhibition or activation of ASK1 activity with either the ASK1-KM-expressing or truncated ASK1-expressing (or wild-type-expressing) adenovirus induced antithetic effects on calcineurin-NFAT signaling, cardiomyocyte apoptosis, and hypertrophic growth suggests that our results likely reflect the physiologic function of ASK1 and not simply an overexpression artifact. Moreover, the observation that ask1−/− mice develop less hypertrophy following angiotensin II infusion, suggesting that ASK1 is a prohypertrophic factor, is somewhat discordant with our results and is difficult to interpret (21). However, the effect of angiotensin II through ASK1 may represent a specific signaling circuit, since ask1−/− showed normal hypertrophy following pressure overload stimulation (58). Additional resolution of these disparate results may require the generation and characterization of ASK1-overexpressing transgenic mice.
ASK1 is regulated by phosphorylation and dephosphorylation at four different amino acids. ASK1 is uniquely activated to induce cell death by phosphorylation of threonine 845 (48), while dephosphorylation of serine 83, serine 967, and serine 1034 results in activation (13, 15, 25, 60). Phosphorylation of serine 967 serves an especially important role in mediating inhibition of ASK1 through 14-3-3 protein recruitment (15, 43, 60). Calcineurin directly dephosphorylated serine 967 within ASK1 both in cells and in vitro, resulting in a loss of 14-3-3 protein binding and activation of ASK1. While purified calcineurin had no effect on dephosphorylation of serine 83 or threonine 845, overexpression of calcineurin in cardiomyocyte cultures, which promoted dephosphorylation of serine 967, actually increased threonine 845 phosphorylation, an effect that would further activate ASK1 (data not shown). Thus, in vivo, calcineurin enhances ASK1 activation through direct dephosphorylation of serine 967 and through an indirect increase in threonine 845 phosphorylation. We speculate that dephosphorylation of serine 967 leads to a conformation change in ASK1 that allows access of a kinase to threonine 845, facilitating greater activation.
ASK1 is also uniquely regulated by oxidative stress through direct interactions with thioredoxin and glutaredoxin (37, 41, 42). Binding of these two proteins inactivates ASK1, but upon oxidation of thioredoxin and glutaredoxin, ASK1 is released and activated (41). Thus, the interaction of thioredoxin and glutaredoxin explains a major mechanism whereby apoptosis is induced through oxidative stress in conjunction with ASK1 activation. Calcineurin is also exceptionally sensitive to oxidative stress, although in this case oxidation results in inactivation of the enzyme (52). Given these observations, redox potential may be very important in ultimately defining the overall effect of stimuli that result in both calcineurin-NFAT and ASK1 activation. For example, agonist stimulation that results in high reactive oxygen species (ROS) generation might initially mobilize both calcineurin and ASK1 signaling, providing both proapoptotic and prosurvival signals, yet gradual calcineurin inactivation through ROS accumulation would allow the proapoptotic effects of ASK1 to dominate. Alternatively, activation of both pathways independent of ROS generation might partially reduce the overall dominance of ASK1 in mediating apoptosis or in antagonizing cardiomyocyte hypertrophy. In conclusion, the interaction and cross talk between calcineurin and ASK1 signaling suggest a novel regulatory paradigm, whereby each factor coordinates the activity of the other, resulting in a feedback circuit that differentially affects cell death and growth depending on the nature, strength, and duration of the stimulation.
Acknowledgments
This work was supported by grants from the National Institutes of Health and by an American Heart Association Established Investigator Grant to J.D.M.
REFERENCES
- 1.Ankarcrona, M., J. M. Dypbukt, S. Orrenius, and P. Nicotera. 1996. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 394:321-324. [DOI] [PubMed] [Google Scholar]
- 2.Asada, A., Y. Zhao, S. Kondo, and M. Iwata. 1998. Induction of thymocyte apoptosis by Ca2+-independent protein kinase C (nPKC) activation and its regulation by calcineurin activation. J. Biol. Chem. 273:28392-28398. [DOI] [PubMed] [Google Scholar]
- 3.Braz, J. C., O. F. Bueno, Q. Liang, B. J. Wilkins, Y. S. Dai, S. Parsons, J. Braunwart, B. J. Glascock, R. Klevitsky, T. F. Kimball, T. E. Hewett, and J. D. Molkentin. 2003. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 111:1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno, O. F., D. J. Lips, R. A. Kaiser, B. J. Wilkins, Y. S. Dai, B. J. Glascock, R. Klevitsky, T. E. Hewett, T. R. Kimball, B. J. Aronow, P. A. Doevendans, and J. D. Molkentin. 2004. Calcineurin Aβ gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ. Res. 94:91-99. [DOI] [PubMed] [Google Scholar]
- 5.Chang, H. Y., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860-1863. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., K. Fujii, L. Zhang, T. Roberts, and H. Fu. 2001. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc. Natl. Acad. Sci. USA 98:7783-7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree, G. R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96:611-614. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl.):S67-S79. [DOI] [PubMed] [Google Scholar]
- 9.Crompton, M. 2000. Mitochondrial intermembrane junctional complexes and their role in cell death. J. Physiol. 529:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai, Y. S., P. Cserjesi, B. E. Markham, and J. D. Molkentin. 2002. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J. Biol. Chem. 277:24390-24398. [DOI] [PubMed] [Google Scholar]
- 11.De Windt, L. J., H. W. Lim, S. Haq, T. Force, and J. D. Molkentin. 2000. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J. Biol. Chem. 275:13571-13579. [DOI] [PubMed] [Google Scholar]
- 12.De Windt, L. J., H. W. Lim, T. Taigen, D. Wencker, G. Condorelli, G. W. Dorn 2nd, R. N. Kitsis, and J. D. Molkentin. 2000. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: an apoptosis-independent model of dilated heart failure. Circ. Res. 86:255-263. [DOI] [PubMed] [Google Scholar]
- 13.Fujii, K., E. H. Goldman, H. R. Park, L. Zhang, J. Chen, and H. Fu. 2004. Negative control of apoptosis signal-regulating kinase 1 through phosphorylation of Ser-1034. Oncogene 23:5099-5104. [DOI] [PubMed] [Google Scholar]
- 14.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 15.Goldman, E. H., L. Chen, and H. Fu. 2004. Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J. Biol. Chem. 279:10442-10449. [DOI] [PubMed] [Google Scholar]
- 16.Hatai, T., A. Matsuzawa, S. Inoshita, Y. Mochida, T. Kuroda, K. Sakamaki, K. Kuida, S. Yonehara, H. Ichijo, and K. Takeda. 2000. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem. 275:26576-26581. [DOI] [PubMed] [Google Scholar]
- 17.He, X., Y. Liu, V. Sharma, R. T. Dirksen, R. Waugh, S. S. Sheu, and W. Min. 2003. ASK1 associates with troponin T and induces troponin T phosphorylation and contractile dysfunction in cardiomyocytes. Am. J. Pathol. 163:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirotani, S., K. Otsu, K. Nishida, Y. Higuchi, T. Morita, H. Nakayama, O. Yamaguchi, T. Mano, Y. Matsumura, H. Ueno, M. Tada, and M. Hori. 2002. Involvement of nuclear factor-κB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation 105:509-515. [DOI] [PubMed] [Google Scholar]
- 19.Hogan, P. G., L. Chen, J. Nardone, and A. Rao. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17:2205-2232. [DOI] [PubMed] [Google Scholar]
- 20.Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. T. Saitoh, Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90-94. [DOI] [PubMed] [Google Scholar]
- 21.Izumiya, Y., S. Kim, Y. Izumi, K. Yoshida, M. Yoshiyama, A. Matsuzawa, H. Ichijo, and H. Iwao. 2003. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ. Res. 93:874-883. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman, T., and A. R. Marks. 2000. Calcineurin is downstream of the inositol 1,4,5-trisphosphate receptor in the apoptotic and cell growth pathways. J. Biol. Chem. 275:6417-6420. [DOI] [PubMed] [Google Scholar]
- 23.Kakita, T., K. Hasegawa, E. Iwai-Kanai, S. Adachi, T. Morimoto, H. Wada, T. Kawamura, T. Yanazume, and S. Sasayama. 2001. Calcineurin pathway is required for endothelin-1-mediated protection against oxidant stress-induced apoptosis in cardiac myocytes. Circ. Res. 88:1239-1246. [DOI] [PubMed] [Google Scholar]
- 24.Kanamoto, T., M. Mota, K. Takeda, L. L. Rubin, K. Miyazono, H. Ichijo, and C. E. Bazenet. 2000. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol. Cell. Biol. 20:196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, A. H., G. Khursigara, X. Sun, T. F. Franke, and M. V. Chao. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang, Q., O. F. Bueno, B. J. Wilkins, C. Y. Kuan, Y. Xia, and J. D. Molkentin. 2003. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 22:5079-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, Q., R. J. Wiese, O. F. Bueno, Y. S. Dai, B. E. Markham, and J. D. Molkentin. 2001. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol. Cell. Biol. 21:7460-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Y., Z. Cseresnyes, W. R. Randall, and M. F. Schneider. 2001. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J. Cell Biol. 155:27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotem, J., R. Kama, and L. Sachs. 1999. Suppression or induction of apoptosis by opposing pathways downstream from calcium-activated calcineurin. Proc. Natl. Acad. Sci. USA 96:12016-12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mano, A., T. Tatsumi, J. Shiraishi, N. Keira, T. Nomura, M. Takeda, S. Nishikawa, S. Yamanaka, S. Matoba, M. Kobara, H. Tanaka, T. Shirayama, T. Takamatsu, Y. Nozawa, and H. Matsubara. 2004. Aldosterone directly induces myocyte apoptosis through calcineurin-dependent pathways. Circulation 110:317-323. [DOI] [PubMed] [Google Scholar]
- 31.Molkentin, J. D. 2004. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 63:467-475. [DOI] [PubMed] [Google Scholar]
- 32.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita, K., M. Saitoh, K. Tobiume, H. Matsuura, S. Enomoto, H. Nishitoh, and H. Ichijo. 2001. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 20:6028-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons, S. A., D. P. Millay, B. J. Wilkins, O. F. Bueno, G. L. Tsika, J. R. Neilson, C. M. Liberatore, K. E. Yutzey, G. R. Crabtree, R. W. Tsika, and J. D. Molkentin. 2004. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J. Biol. Chem. 279:26192-26200. [DOI] [PubMed] [Google Scholar]
- 35.Pu, W. T., Q. Ma, and S. Izumo. 2003. NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro. Circ. Res. 92:725-731. [DOI] [PubMed] [Google Scholar]
- 36.Saito, S., Y. Hiroi, Y. Zou, R. Aikawa, H. Toko, F. Shibasaki, Y. Yazaki, R. Nagai, and I. Komuro. 2000. β-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J. Biol. Chem. 275:34528-34533. [DOI] [PubMed] [Google Scholar]
- 37.Saitoh, M., H. Nishitoh, M. Fujii, K. Takeda, K. Tobiume, Y. Sawada, M. Kawabata, K. Miyazono, and H. Ichijo. 1998. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17:2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanna, B., O. F. Bueno, Y.-S. Dai, B. J. Wilkins, and J. D. Molkentin. 2005. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol. Cell. Biol. 25:865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibasaki, F., and F. McKeon. 1995. Calcineurin functions in Ca2+-activated cell death in mammalian cells. J. Cell Biol. 131:735-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirane, M., and K. I. Nakayama. 2003. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat. Cell. Biol. 5:28-37. [DOI] [PubMed] [Google Scholar]
- 41.Song, J. J., and Y. J. Lee. 2003. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem. J. 373:845-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song, J. J., J. G. Rhee, M. Suntharalingam, S. A. Walsh, D. R. Spitz, and Y. J. Lee. 2002. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J. Biol. Chem. 277:46566-46575. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian, R. R., H. Zhang, H. Wang, H. Ichijo, T. Miyashita, and H. Fu. 2004. Interaction of apoptosis signal-regulating kinase 1 with isoforms of 14-3-3 proteins. Exp. Cell Res. 294:581-591. [DOI] [PubMed] [Google Scholar]
- 44.Taigen, T., L. J. De Windt, H. W. Lim, and J. D. Molkentin. 2000. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 97:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda, K., A. Matsuzawa, H. Nishitoh, and H. Ichijo. 2003. Roles of MAPKKK ASK1 in stress-induced cell death. Cell. Struct. Funct. 28:23-29. [DOI] [PubMed] [Google Scholar]
- 46.Tobiume, K., T. Inage, K. Takeda, S. Enomoto, K. Miyazono, and H. Ichijo. 1997. Molecular cloning and characterization of the mouse apoptosis signal-regulating kinase 1. Biochem. Biophys. Res. Commun. 239:905-910. [DOI] [PubMed] [Google Scholar]
- 47.Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobiume, K., M. Saitoh, and H. Ichijo. 2002. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell Physiol. 191:95-104. [DOI] [PubMed] [Google Scholar]
- 49.Tombal, B., A. T. Weeraratna, S. R. Denmeade, and J. T. Isaacs. 2000. Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells. Prostate 43:303-317. [DOI] [PubMed] [Google Scholar]
- 50.Wang, H. G., N. Pathan, I. M. Ethell, S. Krajewski, Y. Yamaguchi, F. Shibasaki, F. McKeon, T. Bobo, T. F. Franke, and J. C. Reed. 1999. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284:339-343. [DOI] [PubMed] [Google Scholar]
- 51.Wang, T. H., D. M. Popp, H. S. Wang, M. Saitoh, J. G. Mural, D. C. Henley, H. Ichijo, and J. Wimalasena. 1999. Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and -independent pathways in ovarian cancer cells. J. Biol. Chem. 274:8208-8216. [DOI] [PubMed] [Google Scholar]
- 52.Wang, X., V. C. Culotta, and C. B. Klee. 1996. Superoxide dismutase protects calcineurin from inactivation. Nature 383:434-437. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe, T., K. Otsu, T. Takeda, O. Yamaguchi, S. Hikoso, K. Kashiwase, Y. Higuchi, M. Taniike, A. Nakai, Y. Matsumura, K. Nishida, H. Ichijo, and M. Hori. 2005. Apoptosis signal-regulating kinase 1 is involved not only in apoptosis but also in non-apoptotic cardiomyocyte death. Biochem. Biophys. Res. Commun. 333:562-567. [DOI] [PubMed] [Google Scholar]
- 54.Widmann, C., S. Gibson, M. B. Jarpe, and G. L. Johnson. 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79:143-180. [DOI] [PubMed] [Google Scholar]
- 55.Wilkins, B. J., Y. S. Dai, O. F. Bueno, S. A. Parsons, J. Xu, D. M. Plank, F. Jones, T. R. Kimball, and J. D. Molkentin. 2004. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94:110-118. [DOI] [PubMed] [Google Scholar]
- 56.Wilkins, B. J., and J. D. Molkentin. 2002. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J. Physiol. 541:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood, A. M., and D. R. Bristow. 1998. N-Methyl-d-aspartate receptor desensitisation is neuroprotective by inhibiting glutamate-induced apoptotic-like death. J. Neurochem. 70:677-687. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi, O., Y. Higuchi, S. Hirotani, K. Kashiwase, H. Nakayama, S. Hikoso, T. Takeda, T. Watanabe, M. Asahi, M. Taniike, Y. Matsumura, I. Tsujimoto, K. Hongo, Y. Kusakari, S. Kurihara, K. Nishida, H. Ichijo, M. Hori, and K. Otsu. 2003. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc. Natl. Acad. Sci. USA 100:15883-15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi, O., T. Watanabe, K. Nishida, K. Kashiwase, Y. Higuchi, T. Takeda, S. Hikoso, S. Hirotani, M. Asahi, M. Taniike, A. Nakai, I. Tsujimoto, Y. Matsumura, J. Miyazaki, K. R. Chien, A. Matsuzawa, C. Sadamitsu, H. Ichijo, M. Baccarini, M. Hori, and K. Otsu. 2004. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J. Clin. Investig. 114:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, L., J. Chen, and H. Fu. 1999. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc. Natl. Acad. Sci. USA 96:8511-8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao, Y., Y. Tozawa, R. Iseki, M. Mukai, and M. Iwata. 1995. Calcineurin activation protects T cells from glucocorticoid-induced apoptosis. J. Immunol. 154:6346-6354. [PubMed] [Google Scholar]
- 62.Zou, X., T. Tsutsui, D. Ray, J. F. Blomquist, H. Ichijo, D. S. Ucker, and H. Kiyokawa. 2001. The cell cycle-regulatory CDC25A phosphatase inhibits apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:4818-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]