Abstract
Many different viruses activate the extracellular signal-regulated kinase (ERK)/mitogen-activated protein (MAP) kinase signaling pathway during infection and require ERK activation for the efficient execution of their replication programs. Despite these findings, no virus-encoded proteins have been identified that directly modulate ERK activities. In an effort to determine the function of a conserved alphaherpesvirus structural protein called Us2, we screened a yeast two-hybrid library derived from NIH 3T3 cells and identified ERK as a Us2-interacting protein. Our studies indicate that Us2 binds to ERK in virus-infected cells, mediates the incorporation of ERK into the virion, and inhibits the activation of ERK nuclear substrates. The association of Us2 with ERK leads to the sequestration of ERK at the plasma membrane and to a perinuclear vesicular compartment, thereby keeping ERK out of the nucleus. Us2 can bind to activated ERK, and the data suggest that Us2 does not inhibit ERK enzymatic activity. The treatment of cells with U0126, a specific inhibitor of ERK activation, resulted in a substantial delay in the release of virus from infected cells that was more pronounced with a virus deleted for Us2 than with parental and repaired strains, suggesting that both ERK and Us2 activities are required for efficient virus replication. This study highlights an additional complexity to the activation of ERK by viruses, namely, that localization of active ERK can be altered by virus-encoded proteins.
All herpesvirus virions share a common structure: an icosahedral nucleocapsid containing a linear, double-stranded DNA genome surrounded by a lipid envelope embedded with a dozen or so glycoprotein spikes. Between the nucleocapsid and the envelope lies a proteinaceous compartment called the tegument. The tegument is the most complex subvirion compartment, housing at least 14 virus-encoded proteins and an undefined number of cellular proteins. The components of the tegument are delivered to the cytoplasm of infected cells during virus entry and have the opportunity to function prior to de novo virus gene expression. Over the past few years, the complex network of protein-protein interactions required for tegument assembly is starting to be defined (49). By comparison, much less is known about tegument disassembly or the trafficking and functions of most tegument proteins upon entry of virus into cells (20, 44, 50, 51).
Recent work in our laboratory has focused on a pseudorabies virus (PRV) tegument protein, Us2, which is conserved throughout most of the alphaherpesvirus subfamily of the Herpesviridae (10, 67, 75). In addition to PRV, a swine pathogen, the human pathogens herpes simplex virus types 1 and 2 as well as many other viruses of wild and domestic animals encode Us2 homologs (12, 21, 31, 37, 45, 46, 67, 71, 74). Significantly, the Us2 gene is deleted from an attenuated PRV vaccine strain, Bartha, as well as the attenuated equine herpesvirus 1 vaccine strain, RacH, strongly suggesting that Us2 is an important virulence determinant during natural infections (25, 27, 39). A number of studies on Us2 homologs have been published, but none have revealed specific details about Us2 activities (10, 19, 28, 32, 40, 47, 48, 68). To provide clues to Us2 function, we identified cellular proteins that interact with Us2 by screening a yeast two-hybrid library derived from NIH 3T3 cells. We determined that Us2 binds specifically to the extracellular signal-regulated kinase (ERK) a mitogen-activated protein (MAP) kinase.
MAP kinase signaling pathways relay information from the plasma membrane to the nucleus, thereby facilitating a transcriptional response to the extracellular environment. The Raf/MEK/ERK module was the first MAP kinase cascade to be characterized and plays a key role in the regulation of cell proliferation, differentiation, and survival (11, 34, 55, 59, 70). The canonical ERK/MAP kinase cascade is stimulated upon the binding of extracellular growth factors, such as epidermal growth factor, to their respective transmembrane tyrosine kinase receptors. The subsequent autophosphorylation of the cytoplasmic tails of the receptor leads to the recruitment of guanine nucleotide exchange factors that activate Ras through exchange of GDP for GTP. The active, GTP-bound form of Ras binds to Raf, facilitating its phosphorylation and activation by kinases such as Src and PAK. Activated Raf phosphorylates and activates MEK, which in turn phosphorylates and activates ERK. Phosphorylated ERK dimerizes and is translocated to the nucleus, where it phosphorylates and activates a variety of transcription factors (33). Alternatively, active ERK can remain in the cytoplasm and modify targets there (70). To date, more than 50 substrates for ERK have been identified (38). There are two isoforms of ERK, called ERK1 and ERK2 (also known as p44 and p42), which are conserved from worms to humans (36, 72).
ERK activation can result in different outcomes depending on the nature of the extracellular stimulus and the cell type involved. Specificity in signaling can be achieved through the interaction of signaling components with scaffolding and adaptor proteins that regulate and direct the activities of the MAP kinase towards the relevant subset of substrates (42, 66). Examples of spatial regulators of the ERK MAP kinase pathway include KSR (kinase suppressor of Ras), MP1 (MEK partner 1), beta-arrestin, PEA-15 (phosphoprotein enriched in astrocytes 15), and Sef (similar in expression to FGF genes). KSR binds both MEK and ERK in the cytoplasm and targets them to the plasma membrane in response to growth factor stimulation (52). MP1 and beta-arrestin localize active ERK to late and early endosomes, respectively (43, 64). PEA-15 shuttles ERK out of the nucleus and anchors it in the cytoplasm (17). Sef binds to activated MEK bound to ERK and directs the complex to the Golgi apparatus (65). Both PEA-15 and Sef prevent ERK phosphorylation of nuclear substrates without inhibiting the activation of ERK cytoplasmic targets.
In this study, we demonstrate that PRV Us2 binds to ERK, targets it to the plasma membrane and to a perinuclear vesicular compartment, and prevents activation of ERK's nuclear targets. Us2 does not prevent ERK activation, and the data suggest that the Us2-ERK interaction does not inhibit ERK enzymatic activity. Thus, Us2 appears to function as a spatial regulator of ERK signaling. We also demonstrate that PRV infection activates ERK in a Us2-independent manner and that ERK activity is required for the efficient release of progeny virions from infected cells.
MATERIALS AND METHODS
Cells and viruses.
The wild-type (wt) PRV strain Becker and its derivative, PRV174, have been described previously (10). PRV644 is a PRV174 derivative that expresses Us2GAAX from the gG locus. NIH 3T3 cells, obtained from ATCC, were maintained in Dulbecco's modified Eagle's medium containing 10% newborn calf serum (NCS) at 37°C in a 5% CO2 environment. PK15, a kind gift from L. W. Enquist, Princeton University, and MRC-5 cells, generously provided by K. V. Holmes, UCDHSC, were maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum. PK15 cells stably expressing wt Us2 were isolated after transfection with pCC34, a Us2 expression plasmid that also contains the neomycin phosphotransferase gene under the control of the SV40 enhancer/early promoter. Forty-eight hours after transfection, cells were maintained in medium containing 0.5 μg/ml G418 (Sigma, St. Louis, MO) to select for stable incorporation of pCC34. Individual G418-resistant cells were sorted into wells of a 96-well plate by flow cytometry. Clonal lines stably expressing Us2 were identified by indirect immunofluorescence microscopy and subsequently pooled.
Expression plasmids.
The Us2 expression plasmid, pCC34, has been described previously (10). The ERK2-enhanced green fluorescent protein (EGFP) expression construct was kindly provided by N. W. Bunnet, UCSF (13).
Antibodies.
The production of Us2 antiserum was described previously (10). Polyclonal antisera against total ERK1/ERK2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antisera against phospho-ERK, total Elk-1, and phospho-Elk-1 were purchased from Cell Signaling Technology (Beverly, MA).
Yeast two-hybrid analysis.
The PRV Us2 open reading frame (ORF) was cloned into pGBKT7 (Trp) (Clontech, Palo Alto, CA) and used to screen a cDNA library derived from NIH 3T3 cells constructed in pACT2 (Leu) (Clontech; Matchmaker System). Isolates of Saccharomyces cerevisiae strain pJ69-4 (30), expressing the Us2 bait plasmid (yML5), were transformed with 0.5 mg of library DNA. Transformants were plated on 2-by-150-mm dishes of dropout medium lacking Trp and Leu (−Trp/−Leu dropout medium) to determine the transformation efficiency as well as 50-by-150-mm dishes of −Trp/−Leu/−His/−Ade dropout medium to select for two-hybrid interactions. Of the clones able to grow in the absence of histidine and adenine, 105 that were strongly positive for β-galactosidase activity in a colony lift filter assay were selected for further analysis. The cDNAs from 10 of these were identified after isolation of the library plasmids and DNA sequencing using the primer 5′-GTTTAATACCACTACAATGG-3′. Eight of the sequences were identical to mouse vimentin, all of which were out of frame with the GAL4 activation domain. To identify library clones other than vimentin, which are capable of interaction with Us2, we isolated library plasmids from 62 of the 105 positive clones and spotted them onto nitrocellulose and hybridized them to a full-length vimentin cDNA probe. Fifteen cDNA inserts from nonhybridizing library plasmids were sequenced. Six of these encoded ERK1, all of which were in frame with the GAL4 activation domain, five were independent clones having different 5′ ends, and one was a full-length cDNA.
To determine whether PRV Us2 interacted with the ERK2 isoform in yeast, an ERK2-EGFP fusion construct (13) was digested with BamHI to isolate the murine ERK2 ORF. The ERK2 ORF was cloned into the BamHI site of pGAD-C3 (30) in frame with the GAL4 activation domain, creating the plasmid pML32. yML5 was transformed with pML32 as described above and plated on −Trp/−Leu/−His/−Ade dropout medium to select for a two-hybrid interaction.
Immunoprecipitation.
NIH 3T3 cells were grown to 80% confluence in 150-mm-diameter dishes (∼1.5 × 107 cells/dish) and infected with PRV Becker or PRV174 (Us2-null) viruses at a multiplicity of infection (MOI) of 10 PFU/cell. At 16 h postinfection, cells were washed with PBS, scraped into 500 μl of mammalian protein extraction reagent (M-PER) (Pierce, Rockford, IL) containing protease inhibitors (Roche Diagnostics, Germany), and rocked at 4°C for 15 min. The lysates were clarified by centrifugation at 27,000 × g for 15 min. Supernatants were transferred to fresh tubes, and 50 μl of a solution containing 10% NP-40, 5% sodium deoxycholate, and 1% sodium dodecyl sulfate (SDS) was added. Samples were precleared by adding 20 μl of rabbit preimmune serum, rocked at 4°C for 1 h, followed by the addition of 50 μl of immobilized protein G (Pierce, Rockford, IL), and rocked for an additional hour at 4°C. Immune complexes were pelleted by brief centrifugation in a bench-top microcentrifuge at 13,000 rpm. The supernatant was transferred to a fresh microcentrifuge tube containing 5 μg of rabbit anti-ERK antiserum or 5 μg of phospho-specific ERK antiserum and incubated overnight on ice. Fifty microliters of immobilized protein G was added to the sample and rocked at 4°C for 2 h. Immune complexes were pelleted by brief centrifugation at 13,000 rpm. The supernatant was discarded, and the pellet was washed three times successively with 500 μl of wash buffer 1 (20 mM Tris, pH 7.5, 150 mM NaCl, and 1% NP-40), wash buffer 2 (20 mM Tris, pH 8.8, 150 mM NaCl, 1% NP-40, and 0.2% SDS), and wash buffer 3 (20 mM Tris, pH 6.8, 150 mM NaCl, 1% NP-40, and 0.2% SDS) as described previously (3). Immune complexes were resuspended in 50 μl of SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer and boiled for 5 min. Samples were analyzed by SDS-PAGE on 10% gels, followed by Western blotting.
ERK in vitro kinase assay.
NIH 3T3 cells were grown to 80% confluence in 150-mm-diameter dishes and infected with PRV Becker or PRV174 (Us2-null) viruses at an MOI of 10 PFU/cell. At 16 h after infection, cells were washed with ice-cold PBS and scraped into 500 μl of M-PER (Pierce, Rockford, IL) supplemented with 150 mM NaCl, protease inhibitors (Roche Diagnostics, Germany), and phosphatase inhibitors (20 mM NaF, 10 mM β-glycerophosphate, 2 mM EGTA, and 1 mM Na3VO4). Cell lysates were transferred to microcentrifuge tubes, rocked at 4°C for 15 min, and then clarified by centrifugation at 27,000 × g for 15 min. Supernatants were transferred to fresh tubes, and 20 μl of immobilized phospho-ERK antibody (Cell Signaling Technology, Beverly, MA) was added. Samples were rocked overnight at 4°C, and immune complexes were pelleted by brief centrifugation at 13,000 rpm for 30 s. The pellets were washed twice with 500 μl of M-PER lysis buffer and twice with 500 μl of 1× ERK kinase buffer (New England Biolabs, Beverly, MA) and resuspended in a 50-μl kinase reaction mixture consisting of 1× kinase buffer, 200 mM ATP, and 5 μg of Elk-1 fusion protein (Cell Signaling Technology, Beverly, MA). Samples were incubated at 30°C for 40 min, and the reaction was terminated by the addition of 25 μl of 3× SDS-PAGE sample buffer. Purified ERK2 enzyme (New England Biolabs, Beverly, MA) was used in these assays as a positive control. Samples were analyzed by SDS-PAGE and Western blot analysis.
Virus purification.
Three confluent 150-mm dishes of PK15 cells were infected with virus at an MOI of 10. At 16 h postinfection, medium (20 ml/150 mm dish) was collected into 2-by-50-ml conical tubes (30 ml of supernatant/tube) and centrifuged at 3,000 rpm in a Sorvall ST-H750 rotor for 15 min at 4°C to remove cells and cellular debris. The clarified supernatant was layered onto a 7-ml 30% sucrose-PBS cushion (wt/vol) in two Beckman SW28 centrifuge tubes. The tubes were centrifuged in an SW28 rotor at 23,000 rpm for 3 h at 4°C. The supernatant and sucrose cushion were removed, and the virion pellet was resuspended in 1 ml of PBS aided by 10 1-s pulses in a chilled bath sonicator followed by gentle trituration. Virions were centrifuged through a 1-ml 30% sucrose cushion at 28,000 rpm for 90 min at 4°C in an SW55ti rotor. The virion pellet was resuspended in 100 μl of cold PBS and stored in two 50-μl aliquots at −80°C.
Protease treatment of virions.
Purified virions were treated with 10 μg of proteinase K per ml in either the presence or the absence of 1% SDS in a total volume of 50 μl. After incubation for 60 min at room temperature, 5 μl of 200 mM phenylmethylsulfonyl fluoride was added to each sample to inhibit further proteolysis. Samples were loaded onto SDS-PAGE gels immediately and analyzed by Western blotting.
Immunofluorescence microscopy.
NIH 3T3 cells were seeded onto glass coverslips in six-well plates and grown to 30 to 40% confluence. Each well was then transfected with 1 μg of ERK2-EGFP and 1 μg of pCI-neo (empty vector control) or pCC34 (wt Us2) using FuGENE 6 (Roche, Indianapolis, IN) according to the manufacturer's instructions. At 24 h posttransfection, cells were rinsed three times with PBS and then fixed in 4% paraformaldehyde-PBS for 10 min at room temperature. Cells were rinsed with PBS and permeabilized in PBS-1% bovine serum albumin (BSA) containing 0.1% Triton X-100 at room temperature for 3 min. Cells were rinsed three times with PBS and incubated for 45 min with Us2 goat polyclonal antiserum diluted in PBS-1% BSA (1:3,000). Cells were rinsed three times with PBS-1% BSA and incubated for 30 min with Alexa 568-conjugated secondary antibodies (Molecular Probes, Eugene, OR) diluted in PBS-1% BSA. The cells were washed three times with PBS-1% BSA, followed by three washes with PBS. Coverslips were mounted onto glass slides, and digital images were captured using either a Zeiss 510 laser scanning confocal microscope, an Olympus spinning disk confocal microscope, or a Nikon TE200 inverted epifluorescence microscope equipped with digital deconvolution capability.
Elk-1 trans-reporter assay.
Elk-1 activation was measured using the Stratagene PathDetect in vivo signal transduction pathway Elk-1 trans-reporting system (Stratagene, La Jolla, CA). Cells were grown to 40 to 60% confluence in six-well plates, and each well was transfected with 50 ng of GAL4-Elk-1, 1 μg of GAL4-luciferase, 50 ng of constitutively active MEK (positive control), and 1 μg of pCC34 (wt Us2) or pCIneo (empty vector control). Each condition was performed in triplicate and normalized for transfection efficiency using a constitutively active β-galactosidase reporter plasmid. Cells were incubated in medium containing 10% NCS for the first 24 h and in medium containing 0.5% NCS for the final 24 h. Elk-1-dependent transcription (i.e., luciferase expression) was measured using the Stratagene luciferase assay kit, whereas β-galactosidase activity was measured using the Stratagene β-galactosidase assay kit according to the manufacturer's instructions (Stratagene, La Jolla, CA).
RESULTS
Us2 interacts with the MAP kinases ERK1 and ERK2 in yeast.
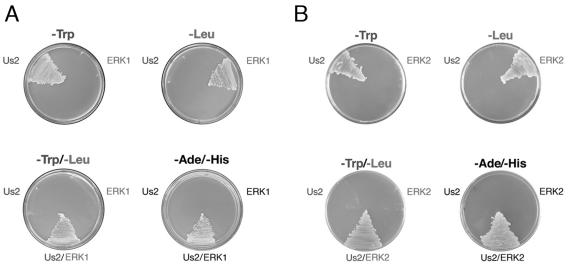
We reasoned that the identification of cellular proteins that interact with Us2 would provide insight into Us2 function. To do this, we employed a yeast two-hybrid system to screen an NIH 3T3 cell cDNA library using PRV Us2 as bait. Five independent, either full- or partial-length cDNAs encoding the MAP kinase ERK1 were identified in the screen. To verify that ERK1 was interacting with Us2 in the yeast two-hybrid assay and not merely capable of transactivating the ADE2 and HIS3 two-hybrid reporter genes alone, we tested the ability of yeast strains expressing only one or the other of the putative binding partners to grow in the absence of adenine and histidine (Fig. 1A). The expression of the appropriate fusion proteins was confirmed by Western blotting (not shown). Only yeast strains that expressed both the Us2 and the ERK1 fusion proteins were capable of growth in the absence of adenine and histidine, confirming the interaction between Us2 and ERK1 in yeast. A similar experiment was performed to demonstrate that Us2 also interacts with the ERK1 isoform ERK2 (Fig. 1B).
FIG. 1.
Us2 interacts with ERK1 and ERK2 in a yeast two-hybrid assay. Verification of protein-protein interactions between Us2 and ERK1 (A) or ERK2 (B). Only yeast strains cotransformed with Us2 and ERK1/ERK2 expression plasmids were able to grow on medium lacking adenine (−Ade) and histidine (−His), indicating a two-hybrid interaction. The ability to grow in the absence of tryptophan (−Trp) indicates selection for the Us2 plasmid. The ability to grow in the absence of leucine (−Leu) indicates selection for the ERK1 and ERK2 plasmids.
ERK interacts with Us2 in infected cells and is packaged into virions in a Us2-dependent manner.
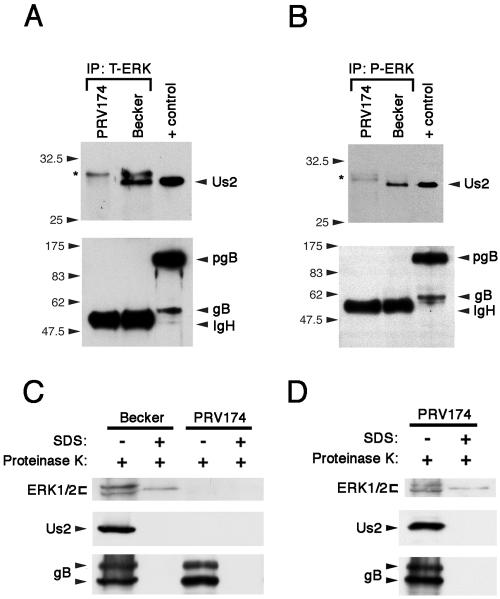
To determine whether ERK interacts with Us2 in mammalian cells, we infected NIH 3T3 cells with the wt PRV strain Becker or the Us2-null virus PRV174. ERK was immunoprecipitated from Becker- and PRV174-infected extracts using polyclonal antiserum, and immunoprecipitates were analyzed by Western blotting for Us2 and glycoprotein gB (Fig. 2A). Whereas Us2 and gB were readily detected in Becker-infected cell extracts (positive control), the ERK antiserum pulled down Us2 from Becker-infected cell extracts, but not the unrelated membrane protein gB. These data indicate that ERK and Us2 interact in infected cells. Furthermore, immunoprecipitation of ERK using phospho-specific antiserum revealed that a considerable portion of Us2 specifically interacts with activated ERK (Fig. 2B). It is noteworthy that we have also demonstrated the interaction of Us2 with ERK in infected PK15 and MRC-5 cells as well as in transiently transfected 293T cells (not shown). NIH 3T3 cells were selected for further analysis because they are most often used for studying the ERK/MAP kinase pathway.
FIG. 2.
Us2 interacts with ERK in PRV-infected cells and directs ERK incorporation into virions. (A) Total ERK (T-ERK) was immunoprecipitated (IP) from NIH 3T3 cells infected with PRV174 (Us2-null mutant) or wt Becker and analyzed by Western blotting using antiserum specific for Us2 (top) and gB (bottom). The immunoglobulin heavy chain (IgH) from the immunoprecipitation cross-reacted with the secondary antiserum used to detect glycoprotein gB. PRV gB is a type I membrane protein that is cleaved in the Golgi from the preprocessed monomer pgB into two smaller subunits that are linked by disulfide bonds. These subunits have molecular masses of 69 kDa and 58 kDa and represent the amino-terminal and carboxy-terminal “halves” of the molecule, respectively (gB). Positive control (+ control) is an extract prepared from PRV Becker-infected cells. The asterisk denotes nonspecific bands that cross-react with the Us2 antiserum. (B) Phosphorylated ERK (P-ERK) was immunoprecipitated (IP) from NIH 3T3 cells infected with PRV174 or Becker and analyzed by Western blotting using antiserum specific for Us2 (top) and gB (bottom). The asterisk denotes nonspecific bands that cross-react with the Us2 antiserum. Positive control (+ control) is an extract prepared from PRV Becker-infected cells. IgH, immunoglobulin heavy chain. (C) Purified virions from Becker and PRV174-infected cell supernatants were isolated as described in Materials and Methods. Treatment of virions with proteinase K in the absence of SDS degraded any proteins not protected by the virion envelope. Proteins found in the tegument are protected by the virion envelope. gB was used as a virus loading control and as a control for the activity of the protease in the absence of SDS. Western blot analysis was performed using antiserum specific for total ERK (top), Us2 (middle), or gB (bottom). +, with; −, without. (D) A PK15 cell line that stably expresses Us2 was infected with PRV174. Virions were purified from infected cell supernatants and treated with proteinase K and/or SDS. Note that the incorporation of Us2 and ERK into the tegument is restored. +, with; −, without.
We have shown previously that Us2 is a component of the virion tegument (10). Upon discovering that Us2 binds to ERK in infected cells, we examined whether ERK was also incorporated into virions. Whereas ERK was readily detected in the tegument of purified Becker virions, virions purified from PRV174-infected cell supernatants did not contain detectable ERK (Fig. 2C). Importantly, PRV174 infection of a Us2-expressing cell line produced virions that contained Us2 and ERK in the tegument (Fig. 2D). These data show that ERK is packaged into PRV virions in a Us2-dependent manner. We noted the presence of a single protease-resistant band that reacted with ERK antiserum in virions treated with SDS and proteinase K; however, the appearance of this band was not dependent on the incorporation of Us2 or ERK into the tegument and it was not present in all of the virion preparations tested.
Us2 expression alters the localization of ERK2.
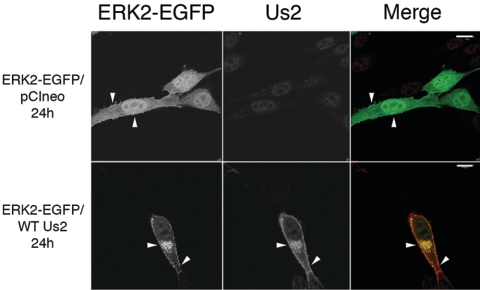
In order to probe the interaction of Us2 with ERK further, we examined the localization of Us2 and ERK2 in transfected cells. To do this, we took advantage of an ERK2-EGFP fusion protein that has been characterized previously (13). It was first important to control for potential interactions between Us2 and unfused EGFP. The expression of wt Us2 had no effect on EGFP localization in transfected NIH 3T3 cells (not shown). By contrast, Us2 expression had profound effects on ERK2-EGFP localization (Fig. 3). In cells transfected with ERK2-EGFP and the empty expression vector, pCIneo, ERK2-EGFP localized throughout the cytoplasm and nucleus of transfected cells, as was expected. In cells transfected with wt Us2 and ERK2-EGFP expression plasmids, ERK2-EGFP colocalized with Us2 and was targeted almost exclusively to the plasma membrane and to a perinuclear cytoplasmic location; the same Us2 staining pattern was observed in cells transfected with Us2 expression plasmid alone (10).
FIG. 3.
Us2 expression alters the subcellular localization of ERK2-EGFP. NIH 3T3 cells were cotransfected with the plasmid combinations indicated on the left of the figure. Twenty-four hours after transfection, cells were stained for Us2. On merged images, the ERK2-EGFP signal is green, and the Us2 signal is red. pCIneo is an empty expression plasmid and has no effect on ERK2-EGFP localization. Arrowheads indicate points of interest. Images were obtained using a Zeiss 510 confocal laser scanning microscope. Scale bars are 15 μm.
Us2-associated ERK retains enzyme activity.
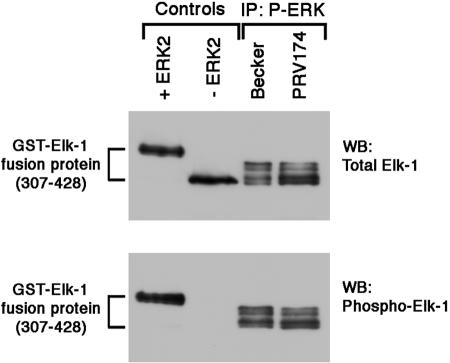
To determine whether Us2 association with phosphorylated ERK inhibited ERK kinase activity, we employed a modified version of an ERK in vitro kinase assay (2). Phosphorylated ERK was immunoprecipitated from extracts prepared from cells infected with Becker or PRV174. Immunoprecipitates were incubated with purified, recombinant Elk-1, a well-characterized ERK substrate, in the presence of ATP and subsequently analyzed by Western blotting using antiserum specific for total Elk-1 or phosphorylated Elk-1. The phosphorylation of Elk-1 by ERK isolated in the presence (Becker) and absence (PRV174) of Us2 was indistinguishable, suggesting that Us2 does not act as an inhibitor of ERK enzymatic activity (Fig. 4).
FIG. 4.
Expression of Us2 does not affect ERK enzymatic activity. Phosphorylated ERK was immunoprecipitated (IP) from NIH 3T3 cells infected with Becker or PRV174. ERK activity was measured using an Elk-1 in vitro kinase assay as described in Materials and Methods. The Elk-1 peptide, comprising amino acids 307 to 428 of full-length Elk-1, is predicted to have eight ERK phosphorylation sites. Samples were analyzed by SDS-PAGE and Western blotting (WB) using antiserum specific to total Elk-1 (top) or phospho-Elk-1 (bottom). In control experiments (left two lanes on blots), purified recombinant active ERK2 (+ERK2) or buffer (−ERK2) was added to the reaction mixtures in place of active ERK immunoprecipitated from infected cell lysates.
Us2 expression inhibits ERK-mediated activation of Elk-1.
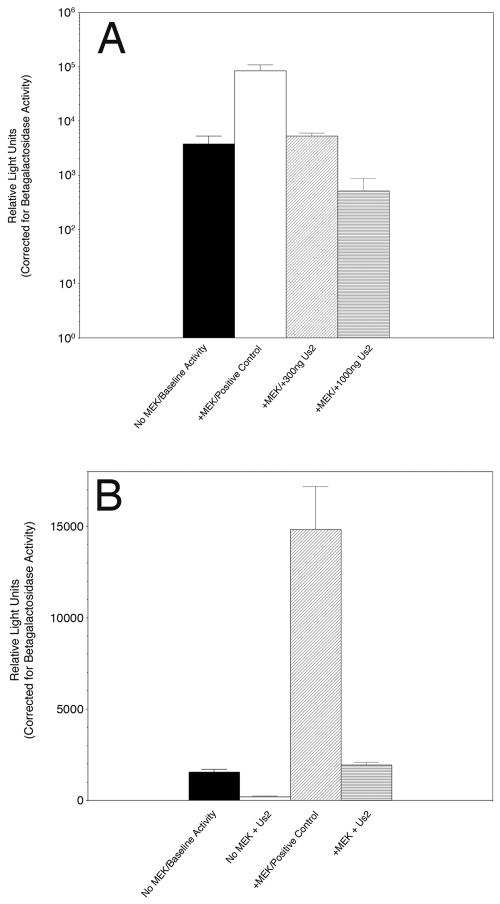
Upon activation and dimerization, ERK translocates to the nucleus, where it phosphorylates transcription factors such as Elk-1 (18). To examine the consequences of Us2 expression for this process, we used a well-established transient transfection assay that monitors in vivo activation and translocation of ERK from the cytoplasm to the nucleus (2, 17). ERK enzymatic activity in the nucleus was measured by an ERK-dependent, Elk1-mediated transactivation of a luciferase reporter construct. The data, obtained in triplicate and normalized for transfection efficiency using a constitutively active β-galactosidase reporter plasmid included in all transfections, are shown in Fig. 5A. As expected, the inclusion of a constitutively active MEK expression plasmid in the cotransfection cocktail resulted in a 22-fold increase in Elk-1 transcription over baseline activity. The presence of increasing amounts of a Us2 expression plasmid resulted in dose-dependent inhibition of Elk-1 transcription activity, such that, when 1,000 ng of Us2 expression plasmid was included in the assay, a 165-fold inhibition of Elk-1-mediated luciferase production was observed. These data indicate that Us2 is a potent inhibitor of ERK activity in the nucleus.
FIG. 5.
Us2 expression blocks ERK-mediated Elk-1 activation of transcription. (A) NIH 3T3 cells were cotransfected with a plasmid, pFA2-Elk1, that expresses the Elk-1 activation domain fused to the GAL4 DNA binding domain, a luciferase reporter plasmid, pFR-Luc, containing a synthetic promoter with five tandem repeats of the GAL4 binding site, and the combinations of plasmids indicated under the x axis. Luciferase activity, which is indicative of Elk1-dependent transcription, is expressed as relative light units. Note that the y axis has a log scale. (B) MRC-5 cells were cotransfected with pFA2-Elk1, pFR-Luc, and the combinations of plasmids indicated under the x axis. Error bars represent the standard deviations between experiments performed in triplicate. Variations in transfection efficiency were normalized by the inclusion of a beta-galactosidase reporter plasmid where expression is under control of a constitutively active promoter.
To test whether the effects of Us2 expression on Elk-1 transcription were cell type or species dependent, we performed the luciferase reporter assays described above with a nontransformed human fibroblast cell line, MRC-5 (Fig. 5B). Us2 inhibited Elk-1 transcription in both the presence and the absence of constitutively active MEK. The degree of inhibition observed was not as great as that seen in NIH 3T3 cells, but it was in line with what has been reported for Sef and PEA-15, which also bind ERK in the cytoplasm (17, 65). In support of these data, PRV Us2 coimmunoprecipitated with human ERK from infected MRC-5 cell lysates (not shown). Taken together, these data are consistent with the idea that Us2 prevents the translocation of active ERK to the nucleus.
ERK is activated early after PRV infection.
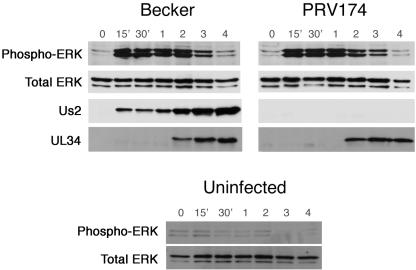
The data so far demonstrate an interaction between Us2 and ERK. To begin to understand the relevance of the Us2/ERK interaction in virus-infected cells, we examined the activation state of ERK during virus infection. For these experiments, it was important to infect cells with purified virus to avoid unwanted stimulation of ERK through serum components present in the inoculum. Becker and PRV174 virions (Us2-null) were purified as described in Materials and Methods. NIH 3T3 cells were grown in medium containing 0.5% serum for 24 h prior to infection to minimize the levels of activated ERK. Cell lysates were prepared from infected cells at various times after infection and analyzed by Western blotting using antiserum against phosphorylated ERK, total ERK, UL34, and Us2 (Fig. 6). In both Becker- and PRV174-infected cells, activation of ERK increased as early as 15 min after the exposure of cells to virus and remained elevated through 3 h postinfection. By 4 h postinfection, the level of active ERK had returned to that seen in uninfected cells. By contrast, mock-infected cells showed no activation of ERK over the course of the experiment. It is noteworthy that Us2 was readily detected in Becker-infected cell extracts at 15, 30, and 60 min after infection but prior to the synthesis of new Us2, which occurs between 1 and 2 h after infection (10), indicating that this material represents Us2 delivered to cells by incoming virions. These data show that early events during PRV infection, which are independent of Us2, activate ERK.
FIG. 6.
PRV infection stimulates ERK activation in an Us2-independent manner. Virions were purified as described in Materials and Methods. NIH 3T3 cells were infected with PRV Becker, the Us2-null mutant, PRV174, or mock infected (uninfected) and harvested at the indicated times after infection. Lysates were analyzed by Western blot analysis using antiserum against phospho-ERK, total ERK, the nonstructural virus protein UL34 (infection control), and Us2.
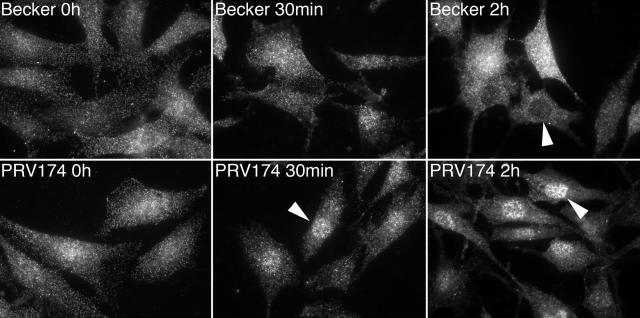
We next examined the localization of activated ERK in cells infected with Becker and PRV174 (Fig. 7). Prior to infection (0 h), active ERK was localized in a diffuse pattern throughout the cell. Thirty minutes after exposure of cells to Becker, active ERK remained localized in a diffuse pattern and, at 2 h postinfection, was notably excluded from the nucleus of many cells. By contrast, as early as 30 min after infection with PRV174, active ERK was detected in the nucleus. Nuclear localization of active ERK in PRV174 was even more evident at 2 h postinfection. These data suggest that the Us2 entering the cell with the virion is capable of sequestering activated ERK in the cytoplasm.
FIG. 7.
Virus-associated Us2 keeps activated ERK out of nucleus. NIH 3T3 cells infected with wt (Becker) or Us2-null (PRV174) viruses were stained for active ERK using phospho-ERK1/2-specific antiserum at the indicated times postinfection. Arrowheads point out the relative presence or absence of activated ERK in the nuclei of infected cells. Digital images were captured using a Nikon TE200 inverted epifluorescence microscope equipped with a cooled charge-coupled device camera. Images were acquired with the aid of the Metamorph software package, such that the full dynamic range of fluorescent signal was obtained for each specimen. Thus, it should be noted that the data shown do not quantify the levels of active ERK1/2 in cells, but rather report on only the subcellular distribution of activated ERK1/2.
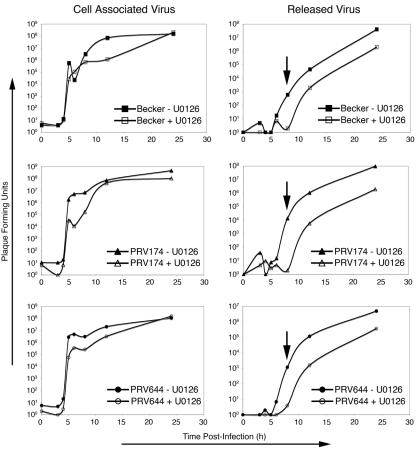
In support of a role for ERK during virus replication, treatment of cells with U0126, a specific inhibitor of ERK activation (16), resulted in a substantial delay in the release of infectious virus, which was particularly acute for the Us2-null virus PRV174 (3 to 4 log units) at 8 h postinfection (Fig. 8, arrows). A delay in the release of the parental Becker strain and PRV644, which ectopically expresses Us2, was also observed but was less severe (∼2 log units) than that seen for PRV174 at 8 h postinfection. The kinetics of appearance of cell-associated infectious virus was unchanged by U0126; however, the amount of virus detected from 6 to 8 h postinfection was reduced by 2 log units in cells infected by PRV 174. The levels of cell-associated virus produced by each strain were similar in the presence and absence of U0126 at 24 h postinfection. These findings suggest that inhibition of ERK activity impinges on the release of infectious virus from cells by either preventing or delaying egress. In the absence of Us2 (PRV174), this effect was more pronounced and was reproducible between experiments.
FIG. 8.
PK15 cells were maintained in medium containing 50 μM U0126 (+U0126) or dimethyl sulfoxide (−U0126) and 0.5% newborn calf serum for 18 h prior to and for 24 h after infection with PRV Becker, PRV174, or PRV644 at 3 PFU per cell. At the indicated times after infection, cells and medium were collected separately and subjected to three freezing (at −80°C) and thawing (at 37°C) cycles and infectious virus was quantified by plaque assay on PK15 cells. Data shown are representative of three independent experiments. Cells from the swine kidney cell line, PK15, were selected for this analysis because PRV replicates to much higher levels in these cells compared to that in NIH 3T3 cells. It should be noted, however, that treatment of NIH 3T3 cells with U0126 also inhibits virus replication.
DISCUSSION
A wide variety of viruses activate the ERK/MAP kinase signaling pathway upon infection; these include adenovirus (7), simian virus 40, herpes simplex virus type 2 (62, 63), human cytomegalovirus (HCMV) (6, 60), Kaposi's sarcoma-associated herpesvirus (KSHV) (54), hepatitis B virus (4), vaccinia virus (14), influenza virus (57), respiratory syncytial virus (35), coxsakievirus B3 (26), Borna disease virus (BDV) (22) and human immunodeficiency virus type 1 (HIV-1) (58). Our data show that robust stimulation of ERK was observed as early as 15 min after exposure of cells to purified PRV virions (Fig. 6), suggesting that early events in the PRV infectious cycle, such as attachment and/or entry, trigger ERK activation. In the case of HCMV, a betaherpesvirus, the interaction of glycoprotein gB with the epidermal growth factor receptor and subsequent epidermal growth factor receptor autophosphorylation likely mediate the activation of ERK. By contrast, a soluble form of glycoprotein gpK8.1A, but not soluble gB, from the gammaherpesvirus KSHV was sufficient to mediate ERK activation (61). Identification of the step in the alphaherpesvirus attachment and entry pathway that leads to ERK activation and identification of the viral and cellular components involved will provide a clearer understanding of the relevance and mechanisms of virus-mediated activation of cellular signal transduction pathways.
Whether ERK activation represents a cell-directed defense against the virus or a specific tactic orchestrated by the virus for its own means is not entirely clear, but it likely depends on the type of virus and the host cell infected. The ERK pathway has been implicated in the regulation of viral gene expression for SV40 (reviewed in reference 53), adenovirus (7, 69), hepatitis B virus (5), HCMV (8, 9, 23, 60), and KSHV (61). Interference with the ERK signaling pathway affects the virus yield for influenza virus (57), respiratory syncytial virus (35), vaccinia virus (1), and coxsackievirus B3 (41). Furthermore, inhibition of ERK activation blocks the spread of BDV between cells (56). Our data indicate that ERK activity also promotes PRV replication and the release of infectious virus from cells and suggest that, when there is a limited pool of active ERK in the cell (e.g., in the presence of U0126), Us2 helps to scavenge what active ERK remains and direct it to where it is needed (Fig. 8). It is tempting to speculate that ERK activity is required for the modification of a cytoplasmic viral or cellular component required for efficient egress of infectious virions; however, further experiments are required to challenge this hypothesis.
The spatial regulation of ERK activity is an emerging concept in the field of signal transduction, and it is remarkable that viruses manipulate ERK in this manner (42, 66). Like Us2, the cell-encoded protein KSR sequesters active ERK at the plasma membrane. Interestingly, we have been unable to identify any significant similarities between Us2 and KSR at the amino acid sequence level. It may be that KSR and Us2 share similar structural features that perform this apparently common function. Other than Us2, no other virus proteins have been identified that regulate ERK localization. However, persistent infection of PC12 cells by BDV results in activation of ERK, while paradoxically inhibiting the neuronal differentiation of these cells upon exposure to nerve growth factor (22). An examination of ERK subcellular localization in BDV-infected PC12 cells revealed that activated ERK was excluded from the nucleus and that nuclear targets of ERK were not phosphorylated. At present, the BDV or BDV-induced products that mediate this effect have not been identified.
Us2 also directs the incorporation of ERK into the tegument of the virion, which has the potential to alter the physiology of a newly infected cell. Notably, HIV-1 virions contain ERK; however, viral components capable of interaction with ERK have not been reported (29). In this case, packaged ERK enhances HIV-1 infectivity and functions in virus assembly and release from infected cells (24, 29, 73). Interestingly, an in silico analysis of PRV virion structural components indicates that several tegument proteins contain consensus phosphorylation sites for ERK. Notably, the PRV homologs for VP1/2, ICP4, VP16, and ICP0 each contain multiple sites. One possibility is that virion-associated ERK modifies the activities of other virion structural components. The role of ERK as a herpesvirus structural component is the focus of future studies.
A large number of cellular proteins, mostly ERK substrates, have been shown to bind to ERK (34). Almost all of these molecules contain a generic MAP kinase binding site called a KIM (kinase interaction motif) that interacts with a conserved acidic site on ERK, known as the common docking domain (15). The KIM consensus sequence is -(R/K)2-(X)2-6-L/I-X-L/I-, where X is any amino acid. An ERK-specific binding motif, again found in many ERK substrates, called the DEF (docking site for ERK, FXFP) has been described and has the consensus FXFP. It is noteworthy that Us2 does not contain either a canonical KIM or DEF, nor is there significant homology between Us2 and other spatial regulators of ERK signaling, such as Sef and PEA-15. Thus, a determination of the sites on Us2 and on ERK that mediate their interaction should yield new information on the regulation of this key signaling pathway.
Acknowledgments
We thank N. Bunnet for kindly supplying plasmids used in this study. Christoph Hengartner provided invaluable advice in setting up the two-hybrid screen. Confocal microscopy was performed in the UCDHSC Light Microscopy Core Facility with the assistance of Steve Fadul. Greg Bird, Jacinta Cooper, Renée Finnen, and Linda van Dyk provided helpful comments on the manuscript.
This work was supported in part by NIH grant AI48626 to B.W.B. M.G.L. was supported in part by NIH training grant AI52066.
REFERENCES
- 1.Andrade, A. A., P. N. Silva, A. C. Pereira, L. P. De Sousa, P. C. Ferreira, R. T. Gazzinelli, E. G. Kroon, C. Ropert, and C. A. Bonjardim. 2004. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 381:437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aplin, A. E., S. A. Stewart, R. K. Assoian, and R. L. Juliano. 2001. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 153:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banfield, B. W., and F. Tufaro. 1990. Herpes simplex virus particles are unable to traverse the secretory pathway in the mouse L-cell mutant gro29. J. Virol. 64:5716-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benn, J., and R. J. Schneider. 1994. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc. Natl. Acad. Sci. USA 91:10350-10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benn, J., F. Su, M. Doria, and R. J. Schneider. 1996. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J. Virol. 70:4978-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J., and M. F. Stinski. 2000. Activation of transcription of the human cytomegalovirus early UL4 promoter by the Ets transcription factor binding element. J. Virol. 74:9845-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J., and M. F. Stinski. 2002. Role of regulatory elements and the MAPK/ERK or p38 MAPK pathways for activation of human cytomegalovirus gene expression. J. Virol. 76:4873-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clase, A. C., M. G. Lyman, T. del Rio, J. A. Randall, C. M. Calton, L. W. Enquist, and B. W. Banfield. 2003. The pseudorabies virus Us2 protein, a virion tegument coomponent, is prenylated in infected cells. J. Virol. 77:12285-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobb, M. H., and E. J. Goldsmith. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843-14846. [DOI] [PubMed] [Google Scholar]
- 12.Colle, C. F., III, and D. J. O'Callaghan. 1995. Transcriptional analyses of the unique short segment of EHV-1 strain Kentucky A. Virus Genes 9:257-268. [DOI] [PubMed] [Google Scholar]
- 13.DeFea, K. A., J. Zalevsky, M. S. Thoma, O. Dery, R. D. Mullins, and N. W. Bunnett. 2000. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 148:1267-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Magalhaes, J. C., A. A. Andrade, P. N. Silva, L. P. Sousa, C. Ropert, P. C. Ferreira, E. G. Kroon, R. T. Gazzinelli, and C. A. Bonjardim. 2001. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J. Biol. Chem. 276:38353-38360. [DOI] [PubMed] [Google Scholar]
- 15.Enslen, H., and R. J. Davis. 2001. Regulation of MAP kinases by docking domains. Biol. Cell 93:5-14. [DOI] [PubMed] [Google Scholar]
- 16.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 17.Formstecher, E., J. W. Ramos, M. Fauquet, D. A. Calderwood, J. C. Hsieh, B. Canton, X. T. Nguyen, J. V. Barnier, J. Camonis, M. H. Ginsberg, and H. Chneiweiss. 2001. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev. Cell 1:239-250. [DOI] [PubMed] [Google Scholar]
- 18.Gille, H., M. Kortenjann, O. Thomae, C. Moomaw, C. Slaughter, M. H. Cobb, and P. E. Shaw. 1995. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goshima, F., D. Watanabe, H. Suzuki, H. Takakuwa, H. Yamada, and Y. Nishiyama. 2001. The US2 gene product of herpes simplex virus type 2 interacts with cytokeratin 18. Arch. Virol. 146:2201-2209. [DOI] [PubMed] [Google Scholar]
- 20.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2005. Entry of pseudorabies virus: an immunogold-labeling study. J. Virol. 79:3200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haanes, E. J., and C. C. Tomlinson. 1998. Genomic organization of the canine herpesvirus US region. Virus Res. 53:151-162. [DOI] [PubMed] [Google Scholar]
- 22.Hans, A., S. Syan, C. Crosio, P. Sassone-Corsi, M. Brahic, and D. Gonzalez-Dunia. 2001. Borna disease virus persistent infection activates mitogen-activated protein kinase and blocks neuronal differentiation of PC12 cells. J. Biol. Chem. 276:7258-7265. [DOI] [PubMed] [Google Scholar]
- 23.Harel, N. Y., and J. C. Alwine. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J. Virol. 72:5481-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemonnot, B., C. Cartier, B. Gay, S. Rebuffat, M. Bardy, C. Devaux, V. Boyer, and L. Briant. 2004. The host cell MAP kinase ERK-2 regulates viral assembly and release by phosphorylating the p6gag protein of HIV-1. J. Biol. Chem. 279:32426-32434. [DOI] [PubMed] [Google Scholar]
- 25.Hermann, S., B. Heppner, and H. Ludwig. 1984. Pseudorabies viruses from clinical outbreaks and latent infections grouped into four major genome types. Curr. Top. Vet. Med. Anim. Sci. 27:387-401. [Google Scholar]
- 26.Huber, M., K. A. Watson, H. C. Selinka, C. M. Carthy, K. Klingel, B. M. McManus, and R. Kandolf. 1999. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 73:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubert, P. H., S. Birkenmaier, H. J. Rziha, and N. Osterrieder. 1996. Alterations in the equine herpesvirus type-1 (EHV-1) strain RacH during attenuation. Zentbl. Vetmed. Reihe B 43:1-14. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki-Ohara, K., T. Iwasaki, D. Watanabe, T. Kurata, and Y. Nishiyama. 2001. Effect of the deletion of US2 and US3 from herpes simplex virus type 2 on immune responses in the murine vagina following intravaginal infection. Vaccine 20:98-104. [DOI] [PubMed] [Google Scholar]
- 29.Jacque, J. M., A. Mann, H. Enslen, N. Sharova, B. Brichacek, R. J. Davis, and M. Stevenson. 1998. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 17:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang, H. K., M. Ono, T. J. Kim, Y. Izumiya, A. M. Damiani, T. Matsumura, M. Niikura, C. Kai, and T. Mikami. 1998. The genetic organization and transcriptional analysis of the short unique region in the genome of nononcogenic Marek's disease virus serotype 2. Virus Res. 58:137-147. [DOI] [PubMed] [Google Scholar]
- 32.Jiang, Y. M., H. Yamada, F. Goshima, T. Daikoku, S. Oshima, K. Wada, and Y. Nishiyama. 1998. Characterization of the herpes simplex virus type 2 (HSV-2) US2 gene product and a US2-deficient HSV-2 mutant. J. Gen. Virol. 79:2777-2784. [DOI] [PubMed] [Google Scholar]
- 33.Khokhlatchev, A. V., B. Canagarajah, J. Wilsbacher, M. Robinson, M. Atkinson, E. Goldsmith, and M. H. Cobb. 1998. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605-615. [DOI] [PubMed] [Google Scholar]
- 34.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 35.Kong, X., H. San Juan, A. Behera, M. E. Peeples, J. Wu, R. F. Lockey, and S. S. Mohapatra. 2004. ERK-1/2 activity is required for efficient RSV infection. FEBS Lett. 559:33-38. [DOI] [PubMed] [Google Scholar]
- 36.Lackner, M. R., K. Kornfeld, L. M. Miller, H. R. Horvitz, and S. K. Kim. 1994. A MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis elegans. Genes Dev. 8:160-173. [DOI] [PubMed] [Google Scholar]
- 37.Leung-Tack, P., J. C. Audonnet, and M. Riviere. 1994. The complete DNA sequence and the genetic organization of the short unique region (US) of the bovine herpesvirus type 1 (ST strain). Virology 199:409-421. [DOI] [PubMed] [Google Scholar]
- 38.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 39.Lomniczi, B., S. Watanabe, T. Ben-Porat, and A. S. Kaplan. 1984. Genetic basis of the neurovirulence of pseudorabies virus. J. Virol. 52:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longnecker, R., and B. Roizman. 1987. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science 236:573-576. [DOI] [PubMed] [Google Scholar]
- 41.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. M. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76:3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luttrell, L. M. 2003. “Location, location, location”: activation and targeting of MAP kinases by G protein-coupled receptors. J. Mol. Endocrinol. 30:117-126. [DOI] [PubMed] [Google Scholar]
- 43.Luttrell, L. M., F. L. Roudabush, E. W. Choy, W. E. Miller, M. E. Field, K. L. Pierce, and R. J. Lefkowitz. 2001. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl. Acad. Sci. USA 98:2449-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 102:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGeoch, D. J., A. Dolan, S. Donald, and D. H. Brauer. 1986. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 14:1727-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGeoch, D. J., H. W. Moss, D. McNab, and M. C. Frame. 1987. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J. Gen. Virol. 68:19-38. [DOI] [PubMed] [Google Scholar]
- 47.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 48.Meindl, A., and N. Osterrieder. 1999. The equine herpesvirus 1 Us2 homolog encodes a nonessential membrane-associated virion component. J. Virol. 73:3430-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrison, E. E., A. J. Stevenson, Y. F. Wang, and D. M. Meredith. 1998. Differences in the intracellular localization and fate of herpes simplex virus tegument proteins early in the infection of Vero cells. J. Gen. Virol. 79:2517-2528. [DOI] [PubMed] [Google Scholar]
- 51.Morrison, E. E., Y. F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller, J., S. Ory, T. Copeland, H. Piwnica-Worms, and D. K. Morrison. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8:983-993. [DOI] [PubMed] [Google Scholar]
- 53.Mumby, M. 1995. Regulation by tumour antigens defines a role for PP2A in signal transduction. Semin. Cancer Biol. 6:229-237. [DOI] [PubMed] [Google Scholar]
- 54.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peyssonnaux, C., and A. Eychene. 2001. The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell 93:53-62. [DOI] [PubMed] [Google Scholar]
- 56.Planz, O., S. Pleschka, and S. Ludwig. 2001. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 75:4871-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 58.Popik, W., J. E. Hesselgesser, and P. M. Pitha. 1998. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 72:6406-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbins, D. J., M. Cheng, E. Zhen, C. A. Vanderbilt, L. A. Feig, and M. H. Cobb. 1992. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc. Natl. Acad. Sci. USA 89:6924-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodems, S. M., and D. H. Spector. 1998. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J. Virol. 72:9173-9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 Induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 79:10308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith, C. C., J. H. Luo, J. C. Hunter, J. V. Ordonez, and L. Aurelian. 1994. The transmembrane domain of the large subunit of HSV-2 ribonucleotide reductase (ICP10) is required for protein kinase activity and transformation-related signaling pathways that result in ras activation. Virology 200:598-612. [DOI] [PubMed] [Google Scholar]
- 63.Smith, C. C., J. Nelson, L. Aurelian, M. Gober, and B. B. Goswami. 2000. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J. Virol. 74:10417-10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teis, D., W. Wunderlich, and L. A. Huber. 2002. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3:803-814. [DOI] [PubMed] [Google Scholar]
- 65.Torii, S., M. Kusakabe, T. Yamamoto, M. Maekawa, and E. Nishida. 2004. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev. Cell 7:33-44. [DOI] [PubMed] [Google Scholar]
- 66.Torii, S., K. Nakayama, T. Yamamoto, and E. Nishida. 2004. Regulatory mechanisms and function of ERK MAP kinases. J. Biochem. (Tokyo) 136:557-561. [DOI] [PubMed] [Google Scholar]
- 67.van Zijl, M., H. van der Gulden, N. de Wind, A. Gielkens, and A. Berns. 1990. Identification of two genes in the unique short region of pseudorabies virus; comparison with herpes simplex virus and varicella-zoster virus. J. Gen. Virol. 71:1747-1755. [DOI] [PubMed] [Google Scholar]
- 68.Weber, P. C., M. Levine, and J. C. Glorioso. 1987. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science 236:576-579. [DOI] [PubMed] [Google Scholar]
- 69.Whalen, S. G., R. C. Marcellus, A. Whalen, N. G. Ahn, R. P. Ricciardi, and P. E. Branton. 1997. Phosphorylation within the transactivation domain of adenovirus E1A protein by mitogen-activated protein kinase regulates expression of early region 4. J. Virol. 71:3545-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Widmann, C., S. Gibson, M. B. Jarpe, and G. L. Johnson. 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79:143-180. [DOI] [PubMed] [Google Scholar]
- 71.Wild, M. A., S. Cook, and M. Cochran. 1996. A genomic map of infectious laryngotracheitis virus and the sequence and organization of genes present in the unique short and flanking regions. Virus Genes 12:107-116. [DOI] [PubMed] [Google Scholar]
- 72.Wu, Y., M. Han, and K. L. Guan. 1995. MEK-2, a Caenorhabditis elegans MAP kinase kinase, functions in Ras-mediated vulval induction and other developmental events. Genes Dev. 9:742-755. [DOI] [PubMed] [Google Scholar]
- 73.Yang, X., and D. Gabuzda. 1999. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J. Virol. 73:3460-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zelnik, V., R. Darteil, J. C. Audonnet, G. D. Smith, M. Riviere, J. Pastorek, and L. J. Ross. 1993. The complete sequence and gene organization of the short unique region of herpesvirus of turkeys. J. Gen. Virol. 74:2151-2162. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, G., R. Stevens, and D. P. Leader. 1990. The protein kinase encoded in the short unique region of pseudorabies virus: description of the gene and identification of its product in virions and in infected cells. J. Gen. Virol. 71:1757-1765. [DOI] [PubMed] [Google Scholar]