Abstract
Viruses typically elicit potent adaptive immune responses, and live-virus-based vaccines are among the most efficient human vaccines known. The mechanisms by which viruses stimulate adaptive immune responses are not fully understood, but activation of innate immune signaling pathways in the early phase of the infection may be of importance. In addition to stimulating immune responses to viral antigens expressed in infected cells, viruses can also provide adjuvant signals to coimmunized protein antigens. Using recombinant Semliki Forest virus (rSFV)-based vaccines, we show that rSFV potently enhanced antibody responses against coimmunized protein antigens in the absence of other exogenously added adjuvants. Elicitation of antibody responses against both virus-encoded antigens and coimmunized protein antigens was independent of the signaling via Toll-like receptors (TLRs) previously implicated in antiviral responses. In contrast, the adjuvant effect of rSFV on coimmunized protein was completely abolished in mice lacking the alpha/beta interferon (IFN-α/β) receptor (IFN-AR1), demonstrating that IFN-α/β signaling was critical for mediating this effect. Antibody responses directed against virus-encoded antigens were intact in IFN-AR1−/− mice, suggesting that other signals are sufficient to drive immune responses against virally encoded antigens. These data provide a basis for the adjuvant effect of rSFV and show that different signals are required to stimulate antibody responses to virally encoded antigens and to antigens administered as purified protein vaccines, together with viral particles.
Viruses and live-virus-based vaccines are generally potent stimulators of adaptive immunity. Activation of early innate immune pathways by viruses may contribute to the generation of adaptive immune responses during virus infection, but the mechanisms behind this remain poorly defined. Many viruses have evolved strategies to interfere with innate immune pathways, which may affect both the immediate control of the infection and the ability of the host to mount adaptive immune responses to the virus. A better understanding of the interplay between the innate and adaptive immune systems in the context of different virus infections is therefore valuable.
A number of viruses have been exploited as vaccine vectors due to their capacities to stimulate strong adaptive immune responses against heterologous antigens (4, 14, 27, 56, 61). This strategy has been explored in the field of human immunodeficiency virus type 1 (HIV-1) vaccine development, where traditional vaccine approaches have failed or are not suitable for safety reasons. Most recombinant viral vaccines stimulate Th1-biased responses, which drive CD8+-T-cell-mediated immunity efficiently (55), while protein-based vaccines, depending on the adjuvant used, generally stimulate Th2-biased immune responses that primarily drive antibody responses (24, 63). A successful vaccine against pathogens such as HIV-1 will likely require the elicitation of both cellular and humoral immune responses, and strategies that can achieve this are therefore desired. In addition to their ability to stimulate strong immune responses against virus-encoded antigens, live viruses and virus-based vaccines have been shown to provide an adjuvant effect on coimmunized protein antigens (7, 9, 15, 31, 52). However, it is not clear if the signals that provide adjuvant activity on the coimmunized protein antigen are the same as those that drive immune responses against virus-encoded antigens. Well-characterized recombinant viruses expressing model antigens are useful tools to address these questions.
There are several possible ways by which viruses can activate adjuvant signals. Many viruses induce cell death, which in turn can lead to leakage of cytoplasmic components into the extracellular environment, where they act as efficient danger signals (59). For example, it has been shown that monosodium urate crystals released from apoptotic cells provide an adjuvant effect on the adaptive immune response (60). Also, structural components of viruses can activate innate immune signaling pathways, which may contribute to shaping the adaptive immune response. An important class of molecules for induction of innate immune signals is the Toll-like receptors (TLRs). TLRs are pattern recognition receptors expressed in a cell-type-specific manner, allowing sensitive detection of incoming pathogens. Viruses may be recognized via their glycoproteins by cell surface-expressed TLRs (6, 12, 36), or they may be detected after uptake, degradation, and exposure of viral genomes to TLRs present in endosomal compartments (8). Specifically, viral single-stranded RNA has been shown to be a ligand for TLR7/8 (16, 26, 45), and unmethylated double-stranded DNA (dsDNA), including bacterial CpG motifs, binds to TLR9 (35, 44). Recognition of viral genomes by TLR7/8/9 has been reported to be a function of plasmacytoid dendritic cells (DCs). TLR3 has also been implicated in viral recognition. This was originally based on the finding that TLR3 binds polyinosine-poly(C) (pI-C) (2), a synthetic analogue of dsRNA and a frequently used mimic of viral replication by-products. However, a role for TLR3 in protective immunity against viruses has not been found (8, 19, 57), except in studies using mouse cytomegalovirus (66). Activation of TLR3 leads to activation of NF-κB and IFN regulatory factor 3 via a TRIF-dependent pathway (17), while signaling via TLR7/8/9 depends on signaling via a pathway that requires myeloid differentiation factor 88 (MyD88). TLR ligands, including pI-C, CpG, and synthetic TLR7/8 agonists, are potent modulators of DC function and effective adjuvants for eliciting adaptive immune responses (13, 18, 50, 58, 69, 70).
In this study, we investigated the adjuvant properties of one-cycle recombinant Semliki Forest virus (rSFV) particles. SFV is a positive-strand RNA virus of the alphavirus genus and a frequently used model virus and vaccine vehicle (5, 22, 28, 41, 46, 64). We demonstrate that rSFV provided a potent adjuvant effect on coimmunized protein antigen both in rabbits and in mice. The adjuvant effect was intact in mice lacking MyD88 and in mice lacking TLR3. Both TLR3−/− and MyD88−/− mice were also fully capable of raising antibody responses against virus-encoded antigen. Thus, our results show that the previously described requirement for MyD88 signaling for the induction of B-cell responses (51) can be overcome by viral infection. In contrast, the adjuvant effect of rSFV on coimmunized protein antigen was completely abolished in mice lacking the IFN-α/β receptor (IFN-AR1−/−), demonstrating that the adjuvant effect on coimmunized protein antigens was dependent on IFN-α/β signaling. Interestingly, the antibody response against virus-encoded antigens was independent of IFN-α/β signaling, demonstrating that other signals are sufficient to stimulate immune responses to antigens expressed in virus-infected cells. These data highlight a clear difference in the requirements for eliciting antibodies to virally encoded antigens and to antigens administered as purified protein antigen, together with viral particles.
MATERIALS AND METHODS
Cells, recombinant viruses, and protein antigens.
BHK-21 cells (ATCC) were cultured in Glasgow minimal essential medium supplemented with 5% fetal calf serum (FCS), 10 mM HEPES, 10% tryptose phosphate, and l-glutamine, penicillin, and streptomycin (Invitrogen, Carlsbad, California). Murine DCs (mDCs) were generated as previously described (23). Briefly, bone marrow cells were isolated from femurs and tibiae, and the red blood cells were lysed. The cells were plated at 3 × 106/ml in DC medium (RPMI 1640 supplemented with 10% FCS, l-glutamine, penicillin, streptomycin, 1 mM sodium pyruvate, 0.02 M HEPES, and 50 μM 2-mercaptoethanol) supplemented with 50 ng/ml of murine granulocyte-macrophage colony-stimulating factor (PeproTech, London, United Kingdom) and harvested at day 6. Primary mouse embryo fibroblasts (MEFs), obtained from 12- to 13-day-old C57BL/6 fetuses, and L929 cells (ATCC) were cultured in Dulbecco's modified Eagle's medium (Sigma, St. Louis, MO) supplemented with 10% FCS, l-glutamine, penicillin, and streptomycin.
Single-round infectious rSFV encoding β-galactosidase (β-Gal) (rSFV-LacZ) or influenza virus nucleoprotein (rSFV-NP) were generated as previously described (62). The titers of the viral stocks were determined according to standard methods (33). UV inactivation was performed using an Amersham UV cross-linker as previously described (28) at 2,000 μJ/cm2 for 1 min to generate the mildly UV-inactivated virus (UV′-rSFV-NP) or 20,000 μJ/cm2 for 30 min for the harshly UV-inactivated virus (UV"-rSFV-NP). Wild-type (wt) SFV type 4 for use in the bioassay for murine IFN-α/β (see below) was generated from the infectious SFV cDNA clone (42). Purified trimeric YU2gp140(−/GCN4) HIV-1 envelope glycoprotein (Env), used in the rabbit immunizations, and YU2gp120, used in the HIV-1 Env enzyme-linked immunosorbent assay (ELISA), were kind gifts from Yuxing Li and Richard Wyatt at the Vaccine Research Center, NIH. The expression and purification of these proteins have been described previously (24). The β-Gal protein used in the mouse immunizations and in the β-Gal ELISA was purchased from Roche.
Animals.
MyD88−/− mice (1), TLR3−/− mice (2), IFN-AR1−/− mice (49), CD4−/− mice (54), and their respective wt controls were kept and bred under pathogen-free conditions at the animal facilities at the Department of Microbiology, Tumor and Cell Biology, Karolinska Institute, and the Swedish Institute for Infectious Disease Control. New Zealand White rabbits, approximately 3-month-old females, were purchased from HB Lidköpings Kaninfarm (Lidköping, Sweden). All animal experiments were approved by the Committee for Animal Ethics in Stockholm, Sweden, and performed according to given guidelines.
Immunizations.
Rabbits (two per group) were immunized two times, with 4 weeks between immunizations, with 25 μg purified HIV-1 Env protein either in 0.5 ml phosphate-buffered saline (PBS) containing 5 × 107 IU rSFV-LacZ or in 0.5 ml PBS. The injections were split between five sites intradermally, two sites intramuscularly, and one site subcutaneously (s.c.). Two control rabbits were immunized with 5 × 107 IU rSFV-LacZ using the same immunization routes. The rabbits were bled 10 days after the second immunization. Mice were immunized using s.c. administration of 100 μl containing 10 or 15 μg β-Gal protein alone in PBS or mixed with 106 to 107 IU rSFV particles, as described in the respective figure legends. Intravenous (i.v.) inoculation of rSFV particles used for immunization experiments or for in vivo IFN-α/β measurements were performed as previously described (28).
ELISA.
For detecting anti-β-Gal antibodies, ELISA plates (Immunosorp, Nunc, Denmark) were coated with 50 μl per well of 1-μg/ml recombinant β-Gal protein (Roche) in carbonate buffer at 4°C overnight. For detection of anti-NP antibodies, the plates were coated with a lysate of rSFV-NP-infected cells. The rSFV-infected cells were lysed 24 h postinfection using a lysis buffer containing 1% NP-40 and protease inhibitors in a volume corresponding to 2 × 106 cells/ml. The lysate was diluted 1:200 in carbonate buffer, and the plates were coated at 4°C overnight. For detecting antibodies against HIV-1 Env, the plates were coated with 100 ng of insect cell-produced YU2gp120, as previously described (25), in 100 μl PBS per well at 4°C overnight. The plates were then incubated for 1 h at 37°C with blocking buffer (PBS containing 15% dry milk and 2% FCS). Sera were diluted in blocking buffer and were incubated in the plates for 2 h at room temperature (RT). Plates coated with comparable lysates from cells infected with rSFV encoding influenza hemagglutinin did not give rise to any signal when incubated with sera from mice immunized with rSFV-NP, showing that the ELISA was specific for NP and not for the nonstructural proteins of rSFV. After the plates were washed (PBS plus 0.2% Tween 20), horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG), anti-mouse-IgG1, anti-mouse IgG2a (Southern Biotech, Birmingham, AL), or anti-rabbit IgG (Sigma) antibody, diluted 1/5,000, was added to the wells. After 1 h of incubation at RT, the plates were washed, and 100 μl fast o-phenylenediamine dihydrochloride substrate (Sigma) was then added for detection of bound antibodies. The optical density (OD) at 450 nm was read using an ELISA reader.
IFN-γ ELISPOT.
IFN-γ enzyme-linked immunspot (ELISPOT) analysis was performed on freshly isolated splenocytes as follows. ELIIP10SSP plates (Millipore Co.) were coated with anti-IFN-γ antibodies (AN18; MabTech, Stockholm, Sweden) in PBS overnight at 4°C. The plates were washed five times with PBS, followed by incubation with complete medium for 2 h at 37°C to block the plates. Spleen cells from individual mice were added at 2 × 105 cells/well in quadruplicate against stimuli of interest: medium alone, 2 μg/ml concanavalin A (Sigma), and 2 μg/ml of NP H-2Db peptide (ASNENMETM; ProImmune Limited, Oxford, United Kingdom). After 20 h of incubation in 5% CO2 at 37°C, the plates were washed six times with PBS-Tween (0.05%). Thereafter a biotinylated anti-IFN-γ antibody, R4-6A2 (MabTech), was added, and the plates were incubated for 2 h at RT. After the plates were washed as described above, an avidin-peroxidase complex (ABC kit; Vector Laboratories) was prepared according to the manufacturer's instructions and added to each well. The plates were incubated for 1 h at RT and were then washed six times with PBS. AEC substrate (Sigma) was prepared according to the manufacturer's instructions and added to the wells. The enzymatic reaction was stopped after 4 min by washing the plates in water. The spots were counted using an ELISPOT reader (Axioplan 2 Imaging; Zeiss) and expressed as the number of spots per 106 splenocytes.
Bioassay for murine IFN-α/β.
A bioassay was employed to determine the total amount of active type I IFN (28). Briefly, flat-bottom 96-well plates were seeded with L929 cells, and the following day, twofold serially diluted samples or IFN-α/β standard (NIAID, NIH; Gu02-901-511) was added to the cells. After overnight incubation, the cells were infected with SFV type 4. Mitochondrial dehydrogenase activity was assayed at 2 days after infection, using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide according to the instructions in Sigma kit CGD-1. The absorbance was read at 570 nm and normalized against 630 nm in a microplate reader (Elx 800 UV; BIO-TEK Instruments, Winooski, Vermont). The data were converted to IU by comparing the samples to the murine IFN-α/β standard (NIAID, NIH).
Apoptosis measurements and DC stimulations.
MEFs were mock infected or infected with rSFV-LacZ particles using a multiplicity of infection of 20 and left in culture for 16 h. The cells were harvested and stained with Annexin V-fluorescein isothiocyanate (FITC) (BD Biosciences PharMingen, San Jose, California) and propidium iodide (PI), and apoptosis induction was monitored by flow cytometry. The mDCs were set up at 1 × 106 in 500 μl of DC medium for stimulation with pI-C (Sigma), rSFV particles, or rSFV-infected MEFs as described in the figure legends. The cells were harvested and analyzed 20 h poststimulation. The phenotypes and activation statuses of the cells were determined by staining them with anti-CD11c-PE, anti-CD11b-APC, and anti-CD40-FITC monoclonal antibodies (PharMingen). Flow cytometric analysis was performed using a FACSCalibur instrument (Becton Dickinson).
RESULTS
Virus particles provide an adjuvant effect on coimmunized protein antigens.
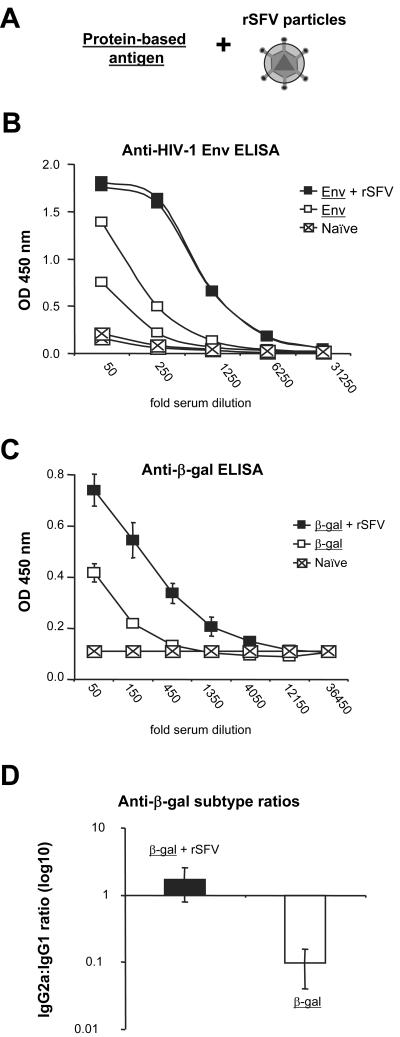
Previous studies have suggested that viral infection or virus-based vaccines can stimulate immune responses against a concurrently administrated unrelated protein (7, 9, 15, 31, 52). To investigate if rSFV exhibited an intrinsic adjuvant effect, we performed a series of immunization experiments in small animals using single-round infectious rSFV particles mixed with protein-based antigen (Fig. 1A). We first immunized rabbits with viral particles mixed with recombinant purified HIV-1 envelope glycoprotein YU2gp140(−/GCN4) (Env) or with Env glycoprotein alone. We found that the antibody response against Env was markedly increased if the protein was coimmunized with rSFV particles (Fig. 1B). Thus, rSFV provides an adjuvant effect on the production of specific antibodies against a coimmunized HIV-1 Env antigen.
FIG. 1.
rSFV confers an adjuvant effect on antibody responses against coimmunized protein. (A) Cartoon illustrating the experimental system. The adjuvant effect of rSFV was investigated by mixing recombinant protein antigens with single-round infectious rSFV particles, and antibody responses against the protein antigens were measured. (B) Rabbits (two per group) were immunized with a mixture of purified HIV-1 envelope glycoprotein (Env) and 5 × 107 IU rSFV, or twice with 25 μg Env alone. The rabbits were prebled before immunization (Naïve). Sera from immunized rabbits were drawn 10 days after the final immunization, and antibodies against Env were analyzed by fivefold serial dilutions starting at a 50-fold dilution. Data from individual rabbits are shown. (C) SV129 mice were immunized s.c. a single time with 107 IU rSFV-NP mixed with 15 μg β-Gal protein (n = 7) or with 15 μg β-Gal protein alone (n = 3). Sera from immunized mice were drawn 12 days after the immunization, and β-Gal-specific IgG was analyzed by threefold serial dilutions starting at a 50-fold dilution. Mean titers from each group are shown. The error bars represent standard deviations. (D) IgG2a:IgG1 ratios in sera from the immunized mice were calculated by dividing the IgG2a with the IgG1 OD values for each individual mouse. Mean values are shown. The protein antigen is underlined.
To investigate the mechanism by which rSFV provides an adjuvant effect, the subsequent experiments were performed in mice. We first immunized SV129 mice with recombinant β-Gal protein mixed with rSFV-NP or with β-Gal protein alone. In agreement with the results obtained in rabbits, coadministration of virus particles with recombinant β-Gal protein elicited higher levels of antibodies against β-Gal than the responses obtained after immunization with β-Gal alone (Fig. 1C). Antibody responses against rSFV-encoded antigens are generally Th1 biased (5, 21, 22), and we therefore investigated if coadministration of rSFV skewed the antibody response against the coimmunized protein toward a Th1 response. We found that the ratio of IgG1 (Th2) to IgG2a (Th1) was inverted when β-Gal was administered alone compared to when it was mixed with rSFV, suggesting that rSFV promoted a Th1 response (Fig. 1D).
The adjuvant effect of rSFV particles is not dependent on MyD88 or TLR3.
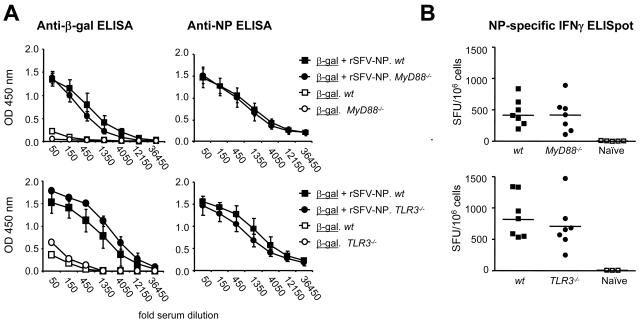
A number of studies have suggested that signaling via TLR3 or TLR7/8 promotes adaptive immune responses during infection with RNA viruses (10, 30, 58, 72), while other studies have shown that signaling via TLRs is not required for the establishment of adaptive immune responses during RNA virus infection (19, 43). To investigate if the adjuvant activity of rSFV particles was dependent on signaling via TLRs, MyD88−/− and TLR3−/− mice were immunized with β-Gal protein mixed with rSFV-NP particles or with β-Gal protein alone. Immune responses against both the virus-encoded antigen and the coimmunized protein antigen were measured. We found that the adjuvant effect provided by rSFV on coimmunized β-Gal protein was intact in both MyD88−/− mice and TLR3−/− mice (Fig. 2A, left). Moreover, the IgG response against the virus-encoded NP was also intact in the MyD88−/− and TLR3−/− mice (Fig 2A, right), demonstrating that signaling via these pathways was not required to generate antibody responses against virus-encoded antigens.
FIG. 2.
Signaling via MyD88 or TLR3 is not required for the adjuvant effect of rSFV. (A) MyD88−/− (n = 6) and TLR3−/− (n = 7) mice and their respective wt control strains (n = 7 each) were immunized s.c. a single time with 107 IU rSFV-NP particles mixed with 15 μg β-Gal protein or with 15 μg β-gal protein alone (n = 3). Sera were drawn 12 days after the immunization, and IgG responses against the coimmunized protein (β-Gal; left) and the virus-encoded antigen (NP; right) were measured using threefold serial dilutions of the sera starting at a 50-fold dilution. Mean OD values from each group are shown. The error bars represent standard deviations. (B) IFN-γ ELISPOT analysis was performed on splenocytes from the immunized MyD88−/− (top) and TLR3−/− (bottom) mice and their respective wt controls. Mean ELISPOT values from quadruplicate wells, stimulated with NP peptide, are shown. Cytokine-producing cells are shown as spot-forming units (SFU)/106 cells. Mean values for the groups are indicated by the bars. The protein antigen is underlined, and the mouse strain is italic.
We also investigated the cellular response against the virus-encoded antigen in MyD88−/− and TLR3−/− mice. Signaling via MyD88 and TLR3 has been suggested to play a role in the induction of T-cell responses against virus-encoded antigens (10, 58, 72). However, by using an NP-specific IFN-γ ELISPOT assay and restimulation of splenocytes with a major histocompatibility complex class class I-restricted NP peptide, we detected similar numbers of IFN-γ-secreting cells in MyD88−/− and TLR3−/− mice and in their respective wt controls (Fig. 2B). Thus, neither the humoral nor the cellular immune response induced by rSFV immunization was compromised in the MyD88−/− or the TLR3−/− mice.
IFN-α/β is required for the adjuvant effect of rSFV on coimmunized protein antigen.
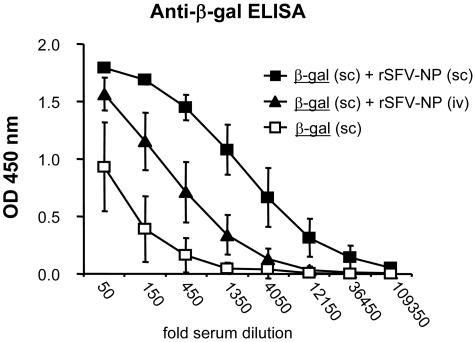
To determine if the adjuvant effect of rSFV depended on locally acting signals or if it could act at distant sites, groups of SV129 wt mice were immunized either with a mixture of β-Gal protein and rSFV-NP particles (s.c.) or with purified β-Gal protein (s.c.) and with rSFV-NP particles (i.v.) at the same time. As in the previous experiments, a control group of mice was also immunized (s.c.) with β-Gal protein alone. The results demonstrated that coadministration of rSFV particles and β-Gal protein to the same local site gave the strongest adjuvant effect. However, an adjuvant effect was also observed when the virus and the protein were injected via different routes (Fig. 3). This led us to ask if the adjuvant effect of rSFV was mediated by soluble molecules, such as type I interferons (IFN-α/β). We have previously shown that rSFV induces high levels of IFN-α/β at early time points after injection into mice (28), and other investigators have shown that IFN-α/β can provide potent adjuvant activity and enhance immune responses against protein antigens (18, 38, 53).
FIG. 3.
Viral particles provide an adjuvant effect to protein antigen administered via a different route. To determine if rSFV particles stimulated an adjuvant effect that could act at distant sites, B6 wt mice (n = 5) were immunized s.c. with 10 μg β-Gal protein mixed with 107 IU rSFV-NP particles or with 10 μg β-Gal protein administered s.c. and 107 IU rSFV-NP particles administered i.v. Control mice were immunized s.c. with 10 μg β-Gal protein alone. Sera were drawn 12 days after immunizations, and β-Gal-specific IgG levels were determined using fivefold serial dilutions starting at a 50-fold dilution. The differences in OD values between the animals that received β-Gal alone s.c. and those that in addition received rSFV-NP i.v. were statistically significant (P < 0.05) for four consecutive serial dilutions. The error bars represent standard deviations.
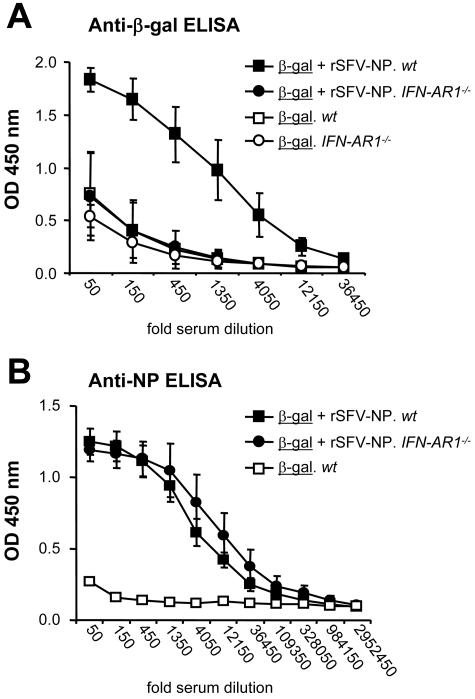
To investigate if virus-induced IFN-α/β mediated the adjuvant effect of rSFV, mice lacking a functional IFN-α/β receptor (IFN-AR1−/−) were employed. Wt SV129 and IFN-AR1−/− mice were immunized with recombinant β-Gal protein mixed with rSFV-NP particles or with recombinant β-Gal protein alone. In agreement with our previous observations, the levels of anti-β-Gal specific IgG in wt mice were significantly enhanced when the protein was coimmunized with rSFV particles compared to when the protein was administered in PBS alone. In contrast, the adjuvant effect of rSFV particles on β-Gal was completely absent in the IFN-AR1−/− mice, using a dose of 106 IU rSFV particles (Fig. 4A) and, in separate experiments, a 10-fold-higher dose of rSFV (data not shown). Since the adjuvant effect of rSFV could be observed only in animals with the ability to respond to IFN-α/β, we conclude that IFN-α/β signaling is required for the adjuvant effect of rSFV on coimmunized protein antigen. In contrast, the antibody responses against the virus-encoded NP antigen were similar in the IFN-AR1−/− mice and the wt control mice (Fig. 4B). Thus, there was a distinct difference in the requirements for IFN-α/β signaling for the response elicited against the virus-encoded antigen and that elicited against the coimmunized protein antigen.
FIG. 4.
IFN-α/β receptor signaling is required for the adjuvant effect of rSFV on coimmunized protein. SV129 wt mice (n = 8) and IFN-AR1−/− mice (n = 9) were immunized with 106 IU of rSFV-NP mixed with 10 μg β-Gal protein each or with 10 μg β-Gal protein alone (n = 3 for each strain). Sera drawn 12 days after the immunization were analyzed for total (A) β-Gal-specific IgG and (B) NP-specific IgG. All samples were analyzed using threefold serial dilutions starting at a 50-fold dilution. The protein antigen is underlined, and the mouse strain is italic. The error bars represent standard deviations.
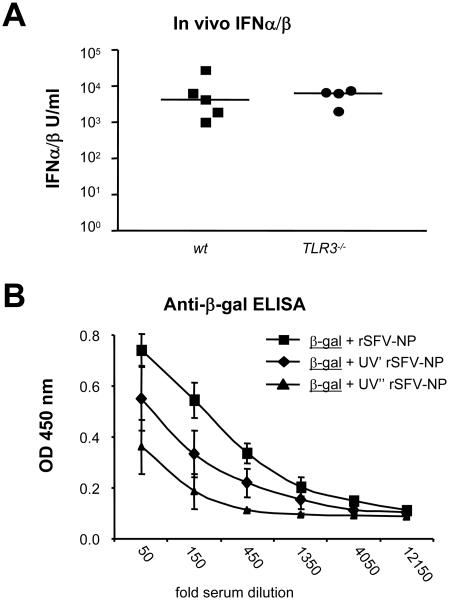
In our previous work, we showed that MyD88−/− mice produced normal levels of IFN-α/β after rSFV immunization (28). Since the adjuvant effects of SFV particles on coimmunized protein were similar in wt, MyD88−/−, and TLR3−/− mice, we asked if TLR3−/− mice were capable of producing IFN-α/β in response to rSFV inoculation. Signaling via TLR3 by the synthetic double-stranded RNA analogue pI-C is known to lead to IFN-α/β production (17), but it is less clear if TLR3 is generally required for induction of IFN-α/β by viruses (29, 43). To investigate if the TLR3−/− mice produced IFN-α/β in response to rSFV, we injected wt and TLR3−/− mice i.v. with rSFV particles and analyzed the sera at 6 h after injection. The results demonstrated that rSFV stimulated high IFN-α/β levels in both wt and TLR3−/− mice, and there was no significant difference in the amplitudes of the responses (Fig. 5A). This is consistent with the majority of virus-stimulated IFN-α/β being induced via a TLR3-independent cytosolic pathway, such as that mediated by retinoic acid-inducible gene I (RIG-I) (34, 47, 71), and with the observation that the adjuvant effect of rSFV is intact in both MyD88−/− and TLR3−/− mice (Fig. 2). To investigate if the adjuvant effect of rSFV required RNA replication or if viral induction of IFN-α/β was sufficient, we used rSFV subjected to different doses of UV light. We have previously described a mild UV treatment resulting in an RNA replication-defective but not entry-defective virus (UV′-rSFV) (28) that induces about 10-fold-lower levels of IFN-α/β than rSFV in vivo. A harsher UV treatment resulted in a virus that was both replication defective and entry defective (UV"-rSFV) and which does not induce any detectable IFN-α/β (data not shown). We found that RNA replication was not required for an adjuvant effect of the viruses on the coimmunized protein but that the adjuvant effect correlated with the ability to induce IFN-α/β. Coimmunization with rSFV stimulated the highest levels of β-Gal antibodies, followed by UV′-rSFV and finally UV"-rSFV (Fig. 5B).
FIG. 5.
IFN-α/β levels in sera of rSFV-infected wt and TLR3−/− mice and adjuvant effects of viruses that induce different levels of IFN-α/β. (A) In vivo IFN-α/β levels were measured to determine if TLR3−/− mice retained the capacity to produce IFN-α/β in response to rSFV infection. IFN-α/β levels in sera from individual wt and TLR3−/− mice at 6 h after injection with 107 IU rSFV particles were measured using a bioassay. Data from individual mice are shown, and the bars indicate mean values. (B) The dose dependence of the adjuvant effect of IFN-α/β was determined by coimmunizing β-Gal protein with 107 IU rSFV that had been subjected to different doses of UV treatment. Mildly UV-inactivated rSFV (UV′ rSFV-NP) has previously been shown to induce lower levels IFN-α/β than rSFV-NP, while harshly UV-inactivated rSFV-NP (UV" rSFV-NP) does not induce any detectable IFN-α/β. Sera were drawn 12 days after the immunizations, and β-Gal-specific IgG levels were determined using fivefold serial dilutions starting at a 50-fold dilution. The differences in OD values between UV′ rSFV-NP and UV" rSFV-NP were statistically significant (P < 0.01) for six consecutive serial dilutions. The error bars represent standard deviations.
Humoral immune responses against rSFV-encoded antigens are intact in the absence of IFN-α/β signaling.
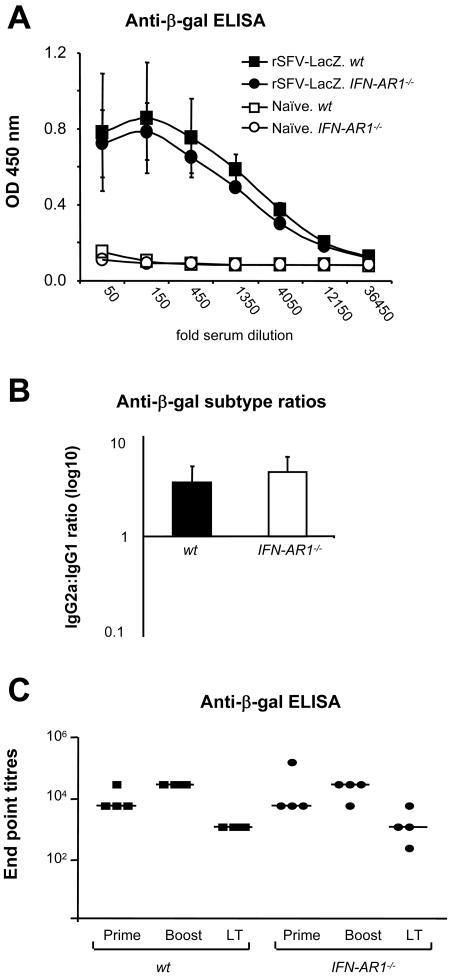
For a more thorough analysis of the induction of the antibody response against virus-encoded antigens in mice lacking the ability to respond to IFN-α/β, we immunized IFN-AR1−/− mice with rSFV particles encoding β-Gal (rSFV-LacZ), the same antigen that was administered in the form of protein in the previous experiments. The first experiment confirmed the observation that IFN-α/β signaling is not required for induction of IgG against a virus-encoded antigen (Fig. 6A). We also investigated if the IgG2a:IgG1 ratio differed in sera from wt and IFN-AR1−/−-immunized mice. We found that the ratios were very similar in wt and IFN-AR1−/− mice, suggesting that IFN-α/β signaling is not critical for the generation of Th1-biased immune responses to the rSFV-encoded antigen (Fig. 6B). In an independent experiment, we asked if the long-term antibody responses stimulated by two consecutive rSFV-LacZ immunizations (given 2 weeks apart) were of the same amplitude in wt and IFN-AR1−/− mice. Total anti-β-Gal IgG was measured 14 days after the first (prime) and second (boost) immunization, and long-term antibody responses were measured 20 weeks after the second immunization (Fig. 6C). No defect in the anti-β-Gal IgG response was observed in IFN-AR1−/− mice compared to wt mice at either time point. These results confirm that IFN-α/β-independent adjuvant signals are induced in virus-infected cells and that these signals are sufficient to promote humoral immune responses against virus-encoded antigens in this system.
FIG. 6.
Antibody responses against virus-encoded antigens are not defective in the absence of IFN-α/β signaling. (A) SV129 wt mice (n = 5) and IFN-AR1−/− mice (n = 5) were immunized once with 106 IU of rSFV-LacZ. After 14 days, total anti-β-Gal specific IgG levels were determined using fivefold serial dilutions starting at a 50-fold dilution. The error bars represent standard deviations. (B) IgG2a:IgG1 ratio between SV129 and IFN-AR1−/− mice from the sample shown in panel A. (C) SV129 (n = 4) and IFN-AR1−/− (n = 4) mice were immunized twice with 106 IU of rSFV-LacZ, 4 weeks apart. Total anti-β-Gal-specific IgG levels were measured in sera 14 days after the first immunization (Prime) and 14 days (Boost) and 20 weeks (long term [LT]) after the second immunization. Endpoint titers for each individual mouse are shown. Geometric means are indicated. The protein antigen is underlined, and the mouse strain is italic.
Activation of bone marrow-derived dendritic cells by rSFV and rSFV-infected cells is dependent on IFN-α/β signaling.
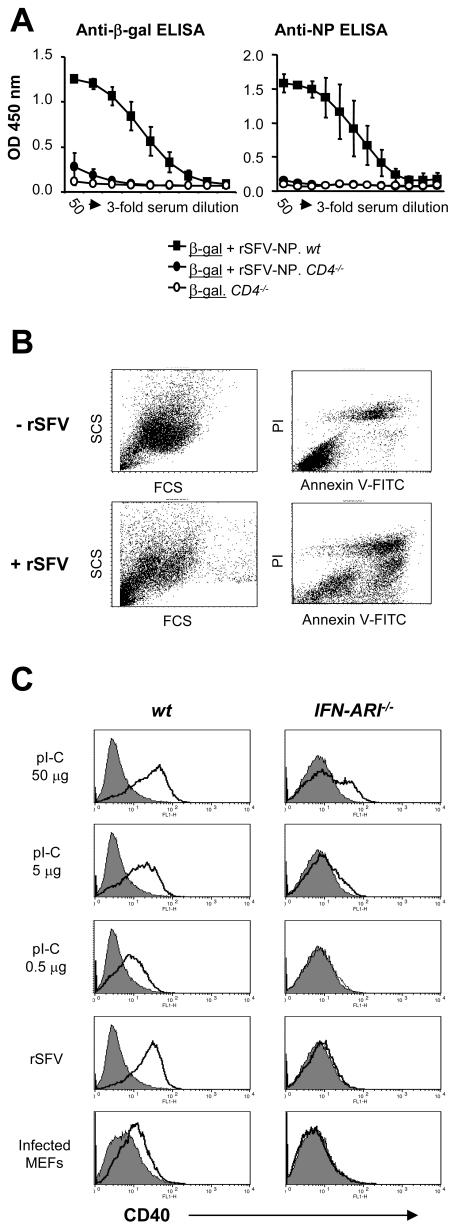
To attempt to understand why antibody responses against virus-encoded antigens are IFN-α/β independent while antibody responses against coadministered protein are strictly IFN-α/β dependent, we first asked if CD4+-T-cell help was required for the response. For most viral infections, including during SFV-based immunizations, T-cell help is required for the elicitation of humoral immunity (67), while some virus infections can induce antibodies independently of T-cell help (40, 54, 65). The ordered structure of the viral antigens and innate immune signals induced during infection are thought to contribute to the elicitation of T-cell-independent antibody production. To investigate if the elicitation of antibody responses against rSFV-encoded NP and coadministered β-Gal protein were dependent on CD4+-T-cell help, we used mice lacking the CD4 molecule (CD4−/− mice). We found that IgG responses against both virus-encoded NP and coadministered β-Gal were critically dependent on the presence of functional CD4+ T cells (Fig. 7A).
FIG. 7.
Role of CD4+ T cells and activation of DCs by rSFV and rSFV-infected cells. (A) CD4+-T-cell requirement for antibody responses elicited against the coadministered protein antigen (β-Gal) and against the virus-encoded antigen (NP). Wt and CD4−/− mice were immunized with a mixture of 106 IU rSFV-NP particles and 10 μg β-Gal protein. Total anti-β-Gal or NP-specific IgG responses were determined using threefold serial dilutions of the respective sera starting at 50-fold dilutions. The error bars represent standard deviations. (B) Apoptosis induction in rSFV-infected and uninfected MEFs was analyzed by flow cytometry using forward scatter (FCS) and side scatter (SCS) analyses (left) and Annexin V-FITC and PI staining (right; ungated cells) at 16 h postinfection. The apoptotic population was Annexin V positive and PI dim. (C) CD40 expression on bone marrow-derived mDCs from SV129 and IFN-AR1−/− mice. Bone marrow-derived DCs (106 per stimulation) were incubated as indicated with 50 μg, 5 μg, or 0.5 μg of pI-C; with rSFV particles at a multiplicity of infection of 20; or with 5 × 105 rSFV-infected MEFs. CD40 expression was analyzed by flow cytometry after 20 h of incubation. CD40 expression on CD11c and CD11b double-positive cells is shown. Unstimulated cells are shown as filled histograms and stimulated cells as overlaid empty histograms. The protein antigen is underlined, and the mouse strain is italic.
Given the important role of DCs in priming naïve CD4+ T cells, we next asked if virus-infected cells could activate DCs in an IFN-α/β-independent manner. Activation of DCs by dsRNA replication intermediates leaking out of dying infected cells could represent one possibility by which immune responses against virus-encoded antigens are stimulated in the absence of IFN-α/β signaling. To investigate this, bone marrow-derived DCs from wt SV129 mice and IFN-AR1−/− mice were exposed to different concentrations of pI-C (a mimic of dsRNA), to cell-free rSFV particles, or to virus-infected apoptotic MEFs. The MEFs were infected with rSFV 8 hours prior to addition to the DC cultures, and the cells were cocultured for 20 h. During this time, a large proportion of the infected cells undergo apoptosis, as shown by an Annexin V-positive, PI-dim population in the infected MEF culture (Fig. 7B), at which time viral dsRNA should be abundant in these cells. The results show that CD40 was markedly upregulated in response to high doses of pI-C (50 μg/ml), and this response was observed, although reduced, in DCs lacking IFN-AR1 compared to wt DCs (Fig. 7C, top row). The upregulation of CD40 in response to pI-C was completely dependent on IFN-α/β as the dose of pI-C was decreased (Fig. 7C, second and third rows from top). Similar data were obtained when CD86 surface expression levels were analyzed (results not shown). It has been shown that cell-associated pI-C is a more potent inducer of interleukin 6 (IL-6) than cell-free pI-C (58). It was therefore possible that rSFV-infected MEFs containing dsRNA replication intermediates would behave like the high dose of pI-C and be less dependent on IFN-α/β for upregulation of CD40. However, when DCs were cocultivated with rSFV-LacZ-infected MEFs, only DCs with an intact IFN-α/β signaling pathway upregulated expression of CD40 (Fig. 7C, bottom row). Cell-free rSFV particles also stimulated DCs to upregulate CD40 in an IFN-α/β-dependent manner (Fig. 7C, fourth row from top). While these data do not explain the IFN-α/β-independent responses observed against virus-encoded antigens in vivo, they provide a mechanism for the IFN-α/β-dependent adjuvant effect of rSFV on coadministered protein antigens.
DISCUSSION
In this study, we investigated the mechanisms by which a model virus stimulates adjuvant activity on adaptive immune responses. We found that rSFV particles potently enhanced antibody responses against coimmunized subunit protein antigen in the absence of other exogenously added adjuvants and that this effect was IFN-α/β dependent. Coimmunized rSFV promoted the production of IgG2a, consistent with the fact that IFN-α is a strongly Th1-inducing cytokine (20). Virus-induced Th1-biased adjuvant effects have also been observed after coadministration of poxviruses with the commercial protein-based hepatitis B surface antigen Engerix-B (31) or with purified HIV-1 envelope glycoproteins (7). Similarly, it has been shown that the Oka varicella vaccine provides Th1-dominant adjuvant signals to the hepatitis B surface antigen during coimmunization (52) and that influenza virus infection converted a nonimmunogenic (tolerogenic) coadministered protein to an immunogenic antigen (9). In the last study, virus infection resulted in DC activation and priming of IL-2- and IFN-γ-producing T cells with specificity for both the viral antigens and the unrelated coadministered protein antigen. Collectively, these studies suggest that a Th1-inducing environment is a general consequence of virus infection and that this can promote antibody responses against unrelated antigens that are present in the surroundings.
The rSFV system used in this study is based on suicidal, one-cycle virus particles that are packaged using a helper system (62). These particles are capable of mediating only a single round of infection, providing a well-defined system to address these questions. SFV is a potent inducer of IFN-α/β (3, 11, 28), and so far, no mechanism for specifically suppressing IFN-α/β induction during SFV infection has been reported. We have previously shown that suicidal rSFV particles induce a rapid and transient IFN-α/β response in mice, which is easily detected in serum at 4 to 6 h after immunization (28). Here we show that both rSFV and UV-inactivated rSFV particles exhibited an adjuvant effect on coimmunized protein. This is consistent with studies using inactivated pseudo-rabies virus particles that exhibited adjuvant effects in the absence of viral replication (15) and with our previous observations that UV-inactivated, entry-competent rSFV particles are capable of inducing an IFN-α/β response (28). In contrast, a recent study by Thompson et al. demonstrating an adjuvant effect of Venezuelan equine encephalitis virus (VEE) on coimmunized protein antigen (68) showed that the effect was abolished upon UV treatment of the virus. It is possible that this reflects differences in how harshly the virus was inactivated, since our data suggest that only entry-competent UV-treated rSFV maintains the ability to induce IFN-α/β (28). Whether the systemic and mucosal adjuvant effect on coimmunized proteins reported for VEE is mediated by IFN-α/β remains to be determined, as this was not investigated in the study by Thompson et al. (68). It will also be interesting to determine if, similarly to VEE, rSFV can promote mucosal immune responses against coimmunized protein antigens and whether there are mechanistic differences between the adjuvant effects of SFV and VEE.
In immunization studies using protein antigens and various TLR ligands, it was recently suggested that CD4+-T-cell-dependent antibody responses require activation of TLRs on B cells (51). The adjuvant effect of rSFV on coimmunized protein was not compromised in mice lacking the adapter protein MyD88 (required for signaling via all TLRs except TLR3 and TLR4) or in mice deficient for TLR3. This is consistent with the fact that both MyD88−/− and TLR3−/− mice produced normal levels of IFN-α/β in response to rSFV infection (28) (Fig. 5A). It is also consistent with the recent demonstration that induction of IFN-α/β by RNA viruses in most cell types is mediated by the cytosolic receptor RIG-I and is independent of TLRs (34, 47). We suggest that IFN-α/β are important mediators of the adjuvant effects of many TLR ligands (18) and that in a viral system, the requirement for TLR signaling can be overcome by the ability of viruses to induce IFN-α/β through alternative pathways (34, 71). Neither MyD88−/− nor TLR3−/− mice were defective in immune responses against virus-encoded antigen. This observation differs from a previous report, in which MyD88 was required for induction of T-cell responses against a different antigen encoded by rSFV (9). Also, previous studies have shown that TLR3 can mediate cross-presentation of viral antigens (58). However, in those experiments, mice were immunized with virus-infected Vero cells (of monkey origin) rather than with infectious rSFV particles to overcome any effects of virus-induced IFN-α/β (58). This approach was taken because IFN-α/β alone has been shown to be sufficient to promote cross-presentation (37). We found that the TLR3−/− mice produced IFN-α/β levels similar to those of wild-type mice upon injection with rSFV (Fig. 5A). Furthermore, in the presence of an active IFN-α/β-signaling pathway, the elicitation of cellular and humoral immune responses against virus-encoded antigens was not compromised in the TLR3−/− mice (Fig. 2).
The IFN-α/β-dependent adjuvant effect of rSFV on coadministered protein is consistent with results reported with other adjuvants. For example, the adjuvant activities of Freund's complete adjuvant and of several TLR ligands are at least partially dependent on IFN-α/β (18, 38). IFN-α/β are potent modulators of DC function, and treatment with IFN-α/β in vitro leads to upregulation of CD40 cell surface expressed on DCs (29, 48). Our results suggest that IFN-α/β induced by rSFV activates DCs that have taken up antigen, locally or at distant sites, enhancing antibody responses against coadministered protein in a CD4+-T-cell dependent manner. It is possible that IFN-α/β also has a direct effect on other cells that contributes to the elicitation of antibody responses in this system. Studies of the human system have shown that IFN-α/β, together with IL-6, affects B cells, leading to their differentiation into antibody-producing plasma cells (32). The importance of IFN-α/β signaling directly to B and T cells was recently investigated in a series of elegant experiments in wt mice using adoptive transfer of T or B cells selectively defective for IFN-α/β signaling (39). These studies established that the direct action of IFN-α/β on both T and B cells is important during the generation of an immune response. Interestingly, it has been shown that SFV infection activates mouse lymphocytes in a non-antigen-specific, IFN-α/β-dependent manner, resulting in upregulation of CD69 and CD86 on both T and B cells (3). This was suggested to bring the cells into a semiactivated state, which may lower the threshold for antigen-mediated activation and thereby facilitate the generation of adaptive immune responses. Thus, IFN-α/β likely modulate the immune responses at several levels, including direct effects on T and B cells.
In our study, we found that the induction of antibody responses against the rSFV-encoded antigens was not dependent on IFN-α/β signaling. This demonstrates that the mechanisms by which humoral immune responses are induced in this coimmunization system can be divided into an IFN-α/β-dependent and an IFN-α/β-independent component. While virus-induced IFN-α/β provides a critical adjuvant signal for the coimmunized protein antigens, virus-encoded antigens benefit from additional, IFN-α/β-independent adjuvant signals. One explanation is that viral dsRNA replication intermediates released from infected cells can stimulate DCs. However, in our in vitro system, rSFV-infected apoptotic MEFs induced maturation of DCs in an IFN-α/β-dependent manner. It is possible that the concentration of dsRNA was not sufficiently high in our in vitro coculture system, since only high concentrations of pI-C induced IFN-α/β-independent maturation of DCs (Fig. 7C). Alternatively, viral or cellular components other than dsRNA that are not appropriately modeled in this in vitro system could be responsible for the IFN-α/β-independent adjuvant effect. It would be interesting to determine if the IFN-α/β-independent adjuvant effect of rSFV requires de novo synthesis of antigen in the infected cell or if a mere association of the antigen with the infected cell is sufficient.
This study is an important step forward in understanding the pathways mediating the adjuvant effects of viral vaccines. The recombinant virus system used here allowed us to independently analyze antibody responses against both coadministered protein and virus-encoded antigens. We demonstrate that virus-induced IFN-α/β can act as a potent adjuvant, suggesting that small-molecule compounds that induce IFN-α/β or recombinant IFN-α/β itself could be developed as effective adjuvants. The ability of viruses to stimulate humoral responses against coimmunized protein subunit antigens could be exploited in the development of effective vaccine regimens to stimulate both potent cellular and humoral immune responses. This may be relevant for development of vaccines against pathogens where both arms of the adaptive immune response need to be mobilized.
Acknowledgments
We thank the personnel at the MTC and the SMI animal facilities for expert technical assistance and Yuxing Li and Richard Wyatt at the Vaccine Research Center at the National Institutes of Health for the generous gift of purified HIV-1 envelope glycoproteins. We also acknowledge Gerry McInerney and Iyadh Douagi for critically reading the manuscript.
This study was supported by grants from the Swedish Research Council and the European Union 5th Framework Programme to P.L. and from the Swedish International Development Cooperation Agency to G.B.K.H.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Alsharifi, M., M. Lobigs, M. Regner, E. Lee, A. Koskinen, and A. Mullbacher. 2005. Type I interferons trigger systemic, partial lymphocyte activation in response to viral infection. J. Immunol. 175:4635-4640. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Berglund, P., M. N. Fleeton, C. Smerdou, and P. Liljeström. 1999. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine 17:497-507. [DOI] [PubMed] [Google Scholar]
- 6.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudet, F., M. Chevalier, T. M. Jourdier, J. Tartaglia, and C. Moste. 2001. Modulation of the antibody response to the HIV envelope subunit by co-administration of infectious or heat-inactivated canarypoxvirus (ALVAC) preparations. Vaccine 19:4267-4275. [DOI] [PubMed] [Google Scholar]
- 8.Bowie, A. G., and I. R. Haga. 2005. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42:859-867. [DOI] [PubMed] [Google Scholar]
- 9.Brimnes, M. K., L. Bonifaz, R. M. Steinman, and T. M. Moran. 2003. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J. Exp. Med. 198:133-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, M., C. Barnfield, T. I. Naslund, M. N. Fleeton, and P. Liljeström. 2005. MyD88 expression is required for efficient cross-presentation of viral antigens from infected cells. J. Virol. 79:2964-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colby, C., and M. J. Morgan. 1971. Interferon induction and action. Annu. Rev. Microbiol. 25:333-360. [DOI] [PubMed] [Google Scholar]
- 12.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta, S. K., V. Redecke, K. R. Prilliman, K. Takabayashi, M. Corr, T. Tallant, J. DiDonato, R. Dziarski, S. Akira, S. P. Schoenberger, and E. Raz. 2003. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J. Immunol. 170:4102-4110. [DOI] [PubMed] [Google Scholar]
- 14.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Wit, M. C., M. C. Horzinek, B. L. Haagmans, and V. E. Schijns. 2004. Host-dependent type 1 cytokine responses driven by inactivated viruses may fail to default in the absence of IL-12 or IFN-alpha/beta. J. Gen. Virol. 85:795-803. [DOI] [PubMed] [Google Scholar]
- 16.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and E. S. C. Reis. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 17.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 18.Durand, V., S. Y. Wong, D. F. Tough, and A. Le Bon. 2004. Shaping of adaptive immune responses to soluble proteins by TLR agonists: a role for IFN-alpha/beta. Immunol. Cell Biol. 82:596-602. [DOI] [PubMed] [Google Scholar]
- 19.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 20.Finkelman, F. D., A. Svetic, I. Gresser, C. Snapper, J. Holmes, P. P. Trotta, I. M. Katona, and W. C. Gause. 1991. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J. Exp. Med. 174:1179-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleeton, M. N., B. J. Sheahan, E. A. Gould, G. J. Atkins, and P. Liljeström. 1999. Recombinant Semliki Forest virus particles encoding the prME or NS1 proteins of louping ill virus protect mice from lethal challenge. J. Gen. Virol. 80:1189-1198. [DOI] [PubMed] [Google Scholar]
- 22.Forsell, M. N., Y. Li, M. Sundback, K. Svehla, P. Liljeström, J. R. Mascola, R. Wyatt, and G. B. Karlsson Hedestam. 2005. Biochemical and immunogenic characterization of soluble human immunodeficiency virus type 1 envelope glycoprotein trimers expressed by Semliki Forest virus. J. Virol. 79:10902-10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X. L. Xu, G. Trinchieri, A. O'Garra, and Y. J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundner, C., Y. Li, M. Louder, J. Mascola, X. Yang, J. Sodroski, and R. Wyatt. 2005. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology 331:33-46. [DOI] [PubMed] [Google Scholar]
- 25.Grundner, C., M. Pancera, J. M. Kang, M. Koch, J. Sodroski, and R. Wyatt. 2004. Factors limiting the immunogenicity of HIV-1 gp120 envelope glycoproteins. Virology 330:233-248. [DOI] [PubMed] [Google Scholar]
- 26.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 27.Hel, Z., J. Nacsa, W. P. Tsai, A. Thornton, L. Giuliani, J. Tartaglia, and G. Franchini. 2002. Equivalent immunogenicity of the highly attenuated poxvirus-based ALVAC-SIV and NYVAC-SIV vaccine candidates in SIVmac251-infected macaques. Virology 304:125-134. [DOI] [PubMed] [Google Scholar]
- 28.Hidmark, A. S., G. M. McInerney, E. K. Nordstrom, I. Douagi, K. M. Werner, P. Liljeström, and G. B. Hedestam. 2005. Early alpha/beta interferon production by myeloid dendritic cells in response to UV-inactivated virus requires viral entry and interferon regulatory factor 3 but not MyD88. J. Virol. 79:10376-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 31.Hutchings, C. L., S. C. Gilbert, A. V. Hill, and A. C. Moore. 2005. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J. Immunol. 175:599-606. [DOI] [PubMed] [Google Scholar]
- 32.Jego, G., A. K. Palucka, J. P. Blanck, C. Chalouni, V. Pascual, and J. Banchereau. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19:225-234. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson, G. B., and P. Liljeström. 2003. Live viral vectors: Semliki Forest virus. Methods Mol. Med. 87:69-82. [DOI] [PubMed] [Google Scholar]
- 34.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 35.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 36.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 37.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 38.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 39.Le Bon, A., C. Thompson, E. Kamphuis, V. Durand, C. Rossmann, U. Kalinke, and D. F. Tough. 2006. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. 176:2074-2078. [DOI] [PubMed] [Google Scholar]
- 40.Lee, B. O., J. Rangel-Moreno, J. E. Moyron-Quiroz, L. Hartson, M. Makris, F. Sprague, F. E. Lund, and T. D. Randall. 2005. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 175:5827-5838. [DOI] [PubMed] [Google Scholar]
- 41.Liljeström, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 9:1356-1361. [DOI] [PubMed] [Google Scholar]
- 42.Liljeström, P., S. Lusa, D. Huylebroeck, and H. Garoff. 1991. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J. Virol. 65:4107-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 44.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McInerney, G. M., N. L. Kedersha, R. J. Kaufman, P. Anderson, and P. Liljeström. 2005. Importance of eIF2{alpha} phosphorylation and stress granule assembly in alphavirus translation regulation. Mol. Biol. Cell 16:3753-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melchjorsen, J., S. B. Jensen, L. Malmgaard, S. B. Rasmussen, F. Weber, A. G. Bowie, S. Matikainen, and S. R. Paludan. 2005. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J. Virol. 79:12944-12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 49.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 50.Oxenius, A., M. M. Martinic, H. Hengartner, and P. Klenerman. 1999. CpG-containing oligonucleotides are efficient adjuvants for induction of protective antiviral immune responses with T-cell peptide vaccines. J. Virol. 73:4120-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364-368. [DOI] [PubMed] [Google Scholar]
- 52.Phumiamorn, S., H. Sato, T. Kamiyama, M. Kurokawa, and K. Shiraki. 2003. Induction of humoral and cell-mediated immunity to hepatitis B surface antigen by a novel adjuvant activity of Oka varicella vaccine. J. Gen. Virol. 84:287-291. [DOI] [PubMed] [Google Scholar]
- 53.Proietti, E., L. Bracci, S. Puzelli, T. Di Pucchio, P. Sestili, E. De Vincenzi, M. Venditti, I. Capone, I. Seif, E. De Maeyer, D. Tough, I. Donatelli, and F. Belardelli. 2002. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169:375-383. [DOI] [PubMed] [Google Scholar]
- 54.Rahemtulla, A., W. P. Fung-Leung, M. W. Schilham, T. M. Kundig, S. R. Sambhara, A. Narendran, A. Arabian, A. Wakeham, C. J. Paige, R. M. Zinkernagel, et al. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353:180-184. [DOI] [PubMed] [Google Scholar]
- 55.Robinson, H. L., and R. R. Amara. 2005. T cell vaccines for microbial infections. Nat. Med. 11:S25-S32. [DOI] [PubMed] [Google Scholar]
- 56.Santra, S., M. S. Seaman, L. Xu, D. H. Barouch, C. I. Lord, M. A. Lifton, D. A. Gorgone, K. R. Beaudry, K. Svehla, B. Welcher, B. K. Chakrabarti, Y. Huang, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 79:6516-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroder, M., and A. G. Bowie. 2005. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 26:462-468. [DOI] [PubMed] [Google Scholar]
- 58.Schulz, O., S. S. Diebold, M. Chen, T. I. Naslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljeström, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887-892. [DOI] [PubMed] [Google Scholar]
- 59.Seong, S. Y., and P. Matzinger. 2004. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4:469-478. [DOI] [PubMed] [Google Scholar]
- 60.Shi, Y., J. E. Evans, and K. L. Rock. 2003. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516-521. [DOI] [PubMed] [Google Scholar]
- 61.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 62.Smerdou, C., and P. Liljeström. 1999. Two-helper RNA system for production of recombinant Semliki Forest virus particles. J. Virol. 73:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spellberg, B., and J. E. Edwards, Jr. 2001. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 64.Sundback, M., I. Douagi, C. Dayaraj, M. N. Forsell, E. K. Nordstrom, G. M. McInerney, K. Spangberg, L. Tjader, E. Bonin, M. Sundstrom, P. Liljeström, and G. B. Karlsson Hedestam. 2005. Efficient expansion of HIV-1-specific T cell responses by homologous immunization with recombinant Semliki Forest virus particles. Virology 341:190-202. [DOI] [PubMed] [Google Scholar]
- 65.Szomolanyi-Tsuda, E., and R. M. Welsh. 1996. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J Exp. Med. 183:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tannis, L. L., A. Gauthier, C. Evelegh, R. Parsons, D. Nyholt, A. Khromykh, and J. L. Bramson. 2005. Semliki Forest virus and Kunjin virus RNA replicons elicit comparable cellular immunity but distinct humoral immunity. Vaccine 23:4189-4194. [DOI] [PubMed] [Google Scholar]
- 68.Thompson, J. M., A. C. Whitmore, J. L. Konopka, M. L. Collier, E. M. Richmond, N. L. Davis, H. F. Staats, and R. E. Johnston. 2006. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc. Natl. Acad. Sci. USA 103:3722-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wille-Reece, U., B. J. Flynn, K. Lore, R. A. Koup, R. M. Kedl, J. J. Mattapallil, W. R. Weiss, M. Roederer, and R. A. Seder. 2005. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. USA. [DOI] [PMC free article] [PubMed]
- 70.Wille-Reece, U., C. Y. Wu, B. J. Flynn, R. M. Kedl, and R. A. Seder. 2005. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J. Immunol. 174:7676-7683. [DOI] [PubMed] [Google Scholar]
- 71.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 72.Zhou, S., E. A. Kurt-Jones, L. Mandell, A. Cerny, M. Chan, D. T. Golenbock, and R. W. Finberg. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 35:822-830. [DOI] [PubMed] [Google Scholar]