Abstract
The tyrosine kinase JAK2 is a key signaling protein for at least 20 receptors in the cytokine/hematopoietin receptor superfamily and is a component of signaling for multiple receptor tyrosine kinases and several G-protein-coupled receptors. In this study, phosphopeptide affinity enrichment and mass spectrometry identified serine 523 (Ser523) in JAK2 as a site of phosphorylation. A phosphoserine 523 antibody revealed that Ser523 is rapidly but transiently phosphorylated in response to growth hormone (GH). MEK1 inhibitor UO126 suppresses GH-dependent phosphorylation of Ser523, suggesting that extracellular signal-regulated kinases (ERKs) 1 and/or 2 or another kinase downstream of MEK1 phosphorylate Ser523 in response to GH. Other ERK activators, phorbol 12-myristate 13-acetate and epidermal growth factor, also stimulate phosphorylation of Ser523. When Ser523 in JAK2 was mutated, JAK2 kinase activity as well as GH-dependent tyrosyl phosphorylation of JAK2 and Stat5 was enhanced, suggesting that phosphorylation of Ser523 inhibits JAK2 kinase activity. We hypothesize that phosphorylation of Ser523 in JAK2 by ERKs 1 and/or 2 or other as-yet-unidentified kinases acts in a negative feedback manner to dampen activation of JAK2 in response to GH and provides a mechanism by which prior exposure to environmental factors that regulate Ser523 phosphorylation might modulate the cell's response to GH.
JAK2 is a member of the Janus family of cytoplasmic tyrosine kinases (JAK1, JAK2, JAK3, and tyk2), critical for transmission of cytokine-induced proliferative, differentiation, and survival signals. Roughly two-thirds of the cytokine/hematopoietin superfamily of receptors activate JAK2, including the receptors for growth hormone (GH), prolactin, erythropoietin (EPO), gamma interferon, and leptin (4, 61). Dysregulation of JAKs has been linked to immune diseases (10) and a variety of cancers (6, 33, 38, 40, 41, 49, 70). Upon binding to the GH receptor, GH causes a particularly rapid, transient, and robust activation of JAK2 (3). Activation of JAK2 is thought to occur when JAK2 molecules are brought into close enough proximity to trans phosphorylate on tyrosine residues located in the activation loop of each kinase (29). Activated JAK2 molecules then phosphorylate themselves, their dimerized JAK2 partner, associated receptor subunits, and a variety of signaling molecules such as signal transducers and activators of transcription 3 and 5 (stat3 and stat5) (25).
Auto- and/or transphosphorylation of JAK2 is therefore an important step in the transduction of cytokine signaling. Murine JAK2 contains 49 tyrosines, of which more than 10 appear to be sites of autophosphorylation (5, 19, 20, 45; L. S. Argetsinger et al., unpublished data). Phosphorylation of some of these tyrosines appears to regulate the activity of JAK2, for example, tyrosine 1007 (20) and more recently identified tyrosine 221 and tyrosine 570 (5, 19). Some phosphorylated tyrosines recruit other signaling molecules that contain motifs of phosphotyrosine binding (SH2 and PTB domains) to JAK2. Phosphorylated tyrosine 1007, which is critical for activation of JAK2, binds the negative regulators SOCS-1 (74), SOCS-3 (57), and phosphatase PTP1B (47); phosphorylated tyrosine 966 binds p70, an SH3 domain-containing protein of unknown function (11); and phosphorylated tyrosine 813 binds SH2-Bβ (39), an adapter protein and enhancer of JAK2 kinase activity (51, 53).
Little is known about the phosphorylation of JAK2 on serines and threonines. Protein kinase C-δ (PKC-δ)-mediated phosphorylation of JAK2 on serine/threonine has been reported to inhibit activation of JAK2 (37). In other proteins, phosphorylation on serines and threonines has a variety of effects. Phosphorylation at serines and threonines can induce conformational changes with a consequent increase or decrease in the catalytic activity of the protein (65; reviewed in reference 42). Serine/threonine phosphorylation of the receptor tyrosine kinases, ErbB-2 (35) and the insulin receptor (48, 63), as well as insulin receptor substrate 1 and 2 (48, 50) and Stat3 (13), decreases tyrosyl phosphorylation of these molecules and downregulates downstream signaling. Serine phosphorylation is reported to modulate the transcriptional activity of Stat3 (68). Phosphorylated serines and threonines can also recruit other signaling proteins: for example, 14-3-3 binds phosphoserine/threonine-containing motifs in its numerous binding partners (reviewed in reference 44). Proteins bind to phosphorylated serines and threonines via a large variety of domains, including WW, FHA, WD40, and Polo-box domains (reviewed in reference 71). To begin to understand how serine/threonine phosphorylation of JAK2 affects signaling, we set out to identify sites of serine/threonine phosphorylation in JAK2 and to determine their function in cytokine signaling.
In this study, serine 523 was identified as a site of phosphorylation in JAK2 using mass spectrometry (MS). Using a phosphospecific antibody directed against phosphorylated serine 523, we demonstrated that phosphorylation of serine 523 can be stimulated by both GH and epidermal growth factor (EGF) and provide evidence that GH-dependent phosphorylation of JAK2 at serine 523 is mediated by extracellular signal-regulated kinases (ERKs) 1 and/or 2 or some other kinase downstream of MEK1. Finally, we show that mutation of serine 523 to alanine increases JAK2 kinase activity and both the basal and GH-stimulated levels of tyrosyl phosphorylation of JAK2 and Stat5b, suggesting that phosphorylation of serine 523 inhibits JAK2 kinase activity. We hypothesize that phosphorylation of serine 523 in JAK2 by ERKs 1 and/or 2 or other, as-yet-unidentified kinases acts in a negative feedback manner to dampen the activation of JAK2 in response to GH and provides a mechanism by which prior exposure to other ERK-activating ligands or environmental factors that regulate Ser 523 phosphorylation might modulate the cell's response to GH.
MATERIALS AND METHODS
Materials.
Recombinant 22,000-Da human GH was a gift from Eli Lilly. Human EGF was from JRH Biosciences (see Fig. 5) or Biosource (see Fig. 8). Recombinant protein A-agarose was from Repligen. Bovine serum albumin (CRG-7) was from Intergen. Bac-to-Bac HT baculovirus expression system, calf serum, and Dulbecco's modified Eagle's medium were from Invitrogen. Aprotinin, leupeptin, and Triton X-100 were from Roche. The enhanced chemiluminescence detection system and nitrocellulose paper were from Amersham Pharmacia Biotech. Protein molecular weight standards were from Santa Cruz and Invitrogen. X-ray film was from Kodak. p81 paper was from Fisher. The QuikChange site-directed mutagenesis kits were from Stratagene. MEK inhibitor UO126 (stock solution, 20 mM in dimethyl sulfoxide [DMSO]) was from Promega. Phorbol 12-myristate 13-acetate (PMA; stock solution, 10 mM in DMSO) was from Sigma. The protein phosphatase pp2A was from Upstate.
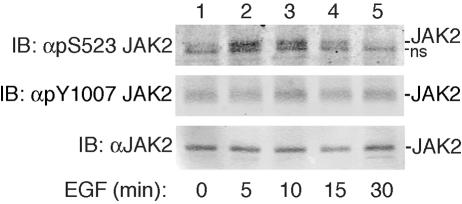
FIG. 5.
EGF promotes the phosphorylation of JAK2 at serine 523. 3T3-F442A cells were stimulated with vehicle or with 125 ng EGF/ml for 5, 10, 15, or 30 min. Cells were lysed, and lysates were immunoblotted with αpS523 JAK2 (top panel), αpY1007/1008 JAK2 (middle panel), or αJAK2 (bottom panel), n = 2. The migration of JAK2 and a nonspecific band (ns) is indicated.
FIG. 8.
Pretreatment with EGF inhibits GH-dependent activation of JAK2. 3T3-F442A cells were treated with 125 ng EGF/ml as indicated or treated with 125 ng EGF/ml or vehicle for 30 min and then treated with vehicle or 50 ng GH/ml as indicated. Cell lysates were blotted with αpY1007/1008 JAK2, αPY, or αJAK2. The intensities of the bands corresponding to phosphorylated JAK2 were quantified, normalized to levels of JAK2, and graphed as the fraction of maximum phosphorylation detected with αpY1007 (A) and αPY (B) (error bars denote ranges of values, n = 2).
Antibodies.
JAK2 antibody (αJAK2) was raised against a peptide corresponding to amino acids 758 to 776 of murine JAK2. The αJAK2 used for immunoprecipitation (at a dilution of 1:1,000) was prepared by our laboratory in conjunction with Pel-Freeze Biologicals (3). The αJAK2 used for Western blotting was from Upstate USA, Inc. (dilution of 1:7,500 for Fig. 2 and 4), or Biosource (αJAK2 monoclonal clone no. 829; dilution of 1:2,000 for Fig. 3 and 5 to 8). Antibody that recognizes a peptide containing phosphorylated serine 523 (αpS523 JAK2) and antibody to a peptide containing phosphorylated tyrosine 570 in JAK2 (αpY570 JAK2) (5) were developed in conjunction with Upstate USA, Inc., and used at a 1:2,000 dilution for immunoblotting. Antibody to phosphorylated tyrosines 1007/1008 in JAK2 (αpY1007/1008 JAK2) was a gift from M. Myers (University of Michigan, Ann Arbor) (19). Antibodies to phospho-p44/42 mitogen-activated protein kinase (αpErk; E10) and total ERK (αErk) were from Cell Signaling and used at a dilution of 1:2,000 for immunoblotting. Antibody to phosphotyrosine (αPY; 4G10) was from Upstate USA, Inc., and used at a dilution of 1:7,500 for immunoblotting. Antibody to Stat5b against amino acids 711 to 727 of murine Stat5b (αStat5b) was from Santa Cruz Biotechnology, Inc., and used at a 1:5,000 dilution for immunoblotting. Antibody to phosphorylated tyrosine 699 of Stat5b (αpStat5b) was from Zymed Laboratories Inc. and was used at a 1:7,500 dilution for immunoblotting. AlexaFluor 680 anti-rabbit antibody from Molecular Probes and IRdye800 anti-mouse antibodies from Rockland were used at a dilution of 1:20,000. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin G antibodies from Santa Cruz were used at a dilution of 1:10,000.
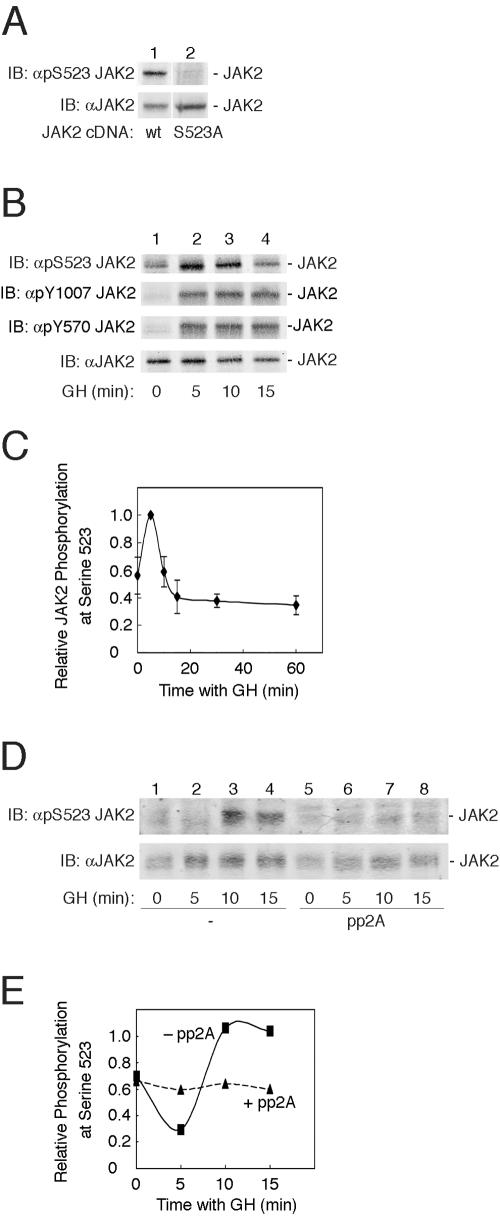
FIG. 2.
GH promotes the phosphorylation of JAK2 at serine 523. (A) 293T cells transfected with cDNA (1 μg) encoding either JAK2 (lane 1) or JAK2 S523A (lane 2) were lysed. The lysates were immunoblotted (IB) with αpSer523 JAK2 (upper panel) or αJAK2 (lower panel) (n = 4). wt, wild type. (B) 3T3-F442A cells were treated with vehicle or with 500 ng GH/ml for 5, 10, or 15 min. The cells were lysed, and lysates were immunoblotted (IB) with αpS523 JAK2 (top panel), αpY1007/1008 JAK2 (second panel), αpY570 JAK2 (third panel), or αJAK2 (bottom panel). (C) Replicates for the experiment shown in panel B were normalized to levels of JAK2. Means ± standard errors of the means are shown for n = 6. (D) 3T3-F442A cells were treated with vehicle (lanes 1 and 5) or with 500 ng GH/ml (lanes 2 to 4 and 6 to 8). Cells were lysed, and lysates were incubated without (lanes 1 to 4) or with (lanes 5 to 8) 0.7 U pp2A at 37°C for 60 min. Cell lysates were immunoblotted with either αpS523 JAK2 (top panel) or αJAK2 (bottom panel). The migration of JAK2 is indicated. (E) Results from the experiment shown in panel D were quantified and normalized to levels of JAK2.
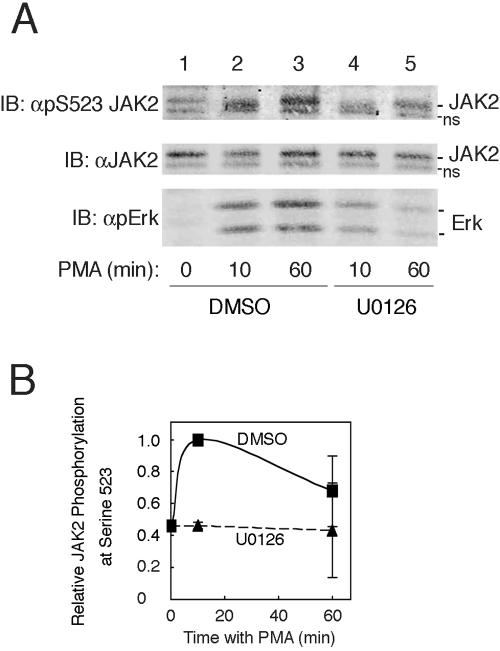
FIG. 4.
MEK1 inhibitor UO126 inhibits PMA-stimulated phosphorylation of JAK2 on serine 523. (A) 3T3-F442A cells were pretreated with DMSO (lanes 1 to 3) or with 10 μM UO126 in DMSO (lanes 4 and 5) for 30 min prior to stimulation with vehicle (lane 1) or with 1 μM PMA for the indicated times (lanes 2 to 5). Cells were lysed, and the lysates were immunoblotted (IB) with αpS523 JAK2 (top panel), αJAK2 (middle panel), or αpErk (bottom panel). The migration of JAK2, a nonspecific band (ns), and the ERKs is indicated. (B) Replicates for the experiment shown in panel A were quantified and normalized to levels of JAK2. Means and the range are shown for n = 2.
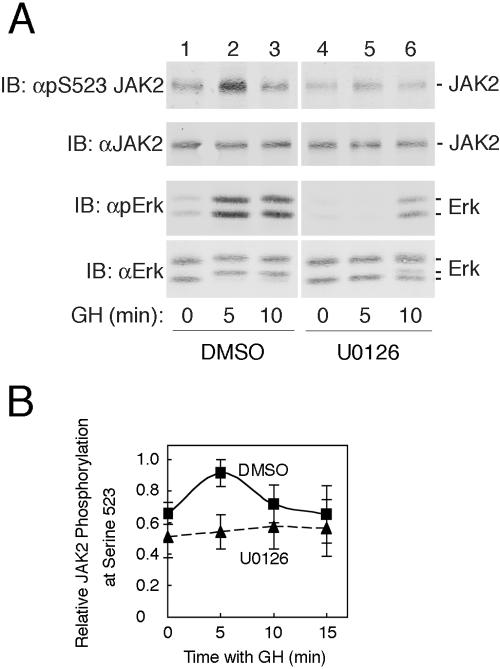
FIG. 3.
MEK1 inhibitor UO126 inhibits GH-stimulated phosphorylation of serine 523 in JAK2. (A) 3T3-F442A cells were incubated with vehicle (lanes 1 to 3) or with 10 μM UO126 (lanes 4 to 6) for 30 min. Cells were then treated with vehicle (lanes 1 and 4) or with 500 ng GH/ml for 5 or 10 min (lanes 2 and 3 and lanes 5 and 6). Cell lysates were immunoblotted (IB) with αpS523 JAK2 (top panel), αJAK2 (second panel), αpErk (third panel), or αErk (bottom panel). The migration of JAK2 and the ERKs is indicated. (B) Replicates for the experiment shown in panel A were quantified and normalized to levels of JAK2. Means ± standard errors of the means are shown for n = 4.
Plasmids.
cDNA encoding rat GH receptor was kindly provided by G. Norstedt (Karolinska Institute, Sweden) (17). cDNAs encoding murine JAK2 or kinase-inactive JAK2 K882E in the prk5 vector were kindly provided by J. Ihle (St. Jude Children's Research Hospital, Memphis, TN) (60). cDNA encoding rat SH2-Bβ was in the prk5myc vector (53). cDNA encoding rat Stat5b in the pRc/CMV vector was a gift of L. Yu-Lee (Baylor College of Medicine, Houston, TX) (43). cDNA encoding JAK2 S523A was created from the JAK2 expression vector above using the QuikChange site-directed mutagenesis kit from Stratagene. The primer (sense strand, mutation in lowercase) used for JAK2 S523A was 5′-CTGATGTTCAGATCgCACCAACATTACAGAGGC-3′. Mutations were verified by DNA sequencing. Amino acids in JAK2 are numbered according to NCBI accession number NP_032439.
Detection of phosphorylation sites by mass spectrometry.
JAK2 was prepared as described previously (5). Briefly, Spodoptera frugiperda (Sf9) cells were infected with baculovirus containing cDNA encoding six-His-tagged JAK2. JAK2 was immunoprecipitated using αJAK2, resolved on 5 to 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels, and stained with Coomassie blue. The JAK2 from 20 immunoprecipitations was pooled for analysis by mass spectrometry. JAK2 was subjected to in-gel reduction and S-carboxyamidomethylation followed by in-gel digestion with trypsin (59). To detect phosphorylation at serine 523, detailed analysis of JAK2 tryptic peptides was performed in the laboratory of O. N. Jensen at the University of Southern Denmark using nanoscale sample preparation methods in combination with matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (REFLEX IV; Bruker Daltonics, Bremen, Germany) and nanoelectrospray quadrupole time of flight tandem mass spectrometry (QTOF-1; Waters/Micromass, Manchester, United Kingdom) (62). The protein digest was loaded onto two nanocolumns (GELoader tips; Eppendorf, Hamburg, Germany) aligned in series, the first containing QIAGEN Fe(III)-nitrilotriacetic acid-immobilized metal affinity chromatography (IMAC; QIAGEN, Valencia, CA) and the second OligoR3 material (Applied Biosystems, Framingham, MA) (62). The IMAC-enriched phosphopeptide mixture was eluted from the Fe(III)-IMAC column with 10 μl dilute ammonia (pH 10.5) and immediately acidified by addition of 10 μl of 5% formic acid. Phosphopeptide mixtures were desalted using an OligoR3 nanocolumn and eluted either directly onto the MALDI target using the MALDI matrix for MALDI mass spectrometry analysis (REFLEX IV; Bruker Daltonics, Bremen, Germany) or by 50% methanol in 1% formic acid directly into a nanoelectrospray needle (Proxeon Biosystems A/S, Odense, Denmark) for nanoelectrospray quadrupole time of flight tandem mass spectrometry (QTOF-1; Waters/Micromass, Manchester, United Kingdom) as described previously (62).
Cell culture and transfection.
The stock of 3T3-F442A fibroblasts was kindly provided by H. Green (Harvard University). 293T and COS-7 cells were from the American Type Culture Collection. 3T3-F442A and 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 8% calf serum, 1 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per ml. COS-7 cells were grown in the same medium using fetal bovine serum rather then calf serum. Both 293T and COS-7 cells were transfected using calcium phosphate precipitation (12) and assayed 24 or 48 h after transfection, respectively. When cells were to be incubated with a ligand, they were incubated overnight in serum-free medium containing 1% bovine serum albumin before addition of ligand (500 ng GH/ml, 125 ng EGF/ml, 1 μM PMA) or vehicle for the time indicated. When the MEK inhibitor UO126 was used, cells were treated with 10 μM UO126 or vehicle for 30 min prior to the addition of ligand.
Cell lysis, immunoprecipitation, and immunoblotting.
Cells were washed three times with chilled PBSV (10 mM sodium phosphate, 137 mM NaCl, 1 mM Na3VO4, pH 7.4) and solubilized in lysis buffer (50 mM Tris, 0.1% Triton X-100, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, pH 7.5) containing 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 25 mM NaF. The solubilized material was centrifuged at 16,750 × g at 4°C for 10 min. For immunoprecipitations, the supernatant (cell lysate) was incubated with the indicated antibody on ice for 2 h. The immune complexes were collected on 30 μl of protein A-agarose for 1 h. The beads were washed three times with lysis buffer and boiled for 5 min in a mixture (80:20) of lysis buffer and SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (250 mM Tris-HCl, 10% SDS, 10% β-mercaptoethanol, 40% glycerol, 0.01% bromophenol blue, pH 6.8). The solubilized proteins were separated on SDS-polyacrylamide gels, transferred to nitrocellulose, immunoblotted with the indicated antibody, and detected using enhanced chemiluminescence or an Odyssey Infrared Imaging System (Li-Cor Biosciences). With the exception of Fig. 2D, results presented were representative of two or more experiments. For experiments monitoring the effect of pp2A, 20 μl of cell lysate was incubated with vehicle or 0.7 units of pp2A at 37°C for 1 h. The reaction was stopped with the addition of SDS-PAGE sample buffer (80:20 ratio), and the reaction mixture was boiled for 5 min. Immunoblots were quantified using Bio-Rad MultiAnalyst software (for enhanced chemiluminescence detection) or Li-Cor Odyssey 2.0 software and normalized for levels of JAK2 or Stat5 as appropriate. As indicated, for some experiments, the results of a representative experiment are quantified and normalized. In other figures, normalized means ± standard errors of the means (or range if n equals 2) are shown for results carried out on different days.
JAK2 kinase assay.
293T cells were transfected with 0.5 mg cDNA for JAK2 or JAK2 S523A and 1.5 μg vector using calcium phosphate precipitation. JAK2 immunoprecipitates were washed and incubated in 50 mM HEPES, 0.5 mM dithiothreitol, 100 mM NaCl, 1 mM NaVO4, 5 mM MnCl2, pH 7.5, at 30°C, and ATP, 10 μCi [γ-32P]ATP (BP Biomedical), and a JAK2 substrate (a Stat5 peptide containing tyrosine 699 [AKAADGY699VKPQIKQVV], prepared by the Protein Structure Facility of the Michigan Biomedical Research Core Facility, University of Michigan) were added to yield a final concentration of 50 μM ATP and the indicated Stat5 peptide concentration. After 20, 40, and 60 min, the vials were centrifuged and an aliquot of the supernatant was spotted on p81 paper. The p81 paper was washed in 75 mM H3PO4, and bound 32P was counted using a Packard scintillation counter.
RESULTS
Mass spectrometry identifies serine 523 in JAK2 as a site of phosphorylation.
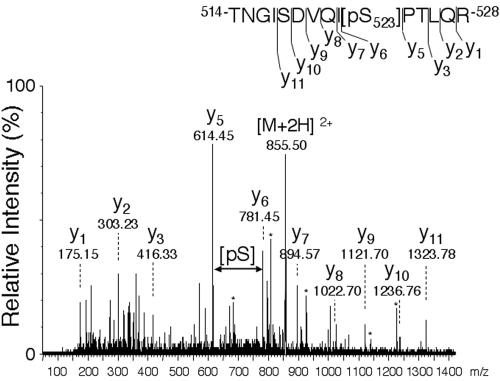
Serine/threonine phosphorylation has been shown to regulate the activity of multiple tyrosine kinases. To begin to determine the role that phosphorylated serines/threonines in JAK2 play in the actions of cytokines, we set out to identify serines and threonines that are phosphorylated in JAK2. To obtain sufficient JAK2 for analysis, murine JAK2 was overexpressed in Sf9 cells. The overexpressed JAK2 was solubilized, highly purified by immunoprecipitation using αJAK2, resolved by SDS-PAGE, and then stained by Coomassie brilliant blue. The protein was reduced and S-alkylated and then in-gel digested using trypsin. Phosphopeptides in the tryptic digest of JAK2 were enriched by Fe(III)-IMAC and then desalted/concentrated using a miniaturized OligoR3 column prior to analysis by mass spectrometry. Peptide samples were analyzed by MALDI MS and nanoelectrospray tandem mass spectrometry. The MALDI MS tryptic peptide mass map of JAK2 displayed an ion signal consistent with phosphorylation of the peptide T514NGISDVQISPTLQR528. Sequencing by nanoelectrospray quadrupole time of flight tandem mass spectrometry confirmed the identity of the peptide and located the phosphorylation site at position 523: T514NGISDVQI[pS]PTLQR528(Fig. 1).
FIG. 1.
JAK2 is phosphorylated on serine 523. JAK2 was isolated from Sf9 cells infected with baculovirus containing the cDNA for murine JAK2. JAK2 was digested with trypsin and analyzed by nanoelectrospray quadrupole time of flight tandem mass spectrometry. The doubly charged ion corresponding to T514NGISDVQI[pS]PTLQR528and the Y-ion series corresponding to the C-terminal peptide ion fragments are indicated. The increment corresponding to the phosphorylated serine (pS) is indicated and corresponds to the molecular weight of a phosphorylated serine residue.
Serine 523 in JAK2 is phosphorylated in response to GH.
To verify that serine 523 of JAK2 is phosphorylated in mammalian cells, we first tested whether antibody directed against phosphoserine 523 (αpS523 JAK2) would recognize JAK2 overexpressed in 293T cells. Cells transiently overexpressing JAK2 or JAK2 S523A were solubilized, and cell lysates were immunoblotted with either αJAK2 or αpS523 JAK2. The αJAK2 blot revealed that the expression of JAK2 and that of JAK2 S523A were similar (Fig. 2A, bottom panel). When the lysates were blotted with αpS523 JAK2, JAK2 was recognized, but not JAK2 S523A, suggesting that overexpressed JAK2 is phosphorylated on serine 523.
We next examined whether endogenous JAK2 was also phosphorylated on serine 523 and whether GH, a potent activator of JAK2, regulates the phosphorylation of serine 523 in JAK2. 3T3-F442A cells were treated with vehicle or with 500 ng GH/ml for 0, 5, 10, or 15 min. Immunoblotting proteins in cell lysates with αpS523 JAK2 demonstrated a low level of phosphorylation of serine 523 in the absence of GH. We believe that this signal is due to a low basal level of phosphorylation of serine 523, since it was detected by both our antibody and the antibody of Ishida-Takahashi et al. (32a) and because it was present in αJAK2 immunoprecipitates but not in precipitates using nonimmune serum (data not shown). GH transiently stimulated the phosphorylation of serine 523 with maximal phosphorylation of serine 523 routinely detected by 5 or 10 min (Fig. 2B, top panel, and 2C). Immunoblotting with αJAK2 demonstrated that the differences in JAK2 phosphorylation were not the result of differences in endogenous JAK2 expression (Fig. 2B, bottom panel). Immunoblotting the lysates with αpY1007/1008 and αpY570 (Fig. 2B, middle panels) demonstrated that GH stimulates tyrosyl phosphorylation of JAK2 within 5 min, as reported previously using αPY (2, 3). When the lysates from GH-stimulated 3T3-F442A cells were treated with protein phosphatase pp2A, detection of JAK2 by αpSer523 was substantially diminished (Fig. 2D and E), indicating that αpS523 JAK2 detects the phosphorylated form of serine 523.
Pretreatment of 3T3-F442A cells with a MEK1 inhibitor, UO126, abolishes GH-stimulated phosphorylation of JAK2 on serine 523.
Inspection of the amino acid sequence flanking serine 523(DVQISPTLQ) suggests that serine 523 is phosphorylated by a proline-directed serine/threonine kinase. Because GH has been shown to rapidly activate the proline-directed serine/threonine kinases ERKs 1 and 2 (1, 9, 46, 69), we examined whether ERKs 1 and/or 2 might be responsible for GH stimulation of phosphorylation of serine 523. Because activated MEK1 phosphorylates and thereby activates ERKs 1 and 2 (58) and MEK1 is activated by GH (66), to block the activation of ERKs 1 and 2, we used UO126, a potent inhibitor of MEK1 (18).
3T3-F442A cells were pretreated for 30 min with either DMSO or 10 μM UO126. We then stimulated the cells with either vehicle or 500 ng GH/ml for 5 or 10 min. Cell lysates were immunoblotted with an anti-active ERK (αpErk) antibody that recognizes the doubly phosphorylated (pThr202, pTyr204) active form of ERKs 1 and 2. GH-stimulated phosphorylation of ERKs 1 and 2 on threonine 202 and tyrosine 204 and UO126 inhibited this stimulation (Fig. 3A, third panel from top). Immunoblotting the lysates with αERK verified that comparable amounts of ERK were present in each lane (Fig. 3A, bottom panel). Immunoblotting the lysates with αJAK2 verified that comparable amounts of JAK2 were loaded in each lane (Fig. 3A, second panel). When the lysates were immunoblotted with αpS523 JAK2, GH was found to stimulate the phosphorylation of serine 523 to maximal levels by 5 min (Fig. 3A, top panel, lanes 1 to 3, and 3B). However, blocking activation of ERKs 1 and 2 with UO126 prior to GH stimulation inhibited GH-dependent phosphorylation of serine 523 (Fig. 3A, top panel, lanes 4 to 6, and 3B). This result suggests that in response to GH, ERKs 1 and/or 2 or an unknown kinase downstream of MEK1 phosphorylates serine 523 in JAK2.
PMA induces phosphorylation of JAK2 on serine 523 via a kinase that lies downstream of MEK1.
Phorbol esters are potent activators of ERKs 1 and 2. Exactly how they activate ERKs 1 and 2 is not known, with some researchers hypothesizing the involvement of PKCs and others invoking the involvement of the phorbol ester binding proteins RasGRPs (7, 8, 67). If GH-dependent phosphorylation of serine 523 is mediated via ERKs 1 and/or 2, we would predict that activation of ERKs 1 and 2 by PMA would also stimulate the phosphorylation of serine 523. When 3T3-F442A cells were treated with 1 μM PMA, phosphorylation of ERKs 1 and 2 was detected by 10 min and persisted for 60 min (Fig. 4A, bottom panel). Blotting with αpS523 JAK2 (Fig. 4A, top panel, and 4B) reveals that PMA also stimulates the phosphorylation of serine 523. Neither PMA nor UO126 affected the levels of JAK2 (Fig. 4A, middle panel). Consistent with PMA stimulation of phosphorylation of serine 523 in JAK2 involving ERKs 1 and/or 2, pretreatment of the cells with the MEK1 inhibitor UO126 (10 μM for 30 min) decreased both PMA-induced activation of ERKs 1 and 2 and phosphorylation of JAK2 on serine 523 (Fig. 4A, lanes 4 and 5, and 4B).
EGF stimulates the phosphorylation of JAK2 on serine 523.
EGF, like PMA and GH, is known to activate ERKs 1 and 2 (52; reviewed in reference 34). We predicted, therefore, that EGF, like PMA and GH, would stimulate the phosphorylation of serine 523. 3T3-F442A cells, which contain endogenous EGF receptors, were treated with vehicle or 125 ng EGF/ml for 5, 10, 15, and 30 min. Cell lysates were prepared, and proteins were blotted with αpS523 JAK2. Similar to what was seen with GH, EGF stimulated the phosphorylation of serine 523 by 5 min (Fig. 5). Serine 523 phosphorylation decreased gradually until it dropped below basal levels by 30 min. As predicted, EGF did not activate JAK2, as assessed by blotting with antibody (αpY1007/1008) to the activating tyrosine in JAK2.
Mutation of serine 523 to alanine in JAK2 increases the activity of JAK2.
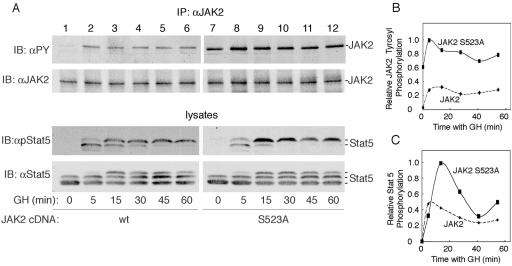
Having established that serine 523 in JAK2 is phosphorylated in response to GH as well as PMA and EGF, we began to investigate the function of phosphorylation of serine 523. Because serine/threonine phosphorylation often has a regulatory function (reviewed in reference 42), we tested whether phosphorylation of JAK2 at serine 523 affects the kinase activity of JAK2. JAK2 or JAK2 S523A was expressed in 293T cells and immunoprecipitated using αJAK2. Kinase activity was then assessed using a Stat5 peptide containing the major JAK2 tyrosyl phosphorylation site at amino acid 699 in Stat5. Immunoblotting with αJAK2 demonstrated that JAK2 and JAK2 S523A were expressed at similar levels (Fig. 6A). The time course for phosphorylation of the Stat5 peptide at the highest (500 μM) Stat5 peptide concentration used demonstrated that the assay was reasonably linear for at least 60 min and that the activity of JAK2 S523A was at least twice as high as the activity of JAK2 (Fig. 6B). When the concentration of Stat5 peptide was varied while the ATP concentration was kept at 50 μM, the ratio of Stat5 peptide phosphorylation in the presence of JAK2 S523A compared to that in the presence of JAK2 was 2.7 ± 0.35 (n = 3) at 50 μM Stat5 peptide, 3.9 ± 0.57 (n = 3) at 150 μM, and 2.7 ± 0.34 (n = 2) at 500 μM. Thus, the activity of JAK2 S523A was consistently higher than the activity of JAK2. Consistent with JAK2 S523A having higher activity than JAK2, Stat5b was more highly phosphorylated when coexpressed with JAK2 S523A than when coexpressed with JAK2 (Fig. 6D).
FIG. 6.
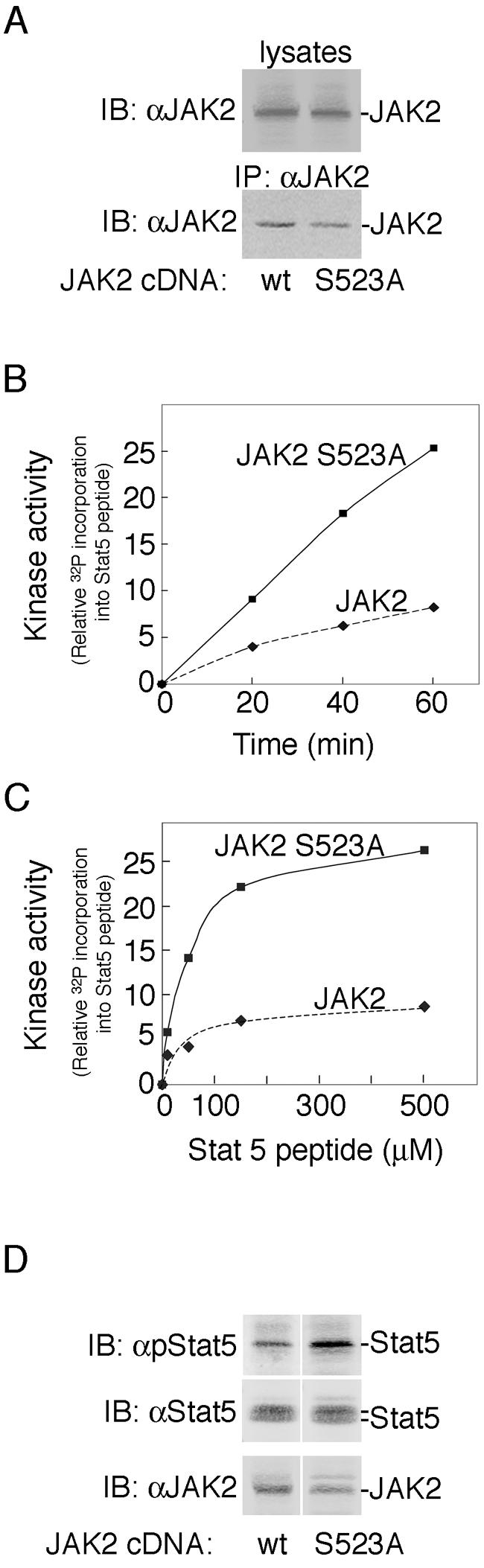
Mutation of serine 523 to alanine in JAK2 elevates JAK2 kinase activity. (A) 293T cells were transfected with cDNA (0.5 μg) encoding JAK2 (lane 1) or JAK2 S523A (lane 2). Cell lysates (top panel) or αJAK2 immunoprecipitates (bottom panel) were immunoblotted with αJAK2. The migration of JAK2 is indicated. wt, wild type. (B) Immunoprecipitated JAK2 or JAK2 S523A was subjected to an in vitro kinase assay for 20, 40, and 60 min using 50 μM ATP and 500 μM of a peptide containing tyrosine 699 of Stat5 as substrate. (C) Immunoprecipitated JAK2 or JAK2 S523A was subjected to an in vitro kinase assay at Stat5 peptide substrate concentrations between 0 and 500 μM. The reaction velocity was determined by averaging the rate of 32P incorporation into the Stat5 peptide at 40 and 60 min, n = 2. (D) COS-7 cells were transfected with cDNAs encoding GH receptor (100 ng), Stat5b (200 ng), and either JAK2 (20 ng) or JAK2 S523A (20 ng). Cells were incubated in serum-free medium overnight and resolved on a 5 to 12% gradient gel. Cell lysates were immunoblotted with αpStat5b, αStat5b, or αJAK2 as indicated, n = 2.
Mutation of serine 523 to alanine in JAK2 increases GH-dependent tyrosyl phosphorylation of JAK2 S523A and Stat5b.
We then examined the effect of mutating serine 523 to alanine on the ability of GH to stimulate the tyrosyl phosphorylation of both JAK2 and Stat5b. COS-7 cells were transfected with cDNA encoding GH receptor, Stat5b, and either JAK2 or JAK2 S523A and treated with vehicle or 500 ng GH/ml. JAK2 in cell lysates was immunoprecipitated with αJAK2. Blotting with αJAK2 revealed that expression levels of JAK2 and JAK2 S523A were comparable (Fig. 7A, second panel from top). When cell lysates were blotted with αPY, tyrosyl phosphorylation of wild-type JAK2 was observed by 5 min. In contrast, with JAK2 S523A, both basal and GH-dependent phosphorylation were increased (Fig. 7A, top panel, and 7B). When cell lysates were blotted with antibody to phosphorylated tyrosine 699 in Stat5b, phosphorylation of tyrosine 699 in Stat5b was detected by 5 min in the presence of wild-type JAK2. In the presence of JAK2 S523A, low-level phosphorylation of Stat5 at tyrosine 699 was detectable at the zero time point (visible in darker exposures) and GH-dependent phosphorylation of Stat5 at tyrosine 699 was enhanced compared to the Stat5 phosphorylation seen in the presence of JAK2 (Fig. 7A, third panel, and Fig. 7C). Unfortunately, the levels of GH receptor required for us to reconstitute GH-dependent activation of exogenous JAK2 were too low for us to assess whether mutation of serine 523 in JAK2 alters phosphorylation of the GH receptor. Taken together, these data suggest that phosphorylation of JAK2 on serine 523 inhibits JAK2 activity.
FIG. 7.
Mutation of serine 523 to alanine in JAK2 enhances GH-dependent phosphorylation of JAK2 and Stat5b. (A) COS-7 cells were transfected with cDNAs encoding GH receptor (100 ng), Stat5b (200 ng), and either JAK2 (10 ng) (lanes 1 to 6) or JAK2 S523A (10 ng) (lanes 7 to 12). Cells were treated with vehicle or 500 ng GH/ml for the indicated times. Cell lysates were immunoprecipitated with αJAK2 and immunoblotted with αPY (top panel) or αJAK2 (second panel). Cell lysates were blotted with αpStat5b (third panel) or αStat5b (bottom panel), n = 2. The migration of JAK2 and Stat5b is indicated. wt, wild type. (B and C) The intensities of the bands corresponding to phosphorylated JAK2 and phosphorylated Stat5b were quantified, normalized for levels of JAK2 and Stat5, and plotted as the fraction of maximum phosphorylation detected with αPY (B) and with αpStat5 (C).
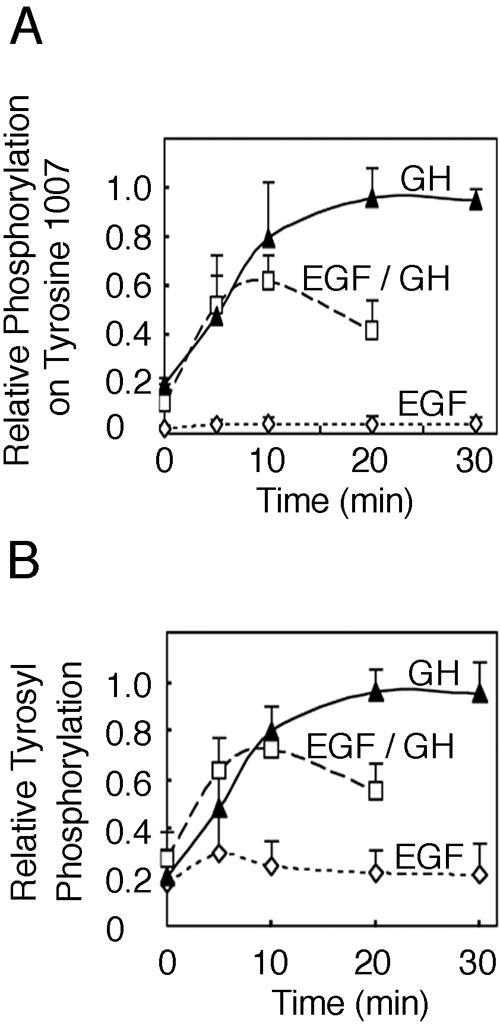
Pretreatment with EGF inhibits GH-dependent activation of JAK2 as measured by phosphorylation at tyrosine 1007 of JAK2.
Because EGF stimulates the phosphorylation of JAK2 at serine 523 and phosphorylation of serine 523 appears to inhibit JAK2 activity, we examined if prior exposure of cells to EGF might dampen the cellular response to GH. 3T3-F442A cells were treated with 125 ng EGF/ml for 30 min and then treated with vehicle or 50 ng GH/ml for 0, 5, 10, or 20 min. For comparison cells were treated with 50 ng GH/ml or 125 ng EGF/ml for 0, 5, 10, 20, or 30 min. Lysates were then immunoblotted with αpY1007/1008 JAK2 and αPY. Previous studies indicated that the tyrosyl-phosphorylated bands that comigrate with JAK2 are primarily if not exclusively JAK2 (3). Tyrosyl phosphorylation that comigrates with JAK2 was quantified, normalized to amounts of JAK2, and graphed. Figures 8A and B, illustrate that EGF did not stimulate overall tyrosyl phosphorylation of JAK2 or tyrosyl phosphorylation of Y1007/1008. When cells stimulated with GH alone were compared to cells pretreated with EGF and then stimulated with GH, the ability of GH to stimulate phosphorylation at tyrosines 1007 and 1008 as well as total tyrosyl phosphorylation of JAK2 (and any proteins that comigrate with JAK2) was similar prior to 10 min. However, by 20 min, pretreatment with EGF suppressed phosphorylation at tyrosines 1007 and 1008 (Fig. 8A) as well as total tyrosyl phosphorylation of JAK2 (and any comigrating proteins) (Fig. 8B) by more than 50%. Thus, pretreatment with EGF appears to dampen the ability of GH to maintain JAK2 activity.
DISCUSSION
Little is known about the sites of tyrosine phosphorylation within JAK2. Even less is known about the sites of serine/threonine phosphorylation within JAK2. We report here the first identification of a site of serine/threonine phosphorylation in JAK2: serine 523. For the initial identification, JAK2 was expressed in a baculoviral expression system and phosphorylation of serine 523 in JAK2 was identified using tandem mass spectrometry. Western blotting with an antibody specific for phosphorylated serine 523 demonstrated that serine 523 is also phosphorylated when JAK2 is overexpressed in mammalian (293T and COS-7) cells. This same antibody was used to demonstrate that GH stimulates the phosphorylation of serine 523 in endogenous JAK2 in 3T3-F442A cells. Phosphorylation of serine 523 in response to GH was rapid but transient with maximal phosphorylation detected at 5 to 10 min and returning towards basal values by 15 min. As reported in reference 32a, Ishida-Takahashi et al. have also used tandem mass spectroscopic analysis to independently identify serine 523 in JAK2 as a site of phosphorylation in activated JAK2 expressed in 293T cells. These findings, using two different phosphospecific antibodies, that overexpressed JAK2 is phosphorylated on serine 523 in COS-7 cells, 293T cells, HEK 293 cells, and Sf9 cells and that endogenous JAK2 is phosphorylated on serine 523 in 3T3-F442A fibroblasts, 32D cells, and mouse spleen (this paper and reference 32a) provide strong evidence that serine 523 in JAK2 is a bona fide site of phosphorylation.
Analysis of the amino acids surrounding serine 523 in JAK2 (DVQISPTLQ) indicates that the kinase that phosphorylates JAK2 at serine 523 is a proline-directed serine/threonine kinase. GH has been shown to activate at least four proline-directed serine/threonine kinases, mitogen-activated protein kinases, ERKs 1 and 2 (1, 9, 46, 69), p38 (28, 78), and Jun N-terminal kinase (77), making these kinases logical candidates. The time course of activation in response to GH of all four of these kinases is compatible with any of them being the kinase that phosphorylates serine 523. When 3T3-F442A cells are treated with the MEK1 inhibitor UO126, GH-dependent phosphorylation of JAK2 at serine 523 is suppressed. This suggests that ERKs 1 and/or 2 (or another kinase downstream of MEK1) phosphorylates serine 523 in response to GH. Consistent with ERKs 1 and/or 2 being able to phosphorylate serine 523, serine 523 was also phosphorylated when 3T3-F442A cells were incubated with the phorbol ester PMA, a potent activator of ERKs 1 and 2 (52, 67). Like GH-induced phosphorylation of serine 523, PMA-induced phosphorylation of serine 523 was blocked by preincubation of the cells with the MEK1 inhibitor. Whether ERKs 1 and/or 2 or another serine/threonine kinase is responsible for the low level of serine 523 phosphorylation seen in serum-deprived 3T3-F442A cells (before GH addition) is not known. Because basal levels of phosphorylation were found to vary from day to day and with time of serum deprivation (data not shown and reference 32a), we suspect that there are other kinases capable of phosphorylating serine 523. Our finding that PMA stimulates the phosphorylation of serine 523 raises the possibility that PKC could be such a kinase. Although our results do not specifically exclude PKC from consideration, Ser523 does not lie in a PKC substrate consensus sequence and PMA-stimulated phosphorylation of serine 523 is inhibited by MEK1 inhibitor to a similar extent as PMA-induced ERK 1 and 2 activity. We also think it unlikely that PKC is the kinase in 3T3-F442A cells that phosphorylates serine 523 in response to GH because we are unaware of any evidence that GH activates PKC in 3T3-F442A cells (unpublished observation).
Because serine phosphorylation is often regulatory in nature, we examined whether phosphorylation of serine 523 might regulate the kinase activity of JAK2. Mutating serine 523 to alanine markedly increased the kinase activity of JAK2, as assessed in an in vitro kinase assay using Stat5 peptide as substrate, suggesting that phosphorylation of serine 523 in vivo inhibits the overall kinase activity of JAK2. It may therefore play a critical role in suppressing basal levels of JAK2 tyrosyl phosphorylation. Consistent with this, basal JAK2 autophosphorylation was increased when serine 523 was mutated. Phosphorylation of serine 523 may also dampen the ability of JAK2 to respond to ligand activation, as suggested by our finding that mutation of serine 523 to alanine increased GH-stimulated tyrosyl phosphorylation of both JAK2 and Stat5b. Based upon these findings, we hypothesize that phosphorylation of serine 523 in JAK2 by ERKs 1 and/or 2 acts in a negative feedback manner to dampen the initial activation of JAK2 in response to GH. Phosphorylation of serine 523 would also provide a mechanism by which prior exposure to other ERK-activating ligands might dampen the cellular response to GH. Ishida-Takahashi et al. (32a) have also found that mutation of serine 523 to alanine results in constitutive JAK2-dependent signaling in the absence of cytokine stimulation. In support of the hypothesis that phosphorylation of serine 523 acts in a negative feedback manner to dampen activation of JAK2 and downstream signaling pathways in response to cytokine binding, they observed that mutating serine 523 to alanine enhanced and prolonged JAK2 activation in response to EPO binding to a hybrid EPO-leptin receptor and increased EPO-dependent stimulation of expression of a Stat3 reporter.
A negative regulatory function for phosphorylation of serine 523 would be consistent with what is known thus far about the role of serine phosphorylation in the regulation of JAK2 and the receptor tyrosine kinases. PKC-δ-dependent phosphorylation of JAK2 on serine and threonine has been reported to abrogate interleukin-3-dependent tyrosyl phosphorylation and activation of JAK2, with inhibition of JAK2 either by PKC-δ-dependent serine/threonine phosphorylation or by interleukin-3 deprivation, leading to differentiation of 32D myeloid progenitor cells (37). Serine/threonine phosphorylation of insulin receptor has been reported to reduce insulin-stimulated tyrosine phosphorylation of the receptor and its targets and inhibit propagation of the insulin receptor signal (48, 63). Protein kinase C-dependent phosphorylation of EGF receptor is reported to decrease autophosphorylation of EGF receptor and reduce tyrosine kinase activity (14, 16). Phosphorylation of the platelet-derived growth factor receptor β on serine 1104 by G protein-coupled receptor kinase 2 (GRK2) is thought to reduce signaling pathways downstream of platelet-derived growth factor receptor at least in part by reducing receptor autophosphorylation and reducing receptor interaction with the Na+/H+ exchanger regulatory factor (NHERF, also known as EBP50) (27).
How phosphorylation of serine 523 inhibits the kinase activity of JAK2 is not known. JAK2 is composed of seven JAK homology domains (JH1 to JH7) (24, 72). The C-terminal JH1 domain is the kinase domain (20, 76). JH2, the pseudokinase domain, is hypothesized to be autoinhibitory (21, 54-56). The entire JH3 domain combined with the second half of the JH4 domain of JAK2 has structural homology to an SH2 domain but does not appear to function as an SH2 domain (26). The first half of the JH4 domain of JAK2 and the JH5 to JH7 domains comprise the FERM (band 4.1, ezrin, radixin, and moesin) domain (23), responsible for binding to the GH receptor (22, 64), erythropoietin receptor (15, 30), gamma interferon receptor (15, 36), and granulocyte-macrophage colony-stimulating factor βc subunit (75). Serine 523 lies in the linker connecting the pseudokinase domain (JH2) to the JH3 domain. Thus, one can envision phosphorylation of serine 523 inducing a conformational change in JAK2 that would strengthen or promote the autoinhibitory action of the JH2 domain on the kinase domain of JAK2. Mutation of serine 523 to alanine would be expected to release phosphorylation-dependent inhibition, resulting in increased kinase activity and tyrosine phosphorylation of JAK2 and its substrates as demonstrated in this report. In support of this general region of JAK2 playing a critical role in regulating the enzymatic activity of JAK2, autophosphorylation of tyrosine 570 in JAK2 similarly appears to inhibit JAK2 activity (5, 19). It is also possible that phosphorylation of serine 523 and/or tyrosine 570 recruits a negative regulatory protein, such as a phosphatase, to JAK2 or prevents the binding of a positive regulatory protein. These possibilities are currently under investigation.
The finding that serine 523 appears to be phosphorylated by ERKs 1 and/or 2 suggests that phosphorylation of serine 523 may be a site of cross talk between ligands such as GH that activate JAK2 and ligands that activate MEK1 and ERKs 1 and/or 2 by a JAK2-independent pathway. Our finding here that EGF stimulates the phosphorylation of JAK2 on serine 523 and decreases the ability of GH to stimulate phosphorylation of the activating tyrosine, tyrosine 1007, in JAK2 as well as the overall tyrosyl phosphorylation of JAK2 provides support for this hypothesis. Interestingly, the cross talk between GH receptors and EGF receptor family members extends to both receptors, even in regards to the role of ERK activation. Thus, GH is thought to stimulate the phosphorylation of ErbB-2 by ERKs 1 and/or 2 and cause a decrease in EGF-induced tyrosyl phosphorylation of ErbB-2 and DNA synthesis (35). The cross talk between EGF family receptors and GH receptors appears multifaceted. GH has also been reported to protect EGF receptor from degradation and thereby potentiate EGF signaling and to decrease EGF binding to its receptor by decreasing the affinity of EGF for its receptor in a MEK1/ERK pathway-dependent manner (31, 32). GH has also been reported to stimulate phosphorylation of the EGF receptor on tyrosines (35, 73). It seems likely that EGF regulation of GH signaling may also be multifaceted, with phosphorylation of serine 523 being just one aspect of that regulation. Similarly, we would anticipate that other ligands that activate receptor tyrosine kinases and ERKs 1 and/or 2, such as platelet-derived growth factor, as well as ligands that activate ERKs 1 and/or 2 by other processes, such as via Gi-coupled receptors, would also stimulate phosphorylation of serine 523 in JAK2 and thereby downregulate GH signaling. The degree to which this would affect GH signaling would depend upon the timing of the stimulation via the various ligands and whether these ligands have other effects on GH signaling.
In summary, we have identified serine 523 in JAK2 as a site of phosphorylation. Using an antibody that specifically recognizes JAK2 phosphorylated at serine 523, we demonstrate that serine 523 is rapidly but transiently phosphorylated in response to GH and EGF and provide evidence that serine 523 is phosphorylated by ERKs 1 and/or 2. We also report that mutating serine 523 to an alanine increases the enzymatic activity of JAK2 as well as basal and GH-stimulated tyrosyl phosphorylation of JAK2 and the JAK2 substrate Stat5b, suggesting that phosphorylation of serine 523 inhibits JAK2 activity. We hypothesize that phosphorylation of serine 523 in JAK2 by ERKs 1 and/or 2 or another kinase downstream of MEK1 acts in a negative feedback manner to dampen the initial activation of JAK2 in response to GH. Phosphorylation of serine 523 also provides a mechanism by which exposure to other ERK-activating ligands or environmental factors that regulate serine 523 phosphorylation might modulate the cell's response to GH.
Acknowledgments
We thank Baljeet Deo and Xiaqing Wang for technical assistance and Barbara Hawkins for assistance with the manuscript.
This work was supported by NIH grant DK34171 (to C.C.-S.) and a grant from the Danish Natural Sciences Research Council (to O.N.J.). cDNA sequencing was supported by the Cellular and Molecular Biology Core and peptide synthesis by the Protein Structure Facility of the Michigan Biomedical Research Core of the Michigan Diabetes Research and Training Center (P60 DK20572), University of Michigan Rheumatologic Diseases Comprehensive Center (P60-AR20557), and the University of Michigan Comprehensive Cancer Center (NIH P30 CA46592). A.M.M.-M. was supported by a Predoctoral Fellowship from the Cellular and Molecular Biology Training Grant T32-GM 07315.
REFERENCES
- 1.Anderson, N. G. 1992. Growth hormone activates mitogen-activated protein kinase and S6 kinase and promotes intracellular tyrosine phosphorylation in 3T3-F442A preadipocytes. Biochem. J. 284:649-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argetsinger, L. S., N. Billestrup, G. Norstedt, M. F. White, and C. Carter-Su. 1996. Growth hormone, interferon-gamma, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J. Biol. Chem. 271:29415-29421. [DOI] [PubMed] [Google Scholar]
- 3.Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. [DOI] [PubMed] [Google Scholar]
- 4.Argetsinger, L. S., and C. Carter-Su. 1996. Mechanism of signaling by growth hormone receptor. Physiol. Rev. 76:1089-1107. [DOI] [PubMed] [Google Scholar]
- 5.Argetsinger, L. S., J.-L. Kouadio, H. Steen, A. Stensballe, O. N. Jensen, and C. Carter-Su. 2004. Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol. Cell. Biol. 24:4955-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter, E. J., L. M. Scott, P. J. Campbell, C. East, N. Fourouclas, S. Swanton, G. S. Vassiliou, A. J. Bench, E. M. Boyd, N. Curtin, M. A. Scott, W. N. Erber, and A. R. Green. 2005. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054-1061. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg, P. M., K. L. Leach, B. Konig, A. Y. Jeng, and N. A. Sharkey. 1985. Receptors for the phorbol ester tumour promoters. Ciba Found. Symp. 116:205-223. [DOI] [PubMed] [Google Scholar]
- 8.Brose, N., and C. Rosenmund. 2002. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 115:4399-4411. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, G. S., T. Miyasaka, L. Pang, A. R. Saltiel, and C. Carter-Su. 1992. Stimulation by growth hormone of MAP kinase activity in 3T3-F442A fibroblasts. J. Biol. Chem. 267:6074-6080. [PubMed] [Google Scholar]
- 10.Candotti, F., L. Notarangelo, R. Visconti, and J. O'Shea. 2002. Molecular aspects of primary immunodeficiencies: lessons from cytokine and other signaling pathways. J. Clin. Investig. 109:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpino, N., R. Kobayashi, H. Zang, Y. Takahashi, S. T. Jou, J. Feng, H. Nakajima, and J. N. Ihle. 2002. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol. Cell. Biol. 22:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung, J., E. Uchida, T. C. Grammer, and J. Blenis. 1997. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol. 17:6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochet, C., G. N. Gill, J. Meisenhelder, J. A. Cooper, and T. Hunter. 1984. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J. Biol. Chem. 259:2553-2558. [PubMed] [Google Scholar]
- 15.Constantinescu, S. N., L. J. Huang, H. Nam, and H. F. Lodish. 2001. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol. Cell 7:377-385. [DOI] [PubMed] [Google Scholar]
- 16.Downward, J., M. D. Waterfield, and P. J. Parker. 1985. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J. Biol. Chem. 260:14538-14546. [PubMed] [Google Scholar]
- 17.Emtner, M., L. S. Mathews, and G. Norstedt. 1990. Growth hormone (GH) stimulates protein synthesis in cells transfected with GH receptor complementary DNA. Mol. Endocrinol. 4:2014-2020. [DOI] [PubMed] [Google Scholar]
- 18.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 19.Feener, E. P., F. Rosario, S. L. Dunn, Z. Stancheva, and M. G. Myers, Jr. 2004. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol. Cell. Biol. 24:4968-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank, S. J., G. Gilliland, A. S. Kraft, and C. S. Arnold. 1994. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology 135:2228-2239. [DOI] [PubMed] [Google Scholar]
- 22.Frank, S. J., W. Yi, Y. Zhao, J. F. Goldsmith, G. Gilliland, J. Jiang, I. Sakai, and A. S. Kraft. 1995. Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J. Biol. Chem. 270:14776-14785. [DOI] [PubMed] [Google Scholar]
- 23.Girault, J. A., G. Labesse, J. P. Mornon, and I. Callebaut. 1999. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem. Sci. 24:54-57. [DOI] [PubMed] [Google Scholar]
- 24.Harpur, A. G., A.-C. Andres, A. Ziemiecki, R. R. Aston, and A. F. Wilks. 1992. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene 7:1347-1353. [PubMed] [Google Scholar]
- 25.Herrington, J., L. S. Smit, J. Schwartz, and C. Carter-Su. 2000. The role of STAT proteins in growth hormone signaling. Oncogene 19:2585-2597. [DOI] [PubMed] [Google Scholar]
- 26.Higgins, D. G., J. D. Thompson, and T. J. Gibson. 1996. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266:383-402. [DOI] [PubMed] [Google Scholar]
- 27.Hildreth, K. L., J. H. Wu, L. S. Barak, S. T. Exum, L. K. Kim, K. Peppel, and N. J. Freedman. 2004. Phosphorylation of the platelet-derived growth factor receptor-beta by G protein-coupled receptor kinase-2 reduces receptor signaling and interaction with the Na+/H+ exchanger regulatory factor. J. Biol. Chem. 279:41775-41782. [DOI] [PubMed] [Google Scholar]
- 28.Hodge, C., J. Liao, M. Stofega, K. Guan, C. Carter-Su, and J. Schwartz. 1998. Growth hormone stimulates phosphorylation and activation of Elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J. Biol. Chem. 273:31327-31336. [DOI] [PubMed] [Google Scholar]
- 29.Hou, S. X., Z. Zheng, X. Chen, and N. Perrimon. 2002. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev. Cell 3:765-778. [DOI] [PubMed] [Google Scholar]
- 30.Huang, L. J., S. N. Constantinescu, and H. F. Lodish. 2001. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 8:1327-1338. [DOI] [PubMed] [Google Scholar]
- 31.Huang, Y., Y. Chang, X. Wang, J. Jiang, and S. J. Frank. 2004. Growth hormone alters epidermal growth factor receptor binding affinity via activation of extracellular signal-regulated kinases in 3T3-F442A cells. Endocrinology 145:3297-3306. [DOI] [PubMed] [Google Scholar]
- 32.Huang, Y., S. O. Kim, J. Jiang, and S. J. Frank. 2003. Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3-F442A cells. Modulation of EGF-induced trafficking and signaling. J. Biol. Chem. 278:18902-18913. [DOI] [PubMed] [Google Scholar]
- 32a.Ishida-Takahashi, R., F. Rosario, Y. Gong, K. Kopp, Z. Stancheva, X. Chen, E. P. Feener, and M. G. Myers, Jr. 2006. Phosphorylation of Jak2 on Ser523 inhibits Jak2-dependent leptin receptor signaling. Mol. Cell. Biol. 26:4063-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James, C., V. Ugo, J. P. Le Couedic, J. Staerk, F. Delhommeau, C. Lacout, L. Garcon, H. Raslova, R. Berger, A. Bennaceur-Griscelli, J. L. Villeval, S. N. Constantinescu, N. Casadevall, and W. Vainchenker. 2005. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434:1144-1148. [DOI] [PubMed] [Google Scholar]
- 34.Jorissen, R. N., F. Walker, N. Pouliot, T. P. Garrett, C. W. Ward, and A. W. Burgess. 2003. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 284:31-53. [DOI] [PubMed] [Google Scholar]
- 35.Kim, S. O., J. C. Houtman, J. Jiang, J. M. Ruppert, P. J. Bertics, and S. J. Frank. 1999. Growth hormone-induced alteration in ErbB-2 phosphorylation status in 3T3-F442A fibroblasts. J. Biol. Chem. 274:36015-36024. [DOI] [PubMed] [Google Scholar]
- 36.Kohlhuber, F., N. C. Rogers, D. Watling, J. Feng, D. Guschin, J. Briscoe, B. A. Witthuhn, S. V. Kotenko, S. Pestka, G. R. Stark, J. N. Ihle, and I. M. Kerr. 1997. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol. Cell. Biol. 17:695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovanen, P. E., I. Junttila, K. Takaluoma, P. Saharinen, L. Valmu, W. Li, and O. Silvennoinen. 2000. Regulation of Jak2 tyrosine kinase by protein kinase C during macrophage differentiation of IL-3-dependent myeloid progenitor cells. Blood 95:1626-1632. [PubMed] [Google Scholar]
- 38.Kralovics, R., F. Passamonti, A. S. Buser, S. S. Teo, R. Tiedt, J. R. Passweg, A. Tichelli, M. Cazzola, and R. C. Skoda. 2005. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352:1779-1790. [DOI] [PubMed] [Google Scholar]
- 39.Kurzer, J. H., L. S. Argetsinger, Y.-J. Zhou, J.-L. Kouadio, J. J. O'Shea, and C. Carter-Su. 2004. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bb. Mol. Cell. Biol. 24:4557-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacronique, V., A. Boureux, V. D. Valle, H. Poirel, C. T. Quang, M. Mauchauffe, C. Berthou, M. Lessard, R. Berger, J. Ghysdael, and O. A. Bernard. 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278:1309-1312. [DOI] [PubMed] [Google Scholar]
- 41.Levine, R. L., M. Wadleigh, J. Cools, B. L. Ebert, G. Wernig, B. J. Huntly, T. J. Boggon, I. Wlodarska, J. J. Clark, S. Moore, J. Adelsperger, S. Koo, J. C. Lee, S. Gabriel, T. Mercher, A. D'Andrea, S. Frohling, K. Dohner, P. Marynen, P. Vandenberghe, R. A. Mesa, A. Tefferi, J. D. Griffin, M. J. Eck, W. R. Sellers, M. Meyerson, T. R. Golub, S. J. Lee, and D. G. Gilliland. 2005. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7:387-397. [DOI] [PubMed] [Google Scholar]
- 42.Lu, K. P., Y. C. Liou, and X. Z. Zhou. 2002. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 12:164-172. [DOI] [PubMed] [Google Scholar]
- 43.Luo, G., and L. Yu-Lee. 1997. Transcriptional inhibition by Stat5. Differential activities at growth-related versus differentiation-specific promoters. J. Biol. Chem. 272:26841-26849. [DOI] [PubMed] [Google Scholar]
- 44.Mackintosh, C. 2004. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem. J. 381:329-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda, T., J. Feng, B. A. Witthuhn, Y. Sekine, and J. N. Ihle. 2004. Determination of the transphosphorylation sites of Jak2 kinase. Biochem. Biophys. Res. Commun. 325:586-594. [DOI] [PubMed] [Google Scholar]
- 46.Moller, C., A. Hansson, B. Enberg, P. E. Lobie, and G. Norstedt. 1992. Growth hormone induction of tyrosine phosphorylation and activation of mitogen activated protein kinases in cells transfected with rat GH receptor cDNA. J. Biol. Chem. 267:23403-23408. [PubMed] [Google Scholar]
- 47.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276:47771-47774. [DOI] [PubMed] [Google Scholar]
- 48.Paz, K., R. Hemi, D. LeRoith, A. Karasik, E. Elhanany, H. Kanety, and Y. Zick. 1997. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J. Biol. Chem. 272:29911-29918. [DOI] [PubMed] [Google Scholar]
- 49.Peeters, P., S. D. Raynaud, J. Cools, I. Wlodarska, J. Grosgeorge, P. Philip, F. Monpoux, L. Van Rompaey, M. Baens, H. Van den Berghe, and P. Marynen. 1997. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15:12) in a myeloid leukemia. Blood 90:2535-2540. [PubMed] [Google Scholar]
- 50.Rui, L., V. Aguirre, J. K. Kim, G. I. Shulman, A. Lee, A. Corbould, A. Dunaif, and M. F. White. 2001. Insulin/IGF-1 and TNFa stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Investig. 107:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rui, L., and C. Carter-Su. 1999. Identification of SH2-Bb as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. USA 96:7172-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rui, L., and C. Carter-Su. 1998. Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bb with PDGF receptor and phosphorylation of SH2-Bb. J. Biol. Chem. 273:21239-21245. [DOI] [PubMed] [Google Scholar]
- 53.Rui, L., L. S. Mathews, K. Hotta, T. A. Gustafson, and C. Carter-Su. 1997. Identification of SH2-Bb as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17:6633-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saharinen, P., and O. Silvennoinen. 2002. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J. Biol. Chem. 277:47954-47963. [DOI] [PubMed] [Google Scholar]
- 55.Saharinen, P., K. Takaluoma, and O. Silvennoinen. 2000. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell. Biol. 20:3387-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saharinen, P., M. Vihinen, and O. Silvennoinen. 2003. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol. Biol. Cell 14:1448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki, A., H. Yasukawa, A. Suzuki, S. Kamizono, T. Syoda, I. Kinjyo, M. Sasaki, J. A. Johnston, and A. Yoshimura. 1999. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 4:339-351. [DOI] [PubMed] [Google Scholar]
- 58.Seger, R., N. G. Ahn, J. Posada, E. S. Munar, A. M. Jensen, J. A. Cooper, M. H. Cobb, and E. G. Krebs. 1992. Purification and characterization of mitogen-activated protein kinase activator(s) from epidermal growth factor-stimulated A431 cells. J. Biol. Chem. 267:14373-14381. [PubMed] [Google Scholar]
- 59.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 60.Silvennoinen, O., B. Witthuhn, F. W. Quelle, J. L. Cleveland, T. Yi, and J. N. Ihle. 1993. Structure of the murine JAK2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc. Natl. Acad. Sci. USA 90:8429-8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smit, L. S., D. J. Meyer, L. S. Argetsinger, J. Schwartz, and C. Carter-Su. 1999. Molecular events in growth hormone-receptor interaction and signaling, p. 445-480. In J. L. Kostyo (ed.), Handbook of physiology, vol. V. Oxford University Press, New York, N.Y. [Google Scholar]
- 62.Stensballe, A., S. Andersen, and O. N. Jensen. 2001. Characterization of phosphoproteins from electrophoretic gels by nanoscale Fe(III) affinity chromatography with off-line mass spectrometry analysis. Proteomics 1:207-222. [DOI] [PubMed] [Google Scholar]
- 63.Takayama, S., M. F. White, and C. R. Kahn. 1988. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J. Biol. Chem. 263:3440-3447. [PubMed] [Google Scholar]
- 64.Tanner, J. W., W. Chen, R. L. Young, G. D. Longmore, and A. S. Shaw. 1995. The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J. Biol. Chem. 270:6523-6530. [DOI] [PubMed] [Google Scholar]
- 65.Tholey, A., A. Lindemann, V. Kinzel, and J. Reed. 1999. Direct effects of phosphorylation on the preferred backbone conformation of peptides: a nuclear magnetic resonance study. Biophys. J. 76:76-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.VanderKuur, J. A., E. R. Butch, S. B. Waters, J. E. Pessin, K.-L. Guan, and C. Carter-Su. 1997. Signalling molecules involved in coupling growth hormone receptor to MAP kinase activation. Endocrinology 138:4301-4307. [DOI] [PubMed] [Google Scholar]
- 67.Verin, A. D., F. Liu, N. Bogatcheva, T. Borbiev, M. B. Hershenson, P. Wang, and J. G. Garcia. 2000. Role of ras-dependent ERK activation in phorbol ester-induced endothelial cell barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L360-L370. [DOI] [PubMed] [Google Scholar]
- 68.Wen, Z., Z. Zhong, and J. E. Darnell, Jr. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241-250. [DOI] [PubMed] [Google Scholar]
- 69.Winston, L. A., and P. J. Bertics. 1992. Growth hormone stimulates the tyrosyl phosphorylation of 42- and 45-kDa ERK-related proteins. J. Biol. Chem. 267:4747-4751. [PubMed] [Google Scholar]
- 70.Xie, S., H. Lin, T. Sun, and R. B. Arlinghaus. 2002. Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene 21:7137-7146. [DOI] [PubMed] [Google Scholar]
- 71.Yaffe, M. B., and S. J. Smerdon. 2004. The use of in vitro peptide-library screens in the analysis of phosphoserine/threonine-binding domain structure and function. Annu. Rev. Biophys. Biomol. Struct. 33:225-244. [DOI] [PubMed] [Google Scholar]
- 72.Yamaoka, K., P. Saharinen, M. Pesu, V. E. Holt III, O. Silvennoinen, and J. J. O'Shea. 2004. The Janus kinases (Jaks). Genome Biol. 5:253-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamauchi, T., K. Ueki, K. Tobe, H. Tamemoto, N. Sekine, M. Wada, M. Honjo, M. Takahashi, T. Takahashi, H. Hirai, T. Tushima, Y. Akanuma, T. Fujita, I. Komuro, Y. Yazaki, and T. Kadowaki. 1997. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature 390:91-96. [DOI] [PubMed] [Google Scholar]
- 74.Yasukawa, H., H. Misawa, H. Sakamoto, M. Masuhara, A. Sasaki, T. Wakioka, S. Ohtsuka, T. Imaizumi, T. Matsuda, J. N. Ihle, and A. Yoshimura. 1999. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao, Y., F. Wagner, S. J. Frank, and A. S. Kraft. 1995. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 270:13814-13818. [DOI] [PubMed] [Google Scholar]
- 76.Zhou, Y. J., E. P. Hanson, Y. Q. Chen, K. Magnuson, M. Chen, P. G. Swann, R. L. Wange, P. S. Changelian, and J. J. O'Shea. 1997. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 94:13850-13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu, T., E. L. K. Goh, D. LeRoith, and P. E. Lobie. 1998. Growth hormone stimulates the formation of a multiprotein signaling complex involving p130Cas and CrkII. Resultant activation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). J. Biol. Chem. 273:33864-33875. [DOI] [PubMed] [Google Scholar]
- 78.Zhu, T., and P. E. Lobie. 2000. Janus kinase 2-dependent activation of p38 mitogen-activated protein kinase by growth hormone. Resultant transcriptional activation of ATF-2 and CHOP, cytoskeletal re-organization and mitogenesis. J. Biol. Chem. 275:2103-2114. [DOI] [PubMed] [Google Scholar]