Abstract
GAS41 is a common subunit of the TIP60 and SRCAP complexes and is essential for cell growth and viability. Here, we report that GAS41 is required for repression of the p53 tumor suppressor pathway during normal cellular proliferation. Either GAS41 small interfering RNA-mediated knockdown of GAS41 expression or specific interruptions of the carboxy-terminal coiled-coil motif of the GAS41 protein activate the p53 tumor suppressor pathway, as evidenced by p53 up-regulation, p53 serine-15 phosphorylation, and p21 transcriptional activation. Activation of the p53 pathway does not result from changes in TIP60 complex assembly or TIP60 coactivator functions for p53, since a TIP60 complex containing a coiled-coil mutant of GAS41 retains the same composition and histone acetyltransferase activity as its wild-type counterpart and since mutant GAS41 does not compromise ectopic p53-dependent transcriptional activation in a reporter gene assay. Finally, we demonstrate that GAS41 is prebound to the promoters of two p53 tumor suppressor pathway genes (p21 and p14ARF) in normal unstressed cells but is dissociated from both promoters in response to stress signals that activate p53. Our data suggest that GAS41 plays a role in repressing the p53 tumor suppressor pathway during the normal cell cycle by a TIP60-independent mechanism.
Epigenetic mechanisms are now widely accepted as important regulators of gene expression (9, 19). Eucaryotic DNA is packaged into a chromatin structure that consists of arrays of core histone-containing nucleosomes organized, along with linker histone H1, into higher-order structures. Tightly packed nucleosomes generate an inherently repressive structure for gene expression by restricting access of various transcription factors and the general transcription machinery. Moreover, DNA modifications, such as CpG island methylation, and histone tail modifications, such as acetylation, methylation, phosphorylation, and ubiquitylation, allow complex epigenetic controls of nuclear activities that include DNA replication, transcription, DNA repair, and recombination (41).
The increasingly large group of chromatin-modifying complexes contains enzymatic activities that covalently modify histone tails. The enzymatic subunits of complexes with histone acetyltransferase (HAT) activity include the H3 HAT GCN5 in Saccharomyces cerevisiae SAGA and human STAGA complexes and the H4 HAT Esa1 in the yeast NuA4 complex (1, 22, 35). In humans, a NuA4-like complex with TIP60 as the H4 HAT has been described, although there is also a similar complex that lacks HAT activity but shares the TRRAP, p400, Epc1, TIP49, TIP48, BAF53, and β-actin components (21, 24). It is not clear whether the last complex has a distinct function or whether it represents an intermediate in the assembly of the TIP60-containing complex. The TIP60 complex was proposed to play a role in DNA repair and apoptosis, and its largest subunit, TRRAP, has been implicated in p53-dependent mdm2 transcriptional activation (3, 24, 27). TIP60 also has been proposed as an essential coactivator for p53-dependent transcriptional activation based on studies involving RNA interference-mediated knockdown of TIP60 or overexpression of a dominant-negative TIP60 mutant (6, 16, 29).
GAS41 was originally identified in the glioblastoma multiforme cell line and implicated in early gliomal tumor development (18). GAS41 has an N-terminal tf2f domain that is conserved in YEATS family members (18, 30). This family includes yeast Yaf9, TAF14, and SAS5, as well as proteins (ENL and AF9) implicated in human cancer. All of these proteins are involved in transcriptional regulation through multisubunit complexes. GAS41 is essential for cell survival and growth and is found as a subunit of the human TIP60 (16, 23, 49) and SRCAP (7) complexes. Yaf9, a yeast ortholog of GAS41, is a common subunit of both the yeast NuA4 HAT complex and the yeast SWR1 chromatin-modifying complex and is required for resistance to UV, DNA-damaging agents, and spindle stress in yeast (30, 48).
An apparent structural and functional conservation between the NuA4 and human TIP60 complexes prompted us to investigate the functional role of GAS41 in p53-dependent transcriptional activation during DNA damage responses. Here, we report that loss of GAS41 function induces up-regulation of two tumor suppressor proteins, p14ARF and p53. Up-regulation of p53 and accompanying serine-15 phosphorylation are induced by mutations in the coiled-coil domain in the GAS41 C terminus and are sufficient to activate p21 gene expression. GAS41 is bound to the promoters of the repressed p14ARF and p21 genes in normal cells, and its dissociation correlates with gene activation. We also provide evidence that the normal function of GAS41 in down-regulation of p14ARF and p21 genes is independent of the TIP60 HAT and coactivator activities. We suggest that GAS41 may be involved in repression of the p53 tumor suppressor pathway during the normal cell cycle in order to prevent aberrant activation of growth inhibitory genes, such as p14ARF and p21.
MATERIALS AND METHODS
Expression vectors, antibodies, and PCR primers.
A mammalian expression plasmid, CbF, driven by a cytomegalovirus promoter was used to express GAS41 in both transient and stably transfected cells. Histidine-tagged fusion proteins of full-length TIP60 and GAS41 were purified on Ni-nitrilotriacetic acid agarose and used to raise rabbit polyclonal antibodies. Affinity-purified antibodies to TIP60 and GAS41 were generated by the AminoLink Plus immobilization kit (Pierce) and used for immunoblotting and chromatin immunoprecipitation (ChIP) assays. Antibodies against TRRAP, TIP49/RUVBL1, and BAF53 were described previously (36, 39, 46). Anti-FLAG antibodies and M2 agarose conjugates were obtained from Sigma. PCR site-directed mutagenesis was conducted using a mutagenesis primer set and DpnI restriction enzyme (12). Mutations were verified by DNA sequencing. Details of individual primer sets and PCR conditions are available upon request. Semiquantitative reverse transcription (RT)-PCR analysis was conducted using 5′-GAACTTCGACTTTGTCACCGAGAC and 3′-TGGAGTGGTAGAAATCTGTCATGCT (p21) primers, 5′-TCTTGGTGACCCTCCGGATT and 3′-CTCCTCAGTAGCATCAGCACGAG (p14ARF) primers, and 5′-CTCAGACACCATGGGGAAGGTGA and 3′-ATGATCTTGAGGCTGTTGTCATA (GAPDH [glyceraldeyhyde-3-phosphate dehydrogenase]) primers. ChIP assays utilized 5′-CAGGCTGTGGCTCTGATTGG and 3′-CCTTCCTCCC TGAAAACAGGC primers for the distal p53 binding region of the p21 promoter, 5′-TCAGAGCCGTTCCGAGATCTT and 3′-CTTAACTGCAGACTGGGACCC primers for the E2F binding region of the p14ARF promoter, and 5′-ATCTTCCTCCCACAGCTCCT and 3′-TTTGCAGCCTCACCTTCTTT primers for the human β-globin gene control.
Cell culture, transfection, and immunoprecipitation.
Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. HEK293T cells were transfected using calcium phosphate and lysed in 0.1% Triton F lysis buffer (39). Cell lysates were immunoprecipitated with anti-FLAG M2 agarose (Sigma) or appropriate antibodies in conjunction with protein A beads. Immunoprecipitates were eluted with either sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer (for denaturation conditions) or an excess of FLAG peptide (for native elution). The immunoprecipitates were then analyzed by immunoblotting with enhanced chemiluminescence detection (Amersham).
Sepharose CL6B gel filtration chromatography and affinity purification.
Nuclear extracts were prepared by a modified Dignam procedure (14) from 293T cells that stably express FLAG-GAS41 or a FLAG-GAS41 mutant and directly applied to a Sepharose CL6B gel filtration column equilibrated with BC200 buffer containing 0.1% NP-40. Fractions corresponding to the TRRAP peak, as determined by immunoblotting, were combined and subjected to M2 agarose affinity purification. After being serially washed with BC300 and BC500 buffer containing 0.1% NP-40, captured FLAG-GAS41 was eluted by the addition of 250 μg/ml of FLAG peptide for 24 h at 4°C.
HAT assay.
HeLa core histones were purified as described previously (45). In vitro chromatin assembly was done as described previously (2). HAT reactions using HeLa core histones (1 μg) or in vitro-assembled nucleosomes (0.3 μg) were carried out as described previously (39).
siRNA design and transfection.
Nontargeting control and GAS41 small interfering RNAs (siRNAs) were purchased from Dharmacon (Lafayett, CO). Sense (CCAAUAGUUUACGGUAAUGUU) and antisense (CAUUACCGUAAACUAUUGGUU) oligonucleotides for GAS41 siRNA were annealed in the reconstitution buffer (20 mM KCl, 6 mM HEPES-KOH, pH 7.5, 0.2 mM MgCl2), and a final concentration of 100 nM siRNA was used in oligofectamin-mediated transfection of U2OS cells. After 48 h, the medium was changed and cells were analyzed for down-regulation of GAS41.
RT-PCR and luciferase expression analysis.
Total RNA was isolated with the RNeasy mini kit (QIAGEN), and 200 to 500 ng of total RNA was used for semiquantitative RT-PCR by the Superscript III one-step RT-PCR kit (Invitrogen). Outputs of two different PCR cycle numbers were analyzed to ensure that amplification was in the linear range. For luciferase assays, H1299 cells were cotransfected with pWWP-Luc (37), control plasmid pRL-CMV, pVP-p53, and plasmids expressing GAS41 and subsequently analyzed by the Dual-Luciferase reporter assay system (Promega).
Retroviral infection.
Retroviral infection of IMR-90 cells was described previously (40). Amphotropic PhoeNX cells were transfected with LXSH plasmid using calcium phosphate, and resultant retroviral supernatants were used to infect IMR90 cells. Transduced cells were selected for resistance to hygromycin (150 μg/ml) for 7 days and then subjected to RT-PCR and ChIP assays.
ChIP assay.
Chromatin immunoprecipitation assays were conducted as described previously (38). Briefly, 107 cells were cross-linked by the direct addition of formaldehyde (final concentration, 1%) to cells for 10 min at room temperature. Fixed cells were harvested and washed twice with swelling buffer (5 mM PIPES [piperazine-N-N′-bis(2-ethanesulfonic acid)], pH 8.0, 85 mM KCl, 0.5% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, and 100 ng of leupeptin and aprotinin per ml). Collected nuclei were resuspended in sonication buffer (0.2% sodium dodecyl sulfate in F buffer, 0.5 mM phenylmethylsulfonyl fluoride, 100 ng of leupeptin and aprotinin per ml) and sonicated on ice to obtain ∼500-bp DNA fragments. Lysates were normalized and diluted with F buffer. Normalized chromatin lysates were incubated overnight with antibody and then with 20 μl of protein A beads for an additional 1 h at 4°C. Beads were washed and eluted, and following cross-link reversal, eluted material was subjected to semiquantitative PCR amplification.
RESULTS
Human GAS41 is a genuine subunit of the TIP60 and SRCAP complexes.
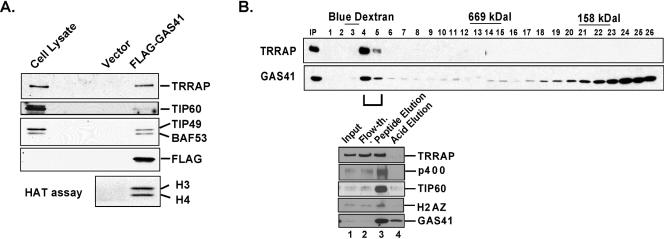
Based on the apparent structural and functional similarities between the human TIP60 and yeast NuA4 complexes, we investigated the functional role of GAS41 in the DNA damage response and p53 tumor suppressor pathway. To confirm that GAS41 is a stable component of the human TIP60 complex under our isolation conditions, interactions between the core subunits of the TIP60 complex and GAS41 were examined both in vitro and in vivo (Fig. 1). FLAG-tagged GAS41 was transiently expressed in 293T cells and immunoprecipitated with M2 agarose (Fig. 1A). The complex containing FLAG-tagged GAS41 was eluted with a FLAG peptide and subjected both to immunoblotting and to a HAT assay with HeLa core histones as a substrate. Known TIP60 complex core subunits TRRAP, TIP60, TIP49, and BAF53 were coimmunoprecipitated with GAS41, and the immunoprecipitate displayed a typical TIP60-mediated H3 and H4 acetyltransferase activity on free core histones.
FIG. 1.
GAS41 is a core subunit of the human TIP60 and SRCAP complexes. (A) Coimmunoprecipitation of GAS41 with core subunits of the TIP60 complex. 293T cells were transfected with empty vector or vector expressing FLAG-GAS41, and whole-cell lysates were immunoprecipitated with M2 agarose beads. After extensive washes, the captured FLAG-GAS41 was eluted with FLAG peptide under nondenaturing conditions. Eluates were analyzed by immunoblotting and HAT assays as indicated. Histone H2A was not separated from H3 in this minigel in which acetylated H2A partially overlapped with H3 (see Fig. 4B for better separation). (B) Sepharose CL6B gel filtration chromatography. Nuclear extracts were prepared from 293T cells stably expressing FLAG-GAS41 and directly applied to the Sepharose CL6B gel filtration column. Each fraction (0.1%) was analyzed by immunoblotting for TRRAP and GAS41. Fractions corresponding to the TRRAP peak (fractions 4 and 5) were combined and subjected to M2 agarose affinity purification. After being washed, captured FLAG-GAS41 was sequentially eluted with FLAG peptide and 0.1 M glycine (pH 2.5) and analyzed by immunoblotting for TRRAP, TIP60, p400, H2AZ, and GAS41.
In a further analysis, a FLAG-tagged GAS41 was stably expressed in 293T cells, and the size distribution of GAS41-containing complexes was analyzed by gel filtration chromatography (Fig. 1B, upper panel). TRRAP appeared exclusively in fractions corresponding to an apparent molecular mass of 2 MDa and a portion of the ectopically (over)expressed GAS41 cofractionated with TRRAP. Affinity purification of FLAG-tagged GAS41 from these fractions resulted in copurification of TRRAP, p400, and TIP60 proteins, indicative of a TIP60 complex (Fig. 1B, bottom panel, lane 3). Recently, GAS41 also has been shown to be a stable subunit of the SRCAP (SWI2/SNF2-related CBP activator protein) complex that is homologous to the yeast SWR1 chromatin-remodeling complex (7). Because the TIP60 and SRCAP complexes share several subunits that include GAS41, we employed H2AZ as an indicator of the SRCAP complex in the anti-FLAG GAS41 immunoprecipitation. Affinity purification of FLAG-tagged GAS41 from the TRRAP-containing fractions also resulted in the copurification of H2AZ (Fig. 1B, bottom panel, lane 3), confirming the previous finding that H2AZ is a stable component of the SRCAP complex. Taken together, these results, with somewhat different methodologies (based on the use of a tagged GAS41), confirm the previous identification of GAS41 as a TIP60-associated protein in the TIP60 complex (16) and as an H2AZ-associated protein in the SRCAP complex (7).
siRNA-mediated knockdown of GAS41 induces p53-dependent p21 gene expression.
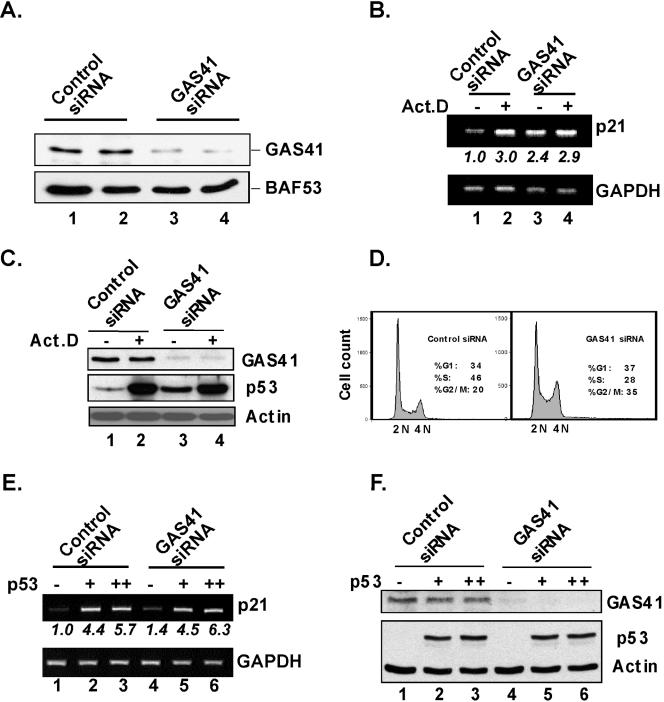
Based on the observation that a Yaf9-null yeast strain is highly susceptible to various stress conditions that include UV radiation and other DNA-damaging agents (48), we asked whether siRNA-induced knockdown of GAS41 resulted in any compromised p53-dependent transcriptional response. Transfection of U2OS cells with GAS41 siRNA consistently and significantly reduced the level of GAS41 (up to 80 percent) compared to transfection with nontargeting control siRNA (Fig. 2A). After transfection of cells with siRNA, actinomycin D-induced p21 gene expression was assessed by RT-PCR (Fig. 2B). Knockdown of GAS41 showed no significant reduction in the actinomycin D-induced p21 gene expression (Fig. 2B, lanes 2 and 4) but, instead, induced a significant up-regulation (2.4-fold) of p21 mRNA in normal U2OS cells in the absence of a DNA-damaging agent (Fig. 2B, lane 3 versus lane 1). Of note, concomitant GAS41 siRNA and actinomycin D treatment did not further induce the level of p21 expression beyond that observed with GAS41 siRNA or actinomycin D alone (Fig. 2B, lane 4 versus lanes 2 and 3). The induction of p21 by GAS41 knockdown is likely mediated by up-regulation of p53 since the knockdown also induced a significant stabilization of p53 in the absence of a DNA-damaging agent (Fig. 2C, lane 3 versus lane 1). We also determined the effect of GAS41 knockdown on cell cycle progression (Fig. 2D). The results clearly show that GAS41-depleted U2OS cells undergo growth arrest and accumulate in G2/M, confirming the essential role of GAS41 in cell proliferation (23).
FIG. 2.
Effects of GAS41 siRNA on p53-dependent p21 gene induction and cell cycle progression. (A) GAS41 siRNA-mediated GAS41 knockdown in U2OS cells. Cells were transfected in duplicate with the indicated siRNAs, and whole-cell lysates were analyzed for relative levels of GAS41 and BAF53. (B) GAS41 siRNA-mediated induction of the p21 gene in U2OS cells. Cells were transfected with the indicated siRNAs and, after 48 h, treated with actinomycin D (10 ng/ml) for 6 h as indicated. Total RNA was analyzed by RT-PCR to estimate the relative expression levels of the p21 and GAPDH mRNAs. Relative levels of p21 gene expression (normalized to GAPDH expression) are shown in italics. (C) Effects of GAS41 siRNA on the levels of GAS41 and p53 in U2OS cells. Cells were transfected with the indicated siRNAs and, after 48 h, treated with actinomycin D (10 ng/ml) for 6 h as indicated. Total cell lysates were analyzed by immunoblotting for GAS41, p53, and actin. (D) Effects of GAS41 siRNA on cell cycle progression. U2OS cells were transfected in duplicate with the indicated siRNAs for 48 h and analyzed for DNA content by fluorescence-activated cell sorter analysis. Relative distributions of cell cycle were obtained from the analysis of FlowJo software. (E) Failure of GAS41 siRNA to induce p21 gene expression in p53-deficient H1299 cells. H1299 cells were transfected with control or GAS41 siRNA and, after 24 h, with empty vector (1 μg [−]) or vector expressing p53 (1 [+] or 2 [++] μg) as indicated. Total RNA was analyzed by RT-PCR for p21 and GAPDH mRNAs. (F) Effects of GAS41 siRNA on the levels of GAS41 and ectopically expressed p53 in H1299 cells. Cells were transfected with control or GAS41 siRNA and, after 24 h, with empty vector (1 μg [−]) or vector expressing p53 (1 [+] or 2 [++] μg). After 48 h, total cell lysates were analyzed by immunoblotting for GAS41, p53, and actin.
To see whether GAS41 siRNA-mediated p21 gene induction requires the p53 tumor suppressor, p53-null H1299 cells were transfected with siRNA in the presence or absence of ectopic p53 gene expression (Fig. 2E). In contrast to what was observed in U2OS cells, GAS41 siRNA had no significant effect on basal p21 gene expression in the absence of ectopic p53 (Fig. 2E, lane 4 versus lane 1). Ectopic p53 induced p21 gene expression in these cells as expected and as a positive control, and GAS41 siRNA did not further enhance the p53-dependent p21 gene expression (Fig. 2E, lanes 5 and 6 versus lanes 2 and 3) and did not alter the levels of ectopic p53 (Fig. 2F, lanes 5 and 6 versus lanes 2 and 3). These latter results are consistent with the failure of GAS41 siRNA to further enhance the level of p21 gene expression induced by actinomycin D (through endogenous p53) in U2OS cells (Fig. 2B). Altogether, these results (Fig. 2B and E) indicate that GAS41 siRNA-induced expression of the p21 gene requires p53. They further suggest that GAS41 depletion may affect upstream events in the p53 tumor suppressor pathway, since there was no significant defect in p53-dependent activation of the p21 gene in GAS41-depleted cells in response to a DNA-damaging agent or ectopic p53 expression. Knockdown of TIP60 has been shown to selectively reduce p21 gene expression in response to ectopic p53 or DNA-damaging agents that induce p53 but not to affect p21 expression in unstressed U2OS cells (6, 29). Therefore, the effect of GAS41 knockdown, which is observed here in normal, nonstressed cells, is distinct from the effect of TIP60 knockdown and is not likely mediated by the modulation of TIP60 HAT activity.
Loss of a coiled-coil motif in the GAS41 C terminus induces activation of the p53 tumor suppressor pathway.
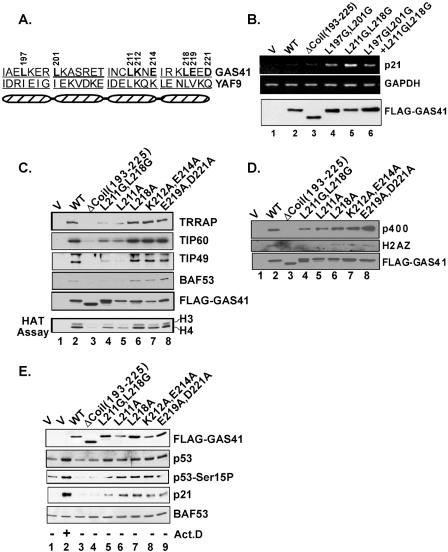
To verify the results of the GAS41 siRNA-mediated knockdown, we sought to identify a GAS41 mutant that could activate the p53 tumor suppressor pathway in a dominant-negative manner. Sequence alignment of YEATS family proteins reveals a highly conserved N-terminal YEATS domain but relatively poor C-terminal sequence conservation. GAS41 and Yaf9 are the most closely related family members with respect to size and sequence conservation (30). A heptad repeat of amino acids is defined as (abcdefg)n, with the a and d positions being predominantly hydrophobic and critical for structural integrity and the e and g positions being mainly charged amino acids that contribute to the interaction specificity (26). The sequence of the coiled-coil region in the GAS41 C terminus matched well with the heptad repeat pattern, and its functional significance was assessed by site-directed mutagenesis (Fig. 3A).
FIG. 3.
Structure-function analysis of the GAS41 coiled-coil motif. (A) Schematic diagram of four heptad repeats in the coiled-coil domain in the GAS41 and YAF9 C termini. The mutated amino acid residues are indicated by position numbers. (B) Disruption of the coiled-coil structure induces p21 gene activation. U2OS cells were transiently transfected with an empty vector (V) or a vector expressing wild-type (WT) or mutant GAS41. At 24 h posttransfection, total RNA and whole-cell extracts were analyzed by RT-PCR and immunoblotting, respectively. (C) Requirement of the GAS41 coiled-coil structure for interaction with the TIP60 complex. 293T cells were transfected with an empty vector or a vector expressing FLAG-GAS41, and whole-cell lysates were immunoprecipitated with M2 agarose beads. Eluates were analyzed by immunoblotting and HAT assays as indicated. (D) Requirement of the GAS41 coiled-coil structure for interaction with p400 and H2AZ. An experiment equivalent to that used for panel C was conducted to analyze interactions of GAS41 mutants with p400 and H2AZ by coimmunoprecipitation. (E) Activation of the p53 tumor suppressor pathway both by GAS41 mutants that are defective in TIP60 and SRCAP complex assembly and by GAS41 mutants that are not. U2OS cells were transiently transfected with the same set of GAS41 mutant vectors as those shown in panel C, and whole-cell lysates were analyzed by immunoblotting as indicated. Cells transfected with an empty vector followed by actinomycin D treatment (10 ng/ml) for 6 h were used as a positive control for activation of the p53 pathway. An immunoblot against BAF53 was used as a loading control.
Deletion of the coiled-coil motif (ΔCoil) did not result in any significant induction of the p21 gene by the mutant GAS41 relative to wild-type GAS41 (Fig. 3B, lane 3 versus lanes 1 and 2) but completely abolished its ability to be incorporated into the TIP60 complex (Fig. 3C, lane 3 versus lane 2). This is consistent with previous studies showing that the Yaf9 C terminus is critical for assembly into the yeast NuA4 complex (48). Interestingly, expression of GAS41 mutants (L197G/L201G and L211G/L218G) with critical heptad leucines changed to glycines induced p21 gene activation (Fig. 3B, lanes 4 and 5 versus lane 1), as was observed in the GAS41 siRNA-mediated knockdown experiment discussed above. In addition, these same alterations of critical heptad leucine residues in these coiled-coil motifs significantly reduced incorporation of GAS41 into a stable TIP60 complex and, consequently, the level of associated HAT activity (Fig. 3C, lane 4 versus lane 2; data not shown for the L197G/L201G mutant). Taken together, these results confirm that a loss of function of GAS41 either by siRNA or by a dominant-negative mutation results in p21 gene activation.
We further analyzed additional mutations of the third and fourth heptad repeats that led to a prominent p21 gene induction by GAS41 to see whether this relates to changes in TIP60 complex assembly or the associated HAT activity. After expression and immunoprecipitation of FLAG-tagged wild-type and mutant GAS41 proteins, the anti-FLAG immunoprecipitates were analyzed for core subunits of the TIP60 complex. The results show that alteration of a single leucine residue at position 211 of the third coil motif is enough to produce a significant reduction of interactions with other components of the TIP60 complex (Fig. 3C, lane 5 versus lane 2). On the other hand, GAS41 mutants bearing a point mutation at the critical leucine residue of the fourth coil (L218A) or at charged residues of the third or fourth coil (K212A/E214A or E219A/D221A) showed normal levels of TIP60 complex assembly and associated TIP60 HAT activity (Fig. 3C, lanes 6 to 8 versus lane 2).
Because GAS41 is also a common subunit of p400 and SRCAP complexes, we attempted to analyze the effects of GAS41 mutations on the respective complexes by analyzing coimmunoprecipitation with p400 and H2AZ (Fig. 3D). As observed for TIP60 complex assembly in Fig. 3C, the coiled-coil region of GAS41 is also required for interactions with p400 and H2AZ (Fig. 3D, lane 3 versus lane 2). The impact of individual GAS41 point mutations on incorporation into the SRCAP complex is similar to that observed for the TIP60 complex (Fig. 3D, lanes 4 to 8 versus 3C, lanes 4 to 8), suggesting that the subunit(s) that directly interacts with GAS41 is shared in the TIP60 and SRCAP complexes.
To investigate the functional significance of these coiled-coil mutations, corresponding GAS41 mutants were transiently expressed in U2OS cells and examined for their ability to activate p53 and to induce p21 (Fig. 3E). The ΔCoil mutant, which showed no incorporation into the TIP60 and SRCAP complexes, produced no significant effects on p53 activation or (as shown above) on p21 induction (Fig. 3E, lane 4 versus lane 1). In contrast, all of the other tested coiled-coil mutants (L211A, L218A, L211G/L218G, K212A/E214A, and E219A/D221A) were capable of activating the p53 tumor suppressor pathway, as evidenced by p53 up-regulation, p53 serine-15 phosphorylation, and p21 induction (Fig. 3E, lanes 5 to 9 versus lane 1). These results suggest that GAS41 defects leading to the activation of p53 do not depend upon an inability of GAS41 mutants to be incorporated into the TIP60 or SRCAP complex or to an alteration of associated TIP60 HAT activity.
Altogether, the results presented in Fig. 3 show p53 and p21 activation both by GAS41 mutants that do get incorporated into the TIP60 and SRCAP complexes and by GAS41 mutants that do not. This GAS41 mutant-mediated activation of p53 and p21 in unstressed cells indicates that wild-type GAS41 may play a repressive role at a point upstream of the p53 tumor suppressor pathway in unstressed proliferating cells.
A coiled-coil GAS41 (K212A/E214A) mutant that activates the p53 pathway shows normal levels of complex assembly and associated HAT activity.
To investigate in more detail the mechanism by which GAS41 mutants activate the p53 tumor suppressor pathway in unstressed cells, we analyzed the GAS41 (K212A/E214A) mutant for more subtle defects in complex assembly, TIP60 HAT activity, and p53-dependent transcriptional activation, since this mutant showed no obvious defects in association with known components of the TIP60 and SRCAP complexes and associated HAT activity in the previous analysis.
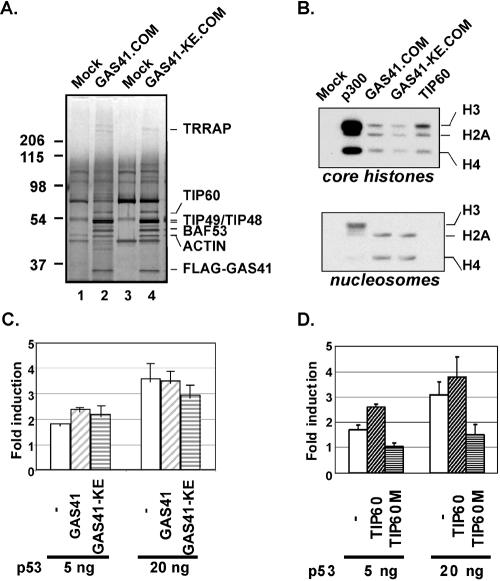
To determine whether the purified complexes containing this GAS41 mutant show any difference in subunit composition relative to the wild-type GAS41 complex, complexes were purified by a two-step procedure involving Sepharose CL6B gel filtration and M2 agarose affinity chromatography and analyzed by silver staining (Fig. 4A). Gel filtration showed that the GAS41 mutant eluted coincidentally with TRRAP, as observed for wild-type GAS41 (Fig. 1B and data not shown). The apparent polypeptide composition of the wild-type GAS41 complex was similar to that observed in the human TIP60 complex purified through a triple-affinity-tagged DMAP1 subunit (16), with typical dense doublet bands of TIP49 and TIP48 along with TRRAP, TIP60, BAF53, and actin. Consistent with the immunoblot analysis results shown in Fig. 3C, the polypeptide patterns of wild-type and mutant GAS41 complexes were the same (Fig. 4A, lane 4 versus lane 2), confirming that incorporation of the GAS41 mutant into the TIP60 and SRCAP complexes is normal.
FIG. 4.
Biochemical analysis of a GAS41 mutant-containing TIP60 complex. (A) Two-step purification of the GAS41-containing TIP60 complex. Nuclear extracts of 293T cells stably expressing FLAG-GAS41 or the FLAG-GAS41 (K212A/E214A) mutant were first fractionated by Sepharose CL6B gel filtration. Fractions corresponding to the TRRAP peak were combined and subjected to M2 agarose affinity purification. After being washed, captured FLAG-GAS41 was eluted with FLAG peptide and analyzed by silver staining. A mock purification was also conducted with a parental 293T cell extract using the same procedures. (B) HAT activities of the GAS41 mutant-containing TIP60 complex on free core histones and nucleosomes. Equivalent amounts of purified complexes containing GAS41 or the GAS41 (K212A/E214A) mutant proteins (A) were assayed for HAT activity using HeLa core histones or in vitro-assembled chromatin. Recombinant p300 (from baculovirus) and TIP60 (from Escherichia coli) were used as controls. (C) Effects of a GAS41 mutant on p53-dependent transcriptional activation. H1299 cells were transfected with pVP-p53 (5 ng or 20 ng), a p21 promoter-driven luciferase plasmid, and 200 ng of empty vector (−) or vector expressing wild-type GAS41 or the GAS41 (K212A/E214A) mutant. Inductions (n-fold) were calculated by dividing the normalized p53-dependent luciferase activity by the basal activity observed in the absence of p53 expression. The data presented are average values from triplicate samples. (D) Effects of a catalytically null TIP60 mutant on p53-dependent transcriptional activation. H1299 cells were transfected with pVP-p53 (5 ng or 20 ng), a p21 promoter-driven luciferase plasmid, and 200 ng of empty vector (−) or vector expressing wild-type TIP60 or the TIP60M (Q377E) mutant. Inductions (n-fold) were calculated by dividing the normalized p53-dependent luciferase activity by the basal activity observed in the absence of p53 expression. The data presented are average values from triplicate samples.
Monomeric TIP60 has a strong HAT activity with free core histone substrates, whereas incorporation into the TIP60 complex is necessary for acetylation of histones H2A and H4 in a nucleosomal substrate (24). To determine whether the GAS41 (K212A/E214A) mutant results in any defect in acetylation of nucleosomal substrates, TIP60 complexes containing wild-type versus GAS41 mutant protein were tested for their ability to acetylate histones in an in vitro-assembled chromatin (Fig. 4B). Both the recombinant TIP60 alone and the GAS41-containing TIP60 complex showed a robust HAT activity with free core histones, but only the complex displayed an ability to acetylate the nucleosomal substrate. Moreover, there was no significant difference between wild-type GAS41 and the mutant GAS41-containing complexes with respect to either free histone or nucleosomal HAT activity. The result is consistent with the observation that Yaf9 is not required either for NuA4 HAT activity or for substrate specificity (48).
Previous studies showed that TIP60 HAT activity is essential for p53-dependent p21 gene activation (6, 16). To determine whether the GAS41 mutant-containing TIP60 complex has any compromised coactivator activity in p53-dependent transcriptional activation, H1299 cells were transfected with a p21 promoter-driven luciferase reporter and vectors expressing either wild-type or mutant GAS41. Overexpression of wild-type GAS41 or the K212A/E214A mutant did not show any stimulatory or inhibitory effect on ectopic p53-dependent p21 gene activation (Fig. 4C). In contrast, overexpression of a catalytically null TIP60 mutant significantly compromised p53-dependent p21 gene activation in a p21 promoter-driven luciferase assay (Fig. 4D). The above results on p21 gene activation by loss of GAS41 function (Fig. 2) also indicate that the dominant-negative GAS41 mutant that activates the p53 tumor suppressor pathway does not counteract p53-dependent transcriptional activation. Taken together, these results suggest that GAS41 does not act as a typical p53 coactivator within the context of the TIP60 complex.
Activation of the p53 tumor suppressor pathway involves dissociation of GAS41 from the p21 and p14ARF promoters.
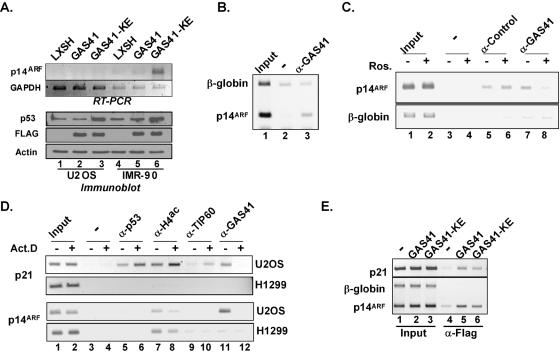
The GAS41 mutant-mediated activation of the p53 signaling pathway suggests that GAS41 may have a specialized function distinct from a typical coactivator function within the TIP60 complex. One attractive idea that could explain how the loss of GAS41 function can lead to activation of the p53 pathway involves a derepression of certain upstream signaling proteins that are normally repressed in proliferating primary cells. The ARF-MDM2-p53-p21 pathway is a key signaling pathway that is activated by abnormal mitogenic signals (42). These signals activate p14ARF, and ARF in turn prevents the MDM2-dependent ubiquitination (and subsequent degradation) of p53, thus leading to p53 stabilization and activation (42). The ARF gene has been shown to be repressed by E2F3b in primary cells, since loss of E2F3b is sufficient to induce the ARF gene and to activate p53 (4). In order to see whether loss of GAS41 function also leads to activation of the p14ARF gene, the GAS41 (K212A/E214A) mutant was stably expressed in both U2OS and primary IMR-90 cells by retroviral transduction, and effects on p14ARF gene expression were investigated (Fig. 5A). Ectopic expression of the GAS41 (K212A/E214A) mutant, but not wild-type GAS41, produced a significant induction of p14ARF gene expression in IMR-90 cells (Fig. 5A, lane 6 versus lane 5) but not in U2OS cells (lane 3 versus lane 2). This result suggested that GAS41 mutant-mediated activation of p53 could be initiated through the ARF-MDM2-p53-p21 signaling pathway. However, based on the fact that U2OS cells with a silenced p14ARF gene (43) showed activation of p53 upon loss of GAS41 function (Fig. 5A, lane 3; Fig. 2B, lane 3; and Fig. 2C, lane 3), GAS41 mutant-mediated p53 activation would not necessarily require activation of the ARF gene.
FIG. 5.
GAS41 binds to the promoters of repressed genes in the p53 tumor suppressor pathway. (A) Induction of p14ARF gene expression by a coiled-coil mutant of GAS41. Retroviruses made from empty vector (LXSH) or from vectors expressing wild-type GAS41 or the GAS41 (K212A/E214A) mutant were used to infect U2OS and primary IMR-90 cells, and infected cells were selected for 7 days in hygromycin. Total RNA was purified and analyzed by RT-PCR for relative levels of p14ARF and GAPDH mRNAs. Total cell lysates were analyzed by immunoblotting for relative levels of p53, FLAG-GAS41, and actin. (B) Binding of GAS41 to the p14ARF gene promoter in unstressed IMR-90 cells. A ChIP assay was performed with anti-GAS41 antibody using proliferating IMR-90 cells. Final eluates were analyzed by PCR against the E2F binding region of the p14ARF gene promoter. As a background control, the β-globin intron region was amplified in the same PCR. (C) Dissociation of prebound GAS41 from the p14ARF promoter upon gene activation. IMR-90 cells were treated in the presence or absence of 20 μM roscovitine for 6 h, and ChIP assays were performed for the p14ARF gene promoter and β-globin intron regions using antibodies against the H2AZ histone variant (as a control) and GAS41. (D) Differential binding of TIP60 and GAS41 to p21 and p14ARF gene promoters. U2OS cells and H1299 cells were grown in the presence or absence of actinomycin D (10 ng/ml) for 6 h, and ChIP assays were performed for the p21 and p14ARF gene promoter regions using antibodies as indicated. (E) Binding of the GAS41 (K212A/E214A) mutant to the p21 and p14ARF gene promoters. Retroviruses expressing wild-type FLAG-GAS41 or the FLAG-GAS41 (K212A/E214A) mutant were used to infect primary IMR-90 cells. After 7 days of hygromycin selection, infected cells were used for the ChIP assay using anti-FLAG antibody.
In order to determine whether GAS41 might be directly involved in regulation of the p14ARF gene, we employed a ChIP assay to determine whether GAS41 is bound to the promoter of the repressed p14ARF gene in normal primary IMR-90 cells (Fig. 5B). The result shows that GAS41 is specifically enriched on the p14ARF gene promoter containing putative E2F binding sites that have been shown to be involved in repression/derepression of the mouse ARF gene (4). However, since the (∼500-bp) DNA fragments used in the ChIP assay likely include other responsive elements, such as pokemon sites (33), it is not yet certain that GAS41 is bound at the E2F sites. Treatment of cells with the cyclin-dependent kinase 2 inhibitor roscovitine induces cell cycle arrest, probably through activation of E2F1 activity (8). Treatment of IMR-90 cells with roscovitine rapidly induced p14ARF gene expression (data not shown) and was used to artificially activate the p14ARF gene in primary cells. A ChIP assay demonstrated dissociation of GAS41 from the p14ARF gene promoter and correlation with roscovitine-induced p14ARF gene activation (Fig. 5C). Since treatment of roscovitine induces activation of p53 without affecting GAS41 stability (data not shown), GAS41 down-regulation is not the mechanism for decreased GAS41 binding on the p14ARF gene promoter. The correlation of gene activation with the dissociation of GAS41 was also observed on the p21 gene promoter (Fig. 5D, lanes 11 and 12), where activation of p53 by actinomycin D resulted in the dissociation of GAS41 from the promoter. Taken together, these results indicate that GAS41 plays a role in gene repression either directly or indirectly (e.g., by recruiting some repressor complex to the promoter).
In order to assess whether prebound GAS41 is part of a TIP60 complex, ChIP assays were performed with U2OS cells in the presence or absence of actinomycin D (Fig. 5D). Actinomycin D induced p53 binding (above a basal level) to the p21 promoter, and this was accompanied by modest increases in TIP60 recruitment and histone H4 hyperacetylation (Fig. 5D, upper panel, lanes 5 to 10). These enrichments depend on activation of p53 since p53-null H1299 cells did not produce similar enrichments. The binding of GAS41 was different from that of TIP60 since activation of the p21 gene correlates with the dissociation of GAS41. The localization of GAS41 on the p53 binding site of the p21 promoter seems to require a preexisting basal level of p53 binding because H1299 cells did not show detectable GAS41 binding in the same region (Fig. 5D, lanes 11 and 12). The ChIP data demonstrate that prebound GAS41, at least, is not strictly correlated with bound TIP60, but it is as yet undetermined whether it is a part of a TIP60-deficient subcomplex, such as the E1A-associated p400 complex (21). Analysis of p14ARF expression indicates that H1299 cells show one of the highest levels of ARF gene expression among cancer cell lines, whereas the ARF gene in U2OS cells is silenced and not responsive to actinomycin D treatment (43). Even though actinomycin D treatment does not activate the p14ARF gene in U2OS cells, it still can induce the dissociation of GAS41 from the p14ARF promoter in these cells (Fig. 5D, lanes 11 and 12); in contrast, H1299 cells show no such regulation of the p14ARF gene promoter, suggesting that p53 is required for regulated recruitment of GAS41 to the promoter.
To investigate the mechanism of GAS41 mutant-mediated p14ARF gene activation, a ChIP assay was used to monitor a possible difference in recruitment of ectopic wild-type versus mutant GAS41 onto the p14ARF gene promoter in primary IMR-90 cells (Fig. 5E). The results showed no significant differences between wild-type and GAS41 (K212A/E214A) mutant proteins with respect to the p14ARF gene promoter. This suggests that the promoter-bound GAS41 mutant loses a repression function by a mechanism that does not involve dissociation of GAS41 from the promoter. Taken together, the results of the ChIP assays indicate a TIP60 complex-independent role in repression of both the p21 and p14ARF genes in the p53 tumor suppressor pathway, possibly by binding to the target promoter either directly or through a distinct complex.
DISCUSSION
We demonstrate here that GAS41 possesses a putative antitumor suppressor activity through its ability to repress the p14ARF and p21 genes. GAS41 resides on the p14ARF and p21 promoters in proliferating cells, and the knockdown of GAS41 or the disruption of its coiled-coil domain induces derepression of the p14ARF and p21 genes and activation of p53. Based on structure-function studies of GAS41, we propose that GAS41-dependent antitumor suppressor activities are mediated by a distinct mechanism(s) that does not involve the TIP60 histone acetyltransferase.
Involvement of GAS41 in the p53 tumor suppressor pathway.
Structural and functional conservation of regulatory proteins and features between yeast and humans have provided insights for studying molecular mechanisms in more-complex human cells. The human TIP60 complex is highly conserved phylogenetically, and because structural homologs of all TIP60 complex subunits can be found in the yeast NuA4 and SWR1 complexes, the TIP60 complex appears to have evolved through a merger of the yeast NuA4 and SWR1 complexes (15, 16). GAS41 and its homolog Yaf9 are core subunits of the human TIP60 and yeast NuA4 complexes, respectively, and targeted disruptions of these genes result in different outcomes for cell viability. Several subunits of the NuA4 complex, including Tra1, Esa1, Arp4, and God1, are essential for yeast cell viability, whereas Yaf9 is dispensable under normal cell growth conditions (30). In contrast, GAS41 is essential for cell viability since targeted disruption of the gene in chicken cells causes cell death (49). A milder GAS41 knockdown through siRNA also revealed a growth arrest phenotype in HeLa cells, demonstrating that GAS41 is essential for cell growth and survival (23). We demonstrate here that GAS41 plays a role in suppressing two principal tumor suppressor genes, p21 and p14ARF, during normal cell growth. Subtle alterations of the coiled-coil structure at the C terminus of GAS41 are sufficient to induce activation of the p21 and p14ARF genes as well as p53. Involvement of GAS41 in the p53 tumor suppressor pathway may explain why GAS41 amplification occurs in certain tumors, such as human glioma (18).
Defects in the TIP60 and SRCAP complexes do not account for the phenotype of GAS41 mutants.
In an attempt to correlate a GAS41 mutant phenotype with defects in the TIP60 and SRCAP complexes, wild-type GAS41 and GAS41(K212A/E214A) mutant proteins were compared with respect to their intracellular association with components (including HAT activity) in the TIP60 and SRCAP complexes and their activities in p53-dependent reporter gene assays. However, in comparison with wild-type GAS41, the GAS41 (K212A/E214A) mutant showed no particular defects in these assays. Therefore, the mechanism underlying GAS41 mutant-mediated activation of the p21 and p14ARF genes cannot be explained by defects in the assembly of the TIP60 or SRCAP complex or by loss of HAT or coactivator functions of the TIP60 complex. However, current results do not exclude the possibility that GAS41 may function to target the TIP60 complex at some other specific genomic locations, analogous to the role of Yaf9 in histone acetylation and gene expression near certain telomeres (48).
In addition to a chromatin-modifying TIP60 HAT activity, the TIP60 complex possesses ATPase and helicase activities that may be attributable to the p400 subunit (21, 27). Interestingly, depletion of p400 has been shown to induce cell cycle arrest and premature senescence in primary human cells (10). Thus, the loss of either p400 or GAS41 appears to have an outcome similar to that of p21 activation and cell cycle arrest. However, the molecular mechanisms may be different because p400 knockdown, unlike GAS41 knockdown, does not effect p53 up-regulation and p53 serine-15 phosphorylation (10). One possible explanation is that if GAS41 is important for the regulation of p400 activity, the GAS41 mutant-induced activation of p53 and the p14ARF and p21 genes could result from the deregulation of p400 activity in the TIP60 or a related p400-containing complex (21). It has yet to be determined whether GAS41 colocalizes with p400 on the p21 gene promoter of unstressed normal cells and how a distinct repressive p400 complex, if one exists, interchanges with a similar TIP60 complex in the process of gene activation.
GAS41-mediated repression of the p14ARF and p21 genes.
The ability of certain GAS41 mutants and GAS41 siRNA to activate p14ARF and p21 in unstressed cells clearly indicates a role for GAS41 in the repression of these genes. Although the repression mechanisms are not understood, they appear to involve promoter occupancy by GAS41 since derepression, in each case, is associated with dissociation from the promoter of prebound GAS41. While the derepression effects of GAS41 mutants are not correlated with an inability to be incorporated into TIP60 and SRCAP complexes, the GAS41 mutant and GAS41 siRNA results do not eliminate the possibility that GAS41 acts in the context of a TIP60 complex that may be dynamic. In this regard, activation of the p21 gene (and possibly the ARF gene) is associated not just with GAS41 dissociation from the promoter but also with increased occupancy by TIP60.
The human TIP60 and p400 complexes have been shown to be recruited to promoters by transcription factors that are required for cellular proliferation and transformation (3, 20, 21, 44). One such transcription factor, E2F1, shares binding sites with other E2F family members and activates or represses target genes, depending on cell cycle status. The ARF gene is a unique E2F family member target gene because it remains constitutively repressed during normal cell cycle progression (4). It is known to be negatively regulated by p53 through a feedback mechanism (43), although there is no clear evidence that p53 directly binds to the p14ARF gene promoter. However, a ChIP assay with p53-null H1299 cells, which show high levels of p14ARF expression, demonstrated no enrichment of GAS41 on either the p14ARF or p21 promoter. Along with our demonstration that mutant GAS41-mediated activation of p21 requires p53, this raises the possibility that p53 could be involved either directly or indirectly in the recruitment of GAS41 and in subsequent repression. Consistent with this possibility, unstressed cells show low levels of constitutively bound p53 on the p21 promoter (17). Several other transcriptional repressors, such as Bmi1, Twist, and pokemon, have been implicated in ARF gene repression and cellular transformation (25, 33, 34), but it remains to be determined whether they play any role in the recruitment of GAS41 to the ARF promoter.
In relation to the regulation of p21 by mutation or down-regulation of GAS41, it is known that ARF (induced by aberrant mitogenic or oncogenic signals) inhibits MDM2-mediated p53 degradation and thus, through p53 stabilization and activation, activates target genes such as p21 (5). Our demonstration that GAS41 down-regulation activates p14ARF expression and the p53 protein is consistent with an indirect effect through effects on p14ARF expression. However, and somewhat surprisingly, our results show that the effect of GAS41 down-regulation on p21 induction is independent of ARF, since it occurs in ARF-deficient U2OS cells. While we did not exclude effects by this mechanism in some situations, these observations nonetheless indicate the existence of a distinct ARF-independent mechanism by which GAS41 down-regulation induces p53 activation and p21 expression. The nature of this mechanism is not yet known. However, p14ARF-independent p53 activation is also observed in response to aberrant oncogenic stress signals. For example, Myc and E2F1 have been shown to induce phosphorylation of p53 at serine-15, accumulation of p53, and up-regulation of p21 following p14ARF knockdown (31). It will be interesting to determine whether GAS41 mutant-dependent p53 activation also requires ATM (or ATR), since ATM/ATR inhibitors have been shown to block Myc- or E2F1-dependent p53 activation (31).
Structure-function analysis of the coiled-coil motif in the GAS41 C terminus.
The prediction of coiled-coil motifs among YEATS family members indicates that GAS41 and Yaf9 are unique members with four consecutive heptad repeats at the C terminus (32). The coiled coil is one of the most common motifs for protein-protein interactions. This is also evident in our GAS41 structure-function studies, which revealed that the coiled coil is essential for GAS41 assembly into the TIP60 and SRCAP complexes. An important finding in the present study is that certain coil mutants apparently do not disturb the protein interactions necessary for assembly of GAS41 into TIP60 and SRCAP complexes but nonetheless result in a loss-of-function phenotype equivalent to that observed when GAS41 is knocked down by siRNA. Studies of the heterodimerization specificity of leucine zippers suggest that charged residues of the heptad repeat can be sufficient to determine the dimerization specificity (47). Importantly, removal of charged residues at the third or fourth coil motif of GAS41 induces p14ARF gene expression and activation of p53 without affecting TIP60 or SRCAP complex assembly, suggesting that these charged residues could be involved in interactions with proteins other than those in the TIP60 or SRCAP complex. Interestingly, two putative interacting partners of GAS41, TACC1 and AF10, have coiled-coil structures that are implicated in the process of oncogenic transformation (13, 28). TACC1 was first identified as a potential oncogene, but the observation that it is down-regulated in several human cancers suggests a role as a tumor suppressor (11). The MLL fusion partner AF10 has a C-terminal leucine zipper motif that is required for the oncogenic activity of the MLL-AF10 fusion protein and for GAS41 interaction (13). Further studies of potential interactions among these proteins will provide further insights into the mechanism by which GAS41 functions to regulate the p14ARF gene and the p53 tumor suppressor. Furthermore, activation of two tumor suppressors by subtle alterations of the GAS41 coiled-coil structure will provide opportunities for rational drug design of cancer therapy using peptidomimetics.
Acknowledgments
We are grateful to Michael D. Cole for providing antibodies and Sohail Malik for critical reading and advice on the manuscript.
This work was supported by an Ellison Medical Foundation/AFAR Senior Postdoctoral Research grant to J.H.P. and by funds from Rockefeller University to R.G.R.
REFERENCES
- 1.Allard, S., R. T. Utley, J. Savard, A. Clarke, P. Grant, C. J. Brandl, L. Pillus, J. L. Workman, and J. Cote. 1999. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 18:5108-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, W., and R. G. Roeder. 2004. Reconstitution and transcriptional analysis of chromatin in vitro. Methods Enzymol. 377:460-474. [DOI] [PubMed] [Google Scholar]
- 3.Ard, P. G., C. Chatterjee, S. Kunjibettu, L. R. Adside, L. E. Gralinski, and S. B. McMahon. 2002. Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Mol. Cell. Biol. 22:5650-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslanian, A., P. J. Iaquinta, R. Verona, and J. A. Lees. 2004. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 18:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates, S., A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden. 1998. p14ARF links the tumour suppressors RB and p53. Nature 395:124-125. [DOI] [PubMed] [Google Scholar]
- 6.Berns, K., E. M. Hijmans, J. Mullenders, T. R. Brummelkamp, A. Velds, M. Heimerikx, R. M. Kerkhoven, M. Madiredjo, W. Nijkamp, B. Weigelt, R. Agami, W. Ge, G. Cavet, P. S. Linsley, R. L. Beijersbergen, and R. Bernards. 2004. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428:431-437. [DOI] [PubMed] [Google Scholar]
- 7.Cai, Y., J. Jin, L. Florens, S. K. Swanson, T. Kusch, B. Li, J. L. Workman, M. P. Washburn, R. C. Conaway, and J. W. Conaway. 2005. The mammalian YL1 protein is a shared subunit of the TRRAP/TIP60 histone acetyltransferase and SRCAP complexes. J. Biol. Chem. 280:13665-13670. [DOI] [PubMed] [Google Scholar]
- 8.Caretti, G., V. Salsi, C. Vecchi, C. Imbriano, and R. Mantovani. 2003. Dynamic recruitment of NF-Y and histone acetyltransferases on cell-cycle promoters. J. Biol. Chem. 278:30435-30440. [DOI] [PubMed] [Google Scholar]
- 9.Carrozza, M. J., R. T. Utley, J. L. Workman, and J. Cote. 2003. The diverse functions of histone acetyltransferase complexes. Trends Genet. 19:321-329. [DOI] [PubMed] [Google Scholar]
- 10.Chan, H. M., M. Narita, S. W. Lowe, and D. M. Livingston. 2005. The p400 E1A-associated protein is a novel component of the p53 → p21 senescence pathway. Genes Dev. 19:196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte, N., E. Charafe-Jauffret, B. Delaval, J. Adelaide, C. Ginestier, J. Geneix, D. Isnardon, J. Jacquemier, and D. Birnbaum. 2002. Carcinogenesis and translational controls: TACC1 is down-regulated in human cancers and associates with mRNA regulators. Oncogene 21:5619-5630. [DOI] [PubMed] [Google Scholar]
- 12.Costa, G. L., J. C. Bauer, B. McGowan, M. Angert, and M. P. Weiner. 1996. Site-directed mutagenesis using a rapid PCR-based method. Methods Mol. Biol. 57:239-248. [DOI] [PubMed] [Google Scholar]
- 13.Debernardi, S., A. Bassini, L. K. Jones, T. Chaplin, B. Linder, D. R. de Bruijn, E. Meese, and B. D. Young. 2002. The MLL fusion partner AF10 binds GAS41, a protein that interacts with the human SWI/SNF complex. Blood 99:275-281. [DOI] [PubMed] [Google Scholar]
- 14.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyon, Y., and J. Cote. 2004. The highly conserved and multifunctional NuA4 HAT complex. Curr. Opin. Genet. Dev. 14:147-154. [DOI] [PubMed] [Google Scholar]
- 16.Doyon, Y., W. Selleck, W. S. Lane, S. Tan, and J. Cote. 2004. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol. Cell. Biol. 24:1884-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinosa, J. M., R. E. Verdun, and B. M. Emerson. 2003. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol. Cell 12:1015-1027. [DOI] [PubMed] [Google Scholar]
- 18.Fischer, U., D. Heckel, A. Michel, M. Janka, T. Hulsebos, and E. Meese. 1997. Cloning of a novel transcription factor-like gene amplified in human glioma including astrocytoma grade I. Hum. Mol. Genet. 6:1817-1822. [DOI] [PubMed] [Google Scholar]
- 19.Fischle, W., Y. Wang, and C. D. Allis. 2003. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15:172-183. [DOI] [PubMed] [Google Scholar]
- 20.Frank, S. R., T. Parisi, S. Taubert, P. Fernandez, M. Fuchs, H. M. Chan, D. M. Livingston, and B. Amati. 2003. MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep. 4:575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs, M., J. Gerber, R. Drapkin, S. Sif, T. Ikura, V. Ogryzko, W. S. Lane, Y. Nakatani, and D. M. Livingston. 2001. The p400 complex is an essential E1A transformation target. Cell 106:297-307. [DOI] [PubMed] [Google Scholar]
- 22.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11:1640-1650. [DOI] [PubMed] [Google Scholar]
- 23.Harborth, J., S. M. Elbashir, K. Bechert, T. Tuschl, and K. Weber. 2001. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 114:4557-4565. [DOI] [PubMed] [Google Scholar]
- 24.Ikura, T., V. V. Ogryzko, M. Grigoriev, R. Groisman, J. Wang, M. Horikoshi, R. Scully, J. Qin, and Y. Nakatani. 2000. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102:463-473. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, J. J., K. Kieboom, S. Marino, R. A. DePinho, and M. van Lohuizen. 1999. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397:164-168. [DOI] [PubMed] [Google Scholar]
- 26.Keating, A. E., V. N. Malashkevich, B. Tidor, and P. S. Kim. 2001. Side-chain repacking calculations for predicting structures and stabilities of heterodimeric coiled coils. Proc. Natl. Acad. Sci. USA 98:14825-14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusch, T., L. Florens, W. H. Macdonald, S. K. Swanson, R. L. Glaser, J. R. Yates III, S. M. Abmayr, M. P. Washburn, and J. L. Workman. 2004. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 306:2084-2087. [DOI] [PubMed] [Google Scholar]
- 28.Lauffart, B., S. J. Howell, J. E. Tasch, J. K. Cowell, and I. H. Still. 2002. Interaction of the transforming acidic coiled-coil 1 (TACC1) protein with ch-TOG and GAS41/NuBI1 suggests multiple TACC1-containing protein complexes in human cells. Biochem. J. 363:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legube, G., L. K. Linares, S. Tyteca, C. Caron, M. Scheffner, M. Chevillard-Briet, and D. Trouche. 2004. Role of the histone acetyl transferase Tip60 in the p53 pathway. J. Biol. Chem. 279:44825-44833. [DOI] [PubMed] [Google Scholar]
- 30.Le Masson, I., D. Y. Yu, K. Jensen, A. Chevalier, R. Courbeyrette, Y. Boulard, M. M. Smith, and C. Mann. 2003. Yaf9, a novel NuA4 histone acetyltransferase subunit, is required for the cellular response to spindle stress in yeast. Mol. Cell. Biol. 23:6086-6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindstrom, M. S., and K. G. Wiman. 2003. Myc and E2F1 induce p53 through p14ARF-independent mechanisms in human fibroblasts. Oncogene 22:4993-5005. [DOI] [PubMed] [Google Scholar]
- 32.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 33.Maeda, T., R. M. Hobbs, T. Merghoub, I. Guernah, A. Zelent, C. Cordon-Cardo, J. Teruya-Feldstein, and P. P. Pandolfi. 2005. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature 433:278-285. [DOI] [PubMed] [Google Scholar]
- 34.Maestro, R., A. P. Dei Tos, Y. Hamamori, S. Krasnokutsky, V. Sartorelli, L. Kedes, C. Doglioni, D. H. Beach, and G. J. Hannon. 1999. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 13:2207-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez, E., V. B. Palhan, A. Tjernberg, E. S. Lymar, A. M. Gamper, T. K. Kundu, B. T. Chait, and R. G. Roeder. 2001. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol. Cell. Biol. 21:6782-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon, S. B., H. A. Van Buskirk, K. A. Dugan, T. D. Copeland, and M. D. Cole. 1998. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell 94:363-374. [DOI] [PubMed] [Google Scholar]
- 37.Nakano, K., T. Mizuno, Y. Sowa, T. Orita, T. Yoshino, Y. Okuyama, T. Fujita, N. Ohtani-Fujita, Y. Matsukawa, T. Tokino, H. Yamagishi, T. Oka, H. Nomura, and T. Sakai. 1997. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J. Biol. Chem. 272:22199-22206. [DOI] [PubMed] [Google Scholar]
- 38.Nikiforov, M. A., S. Chandriani, J. Park, I. Kotenko, D. Matheos, A. Johnsson, S. B. McMahon, and M. D. Cole. 2002. TRRAP-dependent and TRRAP-independent transcriptional activation by Myc family oncoproteins. Mol. Cell. Biol. 22:5054-5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, J., M. A. Wood, and M. D. Cole. 2002. BAF53 forms distinct nuclear complexes and functions as a critical c-Myc-interacting nuclear cofactor for oncogenic transformation. Mol. Cell. Biol. 22:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson, K. D. 2002. DNA methylation and chromatin-unraveling the tangled web. Oncogene 21:5361-5379. [DOI] [PubMed] [Google Scholar]
- 42.Sherr, C. J. 2004. Principles of tumor suppression. Cell 116:235-246. [DOI] [PubMed] [Google Scholar]
- 43.Stott, F. J., S. Bates, M. C. James, B. B. McConnell, M. Starborg, S. Brookes, I. Palmero, K. Ryan, E. Hara, K. H. Vousden, and G. Peters. 1998. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17:5001-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taubert, S., C. Gorrini, S. R. Frank, T. Parisi, M. Fuchs, H. M. Chan, D. M. Livingston, and B. Amati. 2004. E2F-dependent histone acetylation and recruitment of the Tip60 acetyltransferase complex to chromatin in late G1. Mol. Cell. Biol. 24:4546-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utley, R. T., T. A. Owen-Hughes, L. J. Juan, J. Cote, C. C. Adams, and J. L. Workman. 1996. In vitro analysis of transcription factor binding to nucleosomes and nucleosome disruption/displacement. Methods Enzymol. 274:276-291. [DOI] [PubMed] [Google Scholar]
- 46.Wood, M. A., S. B. McMahon, and M. D. Cole. 2000. An ATPase/helicase complex is an essential cofactor for oncogenic transformation by c-Myc. Mol. Cell 5:321-330. [DOI] [PubMed] [Google Scholar]
- 47.Zeng, X., A. M. Herndon, and J. C. Hu. 1997. Buried asparagines determine the dimerization specificities of leucine zipper mutants. Proc. Natl. Acad. Sci. USA 94:3673-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, H., D. O. Richardson, D. N. Roberts, R. Utley, H. Erdjument-Bromage, P. Tempst, J. Cote, and B. R. Cairns. 2004. The Yaf9 component of the SWR1 and NuA4 complexes is required for proper gene expression, histone H4 acetylation, and Htz1 replacement near telomeres. Mol. Cell. Biol. 24:9424-9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann, K., K. Ahrens, S. Matthes, J. M. Buerstedde, W. H. Stratling, and L. Phi-van. 2002. Targeted disruption of the GAS41 gene encoding a putative transcription factor indicates that GAS41 is essential for cell viability. J. Biol. Chem. 277:18626-18631. [DOI] [PubMed] [Google Scholar]