Abstract
The human cytomegalovirus (HCMV) UL37 glycoprotein (gpUL37) is internally cleaved and its products divergently traffic to mitochondria or are retained in the secretory pathway. To define the requirements for gpUL37 cleavage, residues −1 and −3 of the consensus endoplasmic reticulum (ER) signal peptidase I site within exon 3 (UL37x3) were replaced by bulky tyrosines (gpUL37 cleavage site mutant I). Internal cleavage of this UL37x3 mutant was inhibited, verifying usage of the consensus site at amino acids (aa) 193/194. The full-length mitochondrial species of gpUL37 cleavage site mutant I was N glycosylated and endoglycosidase H sensitive, indicating that ER translocation and processing took place prior to its mitochondrial importation. Moreover, these results suggest that internal cleavage of gpUL37 is not necessary for its N glycosylation. Partial deletion or disruption of the UL37 hydrophobic core immediately upstream of the cleavage site resulted in decreased protein abundance, suggesting that the UL37x3 hydrophobic α-helix contributes to either correct folding or stability of gpUL37. Insertion of the UL37x3 hydrophobic core and cleavage site into pUL37M, a splice variant of gpUL37 which lacks these sequences and is neither proteolytically cleaved nor N glycosylated, resulted in its internal cleavage and N glycosylation. Its NH2-terminal fragment, pUL37M-NH2, was detected more abundantly in mitochondria, while its N-glycosylated C-terminal fragment, gpUL37M-COOH, was detected predominantly in the ER in a manner analogous to that of gpUL37 cleavage products. These results indicate that UL37x3 aa 178 to 205 are prerequisite for gpUL37 internal cleavage and alter UL37 protein topology allowing N glycosylation of its C-terminal sequences. In contrast, the NH2-terminal UL37x1 hydrophobic leader, present in pUL37x1, pUL37M, and gpUL37, is not cleaved from mature UL37 protein, retaining a membrane anchor for UL37 isoforms during trafficking. Taken together, these results suggest that HCMV gpUL37 undergoes sequential trafficking, during which it is ER translocated, processed, and then mitochondrially imported.
Human cytomegalovirus (HCMV) is the leading viral cause of congenital defects, including mental retardation and blindness, and the leading nongenetic cause of neurosensory hearing loss in developed countries (7, 8, 17, 35). In addition to its impact on neonatal health, HCMV is a significant cause of morbidity and mortality in immunosuppressed adults, particularly transplant recipients (6, 45).
HCMV gene expression is temporally regulated, and products from the UL37 immediate-early locus are important for viral growth in cultured fibroblasts and in vivo (19, 24, 34, 39, 54). Alternative processing of HCMV UL37 immediate-early pre-mRNA produces a predominant unspliced UL37 exon 1 (UL37x1) RNA and at least 10 alternatively spliced UL37 RNAs (1-3, 23, 26, 48-50). These transcripts encode multiple UL37 protein isoforms (Fig. 1A).
FIG. 1.
A. Schematic representation of UL37 proteins and mutants. The hydrophobic signal (cylinder, aa 1 to 22), juxtaposed basic residues (+, aa 23 to 29), internal hydrophobic core (oval, aa 178 to 196), N-glycosylated sites (branches, aa 206 to 391), C-terminal TM domain (aa 433 to 459), cytosolic tail (aa 460 to 487), and Flag tag are indicated for gpUL37. The sequences spanning the hydrophobic core and adjacent sequences (aa 161 to 205) are enlarged below. The consensus site of ER signal peptidase I cleavage is indicated by an arrow. The UL37 domains in pUL37M and pUL37x1 are indicated. The unmodified N-glycosylation signals in pUL37M are represented by open circles. The mutated sequences in gpUL37 G191Y,G193Y (gpUL37 cleavage site mutant I); in gpUL37 Δaa180-184 (gpUL37 hydrophobic core mutant I); in gpUL37 S189R,G193Y (gpUL37 hydrophobic core mutant II); and in the insertion mutant pUL37M::aa178-205 (pUL37M insertion mutant I) are shown. The predicted internal hydrophobic α-helix for the wild type (wt) and mutant are shown in boldface. Symbols used to indicate the orientations of the residues with respect to cellular membranes: i; inside (cytosolic), H; α-helix region, o; outside (ER lumen). B. Kyte-Doolittle plots for HCMV gpUL37, pUL37M, and pUL37x1. Hydrophobicities of amino acid sequences were plotted using an online Kyte-Doolittle hydropathy prediction program (http://occawlonline.pearsoned.com/bookbind/pubbooks/bc_mcampbell_genomics_1/medialib/activities/kd/kyte-doolittle.htm) with a window size of 19. To highlight conserved and variable regions, figures were aligned to the hydrophobicity plot of full-length gpUL37. The NH2-terminal, internal, and C-terminal hydrophobic cores are indicated by arrows.
The UL37x1 protein (pUL37x1), also known as vMIA and product of the unspliced UL37x1 RNA, is the predominant UL37 isoform produced during permissive HCMV infection (29). pUL37x1 and other UL37 isoforms dually traffic to the endoplasmic reticulum (ER) and to the mitochondrial outer membrane, where they mediate antiapoptotic activity (4, 5, 15, 23, 24, 29-33, 38, 39).
All three known UL37 proteins, pUL37x1, the full-length glycoprotein (gpUL37), and the medium protein (pUL37M), contain the NH2-terminal UL37x1 sequences (11, 23, 26) (Fig. 1A). These sequences include a hydrophobic signal peptide (amino acids [aa] 1 to 22) and juxtaposed basic residues, which serve as a bipartite signal to target UL37 proteins to the ER and mitochondria (29). As it is not currently known whether this signal peptide is cleaved, we examined its retention on mature pUL37x1.
Two short domains (aa 5 to 34 and aa 118 to 147) within UL37x1 are, together, sufficient to confer antiapoptotic activity, and they act by targeting the protein to mitochondria and binding Bax, respectively (5, 23, 24, 31-33, 38). A strongly acidic domain within UL37x1 plays a role in the transactivation of HCMV early gene promoters (14, 55). The full-length gpUL37 further shares exon 2 and part of exon 3 (UL37x3), including 11 of its 17 N-glycosylation sites as well as a C-terminal transmembrane (TM) domain and cytosolic tail with pUL37M (4, 11, 23, 25, 26).
Within the gpUL37 unique sequences are a consensus ER signal peptidase I cleavage site at aa 193/194 and a hydrophobic core spanning aa 178 to 196, which are predicted to fold into an α-helical structure compatible with a membrane-spanning sequence (11, 26, 30). Our previous studies show internal cleavage of gpUL37 in transfected cells (30). Cleavage of proteins by type I signal peptidases occurs at NH2-terminal as well as internal signal sequences on the luminal side of the ER membrane, with the enzymes favoring small, uncharged residues at positions −1 and −3 with respect to the cleavage site (28). The potential UL37x3 cleavage site (aa193/194) is located on the periphery of the hydrophobic α helix, is predicted to abut or extend into the ER lumen, and contains glycine residues at the −1 and −3 positions.
The gpUL37 cleavage products, pUL37NH2 and gpUL37COOH, dissociate and traffic differentially. The NH2-terminal cleavage product (pUL37NH2) is detected in the ER and mitochondria (30). However, it was not clear whether the mitochondrial pUL37NH2 species traffics to the ER prior to its relocation to mitochondria. The C-terminal cleavage product (gpUL37COOH) is N glycosylated and preferentially retained in the secretory pathway. This divergent cleavage product trafficking is in contrast to other internally cleaved HCMV glycoproteins, such as glycoprotein B (gB), whose proteolytic fragments remain joined by disulfide bonds and traffic jointly through the secretory apparatus (9, 16, 44, 46). Cleavage of the UL37 precursor does not require N glycosylation (30). pUL37M lacks UL37x3 aa 178 to 262 and, accordingly, is not internally cleaved. Unexpectedly, pUL37M is not N glycosylated, even though it has 11 consensus N-glycosylation signals (30).
In these studies, we generated gpUL37 mutants to test the requirements for UL37 precursor internal cleavage. We found that internal cleavage occurred at the consensus ER signal peptidase I site and that its mutation resulted in decreased cleavage. The full-length gpUL37 cleavage site mutant was modified by N glycosylation prior to its importation into the mitochondrial outer membrane. On the converse side, insertion of the consensus UL37x3 ER signal peptidase I site and overlapping hydrophobic sequences enabled cleavage of the UL37M isoform. In contrast to the internal UL37x3 site, the UL37x1 hydrophobic leader peptide was not cleaved from the mature protein, allowing for membrane anchoring of UL37 proteins during their subcellular trafficking.
MATERIALS AND METHODS
Cell culture and transfections.
Human diploid fibroblasts (HFFs) and HeLa cells were lipofected as previously described (15, 29, 30) with the following modifications. Briefly, cells (4 × 106 to 8 × 106) were transfected with 20 μg of plasmid DNA expressing wild-type gpUL37 (gpUL37-Flag, p816), gpUL37 hydrophobic core mutant I (gpUL37 Δaa180-184-Flag, p1332), gpUL37 cleavage site mutant I (gpUL37 G191Y,G193Y-Flag, p1333), gpUL37 hydrophobic core mutant II (gpUL37 S189R,G193Y-Flag, p1337), the pUL37M insertion mutant (pUL37M::aa178-205-Flag, p1365), or empty vector DNA (p790, p792) by using Lipofectamine 2000 (Invitrogen) at a ratio of 1.75:1 (lipid:DNA) in OptiMEM. HeLa cells were harvested at 24 h after transfection and fractionated as described below. HFFs were harvested at 24 h after transfection and analyzed by indirect immunofluorescence as detailed below.
Mutant generation.
Mutant expression vectors encoding gpUL37 hydrophobic core mutant I (p1332), gpUL37 cleavage site mutant I (p1333), and gpUL37 hydrophobic core mutant II (p1337) were generated by site-directed mutagenesis of the UL37 open reading frame (ORF) in p816 (15) using the QuikChange kit (Stratagene) following the manufacturer's instructions. The pUL37M insertion mutant (p1365) was generated by deletion of aa 206 to 262 from the gpUL37 ORF in p816. The mutations were verified by DNA sequencing of the mutant plasmids.
Isolation of mitochondria.
Mitochondria were purified on discontinuous sucrose gradients as previously described (15, 29). Briefly, cells in MTE buffer (0.27 M mannitol, 10 mM Tris-HCl, 0.1 mM EDTA, pH 7.4) supplemented with protease inhibitor cocktail (Sigma) were lysed by sonication. Nuclei and cellular debris were removed by centrifugation at 700 × g for 10 min. Mitochondria were obtained by pelleting at 15,000 × g for 10 min, and the postmitochondrial supernatant was used for purification of ER fractions. Crude mitochondria were purified by banding in discontinuous sucrose gradients and by dilution in MTE buffer and pelleting at 15,000 × g for 10 min. Finally, purified mitochondria were resuspended in phosphate-buffered saline (PBS) and stored at −80°C until use.
ER fraction isolation.
ER fractions were isolated as previously described (29, 36). Briefly, the postmitochondrial fraction described above was layered on a sucrose step gradient consisting of 1.3 M, 1.5 M, and 2.0 M sucrose in 10 mM Tris-HCl (pH 7.6) and banded by centrifugation at 100,000 × g for 70 min. The ER fraction at the interface between the supernatant and the 1.3 M sucrose step was collected, diluted with MTE buffer, and pelleted by centrifugation at 100,000 × g for 45 min. The ER membranes were resuspended in PBS and stored at −80°C until use.
Protein concentration determination.
Protein concentrations of the subcellular fractions were determined using a BCA reagent kit (Pierce) as suggested by the manufacturer.
Deglycosylation using PNGase or EndoH.
Endoglycosidase reactions were performed as recommended by the manufacturer (New England BioLabs), as previously described (30). Following denaturation, 10 to 20 μg of ER or mitochondrial protein was digested with 500 U of the desired enzyme (peptide:N-glycosidase F [PNGase] or endoglycosidase H [EndoH]) in the presence of the appropriate buffer for 30 min at 37°C. Deglycosylated proteins were precipitated using 80% cold acetone and resuspended in 1× sodium dodecyl sulfate (SDS) loading buffer for subsequent separation on SDS-10% polyacrylamide gel.
Indirect immunofluorescence staining.
HFFs (1 × 105) were plated onto Lab-Tek II four-chambered cover glass borosilicate slides (Lab-Tek). Twenty-four hours later, cells were lipofected with vectors expressing gpUL37 cleavage site mutant I-Flag (p1333) or gpUL37-Flag (p816). Twenty-four hours after lipofection, cells were fixed with 4% paraformaldehyde in 1× PBS for 30 min. Fixed cells were washed with 1× PBS, permeabilized with 1× PBS plus 0.75% Triton X-100, blocked for 45 min using 1× PBS plus 4% bovine serum albumin (BSA), and sequentially probed with primary antibodies (Ab) at a dilution of 1:250 for 1 to 2 h in 1× PBS plus 4% BSA at room temperature. UL37 NH2-terminal and C-terminal sequences were detected by immunofluorescence staining with Ab1064 or anti-Flag (rabbit anti-Flag M2 antibody; Sigma). The ER and mitochondria were visualized using mouse anti-protein disulfide isomerase (PDI, 1:250; StressGen) and human autoimmune serum against mitochondria (1:250; ImmunoVision) as published (15). The corresponding secondary antibodies were used at 1:250 dilutions in 1× PBS plus 4% BSA. Secondary antibodies used were fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG; Jackson ImmunoResearch), Texas Red (TR) goat anti-mouse IgG (Kirkegaard and Perry Laboratories), and cyanine 5 (Cy5)-conjugated goat anti-human IgG (Jackson ImmunoResearch).
Confocal laser scanning microscopy.
Analyses on transfected cells were performed with a Bio-Rad MRC1024 confocal laser scanning microscope (Center for Microscopy and Image Analysis, GWU, and Children's Mental Retardation and Developmental Disabilities Research Center) which allows for triple excitation. Triple excitation lines at 488, 568, and 647 nm were used for the excitation of FITC, TR, and Cy5, respectively. Emission was measured at 520 (FITC), 615 (TR), and 670 (Cy5) nm. Individual signals were captured sequentially to avoid spurious overlap of the emission signals. Individual optical sections, obtained using z dimensions of between 0.5 and 1 μm, were examined to determine colocalization of UL37 proteins with cellular organelle markers. Optical sections were obtained using a 100× (numerical aperture, 1.35) lens. Images were generated using Adobe Photoshop (version 7.0.1), Bio-Rad plug-ins, and Microsoft PowerPoint 2003.
Western analyses.
Fractionated proteins (10 to 20 μg) were separated by electrophoresis in SDS-10% polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF; Bio-Rad) or nitrocellulose (Hybond-ECL; Amersham Biosciences) membranes using a wet-transfer apparatus at 50 V for 1 h (47). Western analyses were carried out by a chemiluminescent method using the ECL Western blotting detection system (Amersham Pharmacia Biotech). Blots were blocked with 5% milk protein (Bio-Rad) in PBS with 0.005 to 0.1% Tween 20 for 30 min at room temperature or overnight at 4°C. Blotted proteins were reacted with primary Ab, including rabbit anti-UL37x1 aa 27 to 40 (Ab1064 at 1:1,000 or DC35 at 1:6,000), mouse anti-Flag (M2 at 1:2,000; Covance), goat anti-dolichyl phosphate mannose synthase 1 (DPM1) (I-20 at 1:500; Santa Cruz Biotechnology), or mouse anti-glucose regulated protein 75 (GRP75) (SPA-825 at 1:1,000; StressGen Biotechnologies) for 1 h in PBS with 0.005 to 0.1% Tween and 5% milk protein or 1% BSA (Bio-Rad) and with the corresponding horseradish peroxidase-conjugated secondary antibody (1:2500; Bio-Rad). When reprobed, blots were stripped by washing the membranes in the presence of 25 mM Tris-HCl (pH 6.4), 1% SDS, and 10 mM β-mercaptoethanol at 50°C for 20 to 40 min. The stripping buffer was removed by washing two to five times in PBS with or without 0.1% Tween 20 (5 to 10 min) and two to five times with PBS, 0.1% Tween 20, and 5% dried milk (15 min). The blots were then reprobed with the appropriate antibodies. Each blot is representative of a minimum of three independent experiments. Digital images were generated using ScanWizard Pro version 1.21 and imported into Adobe Photoshop version 5.0 LE and Microsoft PowerPoint 2000.
Microsequencing of the pUL37x1 NH2-terminal sequences.
HeLa cells stably expressing pUL37x1 tagged with triple myc peptide at its C terminus (HeLa/UL37x1#3) (23) were lysed in 150 mM NaCl-5 mM EDTA-50 mM Tris-HCl (pH 8.0)-1% Triton X-100 in the presence of protease inhibitors and centrifuged at 10,000 × g at 4°C for 10 min. The supernatants were precleared with ethanolamine-treated Affi-Prep 10 beads (Bio-Rad), then incubated with 9E10 anti-myc antibody (20) covalently linked to Affi-Prep 10 beads and washed with the lysis buffer. Proteins were eluted from beads in nonreducing Laemmli sample buffer and then separated by using SDS-polyacrylamide gel under reducing conditions and transferred onto PVDF membrane. The band containing pUL37x1-myc was stained with amido black and isolated. Sequence analysis of the protein was performed at the Laboratory for Protein Microsequencing and Mass Spectroscopy, University of Massachusetts Medical School (John Leszyk).
RESULTS
Of the HCMV UL37 proteins, gpUL37 is unique in its N glycosylation and internal cleavage. A Kyte-Doolittle hydropathy plot of gpUL37 reveals three predominant hydrophobic regions within the protein: the NH2-terminal signal sequence, a C-terminal anchor, and an internal hydrophobic core spanning aa 178 to 196 (Fig. 1B). The NH2-terminal hydrophobic signal peptide (aa 1 to 22) targets UL37 proteins to the ER (4, 15, 29, 30). The strongly hydrophobic C-terminal TM anchors gpUL37 in microsomes (4). We hypothesized that the internal hydrophobic core at aa 178 to 196, with a peak hydrophobicity score comparable to that of the UL37x1 signal peptide, may act as a third TM domain. Secondary structure prediction software (HMMTOP) revealed a high propensity for α-helical folding in this region. Intriguingly, we also found a consensus site for ER signal peptidase I at aa 191 to 194 within this region, predicting proteolytic cleavage between aa 193 and 194 (30). Another UL37 isoform, pUL37M, contains the NH2-terminal signal sequence and the C-terminal TM anchor but not the internal hydrophobic core at aa 178 to 196. Finally, the predominant UL37 protein, pUL37x1, carries only the NH2-terminal hydrophobic signal sequence.
In order to verify the UL37 internal cleavage site and the requirements for its proteolytic cleavage, we generated UL37 mutants, which disrupt the consensus ER signal peptidase I site or its partially overlapping hydrophobic core (Fig. 1A). Mutations were introduced to specifically disrupt ER signal peptidase I recognition of the potential cleavage site (gpUL37 cleavage site mutant I) by replacing the small, uncharged glycine residues at positions −1 and −3 with bulky tyrosine residues. This mutation did not disrupt the predicted hydrophobic α-helix spanning aa 178 to 196. The second mutant (gpUL37 hydrophobic core mutant I), lacking aa 180 to 184, exclusively disrupted the proposed internal TM domain by shifting it to span UL37x2 encoded residues (starting at aa 164). The third UL37 mutant (gpUL37 hydrophobic core mutant II) introduced a charged residue (R) within the internal hydrophobic core and a single tyrosine residue at the −1 cleavage position. Insertion of the R into the hydrophobic core was disruptive to the α-helix and also shifted the predicted α-helix to upstream sequences within the UL37x2 coded domain (starting at aa 170).
Verification of the UL37x3 site of internal cleavage.
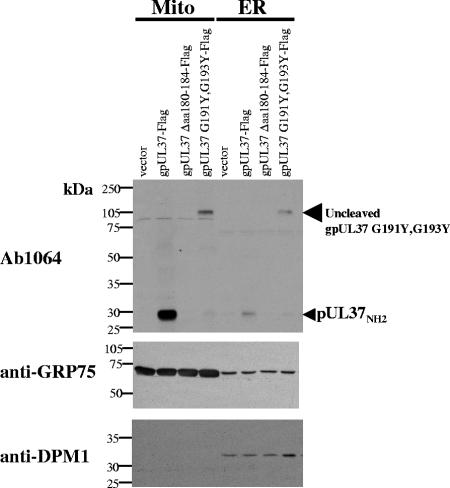
Introduction of a bulky residue (Y) at the critical −1 and −3 positions, of the predicted UL37x3 internal ER signal peptidase I site (aa 193/194) prevented internal cleavage of the mutant gpUL37 (Fig. 2). The gpUL37 cleavage site mutant I was detected by Ab1064, against UL37x1 amino-terminal sequences, as a full-length, uncleaved species of ∼105 kDa in both purified ER and mitochondrial subcellular fractions. The NH2-terminal UL37 cleavage fragment, pUL37NH2, was primarily detected in mitochondrial fractions from control cells expressing the parental gpUL37. Inhibition of gpUL37 internal cleavage by the aa 191 and 193 mutations confirms our earlier prediction of gpUL37 internal cleavage between residues 193 and 194 (30).
FIG. 2.
Full-length gpUL37 cleavage site mutant I, detected by its NH2-terminal sequences, localizes to the ER and mitochondria (Mito). HeLa cells were transfected with expression vectors for gpUL37 G191Y,G193Y-Flag (gpUL37 cleavage site mutant I), gpUL37 Δaa180-184-Flag (gpUL37 hydrophobic core mutant I), or gpUL37-Flag (gpUL37) as previously described (15, 29). Control cells were transfected with empty vector DNA. Transfected cells were fractionated into the ER and mitochondria 24 h after transfection. Fractionated proteins were resolved by SDS-10% PAGE and transferred to PVDF membranes. Blots were probed with Ab1064 (UL37x1, aa 27 to 40; 1:1,000), anti-GRP75 (1:3,500), or anti-DPM1 (1:500). Antibody GRP75 was used as a mitochondrial marker, and anti-DPM1 was used as a marker for the ER. Migration of the molecular weight standards (RPN800; Amersham Biosciences) is indicated on the left side of the blots.
To determine whether the gpUL37 cleavage site mutant I was indeed full length, we examined purified fractions from transfected cells expressing the parental gpUL37 and cleavage site mutant I using their carboxyl-terminal Flag tag (Fig. 3). Uncleaved gpUL37 cleavage site mutant I (∼105 kDa) was also detected using anti-Flag antibody in purified ER and mitochondrial fractions. In this experiment, some cleavage of gpUL37 cleavage site mutant I was detected by the presence of the gpUL37COOH fragment, comigrating with the product of the wild-type gpUL37COOH, suggesting that this mutant is inefficiently internally cleaved compared to wild-type gpUL37.
FIG. 3.
Full-length gpUL37 cleavage site mutant I, detected by its C-terminal Flag tag, localizes to the ER and mitochondria (Mito). HeLa cells were transfected with expression vectors for gpUL37 G191Y,G193Y-Flag (gpUL37 cleavage site mutant I) or gpUL37-Flag (gpUL37) or with empty vector DNA. Transfected cells were harvested, and the ER and mitochondrial proteins were fractionated, resolved, and blotted onto nitrocellulose membranes (Hybond-ECL; Amersham Biosciences) as described in the legend to Fig. 2. Blots were probed with anti-Flag (C-terminal tag, 1:2,000; Covance) or anti-GRP75 (mitochondrial marker, 1:1,000) and reprobed with anti-DPM1 (ER marker, 1:500). Migration of the molecular weight standards is indicated on the left side of the blots. wt, wild type.
We have previously found that gpUL37 protein fragments dually traffic to the secretory apparatus and mitochondria (15, 29, 30). To independently verify the presence of full-length gpUL37 cleavage site mutant I in ER fractions and mitochondria, we used confocal microscopy to examine transfected primary HFFs (Fig. 4). Amino-terminal sequences of gpUL37 cleavage site mutant I, detected by staining with Ab1064, colocalized with ER (PDI) and mitochondrial (autoimmune) markers, similar to the wild-type gpUL37 protein (Fig. 4A and B). In addition, the gpUL37 cleavage mutant I carboxyl-terminal sequences also colocalized with ER and mitochondrial markers (Fig. 4C). In contrast, the wild-type gpUL37COOH was not detected in mitochondrial fractions (Fig. 4D). Although some of the parental gpUL37COOH colocalized with ER markers, most had trafficked into other subcellular compartments, consistent with previous observations of localization in the Golgi apparatus (15). Thus, both the NH2- and C-terminal sequences of gpUL37 cleavage site mutant I were localized in ER and mitochondrial markers, in a pattern distinguishable from that of the parental gpUL37 cleavage fragments.
FIG. 4.
Colocalization of gpUL37 cleavage site mutant I with ER and mitochondria (Mito) in transfected HFFs. HFFs were lipofected with vectors expressing gpUL37 G191Y,G193Y-Flag (gpUL37 cleavage site mutant I) (A and C) or gpUL37-Flag (gpUL37) (B and D), harvested 24 h after lipofection by fixation with 4% paraformaldehyde, and reacted with primary antibodies (1:250). UL37 NH2-terminal and C-terminal sequences were detected by Ab1064 (A and B) or anti-Flag (C and D), respectively, as previously described (15). The ER and mitochondria were visualized by anti-PDI (1:250) and human autoimmune antibody (1:250) and the corresponding secondary antibodies as previously described (15). Shown are optical sections of the individual FITC (green), TR (red), or Cy5 (blue) channels. Overlaid optical sections show colocalization of FITC and TR (yellow), FITR and Cy5 (aquamarine), or all three channels (white). wt, wild type.
Mitochondrial gpUL37 cleavage site mutant I is N glycosylated.
Since migration of full-length gpUL37 cleavage site mutant I (∼105 kDa) exceeded its ORF and retains its 17 consensus N-glycosylation signals, we examined its N glycosylation (Fig. 5). Purified mitochondrial fractions from cells expressing gpUL37 cleavage site mutant I or control wild-type gpUL37 were treated with PNGase. Treatment with PNGase increased the electrophoretic mobility of gpUL37 cleavage site mutant I from ∼105 kDa to ∼60 kDa in mitochondrial (Fig. 5A) and ER fractions (Fig. 5B). The mobility of control pUL37NH2 fragment detected in mitochondria and ER fractions by Ab1064 was not altered by PNGase treatment (Fig. 5A and B), consistent with its lack of N-glycosylation sites. These results strongly suggest that full-length gpUL37 cleavage site mutant I is processed in the ER lumen by N glycosylation prior to its trafficking to mitochondria. Further, this finding also establishes that gpUL37 internal cleavage is not required for its N glycosylation. This result is complementary to our previous finding that N glycosylation of pUL37COOH is not required for UL37 precursor cleavage (30), suggesting that initiation of these events can be separated.
FIG. 5.
Mitochondrial (Mito) gpUL37 cleavage site mutant I is N glycosylated and EndoH sensitive. Mitochondrial (A) and ER (B) fractions of transfected HeLa cells expressing wild-type (wt) gpUL37 or gpUL37 G191Y,G193Y-Flag (gpUL37 cleavage site mutant I) were untreated (-) or treated with PNGase (+) as previously described (30). Control samples were from HeLa cells transfected with empty vector. Deglycosylated protein samples (mitochondria, 10 μg [A]; ER, 20 μg [B]) were separated by SDS-PAGE and subjected to Western blot analysis using Ab1064 (1:1,000). Mitochondrial (C) fractions of transfected HeLa cells expressing wild-type gpUL37, gpUL37 G191Y,G193Y-Flag (gpUL37 cleavage site mutant I), or vector were untreated (-) or treated with EndoH (+). Deglycosylated protein samples (20 μg) were separated by SDS-PAGE and subjected to Western blot analysis using DC35 (1:6,000) or anti-GRP75 (1:1,000).
To determine whether the oligosaccharides in gpUL37 cleavage site mutant I were further processed in the Golgi apparatus prior to mitochondrial importation, purified mitochondria from transfected cells expressing the gpUL37 mutant were reacted with EndoH and probed with DC35, recognizing UL37x1 aa 27 to 40 (Fig. 5C). As previously observed, full-length gpUL37 cleavage site mutant I and its NH2-terminal cleavage fragment were detected in mitochondria as opposed to only the NH2-terminal cleavage fragment in cells expressing wild-type gpUL37. Full-length gpUL37 cleavage site mutant I (∼105 kDa) was sensitive to EndoH treatment, indicating that its oligosaccharides were not further processed. These results are consistent with modification of the UL37 glycoprotein in the ER lumen, but not in the medial or trans-Golgi apparatus, prior to its mitochondrial importation. As anticipated, mitochondrial GRP75 was detected in the banded mitochondrial fractions (Fig. 5C), whereas the ER marker, DPM1, was not (C. D. Williamson and A. M. Colberg-Poley, unpublished results).
gpUL37 hydrophobic core mutant I.
Hydrophobic domains are known to influence the timing and efficiency of protein cleavage and N glycosylation (41). The UL37x3 hydrophobic core is highly conserved in primate CMVs (18, 32). To determine the role of the hydrophobic core in pUL37 proteolytic cleavage and N glycosylation, we generated a gpUL37 mutant which lacks a portion of the UL37x3 internal hydrophobic core (Δaa180-184). However, upon repeated attempts, this gpUL37 mutant was not detectable either as uncleaved protein or as cleaved fragments in purified ER and mitochondrial fractions from transfected HeLa cells (Fig. 2) (Williamson and Colberg-Poley, unpublished). Thus, partial deletion of the internal hydrophobic core sequences appears to either lead to incorrect protein folding or decrease gpUL37 protein stability.
gpUL37 hydrophobic core mutant II.
In an alternative approach to examine the role of the UL37x3 internal hydrophobic core on UL37 protein cleavage and N glycosylation, we generated a single amino acid substitution (S189R) which predictably disrupts the hydrophobic core. This mutant also carries the −1 residue bulky-group substitution (G193Y) in the stable pUL37 cleavage site mutant described above. Surprisingly, gpUL37 hydrophobic core mutant II (∼105 kDa) was dramatically decreased in purified ER fractions and undetectable in mitochondrial fractions from transfected cells even though the parental wild-type pUL37NH2 was readily detected in mitochondria (Fig. 6A). These results are consistent with the decreased stability of gpUL37 hydrophobic core mutant I and underscore the likely importance of these UL37x3 sequences for gpUL37 stability or folding.
FIG. 6.
gpUL37 hydrophobic core mutant II is ER localized, uncleaved, and present in low abundance. HeLa cells were transfected with vectors expressing gpUL37 S189R,G193Y (gpUL37 hydrophobic core mutant II), wild-type (wt) gpUL37, or empty vector and fractionated into ER (A) and mitochondrial (Mito) (B) fractions 24 h after transfection. Fractionated protein samples (anti-UL37x1, aa 27 to 40) were subjected to Western blot analysis using Ab1064 (1:1,000), anti-GRP75 (1:3,500), or anti-DPM1 (1:500). (B) gpUL37 hydrophobic core mutant II in the ER is N glycosylated. ER fractions from transfected HeLa cells expressing gpUL37 hydrophobic core mutant II were untreated (-) or treated with PNGase (+). Control ER fractions were from HeLa cells transfected with empty vector (p790). Deglycosylated protein samples were separated by SDS-PAGE and subjected to Western analysis using Ab1064.
Although gpUL37 hydrophobic core mutant II was greatly reduced in abundance in transfected cells, sufficient levels were detectable in purified ER fractions (Fig. 6A). To test whether it was N glycosylated as gpUL37 cleavage site mutant I was, purified ER fractions from cells expressing gpUL37 hydrophobic core mutant II were treated with PNGase. The mobility of gpUL37 hydrophobic core mutant II was reduced from ∼105 kDa to ∼60 kDa by PNGase treatment, consistent with its modification by N glycosylation.
Internal proteolytic cleavage and N glycosylation of a pUL37M insertion mutant.
pUL37M, which lacks aa 178 to 262 and consequently both the internal hydrophobic core and ER signal peptidase I site, traffics both into the ER and into mitochondria but is neither internally proteolytically cleaved nor N glycosylated (30). To determine whether the UL37x3 domain is sufficient to enable UL37 cleavage, we inserted aa 178 to 205 into pUL37M insertion mutant I. pUL37M insertion mutant I was stably expressed and readily detected in transfected cells (Fig. 7). Moreover, it was cleaved internally and its NH2-terminal fragment, pUL37M-NH2, was readily detected in mitochondria by Ab1064 (Fig. 7A). pUL37M insertion mutant I behaved analogously to wild-type gpUL37 in producing proteolytic fragments not normally observed in pUL37M. The pUL37M-NH2 fragment comigrated with pUL37NH2 from gpUL37, consistent with cleavage at the authentic UL37x3 internal site. These results independently verify use of internal signal peptidase site I at aa 193/194.
FIG. 7.
pUL37M insertion mutant I is internally cleaved, dually traffics, and is N glycosylated. HeLa cells were transfected with vectors expressing gpUL37-Flag, pUL37M::aa178-205 (pUL37M insertion mutant I), or pUL37M for 24 h. Transfected cells were then harvested and fractionated, purifying ER and mitochondrial (Mito) fractions. Fractionated proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and reacted with anti-GRP75 (1:1,000). This blot was then sequentially stripped and reprobed with rabbit Ab1064 (1:1,000) (A) and then with mouse anti-Flag (1:2,000) (B). The migration of molecular weight markers is indicated on the left of the blots. Separately, ER fractions (20 μg) were untreated or treated with PNGase or EndoH as previously described. (C) Deglycosylated protein samples were resolved by SDS-PAGE and subjected to Western analysis using mouse anti-Flag (1:2,000).
To detect the C-terminal fragment from pUL37M insertion mutant I (gpUL37M-COOH), we examined purified ER fractions and mitochondria from transfected cells with anti-Flag antibody (Fig. 7B). The C-terminally tagged cleavage fragment (gpUL37M-COOH) from pUL37M insertion mutant I was detected predominantly in the ER (Fig. 7B). Its apparent molecular mass (∼60 kDa) was greater than that of its ∼28-kDa predicted ORF, suggesting its modification by N glycosylation. In addition to gpUL37M-COOH, the C-terminal fragment from wild-type gpUL37, gpUL37COOH, was detected by its C-terminal Flag tag. As previously observed, the gpUL37COOH was detected predominantly in the purified ER fractions, and its apparent molecular mass was ∼80 kDa. Finally, full-length pUL37M insertion mutant I was detected, and its molecular mass corresponded well with its predicted mass of ∼51 kDa. The parental pUL37M, which is not Flag tagged, was not detected by anti-Flag antibody but was detected by Ab1064.
To determine whether pUL37M insertion mutant I was N glycosylated, we treated ER fractions expressing this mutant with either PNGase or EndoH (Fig. 7C). The apparent molecular mass of the C-terminal fragment of pUL37M insertion mutant I (gpUL37M-COOH) was decreased from ∼50 kDa by PNGase (∼31 kDa) and by EndoH (∼32 kDa) treatment, similar to gpUL37COOH. Again, the parental pUL37M, which is not Flag tagged, was not detected by anti-Flag antibody. Taken together, these results suggest that gpUL37M-COOH is produced by cleavage at the authentic UL37x3 cleavage site and is N glycosylated, mimicking wild-type gpUL37 C-terminal fragment behavior. Furthermore, these results argue that UL37x3 residues 178 to 205 are prerequisite for UL37 protein cleavage, direct downstream UL37 sequences into the ER lumen, and enable N glycosylation of the UL37 precursor.
The NH2-terminal hydrophobic signal sequence of pUL37x1 is not cleaved.
The NH2 terminus of pUL37x1 has a 22-amino-acid-long hydrophobic segment resembling a leader sequence (26). This sequence is invariant in 26 HCMV primary strains (24) and is highly conserved among primate CMVs (18, 32). This amino acid segment is required for translocation of UL37-encoded proteins into the secretory apparatus and into mitochondria (24, 26, 29). To determine whether the pUL37x1 leader peptide is cleaved or not during protein maturation, we examined the NH2-terminal sequence of pUL37x1 by protein microsequencing analysis. For these experiments, we used HeLa/UL37x1 cells constitutively expressing pUL37x1 tagged with a triple myc peptide at its C terminus. Previously, we have shown that the tagged protein traffics to mitochondria both in stably transfected and in HCMV-infected human cells (5, 23). Lysates from HeLa cells stably transfected with pUL37x1-myc were incubated with anti-myc antibody covalently immobilized on beads, and captured proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE). The pUL37x1-myc band, identified by Western blotting with anti-myc antibody in a procedure similar to that previously described (23), was then analyzed by protein microsequencing without prior partial tryptic digestion. This experiment revealed that the band consisted of three pUL37x1 proteins with various NH2-terminal sequences starting at aa 5 (sequence, YVNLLGSVGLLA; 21 pmol), aa 1 (MSPVYVNLLGSV; 12 pmol), or aa 2 (SPVYVNLLGSVG; 7 pmol) of their corresponding NH2 termini (Table 1). Such ragged NH2 termini are commonly observed during microsequencing of cellular proteins and possibly produced by cellular exopeptidases, either as a part of normal intracellular processing or after the release of these enzymes during cell lysis (John Leszyk, personal communication). This result indicates that the first 22 NH2-terminal residues of pUL37x1 (and, by extension, of gpUL37 and pUL37M), are not cleaved. Thus, this hydrophobic core is a noncleavable leader peptide, in agreement with the finding that it is required for mitochondrial targeting and the antiapoptotic activity of pUL37x1 (23, 24, 29), and with the lack of any known proteolytic cleavage signal. Taken together, these results imply that pUL37x1, gpUL37, and pUL37M NH2 termini remain anchored in the membrane by virtue of their uncleaved NH2-terminal sequences while trafficking through the secretory apparatus and to mitochondria.
TABLE 1.
NH2-terminal sequences of pUL37x1/vMIA-myc proteins
DISCUSSION
While dual ER and mitochondrial localization of HCMV UL37 proteins has previously been observed (15, 29, 30), little is known about what regulates their trafficking, nor is it known whether species in the discrete subcellular compartments are interrelated. We set out to determine whether UL37 proteins that traffic into the secretory apparatus are ultimately targeted to mitochondria or whether UL37 proteins in the secretory apparatus and mitochondria are independent species. To that end, we first verified the identity of the UL37x3 internal ER signal peptidase I cleavage site and the requirement of UL37x3 hydrophobic residues (aa 178 to 196) for pUL37 cleavage. Then, we examined the subcellular localization of the gpUL37 mutants. Surprisingly, we found that N glycosylation of the pUL37 precursor occurred in the absence of its cleavage and that full-length, EndoH-sensitive, N-glycosylated UL37 mutant proteins traffic to mitochondria. In addition, we found that the UL37x1 NH2-terminal hydrophobic leader is not cleaved even though the internal UL37x3 consensus ER signal peptidase I site is. This latter result implies that UL37 proteins remain anchored by their leader sequence to cellular membranes during their trafficking through the secretory apparatus and to mitochondria. Taken together, our results suggest that the HCMV gpUL37 is ER translocated, processed, and subsequently mitochondrially imported. Thus, the mitochondrial species of UL37 proteins derive from precursors, which initially translocate to the ER.
Based upon the presence of the UL37x3 consensus ER signal peptidase I site and the sizes of gpUL37 cleavage products, we had predicted that the UL37 precursor would be cleaved between aa 193 and 194 (30). To determine whether this is indeed the case, we inserted bulky tyrosine residues in positions (−1, −3) that were critical with respect to the predicted UL37x3 internal cleavage site. The extent of cleavage of the gpUL37 cleavage site mutant I at the internal site was greatly reduced. Nonetheless, the full-length cleavage site mutant I protein was stable and readily detected in transfected cells. As predicted by HMMTOP, these mutations do not change the overlapping UL37x3 hydrophobic core α-helix (aa 178 to 196). Because mutation of the critical −1 and −3 residues severely compromised gpUL37 cleavage, we concluded that the UL37x3 consensus signal peptidase I site is recognized upon translocation into the ER membrane and that HCMV pUL37 is internally cleaved at aa193/194. Moreover, the identity of the UL37x3 internal cleavage site was independently verified by insertion of UL37x3 aa 178 to 205 into another HCMV UL37 isoform, pUL37M, which normally lacks these sequences and is neither internally cleaved nor N glycosylated (30). Insertion of these UL37 sequences resulted in cleavage of pUL37M insertion mutant I mutant and N glycosylation of its C-terminal fragment.
Hydrophobic domains are known to influence the timing and efficiency of protein cleavage and N glycosylation (41). The UL37x3 ER signal peptidase I site lies within an internal hydrophobic core (aa 178 to 196) (30). This region is highly conserved between primate (chimpanzee and rhesus) CMV and HCMV (18, 32), suggesting its functional importance. In contrast to gpUL37 cleavage site mutant I, the deletion of aa 180 to 184 within the UL37x3 internal hydrophobic core, spanning a leucine-rich motif, reduced the abundance of the gpUL37 hydrophobic core mutant I protein to an undetectable level, suggesting that the alteration of the UL37x3 hydrophobic core resulted in either incorrect folding or instability of the protein. HMMTOP analysis predicted that deletion of aa 180 to 184 would disrupt the UL37x3 hydrophobic core by shifting it some 14 residues within UL37x2 sequences towards the NH2 terminus of gpUL37, which might contribute to the instability of the mutant protein.
To independently examine the importance of the internal UL37x3 hydrophobic core (aa 178 to 196) for gpUL37 processing, we disrupted the core by substitution of a single residue in the helix with a charged residue (R), together with a second point mutation at position −1 (G193Y) of the internal cleavage site. This latter mutation is also present in the stable gpUL37 cleavage site mutant I described above. The level of expression of this UL37 hydrophobic core mutant II was also markedly reduced, suggesting its instability. HMMTOP predicted that S-to-R mutation would shift the α-helix within the UL37x3 hydrophobic core 8 amino acids towards the NH2 terminus, similarly to the UL37 hydrophobic core mutant I. These data suggest that changes in the UL37x3 hydrophobic α-helix contribute to the instability of gpUL37. Previous reports show that introduction of a negatively charged residue into the gamma-aminobutyric acid receptor TM domain resulted in its ER-associated degradation (21) and that an L-to-P substitution in cystic fibrosis TM conductance regulator protein also produced an unstable mutant protein (13). Taken together, our results suggest that the UL37x3 internal hydrophobic core α-helix plays an important role in gpUL37 protein cleavage and stability.
The known UL37 isoforms, pUL37x1, gpUL37, and pUL37M, share UL37x1 and some UL37x3 sequences but differ in the presence of the UL37x3 hydrophobic core. HMMTOP analysis predicts that because of these changes, pUL37x1, gpUL37, and pUL37M should have distinct topologies (Fig. 8). pUL37x1 contains only the UL37x1 hydrophobic signal peptide. Based upon the pUL37x1 microsequencing results, we conclude that its NH2-terminal signal sequence is noncleaved. This conclusion is in agreement with the presence of large bulky groups close to the cleavage site, which predicts it to be a poor substrate for ER signal peptidase I (28), and with the cell death suppressor activity of pUL37x1, which requires the presence of the leader peptide (23, 24). Although the signal peptide is not cleaved it still is sufficient to drive ER translocation of pUL37 proteins (29). In this property, pUL37 proteins are similar to another HCMV membrane protein, US2, whose signal peptide is noncleavable and yet drives US2 ER translocation (22). Furthermore, the UL37 NH2-terminal signal peptide in combination with adjacent basic residues serves as a bipartite signal for mitochondrial targeting of UL37 proteins (29).
FIG. 8.
Topology models of HCMV UL37 proteins. Models of HCMV UL37 isoforms anchored at the ER membrane were generated by combining topology predictions from HMMTOP software, experimental data published herein, and previously published literature (4, 23, 38). The two antiapoptotic domains (ovals), NH2-terminal basic residues (+), internal hydrophobic α-helices (rectangles), ER signal peptidase I (lightning bolt), N-glycosylated residues (branches), C-terminal TM (cylinders), and Flag tag (Flag) are represented on gpUL37. Its unique internal hydrophobic α-helix predictably directs the downstream UL37x3 sequences into the ER lumen. The sequences present in pUL37M, including its unmodified N-glycosylation sites (open circles), and pUL37x1 are also shown. The absence of the internal hydrophobic α-helix results in retention of most of pUL37x1 and pUL37M in the cytosolic side of the ER membrane.
Because of its retention in UL37 proteins, the UL37x1 signal peptide appears to anchor all known UL37 proteins by their NH2 termini to cellular membranes. pUL37x1 is predicted to be membrane anchored by its NH2-terminal leader with its C-proximal domains in the cytosol. Projection of pUL37x1 into the cytosol is consistent with its interaction with cytosolic Bax through its C-terminal antiapoptotic domain at the mitochondrial outer membrane (5, 23, 38).
The HMMTOP analysis predicts the UL37x3 hydrophobic core to direct the downstream UL37x3 sequences into the ER lumen, permitting processing of the consensus N-glycosylation signals. This prediction is supported by the finding that introduction of the UL37x3 internal hydrophobic core into UL37M resulted in its internal cleavage and N glycosylation of its 11 consensus sites, shared with gpUL37. Furthermore, these results suggest that the absence of N glycosylation of pUL37M consensus sites (30) is due to its topology; that is, absence of the UL37x3 internal hydrophobic core results in their retention on the cytosolic side of the ERN membrane, making its sites inaccessible to the ER N glycosylation machinery.
As pUL37M lacks the internal hydrophobic core (aa 178 to 196), its NH2-terminal sequences remain localized on the cytosolic side of the ER membrane up to the UL37x3 TM (aa 433 to 459), which serve as a second membrane anchor. This predicted pUL37M topology is supported by the unanticipated finding of the lack of modification at its 11 consensus N-glycosylation sites. Following insertion of the UL37x3 internal hydrophobic core, pUL37M was internally cleaved and its consensus N-glycosylation sites were modified, indicating that the downstream UL37 sequences, including its N-glycosylation signals, are driven into the ER lumen.
N glycosylation of full-length gpUL37 occurs in the absence of cleavage of NH2-terminal signal peptide. In this property gpUL37 is similar to prolactin, in which in vitro transport studies indicated that signal sequence cleavage is not prerequisite for the N glycosylation (41), and influenza virus neuraminidase, which actually requires an uncleaved signal sequence at the NH2 terminal for its N glycosylation (27). In contrast, signal peptidase cleavage was found to be essential for N glycosylation in the yeast system (12). We had previously found that N glycosylation is not required for internal cleavage of gpUL37 (30). Thus, UL37 internal cleavage and N glycosylation can be dissociated. However, signal sites are reported to be more readily cleaved when the hydrophobic domain is short, so that it maintains proper positioning through the ER translocon (28). Similar to N glycosylation, precursor cleavage predominantly occurs cotranslationally but can be inefficient or delayed depending on the primary sequence of the protein. Predictably, the timing of signal cleavage could affect the retention of protein fragments within the ER. Nonetheless, we had found an unanticipated relationship between stability of the UL37 NH2-terminal fragment and N glycosylation of its C-terminal fragment, gpUL37COOH. In the presence of tunicamycin, an inhibitor of N glycosylation, UL37NH2 was decreased in stability (30). Although the UL37 cleavage and N-glycosylation events can be dissociated, we cannot yet conclude that they are completely independent.
Protein secondary-structure analysis predicts that the C-terminal TM would direct downstream gpUL37 sequences to traverse the ER membrane, orienting its short tail (aa 460 to 487) into the cytosol. This prediction is consistent with the N glycosylation of gpUL37 consensus signals and the finding that the short gpUL37 tail is sensitive to protease digestion (4).
The full-length UL37 mutant glycoproteins localized to both the ER and mitochondria. Since transfer of oligosaccharides to the peptide backbone at consensus N-glycosylation signals occurs in the ER and the mitochondrial UL37 N-glycosylated species are EndoH sensitive, we conclude that the UL37 mutant glycoproteins have been processed posttranslationally in the ER/cis-Golgi apparatus prior to their relocation to mitochondria. These findings are consistent with our previous observations that wild-type gpUL37 is ER translocated and internally cleaved by ER signal peptidase I and that its NH2-terminal product is mitochondrially imported. In contrast to the wild-type gpUL37, the full-length N-glycosylated mutant protein is mitochondrially imported. By extension, our evidence suggests that upon ER cleavage of wild-type gpUL37, its NH2-terminal fragment is also subsequently imported into the mitochondria. Moreover, the dual trafficking of pUL37x1 and pUL37M to the ER and mitochondria would be predicted to occur in the same sequential order. Other mitochondrially associated proteins have been found to traffic sequentially from the ER to mitochondria. Spiro and colleagues identified an N-glycosylated cellular protein which traffics from the ER to the internal mitochondrial membrane (10). More recently, the hepatitis C core protein was found also to target the ER and then traffic to mitochondria (42).
This ER-mitochondrial sequential trafficking model is consistent with the finding that deletion of the hydrophobic residues of the UL37 bipartite leader sequence (aa 2 to 23) blocked both ER and mitochondrial trafficking, while deletion of the juxtaposed basic residues (aa 23 to 34) blocked mitochondrial importation but permitted ER translocation (29).
There are several potential pathways for UL37 protein targeting to mitochondria following ER translocation and modification. In one model, the translocon channel may be transiently opened during the process of translocation and N glycosylation. This could allow the NH2 terminal fragment to be retrotranslocated from the ER into the cytosol, as in the ER-associated degradation pathway (53). A translocon channel component, sec61B, is known to be involved in retrotranslocation of proteins to be targeted for degradation (37, 52). The retrotranslocated UL37 proteins would then be targeted from the cytosolic compartment to mitochondria. However, UL37 proteins have not been detected in the cytosolic compartments of cells (29) (Williamson and Colberg-Poley, unpublished). Furthermore, a pUL37x1 mutant lacking the UL37x1 hydrophobic leader but retaining the juxtaposed basic residues localized to the cytosol and was not mitochondrially imported (29). These finding suggest that UL37 proteins are continuously membrane associated during their trafficking in the cell.
UL37 may be transported by vesicles or by direct contact between the ER and mitochondria through the mitochondrion-associated membrane compartment (15, 51). Contact points between mitochondria and the ER can comprise 5 to 20% of the total mitochondrial network (40). Bridges between mitochondria and the ER serve as conduits to transfer lipids from a subspecialized region of the ER (51). In addition, the hepatitis C core protein has recently been found to colocalize with mitochondrion-associated membrane markers in its trafficking from the ER to mitochondria, where it is peripherally associated to the outer mitochondrial membrane (42).
Recently, Thomas and colleagues (43) proposed a role for the multifunctional sorting protein PACS-2 in regulating ER-mitochondrion communication. As a sorting protein, PACS-2 interacts with ER membrane proteins and manipulates their subcellular trafficking. Moreover, PACS-2 is intimately associated with maintaining ER-mitochondrion contact and homeostasis. Strikingly, PACS-2 depletion (43) displays a phenotype remarkably analogous to that of pUL37x1 expression (5, 33) (Williamson and Colberg-Poley, unpublished). This common phenotype is marked by a transition of association from the ER to mitochondria, resulting in mitochondrial fragmentation, where ensuing punctate mitochondria maintain their membrane potential; induction of the unfolded protein response in the ER; efflux of ER calcium into the cytosol; and importantly, inhibition of apoptosis downstream of caspase-8 activation through blocking of cytochrome c release from the mitochondria. As UL37 isoforms all share cytosolic UL37x1 sequences, it is possible that they interact with PACS-2 or, alternatively, with a chaperone in trafficking to mitochondria.
Acknowledgments
These studies were funded in part by NIH R01 AI057906 and CRI funds to A.M.C.-P. The confocal microscopy imaging was supported by a core grant (1P30HD40677) to the Children's Mental Retardation and Developmental Disabilities Research Center.
REFERENCES
- 1.Adair, R., G. W. Liebisch, and A. M. Colberg-Poley. 2003. Complex alternative processing of human cytomegalovirus UL37 pre-mRNA. J. Gen. Virol. 84:3353-3358. [DOI] [PubMed] [Google Scholar]
- 2.Adair, R., G. W. Liebisch, B. J. Lerman, and A. M. Colberg-Poley. 2006. Human cytomegalovirus temporally regulated gene expression in differentiated, immortalized retinal pigment epithelial cells. J. Clin. Virol. 35:478-484. [DOI] [PubMed] [Google Scholar]
- 3.Adair, R., G. W. Liebisch, Y. Su, and A. M. Colberg-Poley. 2004. Alteration of cellular RNA splicing and polyadenylation machineries during productive human cytomegalovirus infection. J. Gen. Virol. 85:3541-3553. [DOI] [PubMed] [Google Scholar]
- 4.Al-Barazi, H. O., and A. M. Colberg-Poley. 1996. The human cytomegalovirus UL37 immediate-early regulatory protein is an integral membrane N-glycoprotein which traffics through the endoplasmic reticulum and Golgi apparatus. J. Virol. 70:7198-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 101:7988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeckh, M., and W. G. Nichols. 2004. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103:2003-2008. [DOI] [PubMed] [Google Scholar]
- 7.Boppana, S. B., R. F. Pass, W. J. Britt, S. Stagno, and C. A. Alford. 1992. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr. Infect. Dis. J. 11:93-99. [DOI] [PubMed] [Google Scholar]
- 8.Bradford, R. D., G. Cloud, A. D. Lakeman, S. Boppana, D. W. Kimberlin, R. Jacobs, G. Demmler, P. Sanchez, W. Britt, S. J. Soong, and R. J. Whitley. 2005. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J. Infect. Dis. 191:227-233. [DOI] [PubMed] [Google Scholar]
- 9.Britt, W. J., and L. G. Vugler. 1989. Processing of the gp55-116 envelope glycoprotein complex (gB) of human cytomegalovirus. J. Virol. 63:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra, N. C., M. J. Spiro, and R. G. Spiro. 1998. Identification of a glycoprotein from rat liver mitochondrial inner membrane and demonstration of its origin in the endoplasmic reticulum. J. Biol. Chem. 273:19715-19721. [DOI] [PubMed] [Google Scholar]
- 11.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison III, T. Kouzarides, J. A. Martignetti, et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 12.Chen, X., C. VanValkenburgh, H. Liang, H. Fang, and N. Green. 2001. Signal peptidase and oligosaccharyltransferase interact in a sequential and dependent manner within the endoplasmic reticulum. J. Biol. Chem. 276:2411-2416. [DOI] [PubMed] [Google Scholar]
- 13.Choi, M. Y., A. W. Partridge, C. Daniels, K. Du, G. L. Lukacs, and C. M. Deber. 2005. Destabilization of the transmembrane domain induces misfolding in a phenotypic mutant of cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 280:4968-4974. [DOI] [PubMed] [Google Scholar]
- 14.Colberg-Poley, A. M., L. Huang, V. E. Soltero, A. C. Iskenderian, R. F. Schumacher, and D. G. Anders. 1998. The acidic domain of pUL37x1 and gpUL37 plays a key role in transactivation of HCMV DNA replication gene promoter constructions. Virology 246:400-408. [DOI] [PubMed] [Google Scholar]
- 15.Colberg-Poley, A. M., M. B. Patel, D. P. Erezo, and J. E. Slater. 2000. Human cytomegalovirus UL37 immediate-early regulatory proteins traffic through the secretory apparatus and to mitochondria. J. Gen. Virol. 81:1779-1789. [DOI] [PubMed] [Google Scholar]
- 16.Crump, C. M., C. H. Hung, L. Thomas, L. Wan, and G. Thomas. 2003. Role of PACS-1 in trafficking of human cytomegalovirus glycoprotein B and virus production. J. Virol. 77:11105-11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahle, A. J., K. B. Fowler, J. D. Wright, S. B. Boppana, W. J. Britt, and R. F. Pass. 2000. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J. Am. Acad. Audiol. 11:283-290. [PubMed] [Google Scholar]
- 18.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84:17-28. [DOI] [PubMed] [Google Scholar]
- 19.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher, M. J., W. Shen, L. Song, and R. L. Macdonald. 2005. Endoplasmic reticulum retention and associated degradation of a GABAA receptor epilepsy mutation that inserts an aspartate in the M3 transmembrane segment of the alpha1 subunit. J. Biol. Chem. 280:37995-38004. [DOI] [PubMed] [Google Scholar]
- 22.Gewurz, B. E., H. L. Ploegh, and D. Tortorella. 2002. US2, a human cytomegalovirus-encoded type I membrane protein, contains a non-cleavable amino-terminal signal peptide. J. Biol. Chem. 277:11306-11313. [DOI] [PubMed] [Google Scholar]
- 23.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. C. McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayajneh, W. A., A. M. Colberg-Poley, A. Skaletskaya, L. M. Bartle, M. M. Lesperance, D. G. Contopoulos-Ioannidis, N. L. Kedersha, and V. S. Goldmacher. 2001. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology 279:233-240. [DOI] [PubMed] [Google Scholar]
- 25.Hayajneh, W. A., D. G. Contopoulos-Ioannidis, M. M. Lesperance, A. M. Venegas, and A. M. Colberg-Poley. 2001. The carboxyl terminus of the human cytomegalovirus UL37 immediate-early glycoprotein is conserved in primary strains and is important for transactivation. J. Gen. Virol. 82:1569-1579. [DOI] [PubMed] [Google Scholar]
- 26.Kouzarides, T., A. T. Bankier, S. C. Satchwell, E. Preddy, and B. G. Barrell. 1988. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology 165:151-164. [DOI] [PubMed] [Google Scholar]
- 27.Markoff, L., B. C. Lin, M. M. Sveda, and C. J. Lai. 1984. Glycosylation and surface expression of the influenza virus neuraminidase requires the N-terminal hydrophobic region. Mol. Cell. Biol. 4:8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martoglio, B., and B. Dobberstein. 1998. Signal sequences: more than just greasy peptides. Trends Cell Biol. 8:410-415. [DOI] [PubMed] [Google Scholar]
- 29.Mavinakere, M. S., and A. M. Colberg-Poley. 2004. Dual targeting of the human cytomegalovirus UL37 exon 1 protein during permissive infection. J. Gen. Virol. 85:323-329. [DOI] [PubMed] [Google Scholar]
- 30.Mavinakere, M. S., and A. M. Colberg-Poley. 2004. Internal cleavage of the human cytomegalovirus UL37 immediate-early glycoprotein and divergent trafficking of its proteolytic fragments. J. Gen. Virol. 85:1989-1994. [DOI] [PubMed] [Google Scholar]
- 31.McCormick, A. L., C. D. Meiering, G. B. Smith, and E. S. Mocarski. 2005. Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis. J. Virol. 79:12205-12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick, A. L., A. Skaletskaya, P. A. Barry, E. S. Mocarski, and V. S. Goldmacher. 2003. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316:221-233. [DOI] [PubMed] [Google Scholar]
- 33.McCormick, A. L., V. L. Smith, D. Chow, and E. S. Mocarski. 2003. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 77:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 35.Pass, R. F. 2001. Cytomegalovirus, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 36.Paulik, M., D. D. Nowack, and D. J. Morre. 1988. Isolation of a vesicular intermediate in the cell-free transfer of membrane from transitional elements of the endoplasmic reticulum to Golgi apparatus cisternae of rat liver. J. Biol. Chem. 263:17738-17748. [PubMed] [Google Scholar]
- 37.Plemper, R. K., J. Bordallo, P. M. Deak, C. Taxis, R. Hitt, and D. H. Wolf. 1999. Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation. J. Cell. Sci. 112:4123-4134. [DOI] [PubMed] [Google Scholar]
- 38.Poncet, D., N. Larochette, A. L. Pauleau, P. Boya, A. A. Jalil, P. F. Cartron, F. Vallette, C. Schnebelen, L. M. Bartle, A. Skaletskaya, D. Boutolleau, J. C. Martinou, V. S. Goldmacher, G. Kroemer, and N. Zamzami. 2004. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 279:22605-22614. [DOI] [PubMed] [Google Scholar]
- 39.Reboredo, M., R. F. Greaves, and G. Hahn. 2004. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J. Gen. Virol. 85:3555-3567. [DOI] [PubMed] [Google Scholar]
- 40.Rizzuto, R., P. Pinton, W. Carrington, F. S. Fay, K. E. Fogarty, L. M. Lifshitz, R. A. Tuft, and T. Pozzan. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280:1763-1766. [DOI] [PubMed] [Google Scholar]
- 41.Rutkowski, D. T., C. M. Ott, J. R. Polansky, and V. R. Lingappa. 2003. Signal sequences initiate the pathway of maturation in the endoplasmic reticulum lumen. J. Biol. Chem. 278:30365-30372. [DOI] [PubMed] [Google Scholar]
- 42.Schwer, B., S. Ren, T. Pietschmann, J. Kartenbeck, K. Kaehlcke, R. Bartenschlager, T. S. Yen, and M. Ott. 2004. Targeting of hepatitis C virus core protein to mitochondria through a novel C-terminal localization motif. J. Virol. 78:7958-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmen, T., J. E. Aslan, A. D. Blagoveshchenskaya, L. Thomas, L. Wan, Y. Xiang, S. F. Feliciangeli, C. H. Hung, C. M. Crump, and G. Thomas. 2005. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 24:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh, J., and T. Compton. 2000. Characterization of a panel of insertion mutants in human cytomegalovirus glycoprotein B. J. Virol. 74:1383-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spector, S. A., K. Hsia, M. Crager, M. Pilcher, S. Cabral, and M. J. Stempien. 1999. Cytomegalovirus (CMV) DNA load is an independent predictor of CMV disease and survival in advanced AIDS. J. Virol. 73:7027-7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strive, T., E. Borst, M. Messerle, and K. Radsak. 2002. Proteolytic processing of human cytomegalovirus glycoprotein B is dispensable for viral growth in culture. J. Virol. 76:1252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su, Y., R. Adair, C. N. Davis, N. L. DiFronzo, and A. M. Colberg-Poley. 2003. Convergence of RNA cis elements and cellular polyadenylation factors in the regulation of human cytomegalovirus UL37 exon 1 unspliced RNA production. J. Virol. 77:12729-12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su, Y., J. R. Testaverde, C. N. Davis, W. A. Hayajneh, R. Adair, and A. M. Colberg-Poley. 2003. Human cytomegalovirus UL37 immediate early target minigene RNAs are accurately spliced and polyadenylated. J. Gen. Virol. 84:29-39. [DOI] [PubMed] [Google Scholar]
- 49.Tenney, D. J., and A. M. Colberg-Poley. 1991. Expression of the human cytomegalovirus UL36-38 immediate early region during permissive infection. Virology 182:199-210. [DOI] [PubMed] [Google Scholar]
- 50.Tenney, D. J., and A. M. Colberg-Poley. 1991. Human cytomegalovirus UL36-38 and US3 immediate-early genes: temporally regulated expression of nuclear, cytoplasmic, and polysome-associated transcripts during infection. J. Virol. 65:6724-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vance, J. E. 1990. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265:7248-7256. [PubMed] [Google Scholar]
- 52.Wiertz, E. J., D. Tortorella, M. Bogyo, J. Yu, W. Mothes, T. R. Jones, T. A. Rapoport, and H. L. Ploegh. 1996. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384:432-438. [DOI] [PubMed] [Google Scholar]
- 53.Ye, Y., Y. Shibata, C. Yun, D. Ron, and T. A. Rapoport. 2004. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429:841-847. [DOI] [PubMed] [Google Scholar]
- 54.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, H., H. O. al-Barazi, and A. M. Colberg-Poley. 1996. The acidic domain of the human cytomegalovirus UL37 immediate early glycoprotein is dispensable for its transactivating activity and localization but is not for its synergism. Virology 223:292-302. [DOI] [PubMed] [Google Scholar]