Abstract
Endothelial cell proliferation is a critical step in angiogenesis and requires a coordinated response to soluble growth factors and the extracellular matrix. As focal adhesion kinase (FAK) integrates signals from both adhesion events and growth factor stimulation, we investigated its role in endothelial cell proliferation. Expression of a dominant-negative FAK protein, FAK-related nonkinase (FRNK), impaired phosphorylation of FAK and blocked DNA synthesis in response to multiple angiogenic stimuli. These results coincided with elevated cyclin-dependent kinase inhibitors (CDKIs) p21/Cip and p27/Kip, as a consequence of impaired degradation. FRNK inhibited the expression of Skp2, an F-box protein that targets CDKIs, by inhibiting mitogen-induced mRNA. The FAK-regulated degradation of p27/Kip was Skp2 dependent, while levels of p21/Cip were regulated independent of Skp2. Skp2 is required for endothelial cell proliferation as a consequence of degrading p27. Finally, knockdown of both p21 and p27 in FRNK-expressing cells completely restored mitogen-induced endothelial cell proliferation. These data demonstrate a critical role for FAK in the regulation of CDKIs through two independent mechanisms: Skp2 dependent and Skp2 independent. They also provide important insights into the requirement of focal adhesion kinase for normal vascular development and reveal novel regulatory control points for angiogenesis.
Angiogenesis is a highly coordinated process required for normal development and in response to injury (15). A key component of angiogenesis is the tightly regulated proliferation of endothelial cells (25). Loss of normal cell cycle regulation likely contributes to the abnormal vasculature present in many disease states, including vasculopathies, cancer, cardiovascular disease, and proliferative retinopathies. Soluble growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) have been shown to induce endothelial cell proliferation. However, growth factor engagement alone is insufficient to promote endothelial cell growth, which also requires integrin attachment to the extracellular matrix (ECM) and proper cytoskeletal organization (26, 65). This notion is supported by the fact that endothelial cells grown in a saturating amount of bFGF proliferate in a fibronectin concentration-dependent manner (36). Similarly, growth factors are capable of promoting cell growth in endothelial cells plated on fibronectin, but not laminin (52). A large body of work, particularly in fibroblasts, has examined the role of extracellular signal-regulated kinase (ERK) in mediating the joint signal transduction from receptor tyrosine kinases (RTKs) and the ECM. However, in endothelial cells, alterations in the composition of the extracellular matrix can inhibit proliferation despite robust activation of ERK signaling (32). This piece of data implies that additional regulatory molecules are involved in the control of endothelial cell growth mediated by growth factors and integrins.
Soluble growth factors promote the transition through the G1 phase of the cell cycle by inducing the formation of cyclin D-cdk4/6 and cyclin E-cdk2 complexes (78). Formation of these complexes results in activation of these enzymes and subsequent phosphorylation of the retinoblastoma (Rb) protein. The hyperphosphorylated form of Rb is no longer capable of forming inhibitory complexes with E2F transcription factors, resulting in the accumulation of important cell cycle proteins such as cyclin A (20, 81). The G1 cyclin-cdks are regulated by the activity of specific cyclin-dependent kinase inhibitors (CDKIs). The CDKIs consist of two families: the Cip/Kip family, including p21/Cip1, p27/Kip1, and p57/Kip2; and the INK4 family, including p15, p16, p18, and p19 (69). During growth factor-induced transition through the G1 phase of the cell cycle, the levels of p27 and p21 become down-regulated, thereby allowing increased CDK activity, hyperphosphorylation of Rb, and the release of transcriptionally active E2F. However, the levels of both p27 and p21 remain elevated in quiescent cells under serum-free conditions as well as in cells that are maintained in suspension (64, 87). This led us to hypothesize that down-regulation of these CDKIs requires signals from both mitogens and the extracellular matrix in order to promote endothelial cell proliferation and that focal adhesion kinase (FAK) was an excellent candidate to provide such a signal.
Focal adhesion kinase is a non-receptor tyrosine kinase that is localized at focal adhesion sites. FAK is a signal integrator capable of relaying signals from soluble growth factors and cytokines, mechanical stimuli, as well as integrin engagement. Integrin binding has been shown to induce FAK phosphorylation in numerous cell types, resulting in dramatic effects on the actin cytoskeleton, cell migration, and proliferation. Growth factors, including VEGF, have also been shown to rapidly induce tyrosine phosphorylation of FAK (2). This suggests that FAK may be a critical signaling component involved in the regulation of angiogenesis. This notion is further supported by the evidence that the FAK knockout mouse displays an embryonic lethal phenotype at day E8.5 to 9 as a result of numerous abnormalities, including a poorly developed vasculature (34). Recent data using conditional knockout of FAK in endothelial cells have provided evidence that the FAK null phenotype is due to direct effects on the vascular endothelium (67). The loss of FAK in endothelial cells appears to inhibit the ability of endothelial cells to survive, migrate, and proliferate. An earlier study, using microinjection, demonstrated a role for FAK in the migration and proliferation of primary endothelial cells; however, no mechanism was defined (27). We sought to determine the mechanism by which FAK regulates endothelial cell proliferation.
MATERIALS AND METHODS
Cell culture.
Human umbilical vein endothelial cells (HUVECs) and human dermal microvessel endothelial cells (HDMVECs) from pooled donors were purchased from VEC Technologies (Troy, NY). Cells were cultured as previously described (50). Experiments were conducted in the presence of serum-free MCDB-131, and stimulation was performed with the indicated mitogen or complete growth medium containing 20% fetal bovine serum, 1% penicillin/streptomycin, 10 μg/ml heparin, and 60 μg/ml endothelial cell growth supplement (Becton-Dickinson), often referred to for brevity in the text and figures as “serum.”
Western blotting.
Western blot analysis was conducted using the following antibodies: anti-FAK from Upstate, Lake Placid, NY; anti-FAK Y397, anti-FAK Y861, antipaxillin, anti-paxillin Y118, and anti-cyclin D1 from BioSource, Camarillo, CA;, anti-ERK2, anti-phospho-ERK, and anti-phospho-Elk from Santa Cruz Biotechnology, Santa Cruz, CA;, anti-phospho-RbS795 and anti-phospho-JNK from Cell Signaling, Beverly, MA; anti-p21/Cip1 CP74 from NeoMarkers, Fremont, CA; anti-p27/Kip1 from BD Pharmingen, San Diego, CA; and anti-Skp2 from Zymed Laboratories, Cambridge, United Kingdom. Western blotting was performed under the conditions previously detailed (49).
Labeling of actin cytoskeleton.
HUVECs were seeded at 5.6 × 104 into 35-mm dishes containing coverslips coated with 0.2% gelatin. Cells were allowed to adhere and spread overnight and were then placed in serum-free MCDB-131 for 16 h. The cells were then washed with 1× phosphate-buffered saline (PBS) and then fixed with 3.7% formaldehyde for 15 min. Cells were permeabilized with 0.1% Triton X with 1% bovine serum albumin in PBS for 30 min. Filamentous actin was then labeled with Texas red-phalloidin (Molecular Probes) for 30 min as previously described (49). The coverslips were then washed three more times with 1× PBS and mounted on glass slides with Vectashield 4′,6′-diamidino-2-phenylindole (DAPI) mounting medium (Vector Labs). Images were obtained with a fluorescence microscope equipped with a digital camera.
Measurement of cell surface area.
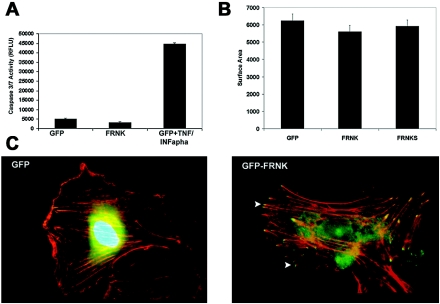
Cells were plated exactly as described for the actin cytoskeleton labeling. Cells were allowed to adhere and spread overnight and then were transduced with adenovirus constructs expressing green fluorescent protein (GFP) (Ad.GFP), FAK-related nonkinase (FRNK; Ad.FRNK), or FRNKS (Ad.FRNKS) in serum-free MCDB-131 for 16 h with a multiplicity of infection (MOI) of 10. The cells were then washed with 1× PBS and fixed with 3.7% formaldehyde. The cells were photographed under bright-field settings. The cell surface area of each cell was then traced, and the surface area was calculated using Image-Pro Plus software. An average of 50 cells was calculated per treatment, and the average surface area was calculated and graphed in Microsoft Excel.
Generation of recombinant adenovirus.
Most viruses described in this study were constructed using the AdEasy adenoviral system (28). GFP-FRNK was generously provided by Allen Samarel (Loyola University Medical Center); Skp2 and the F-box Skp2 mutant adenoviruses were from Mark Bond (University of Bristol). The GFP-expressing adenovirus was from Q-Biogene (Carlsbad, CA). GFP-FAK and GFP-FAKY397F were generated by subcloning the coding region of FAK (with or without the point mutation at amino acid 397) in frame with the 3′-coding region of enhanced GFP (EGFP) into a modified pShuttle vector. Recombinant adenoviruses were generated as previously described (28) and were used at an MOI of between 10 and 20.
Measurement of DNA synthesis.
HUVECs were transduced with Ad.GFP, Ad.FRNK, Ad.FRNKS, Ad.GFP-FAK, Ad.GFP-FAKY397F, Ad.WT (wild type)-Skp2, or Ad.ΔF-box Skp2 under serum-free conditions. After 24 h, complete growth medium, VEGF, or FGF was added as a mitogenic stimulus. Measurement of DNA synthesis by [3H]thymidine and bromodeoxyuridine (BrdU) incorporation was performed as previously described (50).
Real-time PCR.
Total RNA was extracted from 2.5 × 105 HUVECs per treatment with an RNeasy kit and QiaShredder (QIAGEN). First-strand cDNA was synthesized from equal amounts of total RNA using iQ reverse transcriptase (Bio-Rad) according to the manufacturer's instructions. Real-time PCR was performed with primers chosen to extend products under 200 bp with the following characteristics: (i) melting temperature of 58°C; (ii) a product that spans one intron boundary; and (iii) GC content between 40 and 60% and no predicted primer dimer formation. The primers selected are as follows: Skp2, forward, 5′-CCCACGGAAACGGCTGAAGA-3′, and reverse, 5′-CGCTAGGCGATACCACCTCTTACAA-3′; p27/KIP1, forward, 5′-TGCAACCGACGATTCTTCTACTCAA-3′, and reverse, 5′-CAAGCAGTGATGTATCTGATAAACAAGGA-3′); and p21/CIP1, forward, 5′-CGATGCCAACCTCCTCAACGA-3′, and reverse, 5′-TCGCAGACCTCCAGCATCCA-3′. Real-time PCR was then performed on 1 μl of cDNA along with 5 μM as a final concentration for each primer combined with iQ SYBR Green Supermix (Bio-Rad) in a MyiQ real-time detection system (Bio-Rad). Each 20-μl reaction was amplified in thin-wall PCR tubes under the following conditions: 95°C for 2 min followed by 50 cycles of 95°C for 15 s and 72°C for 15 s. Melt-curve analysis was performed following amplification by increasing the temperature in 0.4°C increments starting at 65°C for 85 cycles of 10 s each. The presence of a single PCR product was verified by the presence of a single melting temperature peak and by ethidium bromide staining following electrophoresis on agarose gels. The relative abundance of each gene's message was normalized against actin in each sample (forward primer, 5′-TACCTCATGAAGATCCTCACC-3′; reverse, 3′-TTTCGTGGATGCCACAGGAC-5′) and calculated as  , where CT represents the threshold cycle for each transcript, as previously described (54). In the figures, error bars indicate the standard error of at least three independent experiments. Control experiments run in the absence of reverse transcriptase demonstrated no product amplification. Correct products were also verified by size following electrophoresis on agarose gels.
, where CT represents the threshold cycle for each transcript, as previously described (54). In the figures, error bars indicate the standard error of at least three independent experiments. Control experiments run in the absence of reverse transcriptase demonstrated no product amplification. Correct products were also verified by size following electrophoresis on agarose gels.
Apoptosis assay.
Caspase 3/7 activities were measured by Apo-ONE homogenous caspase 3/7 assay (Promega) as we have previously described (48). A 96-well plate coated with 0.2% gelatin was seeded with 2.0 × 104 cells/well. HUVECs were transduced with Ad.GFP or Ad.FRNK, and the following day, serum-free MCDB-131 was added to the wells. Tumor necrosis factor alpha (TNF-α) and alpha interferon were added as a positive control to induce apoptosis, essentially as described by Alavi et al. (3).
siRNAs.
Validated small interfering RNA (siRNA) sequences targeting p21 (identification no. 1621) and Skp2 (identification no. 1655) were purchased from Ambion, Inc., and p27 (catalog no. D-003472-04) was purchased from Dharmacon Research. The control duplex was directed against luciferase and was purchased from Dharmacon (catalog no. D-002050-01-05). Electroporation was used to transduce 125 ng of siRNA into 7.5 × 104 HUVECs resuspended in 75 μl of siRNA electroporation buffer (Ambion, Inc.). Cells were plated and allowed to recover for 24 h in complete medium prior to any subsequent infection or manipulation. The results shown are from duplexes indicated above. In all cases, similar results were obtained with additional siRNA sequences obtained from the same vendors but directed against different sequences in the same target molecule (data not shown).
Statistical analysis.
All data except those from the real-time PCR are representative experiments; in the case of proliferation experiments, three independent replicates were conducted in each experiment. Similar results were obtained in at least three independent experiments. In real-time PCR experiments, data were pooled from multiple independent experiments. One-way analysis of variance was conducted as appropriate using Statistica software (Tulsa, OK).
RESULTS
FRNK inhibits FAK and paxillin phosphorylation.
To test the role of FAK in growth factor-induced endothelial cell proliferation, we chose a genetic approach of inhibiting FAK activation by expression of the C-terminal portion of FAK referred to as FRNK. This naturally occurring splice variant of FAK functions as a dominant negative by competitively displacing FAK from focal contacts, resulting in reduced tyrosine phosphorylation of FAK as well as the focal adhesion-associated protein paxillin (60). We generated recombinant adenoviruses which were used to transduce cells, one expressing FRNK and one expressing a point mutant of FRNK (C1034S) which is not targeted to the site of focal contacts, these are referred to as Ad.FRNK and Ad.FRNKS respectively. FRNKS is a useful negative control because it disrupts the focal adhesion targeting domain and therefore does not displace FAK from focal contacts (70). The equal expression of both proteins was confirmed by Western blot analysis using a C-terminal-site FAK antibody. Lysates were then prepared, and expression levels were analyzed by Western blotting using a C-terminal FAK antibody (Fig. 1A). The 125-kDa band represents total endogenous FAK contained in the cells, and FRNK is detectable at 41 kDa. The FRNKS is shifted as a result of a triple-hemagglutinin tag present on this construct. To characterize the effect of FRNK expression on endothelial cells, we measured FAK activation following treatment with VEGF on the Y397 autophosphorylation site and the Src-dependent phosphorylation site Y861 (2, 24). Expression of FRNK reduced both Y397 and Y861 below the basal level and inhibited the ability of VEGF to induce FAK Y397 and Y861 phosphorylation (Fig. 1B). Similar results were seen when complete growth medium was used as a stimulus in place of VEGF (see, for example, Fig. 5B). The observed inhibition was dependent on targeting FRNK to focal adhesions, as FRNKS failed to alter the phosphorylation states of either tyrosine residue. Since FAK can phosphorylate paxillin at Y118 (51), we examined the effect of FRNK on paxillin phosphorylation to assess the effects on signaling downstream of FAK. In GFP-expressing cells, mitogen stimulation resulted in the phosphorylation of paxillin at Y118; however, in GFP-FRNK-expressing cells, phosphorylation of paxillin Y118 was inhibited (Fig. 1C).
FIG. 1.

FRNK expression inhibits FAK and paxillin phosphorylation. HUVECs were transduced with Ad.GFP, Ad.FRNK, or Ad. FRNKS for 16 h in serum-free MCDB-131 and then treated with 50 ng/ml VEGF for 10 min, and whole cell lysates were collected and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting using antibodies to total FAK (A) and phosphorylation-specific antibodies to Y397 and Y861 (B). HUVECs were transduced with Ad.GFP or Ad.GFP-FRNK for 16 h in serum-free MCDB-131 and then treated with serum for 10 min and analyzed with total paxillin and Y118 paxillin antibodies (C). pPaxillin, phosphorylated paxillin.
FIG. 5.

Expression of FRNK does not inhibit ERK signaling but does prevent serum-induced degradation of the CDKIs p27 and p21. (A) Serum-starved HUVECs were transduced with Ad.GFP, Ad.FRNK, or Ad.FRNKS and then stimulated with complete growth medium for 16 h and probed with antibodies specific for pJNK, pERK, pElk, and cyclin D1. (B) Serum-starved HUVECs were transduced with Ad.GFP or Ad.GFP-FRNK prior to stimulation with complete growth medium for 16 h. Cell lysates were made after 16 h and probed with antibodies specific for FAK Y397, p27, p21, pRb S795, and ERK2.
FRNK inhibits VEGF- and serum-induced endothelial cell proliferation.
We next directly examined the role of FAK in VEGF-induced cell proliferation. Expression of FRNK inhibited HUVEC [3H]thymidine incorporation in response to VEGF almost completely, while FRNKS was without effect (Fig. 2A). This result was confirmed by BrdU incorporation to ensure that the decrease in [3H]thymidine incorporation was due to a defect in DNA synthesis and not a loss of cell number (Fig. 2B). Given the considerable evidence of endothelial cell heterogeneity, we also tested the effect of interfering with FAK signaling in primary microvessel endothelial cells. We found the requirement for FAK signaling was not specific to large vessel endothelial cells as FRNK also inhibits proliferation of human dermal microvessel endothelial cells in response to VEGF (Fig. 2C). To determine if this effect was specific for VEGF signal transduction or was a more universal requirement of proliferation, we also tested the effect of FRNK expression on S-phase entry induced by complete growth medium containing serum. This is a much stronger mitogenic stimulus with an array of diverse receptor systems likely to be activated in response. As shown in Fig. 2D, FAK signaling was required for S-phase entry and DNA synthesis, even in the presence of serum-containing growth medium. Identical inhibitory results were seen with bFGF as a mitogenic stimulus (data not shown). Taken together, these data indicate that FAK is a critical global regulator of endothelial cell proliferation and that these effects are not dependent on either a specific growth factor or endothelial cell type.
FIG. 2.
FRNK expression inhibits mitogen-induced endothelial cell proliferation. HUVECs (A, B, and D) or HDMVECs were serum starved and transduced with Ad.GFP, Ad.FRNK, or Ad.FRNKS prior to stimulation. The cells were then incubated with 50 ng/ml VEGF (or complete growth medium; Fig. 3D) for 16 h. Cells were then pulsed with [3H]thymidine (H3 counts; A, C, and D) for an additional 3 h prior to scintillation counting. Cells pulsed with BrdU were visualized with an anti-BrdU antibody. BrdU-positive cells were quantified, and the data were graphed as the percentage of positive cells compared to total cell number (B).
FRNK does not disrupt cell spreading or viability in adherent cells.
In order to ensure that the FRNK-expressing cells were not apoptotic as has been reported in rat cardiomyocytes and FAK−/− cells (30, 33), we transduced HUVECs with Ad.GFP and Ad.FRNK and measured apoptosis using a caspase 3/7activity assay. The expression of GFP and FRNK showed equivalent and low levels of caspase 3/7 activity. In contrast, the positive control treatment of TNF-α and alpha interferon greatly increased caspase 3/7 activity (Fig. 3A).
FIG. 3.
Expression of FRNK does not induce apoptosis or disrupt cell spreading. (A) HUVECs were transduced with Ad.GFP or Ad.FRNK-GFP and analyzed at 48 h postinfection for the presence of caspase activity using a fluorescent substrate (Apo-ONE; Promega). Apoptosis was induced with a combination of TNF and alpha interferon (INF-α), as a positive control. RFLU, relative fluorescence units. (B) Adherent HUVECs were plated and transduced with Ad.GFP, Ad.FRNK-GFP, or Ad.FRNKS for 16 h under serum-free conditions. The total average cell surface area was calculated using Image-Pro Plus (Media Cybernetics) software. (C) Adherent HUVECs were placed in serum-free MCDB-131, infected with Ad.GFP or Ad.GFP-FRNK for 16 h, and then washed with 1× PBS and fixed with 3.7% formaldehyde. Cells were stained with Texas red-phalloidin and DAPI, and images of GFP, actin stress fibers, and nuclear staining were captured under ×100 magnification. White arrows indicate localization of GFP-FRNK at focal adhesion sites.
Expression of FRNK has been reported to inhibit spreading and migration in many cell types, including human brain microvessel endothelial cells (HBMECs), particularly when cells are detached into suspension and replated (6). As previous studies have shown that disruption of the actin cytoskeleton and cell shape can influence cell cycle progression (17, 32), we tested the effects of FRNK expression on cell shape, spreading, and cytosleletal organization in adherent endothelial cells. GFP- and FRNK-transduced cells were equally well spread, as determined by calculation of the total cell surface area (Fig. 3B). We analyzed actin staining in cells that expressed either GFP or GFP-FRNK in order to visualize cellular cytoskeleton architecture. We found both GFP and GFP-FRNK-expressing cells contain numerous and well-formed actin stress fibers with FRNK localized at their termini. Interestingly, there appear to be fewer membrane ruffles in FRNK-expressing cells and the stress fibers are more pronounced (Fig. 3C). We also examined the cellular morphology under serum-stimulated conditions and found the two cell populations to be nearly indistinguishable (data not shown). These data are consistent with results recently reported for human pulmonary artery endothelial cells expressing FRNK (31). Together, these results support the conclusion that under the conditions employed, FRNK expression in HUVECs does not disrupt cell adhesion and spreading or dramatically alter cell shape.
FAK Y397 phosphorylation is required but not sufficient for endothelial cell proliferation.
A previous study in fibroblasts found that expression of FAK is sufficient to stimulate proliferation and that phosphorylation of tyrosine 397, the site of autophosphorylation, is critical for this effect (84). To further investigate the role of FAK in endothelial cell proliferation, we examined the effect of overexpressing wild-type FAK. We also investigated the role of the Y397 residue that regulates the activation of FAK (51) and binding of Src, as FRNK expression inhibits the ability of Y397 to be phosphorylated. We created GFP-WT-FAK and GFP-Y397F-FAK adenoviruses and examined their ability to inhibit proliferation in response to mitogens. These viruses were titrated to achieve similar expression levels (Fig. 4). Expression of WT-FAK was not sufficient to induce proliferation in endothelial cells, nor did it have any deleterious or additive effect on the stimulated growth response. Similar results were observed even at expression levels at least 5 times as high as those shown, where FAK 397 phosphorylation was superphysiological (data not shown). In contrast, expression of the Y397F mutant resulted in a nearly complete inhibition of the mitogen-induced BrdU incorporation (Fig. 4). These data implicate signal transduction downstream of FAK 397 phosphorylation as being necessary but not sufficient for endothelial cell proliferation.
FIG. 4.
Expression of an Y397F mutant of FAK also inhibits endothelial cell proliferation. HUVECs were serum starved and transduced with Ad.GFP, Ad.GFP-Y397F-FAK, or Ad.GFP-WT-FAK prior to stimulation. The cells were then incubated with complete growth medium for 16 h. Cells were then pulsed with BrdU and visualized with an anti-BrdU antibody and recorded with a fluorescence digital camera. BrdU-positive cells were quantified, and the data were graphed as the percentage of positive cells compared to total cell number.
Expression of FRNK does not inhibit ERK signaling but prevents serum-induced degradation of the CDKIs p27 and p21.
FAK has been shown to play an important role in the regulation of ERK activation and cyclin D1 expression in other cell types (84, 85); therefore, we tested the effect of FRNK expression on ERK phosphorylation and cyclin D1 accumulation in response to mitogens. Serum induced the phosphorylation of ERK and JNK; however, expression of FRNK did not disrupt these signals (Fig. 5A and B). The nuclear transcription factor Elk, an ERK substrate, was also examined since integrin signaling has been shown to be required for ERK nuclear translocation (4). However, the expression of FRNK did not interfere with VEGF-induced Elk phosphorylation (Fig. 5A). Consistent with these findings, the induction of cyclin D in response to serum was unaltered by FRNK expression.
The ECM has been shown to alter the expression levels of p27 and p21 in some cell types. We therefore examined the effect of FRNK on p27 and p21 expression levels in endothelial cells. We found the levels of both p27 and p21 are elevated in quiescent cells in serum-free media. Upon the addition of mitogens, the levels of these inhibitors are significantly reduced. FRNK expression strongly inhibited the degradation of both p27 and p21 in response to serum (Fig. 5C), such that CDKI levels remained high. Since several reports have documented the ability of these CDKIs to inhibit the activity of the cyclin-CDK complexes (75), we also examined the phosphorylation status of the Rb protein. Consistent with the failure of p27 and p21 to be degraded by serum in the presence of FRNK, the phosphorylation of Rb at S795 was also inhibited (Fig. 5C). These data suggest that FAK signaling can control the levels of cyclin-dependent kinase inhibitors with corresponding effects on CDK substrate phosphorylation and subsequent cell cycle progression.
FRNK does not alter the mRNA expression of p27 or p21.
The levels of both p27 and p21 can be controlled through several different mechanisms, including transcriptional regulation and mRNA stability (23, 42, 44, 66). To investigate the mechanism of FAK-regulated p27 and p21 expression, we used quantitative real-time PCR to measure the mRNA levels in cells expressing GFP (control) or GFP-FRNK. We found stimulation with growth medium produced a modest decrease in p27 mRNA levels; however, this decrease was unaffected by the disruption of FAK signaling (Fig. 6A). Analysis of p21 mRNA revealed a slight elevation following mitogen stimulation; however, no significant changes in the mRNA levels of p21 were detected in mitogen-stimulated cells expressing GFP compared to those expressing GFP-FRNK (Fig. 6B). Similar results were seen by Northern blotting (data not shown). Another possibility was that FAK might be modulating the expression of these proteins through proteasome-mediated degradation (12). To test this possibility, we treated cells with inhibitors to the 26S proteasome. Treatment of asynchronously growing cells with the inhibitors lactacystin and MG-132 resulted in a robust increase in the protein levels of both p27 and p21, suggesting that there is significant turnover of these proteins that is normally mediated through proteasomal degradation (Fig. 7A).
FIG. 6.
FRNK does not alter the mRNA expression of p27 or p21. Real-time PCR was performed on HUVECs expressing GFP (control) or GFP-FRNK for 16 h in serum-free media, at which time complete growth medium was added for an additional 16 h to the cells as indicated. The mRNA was then reversed transcribed and analyzed by quantitative real-time PCR using primers specific to p27 (A) and p21 (B) with a Bio-Rad real-time PCR machine (A and B). Data are normalized to the control value and are reported as percentage of increase ± standard error of three experiments. *, significantly different from serum-free paired controls (P < 0.05). NS, nonsignificant difference from serum-stimulated GFP controls.
FIG. 7.
FRNK inhibits mitogen-induced expression of Skp2 protein and mRNA. Asynchronously growing HUVECs were treated with the proteasome inhibitors lactacystin and MG-132 as shown for 24 h, and then lysates were analyzed with antibodies specific to p27 and p21. Western blot analysis was then performed to analyze the levels of Skp2 protein expression after the addition of complete growth medium for 16 h (B). HUVECs were treated as in panel B, except mRNA was collected instead of protein using an RNeasy kit (QIAGEN). The mRNA was then reversed transcribed, and real-time PCR was performed using primers to Skp2 (C). Data are plotted as mean ± standard error. *, statistically significant difference from GFP; #, statistically significant difference from GFP treated with serum (P < 0.05) (n = 5).
This piece of data prompted us to examine mechanisms involved in the degradation of p27 and p21. Both p27 and 21 can be targeted for proteasomal destruction by the F-box protein Skp2 (13, 21, 77). This led us to investigate a potential role of Skp2 in mediating the FAK-dependent degradation of p27 and p21. The level of Skp2 was typically undetectable in quiescent cells, consistent with the high levels of p27 and p21 observed. The addition of serum for 16 h resulted in an increase in Skp2 (Fig. 7B) levels that parallels the decrease in the levels of p27 and p21 (Fig. 5C). Expression of GFP-FRNK markedly inhibited the expression of Skp2 (Fig. 7B), correlating well with the corresponding failure of p27 and p21 to be degraded (Fig. 5C).
The expression of Skp2 is also highly regulated and can be impacted at both the level of transcription, as well as by posttranscriptional regulation through the anaphase-promoting complex/cyclosome (APC/C) (9, 35, 80). In order to ascertain how Skp2 expression was being regulated in our system, we first examined the levels of Skp2 mRNA using real-time PCR. Interestingly, the addition of growth medium for 16 h resulted in an approximate fourfold increase in the level of Skp2 mRNA. In contrast, induction of Skp2 mRNA was completely inhibited in FRNK-expressing cells (Fig. 7C). These data implicate the levels of mRNA as a critical control point regulated by FAK signaling.
Skp2 regulates the FAK-dependent degradation of p27 but not p21.
To determine if Skp2 regulates the FAK-dependent proteasomal degradation of both p27 and p21, HUVECs were transduced with Ad.GFP or Ad.WT-Skp2 and made quiescent in serum-free media. The cells were then treated with serum for 16 h, and the levels of p27 and p21 were analyzed by immunoblotting. After 16 h of serum stimulation, the levels of p27 and p21 significantly decreased compared to those in quiescent cells, as previously shown in Fig. 5. Skp2 expression resulted in decreased p27 levels in quiescent cells as well as in cells coexpressing GFP-FRNK, but failed to induce the degradation of p21 (Fig. 8A). To further test the role of Skp2 in the degradation of p27 and p21, a similar experiment was performed with cells expressing a dominant-negative form of Skp2 (ΔFbox-Skp2). The mutant form of this protein while still binding p27 could no longer form a complex with Skp1-CUL1, thereby preventing ubiquitination of the target protein (73). Expression of dominant-negative Skp2 inhibited the serum-induced degradation of p27. However, unlike FRNK, expression of dominant-negative Skp2 did not inhibit serum-induced p21 degradation (Fig. 8B). These data provide evidence that Skp2 is required for the majority of p27-mediated degradation. The ability of expressed Skp2 to rescue the FRNK-inhibited p27 degradation suggests that the induction of Skp2 is likely a fundamental control process regulated by FAK. In contrast, the degradation of p21 is independent of Skp2 expression, indicating that focal adhesion kinase can affect cyclin-dependent kinase inhibitor levels through at least two separate mechanisms.
FIG. 8.
Skp2 regulates the FAK-dependent degradation of p27 but not p21. HUVECs were transduced with Ad.GFP, Ad.GFP-FRNK, Ad.WT-Skp2, or Ad.GFP-FRNK and Ad.WT-Skp2 combined in serum-free MCDB-131. The HUVECs were then treated with serum for 16 h and probed for p27, p21, and ERK (A). HUVECs were transduced with Ad.GFP or Ad.ΔF-box Skp2 treated the same as in panel A and then probed for p27, p21, and ERK2 (B). HUVECs in complete growth medium were transduced with Ad.GFP or Ad.GFP-FRNK for 6 h, treated with 10 μM lactacystin for the indicated times, and then lysed and probed for p21 protein levels (C). The net intensity of each band was quantified using a Kodak digital camera along with densitometry software (D).
Since p21 levels were unaffected by Skp2, we considered a hypothesis alternate to impaired proteasomal degradation that might be regulated by FRNK expression. We observed that p21 mRNA levels actually increase in response to mitogens in both control and FRNK-expressing cells: one possibility is that p21 mRNA translation is blocked in serum-treated control cells and that this postulated translational repression is inhibited in cells expressing FRNK. To test this, control cells and GFP-FRNK-expressing cells, in the presence of serum, were treated with lactacystin and examined for p21 protein expression over a time course. As starting mRNA levels are similar (see Fig. 6), we reasoned that in the absence of proteasome activity, protein accumulation over time would be an estimate of translational capacity. This experiment predicts that if FRNK-expressing cells do prevent a translational repression of p21 typically induced by mitogens; higher levels of p21 should be observed in the FRNK-expressing cells compared to in the control. We followed p21 protein accumulation over a 36-h time course and found that p21 protein levels were nearly identical under both conditions (Fig. 8C). The net intensity of the p21 expression was quantified by digital imaging to verify that the changes in p21 expression were numerically similar between control and FRNK-expressing cells (Fig. 8D). Notably, there was no additive effect of FRNK and lactacystin on p21 accumulation at any time point, arguing against any mechanisms regulated by FRNK that were independent of protein degradation. These data, in conjunction with our other findings, are most consistent with the notion that the FAK-dependent regulation of p21 occurs at the level of protein turnover mediated by the 26S proteasome and not at the level of transcription or translation.
Skp2 regulates endothelial cell proliferation through modulation of p27.
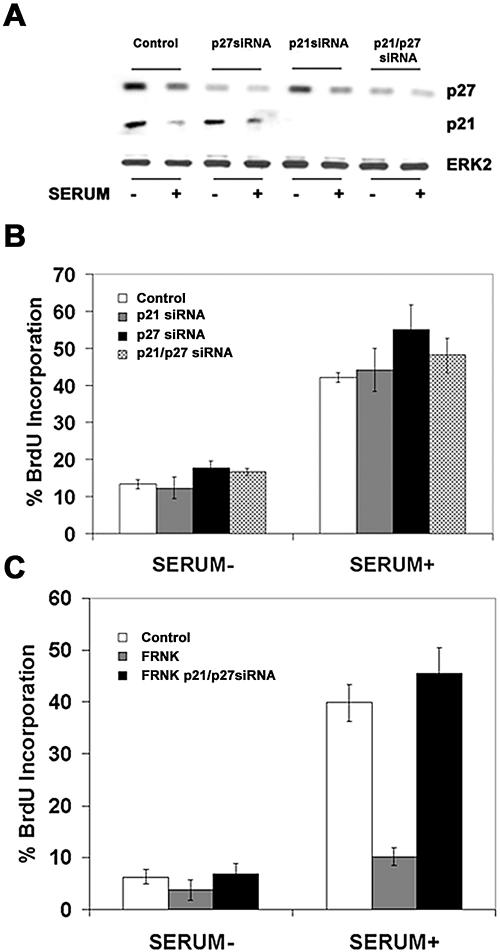
Based on the sensitivity of Skp2 message and protein levels to alterations in FAK signaling, we next investigated the requirement of Skp2 in endothelial cell proliferation using siRNA-mediated knockdown of Skp2. We found that knockdown of Skp2 with siRNA prevented serum-induced degradation of p27 similar to that seen with the mutant Skp2 (Fig. 9A). Furthermore, the knockdown of Skp2 expression completely inhibited the ability of cells to proliferate in response to serum (Fig. 9B). As Skp2 has also been shown to regulate other proteins important for proliferation, including cyclin E1 and myc (38, 55), we used siRNA targeting the combination of Skp2 and p27 to determine if p27 is the key protein degraded by Skp2 for proliferation of endothelial cells. The addition of p27 siRNA prevented accumulation of p27 in quiescent cells as well as cells cotreated with Skp2 siRNA (Fig. 9A). Knockdown of p27 completely restored proliferation in cells that were treated with serum but lacked Skp2 protein (Fig. 9B). This finding suggests that p27 is the principal target of Skp2 in regulating the G1/S-phase transition in endothelial cells.
FIG. 9.
Skp2 regulates endothelial cell proliferation through modulation of p27. HUVECs were electroporated with 150 to 200 ng of siRNA targeted toward luciferase (control), Skp2, or p27 and then allowed to adhere overnight. The following day, the cells were placed in serum-free MCDB-131 for 16 h and then treated with complete growth medium for an additional 16 h. A portion of the cells plated in 35-mm dishes were then lysed and probed for Skp2, p27, or ERK2 (A). A second portion of the cells plated in a 24-well dish were pulsed with BrdU and analyzed for the percentage that were BrdU positive (B).
Knockdown of p21 and p27 restores the ability of FRNK-expressing endothelial cells to proliferate.
Although p21 and p27 are known to be important inhibitors of growth, paradoxically, Cip/Kip proteins have also been shown to be required for the assembly of the cyclin D-cdk complex (19). As an initial experiment, we investigated the role of the CDKI proteins on normal endothelial cell growth control. We found that siRNA duplexes targeted to p21 or p27 specifically knocked down the protein in question without affecting the other, and the knockdown lowered the level of both proteins to levels at or below those seen in serum-treated cells (Fig. 10A). Knockdown of p21, p27, or the combination of both proteins, was insufficient to promote growth in quiescent cells, underscoring the importance of other mitogen-induced events such as the induction of cyclin D. Likewise, knockdown of the proteins with the addition of mitogen did not significantly increase the percentage of cells entering S phase (Fig. 10B). These data demonstrate that knockdown of the CDKIs per se does not confer a proliferative advantage (or disadvantage) in the context of otherwise normal cellular signaling.
FIG. 10.
Knockdown of p21 and p27 restores the ability of FRNK-expressing endothelial cells to proliferate. HUVECs were electroporated with 150 to 200 ng of siRNA targeted toward luciferase (control), p21, and p27. The cells were then treated exactly as described in the legend to Fig. 9 and probed for p27 and p21 (A) and for the percentage of BrdU-positive cells (B). HUVECs were first electroporated with the specified siRNA as in panel A and on the following day during serum starvation were also transduced with Ad.GFP or Ad.GFP-FRNK. The next day, the cells were stimulated with complete growth medium for 16 h, at which point they were pulsed with BrdU and the percentage of BrdU-positive cells was calculated (C).
Our data suggested that alterations in FAK signaling control the levels of two important CDKIs, p21 and p27, through two independent mechanisms. However disruption of FAK signaling could have additional effects on cell cycle proteins such as cyclin E, cdk2, or other inhibitors like p57/Kip2 and INK4 family members. We used siRNA knockdown of both p21 and p27 to test the role of these proteins in mediating the inhibition of cell proliferation seen following expression of GFP-FRNK. We found that siRNA-mediated repression of both p21 and p27 completely restored entry into S phase in response to mitogen stimulation in cells expressing FRNK (Fig. 10C). Notably, individual knockdown of either p21 or p27 displayed intermediate responses, suggesting both CDKIs have growth-suppressive effects (data not shown).
DISCUSSION
Our data indicate that FAK signaling is required for the control of CDKI levels, which are normally lowered in response to growth factors. In contrast to some other cell types, induction of mitogen-activated protein kinase MAPK activities and cyclin D proceeds normally. The regulation imposed by FAK signaling does not occur through changes in the mRNA levels of these CDKIs, but rather appears to be mediated through regulation of protein turnover. Furthermore, the regulatory mechanisms appear to bifurcate as the regulation of p27 was shown to be dependent on Skp2 while p21 degradation occurs independently of Skp2. Our data indicate that the adhesion-dependent, mitogen-stimulated accumulation of Skp2 mRNA is dependent on FAK and demonstrate that Skp2 is critical for endothelial cell proliferation. Finally, we demonstrate that the regulation of these two CDKIs is the critical regulatory event in the inhibition of proliferation by disruption of FAK signaling, as enforced knockdown of both CDKIs restores mitogen-stimulated proliferation.
The coordinated signaling of growth factors and the ECM is believed to be critical for the propagation of adherent endothelial cells. Supporting this notion is the accumulation of progrowth ECM molecules, including a fibronectin-rich interstitial matrix, during the remodeling/proliferative stage of angiogenesis. This is in sharp contrast to the laminin-rich ECM found in the maturation phase of angiogenesis as the cells form capillaries and display negative growth properties (62). One potential mechanism to account for the ability of particular ECM proteins to impart such dramatically different proliferative phenotypes involves signal transduction through distinct integrins and their ability to form focal adhesions and activate FAK (68).
Focal adhesion kinase is a key integrator of signals from growth factors, the ECM, as well as mechanical stimuli. In endothelial cells, FAK becomes highly phosphorylated in response to VEGF, plating on fibronectin, and in response to laminar flow (2, 40, 45). The ability of FAK to become autophosphorylated at Tyr 397 and become activated in response to these diverse stimuli is dependent on cell adhesion. The decreases in the levels of the CDKIs p27 and p21 required for cell cycle progression have been reported to be dependent on cell adhesion as well as growth factor stimulation. Studies along with this one have recently shown that adhesion-dependent degradation of p27 and p21 can be regulated via proteasomal degradation (8, 39, 57). Our findings suggest that the abilities of adherent cells to degrade p27 and p21 in response to many diverse stimuli likely converge at the level of FAK signaling. This finding is important because it provides a possible single explanation for the numerous and diverse findings that cell shape, cell size, adhesion, and mitogens, as well as mechanical forces can affect cell proliferation and/or CDKI levels (5, 36, 46, 79), since all of these environmental stimuli are also known to alter FAK signaling.
Phosphorylation of FAK Y397 appears to be a critical determinant for the cell to transition successfully from G1 to S phase. We do not believe this is a response unique to expression of FRNK, as we see identical results when FAK Y397F is expressed, but not WT-FAK. Moreover, recent data from Shen et al. demonstrate that endothelial cells derived from mice with a floxed FAK allele have defects in BrDU incorporation following deletion of the FAK allele with Cre-recombinase (67), underpinning a critical role for FAK in endothelial cell proliferation. In preliminary experiments, we have attempted to use oligonucleotide-based siRNA to knock down FAK expression. While we have achieved moderate knockdown of FAK protein (approximately 70% inhibited), effects on FAK 397 phosphorylation have been less dramatic (typically 30 to 50% inhibited). Under these conditions, it is important to note that cell cycle progression does not appear to be disrupted. Taken together, these observations suggest that the FAK-dependent signal likely represents a checkpoint-like mechanism with a relatively small threshold (FAK 397 phosphorylation has to be greater than zero but can be less than 50%) in order to trigger CDKI degradation. Mitogen enhancement of FAK phosphorylation may not have to take place, but rather sufficient engagement with the substratum and cellular tension may need to be in place to maintain a level of FAK phosphorylation as a permissive step. Studies are currently under way with vector-based inducible siRNA technologies to further define the lower limit of FAK expression (and/or 397 phosphorylation), as well as conditions which may alter those limits.
Our data demonstrate how crucial the degradation of the Cip/Kip CDKIs is in regulating endothelial growth, as suppression of p27 and p21 was capable of completely restoring proliferation in the presence of disrupted FAK signaling. Conversely, a somewhat surprising finding was that knockdown of p21 and p27 had no effect on normal endothelial cell proliferation, as previous reports have suggested Cip/Kip proteins are required for the assembly of the active cyclin D-cdk complexes (19, 43). Our data would suggest that this function is not critical in endothelial cells, as knockdown of p27 and p21 individually or in tandem did not impede proliferation. Alternatively, it may be that the levels required for cyclin-cdk complex assembly is quite low and below the threshold of our knockdown.
Different cell systems have evolved multiple mechanisms to regulate CDKIs. Mechanisms described to date include nuclear-cytoplasmic shuttling, transcriptional regulation, and mRNA stabilization, as well as proteasomal degradation (7, 11, 14, 37, 41, 42, 59). Previous studies have indicated both p27 and p21 can be regulated through transcriptional mechanisms in endothelial cells (23, 74). For instance, the inhibition of FOXO transcription factors with RNA interference has been shown to decrease p27 levels, thereby promoting cell proliferation (1, 58). While FAK could theoretically alter the activation of FOXO factors through modulation of phosphatidylinositol-3′-kinase (PI 3′-kinase) signaling, our data would suggest this is not occurring, as the decrease in p27 mRNA in response to mitogens occurred normally following disruption of FAK signaling. Therefore, it is also unlikely that PI 3′-kinase-mediated changes in p21 and p27 message are involved, which is in agreement with findings that PI 3′-kinase may not be essential for endothelial cell proliferation (50, 82). Moreover, serum-induced suppression of p27 mRNA levels is not sufficient to substantially alter p27 protein levels when the induction of Skp2 is blocked. Likewise, we have shown that loss of Skp2 expression completely inhibits endothelial cell proliferation, which requires only the knockdown of p27 to be completely restored. Together, these findings underscore the importance of inducing Skp2 for endothelial cell proliferation.
The oncogene Skp2 has been found to be important in the progression of numerous tumors and, based on our data, is critical for the growth of primary endothelial cells as well (10, 22). We have found that induction of Skp2 mRNA accumulation is FAK dependent. This finding provides a biochemical mechanism for the adhesion-dependent accumulation of Skp2 previously described in fibroblasts (16). This finding may also have significant implications in certain cancers such as malignant gliomas, melanomas, and ovarian carcinoma (56, 71, 86)—all of which maintain elevated levels of FAK phosphorylation independently of adhesion (29). This could provide a mechanism for adhesion-independent expression of Skp2 and cell cycle progression in these tumor types. In endothelial cells, our data would suggest that FAK activation alone is not sufficient to stimulate cell cycle proliferation, suggesting a requirement for simultaneous activation of growth factor signals.
Currently little is known about the regulation of Skp2, particularly in nontransformed cells, although reports have suggested that Skp2 regulation is dependent on Ras pathways (53, 63). However, we do not see an inhibition of ERK signaling in FRNK-expressing cells and PI 3′-kinase does not appear to be required for growth in endothelial cells (50, 82). Consistent with this, attempts to rescue the FRNK-induced proliferation block with activated forms of Raf and Akt were ineffective (data not shown). While these data do not discount the possibility that Ras-related signaling may impinge on Skp2 regulation, they do suggest that FAK-derived signals act as an alternate or parallel control mechanism.
Skp2 levels can be regulated by proteasomal degradation through the APC/C complex (9, 80). A recent report on smooth muscle cells suggested that FAK signaling could modulate the levels of Skp2 by controlling protein degradation, as they saw no alterations in Skp2 mRNA levels (12). In contrast, we find that Skp2 mRNA levels are robustly induced by mitogens and that this enhancement is FAK dependent. In addition, disruption of FAK signal transduction did not alter the levels of ectopically expressed Skp2 in endothelial cells, arguing against alterations in the protein degradation machinery as being the primary mechanism controlling Skp2 levels (data not shown). To date, few studies have investigated the Skp2 promoter. To date only the Ets-related transcription factor, GABP, and E2F have been shown to be transcriptional activators of the Skp2 promoter (35, 83). Further study will be required to determine if these transcription factors are mediating the induction of Skp2 mRNA regulated by FAK in endothelial cells.
Although the precise downstream signals through which FAK regulates Skp2/p27 and p21 remain to be clarified, a recent study examining the role of FAK in endothelial permeability has found that expression of FRNK inhibits Rac activity while simultaneously activating Rho (31). Our data also found that expression of FRNK seems to inhibit ruffling while enhancing stress fibers under resting conditions. The possibility that FAK is functioning to regulate small GTPases is intriguing based on evidence that Rho manipulation can control p27 and that Rac can modulate p21 (8, 47). However this hypothesis needs to be approached cautiously, as alterations in Rho and Rac activity are known to regulate FAK activation.
We find that FAK signaling is required for the loss of p21 protein in response to serum. Previous reports have implicated Skp2 as being an important component of p21 degradation: largely based on experiments conducted in vitro. Our data suggest that in primary endothelial cells the regulation of p21 appears to be independent of Skp2 expression as (i) enforced expression of Skp2 (even at supraphysiological levels) was without effect on p21 levels and (ii) expression of an F-box mutant which blocks targeting to the Cul1 E3-ligase did not disrupt the degradation of p21, while completely inhibiting the degradation of p27. These data are consistent with a report in fibroblasts which also suggested regulation of p21 was Skp2 independent (72) and with a recent report that indicates that p21 cannot be ubiquitinated in vivo due to the presence of N-terminal acetylation (18). These data support the notion that p21 proteolysis does not necessarily require ubiquitination (66) and may be explained by the ability of p21 to directly bind to the C8-α subunit of the 20S proteasome (76); however, these possibilities remain to be validated in intact cells.
Our data underscore the significance of cell-type-specific signal transduction. Previous studies investigating FAK and cell cycle progression in fibroblasts have found a requirement for FAK in the regulation of ERK activation and cyclin D expression (85) and a sufficiency of FAK expression to induce cellular proliferation. In contrast, we find ERK activation and cyclin D1 induction proceed normally following disruption of FAK signaling in endothelial cells. It should be noted that we find an absolute dependence on FAK for activation of ERK in primary fibroblasts (data not shown), in agreement with the previous finding. Similarly, our data reveal a role for FAK in regulating mRNA levels of Skp2, while in smooth muscle cells, FAK affects Skp2 stability, not mRNA levels. These data suggest that different cell types have unique cell cycle control networks that could be exploited to develop therapeutics with more selective antiproliferative actions. In addition, our data highlight the significance of proteasomal degradation in the proliferation control of endothelial cells. A clinical study using the proteasome inhibitor bortezomib for the treatment of multiple myeloma found that the drug also impaired angiogenesis (61). Consistent with this, we have found that treatment of HUVECs with the proteasome inhibitor MG-132 inhibits VEGF-induced growth (data not shown). Collectively, our data suggest that targeting the proteasomal degradation of CDKIs may be a useful approach for the treatment of diseases dependent upon endothelial cell proliferation, such as proliferative retinopathies, hemangiomas, and tumor angiogenesis.
Acknowledgments
This work was supported by CA81419 from the National Cancer Institute and the David E. Bryant Trust for Research in Blindness (K.P.). This work also received support through an institutional predoctoral training grant (T32-HL-07194) and an individual predoctoral award from the American Heart Association (P.B.).
We acknowledge the assistance of Hanqui Zheng for his help in establishing conditions for siRNA knockdown of the CDKIs. We thank Mark Bond (University of Bristol) for generously providing the WT-Skp2 and Skp2 adenoviruses with the F-box deleted; Allen Samarel and Maria Heidkamp (Loyola University Medical Center) for the GFP-FRNK adenovirus, and Rebecca Keller (Albany Medical College) for the FRNK and FRNKS adenoviruses. In addition, we acknowledge the support of the Developmental Therapeutics Program of the National Cancer Institute for providing VEGF and endothelial cells.
REFERENCES
- 1.Abid, M. R., K. Yano, S. Guo, V. I. Patel, G. Shrikhande, K. C. Spokes, C. Ferran, and W. C. Aird. 2005. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J. Biol. Chem. 280:29864-29873. (First published 15 June 2005; doi: 10.1074/jbc.M502149200.) [DOI] [PubMed] [Google Scholar]
- 2.Abu-Ghazaleh, R., J. Kabir, H. Jia, M. Lobo, and I. Zachary. 2001. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 360:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alavi, A., J. D. Hood, R. Frausto, D. G. Stupack, and D. A. Cheresh. 2003. Role of Raf in vascular protection from distinct apoptotic stimuli. Science 301:94-96. [DOI] [PubMed] [Google Scholar]
- 4.Aplin, A. E., S. A. Stewart, R. K. Assoian, and R. L. Juliano. 2001. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 153:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assoian, R. K., and M. A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48-53. [DOI] [PubMed] [Google Scholar]
- 6.Avraham, H. K., T. H. Lee, Y. Koh, T. A. Kim, S. Jiang, M. Sussman, A. M. Samarel, and S. Avraham. 2003. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J. Biol. Chem. 278:36661-36668. [DOI] [PubMed] [Google Scholar]
- 7.Baldassarre, G., B. Belletti, P. Bruni, A. Boccia, F. Trapasso, F. Pentimalli, M. V. Barone, G. Chiappetta, M. T. Vento, S. Spiezia, A. Fusco, and G. Viglietto. 1999. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J. Clin. Investig. 104:865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao, W., M. Thullberg, H. Zhang, A. Onischenko, and S. Strömblad. 2002. Cell attachment to the extracellular matrix induces proteasomal degradation of p21CIP1 via Cdc42/Rac1 signaling. Mol. Cell. Biol. 22:4587-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashir, T., N. V. Dorrello, V. Amador, D. Guardavaccaro, and M. Pagano. 2004. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428:190-193. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Izhak, O., S. Lahav-Baratz, S. Meretyk, S. Ben-Eliezer, E. Sabo, M. Dirnfeld, S. Cohen, and A. Ciechanover. 2003. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J. Urol. 170:241-245. [DOI] [PubMed] [Google Scholar]
- 11.Blagosklonny, M. V., P. Giannakakou, L. Y. Romanova, K. M. Ryan, K. H. Vousden, and T. Fojo. 2001. Inhibition of HIF-1- and wild-type p53-stimulated transcription by codon Arg175 p53 mutants with selective loss of functions. Carcinogenesis 22:861-867. [DOI] [PubMed] [Google Scholar]
- 12.Bond, M., G. B. Sala-Newby, and A. C. Newby. 2004. Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. A novel mechanism regulating smooth muscle cell proliferation. J. Biol. Chem. 279:37304-37310. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein, G., J. Bloom, D. Sitry-Shevah, K. Nakayama, M. Pagano, and A. Hershko. 2003. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278:25752-25757. [DOI] [PubMed] [Google Scholar]
- 14.Bottazzi, M. E., X. Zhu, R. M. Bohmer, and R. K. Assoian. 1999. Regulation of p21(cip1) expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J. Cell Biol. 146:1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeliet, P., and R. K. Jain. 2000. Angiogenesis in cancer and other diseases. Nature 407:249-257. [DOI] [PubMed] [Google Scholar]
- 16.Carrano, A. C., and M. Pagano. 2001. Role of the F-box protein Skp2 in adhesion-dependent cell cycle progression. J. Cell Biol. 153:1381-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, C. S., M. Mrksich, S. Huang, G. M. Whitesides, and D. E. Ingber. 1997. Geometric control of cell life and death. Science 276:1425-1428. [DOI] [PubMed] [Google Scholar]
- 18.Chen, X., Y. Chi, A. Bloecher, R. Aebersold, B. E. Clurman, and J. M. Roberts. 2004. N-acetylation and ubiquitin-independent proteasomal degradation of p21(Cip1). Mol. Cell 16:839-847. [DOI] [PubMed] [Google Scholar]
- 19.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeGregori, J., T. Kowalik, and J. R. Nevins. 1995. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell. Biol. 15:4215-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshaies, R. J. 1999. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15:435-467. [DOI] [PubMed] [Google Scholar]
- 22.Drobnjak, M., J. Melamed, S. Taneja, K. Melzer, R. Wieczorek, B. Levinson, A. Zeleniuch-Jacquotte, D. Polsky, J. Ferrara, R. Perez-Soler, C. Cordon-Cardo, M. Pagano, and I. Osman. 2003. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin. Cancer Res. 9:2613-2619. [PubMed] [Google Scholar]
- 23.el-Deiry, W. S., J. W. Harper, P. M. O'Connor, V. E. Velculescu, C. E. Canman, J. Jackman, J. A. Pietenpol, M. Burrell, D. E. Hill, Y. Wang, et al. 1994. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 54:1169-1174. [PubMed] [Google Scholar]
- 24.Eliceiri, B. P., X. S. Puente, J. D. Hood, D. G. Stupack, D. D. Schlaepfer, X. Z. Huang, D. Sheppard, and D. A. Cheresh. 2002. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 157:149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folkman, J. 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1:27-31. [DOI] [PubMed] [Google Scholar]
- 26.Folkman, J., and A. Moscona. 1978. Role of cell shape in growth control. Nature 273:345-349. [DOI] [PubMed] [Google Scholar]
- 27.Gilmore, A. P., and L. H. Romer. 1996. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell 7:1209-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecker, T. P., J. R. Grammer, G. Y. Gillespie, J. Stewart, Jr., and C. L. Gladson. 2002. Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res. 62:2699-2707. [PubMed] [Google Scholar]
- 30.Heidkamp, M. C., A. L. Bayer, J. A. Kalina, D. M. Eble, and A. M. Samarel. 2002. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ. Res. 90:1282-1289. [DOI] [PubMed] [Google Scholar]
- 31.Holinstat, M., N. Knezevic, M. Broman, A. M. Samarel, A. B. Malik, and D. Mehta. 2005. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J. Biol. Chem. 281:2296-2305. (First published 24 November 2005; doi: 10.1074/jbc.M511248200.) [DOI] [PubMed] [Google Scholar]
- 32.Huang, S., C. S. Chen, and D. E. Ingber. 1998. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell 9:3179-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilic, D., E. A. Almeida, D. D. Schlaepfer, P. Dazin, S. Aizawa, and C. H. Damsky. 1998. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 143:547-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 35.Imaki, H., K. Nakayama, S. Delehouzee, H. Handa, M. Kitagawa, T. Kamura, and K. I. Nakayama. 2003. Cell cycle-dependent regulation of the Skp2 promoter by GA-binding protein. Cancer Res. 63:4607-4613. [PubMed] [Google Scholar]
- 36.Ingber, D. E. 1990. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc. Natl. Acad. Sci. USA 87:3579-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph, B., M. Orlian, and H. Furneaux. 1998. p21(waf1) mRNA contains a conserved element in its 3′-untranslated region that is bound by the Elav-like mRNA-stabilizing proteins. J. Biol. Chem. 273:20511-20516. [DOI] [PubMed] [Google Scholar]
- 38.Kim, S. Y., A. Herbst, K. A. Tworkowski, S. E. Salghetti, and W. P. Tansey. 2003. Skp2 regulates Myc protein stability and activity. Mol. Cell 11:1177-1188. [DOI] [PubMed] [Google Scholar]
- 39.King, R. W., R. J. Deshaies, J. M. Peters, and M. W. Kirschner. 1996. How proteolysis drives the cell cycle. Science 274:1652-1659. [DOI] [PubMed] [Google Scholar]
- 40.Kornberg, L., H. S. Earp, J. T. Parsons, M. Schaller, and R. L. Juliano. 1992. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J. Biol. Chem. 267:23439-23442. [PubMed] [Google Scholar]
- 41.Koshiji, M., Y. Kageyama, E. A. Pete, I. Horikawa, J. C. Barrett, and L. E. Huang. 2004. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 23:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kullmann, M., U. Gopfert, B. Siewe, and L. Hengst. 2002. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 16:3087-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 44.Ledford, A. W., J. G. Brantley, G. Kemeny, T. L. Foreman, S. E. Quaggin, P. Igarashi, S. M. Oberhaus, M. Rodova, J. P. Calvet, and G. B. Vanden Heuvel. 2002. Deregulated expression of the homeobox gene Cux-1 in transgenic mice results in downregulation of p27(kip1) expression during nephrogenesis, glomerular abnormalities, and multiorgan hyperplasia. Dev. Biol. 245:157-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, S., M. Kim, Y. L. Hu, S. Jalali, D. D. Schlaepfer, T. Hunter, S. Chien, and J. Y. Shyy. 1997. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J. Biol. Chem. 272:30455-30462. [DOI] [PubMed] [Google Scholar]
- 46.Madri, J. A., B. M. Pratt, and J. Yannariello-Brown. 1988. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am. J. Pathol. 132:18-27. [PMC free article] [PubMed] [Google Scholar]
- 47.Mammoto, A., S. Huang, K. Moore, P. Oh, and D. E. Ingber. 2004. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J. Biol. Chem. 279:26323-26330. [DOI] [PubMed] [Google Scholar]
- 48.McMullen, M. E., P. W. Bryant, C. C. Glembotski, P. A. Vincent, and K. M. Pumiglia. 2005. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J. Biol. Chem. 280:20995-21003. [DOI] [PubMed] [Google Scholar]
- 49.Meadows, K. N., P. Bryant, and K. Pumiglia. 2001. Vascular endothelial growth factor induction of the angiogenic phenotype requires Ras activation. J. Biol. Chem. 276:49289-49298. [DOI] [PubMed] [Google Scholar]
- 50.Meadows, K. N., P. Bryant, P. A. Vincent, and K. M. Pumiglia. 2004. Activated Ras induces a proangiogenic phenotype in primary endothelial cells. Oncogene 23:192-200. [DOI] [PubMed] [Google Scholar]
- 51.Melendez, J., S. Welch, E. Schaefer, C. S. Moravec, S. Avraham, H. Avraham, and M. A. Sussman. 2002. Activation of pyk2/related focal adhesion tyrosine kinase and focal adhesion kinase in cardiac remodeling. J. Biol. Chem. 277:45203-45210. [DOI] [PubMed] [Google Scholar]
- 52.Mettouchi, A., S. Klein, W. Guo, M. Lopez-Lago, E. Lemichez, J. K. Westwick, and F. G. Giancotti. 2001. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol. Cell 8:115-127. [DOI] [PubMed] [Google Scholar]
- 53.Mirza, A. M., S. Gysin, N. Malek, K.-I. Nakayama, J. M. Roberts, and M. McMahon. 2004. Cooperative regulation of the cell division cycle by the protein kinases RAF and AKT. Mol. Cell. Biol. 24:10868-10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison, T. B., J. J. Weis, and C. T. Wittwer. 1998. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. BioTechniques 24:954-958, 960, 962. [PubMed] [Google Scholar]
- 55.Nakayama, K. I., S. Hatakeyama, and K. Nakayama. 2001. Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem. Biophys. Res. Commun. 282:853-860. [DOI] [PubMed] [Google Scholar]
- 56.Natarajan, M., T. P. Hecker, and C. L. Gladson. 2003. FAK signaling in anaplastic astrocytoma and glioblastoma tumors. Cancer J. 9:126-133. [DOI] [PubMed] [Google Scholar]
- 57.Pagano, M., S. W. Tam, A. M. Theodoras, P. Beer-Romero, G. Del Sal, V. Chau, P. R. Yew, G. F. Draetta, and M. Rolfe. 1995. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682-685. [DOI] [PubMed] [Google Scholar]
- 58.Potente, M., B. Fisslthaler, R. Busse, and I. Fleming. 2003. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J. Biol. Chem. 278:29619-29625. [DOI] [PubMed] [Google Scholar]
- 59.Pumiglia, K. M., and S. J. Decker. 1997. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 94:448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson, A., and T. Parsons. 1996. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature 380:538-540. [DOI] [PubMed] [Google Scholar]
- 61.Richardson, P. 2003. Clinical update: proteasome inhibitors in hematologic malignancies. Cancer Treat. Rev. 29(Suppl. 1):33-39. [DOI] [PubMed] [Google Scholar]
- 62.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386:671-674. [DOI] [PubMed] [Google Scholar]
- 63.Sa, G., and D. W. Stacey. 2004. P27 expression is regulated by separate signaling pathways, downstream of Ras, in each cell cycle phase. Exp. Cell Res. 300:427-439. [DOI] [PubMed] [Google Scholar]
- 64.Schulze, A., K. Zerfass-Thome, J. Bergès, S. Middendorp, P. Jansen-Dürr, and B. Henglein. 1996. Anchorage-dependent transcription of the cyclin A gene. Mol. Cell. Biol. 16:4632-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz, M. A., and R. K. Assoian. 2001. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114:2553-2560. [DOI] [PubMed] [Google Scholar]
- 66.Sheaff, R. J., J. D. Singer, J. Swanger, M. Smitherman, J. M. Roberts, and B. E. Clurman. 2000. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell 5:403-410. [DOI] [PubMed] [Google Scholar]
- 67.Shen, T. L., A. Y. Park, A. Alcaraz, X. Peng, I. Jang, P. Koni, R. A. Flavell, H. Gu, and J. L. Guan. 2005. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 169:941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen, Y., and M. D. Schaller. 1999. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol. Biol. Cell 10:2507-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 70.Sieg, D. J., C. R. Hauck, and D. D. Schlaepfer. 1999. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112:2677-2691. [DOI] [PubMed] [Google Scholar]
- 71.Sood, A. K., J. E. Coffin, G. B. Schneider, M. S. Fletcher, B. R. DeYoung, L. M. Gruman, D. M. Gershenson, M. D. Schaller, and M. J. Hendrix. 2004. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am. J. Pathol. 165:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart, S. A., D. Kothapalli, Y. Yung, and R. K. Assoian. 2004. Antimitogenesis linked to regulation of Skp2 gene expression. J. Biol. Chem. 279:29109-29113. [DOI] [PubMed] [Google Scholar]
- 73.Sutterluty, H., E. Chatelain, A. Marti, C. Wirbelauer, M. Senften, U. Muller, and W. Krek. 1999. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1:207-214. [DOI] [PubMed] [Google Scholar]
- 74.Takuwa, N., and Y. Takuwa. 1997. Ras activity late in G1 phase required for p27kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol. Cell. Biol. 17:5348-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanner, F. C., M. Boehm, L. M. Akyurek, H. San, Z. Y. Yang, J. Tashiro, G. J. Nabel, and E. G. Nabel. 2000. Differential effects of the cyclin-dependent kinase inhibitors p27(Kip1), p21(Cip1), and p16(Ink4) on vascular smooth muscle cell proliferation. Circulation 101:2022-2025. [DOI] [PubMed] [Google Scholar]
- 76.Touitou, R., J. Richardson, S. Bose, M. Nakanishi, J. Rivett, and M. J. Allday. 2001. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome. EMBO J. 20:2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tyers, M., and P. Jorgensen. 2000. Proteolysis and the cell cycle: with this RING I do thee destroy. Curr. Opin. Genet. Dev. 10:54-64. [DOI] [PubMed] [Google Scholar]
- 78.Vermeulen, K., D. R. Van Bockstaele, and Z. N. Berneman. 2003. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 36:131-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, F., R. K. Hansen, D. Radisky, T. Yoneda, M. H. Barcellos-Hoff, O. W. Petersen, E. A. Turley, and M. J. Bissell. 2002. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J. Natl. Cancer Inst. 94:1494-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei, W., N. G. Ayad, Y. Wan, G. J. Zhang, M. W. Kirschner, and W. G. Kaelin, Jr. 2004. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428:194-198. [DOI] [PubMed] [Google Scholar]
- 81.Weintraub, S. J., C. A. Prater, and D. C. Dean. 1992. Retinoblastoma protein switches the E2F site from positive to negative element. Nature 358:259-261. [DOI] [PubMed] [Google Scholar]
- 82.Zeng, H., H. F. Dvorak, and D. Mukhopadhyay. 2001. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) receptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 276:26969-26979. [DOI] [PubMed] [Google Scholar]
- 83.Zhang, L., and C. Wang. 5 December 2005, posting date. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene [Online.] doi: 10.1038/sj.onc.1209286. [DOI] [PMC free article] [PubMed]
- 84.Zhao, J., R. Pestell, and J. L. Guan. 2001. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol. Biol. Cell 12:4066-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao, J. H., H. Reiske, and J. L. Guan. 1998. Regulation of the cell cycle by focal adhesion kinase. J. Cell Biol. 143:1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu, N. W., C. M. Perks, A. R. Burd, and J. M. Holly. 1999. Changes in the levels of integrin and focal adhesion kinase (FAK) in human melanoma cells following 532 nm laser treatment. Int. J. Cancer 82:353-358. [DOI] [PubMed] [Google Scholar]
- 87.Zhu, X., M. Ohtsubo, R. M. Bohmer, J. M. Roberts, and R. K. Assoian. 1996. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 133:391-403. [DOI] [PMC free article] [PubMed] [Google Scholar]