Abstract
Carotenoids contribute to energy transduction in the light harvesting complexes and serve in protection from excess light fluence. Because of the importance of carotenoids, the genes encoding enzymes of carotenoid biosynthesis in higher plants are potential targets for herbicides. To obtain further insight into tobacco carotenoid biosynthesis and to investigate and prioritize potential herbicide targets in the pathway, the effects of changed phytoene synthase (PSY) and phytoene desaturase (PDS) gene expression were studied in transgenic tobacco (Nicotiana tabacum Petit Havana SR1) plants. Genes for both enzymes were cloned from tobacco, and surprisingly two functional PSY genes were found. Transgenic tobacco plants constitutively expressing these genes in both sense and antisense orientations were examined regarding phenotype, carotenoid content and transcript levels of carotene biosynthesis genes. Overexpression of either psy gene resulted in severe phenotypic effects including dwarfism, altered leaf morphology, and pigmentation. A correlation among phenotype, transcript level, and metabolic profile was demonstrated by comparison of hemizygous and homozygous plants from the same transformation event. Antisense expression of PSY and PDS also caused lethal phenotypes. Transcript levels of other carotene biosynthesis genes remained unaltered in the transgenic mutant. Phytoene accumulated in plants expressing antisense RNA to pds. However, elevated levels of phytoene were detected suggesting an increase in metabolic flux into this pathway.
Carotenoids, both carotenes and xanthophylls, the oxygenated derivatives of carotenes, are colored pigments common to all photosynthetic organisms. The unsaturated C40 hydrocarbons not only give color to fruits and flowers but have multiple functions in photosynthesis. They participate in light harvesting in photosynthetic membranes and protect the photosynthetic apparatus from excessive light energy by quenching triplet chlorophylls and singlet oxygen (Siefermann-Harms, 1987). Furthermore, carotenoids provide a structural component of some pigment-protein complexes (Moskalenko and Karapetyan, 1996).
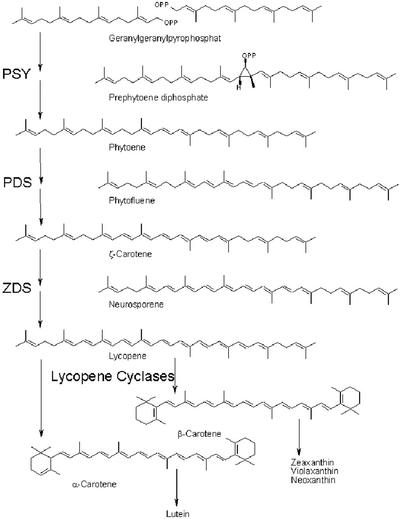
In higher plants, carotenoids are synthesized in the plastids from isopentenylpyrophosphate precursors originating from the glyceraldehyde phosphate/pyruvate pathway (Lichtenthaler et al., 1997; Cunningham and Gantt, 1998). The first committing step of carotenoid biosynthesis is a head-to-head coupling of two molecules of geranylgeranylpyrophosphate (GGPP) to yield colorless phytoene by phytoene synthase (PSY), with prephytoene diphosphate as an intermediate (Fig. 1). Subsequently, four additional double bonds are introduced by desaturases producing the colored carotenes phytofluene, ζ-carotene, neurosporene, and lycopene. Lycopene is cyclized twice by two individual cyclases, yielding α- and β-carotene, which subsequently are further processed to different xanthophylls, such as lutein, violaxanthin, and zeaxanthin.
Figure 1.
Biosynthesis of carotenes in plants.
The gene encoding PSY has been cloned from a variety of organisms, such as bacteria, algae, and plants. Whereas most plants have a single gene, two differentially expressed genes exist in tomato (Lycopersicon esculentum). psy1, which had been identified as a fruit-ripening–related gene named pTOM5 (Slater et al., 1985; Bartley et al., 1991), contributes to phytoene formation in tomato chromoplasts (Giuliano et al., 1993), whereas psy2 was related to carotenoid biosynthesis in mature leaves (Bartley and Scolnik, 1993). Because antisense expression of psy1 alters carotenoids in the fruit but not the leaves and because yellow fruit mutant contains a defective psy1 gene, the results from genetic experiments suggest that PSY1 functions in the fruit and PSY2 functions primarily in leaf tissue (Bramley et al., 1992; Fraser et al., 1999).
The high economic value of carotenoids as a nutritional source of vitamin A and coloring agents in foods resulted in several attempts to increase carotene biosynthesis in planta. Seed-specific overexpression of bacterial PSY targeted to the plastid led to a 50-fold increase in carotenoid levels in the mature seeds of Brassica napus (canola) (Shewmaker et al., 1999). Increasing carbon flux into the carotenoid pathway can produce other phenotypes because the GGPP is an intermediate common to many pathways. For example, tomato plants with constitutive overexpression of the fruit-related PSY1 showed dwarfism and reduced gibberellin levels because of redirecting GGPP from the respective pathway (Fray et al., 1995).
In plants, algae, and cyanobacteria, the desaturation steps are performed by two individual enzymes. Phytoene desaturase (PDS) introduces two double bonds at symmetrical positions C11 and C11′ of phytoene and ζ-carotene desaturase (ZDS) at symmetrical positions C7 and C7′ of ζ-carotene. The deduced peptide sequences of PDS genes show a high degree of similarity between different plant species as is the case for the ZDS genes. It is interestingly that PDS genes show 33% to 35% similarity to ZDS genes and can be grouped together in phylogenetic analysis, indicating a common ancestor of both genes (Albrecht et al., 1995; Hirschberg et al., 1997). The desaturases are inactive when soluble (Bonk et al., 1997). They associate with the membrane via binding to FAD. The FAD appears to feed an electron transport chain involving quinones and the plastid terminal oxidase (PTOX), which ultimately reduces oxygen (Mayer et al., 1990; Norris et al., 1995; Al-Babili et al., 1996; Carol et al., 1999; Wu et al., 1999; Josse et al., 2000; Carol and Kuntz, 2001).
The protective function of carotenoids depends on the complete conjugated double-bond system. As a consequence, any step in carotene biosynthesis (Fig. 1) up to lycopene formation is a potential target for a herbicidal inhibitor (Böger and Sandmann, 1998). To illuminate the mechanisms underlying carotene biosynthesis and to further evaluate the use of the respective enzymes for herbicide discovery, we up- and down-regulated the PSY and PDS from tobacco (Nicotiana tabacum). Overexpression of PSY resulted in a dwarf phenotype with severe effects on leaf morphology, flowers, capsules, and seedlings. The down-regulation led to a decrease in carotenoid levels and a lethal phenotype in progeny with multiple insertions of the transgene into the genome. For PDS, the down-regulation led to an accumulation of the immediate precursor phytoene. Decreasing PDS levels resulted in lethal phenotypes in homozygous seedlings.
RESULTS
Cloning the Genes of PSY and PDS from Tobacco
The coding sequences of tobacco PSY and PDS were cloned with PCR-based methods. For psy, two different genes have been found. The mRNA of psy1 encodes a protein with a deduced peptide sequence of 440 amino acids. The protein shows high sequence similarity to the peptide sequence of tomato PSY2 (93% identity). The mRNA of psy2 encodes a slightly shorter peptide sequence of 410 amino acids with 89% identity to the tomato PSY2. Psy1 and 2 show 86% identity in both nucleotide and amino acid sequence. Major differences can be found in the amino-terminal sequences. Both sequences share conserved sequence motifs found for polyisoprene synthases (squalene synthates and PSY) as noted in the BLOCKS database (Henikoff and Henikoff, 1991). Northern blot analysis revealed that the expression level of tobacco psy1 was much higher than the almost undetectable psy2 (data not shown).
The mRNA of pds encodes a protein with a deduced peptide sequence of 582 amino acids. The protein shows high sequence similarity to the protein sequences of pepper (Capsicum annuum) and tomato PDS (95% and 94% identity, respectively). An FAD/NAD-binding motif is found near the NH2-terminal end of the protein, as in other PDSs from various organisms (Bartley and Sandmann, 1994).
Overexpression of PSY1 and 2 Led to a Severe Dwarf Phenotype and an Accumulation of Phytoene
The coding sequences for tobacco PSY1, PSY2, and PDS were expressed both in sense (PSY1+, PSY2+, PDS+) and in antisense (PSY1−, PSY2−, PDS−) orientation under control of a duplicated 35S RNA promoter in tobacco. Transgenic plants obtained by Agrobacterium tumefaciens-mediated transformation of protoplasts as well as leaf discs were sent to the greenhouse; 6 to 7 weeks thereafter the contents of different carotenes and xanthophylls were determined by HPLC.
Among 44 lines overexpressing psy1, 10 showed various degrees of significant phenotypic changes in comparison with wild type or control plants transformed with the empty vector. The leaves of the most affected plants showed abnormal pigmentation, sometimes looking like parchment, and were very hairy (Fig. 2A). The sides of the leaves typically rolled up to the middle vein. Very young leaves sometimes were colored bright orange, but then rapidly turned green. Some plants exhibited a strong dwarf phenotype (Fig. 2G), others developed numerous buds but failed to flower (Fig. 2B), and others flowered but lacked the typical pink flower color of wild-type plants and were white to very pale pink instead (Fig. 2C). Upon selfing, the plants developed orange capsules instead of normal green capsules (Fig. 2D). Among the 40 transgenic plants overexpressing psy2, six resembled the effected PSY1+, plants but the effects were much weaker. Carotenoid and chlorophyll contents of all transgenic lines overexpressing psy1 or psy2 were determined by HPLC and compared with control plants. PSY1+ plants exhibiting a morphological phenotype accumulated phytoene between 13 and 60 nmol g−1 plant material based on fresh weight, whereas phytoene did not accumulate in the controls (Table I). Some plants showed an increase in both carotenoid content up to 127% and chlorophyll content up to 116%; others showed reduced chlorophyll content down to 40% in parallel with an unchanged to reduced carotenoid content down to 54%. The ratio of chlorophyll to carotenoid content was determined as a measurement for all the plants. The chlorophyll to carotenoid ratio decreased from 4.5 ± 0.1 mol mol−1 to an average of 3.9 ± 0.2 mol mol−1 among the affected plants with one line having a minimum of 2.6. Measurement of pigment content of orange capsules in comparison with green capsules revealed a strong accumulation of phytoene and lycopene together with slightly reduced xanthophyll content. The orange capsules showed reduced chlorophyll content down to 6% and an increase in total carotenoids to 400%.
Figure 2.
Ectopic expression of PSY1 in tobacco leads to severe phenotypic effects. A, Leaf of line PSY1+10 showing abnormal pigmentation and curling of the leaves. B, Buds of line PSY1+32 arrested in flower development. C, White flowers of lines PSY1+27, 29, and 31 (right, bottom to top) in comparison with flower of control plant (left). D, Orange capsules of line PSY1+27 in comparison with mounted capsule of control plant (red arrow). E, Seedlings from selfing line PSY1+31 developing on LS medium containing kanamycin segregate into different phenotypes (wild type, type G [green], type O [orange primary leaves]), aged 2 weeks. F, Homozygous seedlings of line PSY1+18 (F2 generation). G, Dwarf phenotype of primary transformant PSY1+10 7 weeks after setting to the greenhouse (left) in comparison with control plant with empty transformation vector (right). H, F1 generation of line PSY1+18, aged 7 weeks, segregating into type G (middle) and type O (right) plants in comparison with control plant (left).
Table I.
Phytoene accumulation and chlorophyll to carotenoid ratios of selected transgenic tobacco plants expressing PSY1 in comparison with gene expression and phenotype data
| Line | Total Chlorophylls | Total Carotenoids | Phytoene | Chlorophyll to Carotenoid Ratio | Expression Level psy1 | Phenotypea |
|---|---|---|---|---|---|---|
| nmol/g−1 | nmol/g−1 | nmol/g−1 | mol/mol−1 | % control | ||
| PSY1+04 | 1,672 ± 134 | 440 ± 32 | 21 ± 4 | 3.8 ± 0.2 | n.d.b | lp |
| PSY1+10 | 1,332 ± 107 | 330 ± 23 | 29 ± 7 | 4.0 ± 0.2 | 501 ± 129 | lp, dw, fn |
| PSY1+18 | 1,449 ± 116 | 347 ± 24 | 20 ± 4 | 4.2 ± 0.2 | 900 ± 242 | lp, co |
| PSY1+27 | 1,771 ± 142 | 506 ± 35 | 22 ± 4 | 3.5 ± 0.1 | 1,078 ± 279 | lp, fw, co |
| PSY1+29 | 2,309 ± 184 | 566 ± 40 | 12 ± 3 | 4.1 ± 0.2 | n.d. | lp, fw, co |
| PSY1+31 | 1,947 ± 156 | 483 ± 34 | 13 ± 3 | 4.0 ± 0.2 | 650 ± 174 | lp, fw, co |
| PSY1+32 | 2,176 ± 174 | 542 ± 38 | 18 ± 4 | 4.0 ± 0.2 | n.d. | lp, fr, co |
| PSY1+48 | 1,708 ± 137 | 393 ± 28 | 13 ± 3 | 4.3 ± 0.3 | n.d. | lp |
| PSY1+49 | 2,246 ± 180 | 552 ± 39 | 22 ± 4 | 4.1 ± 0.2 | n.d. | lp, co |
| PSY1+58 | 783 ± 626 | 296 ± 21 | 62 ± 12 | 2.6 ± 0.1 | 774 ± 202 | lp, co |
| PSY1+ (mean)c | 1,739 ± 445 | 446 ± 99 | 23 ± 14 | 3.9 ± 0.2 | ||
| Control (mean) | 1,961 ± 251 | 437 ± 26 | 0 ± 0 | 4.5 ± 0.1 | 100 ± 57 | Wild type |
lp, Leaf pigmentation and rolling; dw, dwarf; fn, no mature flowers; fw, flowers white; fr, flower development strongly reduced; co, capsules orange.
n.d., Not determined.
Mean value of the 10 plants shown above.
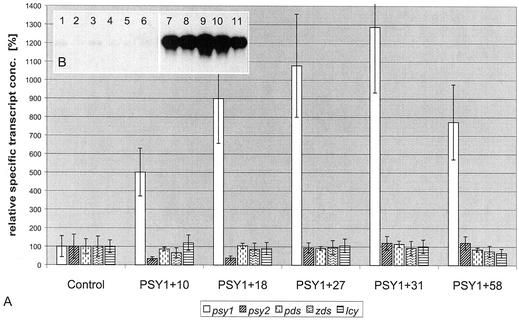
Transcript levels of transgenic plants expressing psy1 and 2 were determined using slot blots, northern blots, and quantitative reverse transcriptase (RT)-PCR. Slot-blot analysis showed a significant overexpression of psy for 60% of the transgenic plants (data not shown). Some of these were subjected to further analysis by northern blot and showed a strong increase in psy1 transcript levels compared with control plants (Fig. 3B). For a set of five plants, a detailed analysis was made by quantification of transcript levels of carotene biosynthesis genes psy1 and 2, pds, zds, and lcyb to show possible reactions of these genes in response to the overexpression of psy1. For this purpose zds from tobacco was cloned with a similar RACE protocol as used for cloning of PSY, and lcyb was cloned by PCR using the sequence published by Cunningham et al. (1996). Transcript levels of the genes showed no significant difference in comparison with levels in control plants, although there was an increase in psy1 transcript level by a factor ranging from 5 to 13 (Fig. 3A). Determination of transcript levels of transgenic lines expressing psy2 revealed similar results (data not shown).
Figure 3.
Constitutive overexpression of tobacco PSY1 in tobacco. Poly(A+) RNA was isolated from mature leaves of 7-week-old transgenic tobacco plants from the greenhouse. A, mRNA was subjected to RNA gel-blot analysis and hybridized with probes derived from psy1. Lanes 1 to 6, Control lines pSS05, 07, 09, 17, 20, and 26; lanes 7 to 11, lines PSY1+18, 27, 43, 29, and 32. B, mRNA of lines PSY1+10, 18, 27, and 58 was subjected to quantitative RT-PCR using specific primers and probes for quantification of carotenoid biosynthesis genes psy1, psy2, pds, zds, and lcyb based on calibration with in vitro transcribed RNA of the respective genes. Error bars represent 2 sd as calculated from errors of quantitative RT-PCR, dilution, and determination of total mRNA concentration (conc.).
Correlation of Metabolic and Phenotypic Effects with Transcript Levels in Progeny of Transgenic Plants Overexpressing PSY
After self-fertilization and harvesting capsules from selected transgenic lines, seeds were regenerated with kanamycin to generate homozygous plants overexpressing psy1 and psy2. The most severely affected PSY1+ and all PSY2+ plants segregated in a ratio of 1:15 (wild type:transgenic) or lower, indicating two or more unlinked copies of the transgene having integrated into the genome. Seedlings of some weaker affected plants, however, showed a segregation pattern expected for a single insertion. About one-fourth of the seedlings exhibited slightly orange primary leaves and produced stunted plants (type O), whereas another one-fourth was kanamycin sensitive (wild type), and one-half showed a normal green phenotype and produced plants resembling their parent (type G), resulting in a segregation pattern of 1:2:1 (wild type:type G:type O; Fig. 2E). Type O progeny of lines PSY1+18 and PSY1+31 just reached 15% to 20% of the height of control plants (Fig. 2H). They did not develop normal buds, and just one single flower of line PSY1+18 gave rise to a capsule. All seedlings of this capsule showed the type O phenotype and were, therefore, homozygous (Fig. 2F). Type G plants of generation F1 showed the segregation into wild type, type G, and type O seedlings and were, therefore, hemizygous. Hemi- and homozygous progeny of line PSY1+18 had elevated psy1 transcript levels, and these levels were doubled in homozygous plants compared with hemizygous plants. The carotenoid content of hemizygous plants was increased, whereas chlorophyll content remained unchanged. In homozygous plants, however, both total carotenoid content and chlorophyll content decreased to about 50% and 41%, respectively, and phytoene accumulated. Thus, pigment content and visible phenotype reflected the increased transcript levels of these plants.
Antisense Expression of PSY1 in Transgenic Plants Resulted in Deleterious Effects in Progeny Having Multiple Insertions of the Transgene.
In contrast to transgenic plants overexpressing psy1 and 2, antisense expression of these genes resulted in no significant phenotype in primary transformants. Measuring pigment content revealed slightly decreased carotenoid content as expressed by increased chlorophyll to carotenoid ratios for six of 58 transgenic plants expressing antisense RNA to psy1 (Table II). The most prominently affected plants, PSY1−17 and 39, had relative carotenoid contents of 48% and 51% and chlorophyll to carotenoid ratios of 5.1 and 5.8 mol mol−1, respectively, compared with 4.5 mol mol−1 for control plants. Among 26 plants expressing antisense RNA to psy2, none showed either phenotypic or metabolic differences in comparison with control plants.
Table II.
Decreased carotenoid contents and chlorophyll to carotenoid ratios of selected transgenic tobacco plants expressing antisense RNA to PSY1
| Line | Total Chlorophylls | Total Carotenoids | Chlorophyll to Carotenoid Ratio |
|---|---|---|---|
| nmol/g−1 | nmol/g−1 | mol/mol−1 | |
| PSY1−06 | 1,895 ± 113 | 387 ± 27 | 4.9 ± 0.3 |
| PSY1−15 | 1,415 ± 113 | 297 ± 21 | 4.8 ± 0.3 |
| PSY1−17 | 1,062 ± 85 | 210 ± 15 | 5.1 ± 0.3 |
| PSY1−20 | 1,829 ± 146 | 383 ± 27 | 4.8 ± 0.3 |
| PSY1−22 | 1,778 ± 142 | 372 ± 26 | 4.8 ± 0.3 |
| PSY1−39 | 1,291 ± 103 | 221 ± 16 | 5.8 ± 0.3 |
| Control (mean) | 1,961 ± 251 | 437 ± 26 | 4.5 ± 0.1 |
Based on the segregation pattern, we found that all lines with increased chlorophyll to carotenoid ratio had two or more insertions of the transgene into the genome. Among these lines PSY−39 showed a complex segregation pattern. Ten of the kanamycin-resistant seedlings were set to soil in the greenhouse and seven showed pale to white primary leaves but normal secondary leaves and were significantly reduced in size compared with control plants (Fig. 4, A and B). When growing, the seedlings developed into different phenotypes. Presumably because of different dosages of the antisense transgene, all degrees of effects were found in these plants. The most severely affected plant turned completely white and died within 2 weeks after being set to soil (Fig. 4C). Another plant developed very pale leaves with veins that were light green, was reduced in height to about 25% of control or wild-type plants, and developed no buds or flowers (Fig. 4C). Finally, leaves turned completely white. When measuring carotenoid contents of this plant, a decrease in total carotenoids to 17% was found accompanied by a decrease in chlorophyll content to 30%, resulting in a chlorophyll to carotenoid ratio of about 8.0 mol mol−1 (177%). Other plants showed less severe effects with slightly decreased pigment concentrations and chlorophyll to carotenoid ratios between 4.5 (100%) and 5.0 (110%).
Figure 4.
Expression of antisense RNA to PSY1 (PSY1−) and PDS (PDS−) in transgenic tobacco results in abnormal pigmentation and lethality in progeny. A, F1 seedlings of line PSY1−39, aged 2 weeks, showing reduced size and pale primary leaves when compared with seedlings of control plants bearing the empty transformation vector. B, F1 seedlings of control line pSS33, aged 2 weeks. C, F1 progeny 9 of line PSY1−39 are lethal. D, F1 progeny 6 of line PSY1−39, aged 7 weeks, reduced in size and showing pale leaves (left) in comparison with control plant (right). E, White capsules of line PDS−13. F, White capsule of line PDS−13 (right) in comparison with capsule of control plant (left). G, White spot on leaf of line PDS−24, aged 6 weeks. H, Leaf of line PDS−30. I, Leaf of line PDS−47. J, Seedling of line PDS−13 5 d after setting to soil. K, F1 seedlings of line PDS−13 developing on LS medium containing kanamycin segregate into wild type (just primary leaves), type I (fully green), and type II (bleaching secondary leaves) seedlings.
Ectopic Expression of PDS Had No Effect on Transgenic Tobacco Plants
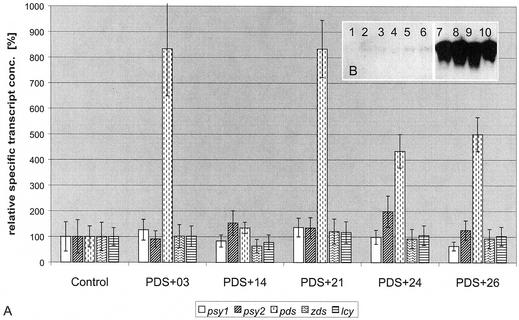
Among 52 independent PDS+ lines with confirmed increases in PDS transcript levels, none showed either phenotypic or metabolic effects. Some individual lines were analyzed in northern blot experiments. Although only very low PDS transcript levels were detectable in leaves of control plants, the lines expressing sense mRNA for the respective genes showed a strong increase in transcript levels (Fig. 5B). For a set of five plants with elevated pds, transcript levels of carotene biosynthesis genes psy1 and 2, pds, zds, and lcyb were determined and found to be unchanged (Fig. 5A).
Figure 5.
Constitutive overexpression of tobacco PDS in tobacco. Poly(A+) RNA was isolated from mature leaves of 7-week-old transgenic tobacco plants from the greenhouse. A, mRNA was subjected to RNA gel-blot analysis and hybridized with probes derived from pds. Lanes 1 to 6, Control lines pSS05, 07, 09, 17, 20, and 26; lanes 7 to 10, lines PDS+21, 31, 37, and 47. B, mRNA of lines PDS+03, 14, 21, 24, and 26 was subjected to quantitative RT-PCR using specific primers and probes for quantification of carotenoid biosynthesis genes psy1, psy2, pds, zds, and lcyb based on calibration with in vitro transcribed RNA of the respective genes. Error bars represent 2 sd as calculated from errors of quantitative RT-PCR, dilution, and determination of total mRNA concentration.
Down-Regulation of PDS Led to an Accumulation of Phytoene in Leaves of Tobacco
From 55 independent lines with PDS−, six showed an accumulation of phytoene, ranging from 50 to 1,400 nmol g−1 plant material (Table III). In wild-type SR1 and in control plants bearing the empty transformation vector, no phytoene could be detected. The total carotenoid content in these plants was higher than in control plants that have an average total concentration of about 440 nmol g−1. The phytoene content was related to the content of total residual carotenoids. The resulting phytoene to carotenoid-phytoene ratio gives a measurement of the proportion of precursors accumulating because of inhibition of PDS in comparison with those that can still be processed. The highest phytoene concentration of 1,399 nmol g−1 plant material was measured in line PDS−47, resulting in a phytoene to carotenoid − phytoene ratio of 3.91 mol mol−1.
Table III.
Phytoene accumulation, phy/(car-phy) ratios and phenotypes of selected transgenic tobacco plants expressing antisense RNA to PDS
| Line | Total Chlorophylls | Total Carotenoids | Phytoene | Phytoene to Carotenoid − Phytoene Ratio | Phenotype |
|---|---|---|---|---|---|
| nmol/g−1 | nmol/g−1 | nmol/g−1 | mol/mol−1 | ||
| PDS−13 | 1,433 ± 115 | 625 ± 64 | 289 ± 58 | 0.86 | White capsules |
| PDS−24 | 1,783 ± 143 | 535 ± 43 | 133 ± 27 | 0.33 | White spots |
| PDS−30 | 1,841 ± 147 | 868 ± 124 | 469 ± 94 | 1.17 | White areas |
| PDS−35 | 2,347 ± 188 | 561 ± 39 | 50 ± 10 | 0.10 | Like wild type |
| PDS−47 | 1,854 ± 148 | 1,756 ± 280 | 1,399 ± 280 | 3.91 | White veins |
| PDS−54 | 2,166 ± 173 | 528 ± 42 | 48 ± 10 | 0.10 | Like wild type |
| Control (mean) | 1,961 ± 251 | 437 ± 26 | 0 ± 0 | 0.00 | Like wild type |
Notably, the phenotype of those phytoene-accumulating lines showed some differences regarding bleaching. While lines PDS−35 and 54 having the slightest phytoene accumulation looked like control plants, line PDS−24 showed some white spots of 3 to 5 mm in diameter (Fig. 4F). The even more phytoene-accumulating lines showed white areas in between the veins (PDS−30, Fig. 4G) and white veins and leaf tips (PDS−47, Fig. 4H). Bleaching appeared 4 to 5 weeks after setting the plants to the greenhouse. One exception was line PDS−13 that produced white capsules (Fig. 4, D and E) but showed a completely normal leaf phenotype.
In the F1 Generation of Line PDS−13, White Kanamycin-Resistant Seedlings Were Not Viable Either in Sterile Culture or in the Greenhouse
To examine the phenotype in homozygous plants, the phytoene-accumulating lines were allowed to self. For most of the lines the segregation indicated two or more insertions of the transgene into the genome, but progeny of line PDS−13 segregated into three phenotypes in a 1:2:0.9 ratio. One phenotype was kanamycin sensitive, representing the wild type. Heterozygous plants were phenotypically normal during vegetative growth and developed white capsules. Homozygous plants were initially green but turned white by the four-leaf stage. The seedlings bleached out from the shoot axes to the leaf tips of the primary and secondary leaves. Finally, these seedlings turned completely white. In sterile culture, the chlorotic seedlings developed up to eight leaves at the most. When set to the greenhouse these seedlings died within 1 week (Fig. 4I). It is interestingly that in sterile culture primary transformant PDS−27 showed a phenotype like the chlorotic seedlings from line PDS−13, but we were not able to grow this plant or set it to the greenhouse to get seeds. The bleaching was also observed in lines with multiple insertions, indicating that this phenotype is not caused by an insertion effect.
To measure carotenoid contents in seedlings from line PDS−13, we pooled green ones and white ones separately and used them for HPLC analysis. In parallel, we pooled seedlings of control plants. The green seedlings of line PDS−13 showed a strong accumulation of phytoene to 1,033 nmol g−1 plant material, accounting for a phytoene to carotenoid − phytoene ratio of 8 mol mol−1. In the white seedlings, phytoene was the only carotene with an amount of 681 nmol g−1 plant material and a phytoene to carotenoid − phytoene ratio of 49 mol mol−1. Notably, small amounts of xanthophylls were still detectable with a relative concentration of about 10% in comparison with control seedlings.
When Expressing Antisense RNA to psy1, psy2, and pds, Transcript Levels of Other Carotene Biosynthesis Genes Remain Unchanged
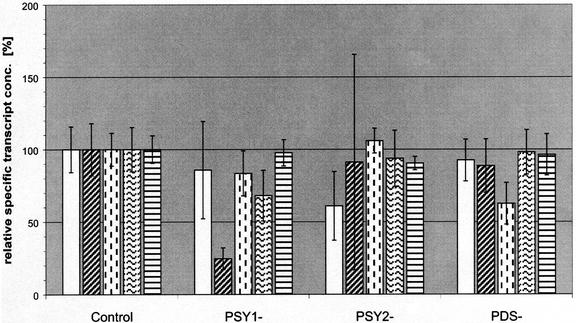
Expression of antisense RNA to pds in transgenic tobacco plants led to severe phenotypic and metabolic changes even in the parental generation, whereas for psy1 generating F1 progeny was a presumption for phenotypic effects. Antisense expression of psy2 resulted in no deviations in comparison with control plants. There might be some kind of transcriptional regulation to overcome the effects of antisense expression of the respective genes in leaves of tobacco. To illuminate this, each five plants expressing antisense RNA to psy1, psy2, and pds were taken for detailed analysis of transcript levels by quantitative RT-PCR. It has to be mentioned that probes and primers used for quantitative RT-PCR are able to bind to sense RNA as well as antisense RNA and will thus amplify both RNA types in these plants expressing antisense RNA to one of the genes. The transcript levels of all carotene biosynthesis genes showed no significant changes (see error bars) but with the following exceptions (Fig. 6). In plants expressing antisense RNA to psy1 the transcript level of psy2 was reduced to about 25%. The opposite effect was much weaker because in PSY2− plants the transcript level of psy1 was reduced to just about 65%.
Figure 6.
Unaffected transcript levels of carotenoid biosynthesis genes despite antisense expression of psy1, psy2, and pds. Poly(A+) RNA was isolated from mature leaves of 7-week-old transgenic tobacco plants from the greenhouse. mRNA of lines PSY1−02, 03, 12, 15, and 39; lines PSY2−04, 06, 17, 22, and 32; and PDS−13, 24, 30, 35, and 47 was subjected to quantitative RT-PCR using specific primers and probes for quantification of carotenoid biosynthesis genes psy1, psy2, pds, zds, and lcyb based on calibration with in vitro transcribed RNA of the respective genes. Transcript concentrations (conc.) are represented as mean values of each set (PSY1−, PSY2−, and PDS−) of five plants. Error bars represent 2 sd as calculated from errors of quantitative RT-PCR, dilution, and determination of total mRNA concentration.
DISCUSSION
Based on high sequence conservation among either psy genes or zds genes from different plant species and a known fragment of the Nicotiana benthamiana pds gene, we were able to clone the respective genes from tobacco (N. tabacum) by using various PCR-based methods. The cloned genes encode proteins with homologies higher than 90% identity to the respective genes from other plant species and share conserved sequence motifs.
The high sequence similarities, but more importantly the accumulation of phytoene in PSY-expressing transgenic lines and in plants expressing antisense RNA to pds, indicate that the genes encode functional PSYs and PDS. For ZDS, this remains to be demonstrated.
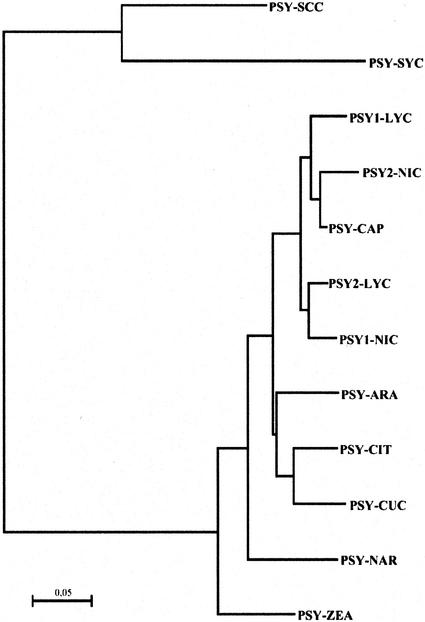
The finding of two PSY genes in tobacco was surprising because most plants, including Arabidopsis, with a completely sequenced genome have only one gene. So far the only other species with two psy genes known is tomato. In tomato, the two enzymes are related to different organs, psy1 to the fruit and psy2 to the leaf. For the closely related pepper, which also produces carotenoids in the fruits, however, only one psy gene has been found (Römer et al., 1993; Ha et al., 1999). Phylogenetic analysis of psy genes from different plant species and two cyanobacteria revealed grouping of tobacco psy1 and 2 with tomato psy2 and 1, respectively (Fig. 7). One can assume a duplication event of an ancestral psy gene that took place prior to separation of Lycopersicon and Nicotiana into different genera.
Figure 7.
Phylogenetic tree of PSYs from different plant and cyanobacterial origin. Deduced peptide sequences of different PSYs were aligned using ClustalX 1.8 (Thompson et al., 1997) and a phylogenetic tree was drawn using the neighbor-joining method (Saitou and Nei, 1987). SYC, Synechocystis sp. (accession no. P37294); SCC, Synechococcus sp. (accession no. P37269); LYC, L. esculentum (PSY1, accession no. P08196; PSY2, accession no. P37273); NIC, N. tabacum; CAP, C. anuum (accession no. P37272); ARA, Arabidopsis (accession no. AF009954; F. Castrignano and G. Giuliano, unpublished data); CIT, Citrus × paradisi (accession no. AF192852); CUC, Cucumis melo (accession no. P49293); NAR, N. pseudonarcissus (accession no. P53797); ZEA, Zea mays (accession no. P49085).
Overexpression of both psy genes resulted in a severe dwarf phenotype along with changes in pigment composition and an accumulation of phytoene. When comparing hemi- and homozygous plants of individual transgenic lines, the severeness of both phenotype and metabolite changes correlated with transcript levels of overexpressed psy gene. Whereas a moderate expression level just led to slightly increased carotenoid content, a strong increase in psy expression led to a dwarf phenotype and a decrease in total pigment content, along with accumulation of phytoene. Other researchers have attributed the dwarf phenotype in psy-overexpressing plants to reduced gibberellin levels that result from an increased utilization of GGPP into phytoene at the expense of the gibberellin and other pathways, e.g. biosynthesis of phytol, tocopherol, phylloquinone, and plastoquinone (Fray and Grierson, 1993; Fray et al., 1995; Shewmaker et al., 1999).
Antisense expression of PSY in transgenic tobacco plants resulted in an increase in chlorophyll to carotenoid content in some PSY1− plants but no differences in visible phenotype compared with control plants. Comparable results were reported for viral expression of antisense RNA to bacterial psy in N. benthamiana, which resulted in no phenotype apart from effects due to viral infection (Kumagai et al., 1995). The expression of antisense RNA to tomato psy1 resulted in inhibition of carotenoid biosynthesis in flowers and fruits but had no effect in leaves (Bramley et al., 1992; Jones et al., 1998).
Plants that had reduced levels of psy had reduced levels of both carotenoids and chlorophyll. Earlier experiments had shown that carotenoids are required not only for photoprotection but also for the functional assembly of the pigment-protein complexes of the light-harvesting complexes and the buildup of primary thylakoids (Karapetyan et al., 1991; Bolychevtseva et al., 1995). The lack of chlorophyll can then be attributed to the reduced carotenoid content.
Phenotypes were observed for psy1 antisense expression but not for psy2. This can be attributed to the different expression levels of the genes. Antisense expression of psy1 results in a strong decrease of the total amount of PSY, whereas antisense expression of psy2 results in only the decrease of the already low amount of psy2 with psy1 mostly remaining. The observed cross-antisensing is obviously not sufficient to decrease the psy1 in psy2 antisense plants.
Unlike PSY+ plants, no transgenic lines overexpressing pds showed any phenotypic or metabolic effects, although there was a strong increase in transcript levels in most analyzed lines, implying that PDS is regulated post-transcriptionally. This notion is supported by studies indicating that PDS is activated from soluble inactive forms by flavinylation and membrane association (Al Babili et al., 1996; Bonk et al., 1997). This activation may be linked to and eventually regulated by an electron transport chain that involves plastoquinone and PTOX (Carol et al., 1999; Wu et al., 1999; Carol and Kuntz, 2001). Taking these results into account, one might infer that an increase in expression of pds could lead to increased transcript level and even protein level without having any effect on carotenoid biosynthesis.
Antisense expression of pds in transgenic tobacco plants led to an accumulation of the direct precursor phytoene to different extents. These differences could be due in part to position effects (Wilson et al., 1990). Homozygous antisense expression in line PDS−13 led to a lethal phenotype, as did some progeny of lines with multiple insertions of the transgene, excluding insertion effects as the reason for the phenotype. Along with phytoene accumulation, there was a concomitant decrease in β-carotene, xanthophylls, and chlorophylls. In general, we found similar reduction rates for xanthophylls and chlorophylls. The decrease in xanthophylls is a direct consequence of the reduced synthesis of higher desaturated carotenes. The decrease in chlorophyll content, however, fits the same reason as for antisense expression of psy1. However, the increase in total carotenoids in plants accumulating phytoene might indicate some kind of up-regulation by an increased flow of metabolites into the carotenoid biosynthesis pathway.
Regulation of carotenoid biosynthesis was examined in greatest detail in chromoplasts of ripening fruits from tomato, pepper, and daffodil (Narcissus pseudonarcissus). Concerning regulation in leaves and seedlings, there are contradictory results. Increased psy and pds transcript levels were found in tomato seedlings treated with the PDS inhibitor norflurazon (Giuliano et al., 1993; Scolnik and Giuliano, 1994) and plants of the tomato mutant ghost bearing a mutation in the tomato gene encoding PTOX (Scolnik et al., 1987; Josse et al., 2000), indicating an end product or stress-dependant regulation. The same conclusion was made for transgenic tobacco plants having a chimeric PDS promoter/β-glucuronidase fusion that upon norflurazon treatment yielded higher β-glucuronidase activity (Corona et al., 1996). Unchanged expression levels were reported for norflurazon-treated Arabidopsis plants (Wetzel and Rodermel, 1998), in the mutant IMMUTANS (Wetzel et al., 1994), and for Capsicum annum plants treated with different inhibitors for PDS alone or PDS and ZDS together. The transcript levels of psy, pds, zds, and PTOX remained constant (Simkin et al., 2000). For tobacco, we found unchanged transcript levels for all examined carotenoid biosynthesis genes (psy1, psy2, pds, zds, lcyb) although there were severe depletions in carotenoid biosynthesis comparable with those by norflurazon treatment. The increase in total carotenoids due to inhibition of phytoene desaturation by different approaches thus implies a regulation that obviously is performed by different modes in tomato on one side and tobacco, pepper, and Arabidopsis on the other. For the latter, a post-transcriptional regulation can be favored and remains to be elucidated.
Blocking of the pathway leading to carotenes at the step of PSY and PDS led to strong effects both at the metabolic and at the phenotypic level. It was demonstrated that antisense expression of both enzymes can lead to lethal phenotypes, and expression of PSY imposes severe damage to transgenic tobacco plants. Both enzymes can be regarded as herbicide targets, which was clearly known before for PDS, because herbicides blocking this step are known but not for PSY, for which there are no known inhibitors and so far no plants with a block at that step.
MATERIALS AND METHODS
Cloning of PSY, PDS, and ZDS
The tobacco (Nicotiana tabacum cv Petit Havana SR1) psy was cloned by amplification of a partial coding sequence using primers specific for the psy sequence of Arabidopsis (Castignano and Giuliano, 1997). Tobacco cDNA (cDNA synthesis kit; Stratagene, Heidelberg) was prepared from total RNA (RNeasy plant; Qiagen, Hilden, Germany) extracted from 4-week-old seedlings. The cDNA was ligated to marathon adaptors (CLONTECH, Palo Alto, CA). Using the Arabidopsis specific primers (5′-TAT GCT AAG ACG TTT TAT CTT GGA AC and 5′-CCA TAC AGG CCA TCT GCT AGC) two fragments were amplified and sequenced according to the method of Sanger and Coulson (1974). 5′- and 3′-terminal fragments were cloned based on a RACE protocol by amplification with each gene-specific primer (5′-CCA TCG ACT AGC TCA TCC GTT CTC CTG CAC C and 5′-AAG CCG GTC TTC CCA CCT ATC TAA GGC TTG G for the 5′-terminal fragment of psy1 and 2, respectively, and 5′-AGT AGG ACT GAT GAG TGT TCC AGT TAT GGG TAT TGC ACC for the 3′-terminal fragment of both psy1 and 2) and primer ap1 (CLONTECH). Resulting fragments were sequenced, and psy1 and psy2 were assembled according to overlapping sequences.
The tobacco pds was cloned based on a 369-bp sequence from Nicotiana benthamiana phytoene dehydrogenase precursor mRNA, partial coding sequence (Kumagai et al., 1995; accession no. U19262) by using a RACE protocol. 5′-Terminal and 3′-terminal fragments of pds were amplified with each gene-specific primer (5′-CCA CCT TTT GAC TCA ATA TGT TCC ACA ATC GGC and 5′-GTC AAA AGG TGG CCA AGT CAG ACT AAA CTC ACG, respectively) and ap1 primer (CLONTECH). The resulting fragments were sequenced and pds was assembled according to overlapping sequences.
The tobacco zds was cloned by amplification of a 3′-terminal portion of the gene from cDNA using an Arabidopsis forward primer (5′-GAG CTG GAC TTG CAG GCA TGT CG) and an oligo(dT)18 primer. The resulting fragment was sequenced. A 5′-terminal fragment of ZDS was amplified from cDNA ligated to marathon adaptors (see above) by using one gene-specific primer (5′-TCC ACC TCA TGT CCT TGA TCC AAG AGC TCC) and primer ap1 (CLONTECH). The resulting fragment was sequenced and the sequence of ZDS was assembled according to overlapping sequences.
Sequences of psy1, pds, and zds were confirmed by amplification of the genes from a different cDNA with primers binding at the very ends of the completely assembled sequences (5′-AGA AAC CCA GAA AGA ACA ACA GGT TTT G and 5′-CTC ACT TGA GGG TTT GAT GAG TGT GG for psy1, 5′-GGC CTT TCC ACC ACA AAT TTC CAG and 5′-GCA CAT ATT TTG TGT AAC ATT TCT CGT ATT TGG for pds, and 5′-CTG GCA TCT ATC TGC CAA ATT TCC and 5′-TCT TCT CAA TGA ATG ATG AGC AAT ACG ATC C for zds). The resulting fragments were sequenced.
Plasmid Constructions
The tobacco psy1- and pds-coding sequences were amplified from tobacco SR1 cDNA using primers generating an XmaI restriction fragment after cleavage with XbaI (5′-TTC CCG GGT TGT TTC ATG AGC ATG and 5′-TTC CCG GGT CAT TCA TGT CTT TGC for psy1 and 5′-TTC CCG GGC TCA GTA AAA TGC C and 5′-TAC CCG GGC TAA ACT ACG CTT GC for pds). The tobacco psy2-coding sequence was amplified from tobacco SR1 cDNA using primers generating an EcoRI restriction fragment after cleavage with EcoRI. The resulting XmaI and EcoRI fragments were ligated into the XmaI and EcoRI restriction site of the binary plant expression vector pSS (Voss et al., 1995), resulting in vectors pPSY1+ and pPSY1− for psy1, pPSY2+ and pPSY2− for psy2, and pPDS+ and pPDS− for pds. The superscripts + and − indicate the orientation of the transgene as sense and antisense, respectively, as identified by restriction analyses. pSS is a derivative of the plasmids pPCV002 and pRT101 (Koncz and Schell, 1986; Töpfer et al., 1987) and contains a double 35S RNA promoter in combination with the termination sequence of the 35S RNA. In parallel, zds and lcyb (Cunningham et al., 1996; accession no. X81787) were amplified from cDNA with primers generating an XmaI restriction fragment after cleavage with XmaI (5′-AAC CCG GGA TAG CAC GAT TCA ATG and 5′-AAC CCG GGA TTT CCA GTC ATC AGA C for zds and 5′-TTC CCG GGT GTT GGA AGA TAT GG and 5′-TTC CCG GGT TCC TGG TAA GTC ATT C for lcyb). XmaI and EcoRI fragments of carotene biosynthesis genes psy1, psy2, pds, zds, and lcyb were ligated into XmaI and EcoRI-digested and -dephosphorylated vector pGEM7Zf(+). The orientation of the transgenes was identified by restriction analysis with HindIII (Promega, Mannheim, Germany). The resulting plasmids were used for generating RNA by in vitro transcription (RiboMax large-scale RNA production system, Promega). All plasmid manipulations were carried out according to the methods described by Sambrook et al. (1989).
Plant Transformation
N. tabacum cv Petit Havana SR1 (Maliga et al., 1973) was transformed with the constructs pPSY1±, pPSY2±, and pPDS± by Agrobacterium tumefaciens-mediated gene transfer according to the method of Horsch et al. (1985). Vector pSS was used to generate kanamycin-resistant control plants.
In parallel, transformation was performed by cocultivation of regenerating tobacco protoplasts with A. tumefaciens (Fischer and Hain, 1995). Leaf protoplasts of tobacco were isolated from sterile shoot culture grown on half-concentrated LS medium (Linsmaier and Skoog, 1965) according to the method of Nagy and Maliga (1976). Protoplasts at a concentration of 1 × 106/6 mL K3 medium (Nagy and Maliga, 1976) were cultured for 3 d in the dark. Regenerating protoplasts were mixed with 100 to 200 Agrobacteria (grown in YEB medium) per protoplast and cocultured for 3 d at 26°C and 3,000 lux. Plant cells were washed twice with W5 medium (0.125 m CaCl2, 0.155 m NaCl, 5 mm KCl, 5 mm Glc, pH 5.6) and resuspended in K3 medium containing 1 mg L−1 naphthylacetic acid, 0.2 mg L−1 kinetin, and 500 mg L−1 cefotaxim. Plant cell suspensions were incubated for 1 d under the conditions described above. The plant cells were beaded in agarose (Shillito et al., 1983) and agarose beads were kept under K3 medium. Medium was replaced every weak with fresh K3 medium and osmotic pressure was decreased by 0.1 m Suc every 2 weeks. Selection of transformants was started when refreshing medium for the first time by adding 100 mg L−1 kanamycin acid sulfate (Sigma, Deisenhofen, Germany).
Regeneration of Plants
SR1 wild-type and transgenic tobacco plants from leaf disc transformation were regenerated on LS medium (Linsmaier and Skoog, 1965), and calli from protoplast cocultivation were regenerated on selective (100 mg/L kanamycin acid sulfate) or nonselective LS medium containing 0.5 mg L−1 benzylaminopurine. Kanamycin-resistant tobacco shoots were selected on LS medium containing 100 mg L−1 kanamycin. After rooting, shoot tips were cut off and transferred to fresh LS medium in sterile culture. Residual shoots were set to soil and grown in the greenhouse. The individual transformants were named according to the transformed plasmid and consecutively numbered.
Seeds of transgenic tobacco were germinated on LS medium containing 200 mg L−1 kanamycin. After 3 weeks, kanamycin-resistant F1 seedlings were transferred to soil.
Carotenoid Content
One fully developed leaf was cut off and immediately frozen in liquid nitrogen. Plant material was disrupted with a mortar and pestle and stored for further analysis. An aliquot of 0.4 g was extracted with 3 mL of acetone. Filtered extract (25 μL) was measured on a Prontosil 200–5 C30 5-μm column (Macherey & Nagel, Dueren, Germany) with a length of 15 cm using a 30-min gradient of ethyl acetate (30% to 60%), followed by a 5-min gradient of ethyl acetate (60% to 90%), and followed by an isocratic solvent of ethyl acetate (90%) in methanol:water (9:1 [v/v]) at a flow rate of 1 mL min−1. Identification of carotenoids was carried out using their retention time relative to known standards with detection at 280-, 350-, 400-, and 450-nm wavelength. The absorption spectra of individual peaks were taken and compared with published data (Britton, 1995).
RNA Isolation and Northern-Blot Analysis
Poly(A+) RNA was purified from frozen tobacco material by the use of Dynabeads oligo(dT)25 (Dynal, Oslo) and used in northern hybridization as indicated in the figure legends. RNA was separated on a denaturing 1.5% agarose/formaldehyde gel and transferred to nitrocellulose filters according to the method of Sambrook et al. (1989). As probes, psy and pds fragments were labeled by random primed labeling (Stratagene) to a specific activity >8 × 108 cpm μg−1 DNA. Hybridization was for 16 h at 42°C, and washes were at 50°C in 0.1× SSC and 0.1% SDS. X-ray films were exposed for 3 d at −70°C using intensifier screens.
Quantitative RT-PCR
Quantitative RT-PCR was performed using the Light Cycler Instrument (Roche, Mannheim, Germany) with hybridization probes based on fluorescence resonance energy transfer and the Light Cycler RNA Amplification Kit Hybridization Probes (Roche). Primers and probes were designed for quantification of fragments of about 300 bp each in the C-terminal region of psy1, psy2, pds, zds, and lcyb. In vitro transcribed RNA (ivRNA) of the genes was quantified using SYBR Green II (Sigma) in a fluorometric assay (Schmidt and Ernst, 1995). Dilutions of 10−3 to 10−8 representing ivRNA concentrations of about 2 ng μL−1 to 20 fg μL−1 were used in quantitative RT-PCR for calibration and for calculating relative contents of specific transcripts in mRNA preparations from transgenic plants in comparison with control plants. The contents were related to total mRNA concentration determined by SYBR Green II quantification (see above). The specificity of each pair of probes was shown by cross-test with ivRNA of other genes that resulted in no detection (data not shown). The contamination with genomic DNA was excluded by performing the quantitative RT-PCR without the initial step of 10 min at 55°C, thus excluding the reverse transcription. A negligible contamination of 0.04% at the most was determined. The primer and probes used (with fluorescein label [F], LC-Red640 label [L], phosphorylation [p]) were as follows: for psy1, 5′-GTT ACT ATG TTG CTG GTA CTG TAG GA, 5′-GCT TCA ATC TCG TCC AAT ATC TTG, 5′-TGG ATG AGT CAG AGA AAG GTG TCA CAG A-F, 5′-L-TGG ACT CTG CTA GTA GAT GGC CTG T-p; for psy2, 5′-TCA GAG ATG TTG GAG AAG ATG C, 5′-GCT TCA ATC TCG TCC AAT ATC TTG, 5′-CAG AGG AAG GAG TTA CAC AAC TGA GCT-F, 5′-L-AGC TAG CAG ATG GCC TGT ATG GG-p; for pds, 5′-AGG AAT ATT ACA ACC CCA ATC, 5′-CGT AAT CCT GTA CAA TAG CTT G, 5′-TAT TTA GCT GGT GAC TAC ACG AAA CAG AA-F, 5′-L-AGT ACT TGG CTT CAA TGG AAG GTG CTG-p; for zds, 5′-CTT GCA TTG GCA TCT CCT GAA G, 5′-TAT GCA GAT GCT TGC CTA CCT G, 5′-GCA TGT AAG GGT CAC CAG GTG TAA GGA CA-F, 5′-L-ATT GAA GCA ATG AGC CTT GGC CCT C-p; for lcyb, 5′-TTG AAG AGG ACG AGC ATT GTG TAA, 5′-CAT CGA AAA ACC TTC TTG TAG CG, 5′-TGT TGA GGG ATG AAC CAG ACC AGC T-F, 5′-L-ACC ACC AGT TCC AAC AAC TCT CTG AGG-p.
ACKNOWLEDGMENTS
We would like to thank Neil Hoffman for critical reading of the manuscript and Claudia Szadkowski, Gabi Lachner, Dorit Boetzel, and Ippazio Cicerello for their great technical assistance in various aspects of this work.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010573.
LITERATURE CITED
- Al-Babili S, von Lintig J, Haubruck H, Beyer P. A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissuschromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J. 1996;9:601–612. doi: 10.1046/j.1365-313x.1996.9050601.x. [DOI] [PubMed] [Google Scholar]
- Albrecht M, Klein A, Hugueney P, Sandmann G, Kuntz M. Molecular cloning and functional expression in E. coliof a novel plant enzyme mediating ζ-carotene desaturation. FEBS Lett. 1995;372:199–202. doi: 10.1016/0014-5793(95)00978-i. [DOI] [PubMed] [Google Scholar]
- Bartley GE, Sandmann PA. Molecular biology of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:287–301. [Google Scholar]
- Bartley GE, Scolnik PA. cDNA cloning, expression during development, and genome mapping of psy2, a second tomato gene encoding phytoene synthase. J Biol Chem. 1993;268:25718–25721. [PubMed] [Google Scholar]
- Bartley GE, Viitanen PV, Bacot KO, Scolnik PA. A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. J Biol Chem. 1991;267:5036–5039. [PubMed] [Google Scholar]
- Böger P, Sandmann G. Carotenoid biosynthesis inhibitor herbicides: mode of action and resistance mechanisms. Pestic Outlook. 1998;9:29–35. [Google Scholar]
- Bolychevtseva YV, Ramhimberdieva MG, Karapetyan NV, Popov VI, Moskalenko AA, Kuznetsova NY. The development of carotenoid-deficient membranes in plastids of barley seedlings treated with norflurazon. J Photochem Photobiol Biol. 1995;27:153–160. [Google Scholar]
- Bonk M, Hoffmann B, von Lintig J, Schledz M, Al-Babili S, Hobeika E, Kleinig H, Beyer P. Chloroplast import of four carotenoid biosynthetic enzymes in vitro reveals differential fates prior to membrane binding and oligomeric assembly. Eur J Biochem. 1997;247:942–950. doi: 10.1111/j.1432-1033.1997.00942.x. [DOI] [PubMed] [Google Scholar]
- Bramley P, Teulieres C, Blain I, Bird C, Schuch W. Biochemical characterization of transgenic tomato in which carotenoid biosynthesis has been inhibited through the expression of antisense RNA to pTOM5. Plant J. 1992;2:343–349. [Google Scholar]
- Britton G. Carotenoids. 1B: Spectroscopy. Basel: Birkhäuser Verlag; 1995. [Google Scholar]
- Carol P, Kuntz M. A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci. 2001;6:31–36. doi: 10.1016/s1360-1385(00)01811-2. [DOI] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M. Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell. 1999;11:57–68. doi: 10.1105/tpc.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona V, Aracri B, Kosturkova G, Bartley GE, Pitto L, Giorgetto L, Scolnik PA, Giuliano G. Regulation of a carotenoid biosynthesis gene promoter during plant development. Plant J. 1996;9:505–512. doi: 10.1046/j.1365-313x.1996.09040505.x. [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Pogson B, Sun Z, McDonald KA, DellaPenna D, Gantt E. Functional analysis of the β and ε lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell. 1996;8:1618–1626. doi: 10.1105/tpc.8.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Hain R. Tobacco protoplast transformation and use for functional analysis of newly isolated genes and gene constructs. Methods Cell Biol. 1995;50:401–410. doi: 10.1016/s0091-679x(08)61046-8. [DOI] [PubMed] [Google Scholar]
- Fraser PD, Kiano JW, Truesdale MR, Schuch W, Bramley PM. Phytoene synthase-2 enzyme activity in tomato does not contribute to carotenoid biosynthesis in ripening fruit. Plant Mol Biol. 1999;40:687–698. doi: 10.1023/a:1006256302570. [DOI] [PubMed] [Google Scholar]
- Fray RG, Grierson D. Identification and genetic analysis of normal an mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol. 1993;22:589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Branley PM, Grierson D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. [Google Scholar]
- Giuliano G, Bartley GE, Scolnik PA. Regulation of carotenoid biosynthesis during tomato development. Plant Cell. 1993;5:379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S-H, Lee S-W, Kim J-G, Hwang Y-S. Expression patterns of genes involved in carotenoid biosynthesis in pepper. Agric Chem Biotechnol. 1999;42:92–96. [Google Scholar]
- Henikoff S, Henikoff JG. Automated assembly of protein blocks for database searching. Nucleic Acids Res. 1991;19:6565–6572. doi: 10.1093/nar/19.23.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J, Cohen M, Harker M, Lotan T, Mann V, Pecker I. Molecular genetics of the carotenoid biosynthesis pathway in plants and algae. Pure Appl Chem. 1997;69:2151–2158. [Google Scholar]
- Horsch R, Fry J, Hoffmann N, Eichholtz D, Rogers S, Fraley R. A simple method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Jones CG, Scothern GP, Lycett GW, Tucker GA. The effect of transgene architecture on co-ordinated gene silencing. Planta. 1998;204:499–505. [Google Scholar]
- Josse E-M, Simkin AJ, Gaffé J, Laboré A-M, Kuntz M, Carol P. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol. 2000;123:1427–1436. doi: 10.1104/pp.123.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapetyan NV, Bolychevtseva YV, Rakhimberdieva MG. The necessity of carotenoids for the assembly of active photosystem II reaction centers. In: Duglas RH, Moan J, Rönto G, editors. Light in Biology and Medicine. Vol. 2. New York: Plenum Press; 1991. pp. 45–54. [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Kumagai MH, Donson J, della-Cioppa G, Harvey D, Hanley K, Grill LK. Cytoplasmic inhibition of carotenoid biosynthesis with viral derived RNA. Proc Natl Acad Sci USA. 1995;92:1679–1683. doi: 10.1073/pnas.92.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Schwender J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plants proceeds via a mevalonate independent pathway. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. [Google Scholar]
- Maliga PS, Breznovitis A, Marton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nature New Biol. 1973;244:29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Mayer PM, Beyer P, Kleinig H. Quinone compounds are able to replace molecular oxygene as a terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus. Eur J Biochem. 1990;191:359–363. doi: 10.1111/j.1432-1033.1990.tb19130.x. [DOI] [PubMed] [Google Scholar]
- Moskalenko AA, Karapetyan NV. Structural role of carotenoids in photosynthetic membranes. Z Naturforsch. 1996;51c:763–771. [Google Scholar]
- Nagy JI, Maliga P. Callus induction and plant regeneration from mesophyll protoplasts of Nicotiana sylvestris. Z Pflanzenphysiol. 1976;78:453–455. [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D. Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell. 1995;7:2139–2149. doi: 10.1105/tpc.7.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer S, Hugueney P, Bouvier F, Camara B, Kuntz M. Expression of the genes encoding the early carotenoid biosynthetic enzymes in Capsicum anuum. Biochem Biophys Res Commun. 1993;196:1414–1421. doi: 10.1006/bbrc.1993.2410. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei N. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1974;94:441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Schmidt DM, Ernst JD. A fluorometric assay for the quantification of RNA in solution with nanogram sensitivity. Anal Chem. 1995;232:144–146. doi: 10.1006/abio.1995.0025. [DOI] [PubMed] [Google Scholar]
- Scolnik PA, Giuliano G. Regulation of carotenoid biosynthesis genes during plant development. Pure Appl Chem. 1994;66:1063–1068. [Google Scholar]
- Scolnik PA, Hinton P, Greenblatt IM, Giuliano G, Delanoy MR, Spector DL, Pollock D. Somatic instability of carotenoid biosynthesis in the tomato ghost mutant and its effect on plastid development. Planta. 1987;171:11–18. doi: 10.1007/BF00395063. [DOI] [PubMed] [Google Scholar]
- Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY. Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J. 1999;20:401–412. doi: 10.1046/j.1365-313x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Shillito RD, Paszkowski J, Potrykus I. Agarose plating and bead type culture technique enable and stimulate development of protoplast-derived colonies in a number of plant species. Plant Cell Rep. 1983;2:244–247. doi: 10.1007/BF00269151. [DOI] [PubMed] [Google Scholar]
- Siefermann-Harms D. The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol Plant. 1987;69:561–568. [Google Scholar]
- Simkin AJ, Breitenbach J, Kuntz M, Sandmann G. In vitro and in situ inhibition of carotenoid biosynthesis in Capsicum anuumby bleaching herbicides. J Agric Food Chem. 2000;48:4676–4680. doi: 10.1021/jf0006426. [DOI] [PubMed] [Google Scholar]
- Slater A, Maunders MJ, Edwards K, Schuch W, Grierson D. Isolation and characterization of cDNA clones for tomato polygalacturonase and other ripening-related proteins. Plant Mol Biol. 1985;5:137–147. doi: 10.1007/BF00015677. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss HH. A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res. 1987;14:5890. doi: 10.1093/nar/15.14.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A, Niersbach M, Hain R, Hirsch HJ, Liao YC, Kreuzaler F, Fischer R. Reduced virus infectivity in N. tabaccumsecreting a TMV-specific full-size antibody. Mol Breed. 1995;1:39–50. [Google Scholar]
- Wetzel CM, Jiang C-Z, Meehan LJ, Voytas DF, Rodermel SR. Nuclear-organelle interactions: The immutans variegation mutant of Arabidopsis is a plastid autonomous and impaired in carotenoid biosynthesis. Plant J. 1994;6:161–175. doi: 10.1046/j.1365-313x.1994.6020161.x. [DOI] [PubMed] [Google Scholar]
- Wetzel CM, Rodermel SR. Regulation of phytoene desaturase expression is independent of leaf pigment content in Arabidopsis thaliana. Plant Mol Biol. 1998;37:1045–1053. doi: 10.1023/a:1006021522259. [DOI] [PubMed] [Google Scholar]
- Wilson C, Bellen HJ, Gehring WJ. Position effects on eukaryotic gene expression. Annu Rev Cell Biol. 1990;6:679–714. doi: 10.1146/annurev.cb.06.110190.003335. [DOI] [PubMed] [Google Scholar]
- Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S. The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell. 1999;11:43–55. doi: 10.1105/tpc.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]