Abstract
Vaccinia virus, a poxvirus, produces structurally distinct forms of virions for which the immediate events following cell entry are ill-defined. We provide evidence that intracellular mature virus (IMV) enters both permissive and nonpermissive T-cell lines and that introduction of CCR5 into nonpermissive mouse fibroblasts or human primary T cells renders the cells permissive for vaccinia replication. Notably, T cells expressing CCR5 in which tyrosine 339 in the intracellular region is replaced by phenylalanine no longer support virus replication or virus-inducible activation of specific host cell signaling effectors IRS-2, Grb2, and Erk1/2. We show that following IMV entry into the cell, the intact but not the tyrosine-deficient CCR5 is rapidly internalized and colocalizes with virus. This colocalization precedes virus-inducible signaling and replication.
Vaccinia virus (VACV) is the most intensively studied member of the poxvirus family, because of its role as the vaccine agent responsible for the eradication of smallpox. Like other poxviruses, VACV is a large DNA virus that has an autonomous transcription system and replicates its DNA in the host cell cytoplasm, largely independently of the nucleus, although evidence suggests that the virus utilizes host cell proteins for transcription of intermediate and late genes (7, 11, 27). VACV produces several distinct forms of viral particles, which include the intracellular mature virus (IMV), the intracellular enveloped virus, the cell-associated enveloped virus, and the extracellular enveloped virus (EEV) (6, 31, 42). The VACV replication cycle takes place entirely in the host cell cytoplasm and comprises a sequence of discrete steps that begin with the entry of the viral particles and culminate with morphogenesis, release, and egress. Although identification of all components of the viral entry/fusion complex which contribute to VACV binding and entry into cells remains incomplete and is complicated by the existence of low-affinity cell surface interactions with glycosaminoglycans that are cell type specific (8, 23, 24, 44), evidence for conserved membrane proteins, A28 and H2, determining poxvirus cell penetration does suggest a mechanism for cell entry, at least in cell lines (40). Until recently, no cell surface molecules on primary cells or cell lines have been demonstrated to be essential for poxvirus infection. However, new evidence for the inducible expression of a receptor on primary T cells determining VACV binding and entry has been provided (7). Notably, activated T cells, but not resting T cells, express this uncharacterized virus receptor.
It is becoming increasingly apparent that chemokines, originally viewed as inflammatory mediators, interact with their cognate serpentine G-protein-coupled receptors to activate many diverse developmental and immunological operations, including viral clearance. Not surprisingly, therefore, viruses have evolved immune evasion strategies that subvert the chemokine system. Human immunodeficiency virus and simian immunodeficiency virus use CCR5 and CXCR4 as coreceptors to mediate their entry (41), while respiratory syncytial virus employs the fractalkine receptor (CX3CR1) to initiate infection (43). Poxviruses employ strategies to modulate chemokine activity, including virus-encoded chemokine-binding proteins, receptor homologs, and ligand mimics (18, 28, 39). Interestingly, the potential for the involvement of certain chemokine receptors in poxviral infection was suggested in studies utilizing the rabbit poxvirus, myxoma virus. Specifically, CCR5 was implicated in mediating cell target susceptibility to infection in BGMK cells (19), later shown to correlate with intracellular signaling (17). Myxoma virus infection of mouse fibroblasts elicits downstream signaling events that are pertussis toxin insensitive and that may utilize CCR5, starting with tyrosine phosphorylation of the receptor itself and including activation of the Jak-Stat pathway and the IRS proteins, as well as the recruitment of p56lck (25). Certainly, there is considerable evidence for poxvirus activation of signaling effectors required to support viral replication (1, 12, 17, 38). The mechanism(s) whereby poxviruses activate these signaling effectors is unknown, and the notion that intracellular virus or viral proteins may activate transmembrane receptors to initiate these signaling events prompted the studies described herein. Accordingly, we undertook to investigate whether modulating CCR5 expression and activation influences the permissive phenotype in the context of infecting VACV IMV particles.
MATERIALS AND METHODS
Cells and virus.
Murine NIH 3T3.CD4.CCR5 and NIH 3T3.CD4.neo fibroblasts, generated from NIH 3T3 parental cells and distinguished by the unique presence of a neomycin resistance cassette in the NIH 3T3.CD4.neo variant, were obtained from D. Littman (New York University). Phoenix cells were a gift from Josef Penninger (Institute of Molecular Biotechnology, Austrian Academy of Sciences, Vienna, Austria). All cells were maintained in Dulbecco's modified Eagle's medium (Gibco-BRL), supplemented with 10% fetal calf serum (FCS) (Gibco-BRL), 100 U/ml penicillin (Gibco-BRL), and 100 mg/ml streptomycin (Gibco-BRL), and grown at 37°C in an atmosphere of 5% CO2.
T cells were purified from peripheral blood (healthy adult donors) by Ficoll-Hypaque (Amersham Biosciences) density gradient separation, the leukocytes collected at the interphase were incubated with an anti-CD32 blocking antibody, and then T cells were purified by negative selection using a StemCell Technologies magnetic bead separation system and a cocktail of antibodies directed against CD19, CD56, and CD66b, per the manufacturer's instructions. Purified T cells were cultured in Dulbecco's modified Eagle's medium with 10% FCS, 100 U/ml penicillin, and 100 mg/ml streptomycin overnight.
Human PM1 T cells were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program and were maintained in RPMI 1640 (Gibco-BRL) with 10% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 25 μg/ml Plasmocin (InvivoGen).
VACV65 (modified Western reserve strain) containing an Escherichia coli β-galactosidase cassette driven by a late viral promoter was a gift from Grant McFadden (Robarts Research Institute, University of Western Ontario). Purified IMV was harvested from infected green monkey kidney cells (BGMK cells) that were subjected to two freeze-thaw cycles, sonication, and Dounce homogenization. Supernatants containing crude viral preparations were collected by centrifugation of the cell homogenates at 10,000 rpm for 1 h at 4°C. Since the EEV form accounts for approximately 1% of progeny, no attempt was made to fractionate EEV and IMV. (Mock infections were conducted using BGMK culture supernatants that were subjected to the same process, but the BGMK cells were not infected with VACV). Viral titers were determined by duplicate plaque assays. Specifically, 1:10 serial dilutions of stock virus were adsorbed onto BGMK cells for 1 h, and the cultures were incubated for 48 h and then stained for X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to reveal plaques.
A5L-EGFP VACV (where EGFP is enhanced green fluorescent protein) was a gift from Geoffrey Smith (Department of Virology, Faculty of Medicine, Imperial College of London) (9). A5L-EGFP VACV was grown in HeLa cells and purified by sedimentation through a 36% sucrose cushion (20), and the number of virus particles was determined from the optical density at 260 nm (1 U = 1.2 × 1010 particles) (30). Using this value, the particle:PFU ratio of A5L-EGFP VACV was estimated to be 50:1.
Measurements of viral infectivity.
The extents of VACV infection in different cell types were determined by detecting vaccinial-associated β-galactosidase activity by means of X-Gal staining as described previously (22). Images were captured with a Leica DMIL inverted microscope (Leica, Willowdale, Canada) equipped with a Zeiss digital AxioCam camera using Axio Vision 2.05 software. Acquired images were processed with Adobe Photoshop version 7 (Adobe Systems, Inc., San Jose, CA).
In all of our studies, β-galactosidase activity was directly proportional to the extent of viral replication, as determined from replicate plaque assays undertaken to determine the titer of the β-galactosidase cassette-containing vaccinia strain by using an X-Gal colorimetric staining assay.
Additionally, to examine the effects of the pharmacological inhibitors herbimycin A (Calbiochem), pertussis toxin (Calbiochem), TAK 779 (Serono Pharmaceuticals), and AG490 (Calbiochem) on the extent of viral infection, cells were pretreated with various doses of theses inhibitors and then infected with VACV as described above. The effects of these inhibitors on viral replication were measured using a β-galactosidase colorimetric assay.
Analysis of CCR5 surface expression by flow cytometry.
Cell surface expression of human CCR5 was determined by flow cytometry using anti-human CCR5 (BD Pharmingen) and fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG) (eBioscience). Staining was conducted according to the manufacturer's protocol. Cells were gated based on forward and side scatter. Flow cytometric data were obtained using FACSCalibur (BD Pharmingen) and analyzed using CELLQuest software. Notably, the anti-human CCR5 antibody recognizes ectopically expressed intact CCR5, CCR5 in which tyrosine 339 is replaced by phenylalanine (the CCR5.Y339F mutant), the CCR5.Y307F mutant, and CCR5 in which all three intracellular tyrosine residues (127, 307, and 339) are replaced by phenylalanine (the CCR5.YΔ3F mutant).
Cell lysis and immunoblotting.
To prepare whole-cell lysates, 107 cells were infected with the equivalent of 108 infectious units (multiplicity of infection [MOI] of 10) of VACV for the indicated times. Cells were washed in cold phosphate-buffered saline and lysed as described previously (25). Immunoprecipitations and immunoblotting using enhanced chemiluminescence were performed as described previously (25), using the following antibodies: 4G10 (UBI), anti-IRS-2 (Upstate Biotech), anti-Erk1/2 (Santa Cruz), anti-phospho-Erk1/2 (Santa Cruz), anti-Grb2 (Santa Cruz), and anti-Jak2 (Santa Cruz).
siRNA transfection.
Small interfering RNA (siRNA) duplexes were synthesized and purified using products from QIAGEN, Inc. The CCR5 target sequences were siRNA1 (5′-CUCUUGACAGGGCUCUAUU-3′) and siRNA2 (5′-AAGCCAGGACGGUCACCUU-3′). NIH 3T3.CD4.CCR5 cells (2 × 106) were transfected with a mixture of 10 μg siRNA1 and 5 μg siRNA2 or a control, scrambled siRNA by electroporation (BTX T820 electroporator). The settings were 475 V, 1 millisecond, and four pulses. At 24 and 48 h after transfection, cell surface CCR5 expression was determined by flow cytometry using the anti-human CCR5 antibody (BD Pharmingen).
CCR5 mutagenesis.
pEF-BOS-CCR5, carrying the human CCR5 gene, was obtained from Martin Opperman (University of Gottingen, Germany). Human CCR5 was subcloned into pUMFG retroviral vector (a gift from Jeffery Medin, Division of Experimental Therapeutics, Toronto General Research Institute). CCR5 mutagenesis was performed on the pEF-BOS-CCR5 and pUMFG-CCR5 templates. Initially, single Y127F, Y307F, and Y339F mutations were introduced using a QuickChange site-directed mutagenesis kit (Stratagene) and the appropriate mutagenic primers: for the Y127F mutation, (forward) CTC CTG ACA ATC GAT AGG TTC CTG GCT GTC GTC C and (reverse) GGA CGA CAG CCA GGA ACC TAT CGA TTG TCA GGA G; for the Y307F mutation, (forward) CGG GGA GAA GTT CAG AAA CTT CCT CTT AGT CTT CTT CC and (reverse) GGA AGA AGA CTA AGA GGA AGT TTC TGA ACT TCT CCC CG; and for the Y339F mutation, (forward) GCG AGC AAG CTC AGT TTT CAC CCG ATC CAC TGG GG and (reverse) CCC CAG TGG ATC GGG TGA AAA CTG AGC TTG CTC GC.
The CCR5.YΔ3F mutant was generated in three subsequent rounds of mutagenesis, using the relevant primer sets and the following cycling parameters: denaturation at 95°C for 30 s, followed by 16 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 1 min, and elongation at 68°C for 14 min, and a final extension at 68°C for 7 min. After each round of mutagenesis, the location of the appropriate nucleotide changes and the lack of additional mutations were confirmed by sequencing (ACGT Corporation).
Introduction of CCR5 and CCR5.Y307F, CCR5.Y339F, and CCR5.YΔ3F mutants into cells.
NIH 3T3.CD4.neo cells were transfected with 5 μg of either intact or mutant CCR5 cDNA by use of Fugene 6 transfection reagent, according to the manufacturer's protocol (Roche). T cells purified from human peripheral blood were transfected with 30 μg of either intact or mutant CCR5 cDNA by electroporation (BTX T820 electroporator at 600 V, 99 microseconds, and 10 pulses). At 3 h posttransfection, cell surface CCR5 expression was determined by flow cytometry using the anti-human CCR5 antibody (BD Pharmingen). The amphotropic packaging cell line Phoenix was transfected by the calcium phosphate-chloroquine method. At 48 h posttransfection, the viral supernatant was collected and used for transfection of PM1 cells as described previously (17a). Positive transfectants were sorted with a fluorescence-activated cell sorter by use of anti-human CCR5 antibody.
Immunohistochemical analysis and confocal microscopy.
PM1, PM1.CCR5, and PM1.CCR5.Y339F cells were infected with A5L-EGFP VACV and stained for CCR5 by immunohistochemistry as described previously (49). Specifically, CCR5 was visualized using an anti-mouse Cy3 antibody (Amersham Biosciences, Cardiff, United Kingdom) directed against the mouse anti-human CCR5 antibody. Images were collected using an upright Leica SP2 confocal laser-scanning microscope (Leica Microsystems Heidelberg GmbH, Mannheim, Germany), a 100× oil immersion lens (1.4 numerical aperture), and a ×4 digital zoom. Laser excitations were 488 nm (Ar/Kr) and 543 nm (He/Ne), attenuated to 10% and 50%, respectively, by way of an acoustic-optical transmission filter. Sequential scan mode was used to eliminate cross talk of detected signals, which were filtered between 500 to 530 nm and 560 to 660 nm. Image resolution was 512 dpi by 512 dpi (12 bit), and line averaging (4×) was used. Optical sections were collected at 0.5-μm intervals through the entire cell.
Reverse-transcription PCR.
Cells (107) were infected with VACV at an MOI of 10 and then lysed at various times postinfection by using QIAGEN QIA-shredder columns, and RNA was isolated using a QIAGEN RNeasy minikit (per the manufacturer's instructions). cDNA was synthesized by annealing 2 μg of RNA at 65°C for 5 min in the presence of oligo(dT), and reverse transcription was performed at 25°C for 10 min, 37°C for 50 min, and 70°C for 15 min.
PCR was performed using the following primer sets: 005R primers, (forward) 5′-TTA TCT GAT GTT GTT GTT GTT CGC-3′ and (reverse) 5′-GTT TAG TTC GTC GAG TGA AC-3′; 078R primers, (forward) 5′-CGA TAA ACT GCG CCA AAT TG-3′ and (reverse) 5′-CAT AAT AGC CAA ATG CTG ATG-3′; and 047R primers, (forward) 5′-ATT CTC ATT TTG CAT CTG CTC-3′ and (reverse) 5′-CTA GAA GCT ACA TTA TCG CG-3′.
RESULTS
Ectopic expression of CCR5 confers a permissive phenotype for VACV replication.
We have previously shown that NIH 3T3.CD4.CCR5 cells are permissive for myxoma virus infection (25). Evidence that the serine-threonine kinase PAK-1 plays an important role in myxoma virus infection of 3T3 cells, specifically, distinguishing permissive NIH 3T3.CD4.CCR5 cells from nonpermissive NIH 3T3.CD4.neo cells, has been provided (17). In a first series of experiments, we infected NIH 3T3.CD4.CCR5 cells with a different poxvirus, VACV, which expresses the E. coli β-galactosidase gene under the control of a late viral promoter and identified viral infection (Fig. 1). To investigate the contribution of cell surface expression of CCR5 to viral infectivity, we performed studies in which the expression of CCR5 in NIH 3T3.CD4.CCR5 cells was blocked by use of CCR5-specific siRNA duplexes. Transfection of CCR5-specific RNA duplexes, but not scrambled siRNA, in NIH 3T3.CD4.CCR5 cells resulted in significant downregulation of cell surface-expressed CCR5 at 48 h (Fig. 1a). Notably, cells in which CCR5 expression was knocked down showed a dramatic decrease in viral infection (Fig. 1b and c). In parallel, we introduced CCR5 into NIH 3T3.CD4.neo cells by transfection to investigate the contribution of CCR5 expression to their permissive phenotype. NIH 3T3.CD4.neo cells are not permissive for VACV infection. Whereas transfection of vector alone had no effect, cell surface expression of CCR5 in the NIH 3T3.CD4.neo cells rendered them permissive for VACV infection (Fig. 2).
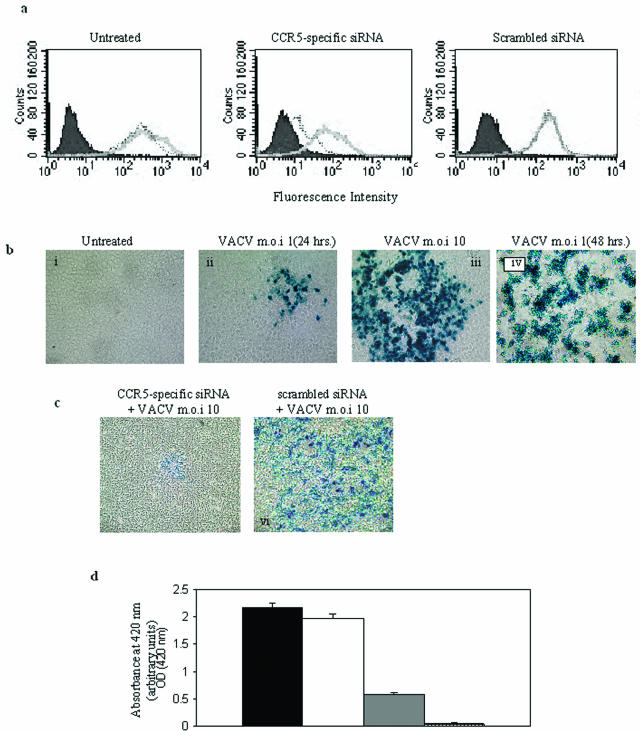
FIG. 1.
Knockdown of CCR5 in NIH 3T3.CD4.CCR5 cells reduces vaccinia virus infection. (a) NIH 3T3.CD4.CCR5 cells were left untreated or transfected with either CCR5-specific siRNA or the control, scrambled siRNA. At 24 (gray solid line) and 48 (dotted line) h posttransfection, cell surface CCR5 expression was determined by flow cytometric analysis using anti-CCR5-specific antibodies. Filled (negative) cytograms correspond to IgG reagents alone. (b) NIH 3T3.CD4.CCR5 cells were either left untreated (i) or infected with VACV at an MOI of 1 (ii and iv) or 10 (iii). Twenty-four (i, ii, and iii) or 48 (iv) h postinfection, the monolayers were fixed and stained with X-Gal to determine LacZ expression. (c) NIH 3T3.CD4.CCR5 cells were transfected with either CCR5-specific siRNA (v) or the control, scrambled siRNA (vi). Forty-eight hours posttransfection, monolayer cultures were infected with VACV at an MOI of 10. Twenty-four hours postinfection, the monolayers were fixed and stained with X-Gal to determine LacZ expression. (d) The NIH 3T3.CD4.CCR5 infected cells shown in panel B were also analyzed for β-galactosidase activity of cell lysates by means of a colorimetric assay (see Materials and Methods). Black column, NIH 3T3.CD4.CCR5 cells infected with VACV; white column, NIH 3T3.CD4.CCR5 cells transfected with the control, scrambled siRNA, and then VACV infected; gray column, NIH 3T3.CD4.CCR5 cells transfected with CCR5-specific siRNA and then VACV infected; striped column, uninfected NIH 3T3.CD4.CCR5 cells. The means ± standard errors from quadruplicate assays, representative of two independent experiments, are shown.
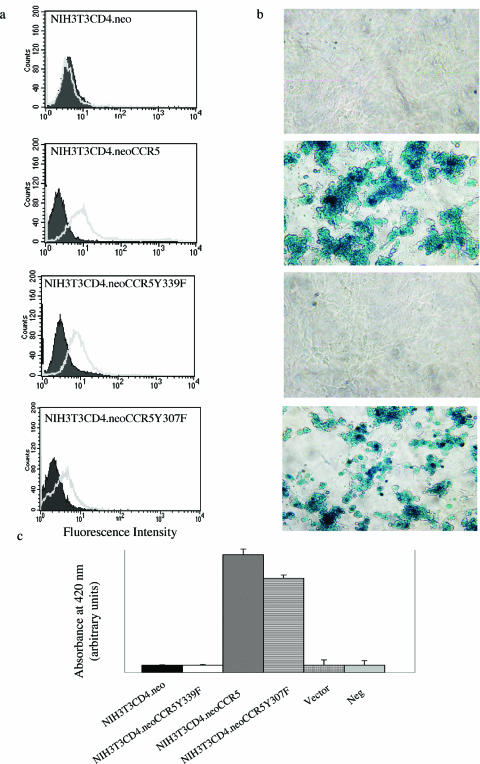
FIG. 2.
Introduction of CCR5 into NIH 3T3.CD4.neo cells renders cells permissive for vaccinia virus infection. CCR5 cDNA, CCR5.Y307F mutant cDNA, CCR5.Y339F mutant cDNA, or vector alone was introduced by transfection into NIH 3T3.CD4.neo cells. (a) Cell surface CCR5 or mutant CCR5 expression was determined by flow cytometry using anti-CCR5-specific antibodies that do not distinguish between intact or mutant CCR5 (gray solid line). Filled (negative) cytograms correspond to IgG reagents alone. (b) Cells were infected with VACV at an MOI of 10. Cells were fixed, and X-Gal staining was performed. (c) LacZ activity was measured 16 h postinfection by using a β-galactosidase colorimetric assay. Neg, negative. The means ± standard errors from quadruplicate assays are shown, representative of two independent experiments.
In an earlier study, we provided evidence for myxoma virus-inducible CCR5 tyrosine phosphorylation and activation of tyrosine kinase signal transduction associated with a permissive phenotype (25). Tyr (Y)-127 lies in the second intracellular loop of the receptor in the DRY motif, highly conserved among CC chemokine receptors and implicated in mediating chemokine receptor signal transduction. For CCR2, the homologous tyrosine residue of the DRY motif (Y139) is the primary target for Jak2-mediated phosphorylation, and in the context of the CCR2b/CCL2 interaction, Jak2 is required for G-protein coupling. Mutating Y139 of CCR2b to a phenylalanine (F) results in an inactive receptor (the CCR2b.Y139F mutant), unable to recruit Jak2 and incapable of G-protein activation (32). This mutant receptor confers a loss-of-function dominant-negative phenotype and dimerizes to form nonfunctional complexes with CCR2b partners containing the functional tyrosine (34). In the context of CCR5, mutation of the DRY motif was shown to result in a nonfunctional receptor with somewhat reduced surface expression, incapable of Gα-subunit binding and signaling (15, 45). The other two intracellular tyrosine residues of CCR5, Y307 and Y339, reside in the C-terminal tail of the receptor. While Y307 is conserved among CC chemokine receptors, Y339 is unique to CCR5 and CCR4.
In the context of VACV infection, NIH 3T3.CD4.neo cells expressing the CCR5.YΔ3F mutant or the CCR5.Y339F mutant are nonpermissive for viral infection, whereas cells expressing the CCR5.Y307F mutant are permissive (Fig. 2). Notably, we observe that NIH 3T3.CD4.CCR5 cells that overexpress the CCR5.Y339F or CCR5.YΔ3F mutant do not support VACV infection, whereas cells coexpressing the CCR5.Y307F mutant remain permissive for virus infection (data not shown). Viewed altogether, these data suggest that in cells for which CCR5 confers a permissive phenotype for infection, tyrosine 339 is critical.
Different leukocyte populations are variably permissive for VACV infection, with T lymphocytes exhibiting poor infectivity (36). Accordingly, we examined the effect of ectopic CCR5 expression on VACV replication in primary T cells derived from human peripheral blood lymphocytes and in a human T-cell line, PM1. In contrast to nonpermissive naïve T cells and parental PM1 cells, which both lack CCR5 expression, ectopic expression of intact CCR5 confers permissiveness for VACV infection, whereas ectopic expression of the CCR5.YΔ3F or CCR5.Y339F mutant fails to affect permissiveness (Fig. 3 and 4).
FIG. 3.
Introduction of CCR5 but not the CCR5.Y339 mutant into primary (1°) human T cells renders cells permissive for vaccinia virus infection. cDNA for intact CCR5, the CCR5.Y339F mutant, or vector alone was introduced by electroporation into primary human T cells. (a) Cell surface CCR5 or mutant CCR5 expression was determined by flow cytometry. Gray solid lines represent cell surface CCR5/CCR5.Y339F mutant expression, while filled (negative) cytograms correspond to IgG reagents alone. (b) Cells were infected with VACV at an MOI of 10, and then after 16 h the cells were lysed and centrifuged at 1,500 rpm for 5 min and the supernatants were transferred onto BGMK cell monolayers. Sixteen hours later, the monolayers were fixed and X-Gal staining was performed. (c) Cells were infected with VACV at the MOIs indicated. After 16 h, cells were lysed and LacZ activity was measured using a β-galactosidase colorimetric assay. White columns, primary T cells; black columns, T cells expressing intact CCR5; striped columns, T cells expressing the CCR5.Y339F mutant; gray columns, T cells transfected with pEF.BOS vector alone. The data are representative of three independent experiments.
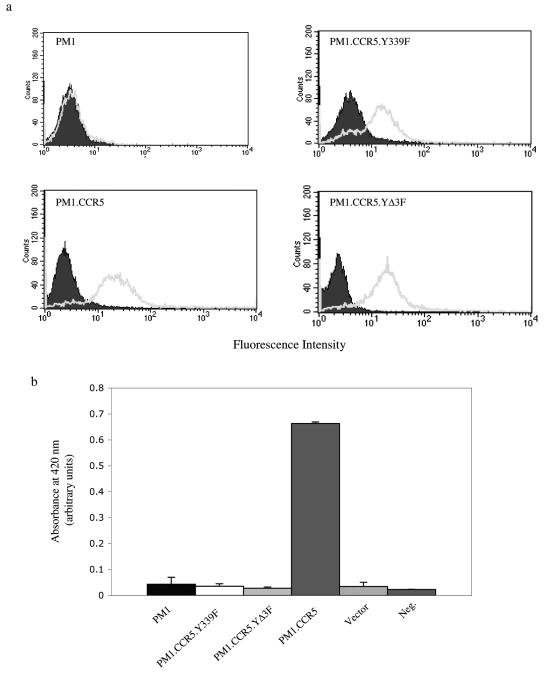
FIG. 4.
Introduction of CCR5 but not the CCR5.Y339 or CCR5.YΔ3F mutant into PM1 T cells renders cells permissive for vaccinia virus infection. cDNA for intact CCR5, the CCR5.Y339F mutant, the CCR5.YΔ3F mutant, or vector alone was introduced by retroviral transduction into a PM1 human T-cell line. (a) Cell surface CCR5 or mutant CCR5 expression was determined by flow cytometry. Gray solid lines represent cell surface CCR5/CCR5.Y339F mutant/CCR5.YΔ3F mutant expression, while filled (negative) cytograms correspond to IgG reagents alone. (b) Cells were infected with VACV at an MOI of 10, and then after 16 h the cells were lysed and LacZ activity was measured using a β-galactosidase colorimetric assay. Neg., negative. The means ± standard errors from quadruplicate assays are shown, representative of two independent experiments.
To demonstrate the restricted replication of VACV in cells expressing the CCR5.Y339F mutant, we performed a one-step growth curve, specifically, transferring lysates from cells inoculated with VACV to BSC-1 cells for plaque evaluation. The data in Fig. 5 indicate that VACV replication is restricted in cells expressing the CCR5.Y339F mutant but not in cells expressing CCR5, consistent with the infection data shown in Fig. 4.
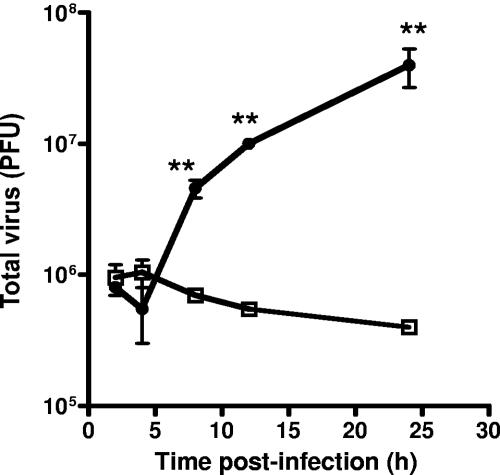
FIG. 5.
Replication of VACV is restricted in PM1 cells expressing the CCR5.Y339F mutant but not CCR5. PM1.CCR5 or PM1.CCR5.Y339F cells were infected at an MOI of 10 with VACV. At the indicated times, cells were harvested and lysed by three successive freeze-thaw cycles. Viral titers were determined by plaque assay with BSC-1 cells. □, PM1.CCR5.Y339F; •, PM1.CCR5. Values are the means ± standard errors. The data are representative of two independent experiments. **, P < 0.05.
Absence of VACV late gene transcription in nonpermissive cells.
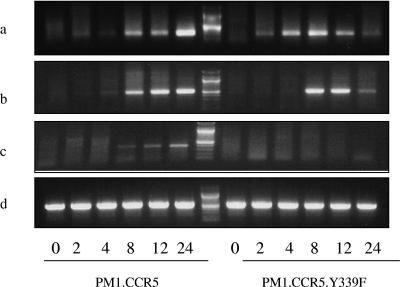
To examine at which stage viral replication is affected in cells expressing the CCR5.Y339F mutant, we investigated the kinetics of VACV early, intermediate, and late gene transcription. Cells expressing the intact CCR5 or the CCR5.Y339F mutant were inoculated with an MOI of 10 of VACV, and at 2, 4, 8, 12, and 24 h, cells were harvested for RNA extraction. Primers specific for VACV genes 005R (VACV growth factor), 078R (VACV late transcription factor 1), and 047R (VACV 11-kDa DNA-binding protein) were designed, representing well-characterized VACV early, intermediate, and late genes, respectively (2, 4). PCR data revealed that virus early gene expression was intact in cells expressing the intact or mutant receptor, with the 005R transcript detectable at 2 h postinoculation (Fig. 6a). Notably, expression of the intermediate gene transcript, 078R, was apparent at 2 h postinoculation in cells expressing intact CCR5, yet delayed to 4 to 8 in PM1 cells expressing the CCR5.Y339F mutant (Fig. 6b). Moreover, whereas expression of the late gene transcript, 047R, was initiated between 2 and 4 h postinoculation in PM1.CCR5 cells, we observed no evidence of this transcript in cells expressing the CCR5.Y339F mutant (Fig. 6c).
FIG. 6.
Absence of VACV late gene transcription fails in PM1.CCR5.Y339F cells. PM1.CCR5.Y339F and PM1.CCR5 cells were infected with VACV at an MOI of 10. At 0, 2, 4, 8, 12, and 24 h postinoculation (shown at bottom), cells were harvested for RNA extraction. Reverse-transcription PCR for VACV genes 005R (a), 078R (b), and 047R (c), representing vaccinia virus early, intermediate, and late genes, respectively, was performed. A probe specific for human β-actin was included to monitor transcription of a cellular mRNA (d).
A CCR5 antagonist inhibits VACV infection.
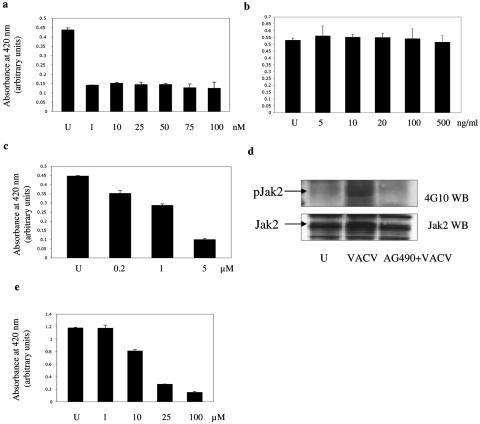
In subsequent studies, we employed the CCR5 antagonist TAK 779 (3). This small chemical molecule binds a cavity between transmembrane helices 1, 2, 3, and 7 of CCR5, near the extracellular surface (13), and may induce a conformational change in CCR5 that perturbs the ligand binding sites and inhibits ligand-induced signaling. We observed a marked reduction in VACV infection in PM1.CCR5 cells pretreated with TAK 779 (Fig. 7a). The dose required to maximally inhibit VACV infection, 1 nM, is in keeping with the 50% effective concentration of 1.2 nM and 90% effective concentration of 5.7 nM reported for human immunodeficiency virus (3). These data further suggest that CCR5 activation is necessary to permit VACV infection and that TAK 779 may interfere with critical CCR5-mediated signaling events that are required for viral infection.
FIG. 7.
Pharmacological inhibition of vaccinia virus infection in PM1.CCR5 cells. PM1.CCR5 cells were either left untreated (U) or treated for 1 h with various doses of TAK 799 (a), pertussis toxin (b), or herbimycin A (c) or for 16 h with AG490 (e) prior to VACV adsorption. Cells were infected at an MOI of 10, and LacZ activity was measured 16 h postinfection by using a β-galactosidase colorimetric assay. (d) PM1.CCR5 cells were left untreated (U), infected with VACV at an MOI of 10 for 1 min, or treated with AG490 at 100 μM concentration for 16 h prior to infection with vaccinia at an MOI of 10 for 1 min. Cell lysates were immunoprecipitated with anti-Jak2 antibodies. Lysates were resolved by SDS-PAGE and immunoblotted (WB) with antiphosphotyrosine (4G10) antibodies. The blot was stripped and reprobed for Jak2. The data are representative of two independent experiments.
VACV induces tyrosine phosphorylation of cellular intermediates.
Chemokine activation of CCR5 results in the engagement of G-protein-coupled events as well as G-protein-independent, tyrosine phosphorylation signaling effectors (reviewed in reference 51). Accordingly, we examined viral infection under conditions where either G-protein or phosphotyrosine signaling pathways are inhibited. Specifically, in dose-response experiments, PM1.CCR5 cells were pretreated with the tyrosine kinase inhibitor herbimycin A or with pertussis toxin. Cells were then infected with VACV, and 16 h postinfection, cell lysates were assayed for viral infection. In contrast to treatment with pertussis toxin, which had no effect, herbimycin A inhibited VACV infection in a dose-dependent manner (Fig. 7b and c). These data are in agreement with published reports that the activation of tyrosine kinase but not G-protein-coupled signal transduction contributes to both myxoma and VACV infection (17, 18, 24, 25).
When whole-cell lysates from VACV-infected PM1.CCR5 cells were resolved by SDS-PAGE and immunoblotted with antibodies against phosphotyrosine, a general increase in VACV-dependent induction of tyrosine phosphorylation in several protein bands was consistently observed (data not shown). Notably, cell lysates from infected PM1.CCR5.Y339F cells exhibited no increase in tyrosine phosphorylation. Chemokine activation of CCR5 triggers the rapid phosphorylation of receptor-associated Jak proteins (50), and CCR5-CCL5 interactions have been known to recruit Jak1 (33), Jak2, and Jak3 (50) in a cell-specific manner. Examination of the phosphorylation status of different Jak proteins revealed a VACV-dependent induction of Jak2 tyrosine phosphorylation within 1 min of viral adsorption in PM1.CCR5 cells (Fig. 7d). To examine the role of Jak2 activation in mediating VACV infection, PM1.CCR5 cells were pretreated with the Jak2 inhibitor AG490 (tyrophostin B42, 50% inhibitory concentration of ∼10 μM [26, 29]) prior to VACV adsorption. AG490 inhibited VACV-inducible phosphorylation of Jak2 (Fig. 7d). In parallel, a dose-dependent inhibition of VACV infection was observed (Fig. 7e). These results are consistent with earlier findings of myxoma virus activation of the Jak-Stat pathway (25).
VACV induces tyrosine phosphorylation of signaling effectors in permissive cells.
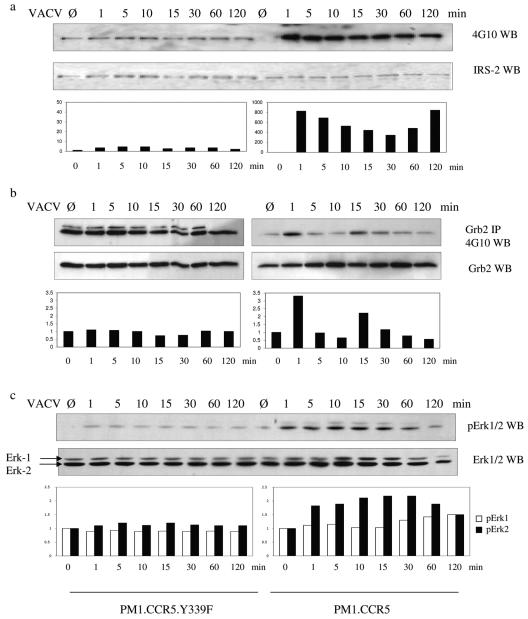
We examined the effects of VACV infection on tyrosine phosphorylation of IRS-2, a downstream effector of Jak proteins. IRS proteins participate in signal transduction by providing tyrosine-phosphorylated motifs for direct binding to the SH2 domains of various signaling proteins. The data in Fig. 8a show that VACV infection of PM1.CCR5 cells results in a rapid phosphorylation of IRS-2 at 1 min that is sustained for at least 120 min postinfection. Expression of the CCR5.Y339F mutant in these cells results in no discernible VACV-induced phosphorylation of IRS-2 above baseline levels (Fig. 8a). Since IRS proteins associate with phosphatidylinositol 3-kinase, SHP2, Fyn, Nck, and Grb2 (48), we infer that VACV activation of IRS-2 has the potential for engagement of a diverse range of signaling cascades. Indeed, we provide evidence for VACV-dependent induction of tyrosine phosphorylation of the adaptor protein Grb2 in permissive PM1.CCR5 cells by 1 min postinfection (Fig. 8b). By use of replicate experiments, we observed that this VACV-inducible phosphorylation of Grb2 is transient, diminishing by 5 min postinfection and then increasing once again between 12 and 15 min postinfection. Notably, with PM1.CCR5.Y339F cells, we consistently observed basal levels of phosphorylation of Grb2 that were not increased further by VACV infection (Fig. 8b). Grb2 activation may have very specific implications for VACV infection, especially since its phosphorylation has been shown to reduce SH3-dependent binding to Sos (21) and is thus thought to represent a potential regulatory point for signal transduction that may regulate the specificity of SH3 domain interactions (5). The viral protein A36R, responsible for actin polymerization during viral egress, has been shown to interact with Grb2 (38), followed by a colocalization with N-WASP and the actin-related protein complexes 2 and 3 (Arp2/3) (14). Since protein tyrosine phosphorylation was shown to be required for the formation of membrane protrusions mediating virus entry into HeLa cells (24), the tyrosine phosphorylation of Grb2 observed to occur in PM1.CCR5 cells may be linked to actin reorganization upon viral entry.
FIG. 8.
Vaccinia virus induces tyrosine phosphorylation of IRS-2, Grb2, and Erk1/2 in permissive but not in nonpermissive cells. PM1.CCR5 or PM1.CCR5.Y339F cells were left untreated (Ø) or infected with VACV at an MOI of 10 for the times indicated. Cell lysates were either (a) resolved by SDS-PAGE, immunoblotted (WB) with 4G10 antiphosphotyrosine antibody, and then stripped and reprobed for IRS-2; (b) immunoprecipitated (IP) with an anti-Grb2 antibody, after which the solubilized immunoprecipitate was resolved by SDS-PAGE and cells were immunoblotted with 4G10 antibody and then stripped and reprobed for Grb2; or (c) resolved by SDS-PAGE, immunoblotted with anti-phospho-Erk1/2 antibody, and then stripped and reprobed for Erk1/2. The data are representative of two independent experiments.
In addition to its SH2 domain, which can associate with IRS-2, the Grb2 adaptor molecule contains multiple SH3 domains, capable of binding downstream signaling proteins involved in the regulation of cell metabolism, growth, and differentiation. Grb2 is known to participate in the Ras cascade, due to its association with the guanine nucleotide exchange factor Sos, and thus may link signaling effectors to the mitogen-activated protein kinase (MAPK) pathway. Indeed, we were able to demonstrate a VACV-induced increase in extracellular signal-regulated kinase (Erk) tyrosine phosphorylation in the permissive PM1.CCR5 cells. Figure 8c shows that in the permissive PM1.CCR5 cells, VACV-inducible phosphorylation of Erk1/2 is present by 1 min postinfection and reaches a maximum at 15 min postinfection. In comparison, we consistently observed basal levels of phosphorylation of Erk1/2 that were not increased further by VACV infection in the PM1.CCR5.Y339F cells (Fig. 8c). This activation of the MAPK pathway appears to be virus specific since U0126, a MAPK and an Erk1/2 inhibitor, has been shown to attenuate VACV infection but increase myxoma virus infection in permissive 3T3 cell lines (17). Furthermore, the role for activation of intracellular signaling events in permissive cells was demonstrated by a myxoma-dependent activation of the Ser/Thr kinase PAK-1 (17). Activation of this intermediate was greatly reduced in nonpermissive NIH 3T3.CD4.neo cells, and the expression of the autoinhibitory domain of PAK-1 in permissive cells was shown to inhibit myxoma infection, suggesting a regulatory role for this kinase in determining the extent of poxviral infection. As with IRS-2 and Grb2, there was no discernible VACV-inducible phosphorylation of either Erk1 or Erk2 in the nonpermissive PM1.CCR5.Y339F cells (Fig. 8c).
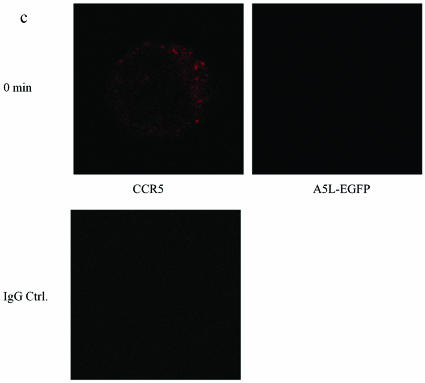
VACV colocalizes with CCR5 in permissive cells.
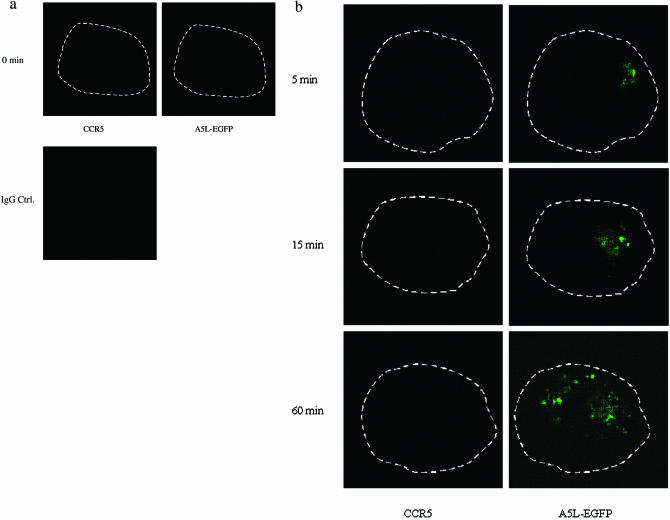
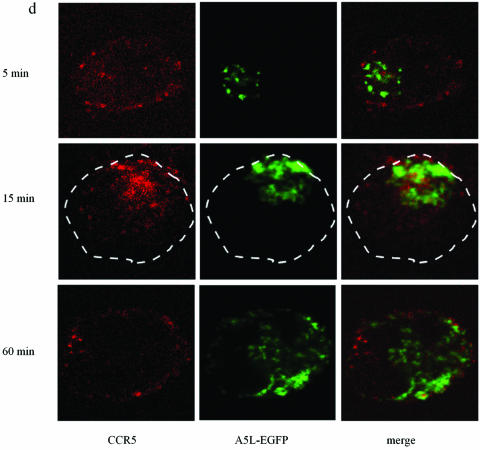
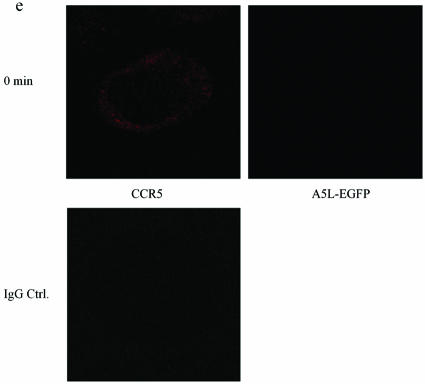
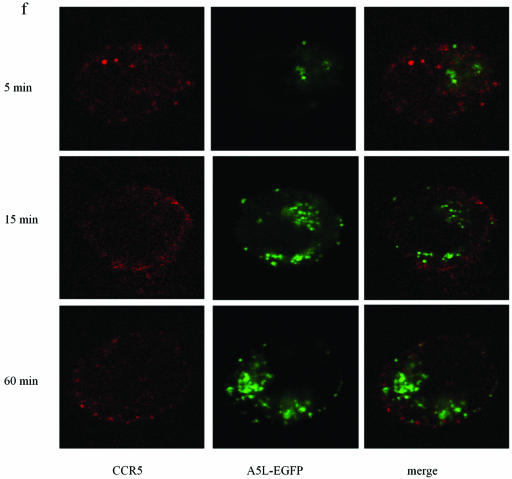
Using A5L-EGFP IMV, we examined VACV entry into both permissive and nonpermissive cells, while at the same time visualizing the movement/location of CCR5, by using confocal microscopy. Cells were infected on ice at an MOI of 10 with IMV infectious particles and then warmed to 37°C for the times indicated in the series of panels in Fig. 9. As shown, VACV enters the nonpermissive PM1 cells within 5 min of warming to 37°C and is distributed in the cell for up to 60 min (Fig. 9b). Since CCR5 is not expressed in these cells, staining with anti-mouse Cy3 antibody directed against mouse anti-human CCR5 antibody revealed no CCR5. By contrast, PM1.CCR5 cells expressed CCR5 at the cell surface, visualized using red Cy3 antibody (Fig. 9c and d). When PM1.CCR5 cells are infected with VACV, the virus enters the cells within 5 min of warming to 37°C. Notably, at 5 min, CCR5 remains externally expressed. By 15 min, both the virus and CCR5 colocalize inside the cell. By 60 min, the majority of CCR5 relocates to the cell surface. In Fig. 9e and f, the data presented illustrate that the CCR5.Y339F mutant remains externally expressed in the nonpermissive PM1.CCR5.Y339F cells for up to 60 min postinfection/warming to 37°C. As for the nonpermissive PM1 cells, VACV enters the PM1.CCR5.Y339F cells on warming to 37°C, rapidly, and remains distributed throughout the cell for up to 60 min. The data indicate that VACV enters both permissive and nonpermissive cells, and yet the sequence of signaling events described in Fig. 8 and the permissive phenotype are associated with intracellular colocalization of VACV and CCR5.
FIG.9.
CCR5 and IMV vaccinia virus colocalize in permissive PM1.CCR5 T cells. PM1 (a and b), PM1.CCR5 (c and d), or PM1.CCR5.Y339F (e and f) cells were infected with A5L-EGFP IMV vaccinia virus for 1 h at 4°C, and the cells were warmed to 37°C, fixed and stained for CCR5 (Cy3 antibody) at the time points indicated, and then analyzed by confocal microscopy, as described in Materials and Methods. The cell boundary is outlined in panels a, b, and d as a dashed line. The data are representative of two independent experiments. Optical sections were collected at 0.5-μm intervals through the entire cell. Ctrl., control.
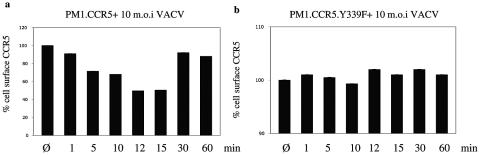
In a final series of experiments, we compared the abilities of ectopically expressed CCR5 and the CCR5.Y339F mutant to internalize following infection of cells with IMV infectious particles. In agreement with the confocal microscopy studies depicted in Fig. 9, flow cytometric analysis of cell surface-expressed CCR5 revealed that VACV infection leads to the rapid internalization of CCR5 within minutes of exposure to virus, which is maximal at 12 to 15 min postinfection, and that CCR5 returns to the cell surface by 30 min (Fig. 10a). By contrast, cells expressing the CCR5.Y339F mutant do not internalize the mutant receptor in response to VACV infection (Fig. 10b).
FIG. 10.
CCR5 internalizes in response to vaccinia virus in PM1.CCR5 but not PM1.CCR5.Y339F cells. (a) PM1.CCR5 cells were either left untreated (Ø) or infected with VACV at an MOI of 10 for the times indicated, and then ectopically expressed CCR5 was quantitated by flow cytometry using anti-human CCR5 antibody and fluorescein isothiocyanate-conjugated anti-mouse IgG. (b) Similarly, PM1.CCR5.Y339F cells were either left untreated or infected with VACV for the times indicated, and cell surface CCR5Y339F mutant expression was quantitated by flow cytometry. The percentages of cell surface CCR5 or CCR5.Y339F mutant expression were determined relative to total ectopic expression in untreated cells. The data are representative of two independent experiments.
DISCUSSION
Viewed altogether, these results allow us to infer that not only is tyrosine phosphorylation of CCR5 important for VACV infection in the context of permissive mouse fibroblasts and T cells expressing CCR5, but in particular Y-339 in the C-terminal tail of the receptor is crucial. Interestingly, from other studies we have evidence that Y-339 in CCR5 is also critical for CCL5-inducible activation of various signaling effectors, including Erk1/2 (unpublished). The fact that NIH 3T3.CD4.CCR5 cells are rendered less permissive by knockdown of CCR5 and NIH 3T3.CD4.neo cells are rendered permissive by ectopic expression of CCR5 but not a tyrosine phosphorylation CCR5 mutant (the CCR5.Y339F mutant) argues against the notion of permissiveness being entirely dependent on the lineage derivation of the NIH 3T3.CD4.CCR5 cells, independent of ectopic expression of the specific transfected receptors, as has been suggested previously (17). Phosphorylation of the CCR5 Y-339 receptor residue may be an early event in a virus-induced signaling cascade that accompanies infection, and Y-339 may be responsible for recruitment of signaling intermediates that we have shown to be induced by vaccinia at early times postinfection. Subsequent activation of crucial downstream kinases such as PAK-1 and other serine/threonine kinases may, in turn, promote some early aspect of viral infection. The implications are that viral activation of CCR5 activates signaling events that involve tyrosine phosphorylation, thereby rendering a cell permissive for viral infection. Our data infer that in these 3T3 mouse fibroblasts, expression of CCR5 contributes to the permissive phenotype. Moreover, we provide evidence that expression of CCR5 in primary human T cells or an immortalized human T-cell line renders these T cells permissive for VACV replication and that Y-339 is critical. Interestingly, recent data suggested that activated T cells, but not resting T cells, are permissive for VACV infection (10). Notably, susceptibility to infection was associated, minimally, with expression of a binding receptor that is induced de novo upon T-cell activation. When primary human T cells derived from peripheral blood mononuclear cells were activated by exposure to phytohemagglutinin and interleukin-12 (16), we observed the inducible de novo ectopic expression of CCR5 (data not shown). Although the studies described herein clearly demonstrate that CCR5 is not required for viral binding and entry, our data do not exclude the possibility that in addition to the de novo expression of a binding receptor, activated T cells express a signaling receptor, CCR5, which is not expressed on resting T cells and which renders the activated T cells permissive for viral replication. Since VACV replicates in cells and cell lines that lack CCR5, it is intriguing to speculate that virus activation of different receptors may occur to initiate host signaling events.
Of note, the myxoma virus infection defect in NIH 3T3.CD4.neo cells occurs at an intracellular stage following virion binding, entry, and early gene expression but prior to the later events of DNA replication and late gene expression (17). Our data provide evidence that VACV enters both permissive and nonpermissive T-cell lines and that replication is restricted in nonpermissive cells, specifically at the stage of late viral gene expression. We show that VACV early gene expression is evident for up to 24 h postinfection in both permissive and nonpermissive cells. Early VACV gene expression is unique in that it does not require the de novo synthesis of DNA or proteins, since the template, viral RNA polymerase, and early transcription factors are present within the infectious virus particle (7). New progeny IMVs are generated yet are not released from infected cells until lysis, except as EEV (42). Thus, new rounds of early VACV gene expression may persist in infected cells, prior to progeny release, deriving from the new progeny. With nonpermissive cells, we observed that infectious virus may be harvested for up to 24 h postinfection (Fig. 5), implying that not all IMV cores enter the viral replication cycle and/or that entry may be asynchronous or delayed. Thus, prolonged early VACV gene expression in nonpermissive cells may reflect this asynchronous commencement of early viral replication. The products of early gene transcription are required for intermediate VACV gene expression (35, 37, 46, 47). Accordingly, protracted intermediate VACV gene expression reflects the corresponding broad time frame for early gene expression.
In the context of CCR5, permissiveness for virus replication correlates with internalization of the receptor and the rapid virus-inducible tyrosine phosphorylation of a number of different signaling effectors. By use of time course studies, we have provided evidence for the intracellular peripheral colocalization of VACV and CCR5 in permissive cells. Since VACV enters cells regardless of whether or not CCR5 is ectopically expressed, CCR5 is not associated with viral entry. In nonpermissive cells, the apparent block in viral replication we observed coincides with a block in late gene expression. It is intriguing to speculate that IMV colocalization with CCR5 at the plasma membrane results in activation of CCR5 at Y339 either through direct contact between IMV core proteins and CCR5 or by mediation due to IMV interacting with other cellular factors that are recruited to the intracellular region of CCR5. CCR5 activation leads to a cascade of tyrosine phosphorylation signaling events that, apparently, prescribe cellular events that are necessary for late VACV gene expression. Whether conserved amino acid motifs in the intracellular domains of different receptors are associated with viral activation or whether a broad spectrum of tyrosine kinases that can associate with different receptors and invoke their activation are rendered kinase active by viral factors remains to be determined and is the subject of our ongoing investigations.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research to E.N.F. (MOP42564) and NIH grants CA77816 and CA94079 to L.C.P. R.R. and T.T.M. are recipients of CIHR doctoral research awards.
REFERENCES
- 1.Andrade, A. A., P. N. Silva, A. C. Pereira, L. P. De Sousa, P. C. Ferreira, R. T. Gazzinelli, E. G. Kroon, C. Ropert, and C. A. Bonjardim. 2004. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 381:437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 3.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldick, C. J., Jr., and B. Moss. 1993. Characterization and temporal regulation of mRNAs encoded by vaccinia virus intermediate-stage genes. J. Virol. 67:3515-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin, C. W., D. A. Linseman, and D. A. Jones. 1994. Platelet-derived growth factor stimulates phosphorylation of growth factor receptor-binding protein-2 in vascular smooth muscle cells. J. Biol. Chem. 269:31346-31349. [PubMed] [Google Scholar]
- 6.Boulter, E. A. 1969. Protection against poxviruses. Proc. R. Soc. Med. 62:295-297. [PMC free article] [PubMed] [Google Scholar]
- 7.Broyles, S. S. 2003. Vaccinia virus transcription. J. Gen. Virol. 84:2293-2303. [DOI] [PubMed] [Google Scholar]
- 8.Carter, G. C., M. Law, M. Hollinshead, and G. L. Smith. 2005. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 86:1279-1290. [DOI] [PubMed] [Google Scholar]
- 9.Carter, G. C., G. Rodger, B. J. Murphy, M. Law, O. Krauss, M. Hollinshead, and G. L. Smith. 2003. Vaccinia virus cores are transported on microtubules. J. Gen. Virol. 84:2443-2458. [DOI] [PubMed] [Google Scholar]
- 10.Chahroudi, A., R. Chavan, N. Koyzr, E. K. Waller, G. Silvestri, and M. B. Feinberg. 2005. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J. Virol. 79:10397-10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condit, R. C., and E. G. Niles. 2002. Regulation of viral transcription elongation and termination during vaccinia virus infection. Biochim. Biophys. Acta 1577:325-336. [DOI] [PubMed] [Google Scholar]
- 12.de Magalhaes, J. C., A. A. Andrade, P. N. Silva, L. P. Sousa, C. Ropert, P. C. Ferreira, E. G. Kroon, R. T. Gazzinelli, and C. A. Bonjardim. 2001. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J. Biol. Chem. 276:38353-38360. [DOI] [PubMed] [Google Scholar]
- 13.Dragic, T., A. Trkola, D. A. Thompson, E. G. Cormier, F. A. Kajumo, E. Maxwell, S. W. Lin, W. Ying, S. O. Smith, T. P. Sakmar, and J. P. Moore. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. USA 97:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401:926-929. [DOI] [PubMed] [Google Scholar]
- 15.Huttenrauch, F., A. Nitzki, F. T. Lin, S. Honing, and M. Oppermann. 2002. Beta-arrestin binding to CC chemokine receptor 5 requires multiple C-terminal receptor phosphorylation sites and involves a conserved Asp-Arg-Tyr sequence motif. J. Biol. Chem. 277:30769-30777. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki, M., T. Mukai, P. Gao, W. R. Park, C. Nakajima, M. Tomura, H. Fujiwara, and T. Hamaoka. 2001. A critical role for IL-12 in CCR5 induction on T cell receptor-triggered mouse CD4(+) and CD8(+) T cells. Eur. J. Immunol. 31:2411-2420. [DOI] [PubMed] [Google Scholar]
- 17.Johnston, J. B., J. W. Barrett, W. Chang, C. S. Chung, W. Zeng, J. Masters, M. Mann, F. Wang, J. Cao, and G. McFadden. 2003. Role of the serine-threonine kinase PAK-1 in myxoma virus replication. J. Virol. 77:5877-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405-1413. [DOI] [PubMed] [Google Scholar]
- 18.Lalani, A. S., J. W. Barrett, and G. McFadden. 2000. Modulating chemokines: more lessons from viruses. Immunol. Today 21:100-106. [DOI] [PubMed] [Google Scholar]
- 19.Lalani, A. S., J. Masters, W. Zeng, J. Barrett, R. Pannu, H. Everett, C. W. Arendt, and G. McFadden. 1999. Use of chemokine receptors by poxviruses. Science 286:1968-1971. [DOI] [PubMed] [Google Scholar]
- 20.Law, M., and G. L. Smith. 2004. Studying the binding and entry of the intracellular and extracellular enveloped forms of vaccinia virus. Methods Mol. Biol. 269:187-204. [DOI] [PubMed] [Google Scholar]
- 21.Li, S., A. D. Couvillon, B. B. Brasher, and R. A. Van Etten. 2001. Tyrosine phosphorylation of Grb2 by Bcr/Abl and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling. EMBO J. 20:6793-6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Y., R. L. Hall, and R. W. Moyer. 1997. Transient, nonlethal expression of genes in vertebrate cells by recombinant entomopoxviruses. J. Virol. 71:9557-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locker, J. K., A. Kuehn, S. Schleich, G. Rutter, H. Hohenberg, R. Wepf, and G. Griffiths. 2000. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11:2497-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masters, J., A. A. Hinek, S. Uddin, L. C. Platanias, W. Zeng, G. McFadden, and E. N. Fish. 2001. Poxvirus infection rapidly activates tyrosine kinase signal transduction. J. Biol. Chem. 276:48371-48375. [DOI] [PubMed] [Google Scholar]
- 26.Meydan, N., T. Grunberger, H. Dadi, M. Shahar, E. Arpaia, Z. Lapidot, J. S. Leeder, M. Freedman, A. Cohen, A. Gazit, A. Levitzki, and C. M. Roifman. 1996. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379:645-648. [DOI] [PubMed] [Google Scholar]
- 27.Moss, B. 2001. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, p. 2849-2883, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 28.Murphy, P. M. 2001. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2:116-122. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen, M., K. Kaltoft, M. Nordahl, C. Ropke, C. Geisler, T. Mustelin, P. Dobson, A. Svejgaard, and N. Odum. 1997. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc. Natl. Acad. Sci. USA 94:6764-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parrish, S., and B. Moss. 2006. Characterization of a vaccinia virus mutant with a deletion of the D10R gene encoding a putative negative regulator of gene expression. J. Virol. 80:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payne, L. G., and E. Norrby. 1978. Adsorption and penetration of enveloped and naked vaccinia virus particles. J. Virol. 27:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Frade, J. M., M. Mellado, and A. C. Martinez. 2001. Chemokine receptor dimerization: two are better than one. Trends Immunol. 22:612-617. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Frade, J. M., A. J. Vila-Coro, A. M. de Ana, J. P. Albar, A. C. Martinez, and M. Mellado. 1999. The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. Proc. Natl. Acad. Sci. USA 96:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Frade, J. M., A. J. Vila-Coro, A. Martin, M. Nieto, F. Sanchez-Madrid, A. E. Proudfoot, T. N. Wells, A. C. Martinez, and M. Mellado. 1999. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J. Cell Biol. 144:755-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosales, R., N. Harris, B. Y. Ahn, and B. Moss. 1994. Purification and identification of a vaccinia virus-encoded intermediate stage promoter-specific transcription factor that has homology to eukaryotic transcription factor SII (TFIIS) and an additional role as a viral RNA polymerase subunit. J. Biol. Chem. 269:14260-14267. [PubMed] [Google Scholar]
- 36.Sanchez-Puig, J. M., L. Sanchez, G. Roy, and R. Blasco. 2004. Susceptibility of different leukocyte cell types to vaccinia virus infection. Virol. J. 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanz, P., and B. Moss. 1998. A new vaccinia virus intermediate transcription factor. J. Virol. 72:6880-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaplehorn, N., A. Holmstrom, V. Moreau, F. Frischknecht, I. Reckmann, and M. Way. 2002. Grb2 and Nck act cooperatively to promote actin-based motility of vaccinia virus. Curr. Biol. 12:740-745. [DOI] [PubMed] [Google Scholar]
- 39.Seet, B. T., and G. McFadden. 2002. Viral chemokine-binding proteins. J. Leukoc. Biol. 72:24-34. [PubMed] [Google Scholar]
- 40.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 78:2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons, G., J. D. Reeves, S. Hibbitts, J. T. Stine, P. W. Gray, A. E. Proudfoot, and P. R. Clapham. 2000. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol. Rev. 177:112-126. [DOI] [PubMed] [Google Scholar]
- 42.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 43.Tripp, R. A., L. P. Jones, L. M. Haynes, H. Zheng, P. M. Murphy, and L. J. Anderson. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2:732-738. [DOI] [PubMed] [Google Scholar]
- 44.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79:877-887. [DOI] [PubMed] [Google Scholar]
- 45.Venkatesan, S., A. Petrovic, M. Locati, Y. O. Kim, D. Weissman, and P. M. Murphy. 2001. A membrane-proximal basic domain and cysteine cluster in the C-terminal tail of CCR5 constitute a bipartite motif critical for cell surface expression. J. Biol. Chem. 276:40133-40145. [DOI] [PubMed] [Google Scholar]
- 46.Vos, J. C., M. Sasker, and H. G. Stunnenberg. 1991. Promoter melting by a stage-specific vaccinia virus transcription factor is independent of the presence of RNA polymerase. Cell 65:105-113. [DOI] [PubMed] [Google Scholar]
- 47.Vos, J. C., M. Sasker, and H. G. Stunnenberg. 1991. Vaccinia virus capping enzyme is a transcription initiation factor. EMBO J. 10:2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White, M. F. 1998. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Recent Prog. Horm. Res. 53:119-138. [PubMed] [Google Scholar]
- 49.Wolffe, E. J., A. S. Weisberg, and B. Moss. 1998. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology 244:20-26. [DOI] [PubMed] [Google Scholar]
- 50.Wong, M., S. Uddin, B. Majchrzak, T. Huynh, A. E. Proudfoot, L. C. Platanias, and E. N. Fish. 2001. Rantes activates Jak2 and Jak3 to regulate engagement of multiple signaling pathways in T cells. J. Biol. Chem. 276:11427-11431. [DOI] [PubMed] [Google Scholar]
- 51.Wong, M. M., and E. N. Fish. 2003. Chemokines: attractive mediators of the immune response. Semin. Immunol. 15:5-14. [DOI] [PubMed] [Google Scholar]