Abstract
A comprehensive set of clustered charged-to-alanine mutations was generated that systematically alter TUB1, the major α-tubulin gene of Saccharomyces cerevisiae. A variety of phenotypes were observed, including supersensitivity and resistance to the microtubule-destabilizing drug benomyl, lethality, and cold- and temperature-sensitive lethality. Many of the most benomyl-sensitive tub1 alleles were synthetically lethal in combination with tub3Δ, supporting the idea that benomyl supersensitivity is a rough measure of microtubule instability and/or insufficiency in the amount of α-tubulin. The systematic tub1 mutations were placed, along with the comparable set of tub2 mutations previously described, onto a model of the yeast α–β-tubulin dimer based on the three-dimensional structure of bovine tubulin. The modeling revealed a potential site for binding of benomyl in the core of β-tubulin. Residues whose mutation causes cold sensitivity were concentrated at the lateral and longitudinal interfaces between adjacent subunits. Residues that affect binding of the microtubule-binding protein Bim1p form a large patch across the exterior-facing surface of α-tubulin in the model. Finally, the positions of the mutations suggest that proximity to the α–β interface may account for the finding of synthetic lethality of five viable tub1 alleles with the benomyl-resistant but otherwise entirely viable tub2-201 allele.
INTRODUCTION
Microtubules are ubiquitous cytoskeletal structures, made up of heterodimers of α and β-tubulin (Hyams and Lloyd, 1994). Microtubules have been studied in many eukaryotes, both in vitro and in vivo, with the aim of defining both the functions of microtubules and the spatial and temporal regulation of those microtubule functions. As in all eukaryotic cells, microtubules are necessary for chromosome movement in Saccharomyces cerevisiae. Unlike most other eukaryotes, the only other functions known to depend on microtubules in yeast are the movement of nuclei to the bud neck before mitosis and the fusion of nuclei after mating (Huffaker et al., 1988; Jacobs et al., 1988). The high degree of conservation among tubulin proteins (Little and Seehaus, 1988; Burns, 1991), together with the relative simplicity of the yeast microtubule cytoskeleton, make S. cerevisiae a suitable organism for studying the microtubule cytoskeleton.
TUB1 is the major gene encoding α-tubulin in S. cerevisiae (Schatz et al., 1986a,b), and TUB2 is the only gene encoding the β-tubulin gene (Neff et al., 1983). TUB3 is a second gene encoding α-tubulin; it is expressed at lower levels than TUB1 (Schatz et al., 1986a,b). TUB1 and TUB2 are essential genes, whereas a strain carrying a deletion of TUB3 is viable. tub3 null mutations, and most tub1 mutations, show a characteristic supersensitivity to the benzimidazole microtubule drug benomyl (Neff et al., 1983; Schatz et al., 1986b). Deletion of TUB1 produces a dominant phenotype resulting from haploinsufficiency; heterozygotes tend to become trisomic for the wild-type chromosome XIII, which presumably provides a growth advantage because of an additional copy of both TUB1 and TUB3 (Schatz et al., 1986b). The two genes encoding α-tubulin in yeast are very similar, producing protein products that are ∼90% identical, making them much more similar to each other than to α-tubulin proteins from other organisms, to which they are ∼70% identical (Little and Seehaus, 1988). Either α-tubulin gene, TUB1 or TUB3, can compensate for loss of the other, if expressed at high enough levels. Therefore, the differences between TUB1 and TUB3 appear to be largely quantitative, resulting from higher levels of TUB1 expression, rather than qualitative (i.e., sequence) differences in the proteins (Schatz et al., 1986b).
Clustered charged-to-alanine scanning mutagenesis has been used to mutagenize the surface of proteins systematically (Bass et al., 1991; Bennett et al., 1991; Gibbs and Zoller, 1991). This strategy has been used previously to mutagenize the two other major cytoskeletal proteins in S. cerevisiae, actin and β-tubulin (Wertman et al., 1992; Reijo et al., 1994). In the case of ACT1 (the single yeast gene encoding actin), such a program of mutagenesis produced many conditionally lethal alleles, which had previously proven quite difficult to isolate (Wertman et al., 1992). In the case of TUB2 (the sole β-tubulin gene), many new conditional alleles were obtained, some displaying previously unreported phenotypes (Reijo et al., 1994). Both of these sets of mutants are proving to be useful in studies of the cytoskeleton in yeast (Pasqualone and Huffaker, 1994; Amberg et al., 1995; Cali et al., 1998).
Recently the structure of bovine tubulin has been solved at atomic resolution (Nogales et al., 1998b). This allows the results of years of study of the tubulin genes and the function of microtubules in yeast to be analyzed in light of the three-dimensional structure of the tubulin protein. In this paper we map nearly all the known systematic mutations in TUB1 and TUB2 to a model of yeast tubulin based on the bovine structure. As had been found with actin and cofilin (Wertman et al., 1992; Lappalainen et al., 1997), this mapping has indicated regions of the tubulin surface implicated in particular phenotypes, namely cold sensitivity and benomyl resistance. In addition, we have found evidence for the involvement of particular residues in binding of the microtubule ligand Bim1p (Schwartz et al., 1997), and others have found similar evidence for other proteins that bind tubulins (Feierbach et al., 1999).
MATERIALS AND METHODS
Media and Genetic Manipulations
Yeast media and techniques are as described (Rose et al., 1990), except that YPD medium was supplemented with adenine sulfate to a concentration of 40 mg/l. Minimal medium was supplemented only with the required amino acids, rather than using synthetic complete medium, unless specified. Permissive temperature for all experiments was 25°C. Sporulation was induced by diluting a liquid culture 100-fold into sporulation medium described by Kassir and Simchen (1991). Benomyl, a generous gift from DuPont (Wilmington, DE), was kept as a 10 mg/ml stock in dimethylsulfoxide at −20°C. It was added to warm medium, with vigorous swirling to prevent precipitation of the benomyl, just before pouring plates. Bacterial media and techniques were as described (Sambrook et al., 1989). Ampicillin was used at a final concentration of 50 μg/ml.
Strains and Plasmids
Plasmids are listed in Table 1, along with details of their construction. Yeast strains were derived from YPH102 and YPH250 (Sikorski and Hieter, 1989) and are listed in Table 2. YPH102 was transformed with a TUB1 CEN plasmid, pRB326. The resulting strain, KRY73, was transformed with a tub1 deletion construct from pRB2067, to give DBY6590. DBY6591 was constructed by transforming YPH250 with the TUB1-LYS2 construct from pRB2070. DBY6590 was crossed to DBY6591 to give the diploid KRY76. KRY76 was dissected to remove the trp1-Δ1 allele, and two resulting spores, DBY6592 and DBY6593, were crossed, yielding DBY6596. DBY6596 was the recipient strain for the Ala-scan alleles, and its genomic configuration was verified by Southern blot analysis. Mating type tester strains DBY786 and DBY789 were essentially congenic with S288C. Bacterial strains used for transformation and propagation of plasmid DNA were either DH5α or DH5αF′ (Sambrook et al., 1989).
Table 1.
Plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| pUC119 | pUC19 with M13 origin of replication | Sambrook et al., 1989 |
| pRB306 | TUB1 in pRB322 | Schatz et al., 1986b |
| pRB326 | TUB1 in YCp50 | Schatz et al., 1986b |
| pJJ217 | HIS3 in pUC18 polylinker | Jones and Prakash, 1990 |
| pJJ282 | LEU2 in pUC18 polylinker | Jones and Prakash, 1990 |
| pRS317 | LYS2 in pRSS56 | Sikorski and Hieter, 1989 |
| pRB2063 | TUB1 fragment (SphI–SacI) from pRB306 inserted into SphI–SacI-cut polylinker of pUC119 | This study |
| pRB2065 | BamHI–SalI fragment from pJJ282 (LEU2 gene) Klenow filled and inserted into the HpaI site of pRB2063, downstream of TUB1; LEU2 is oriented in the same direction as TUB1 | This study |
| pRB2067 | SalI–KpnI (partial) fragment from pJJ217 containing the entire HIS3 gene and flanking regions, inserted into XhoI–KpnI vector fragment from pRB2063; HIS3 is in the same orientation that TUB1 was; note that this disruption construct removes the first 763 bp of the 1580-bp ORF downstream of TUB1 (YML086c) | This study |
| pRB2070 | PvuII fragment from pRS317 containing LYS2 gene inserted into HpaI site of pRB2063; LYS2 is oriented in the same direction as TUB1 | This study |
| pRB2666 | BglII fragment containing TUB3 from pRB300 ligated into BamHI site of pUC119 | This study |
| pRB2668 | SalI–SmaI containing HIS3 fragment from pJJ217 ligated into XhoI–NdeI (Klenow-filled) vector fragment from pRB2666; HIS3 is in the same orientation as TUB3 was | This study |
| pRB2254 through pRB2412 | pRB2065 mutagenized via site-directed mutagenesis, yielding three isolates of each Ala-scan mutation, tub1-801 through tub1-853 | This study |
Table 2.
Yeast strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| YPH102 | MAT∝ ade2-101 his3-Δ200 lys2-801 leu2-Δ1 ura3-52 | Sikorski and Hieter, 1989 |
| YPH250 | MATa ade2-101 his3-Δ200 lys2-801 leu2-Δ1 ura3-52 trp1-Δ1 | Sikorski and Hieter, 1989 |
| DBY786 | MAT∝ trp1-1 | This laboratory |
| DBY789 | MATa trp1-1 | This laboratory |
| KRY73 | MAT∝ ade2-101 his3-Δ200 lys2-801 leu2-Δ1 ura3-52 | This study |
| plasmid: pRB326 | ||
| DBY6590 | MAT∝ ade2-101 his3-Δ200 lys2-801 leu2-Δ1 ura3-52 tub1Δ::HIS3 | This study |
| plasmid: pRB326 | ||
| DBY6591 | MATa ade2-101 his3-Δ200 lys2-801 leu2-Δ1 ura3-52 trp1-Δ1 TUB1-LYS2 | This study |
| KRY76 (DBY6590 × 6591) | MATa/∝ ade2-101/ade2-101 his3-Δ200/his3-Δ200 lys2-801/lys2-801 leu2-Δ1/leu2-Δ1 ura3-52/ura3-52 trp1-Δ1/TRP1 TUB1-LYS2/tub1Δ::HIS3 | This study |
| plasmid: pRB326 | ||
| DBY6592 | MAT∝ lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1-LYS2 | This study |
| plasmid: pRB326 | ||
| DBY6593 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1Δ::HIS3 | This study |
| plasmid: pRB326 | ||
| DBY6596 | MATa/∝ lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 ura3-52/ura3-52 TUB1-LYS2/tub1Δ::HIS3 | This study |
| plasmid: pRB326 | ||
| DBY6598 | MAT∝ lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1-LYS2 tub3Δ::HIS3 | This study |
| plasmid: pRB326 | ||
| DBY6599 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1-LYS2 tub3Δ::HIS3 | This study |
| plasmid: pRB326 | ||
| DBY6600 | MATa/∝ lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 ura3-52/ura3-52 TUB1-LYS2/TUB1-LEU2 | This study |
| plasmid pRB326 | ||
| DBY6601–6653 | Like DBY6600, but with tub1 Ala-scan alleles, -801 to -853, respectively | This study |
| DBY6654 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1–LEU2 | This study |
| DBY6655 | MAT∝ lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1–LEU2 | This study |
| DBY6656–6761 | Like DBY6654 and 6655, but with tub1 Ala-scan alleles, -801 to -853, respectively (2 each, 1 of each mating type) | This study |
| DBY6762 | MATa/∝ lys2-801/lys2-801 ade2-101/ade2-101 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 ura3-52/ura3-52 TUB1-LYS2/TUB1-LEU2 tub3Δ::HIS3/TUB3 | This study |
| plasmid: pRB326 | ||
| DBY6816 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1–LEU2 tub3Δ::HIS3 | This study |
| DBY6822 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-803–LEU2 tub3Δ::HIS3 | This study |
| DBY6824 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-804–LEU2 tub3Δ::HIS3 | This study |
| DBY6826 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-805–LEU2 tub3Δ::HIS3 | This study |
| DBY6828 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-806–LEU2 tub3Δ::HIS3 | This study |
| DBY6830 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-807–LEU2 tub3Δ::HIS3 | This study |
| DBY6838 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-811–LEU2 tub3Δ::HIS3 | This study |
| DBY6840 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-812–LEU2 tub3Δ::HIS3 | This study |
| DBY6846 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-815–LEU2 tub3Δ::HIS3 | This study |
| DBY6854 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-819–LEU2 tub3Δ::HIS3 | This study |
| DBY6866 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-825–LEU2 tub3Δ::HIS3 | This study |
| DBY6868 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-826–LEU2 tub3Δ::HIS3 | This study |
| DBY6878 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-831–LEU2 tub3Δ::HIS3 | This study |
| DBY6880 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-832–LEU2 tub3Δ::HIS3 | This study |
| DBY6882 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-833–LEU2 tub3Δ::HIS3 | This study |
| DBY6886 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-835–LEU2 tub3Δ::HIS3 | This study |
| DBY6894 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-839–LEU2 tub3Δ::HIS3 | This study |
| DBY6898 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-841–LEU2 tub3Δ::HIS3 | This study |
| DBY6902 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-843–LEU2 tub3Δ::HIS3 | This study |
| DBY6916 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-850–LEU2 tub3Δ::HIS3 | This study |
| DBY6918 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-851–LEU2 tub3Δ::HIS3 | This study |
| DBY6920 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 tub1-852–LEU2 tub3Δ::HIS3 | This study |
| DBY7830 | MAT∝ lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1-LYS2 tub2-201 | Schwartz et al., 1997 |
| plasmid: pRB326 | ||
| DBY7051 | MATa ACT1::HIS his3-Δ200 leu2-3,112 ura3-52 ade4 tub2-201 | This laboratory |
| DBY8154 | MATa lys2-801 ade2-101 his3-Δ200 leu2-Δ1 ura3-52 TUB1-LEU2 tub2-201 | This study |
To construct the tub3Δ strain, DBY6592 was transformed with the gel-purified 2.8-kb BstEII–HindIII fragment from pRB2668, obtained from a BstEII digest followed by a partial HindIII digest, to give DBY6598. The proper genomic organization of the strain was verified by Southern blot analysis. To obtain a congenic strain of the opposite mating type, DBY6598 was mated to DBY6654, yielding the haploid His+ Lys+ Ura+ segregant DBY6599.
Two haploids with each tub1 mutant, one of each mating type, were mated to a tub3Δ strain; MATa tub1 strains were mated to DBY6598, and MATα tub1 strains were mated to DBY6599. The tub1 tub3Δ double mutants (DBY6816 through DBY6920) are haploid progeny of these crosses.
DNA Manipulation
All restriction enzymes, T4 ligase, T7 polymerase, and Klenow were from New England Biolabs (Beverly, MA). Calf intestinal phosphatase was from Boehringer Mannheim (Indianapolis, IN). Taq polymerase was from Perkin Elmer-Cetus (Foster City, CA) or Life Technologies (Santa Clara, CA). Buffers were either used as supplied or remade according to the manufacturer's specifications. Nucleotides were from Pharmacia (Alameda, CA). Oligonucleotide primers were supplied by Genset (La Jolla, CA).
Site-directed Mutagenesis
Methods were modified from those of Kunkel et al. (1987) and Sambrook et al. (1989) and are described in detail elsewhere (Miller et al., 1996). Briefly, an oligonucleotide bearing the desired mutation (see Table 3) was annealed to a single-stranded DNA template, pRB2065, containing a high frequency of uracil misincorporations. Oligonucleotides were designed with at least 12 bp of homology on either side of the altered nucleotides. Synthesis of the second strand, primed by the mutant oligonucleotide, was done in vitro, and the product was transformed into an Escherichia coli strain able to recognize and repair the uracil misincorporations. The repair process resulted in coding information from the template (wild-type) strand being replaced with the coding information from the mutant strand. Resultant plasmids were screened for the introduction or loss of a restriction site to identify those with the desired mutation.
Table 3.
Summary of mutations
| tub1 allele | Amino acid substitutions | DNA sequence changesa | Diagnostic restriction sites | Phenotypeb |
|---|---|---|---|---|
| 801 | R2A, E3A | GCTGCA | Gain PstI | Cs, Ts, BenSS |
| AGAGAA | ||||
| 802 | E27A, H28A | GCAGCC | Gain BbvI | RL |
| GAGCAC | ||||
| 803 | K31A, D33A | GCGCCGGCT | Gain HinP1I, | BenSS |
| AAGCCGGAT | Lose BsaBI | |||
| 804 | H35A, E37A, D38A | GCTCTAGCAGCT | Gain PvuII, | BenSS |
| CATCTAGAAGAT | Lose BsaBI | |||
| 805 | K42A, K44A | GCGCCGGCG | Gain HinP1I | BenSS |
| AAGCCGAAG | ||||
| 806 | E47A, E48A | GCAGCT | Gain PvuII | BenR |
| GAAGAG | ||||
| 807 | H55A, E56A | GCTGCA | Gain BbvI | BenSS |
| CATGAA | ||||
| 808 | K61A, R65A | GCGTTCGTTCCAGCT | Gain PvuII | RL |
| AAGTTCGTTCCAAGG | ||||
| 809 | D70A, E72A | GCTTTAGCG | Gain HinP1I | Slow growth, |
| GATTTAGAG | BenSS | |||
| 810 | D77A, E78A, R80A | GCAGCAGTCGCT | Gain BbvI | BenSS |
| GACGAAGTCCGT | ||||
| 811 | K85A, D86A | GCTGCC | Gain BbvI | WT |
| AAGGAC | ||||
| 812 | H89A, E91A | GCTCCAGCA | Gain BpmI | BenSS |
| CATCCAGAA | ||||
| 813 | K97A, E98A, D99A | GCGGCGGCC | Gain NotI | Slow growth, |
| AAGGAGGAC | BenSS | |||
| 814 | R106A, H108A | GCTGGCGCT | Gain PvuII | Slow growth, |
| AGAGGCCAT | BenSS | |||
| 815 | R113A, E114A | GCAGCA | Gain BbvI | WT |
| AGAGAA | ||||
| 816 | D118A, D121A, R122A | GCTGTTCTGGCTGCG | Gain BbvI | Cs, BenSS |
| GATGTTCTGGATAGG | ||||
| 817 | R124A, K125A | GCAGCA | Gain BbvI | BenSS |
| AGAAAA | ||||
| 818 | D128A, D131A | GCCCAATGTGCA | Gain BsgI | Cs, BenSS |
| GACCAATGTGGAT | ||||
| 819 | T146A | GCA | Gain BsgI, | BenSS |
| ACT | BspMI | |||
| 820 | E156A, E157A | GCAGCA | Gain BbvI | DL |
| GAAGAA | ||||
| 821 | E161A, K164A, K165A | GCATACGGTGCGGCA | Gain Fnu4HI | BenSS |
| GAATACGGTAAGAAA | ||||
| 822 | K167A, E169A | GCGCTGGCA | Gain HinP1I | Slow growth, |
| AAGCTGGAA | BenSS | |||
| 823 | E197A, H198A, D200A | GCAGCTGCAGCT | Gain PstI, PvuII | RL |
| GAACATGCAGAT | ||||
| 824 | D206A, E208A | GCGAATGCG | Gain NruI, | Cs, BenSS |
| GATAATGAG | BsmI | |||
| 825 | D212A, K215A, R216A | GCCATGTGCGCAGCA | Gain BbvI | BenR |
| GACATGTGCAAAAGA | ||||
| 826 | D219A, R222A | GCTATCCCAGCA | Lose EcoRV | BenR |
| GATATCCCAAGA | ||||
| 827 | R244A, D246A | GCATTCGCG | Gain BstUI | Slow growth, |
| AGATTCGAG | BenSS | |||
| 828 | D252A, E255A | GCTTTGAACGCG | Gain MluI | DL |
| GATTTGAACGAA | ||||
| 829 | R265A, H267A | GCAATTGCT | Gain MunI, | Slow growth, |
| AGAATTCAT | Lose EcoRI | BenSS | ||
| 830 | K279A, K281A | GCATCAGCT | Gain PvuII | BenSS |
| AAATCAAAG | ||||
| 831 | H284A, E285A | GCTGCG | Gain Fnu4HI, | Cs, BenSS |
| CATGAG | Lose HinfI, PleI | |||
| 832 | K305A, D307A | GCGTGTGCA | Gain ApaLI | BenR |
| AAGTGTGAT | ||||
| 833 | R309A, D310A, K312A | GCAGCTGGTGCA | Gain PvuII, | BenSS |
| AGAGATGGTAAA | Fnu4HI | |||
| 834 | R321A, D323A | GCGGGTGCT | Gain BstUI | Slow growth, |
| AGGGGTGAT | BenSS | |||
| 835 | R327A, D328A, | GCTGTTCAAGCA | Gain PvuII | WT |
| R331A | GATGTTCAAAGA | |||
| 836 | E334A, K337A | GCGCAGGTGGCA | Gain BstUI, | Cs, Ts, BenSS |
| GAGCAGGTGAAA | HinP1I | |||
| 837 | K339A, K340A | GCGGCG | Gain Fnu4HI, | BenSS |
| AAGAAG | BstUI, Lose BbsI | |||
| 838 | D373A, R374A | GCTGCA | Gain PstI, SfcI | BenSS |
| GATAGG | ||||
| 839 | E387A, K390A, R391A | GCAGCTTGGGCGGCA | Gain PstI | BenR |
| GAGGCTTGGAAGAGA | ||||
| 840 | D393A, R394A, K395A, D397A | GCTGCAGCATTCGCT | Gain PstI, | RL |
| GATAGAAAATTCGAT | BsmI, SfcI, Lose ClaI | |||
| 841 | D393A, R394A | GCTGCA | Gain PstI | BenR |
| GATAGA | ||||
| 842 | K395A, D397A | GCATTCGCT | Gain BsmI | Cs, Ts, BenSS |
| AAATTCGAT | ||||
| 843 | K395A | GCA | Gain BsmI | WT |
| AAA | ||||
| 844 | D397A | GCA | Gain AseI | BenSS |
| GAT | ||||
| 845 | K402A, R403A | GCAGCT | Gain PvuII | Slow growth, BenSS |
| AAACGT | BenSS | |||
| 846 | E412A, E415A | GCAGGTATGGCA | Gain BsgI, | Cs, Ts, BenSS |
| GAAGGTATGGAA | BspMI | |||
| 847 | E416A, E418A | GCAGGTGCA | Gain BsmI, | Slow growth, |
| GAAGGTGAA | BspMI, Lose EcoRI | BenSS | ||
| 848 | E421A, R423A, | GCAGCTGCAGCAGCT | Gain PstI, PvuII | Slow growth, |
| E424A, D425A | GAAGCTAGAGAAGAT | BenSS | ||
| 849 | E430A, R431A, D432A | GCTGCAGCT | Gain PstI | Slow growth, |
| GAAAGAGAT | BenSS | |||
| 850 | E435A, D439A | GCGGTGGGTGCCGCC | Gain BstUI | BenSS |
| GAAGTGGGTGCCGAC | ||||
| 851 | E443A, E444A, E445A, | GCTGCAGCGGCA | Gain PstI | BenSS |
| E446A | GAGGAAGAGGAA | |||
| 852 | F447A | GCT | Gain HindIII | BenSS |
| TTT | ||||
| 853 | E184A | GCG | Gain HinP1I | Slow growth, |
| GAG | BenSS |
The mutant sequence is above the corresponding wild-type sequence. Altered nucleotides are in bold; altered codons are underlined.
BenR, benomyl resistant; BenSS, benomyl supersensitive; Cs, cold-sensitive; DL, dominant lethal; RL, recessive lethal; Slow growth, impaired growth at all temperatures; Ts, heat-sensitive; WT, wild-type.
Construction of tub1 Strains
tub1 mutant plasmids, digested with SphI and SacI to release the fragment containing the tub1 and downstream LEU2 integration, were transformed into DBY6596, and Leu+ transformants were selected. DBY6596 is a heterozygous diploid with a HIS3-marked tub1 deletion (tub1Δ::HIS3) on one chromosome XIII; on the homologue, the wild-type TUB1 locus marked by LYS2 integrated downstream. In this diploid, a TUB1 centromeric plasmid, pRB326, is maintained to prevent the aneuploidy that results from propagating strains heterozygous for a tub1 deletion (Schatz et al., 1986b). The transforming tub1-LEU2 fragment could integrate by homologous recombination at any of the three TUB1 loci (two chromosomal and one plasmid) in the recipient strain. The desired integration event was recovered by screening Leu+ transformants that had become His− as a result of the replacement of the tub1Δ::HIS3 allele. This screening also eliminated any unwanted products of illegitimate recombination (i.e., integrations at loci other than TUB1).
The resultant diploids are heterozygous for the tub1 mutation. In addition, both the wild-type and mutant TUB1 loci are marked by LYS2 and LEU2, respectively. These auxotrophic markers were inserted ∼1 kb downstream of TUB1 and therefore remain tightly linked to TUB1 during meiotic segregation, allowing for convenient identification of tub1-containing haploid segregants. These markers can also readily reveal any chromosome XIII aneuploidy, because Lys+ and Leu+ do not segregate 2:2 under those conditions. It should be noted that insertion of these auxotrophic marker genes disrupts the open reading frame downstream of TUB1. This gene, ALO1, encodes d-arabinono-1,4-lactone oxidase, which catalyzes the biosynthesis of d-erythroascorbic acid in yeast (Huh et al., 1998). This disruption had no microtubule phenotype on its own, and all tub1 alleles were compared with the appropriate control, a strain with a wild-type TUB1 gene marked by the downstream auxotrophic marker insertion in ALO1.
To assess viability in the absence of the plasmid-borne TUB1 gene, the heterozygous diploids and the dissection plates containing haploid segregants were replica plated to medium containing 5-fluoroorotic acid (5-FOA), which selects against the URA3-marked pRB326 plasmid. Each dissection plate was also replica plated to mating-type tester lawns and onto media to test for the various auxotrophic markers. The auxotrophic markers indicate which haploid segregants carry pRB326 and whether they carry the wild-type (Lys+) or mutant (Leu+) TUB1 gene.
Each mutant was constructed twice independently. Both independent isolates of each haploid mutant, and occasionally the heterozygous diploid parent, were verified by PCR amplification of the TUB1 gene followed by a restriction digest to verify the presence of the restriction site introduced by that particular mutation (Table 3). This test also confirmed the absence of the wild-type TUB1 gene in the haploids.
Yeast Transformation
Yeast were transformed using electroporation (Becker and Guarente, 1991), using ∼1–2 μg of DNA per transformation. For constructing parental strains and for making the first isolate of each Ala-scan mutant, the DNA usually came from a gel-purified fragment. The DNA was purified using the Qiaex (Qiagen, Chatsworth, CA) DNA purification system. For the second isolate of each mutation (and sometimes for the first isolate as well), the DNA was digested from a crude minipreparation of DNA, ethanol precipitated, and transformed directly without purification of the transforming fragment.
Colony PCR to Verify Mutation
Genomic DNA was amplified directly from yeast colonies by resuspending a colony directly into 5 μl of water in a PCR tube and immediately placing it on ice. A mixture of all the other components was prepared on ice. The mixture was composed such that adding 25 μl to the 5 μl of cells would produce a final concentration of 0.25 μM of each primer, 2.5 U of Taq polymerase, 1× Taq buffer (10 mM Tris-HCl, pH 8.5, 50 mM KCl, and 1.5 mM MgCl2), and 0.1 μg/μl BSA. Immediately after dividing the mixture into each reaction tube while on ice, the tubes were vortexed very briefly, mineral oil was added, and tubes were placed into a preheated (>90°C) DNA Thermal Cycler (Perkin Elmer-Cetus). The cycling conditions varied but worked best with 94°C, 4 min, 30 cycles (92°C, 1 min; 55°C, 1 min; and 72°C, 2 min); 72°C, 20 min; and a 4°C soak.
Half of the reaction product (15 μl) was digested with the appropriate restriction enzyme (see Table 3) in a final reaction volume of 20 μl, and the entire reaction was run on an agarose gel to verify the presence or absence of the restriction site resulting from the particular tub1 mutation.
DNA Sequencing of tub2-201
tub2-201 DNA was PCR amplified from strains DBY7051 (twice) and DBY8154 (once) using Taq polymerase (Life Technologies, Rockville, MD) and primers KStub2-01 (5′-GCGCCATGGAGATGAGAGAAATCATTCAT-3′) and KStub2-02 (5′-GCGCCATGGTTATTCAAAATTCTCAGTGATTGG-3′), which anneal to the first and last 18 nucleotides of the TUB2 open reading frame. Products from independent amplifications were cloned into the NcoI site of pACTII (Bai and Elledge, 1996), resulting in three tub2-201-containing plasmids. The 5′- and 3′-most ends of the tub2-201 open reading frame were PCR amplified using primers KStub2-03 (5′-GCGGGATCCGTAGTGGTGAGGCAATTGG-3′), KStub2-04 (5′-GCGTCTAGACGAGATCAAAAGC-GTACC-3′), KStub2-05 (5′-GCGGGATCCCCCCAACAATGTGCA-AAC-3′), and KStub2-06 (5′-GCGTCTAGAGTTTATTTTGCTCCA-AGTGC-3′). Purified tub2-201 DNA (plasmid or PCR product) was sequenced with ThermoSequenase dye terminator cycle sequencing mix (Amersham, Arlington Heights, IL) and an ABI PRISM 373XL DNA sequencer (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions.
Immunofluorescence Microscopy
Visualization of microtubules with indirect immunofluorescence and 4′,6′-diamidino-2-phenylindole (DAPI) staining to visualize nuclei were performed as described previously (Schwartz et al., 1997).
Growth Rate Measurement
Growth rates were determined by diluting 1 ml of a dense YPD culture into 24 ml of YPD, incubating with agitation for 2–3 h to allow cells to begin growing exponentially, and then splitting the culture three ways: 8 ml of culture into 17 ml of YPD to be incubated at 11, 25, and 37°C. Readings were taken periodically with a Klett (New York, NY) colorimeter, and growth was plotted using Cricket Graph 1.3.2 (Cricket Software, Malvern, PA). The equation of the growth curve was used to calculate a doubling time for each strain.
Bud Index Determination
Cells were diluted from a saturated culture and incubated 3–4 h to allow cells to begin exponential growth. The cultures were then split and placed at 25 and 11°C. After two wild-type doubling times, the cells were counted using a hemacytometer. The bud index was simultaneously obtained by counting at least 200 cells and categorizing them as unbudded, small-budded, medium-budded (a bud diameter greater than one-fourth of the diameter of the mother cell body), or large-budded (a bud diameter greater than one-half of the diameter of the mother cell body).
Spot Replica Plating
Cells were grown in 2 ml of YPD for 36–48 h. to allow all cultures to arrive at approximately the same density. All cultures were diluted into wells of a microtiter dish, producing 10−1 and 10−3 dilutions of each culture. These dilutions were plated using a 12-channel multipipettor, dispensing 3 μl per spot. Plates were observed and photographed daily to record data.
Molecular Modeling
The structure of the α–β-tubulin heterodimer from bovine brain was obtained by electron crystallography of zinc-induced tubulin sheets stabilized with taxol (Nogales et al., 1998b). Tub1p and Tub2p sequences were modeled with the LOOK software package (Molecular Applications Group, Palo Alto, CA) using the coordinates from the solved bovine tubulin structure as a template. Five hundred rounds of energy minimization refinements were used. GTP and GDP, but not taxol, were included in the model. The C termini of Tub1p (residues 442–447) and Tub2p (residues 428–457) were not included in the model, because they are not resolved in the current bovine tubulin structure (Nogales et al., 1998b). The resulting yeast tubulin model was quite similar to the bovine tubulin crystal structure: the root mean square deviation for 2601 polypeptide backbone atoms was 1.21 Å as calculated with Swiss-PdbViewer (Guex and Peitsch, 1997). The coordinates of this yeast tubulin model, along with interactive views of the model, are available at the Botstein laboratory web site: http://genome-www.stanford.edu/group/botlab/tubulin.
RESULTS AND DISCUSSION
Design and Construction of Charged-to-Ala Scan Mutations
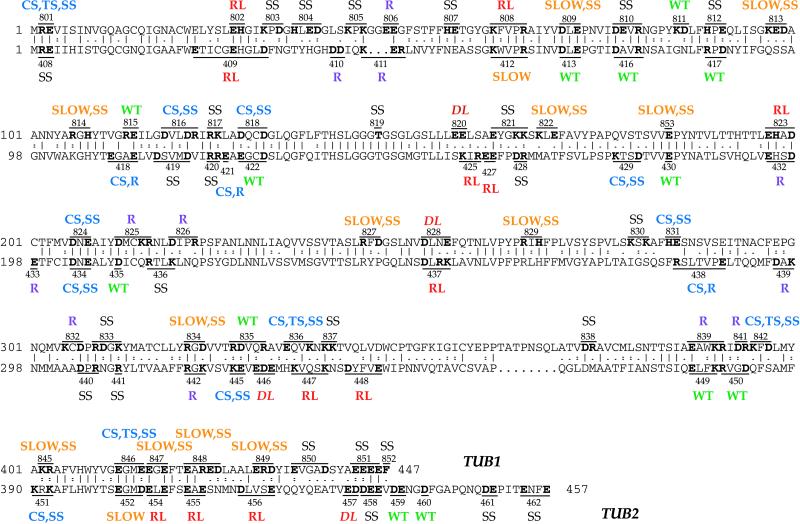
Mutations were designed by examining the protein sequence of Tub1p, inspecting each window of five amino acids for two or more “charged” residues (we included H as well as K, R, D, and E). Mutations were designed to replace all of these “clustered charged” residues with alanine while minimizing the total number of mutations. As a result, each mutation changed two to four residues from charged to alanine. Only 10 charged residues were not included within any of the alanine-scanning mutations, and only one of these (H407) was within five amino acids of another charged residue. Residue E184, although not within a charged cluster, was mutated because it is in the middle of an extremely well-conserved sequence present in all tubulins (α, β, and γ): VVEPYN. tub1-842 was subdivided into two additional mutations, changing K395 and D397 separately (tub1-843 and tub1-844). Based on sequence conservation, these two residues may be important for α-tubulin-specific functions (Cleveland et al., 1990; Burns, 1991), and in addition, K395 has been shown to be an important residue for microtubule assembly (Sherman et al., 1983; Szasz et al., 1986).
Other noncharged residues were also mutated to alanine. T146 (mutated to A in tub1-819) is part of the tubulin signature sequence (GGGTGSG) (Bairoch, 1991), an invariant sequence found in every tubulin gene discovered to date. This sequence is now known to form part of the GTP-binding site by interacting with the nucleotide's phosphates (Nogales et al., 1998a,b). tub1-852 changes the last residue of Tub1p, a phenylalanine, to an alanine. This C-terminal residue is the subject of post-translational modification in vertebrate α-tubulins (Arce et al., 1978; Argarana et al., 1978; Barra et al., 1988).
During the construction of these mutations, two sequencing errors were discovered in the published TUB1 sequence (Schatz et al., 1986a). The first changes Leu-94(CTC) to Ile-94(ATC), and the second changes Thr-326 Gly-327(ACG GGA) to Thr-326 Arg-327(ACA AGA). Both of these amino acid changes increase the identity of Tub1p with Tub3p. Both of these nucleotide changes were also found by the systematic sequencing effort, as reported in the chromosome XIII sequence (Bowman et al., 1997).
A total of 53 mutations were constructed by site-directed mutagenesis and then integrated into the TUB1 locus by homologous recombination, resulting in tub1/TUB1 heterozygotes (see MATERIALS AND METHODS). Each mutant was constructed twice independently and was subjected to the same initial phenotypic characterization to be sure the phenotypes of the two isolates agreed. This guarded against misinterpretation attributable to second-site mutations introduced sometime during the mutagenesis procedure, because the likelihood of this occurring in two independent isolates is minimal. A TUB1 centromeric plasmid (pRB326) was maintained in these strains during their construction to prevent aneuploidy, which results from propagating strains heterozygous for tub1 null alleles (Schatz et al., 1986b). Once the mutants were constructed, the phenotypes of these diploids and their haploid progeny were assessed in the absence of the plasmid-borne TUB1 by plating on 5-FOA, which selects against the URA3-marked pRB326 plasmid.
tub1 Mutant Phenotypes
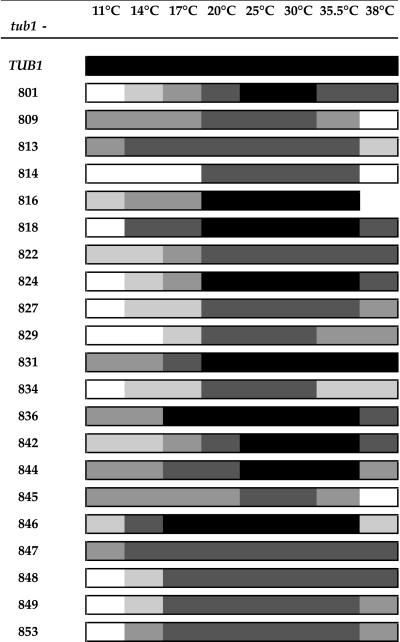
The phenotype of the tub1 haploids was characterized by testing for growth at different temperatures ranging from 11 to 37°C, and on media containing benomyl concentrations ranging from 2 to 50 μg/ml. Benomyl is a member of the benzimidazole family of microtubule-destabilizing drugs and is known to affect the growth of many tubulin mutants in yeast (Thomas et al., 1985; Schatz et al., 1988), although its exact mechanism of action is unknown. Growth rates in liquid medium were determined for each viable mutant at 11, 25, and 37°C. Each mutant was then classified as cold-sensitive (Cs) if growth was impaired at 11°C, temperature-sensitive (Ts) if growth was impaired at 37°C, and slow-growing if growth was impaired at 25°C as well as at extreme temperatures. In addition, each mutant was classified as benomyl-supersensitive if growth was impaired relative to wild type on media containing benomyl and benomyl-resistant if growth was better than wild type on media containing benomyl.
The phenotypes resulting from each tub1 mutation are shown in Figure 1 and Table 3. Two were dominant lethal mutations, because the diploid transformants, heterozygous for the mutation, were unable to grow without additional copies of TUB1 (pRB326) and therefore died on 5-FOA. Four were recessive lethal mutations; the haploid tub1 segregants failed to grow without the TUB1 plasmid on 5-FOA. Twenty-one alleles produced a conditional growth phenotype (Cs, both Cs and Ts, or impaired over the entire temperature range). Growth of each of these mutants at various temperatures is shown in Figure 2. Almost all of the mutations show an altered sensitivity to benomyl. Wild-type haploids (e.g., DBY6654 and DBY6655) grew well on concentrations of benomyl up to 20 μg/ml and grew weakly on a concentration of 30 μg/ml. Six strains, those containing the alleles tub1-806, -825, -826, -832, -839, and -841, grew well on 30 μg/ml benomyl and were therefore benomyl resistant. Despite previous extensive mutagenesis of TUB1 (Schatz et al., 1988), these are the first benomyl-resistant tub1 alleles isolated. These strains were not completely insensitive to benomyl, however, because they failed to grow on 75 μg/ml benomyl. Most of the other tub1 mutants were more benomyl sensitive than wild type, failing to grow on 20 μg/ml benomyl. We placed the tub1 mutants into five categories based on their benomyl sensitivities relative to wild type and the benomyl-supersensitive mutant tub3Δ (which failed to grow on 10 μg/ml benomyl). The categories were, in descending degree of sensitivity: 1) more sensitive than tub3Δ, 2) approximately equal in sensitivity to tub3Δ, 3) less sensitive than tub3Δ but more sensitive than wild type, 4) approximately equal in sensitivity to wild type, and 5) less sensitive than wild type (i.e., benomyl-resistant) (Table 4). Because nearly all of these systematic tub1 mutations alter benomyl sensitivity, it appears that microtubules are exquisitely sensitive to changes in Tub1p sequence.
Figure 1.
Phenotypes of TUB1 and TUB2 Ala-scan mutants. The positions of the mutations and their resulting phenotypes are shown for both TUB1 (top) and TUB2 (bottom). The allele number of each mutation is shown, and the altered residues are in bold. SS, benomyl-supersensitive; R, benomyl-resistant; WT, wild-type; CS, cold-sensitive; TS, heat-sensitive; SLOW, slow growth at all temperatures. The TUB2 Ala-scan data is reproduced from Reijo et al. (1994), and phenotype definitions are adapted to be identical to those used in describing the TUB1 mutants.
Figure 2.
Growth of tub1 mutants at various temperatures. The growth of each mutant was compared with the growth of the wild-type control across a range of temperatures. Growth ranges from wild-type growth (heavily shaded) to no growth (unshaded).
Table 4.
tub1 and tub1 tub3 mutant phenotypes
| Category of benomyl sensitivitya | tub1 allele | tub1 phenotypeb | tub1 tub3Δ phenotypeb |
|---|---|---|---|
| 1 | 801 | BenSS, Cs, Ts | SL |
| 809 | BenSS, slow | SL | |
| 813 | BenSS, slow | SL | |
| 814 | BenSS, slow | SL | |
| 817 | BenSS | SL | |
| 822 | BenSS, slow | SL | |
| 824 | BenSS, Cs | SL | |
| 827 | BenSS, slow | SL | |
| 829 | BenSS, slow | SL | |
| 833 | BenSS | BenSS, slow | |
| 834 | BenSS, slow | SL | |
| 836 | BenSS, Cs, Ts | SL | |
| 2 | 810 | BenSS | SL |
| 816 | BenSS, Cs | SL | |
| 818 | BenSS, Cs | SL | |
| 819 | BenSS | BenSS, slow | |
| 830 | BenSS | SL | |
| 837 | BenSS | SL | |
| 845 | BenSS, slow | SL | |
| 846 | BenSS, Cs, Ts | SL | |
| 849 | BenSS, slow | SL | |
| 853 | BenSS, slow | SL | |
| 3 | 803 | BenSS | BenSS, slow |
| 804 | BenSS | BenSS, Cs | |
| 805 | BenSS | BenSS, slow | |
| 807 | BenSS | BenSS, slow | |
| 812 | BenSS | BenSS, Ts | |
| 821 | BenSS | SL | |
| 831 | BenSS, Cs | BenSS, slow | |
| 838 | BenSS | SL | |
| 842 | BenSS, Cs, Ts | SL | |
| 844 | BenSS, Cs, Ts | SL | |
| 847 | BenSS, slow | SL | |
| 848 | BenSS, slow | SL | |
| 850 | BenSS | BenSS, slow | |
| 851 | BenSS | BenSS, slow | |
| 852 | BenSS | BenSS | |
| 4 | TUB1 | WT | BenSS |
| 811 | WT | BenSS | |
| 815 | WT | BenSS | |
| 835 | WT | BenSS | |
| 843 | WT | BenSS, Cs | |
| 5 | 806 | BenR | BenSS |
| 825 | BenR | BenSS | |
| 826 | BenR | BenSS | |
| 832 | BenR | BenSS | |
| 839 | BenR | BenSS | |
| 841 | BenR | BenSS, slow |
1, more sensitive than tub3Δ; 2, sensitivity equal to tub3Δ; 3, less sensitive than tub3Δ but more sensitive than wild type; 4, sensitivity equal to wild type; 5, less sensitive than wild type (benomyl-resistant).
BenR, benomyl resistant; BenSS, benomyl supersensitive; Cs, cold-sensitive; SL, synthetic lethal; slow, slow growth at all temperatures; Ts, heat-sensitive; WT, wild-type.
Synthetic Lethality with Deletions of TUB3
To determine the phenotype of each tub1 allele in the absence of any wild-type α-tubulin, each was combined with a deletion of the minor α-tubulin gene TUB3. The resulting phenotypes are shown in Table 4. None of the lethal tub1 mutants was rescued by removing TUB3. Remarkably, more than half of the viable tub1 mutants (26 of 47) were synthetically lethal in combination with tub3Δ. Furthermore, many of the remaining viable tub1 tub3Δ double mutants were severely growth impaired. TUB3 obviously plays a vital role in the microtubule function of most of the tub1 mutants.
Categorizing the tub1 mutants according to their degree of benomyl supersensitivity was a very good predictor of the viability of the tub1 tub3Δ double mutant. Alongside each mutant allele in Table 4 are any temperature and/or cold sensitivities produced by that allele, as well as its tub3Δ double mutant phenotype. Almost without exception, the most benomyl-supersensitive and growth-impaired tub1 alleles are also the alleles that are synthetically lethal with tub3Δ. The most parsimonious explanation for this observation is that the phenotypic effect of removing TUB3 from tub1 strains is merely additive, so that the most severe tub1 alleles will not be able to tolerate it, whereas the less severe alleles will. The exceptions to this rule may indicate TUB1- or TUB3-specific functional regions or may indicate regions where a particular residue is required, but only in a subset of α-tubulin subunits. For example, the tub1-844 mutant, although just slightly more benomyl-supersensitive than the wild-type, does not survive in the absence of TUB3. The residue altered by this allele may be crucial for microtubule function, but the few monomers encoded by TUB3 are sufficient to allow microtubule function.
Microtubule and Nuclear Phenotypes of Cold-sensitive tub1 Mutants
The cold-sensitive tub1 mutants were further examined by visualizing microtubules themselves with indirect immunofluorescence. Previously, tub1 mutants were found to fall in three classes. Class 1 mutants had very few or no detectable microtubules, class 2 mutants had extra and/or longer microtubules, and class 3 mutants had relatively normal numbers of microtubules, sometimes disorganized. (Schatz et al., 1988). This system provided a convenient classification scheme for organizing the current set of tub1 mutants as well.
The microtubules were observed after fixing cells grown at the nonpermissive temperature. In general, the observed phenotypes agreed very well with the tub1 mutant phenotype categories defined by Schatz et al. (1988). Several of the mutants displayed reduced or no staining at the restrictive temperature, indicating that they have impaired microtubule formation or stability. Many mutants, other than having an absence of long spindles, as would be predicted from the defect in nuclear division seen by DAPI staining (see below), displayed no other distinct microtubule phenotypes. One mutant, tub1-831, has brighter, more numerous, and longer than normal extranuclear microtubules throughout the cell cycle. This mutant phenotype is similar to the class 2 mutant microtubule phenotype described by Schatz et al. (1988). Table 5 shows the microtubule phenotypes of each of the tub1 mutants.
Table 5.
Microtubule phenotypes of tub1 mutants and tub1 tub3Δ double mutants
| tub1 mutant | Microtubule class | Growth phenotype | tub1 tub3Δ mutant | Microtubule class | Growth phenotype |
|---|---|---|---|---|---|
| 800 (wild-type) | 3 | WT | 800 tub3Δ | 3 | BenSS |
| 801 | 3 | BenSS, Cs, Ts | 803 tub3Δ | 1 | BenSS, slow |
| 806 | 3 | BenR | 805 tub3Δ | 3 | BenSS, slow |
| 809 | 1 | BenSS, slow | 806 tub3Δ | 3 | BenSS |
| 813 | 3 | BenSS, slow | 807 tub3Δ | 1 | BenSS, slow |
| 814 | 3 | BenSS, slow | 812 tub3Δ | 3 | BenSS, Ts |
| 822 | 3 | BenSS, slow | 819 tub3Δ | 3 | BenSS, slow |
| 827 | 1 | BenSS, slow | 825 tub3Δ | 3 | BenSS |
| 829 | 3 | BenSS, slow | 831 tub3Δ | 3 | BenSS, slow |
| 831 | 2 | BenSS, Cs | 833 tub3Δ | 3 | BenSS, slow |
| 834 | 1 | BenSS, slow | 850 tub3Δ | 3 | BenSS, slow |
| 845 | 3 | BenSS, slow | 851 tub3Δ | 1 | BenSS, slow |
| 846 | 3 | BenSS, Cs, Ts | 852 tub3Δ | 3 | BenSS |
| 847 | 3 | BenSS, slow | |||
| 848 | 3 | BenSS, slow | |||
| 849 | 3 | BenSS, slow | |||
| 853 | 3 | BenSS, slow |
Microtubules from mutant cells grown for two generations at 11°C were visualized using indirect immunofluorescence. Intensity was scored in comparison with wild type (TUB1-800), which was included in every experiment. Mutants are divided into three categories according to their microtubule phenotypes: 1) very diminished or no microtubules, 2) excess microtubules, and 3) normal microtubules, sometimes fainter than usual. Growth phenotypes are shown for comparison, and abbreviations are as listed in Table 4.
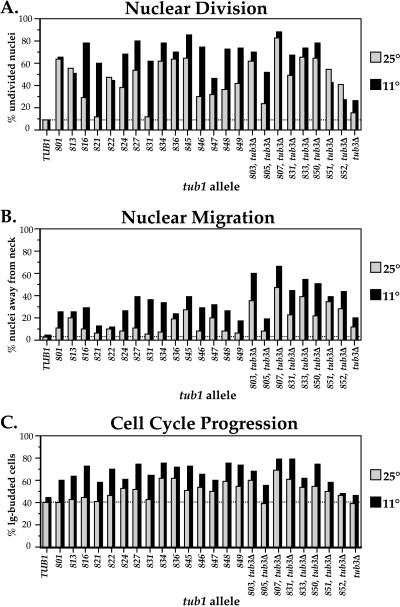
Many of the cold-sensitive mutants were examined at the restrictive temperature (11°C) for nuclear phenotypes resulting from disrupted microtubules. Two primary microtubule defects, nuclear migration and nuclear division, were quantified using the DNA dye DAPI to visualize nuclei, and cell cycle progression was assessed by determining the distribution of bud sizes. Nuclear migration requires extranuclear microtubules (Huffaker et al., 1988). In wild-type cells, nuclear migration happens early in the cell cycle, so large-budded cells are almost never observed without the nucleus at or extended through the bud neck. The other microtubule defect that was visualized with DAPI staining is in nuclear division, a process requiring intranuclear microtubules (Huffaker et al., 1988). In tubulin mutants, when chromosomes do not separate because of a nonfunctional spindle, the nucleus does not divide. This is easily visualized as large-budded cells containing a single DAPI-staining region, rather than the two distinct DAPI-staining regions seen in wild-type large-budded cells.
The tub1 mutants accumulate large-budded cells to varying degrees and show a variety of nuclear phenotypes, shown in Figure 3. Some mutants become dramatically worsened at 11°C; whereas some are equally affected at both temperatures. Some alleles produce defects predominantly in either nuclear division or nuclear migration, whereas some alleles affect both processes to the same extent. Analysis of these phenotypes leads to several general conclusions. First, the accumulation of large-budded cells, although not absolute, is significant in most of the tub1 mutants, probably arising from a delay in mitosis. Second, the tub3Δ mutant has both a nuclear migration and a detectable nuclear division defect that is more severe at 11°C. This ability to distinguish a tub3Δ strain from the wild type demonstrates the sensitivity of this assay and reveals two new, albeit subtle, phenotypes for the tub3Δ mutant. Third, the tub1 tub3Δ double mutants have generally more severe defects in nuclear migration than tub1 mutants, in contrast with nuclear division, which is affected to the same degree. Last, the defects in nuclear division resulting from the tub1 Ala-scan mutations are more dramatic than the defects in nuclear migration, in concordance with the results obtained from analysis of the tub2 Ala-scan mutants.
Figure 3.
Nuclear phenotypes and cell cycle progression of tub1 mutants. Large-budded cells were examined both by ascertaining their frequency in a growing population of cells and by staining their nuclei with DAPI. Cells were examined both at 25°C and after a shift to 11°C for two generations. In A, the percentage of large-budded cells with an undivided nucleus was determined; in B, the percentage of large-budded cells with a nucleus away from its normal position near the bud neck was determined; and in C, the percentage of large-budded cells (bud diameter greater than one-half of the mother cell's body) in the population was determined.
Comparison of tub1 and tub2 Mutant Phenotypes
Because microtubules are made of heterodimers of α- and β-tubulin, it is worthwhile to compare the set of TUB1 Ala-scan mutants and the set of TUB2 Ala-scan mutants (Reijo et al., 1994). It should be noted that direct comparisons between tub1 and tub2 mutant phenotypes are complicated by the existence of a second α-tubulin gene (TUB3). Nonetheless, there are several notable comparisons. First, both the TUB1 and TUB2 systematic mutageneses produced a few dominant lethal alleles. There were more recessive lethal alleles in TUB2, which is not surprising, given that TUB2 is the sole β-tubulin gene, whereas TUB3 provides a nontrivial fraction of the cell's α-tubulin. Second, there are more tub2 benomyl-resistant mutants. Mutations that simultaneously result in benomyl resistance and cold sensitivity were found only in TUB2. In TUB1, all of the benomyl-resistant mutants show wild-type growth at all temperatures. Finally, a much higher percentage of tub1 mutants are benomyl supersensitive, leaving only three tub1 mutants with a normal growth phenotype, many fewer than the number of normally growing tub2 mutants.
The results of comparing analogous mutations in TUB1 and TUB2 are shown in Figure 1. Although the identical mutation was rarely made, it is nonetheless notable how dissimilar the results are across the entire length of the proteins. Six of the tub1 mutations (tub1-801, -808, -809, -824, -847, and -853) are absolutely identical to their cognate tub2 mutation, yet none of these shares the same phenotype with its tub2 counterpart. There are three lethal tub1 mutations that seem to be in essential regions shared between the two proteins (see tub1-802, -820, and -828), but in other cases (tub1-823 and tub2-454) the exact counterpart of a lethal mutation in one gene has a viable phenotype in the other gene. Furthermore, the dominant lethal TUB1 mutations are in the N-terminal half of the protein, whereas the dominant lethal TUB2 mutants are in the C-terminal half. This illustrates what appears to be an essentially complete functional divergence between the two components of the tubulin heterodimer, even though their three-dimensional structure is nearly identical and their primary sequence is very similar. This seems a natural consequence of the polarity of both the tubulin dimer and the microtubule and of the different involvement of α- and β-tubulin in the interaction with other cellular components.
Structure–Function Relationships
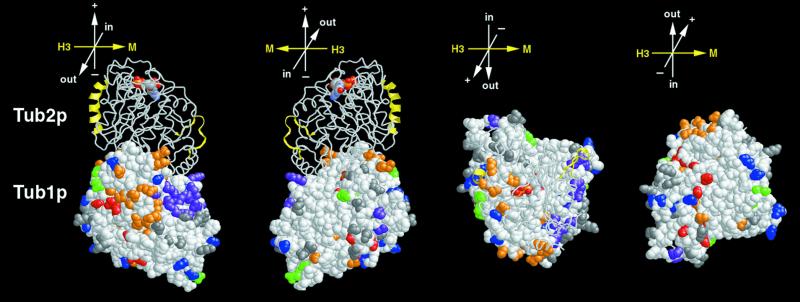
The amino acids altered by tub1 and tub2 systematic mutations were mapped onto a three-dimensional model (see MATERIALS AND METHODS) of the yeast tubulin dimer. Most of the mutated residues are distributed relatively evenly across the protein surface. Figure 4 illustrates this distribution for Tub1p; the distribution of mutations on Tub2p is similar. The longitudinal interfaces and the microtubule-interior face of each monomer have regions (10–15 Å in diameter) that are devoid of charged residues. A small fraction of the mutated residues are buried in the structure, including Tub1p residues R65, D70, T146, E169, D206, and R321 and Tub2p residues R62, D67, E123, E128, D203, and R318.
Figure 4.
Distribution of alanine-scanning mutations in Tub1p. Side chains of amino acids altered by tub1 mutations are colored as in Figure 1: Green, wild-type; red, lethal; blue, cold-sensitive; orange, slow growth; gray, benomyl-supersensitive; purple, benomyl-resistant. Tub2p shown as a backbone trace, and Tub1p is shown as space-filled atoms. Lateral interaction elements, α-helix H3 and M loop, are shown in yellow in Tub2p (Nogales et al., 1999). Orientation axes: + and − show microtubule orientation; H3 and M show lateral sides marked by helix H3 α-helix and M loop, respectively; in and out represent the inside and the outside of the microtubule, respectively. Structure drawn using RASMOL (Sayle and Milner-White, 1995).
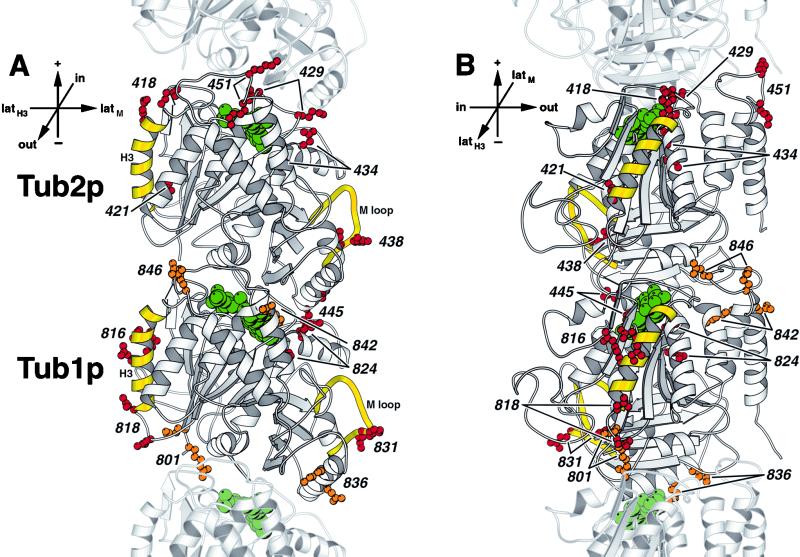
The distribution of mutations in different phenotypic classes was examined. Tubulin mutations often result in the unusual phenotype of cold sensitivity. As shown in Figure 5, the distribution of the cold-sensitive alleles is striking; all these alleles, in both Tub1p and Tub2p, lie either at or near the lateral contacts between protofilaments or in longitudinal contacts between tubulin monomers along the protofilament. In other words, virtually every cold-sensitive tubulin Ala-scan mutation is physically in a position to disrupt the polymerized microtubule structure by altering tubulin–tubulin contacts. This is consistent with the fact that microtubules are known to be cold-sensitive structures.
Figure 5.
Mutations resulting in cold sensitivity are located in areas of the tubulin protein involved in longitudinal or lateral contacts in the microtubule polymer. (A) View from the outside of the microtubule. (B) View from the side of the protofilament. The side chains of amino acids mutated in cold-sensitive alleles (red) and temperature- and cold-sensitive alleles (orange) are shown on Tub2p (top) and Tub1p (bottom) of the yeast tubulin heterodimer. These amino acids are labeled by their allele number. Lateral interaction elements, α-helix H3 and M-loop, are shown in yellow (Nogales et al., 1999). GTP and GDP are shown in green. Adjacent monomers of the protofilament are shown in lighter gray. Orientation axes: + and − show microtubule orientation; latH3 and latM show lateral sides marked by helix H3 and M loop, respectively; in and out represent the inside and the outside of the microtubule, respectively. Structure drawn using MOLSCRIPT (Kraulis, 1991).
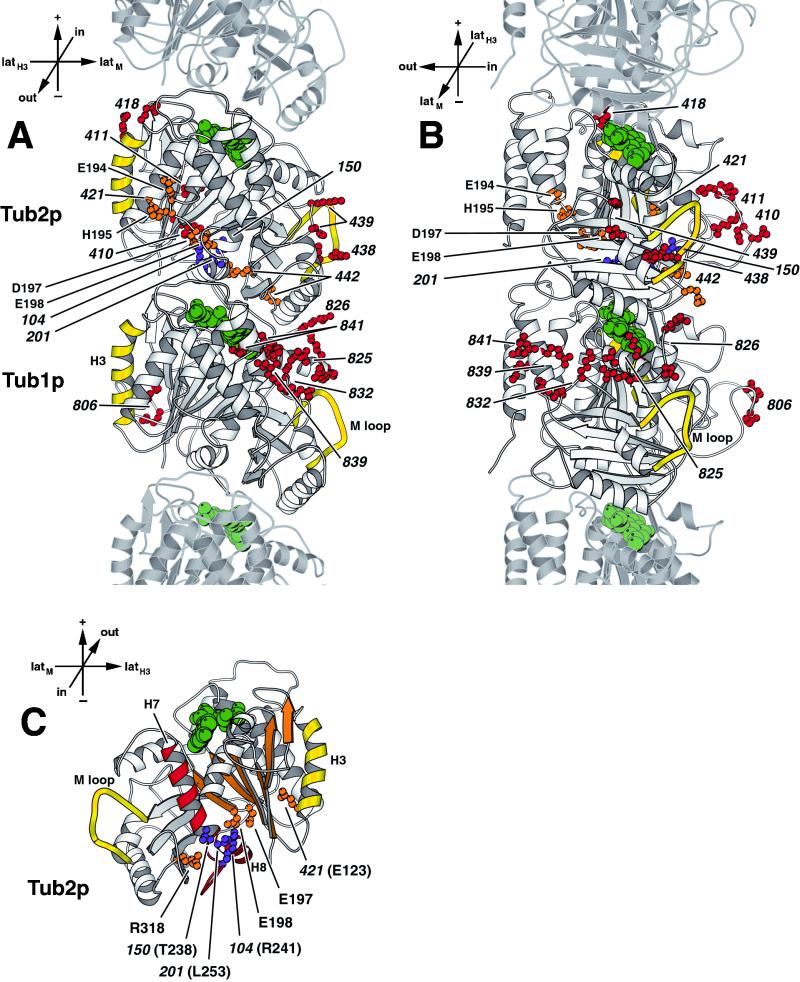
The mutations producing a benomyl-resistant phenotype were mapped onto the tubulin model. Benomyl-resistant mutations were split into two classes; five tub2 mutants were resistant to very high concentrations of benomyl (75 or 80 μg/ml), and the remainder were resistant only to lesser concentrations. In wild-type Tub2p each of the five (Figure 6, orange) contain component charged residues (123, 197, 198, and 318) in a pocket relatively deep in the tubulin structure (unusual for charged residues). This pocket, situated between the β-sheet S1–S6, helix H8 and the core helix H7, is also the site of several other highly benomyl-resistant mutations, tub2-201 (L253V), tub2-104 (R241H), and the benomyl-dependent tub2-150 (T238A) (Figure 6, purple; Thomas, 1984; Thomas et al., 1985; Machin et al., 1995). It seems possible, although we have no more direct evidence, that this pocket might be associated with either benomyl binding or a conformational response to benomyl binding. Consistent with this possibility, residue 198 is found to be changed in many benomyl-resistant mutations in the β-tubulin-encoding genes of a variety of species other than S. cerevisiae (e.g., Fujimura et al., 1992; Jung et al., 1992; Buhr and Dickman, 1994; Park et al., 1997).
Figure 6.
Location of benomyl-resistant tub1 and tub2 alleles. (A) View from the outside of the microtubule. (B) View from the side of the protofilament. (C) Cutaway view of the core of β-tubulin, with the interior-facing loop (residues 24–62) removed, β-sheet S1–S6 is shown in orange, and the core helix H7, helix H8, and the intervening T7 loop are shown in red (Nogales et al., 1998b). The side chains of amino acids mutated in alleles resistant to only 40 μg/ml benomyl (red) and alleles resistant to at least 80 μg/ml benomyl (orange) are shown on Tub2p and Tub1p of the yeast tubulin heterodimer, labeled by their allele number. The amino acids mutated in tub2 alleles 431 (E194A, H195A, and E198A), 432 (E194A and D197A), and 433 (E198A) are individually labeled by their residue name and number. The amino acids mutated in tub2 alleles 201, 104, and 150 (Thomas et al., 1985; Machin et al., 1995) are shown in purple. Lateral interaction elements, α-helix H3 and M loop, are shown in yellow (Nogales et al., 1999). GTP and GDP are shown in green. Adjacent monomers of the protofilament are shown in lighter gray. Orientation axes: + and − show microtubule orientation; latH3 and latM show lateral sides marked by helix H3 α-helix and M loop, respectively; in and out represent the inside and the outside of the microtubule, respectively. Structure drawn using MOLSCRIPT (Kraulis, 1991).
The second class of benomyl-resistant alleles includes both tub1 and tub2 alleles. Whereas these benomyl-resistant tub2 mutations are distributed across Tub2p, all of the benomyl-resistant tub1 alleles except one cluster to a face of Tub1p near the lateral protofilament contact but exposed to the outer surface of the microtubule. Because an increased level of α-tubulin can cause benomyl resistance (Schatz et al., 1986b), it is a formal possibility that these mutations might increase the quantity of functional α-tubulin. Alternatively, they might disrupt the binding of a protein that acts to destabilize microtubules. For example, deletion of the DYN1 or KIP1 genes encoding microtubule motor proteins results in benomyl resistance and increased microtubule length (Cottingham and Hoyt, 1997). Interestingly, several benomyl-resistant mutations in both tub1 and tub2 map to loops that would be in the interior of an assembled microtubule (Figure 6, see tub1-806 and tub2-410 and -411).
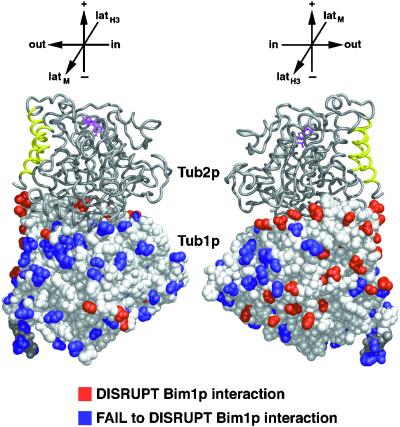
Residues Affecting Bim1p Binding
We previously described a microtubule-binding protein, Bim1p, and demonstrated that it bound to Tub1p in the two-hybrid system and decorated microtubules in vivo. All of the tub1 Ala-scan alleles were tested for binding to Bim1p in the two-hybrid system, and tub1 mutations that disrupted binding were found to cluster at the C terminus of Tub1p (Schwartz et al., 1997). The tub1 mutations that disrupt Bim1p binding are mapped on the structure of α-tubulin in Figure 7. Most of these mutations cluster in a region facing outward in the assembled microtubule; however, there are several (tub1-809, -813, -842, and -846) that were located at the intradimer interface. Perhaps an α–β dimer must be formed to achieve a positive two-hybrid interaction with Bim1p. Two-hybrid interactions were not tested between Bim1p and the Tub2p Ala-scan mutants, because the ectopic expression of Tub2p from these plasmids was lethal, as expected (Burke et al., 1989; Weinstein and Solomon, 1990).
Figure 7.
Two-hybrid interaction footprint of Bim1p on Tub1p. Bim1p was tested for interaction with each of the Tub1p mutant proteins using the two-hybrid system. Those tub1 mutations that abolished the two-hybrid interaction are shown in red; those tub1 mutations that still interacted are shown in blue (Schwartz et al., 1997). Structure drawn using MIDAS (Ferrin et al., 1988).
A similar approach has been used to delineate the interaction footprint of another Tub1p-binding protein, Alf1p (Feierbach et al., 1999), which lies at a site overlapping but not identical to the Bim1p footprint at the C terminus of Tub1p, on the outside face of the microtubule. Motor proteins, such as dynein and kinesin, have also been shown to bind β-tubulin, also at the C terminus (Goldsmith et al., 1995; Hirose et al., 1995; Hoenger et al., 1995).
Double Mutant Analysis
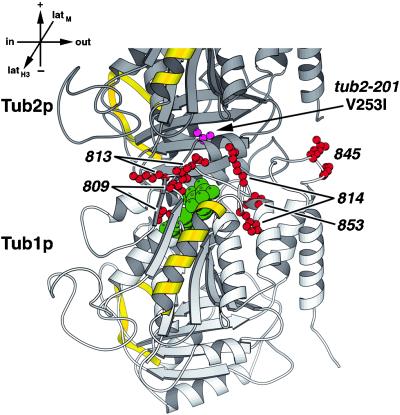
Another approach to examine interactions between two proteins is by differential genetic interactions. We tested the genetic interactions between each of the tub1 Ala-scan mutants and a benomyl-resistant mutant, tub2-201. The tub2-201 mutation confers a high level of benomyl resistance but no other discernible phenotypes (Thomas, 1984). When sequenced, tub2-201 contained a Leu to Val change at position 253, which maps to the interior side of helix H8 at the intradimer interface (see Figures 6 and 8). DBY7830, which contains tub2-201 back-crossed into the DBY6654 strain background (Schwartz et al., 1997), was crossed to each of the tub1 Ala-scan mutants. The double mutants displayed a range of phenotypes, from wild-type growth to synthetic lethality. On media containing benomyl, the double mutants generally showed additive effects; i.e., they displayed the benomyl resistance of tub2-201; but somewhat diminished commensurate with the degree of benomyl sensitivity of the single tub1 mutant. The tub1 mutations that were synthetically lethal in combination with tub2-201 are shown in Figure 8. These tub1 alleles (-809, -813, -814, -845, and -853) cluster at the intradimer interface. This suggests that tub2-201, although nearly wild-type in phenotype, may cause a distortion of its intradimer interface. When tub2-201 is combined with tub1 mutations at the intradimer interface, the α–β dimer interaction may become so unstable as to cause death. In support of this hypothesis, tub1-814 contains a mutant residue 106 that is known to be important for stabilizing the intradimer interaction in yeast (Vega et al., 1998).
Figure 8.
tub1 alleles that are synthetically lethal with tub2-201 localize to the intradimer interface. Amino acids mutated in these tub1 alleles (red) are shown on Tub1p (bottom). Tub2p is partly shown above with the tub2-201 mutant residue shown in magenta. Lateral interaction elements, α-helix H3 and M loop, are shown in yellow (Nogales et al., 1999). GTP is shown in green. The view is from the side of the heterodimer. Orientation axes: + and − show microtubule orientation; latH3 and latM show lateral sides marked by helix H3 and M loop, respectively; in and out represent the inside and the outside of the microtubule, respectively. Structure drawn using MOLSCRIPT (Kraulis, 1991).
Conclusion
The TUB1 mutations described here complete the systematic mutagenesis of the genes encoding the major subunits of the yeast cytoskeleton. Although there are two α-tubulin-encoding genes, TUB1 and TUB3 are so similar that it is likely that it will be possible to study virtually all structure–function relationships with associated proteins using the mutations already in hand. Mapping of the mutations to the surface will provide, for tubulin, genetic tools for structure–function analysis analogous to those that have served so well in the case of actin, in which mutations derived from a similar systematic mutagenesis scheme have served to connect residues on the actin three-dimensional structure to binding regions for a great variety of ligands. To illustrate this, the new tub1 mutants described here have allowed us to understand better the interaction of α- with β-tubulin and α-tubulin with Bim1p and to suggest the location of the benomyl-binding site by placing tub2 mutations on the structure.
Supplementary Material
ACKNOWLEDGMENTS
We thank Charles Scafe for assistance with molecular modeling and Tim Stearns and Becket Feierbach for helpful discussions. This work was supported by a grant from the National Institutes of Health (GM-46406) to D.B. and a National Institutes of Health Postdoctoral Fellowship to K.R.A.
Footnotes
A complete data set for this article is available at www.molbiolcell.org.
REFERENCES
- Amberg DC, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. Nat Struct Biol. 1995;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Arce CA, Hallak ME, Rodriguez JA, Barra HS, Caputto R. Capability of tubulin and microtubules to incorporate and to release tyrosine and phenylalanine and the effect of the incorporation of these amino acids on tubulin assembly. J Neurochem. 1978;31:205–210. doi: 10.1111/j.1471-4159.1978.tb12449.x. [DOI] [PubMed] [Google Scholar]
- Argarana CE, Barra HS, Caputto R. Release of [14C]tyrosine from tubulinyl-[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol Cell Biochem. 1978;19:17–21. doi: 10.1007/BF00231230. [DOI] [PubMed] [Google Scholar]
- Bai C, Elledge SJ. Gene identification using the yeast two-hybrid system. Methods Enzymol. 1996;273:331–347. doi: 10.1016/s0076-6879(96)73029-x. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991;19:2241–2246. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra HS, Arce CA, Argarana CE. Posttranslational tyrosination/detyrosination of tubulin. Mol Neurobiol. 1988;2:133–153. doi: 10.1007/BF02935343. [DOI] [PubMed] [Google Scholar]
- Bass SH, Mulkerrin MG, Wells JA. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci USA. 1991;88:4498–4502. doi: 10.1073/pnas.88.10.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DM, Guarente L. High-efficiency transformation of yeast by electroporation. In: Guthrie C, Fink GR, editors. Methods in Enzymology, Guide to Yeast Genetics and Molecular Biology. Vol. 194. San Diego: Academic Press; 1991. pp. 182–187. [DOI] [PubMed] [Google Scholar]
- Bennett WF, Paoni NF, Keyt BA, Botstein D, Jones AJS, Presta L, Wurm FM, Zoller MJ. High resolution analysis of functional determinants on human tissue-type plasminogen activator. J Biol Chem. 1991;266:5191–5201. [PubMed] [Google Scholar]
- Bowman S, et al. The nucleotide sequence of Saccharomyces cerevisiae chromosome XIII. Nature. 1997;387:90–93. [PubMed] [Google Scholar]
- Buhr TL, Dickman MB. Isolation, characterization, and expression of a second beta-tubulin-encoding gene from Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol. 1994;60:4155–4159. doi: 10.1128/aem.60.11.4155-4159.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gasdaska P, Hartwell L. Dominant effects of tubulin overexpression in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:1049–1059. doi: 10.1128/mcb.9.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RG. Alpha-, beta-, and gamma-tubulins: sequence comparisons and structural constraints. Cell Motil Cytoskeleton. 1991;20:181–189. doi: 10.1002/cm.970200302. [DOI] [PubMed] [Google Scholar]
- Cali BM, Doyle TC, Botstein D, Fink GR. Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1873–1889. doi: 10.1091/mbc.9.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Joshi HC, Murphy DB. Tubulin site interpretation. Nature. 1990;344:389. doi: 10.1038/344389a0. [DOI] [PubMed] [Google Scholar]
- Cottingham FR, Hoyt MA. Mitotic spindle positioning in Saccharomyces cerevisiae is accomplished by antagonistically acting microtubule motor proteins. J Cell Biol. 1997;138:1041–1053. doi: 10.1083/jcb.138.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B, Nogales E, Downing KH, Stearns T. Alf1p, a CLIP-170 domain-containing protein, is functionally and physically associated with alpha-tubulin. J Cell Biol. 1999;144:113–124. doi: 10.1083/jcb.144.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrin TE, Huang CC, Jarvis LE, Langridge R. The MIDAS Display System. J Mol Graph. 1988;6:13–37. [Google Scholar]
- Fujimura M, Oeda K, Inoue H, Kato T. A single amino-acid substitution in the beta-tubulin gene of Neurospora confers both carbendazim resistance and diethofencarb sensitivity. Curr Genet. 1992;21:399–404. doi: 10.1007/BF00351701. [DOI] [PubMed] [Google Scholar]
- Gibbs CS, Zoller MJ. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991;266:8923–8931. [PubMed] [Google Scholar]
- Goldsmith M, Yarbrough L, van der Kooy D. Mechanics of motility: distinct dynein binding domains on alpha- and beta-tubulin. Biochem Cell Biol. 1995;73:665–671. doi: 10.1139/o95-074. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Hirose K, Lockhart A, Cross RA, Amos LA. Nucleotide-dependent angular change in kinesin motor domain bound to tubulin. Nature. 1995;376:277–279. doi: 10.1038/376277a0. [DOI] [PubMed] [Google Scholar]
- Hoenger A, Sablin EP, Vale RD, Fletterick RJ, Milligan RA. Three-dimensional structure of a tubulin-motor-protein complex. Nature. 1995;376:271–274. doi: 10.1038/376271a0. [DOI] [PubMed] [Google Scholar]
- Huffaker TC, Thomas JH, Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988;106:1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Lee BH, Kim ST, Kim YR, Rhie GE, Baek YW, Hwang CS, Lee JS, Kang SO. d-Erythroascorbic acid is an important antioxidant molecule in Saccharomyces cerevisiae. Mol Microbiol. 1998;30:895–903. doi: 10.1046/j.1365-2958.1998.01133.x. [DOI] [PubMed] [Google Scholar]
- Hyams JS, Lloyd CW. Microtubules. In: Harford JB, editor. Modern Cell Biology. Vol. 13. New York: Wiley-Liss; 1994. [Google Scholar]
- Jacobs CW, Adams AE, Szaniszlo PJ, Pringle JR. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1988;107:1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JS, Prakash L. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast. 1990;6:363–366. doi: 10.1002/yea.320060502. [DOI] [PubMed] [Google Scholar]
- Jung MK, Wilder IB, Oakley BR. Amino acid alterations in the benA (beta-tubulin) gene of Aspergillus nidulans that confer benomyl resistance. Cell Motil Cytoskeleton. 1992;22:170–174. doi: 10.1002/cm.970220304. [DOI] [PubMed] [Google Scholar]
- Kassir Y, Simchen G. Monitoring meiosis and sporulation in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:94–110. doi: 10.1016/0076-6879(91)94009-2. [DOI] [PubMed] [Google Scholar]
- Kraulis PJ. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lappalainen P, Fedorov EV, Fedorov AA, Almo SC, Drubin DG. Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 1997;16:5520–5530. doi: 10.1093/emboj/16.18.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M, Seehaus T. Comparative analysis of tubulin sequences. Comp Biochem Physiol. 1988;90:655–670. doi: 10.1016/0305-0491(88)90320-3. [DOI] [PubMed] [Google Scholar]
- Machin NA, Lee JM, Barnes G. Microtubule stability in budding yeast: characterization and dosage suppression of a benomyl-dependent tubulin mutant. Mol Biol Cell. 1995;6:1241–1259. doi: 10.1091/mbc.6.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Doyle TC, Bobkova E, Botstein D, Reisler E. Mutational analysis of the role of hydrophobic residues in the 338–348 helix on actin in actomyosin interactions. Biochemistry. 1996;35:3670–3676. doi: 10.1021/bi952645v. [DOI] [PubMed] [Google Scholar]
- Neff NF, Thomas JH, Grisafi P, Botstein D. Isolation of the beta-tubulin gene from yeast and demonstration of its essential function in vivo. Cell. 1983;33:211–219. doi: 10.1016/0092-8674(83)90350-1. [DOI] [PubMed] [Google Scholar]
- Nogales E, Downing KH, Amos LA, Lowe J. Tubulin and FtsZ form a distinct family of GTPases. Nat Struct Biol. 1998a;5:451–458. doi: 10.1038/nsb0698-451. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998b;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- Park SY, Jung OJ, Chung YR, Lee CW. Isolation and characterization of a benomyl-resistant form of beta- tubulin-encoding gene from the phytopathogenic fungus Botryotinia fuckeliana. Mol Cells. 1997;7:104–109. [PubMed] [Google Scholar]
- Pasqualone D, Huffaker TC. STU1, a suppressor of a beta-tubulin mutation, encodes a novel and essential component of the yeast mitotic spindle. J Cell Biol. 1994;127:1973–1984. doi: 10.1083/jcb.127.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijo RA, Cooper EM, Beagle GJ, Huffaker TC. Systematic mutational analysis of the yeast beta-tubulin gene. Mol Biol Cell. 1994;5:29–43. doi: 10.1091/mbc.5.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sayle RA, Milner-White EJ. RASMOL: biomolecular graphics for all. Trends Biochem Sci. 1995;20:374. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]
- Schatz PJ, Pillus L, Grisafi P, Solomon F, Botstein D. Two functional alpha-tubulin genes of the yeast Saccharomyces cerevisiae encode divergent proteins. Mol Cell Biol. 1986a;6:3711–3721. doi: 10.1128/mcb.6.11.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz PJ, Solomon F, Botstein D. Genetically essential and nonessential alpha-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol. 1986b;6:3722–3733. doi: 10.1128/mcb.6.11.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz PJ, Solomon F, Botstein D. Isolation and characterization of conditional-lethal mutations in the TUB1 alpha-tubulin gene of the yeast Saccharomyces cerevisiae. Genetics. 1988;120:681–695. doi: 10.1093/genetics/120.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Richards K, Botstein D. BIM1 encodes a microtubule-binding protein in yeast. Mol Biol Cell. 1997;8:2677–2691. doi: 10.1091/mbc.8.12.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G, Rosenberry TL, Sternlicht H. Identification of lysine residues essential for microtubule assembly. Demonstration of enhanced reactivity during reductive methylation. J Biol Chem. 1983;258:2148–2156. [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz J, Yaffe MB, Elzinga M, Blank GS, Sternlicht H. Microtubule assembly is dependent on a cluster of basic residues in alpha-tubulin. Biochemistry. 1986;25:4572–4582. doi: 10.1021/bi00364a018. [DOI] [PubMed] [Google Scholar]
- Thomas JH. Genes Controlling the Mitotic Spindle and Chromosome Segregation in Yeast. Ph.D. Thesis. Cambridge, MA: Massachusetts Institute of Technology; 1984. [Google Scholar]
- Thomas JH, Neff NF, Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985;111:715–734. doi: 10.1093/genetics/111.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega LR, Fleming J, Solomon F. An alpha-tubulin mutant destabilizes the heterodimer: phenotypic consequences and interactions with tubulin-binding proteins. Mol Biol Cell. 1998;9:2349–2360. doi: 10.1091/mbc.9.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein B, Solomon F. Phenotypic consequences of tubulin overproduction in Saccharomyces cerevisiae: differences between alpha-tubulin and beta-tubulin. Mol Cell Biol. 1990;10:5295–5304. doi: 10.1128/mcb.10.10.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman KF, Drubin DG, Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.