Abstract
Marburg virus (MARV) and Ebola virus (EBOV), members of the viral family Filoviridae, cause fatal hemorrhagic fevers in humans and nonhuman primates. High viral burden is coincident with inadequate adaptive immune responses and robust inflammatory responses, and virus-mediated dysregulation of early host defenses has been proposed. Recently, a novel class of innate receptors called the triggering receptors expressed in myeloid cells (TREM) has been discovered and shown to play an important role in innate inflammatory responses and sepsis. Here, we report that MARV and EBOV activate TREM-1 on human neutrophils, resulting in DAP12 phosphorylation, TREM-1 shedding, mobilization of intracellular calcium, secretion of proinflammatory cytokines, and phenotypic changes. A peptide specific to TREM-1 diminished the release of tumor necrosis factor alpha by filovirus-activated human neutrophils in vitro, and a soluble recombinant TREM-1 competitively inhibited the loss of cell surface TREM-1 that otherwise occurred on neutrophils exposed to filoviruses. These data imply direct activation of TREM-1 by filoviruses and also indicate that neutrophils may play a prominent role in the immune and inflammatory responses to filovirus infections.
Marburg virus (MARV) and Ebola virus (EBOV), members of the family Filoviridae, are best known as causative agents of acute hemorrhagic fevers, with viruses in both the Marburg virus and Ebola virus genera responsible for mortality rates of up to 88% in outbreaks involving hundreds of persons (4, 9, 41, 43). Subversion of the innate immune system appears to be among the factors in the profound virulence of these viruses in humans and other primates. Evidence includes the observation that markers of early innate and adaptive responses were lacking in EBOV patients for whom the infection would ultimately prove fatal, whereas survivors had detectable virus-specific immunoglobulin M, along with transient elevations in interleukin-6 (IL-6), IL-1β, tumor necrosis factor α (TNF-α), and other cytokines (1, 2, 28). Mechanistic correlates have included the observation that monocytes and dendritic cells (DC) are among the first to be infected by filoviruses in nonhuman primates (NHP) (16, 38). Moreover, one of the filoviral proteins (VP35) has been shown to be an interferon antagonist (3, 5) and dysfunctions of DC occurred upon their infection with filoviruses (5, 29), cumulatively suggesting direct negative impact on innate immunity and indirect diminution of adaptive immunity (5).
Dysregulation of inflammation is also suggested as a factor in filoviral pathogenesis. In terminal stages of disease, high viral burden in blood and organs coincides with vascular leakage and what has been called a “cytokine storm.” Some of this is attributed to the infection of monocytes and macrophages, which, unlike DC (5, 29), respond to filoviral infection by producing proinflammatory cytokines (13). Clinically, increases and shifts in neutrophils have been observed during disease (14, 30). In one set of studies (44), it was asserted that it was not the membrane-bound and virion-associated form of the viral glycoprotein (GP) that interacted with neutrophils but rather the soluble variant of EBOV GP (sGP), a finding called into uncertainty (31) and not necessarily pertinent to MARV, in which no sGP form is known (42). Thus, our investigations of the decisive early events of filoviral infection, when innate and adaptive immunity appear to be subverted and overwhelmed, and the later events of infection, when inflammation appears to exacerbate disease, led us to an examination of neutrophils as one of the pivotal cell types in these processes.
Polymorphonuclear neutrophils (PMN) constitute a major fraction of blood leukocytes and a critical arm of host defense. Upon activation, PMN migrate to inflamed sites, where they internalize and eradicate invading pathogens through an arsenal of cytotoxic agents in preformed granules. Activated neutrophils produce inflammatory cytokines, chemokines, cytolytic granules, and reactive oxygen intermediates and phagocytose infected cells (15, 24, 25, 39). Of the several neutrophil effector functions newly identified (8, 12, 26, 27, 32), one recent discovery is the presence of a receptor expressed on neutrophils, the triggering receptors expressed in myeloid cells (TREMs). TREMs are an emerging class of immunorecognition receptors encoded in a cluster on chromosome 6 in humans (6, 10). These receptors are capable of regulating various immunological events in both innate and adaptive immune cells, particularly neutrophils. TREM-1 has been implicated in the amplification of septic shock by enhancing the Toll-like receptor (TLR)-mediated production of proinflammatory cytokines (7, 10). TREM-1 signals myeloid cell activation by association with the transmembrane portion of DAP12 (10), signaling a cytosolic immunoreceptor tyrosine-based activation motif (ITAM). Activation of DAP12-coupled receptors results in transient tyrosine phosphorylation of ITAM chains, recruits and promotes activation of the tyrosine kinase Syk, and subsequently leads to activation of extracellular signal-regulated kinases 1/2 (ERK1/2) (33), resulting in the secretion of primary neutrophil cytokines and chemokines (6, 10).
Here, we studied directly the in vitro interaction of human neutrophils with MARV and EBOV. We report that although productive filovirus replication was not observed in human neutrophils, these cells rapidly activated TREM-1 molecules and the transient tyrosine phosphorylation of its adaptive receptor DAP12, mobilized intracellular calcium, and induced the production of proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α]), which were significantly down-regulated by a specific peptide derived from TREM-1.
MATERIALS AND METHODS
Human neutrophil isolation.
Enriched human neutrophils from healthy donors were isolated as described previously (15, 24, 25, 39). Briefly, neutrophils were purified from EDTA anticoagulated venous blood by centrifugation on a Histopaque gradient and dextran sedimentation following removal of erythrocytes by hypotonic water lysis. Isolated neutrophils were then washed with phosphate-buffered saline (PBS) and resuspended in Hank's balanced salt solution (HBSS) with Ca2+/Mg2+ (Biosource, Camarillo, CA) for use in the various experiments. The purity of neutrophils was typically more than 98% as determined by flow cytometry using anti-CD67 antibody.
Viruses used.
MARV is a single species taxonomically (Lake Victoria marburgvirus), but considerable variation has been observed among different isolates. The Musoke and Ravn isolates (indicated as MARVMusoke and MARVRavn), which are 22% nonidentical in the derived amino acid sequences of their GPs, were as described previously (22, 23). We obtained a variant called Ci67 (MARVCi67) from Werner Slenczka and Stephan Becker; this virus was isolated in Marburg, Germany, during the first MARV outbreak (41), has the same GP amino acid sequence as the more familiar Popp variant obtained in Frankfurt during the same outbreak, and differs from the Musoke isolate by 7% in its GP. Unless otherwise noted, the EBOV used in these studies was Zaire ebolavirus (strain Kikwit, 1995), provided by Peter Jahrling (16). In a few studies, where noted, we included Reston ebolavirus, provided by Peter Jahrling and Thomas Geisbert. For experiments with inactivated virus, the viruses were concentrated by centrifugation, resuspended, and further purified by centrifugation on an isopycnic sucrose gradient before irradiation (10 million rad, 60Co source) and safety testing; the irradiation not only guaranteed complete inactivation of infectivity (22) but also was in sufficient excess to result in an absence of new antigen synthesis by Vero cells, as measured by indirect immunofluorescence in cells exposed 10 to 14 days earlier at high particle/cell ratios (these observations are part of the safety testing). Inactivated viruses were removed from sucrose by resuspension in lipopolysaccharide (LPS)-free phosphate-buffered saline (PBS), ultracentrifugation, and resuspension to the original volume. The inactivated viruses represented approximately 100-fold concentrations by volume and titer compared with live virus supernatants. Where alphaviruses such as Venezuelan equine encephalitis virus (VEEV) were used as nonfilovirus controls, the viruses to be inactivated were prepared, sucrose gradient purified, and inactivated in a manner similar to that for MARV and EBOV (36).
Infection of human neutrophils with viruses.
All live and gamma-irradiated virus stocks used in our studies were analyzed by a Limulus amoebocyte lysate assay for endotoxin content and found to contain <0.03 IU of endotoxin/μg total protein (Bio Whittaker, Walkersville, MD). Neutrophils were treated at a multiplicity of infection (MOI) of 1 with MARVCi67, MARVMusoke, MARVRavn, EBOVZaire, or EBOVReston. All experiments using live viruses were conducted in biosafety level 4 laboratories at the U.S. Army Medical Research Institute for Infectious Diseases. Briefly, neutrophils (1 × 106 to 5 × 106) or Vero cells as control cells were incubated with filoviruses. After 1 h at 37°C in a 5% CO2 incubator, the unadsorbed viruses were washed, and neutrophils or Vero cells were resuspended in fresh HBSS with Ca2+/Mg2+ or complete Eagle minimum essential medium. Cell supernatants were removed after 6, 24, and 48 h and evaluated for viral titer by plaque assay (22).
Activation of human neutrophils by filoviruses.
Neutrophils were treated with inactivated (1 μg/100 μl) or live (MOI = 1) filoviruses as indicated for 1 or 6 h. For inactivated viruses, the 1-μg/100-μl concentrations used for neutrophil treatment were determined by empirical titration as shown in Results. By inference from preinactivation infectivity titers (see above), we estimated that the 1 μg of inactivated virus used in most experiments contained the equivalent of approximately 107 PFU, such that the number of filovirus PFU equivalents used per cell in the inactivated preparations was approximately 2- to 10-fold higher than that used with live virus treatments at an MOI of 1 (variations depending on cell numbers). We note that for live viruses, the particle/PFU ratio for filoviruses has been estimated previously at around 30 (17). VEEV was used as a control in all experiments.
Flow cytometry.
Human neutrophils (1 × 106 to 2 × 106) were placed in a V-bottom 96-well plate and stimulated with either live or inactivated virus as described above. At the end of each incubation, cells were washed and subsequently stained with CD11b-fluorescein isothiocyanate (FITC), CD45-FITC, CD67-FITC (Biosource, Camarillo, CA), CD18-FITC (Dako, Carpinteria, CA), CD62L-FITC (BD Biosciences, San Diego, CA), and TREM-1 phycoerythrin (PE) (R&D, Minneapolis, MN). All samples were analyzed by a laser FACSCalibur (BD, La Jolla, CA).
DAP12 phosphorylation.
Phosphorylated DAP12 was detected by Western blotting as previously described (6). Briefly, human neutrophils (107/100 μl of HBSS with Ca2+/Mg2+) were incubated with inactivated MARVCi67 (iMARVCi67) (1 μg/100 μl) or iEBOVZaire (1 μg/100 μl) for 0, 1, 5, or 10 min at 37°C. Pervanadate (sodium orthovanadate plus H2O2; Sigma, St. Louis, MO) was used as a positive control (33). After stimulation, neutrophils were lysed and lysates were immune precipitated using polyclonal anti-DAP12 antibody (kindly provided by D. McVicar, NCI, Frederick, MD), separated on a 10% Bis-Tris NuPAGE gel (Invitrogen, Carlsbad, CA), and transferred onto activated polyvinylidene difluoride membranes (Millipore, Billerica, MA). Membranes were treated with mouse antiphosphotyrosine monoclonal antibody 4G10 (Upstate, Lake Placid, NY). Washed membranes were incubated with anti-mouse immunoglobulin G-horseradish peroxidase (HRP) and developed with Super Signal Substrate (Pierce, Rockford, IL) according to the manufacturer's instructions. Total DAP12 was detected using a rabbit anti-human DAP12 polyclonal antibody according to the manufacturer's instructions (Cell Sciences, Canton, MA). Additionally, ERK1/2 was determined in filovirus-activated neutrophils using specific anti-ERK1/2 phosphotyrosine (Cell Signaling, Beverly, MA) or total ERK1/2 polyclonal antibody (Cell Signaling, Beverly, MA). Escherichia coli LPS (Sigma, St. Louis, MO) was used as a positive control (300 ng/100 μl). The membranes were then probed with a secondary anti-rabbit-HRP (MP Biomedicals, Irvine, CA). Membranes were then stripped and reprobed with an anti-β-actin (Chemicon, Temecula, CA). Chemiluminescence was detected according to the manufacturer's instructions.
Calcium flux assay.
Freshly isolated neutrophils (5 × 106/ml) were resuspended in HBSS with Ca2+/Mg2+ with 1% fetal calf serum in a light-blocked container and loaded with 2.5 μg/ml of Fluo-4AM, 5 μg/ml of Fura-2AM, and 0.5% pluronic acid (Molecular Probes, Eugene, OR) for 30 min at 37°C. Cells were then washed twice in ice-cold HBSS with Ca2+/Mg2+ before being resuspended at 107 cells/ml in cation-supplemented HBSS with Ca2+/Mg2+. Cells were then diluted 1:10 in 37°C supplemented HBSS with Ca2+/Mg2+ just prior to use. Neutrophils were stimulated with inactivated viruses as described above followed by ionomycin to establish maximum flux. Ratiometric data were collected on a FACSCalibur (BD Systems, La Jolla, CA) and then analyzed using FACS Press (Ray Hicks) and Excel (Microsoft, Redland, WA).
Confocal microscopy.
Confocal microscopy was conducted as previously described (34). Briefly, neutrophils were stimulated with live (MOI = 10 to 100) or inactivated (1 μg/100 μl) MARVCi67 for 6 h at 37°C. Cells were washed and fixed with 1% formaldehyde. Treated and untreated neutrophils were permeabilized with 1% Triton X. Fluorescent markers used included 5D7 Alexa 568 antibody against MARVGP (22), phalloidin Alexa 488, Hoechst dye (Molecular Probes, Eugene, OR), and anti-TREM-1 Alexa 488. Stained cells were then visualized with a Bio-Rad 2000 MP confocal multiphoton system attached to a Nikon TE300 inverted microscope. To compare cells in the presence and absence of MARV exposure, image acquisition and subsequent contrast enhancement were performed identically for the two conditions.
ELISA.
Neutrophils (107) were stimulated with either live or inactivated filoviruses as described above for 1 or 6 h at 37°C. Supernatants and cell lysates were separately assayed by enzyme-linked immunosorbent assay (ELISA) kits for IL-1R antagonist (IL-1Ra), IL-6, IL-12, IL-17, TNF-α, macrophage inflammatory protein 1α (MIP-1α), MIP-1β (Biosource, Camarillo, CA), IL-8, RANTES, soluble TREM-1, anti-TREM-1 (R&D Systems, Minneapolis, MN), IFN-α, and IL-1β (Endogen, Rockford, IL) according to manufacturers' instructions. Cell culture supernatants were assayed for myeloperoxidase (MPO) and lactoferrin (OXIS, Portland, OR). For some cytokines (e.g., IL-1β and TNF-α) found in greatest abundance in cytoplasmic granules, virus-activated or nonactivated cells were lysed for cytokine measurement (24). Quantitative data are presented as average concentrations in units/ml. To detect soluble TREM-1 (sTREM-1), supernatants were added to an anti-TREM-1-coated, 1% bovine serum albumin-blocked polystyrene ELISA plate for 2 h. A recombinant human TREM-1 (R&D Systems, Minneapolis, MN) was used to generate a standard curve. Each incubation step was followed by five washes in PBS plus 0.1% Tween 20. Detection of captured TREM-1 was facilitated by 400 ng/ml of rabbit anti-TREM-1 biotin (R&D Systems, Minneapolis, MN) antibody. Streptavidin-HRP (R&D Systems, Minneapolis, MN), diluted 1:200, was then added, and samples were incubated for 20 min. For development, the two-component 3,3′,5,5′ tetramethyl benzidine substrate system was used (Pierce, Rockford, IL). The reaction, which was allowed to proceed for 20 min, was halted with a stop solution and read at 450 nm on a UVmax spectrophotometer.
Inhibition of proinflammatory cytokines by TREM-1 peptide.
The inhibition of proinflammatory responses induced by septic shock was described previously (20). Briefly, neutrophils were pretreated with a 17-mer TREM-1 binding peptide (100 ng/ml) called LP17 or a sequence-scrambled control (20) for 10 min. Subsequently, cells were incubated with live or inactivated filoviruses as described above for 6 h at 37°C. Supernatants were then harvested and the cells were lysed. Proinflammatory cytokines in the supernatants and cell lysates were assayed by ELISA.
GP binding to TREM-1.
To assess the possible binding of GP to TREM-1, MARVCi67 GP or nucleoproteins (NP) (as a control) were expressed in Vero cells by using a Venezuelan equine encephalitis replicon system as described previously (23). Briefly, Venezuelan equine encephalitis replicons were packaged into viral replicon particles (VRPs) (37). The resulting VRPs, capable of one-step infection, along with expression of MARVCi67 GP or NP, were purified and titrated by immunofluorescence (37). Replicons were used to infect Vero cells (MOI = 5). Vero cells expressing MARVCi67 GP or NP and mock-infected cells were then harvested and subsequently pretreated with TREM-1 Fc fusion protein (FP) (100 ng/ml; R&D Systems, Minneapolis, MN) for 1 h at 4°C. Cells were washed, stained with anti-TREM-1 PE or an isotype-matched control, and analyzed by a FACSCalibur. Moreover, inhibiting the binding of filoviruses to neutrophils was also performed on neutrophils. Briefly, iMARVCi67 (1 μg/100 μl) was incubated with TREM-1 Fc FP (100 ng/ml) for 30 min at 4°C. Subsequently, neutrophils were incubated with MARVCi67 or MARVCi67-TREM-1 FP for 6 h at 37°C. Treated and untreated neutrophils were then washed, stained with anti-TREM-1 PE antibody, and analyzed by a FACSCalibur.
RESULTS
Adsorption and internalization of filoviruses by human neutrophils.
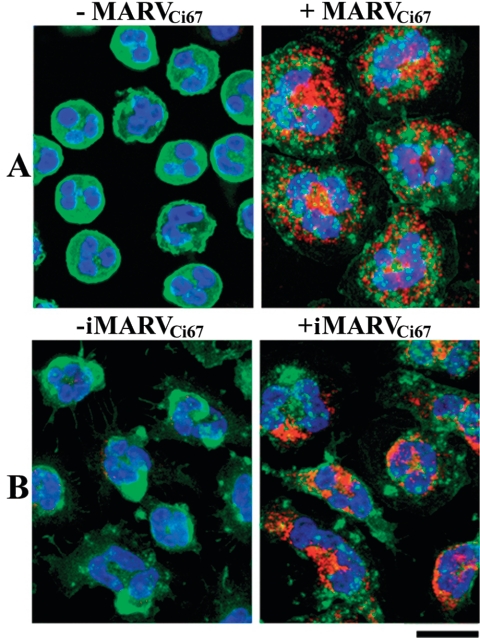
Because of the relatively short life span of neutrophils (40), we did not expect filoviruses to productively infect these cells. Nevertheless, we examined this possibility and confirmed no productive viral replication in human neutrophils exposed to live MARV or EBOV (MOI = 1), as measured by plaque assay of supernatants 1, 6, 24, or 48 h postinfection; in contrast, parallel culture of Vero cells produced 100- to 1,000-fold increases in viral titers (data not shown). Similarly, quantitative RT-PCR did not indicate viral replication in neutrophils (data not shown). However, adsorption and uptake of viral antigen by neutrophils was demonstrable by confocal microscopy at 6 h after addition of either inactivated or live MARVCi67 (Fig. 1). Thus, human neutrophils bound and internalized viral antigen and presumptively ingested whole viral particles but did not produce new virus in vitro.
FIG. 1.
Confocal analysis of human neutrophils exposed to filoviruses. Neutrophils were treated with live (A) or inactivated (B) MARVCi67 for 6 h at 37°C. Treated and untreated cells were harvested, washed extensively with PBS, permeabilized, stained with anti-5D7 Alexa 568 (red), phalloidin Alexa 488 (green), and Hoechst dye (blue) for 1 h at 4°C, and visualized with a Bio-Rad 2000 MP confocal multiphoton system attached to a Nikon TE300 inverted microscope.
Engagement of TREM-1 and human neutrophil activation.
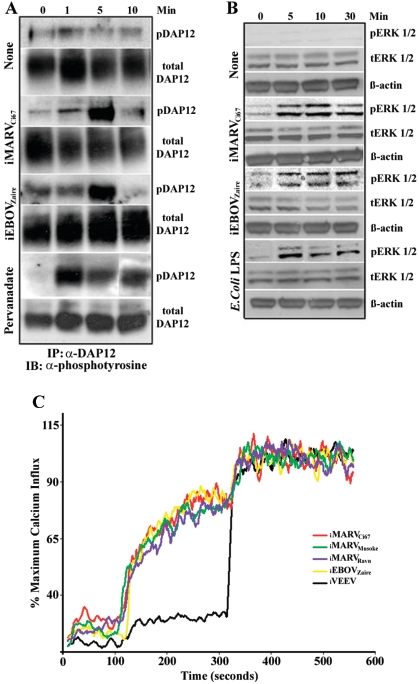
It was previously shown that engagement of TREM-1 with a stimulus (e.g., E. coli LPS) delivers activation signals, which in turn induce cytokines and chemokines in synergy with (or without) TLR stimulation (7, 21). Accordingly, we examined TREM-1 expression after neutrophil interaction with endotoxin-free live or inactivated filoviruses. Data showed that TREM-1 was down-regulated from the surfaces of neutrophils within 1 h (not shown) and 6 h of live (Fig. 2A) or inactivated (Fig. 2A and B) filovirus interaction. Additionally, we observed that TREM-1 down-regulation corresponded with the timeframe of neutrophil-filovirus interaction (Fig. 2C) and was dose dependent (Fig. 2D). Importantly, similarly prepared alphavirus controls (e.g., VEEV) did not induce any TREM-1 phenotype change on neutrophils (Fig. 2A). Moreover, it was demonstrated in other systems that TREM-1 activates downstream signaling cascades in association with its adaptor protein DAP12 (6). Here, in response to exposure to inactivated filoviruses (e.g., iMARVCi67 and iEBOVZaire) or the positive control pervanadate, DAP12 was activated in neutrophils as indicated by its transient tyrosine phosphorylation (Fig. 3A). Consistent with the involvement of TREM-1/DAP12 in the filovirus-mediated neutrophil stimulation, activation of ERK1/2 following exposure to iMARVCi67 or iEBOVZaire was observed (Fig. 3B). Furthermore, analysis of intracellular calcium ([Ca2+]i) mobilization clearly showed that filovirus activated-neutrophils mobilized [Ca2+]i instantaneously upon the addition of filoviruses (Fig. 3C). As seen in Fig. 3C, VEEV did not induce [Ca2+]i in neutrophils (Fig. 3C).
FIG. 2.
TREM-1 activation on human neutrophils by filoviruses. (A) Neutrophils were treated with live or inactivated filoviruses, VEEV as a control, or no virus for 6 h at 37°C. Treated (red line) and untreated (blue line) cells were harvested, washed, stained with anti-TREM-1 PE or an isotype match (black line) for 1 h at 4°C, and analyzed by a FACSCalibur. Experiments were repeated at least 10 times with similar results. (B) Treated (right panel) or untreated (left panel) neutrophils incubated with inactivated MARVCi67 for 6 h at 37°C. Cells were fixed, permeabilized, and stained with anti-TREM-1 Alexa 488 (green) and Hoechst dye (blue). Cells were visualized with a confocal multiphoton system. Treated and untreated neutrophils were incubated with iMARVCi67 in a time-dependent (C) and dose-dependent (D) manner.
FIG. 3.
Activation of DAP12. (A) Freshly isolated neutrophils were treated with iMARVCi67 or iEBOVZaire for the indicated time points at 37°C. DAP12 was immunoprecipitated (IP) and immunoblotted (IB) with an antiphosphotyrosine antibody. Pervanadate was used as a positive control. The immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with antiphosphotyrosine. (B) Lysates for detection of ERK1/2 were separated under reducing conditions on Tris-Gly gel and blotted with anti-ERK1/2 phosphotyrosine or total ERK1/2 polyclonal antibodies. Stripped blots were reprobed with an anti-β-actin monoclonal antibody. E. coli LPS was used as a positive control. (C) Intracellular calcium mobilization was assayed in human neutrophils which were treated with inactivated filovirus or VEEV and analyzed by a FACSCalibur. Experiments were performed three times with similar results.
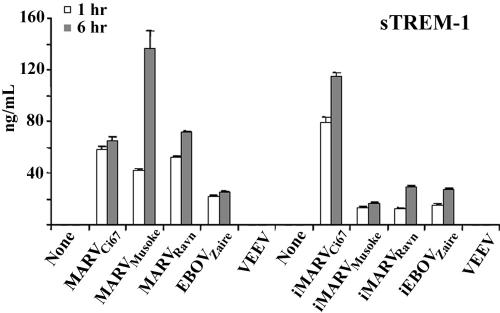
Soluble TREM-1.
Previously, sTREM-1 was shown to be significantly elevated in plasma or bronchial lavage fluids of patients with bacterial sepsis (18-20). Here, sTREM-1 was shed at elevated levels in the supernatants of neutrophils incubated with live or inactivated filoviruses (Fig. 4). VEEV as a control did not induce sTREM-1 in neutrophils (Fig. 4).
FIG. 4.
Soluble TREM-1. Neutrophils were incubated with live or inactivated filoviruses or VEEV for 1 or 6 h at 37°C. Afterwards, cell supernatants were harvested and sTREM-1 was detected by ELISA as described in Materials and Methods. Experiments were repeated at least three times with similar results.
Filovirus-associated cytokine secretion and phenotypic changes.
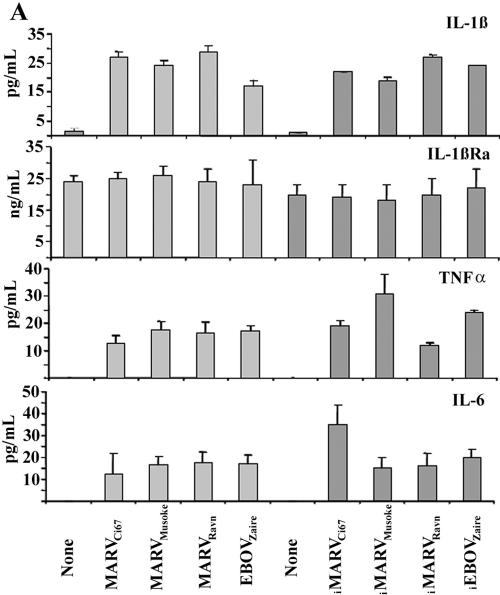
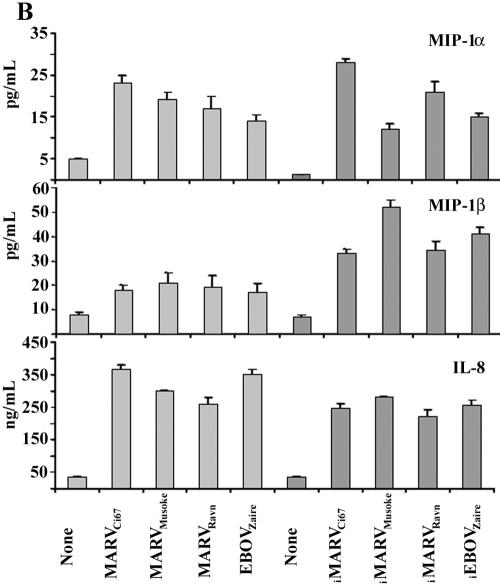
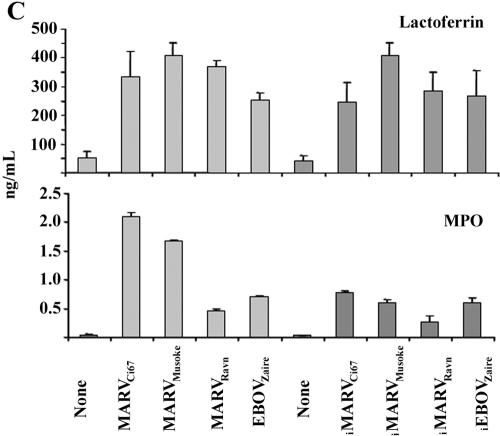
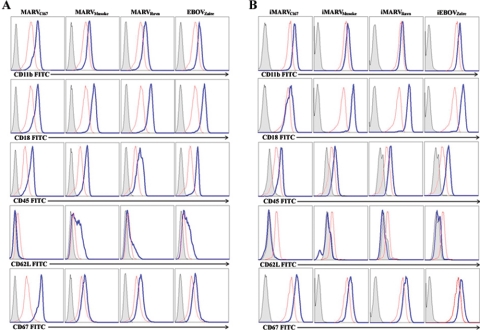
It is well documented and affirmed by positive controls in our experiments (not shown) that binding of TREM-1 to microbial stimuli (e.g., E. coli LPS) amplifies proinflammatory cytokines and chemokines in activated cells (6, 7). Similarly, human neutrophils exposed to live or inactivated MARV or EBOV within 1 h (not shown) and 6 h produced high levels of IL-1β, TNF-α, IL-6 (Fig. 5A), IL-8, MIP-1α/β (Fig. 5B), MPO, and lactoferrin (Fig. 5C) but not IFN-α, IL-17, IL-12, TGF-β, or RANTES (not shown). Interestingly, neutrophils expressed IL-1Ra constitutively, and this was not affected after incubating these cells with various live or inactivated filoviruses (Fig. 5A). Additionally, neutrophils exposed to live or inactivated filoviruses displayed significant upregulation of CD11b, CD18, CD45, and CD67 and down-regulation of CD62L within 1 h (data not shown) and 6 h (Fig. 6). In contrast, treatment with VEEV did not induce any cell surface changes or cytokine production by human neutrophils (not shown).
FIG.5.
Cytokine analysis. Neutrophils were treated with filoviruses or no virus for 6 h. Cell supernatants and lysates were collected and analyzed for proinflammatory cytokines, chemokines, MPO, and lactoferrin by ELISA. Experiments were performed at least three times with similar results.
FIG. 6.
Phenotypic change. Neutrophils were treated with live (A) or inactivated (B) filoviruses for 6 h at 37°C. Treated (blue line) and untreated (red line) neutrophils were washed and stained with corresponding antibodies and isotype matches (filled gray histogram). Cells were fixed and analyzed by a FACSCalibur. Experiments were performed at least 10 times with similar results.
Inhibition of inflammatory responses by TREM-1 peptide.
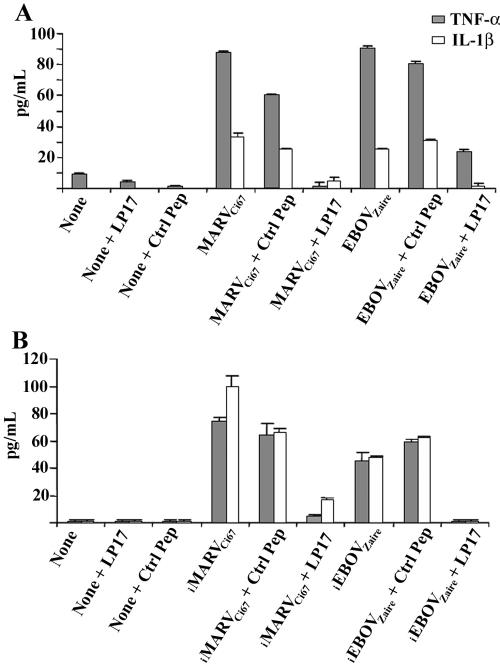
It has been shown that a synthetic peptide called LP17, mimicking a conserved domain of the soluble form of TREM-1, inhibits inflammatory responses in vivo and in vitro (20). Here, treating neutrophils with LP17, but not the control, abrogated the production of TNF-α and IL-1β that was otherwise induced upon interaction of these cells with live (Fig. 7A) or inactivated (Fig. 7B) MARVCi67 and EBOVZaire.
FIG. 7.
Inhibition of inflammatory cytokines by TREM-1 peptide. Neutrophils were first pretreated with synthetic peptide LP17 or the control peptide for 1 h at 37°C. Subsequently, cells were treated with live (A) or inactivated (B) MARVCi67 or EBOVZaire for 6 h at 37°C. Cell supernatants were harvested, and cells were lysed with 1% Triton X. IL-1β and TNF-α levels were determined by ELISA. Experiments were performed at least three times with similar results.
TREM-1 binding to MARV GP.
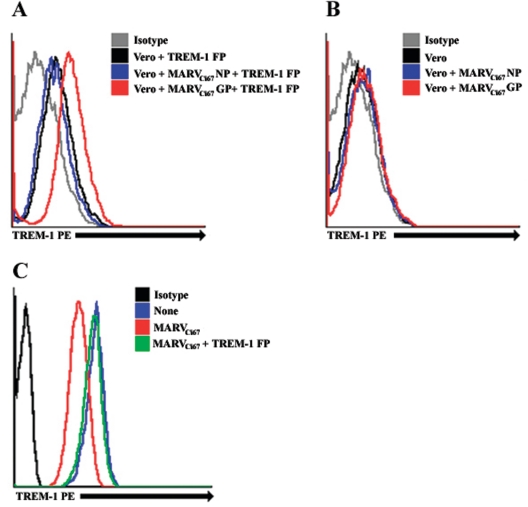
The engagement of TREM-1 by filoviruses and subsequent neutrophil activation could be due to direct binding between TREM-1 and GP, the only viral protein known with certainty to be available on virion surfaces, or possibly to initial binding to other moieties associated with TREM-1 or GP. To illuminate any direct binding of filovirus GP to TREM-1, Vero cells were infected with VRPs expressing MARVCi67 GP or, as a control, NP. Vero cells expressing MARVCi67 GP, Vero cells expressing NP, or mock-infected cells were incubated with or without TREM-1 Fc FP and subsequently stained with anti-TREM-1 PE antibody. The data revealed that while Vero cells expressing MARV NP (Fig. 8A) or mock-infected Vero cells (Fig. 8A) did not show significant binding to TREM-1 Fc FP, the majority of Vero cells expressing MARV GP bound to TREM-1 Fc FP as visualized by anti-TREM-1 antibodies (Fig. 8A). By contrast, cells (GP, NP, or mock infected) which were not pretreated with TREM-1 Fc FP were not bound by anti-TREM-1 (Fig. 8B). These data imply a direct binding of TREM-1 protein to GP expressed on Vero cells and thus may explain the activation of neutrophils, which express TREM-1 on their surfaces, upon exposure to filoviruses. In further support of direct binding, as shown in Fig. 8C, we found that TREM-1 Fc FP competitively inhibited the capacity of inactivated MARVCi67 to engage and down-regulate expression of TREM-1 on human neutrophils.
FIG. 8.
Glycoprotein-TREM-1 binding. (A and B) Vero cells were transfected with VRPs expressing MARVCi67 GP or NP. Transfected and control Vero cells were pretreated with TREM-1 Fc FP for 1 h at 4°C. Washed cells were incubated with anti-TREM-1 PE and subsequently analyzed by a FACSCalibur. Experiments were repeated three times with similar results. (C) iMARVCi67 was preincubated with TREM-1 Fc FP for 30 min at 4°C. Neutrophils were incubated with iMARVCi67 or iMARVCi67-TREM-1 FP for 6 h at 37°C. Treated and untreated neutrophils were then stained with anti-TREM-1 PE antibody and analyzed by a FACSCalibur. Experiments were repeated three times with similar results.
DISCUSSION
During MARV and EBOV infections in humans and nonhuman primates, poorly understood failures in the complex interrelationships between the innate immunity, adaptive immunity, and inflammation of the host responses result in extraordinarily high mortality rates. Here, we describe previously unknown and potentially important interactions between filoviruses and neutrophils, an abundant population of blood cells better known for their role in resistance to bacteria (32, 39). The consequences of the interaction between filoviruses and neutrophils could be favorable or unfavorable to the host depending upon the stage of infection, the microenvironment in which the interaction occurs, and the interplay with other virus associated immune perturbances already described (1-3, 7, 13, 14, 16, 28, 38).
Within minutes of encountering MARV or EBOV, human neutrophils were observed to initiate a cascade of cellular events that included DAP12-transient phosphorylation, activation of ERK1/2, calcium influx, and shedding of soluble TREM-1. By 1 h (and more fully observable by 6 h), neutrophils released significant levels of primary cytokines (e.g., IL-1β, TNF-α, and IL-6), chemokines (e.g., IL-8 and MIP-1α/β), MPO, and lactoferrin but not IFN-α, IL-17, IL-12, TGF-β, or RANTES. Consistent with the rapidity of these responses, viral replication was nonessential; thus, inactivated MARV and EBOV were both effective in neutrophil activation. Differences between the effects of inactivated and live viruses were unremarkable, tending to be within expected and observed variations between experiments and among neutrophil donors. Initiation of these TREM-1 activation events is presumptively ascribed here to direct interaction between filoviral GPs and TREM-1, a 30-kDa glycoprotein expressed on the surfaces of mature neutrophils (10).
The ligand or pattern recognized by TREM-1 is not yet known, but its association with inflammation and sepsis and its distribution on myeloid cells, including not only neutrophils but also CD14high monocytes/macrophages and lung alveolar macrophages (10), suggest a critical role in the regulation of inflammatory immune responses upon pathogen infection. Accordingly, it was previously reported (7) that neutrophils and monocytes incubated with heat-inactivated Staphylococcus aureus, Pseudomonas aeruginosa, lipoteichoic acid, and LPS (but not bacillus Calmette-Guérin) respond with significantly increased levels of TREM-1 on neutrophils, a finding we also observed with respect to neutrophils and LPS (data not shown). In contrast, we consistently observed a decrease in expression of TREM-1 in human neutrophils exposed to filoviruses, along with the previously described increase in soluble TREM-1 in neutrophil supernatants. While this partial discordance adds further credence to our efforts to eliminate LPS from all reagents used, it poses a question of whether the binding of filoviruses to neutrophils is qualitatively different from the binding of certain bacterial products. Nevertheless, in our experiments, neutrophils exposed to MARV or EBOV did respond with cytokine secretion profiles similar to those described previously for bacterial products (7, 10).
Early in infection, we surmise that the proinflammatory burst of cytokines by neutrophils—localized and in response to a relatively small viral burden—may help augment innate and adaptive immune responses, which aligns in part with the reported coincidence (1, 2, 28) between early proinflammatory cytokine levels and patient survival in EBOV infection. However, if virus replication remains unchecked, neutrophils are soon confronted with high levels of virus—often 108 PFU/ml or higher in blood and organs of NHP (16, 23)—and the inflammation from widespread cytokine release may contribute to many of the symptoms observed. For context, the relationship between these results and other previous filovirus reports may be helpful. For example, it was reported that the soluble truncated form of EBOV GP called sGP, but not full-length GP that is found on virion and cell surfaces and is identical to sGP in the amino half of GP, reacted with neutrophils via CD16b, the neutrophil-specific form of Fcγ receptor III (44). Presuming the sGP-neutrophil observations are ultimately confirmed, they differ from this report which finds that sGP may be present in live EBOV supernatant preparations but is absent from purified virions and from MARV, where no homolog of sGP is known. Nevertheless, such data emphasize the fact that GP processing in filoviruses is complex, with soluble products emanating from incomplete establishment of disulfide linkage between the GP cleavage products GP1 and GP2 and by ectodomain cleavage and shedding (11). A separate area of interest is the attempt by some to define a singular explanation for the profound virulence of filoviruses in primates. In this respect, the Reston species of EBOV is sometimes taken as a pivotal example because it is as lethal as other EBOV species in NHP but has caused only a few known human infections, none with serious disease. We found Reston ebolavirus to cause activation of TREM-1 on human neutrophils in a manner indistinguishable from MARV and EBOVZaire. We note that Reston ebolavirus remains both a biosafety level 4 agent and a select agent precisely because there is little confidence in its nonvirulence for humans. More generally, we surmise that filovirus virulence will prove to be multifactorial, and we do not presuppose that the interactions between filoviruses and neutrophils are all that determine the outcome of infection.
We have documented here several new aspects of filovirus pathogenesis as they relate to the consequences of the encounter between filoviruses and neutrophils. The implications are relevant not only to the understanding of disease but to potential applications in both vaccination and therapy. If neutrophil participation proves important in the early communication between innate and adaptive responses to MARV and EBOV, it may be possible to improve the potency of existing vaccine candidates by regulated recruitment of neutrophils to the site of antigen delivery (35). Similarly, the search for therapeutic interventions effective in the hours after filovirus exposure may now include consideration of neutrophils. Finally, it may prove fruitful to ameliorate filovirus disease by finding ways to dampen the initiation or effects of profuse neutrophil activation. In this respect, it is provocative that soluble TREM-1 and a peptide antagonist derived from TREM-1 both appear to interfere in vitro with filoviral activation of neutrophils. The peptide, shown previously to have therapeutic potential in a murine model of bacterial sepsis (7), signals the possibility of a common pathway that may be accessible to treatment. Thus, these and the many other efforts to understand the complexities of host responses during filoviral infection are rooted in the hypothesis that such data will have practical consequences in the development of medical countermeasures against MARV and EBOV. Here, we conclude that interactions between filoviruses and neutrophils may be part of the problem and also part of the solution.
Acknowledgments
The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as an official Department of the Army, National Cancer Institute (NCI) at Fredrick position, policy, or decision unless designated by other documentation.
This work was supported in part by NIH grant AI 59590-01, by NCI N01-CO-12400, and by the Defense Threat Reduction Agency (DTRA-JSTO-CBD).
We thank D. McVicar, G. Whittaker, L. Lanier, J. Hamerman, M. K. Hart, and Mohamed Aitichou for fruitful discussions. We also thank J. Wells, J. Johnson, D. Negley, L. Lofts, S. Harrison, L. Prugar, J. Geisbert, and R. Ortiz for their excellent technical assistance.
REFERENCES
- 1.Baize, S., E. M. Leroy, A. J. Georges, M. C. Georges-Courbot, M. Capron, I. Bedjabaga, J. Lansoud-Soukate, and E. Mavoungou. 2002. Inflammatory responses in Ebola virus-infected patients. Clin. Exp. Immunol. 128:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baize, S., E. M. Leroy, M. C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. [DOI] [PubMed] [Google Scholar]
- 3.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borio, L., T. Inglesby, C. J. Peters, A. L. Schmaljohn, J. M. Hughes, P. B. Jahrling, T. Ksiazek, K. M. Johnson, A. Meyerhoff, T. O'Toole, M. S. Ascher, J. Bartlett, J. G. Breman, E. M. Eitzen, Jr., M. Hamburg, J. Hauer, D. A. Henderson, R. T. Johnson, G. Kwik, M. Layton, S. Lillibridge, G. J. Nabel, M. T. Osterholm, T. M. Perl, P. Russell, and K. Tonat. 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 287:2391-2405. [DOI] [PubMed] [Google Scholar]
- 5.Bosio, C. M., M. J. Aman, C. Grogan, R. Hogan, G. Ruthel, D. Negley, M. Mohamadzadeh, S. Bavari, and A. Schmaljohn. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 188:1630-1638. [DOI] [PubMed] [Google Scholar]
- 6.Bouchon, A., J. Dietrich, and M. Colonna. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991-4995. [DOI] [PubMed] [Google Scholar]
- 7.Bouchon, A., F. Facchetti, M. A. Weigand, and M. Colonna. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103-1107. [DOI] [PubMed] [Google Scholar]
- 8.Buonocore, S., M. Surquin, A. Le Moine, D. Abramowicz, V. Flamand, and M. Goldman. 2004. Amplification of T-cell responses by neutrophils: relevance to allograft immunity. Immunol. Lett. 94:163-166. [DOI] [PubMed] [Google Scholar]
- 9.Bwaka, M. A., M. J. Bonnet, P. Calain, R. Colebunders, A. De Roo, Y. Guimard, K. R. Katwiki, K. Kibadi, M. A. Kipasa, K. J. Kuvula, B. B. Mapanda, M. Massamba, K. D. Mupapa, J. J. Muyembe-Tamfum, E. Ndaberey, C. J. Peters, P. E. Rollin, and E. Van den Enden. 1999. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J. Infect. Dis. 179:S1-S7. [DOI] [PubMed] [Google Scholar]
- 10.Colonna, M., and F. Facchetti. 2003. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J. Infect. Dis. 187(Suppl. 2):S397-S401. [DOI] [PubMed] [Google Scholar]
- 11.Dolnik, O., V. Volchkova, W. Garten, C. Carbonnelle, S. Becker, J. Kahnt, U. Stroher, H. D. Klenk, and V. Volchkov. 2004. Ectodomain shedding of the glycoprotein GP of Ebola virus. EMBO J. 23:2175-2184. [DOI] [PMC free article] [PubMed]
- 12.Ellis, T. N., and B. L. Beaman. 2004. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 112:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann, H., H. Bugany, F. Mahner, H. D. Klenk, D. Drenckhahn, and H. J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher-Hoch, S. P., G. S. Platt, G. H. Neild, T. Southee, A. Baskerville, R. T. Raymond, G. Lloyd, and D. I. Simpson. 1985. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola). J. Infect. Dis. 152:887-894. [DOI] [PubMed] [Google Scholar]
- 15.Gale, R. P., and J. Zighelboim. 1975. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J. Immunol. 114:1047-1051. [PubMed] [Google Scholar]
- 16.Geisbert, T. W., L. E. Hensley, T. Larsen, H. A. Young, D. S. Reed, J. B. Geisbert, D. P. Scott, E. Kagan, P. B. Jahrling, and K. J. Davis. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163:2347-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisbert, T. W., and P. B. Jahrling. 1995. Differentiation of filoviruses by electron microscopy. Virus Res. 39:129-150. [DOI] [PubMed] [Google Scholar]
- 18.Gibot, S. 2006. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia and severe sepsis. Semin. Respir. Crit. Care Med. 27:29-33. [DOI] [PubMed] [Google Scholar]
- 19.Gibot, S., A. Cravoisy, B. Levy, M. C. Bene, G. Faure, and P. E. Bollaert. 2004. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N. Engl. J. Med. 350:451-458. [DOI] [PubMed] [Google Scholar]
- 20.Gibot, S., M. N. Kolopp-Sarda, M. C. Bene, P. E. Bollaert, A. Lozniewski, F. Mory, B. Levy, and G. C. Faure. 2004. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 200:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamerman, J. A., N. K. Tchao, C. A. Lowell, and L. L. Lanier. 2005. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 6:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hevey, M., D. Negley, J. Geisbert, P. Jahrling, and A. Schmaljohn. 1997. Antigenicity and vaccine potential of Marburg virus glycoprotein expressed by baculovirus recombinants. Virology 239:206-216. [DOI] [PubMed] [Google Scholar]
- 23.Hevey, M., D. Negley, P. Pushko, J. Smith, and A. Schmaljohn. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28-37. [DOI] [PubMed] [Google Scholar]
- 24.Kuhns, D. B., D. A. Long Priel, and J. I. Gallin. 1997. Endotoxin and IL-1 hyporesponsiveness in a patient with recurrent bacterial infections. J. Immunol. 158:3959-3964. [PubMed] [Google Scholar]
- 25.Kuhns, D. B., E. L. Nelson, W. G. Alvord, and J. I. Gallin. 2001. Fibrinogen induces IL-8 synthesis in human neutrophils stimulated with formyl-methionyl-leucyl-phenylalanine or leukotriene B(4). J. Immunol. 167:2869-2878. [DOI] [PubMed] [Google Scholar]
- 26.Lehrer, R. I. 2004. Primate defensins. Nat. Rev. Microbiol. 2:727-738. [DOI] [PubMed] [Google Scholar]
- 27.Lekstrom-Himes, J. A., D. B. Kuhns, W. G. Alvord, and J. I. Gallin. 2005. Inhibition of human neutrophil IL-8 production by hydrogen peroxide and dysregulation in chronic granulomatous disease. J. Immunol. 174:411-417. [DOI] [PubMed] [Google Scholar]
- 28.Leroy, E. M., S. Baize, P. Debre, J. Lansoud-Soukate, and E. Mavoungou. 2001. Early immune responses accompanying human asymptomatic Ebola infections. Clin. Exp. Immunol. 124:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahanty, S., K. Hutchinson, S. Agarwal, M. McRae, P. E. Rollin, and B. Pulendran. 2003. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 170:2797-2801. [DOI] [PubMed] [Google Scholar]
- 30.Martini, G. A., and R. Siegert. 1971. Marburg virus disease. Springer-Verlag, Berlin, Germany.
- 31.Maruyama, T., M. J. Buchmeier, P. W. H. I. Parren, and D. R. Burton. 1998. Ebola virus, neutrophils, and antibody specificity. Science 282:845a. [Google Scholar]
- 32.Mayer-Scholl, A., P. Averhoff, and A. Zychlinsky. 2004. How do neutrophils and pathogens interact? Curr. Opin. Microbiol. 7:62-66. [DOI] [PubMed] [Google Scholar]
- 33.McVicar, D. W., L. S. Taylor, P. Gosselin, J. Willette-Brown, A. I. Mikhael, R. L. Geahlen, M. C. Nakamura, P. Linnemeyer, W. E. Seaman, S. K. Anderson, J. R. Ortaldo, and L. H. Mason. 1998. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J. Biol. Chem. 273:32934-32942. [DOI] [PubMed] [Google Scholar]
- 34.Mohamadzadeh, M., S. Olson, W. V. Kalina, G. Ruthel, G. L. Demmin, K. L. Warfield, S. Bavari, and T. R. Klaenhammer. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 102:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173-182. [DOI] [PubMed] [Google Scholar]
- 36.Pratt, W. D., P. Gibbs, M. L. Pitt, and A. L. Schmaljohn. 1998. Use of telemetry to assess vaccine-induced protection against parenteral and aerosol infections of Venezuelan equine encephalitis virus in non-human primates. Vaccine 16:1056-1064. [DOI] [PubMed] [Google Scholar]
- 37.Pushko, P., M. Bray, G. V. Ludwig, M. Parker, A. Schmaljohn, A. Sanchez, P. B. Jahrling, and J. F. Smith. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142-153. [DOI] [PubMed] [Google Scholar]
- 38.Ryabchikova, E. I., L. V. Kolesnikova, and S. V. Luchko. 1999. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J. Infect. Dis. 179(Suppl. 1):S199-S202. [DOI] [PubMed] [Google Scholar]
- 39.Segal, A. W. 2005. How neutrophils kill microbes. Annu. Rev. Immunol. 23:197-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serhan, C. N., and J. Savill. 2005. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6:1191-1197. [DOI] [PubMed] [Google Scholar]
- 41.Slenczka, W. G. 1999. The Marburg virus outbreak of 1967 and subsequent episodes. Curr. Top. Microbiol. Immunol. 235:49-75. [DOI] [PubMed] [Google Scholar]
- 42.Volchkov, V. E., V. A. Volchkova, U. Stroher, S. Becker, O. Dolnik, M. Cieplik, W. Garten, H. D. Klenk, and H. Feldmann. 2000. Proteolytic processing of Marburg virus glycoprotein. Virology 268:1-6. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 2005. Marburg haemorrhagic fever, Angola. Wkly. Epidemiol. Rec. 80:158-159. [PubMed] [Google Scholar]
- 44.Yang, Z., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034-1037. [DOI] [PubMed] [Google Scholar]