Abstract
Bright/ARID3a/Dril1, a member of the ARID family of transcription factors, is expressed in a highly regulated fashion in B lymphocytes, where it enhances immunoglobulin transcription three- to sixfold. Recent publications from our lab indicated that functional, but not kinase-inactive, Bruton's tyrosine kinase (Btk) is critical for Bright activity in an in vitro model system, yet Bright itself is not appreciably tyrosine phosphorylated. These data suggested that a third protein, and Btk substrate, must contribute to Bright-enhanced immunoglobulin transcription. The ubiquitously expressed transcription factor TFII-I was identified as a substrate for Btk several years ago. In this work, we show that TFII-I directly interacts with human Bright through amino acids in Bright's protein interaction domain and that specific tyrosine residues of TFII-I are essential for Bright-induced activity of an immunoglobulin reporter gene. Moreover, inhibition of TFII-I function in a B-cell line resulted in decreased heavy-chain transcript levels. These data suggest that Bright functions as a three-component protein complex in the immunoglobulin locus and tie together previous data indicating important roles for Btk and TFII-I in B lymphocytes.
The transcription factor Bright (B-cell regulator of immunoglobulin H [IgH] transcription)/ARID3a/Dril1 is a B-cell-specific protein first discovered in a mature mouse B-cell line, BCg3R1-d, as a mobility-shifted protein complex that caused three- to sixfold increases in μ heavy-chain mRNA levels in response to stimulation with a T-dependent antigen and interleukin-5 (42, 43). Bright binds to A+T-rich regions of the intronic heavy-chain enhancer previously identified as matrix association regions and to regions 5′ of some variable heavy-chain (VH) promoters, including the V1 S107 family gene (15, 43). The cDNA for Bright was isolated in 1995, and the protein was shown to interact with DNA as a multimeric complex that included multiple copies of Bright (15). The Bright protein structure consists of an acidic N-terminal domain of unknown function, a DNA-binding A+T-rich interaction domain (ARID), a putative transactivation domain, a protein interaction domain containing a helix-turn-helix region, and a small carboxyl-terminal domain with no assigned function.
Earlier studies indicated that Bruton's tyrosine kinase (Btk), the defective enzyme in X-linked immunodeficiency disease in both mice and humans, is a component of the Bright DNA-binding complex (28, 44). X-linked immunodeficiency disease in mice, or X-linked agammaglobulinemia (XLA) in humans, results in blocks in B-lymphocyte development that ultimately lead to a deficient production of serum antibodies (9, 33, 40). Patients with XLA are unable to fight normal bacterial infections without frequent intravenous Ig treatments. Although Btk was identified as the genetic defect in XLA many years ago, the mechanism by which Btk deficiencies lead to early blocks at the pro-B- to pre-B-lymphocyte stages in humans remains unclear.
Recently, an in vitro model system using an Ig reporter gene was developed to determine if Btk contributed to Bright function (32). In this model system, Btk kinase activity was critically required for Bright-dependent transactivation of the Ig heavy-chain promoter. However, Bright itself was not appreciably phosphorylated (44). These data led to the hypothesis that a third protein, a Btk substrate, was associated with the Bright/Btk complex (32).
Multiple substrates for Btk have been identified and include BAM11, STAT5a, G proteins, and the Btk-associated protein (135 kDa; BAP135) first identified as a Btk substrate in activated human B cells (2, 13, 18, 20, 24, 41, 45). Later studies proved that BAP135 was identical to the transcription factor TFII-I (35). TFII-I is a ubiquitously expressed protein proposed to function as both a basal transcription factor facilitating communication between basal machinery at the core promoter and a transcription activator contributing to protein complexes assembled at upstream sites (36). There are multiple isoforms of TFII-I produced by alternative splicing that include Δ, α, β, and γ forms of 957, 977, 978, and 998 amino acids, respectively, and these proteins are differentially expressed in various cell types (reviewed in reference 34). Each isoform contains a leucine zipper sequence at the N-terminal region and six direct helix-loop-helix I-repeats. Two nuclear localization signals, a DNA-binding domain, and a C-terminal activation domain have also been identified within these forms (34). The N-terminal domain, including the first 90 amino acids, was shown to be important both for the formation of dimers and for interaction with Btk (37).
TFII-I has numerous potential phosphorylation sites and likely contributes to the transcription of multiple genes in many tissues. Tyrosine residues 248 and 249 are documented phosphorylation sites for Btk in vitro and, therefore, may be important in B lymphocytes (12, 29, 37, 45), while Y248 was indicated as a phosphorylation substrate for JAK2 and src family kinases (23). Other signaling molecules, such as mitogen-activated protein kinase, phosphorylate TFII-I at S633 (22). Mutation of this serine, S633A, yielded a form of TFII-I that was ineffective in inducing c-fos promoter activation in transient transfections (21). In another study, TFII-I associated with a protein called BAM11 and contributed to transcriptional coactivation of a reporter construct (16). In this case, however, the phosphorylation status of TFII-I was not directly examined. Due to the complexity of the protein and the numerous serine/threonine and tyrosine phosphorylation sites within TFII-I, it has been difficult to demonstrate which phosphorylation sites are most critical for the regulation of individual pathways.
Our previous studies suggested that the Bright/Btk complex required a third protein for transcription activation (32) and that this protein was likely to be a Btk substrate. Preliminary evidence from a hamster cell line demonstrated that a 107-kDa protein that coprecipitated with transiently transfected Bright/Btk also cross-reacted with anti-TFII-I antibody (32). We therefore sought to determine if Bright associated with endogenous TFII-I in B lymphocytes and whether such an association explained the requirement for a third protein in our Ig model system. The data described herein define regions of Bright required for an interaction with TFII-I and suggest that TFII-I is functionally important for Bright activity. Moreover, the tyrosine residues previously identified as Btk substrates are critical for Bright function in this system.
MATERIALS AND METHODS
Plasmids.
The Ig reporter construct contained the S107 V1 heavy-chain genomic sequence from −574 base pairs 5′ of the transcription start site through the leader sequence, first intron, and 146 bases of the coding sequence, along with a 1-kb XbaI fragment containing the intronic μ enhancer (32). The full-length human Bright expression construct was described previously (28). Each of the mutant forms of Bright was constructed using a recombinant two-step PCR technology. A mutant mouse Bright protein with an N-terminal deletion (Δ216) was provided by P. Tucker (University of Texas, Austin, TX) (15). The Btk-green fluorescent protein (GFP) fusion construct was a gift from William Rodgers (Oklahoma Medical Research Foundation, Oklahoma City, OK). Wild-type TFII-I and mutant constructs were described previously and include a dominant negative form (p70) and mutant forms with a leucine zipper deletion (ΔN90), a nuclear localization sequence deletion (ΔNLS1), and a Btk phosphorylation site mutation (YY248/249FF) (37). A GFP expression plasmid, pmaxGFP (Amaxa, Gaithersburg, MD), was used in some cases.
Cell culture and transient transfections.
The mouse B-cell line BCg3R-1d and the human B-cell lines CL01 and Raji were grown in RPMI 1640 supplemented with 5% fetal calf serum as previously described (42). The T-cell hybridoma line KD3B5.8 (a gift of D. Farris, Oklahoma Medical Research Foundation) and Cos-7 cells (ATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (Life Technologies Inc., Gaithersburg, MD) with 10% heat-inactivated fetal calf serum (Atlanta Biologicals, Norcross, GA), 5 × 10−5 M β-mercaptoethanol (Sigma, St. Louis, MO), 10 mM sodium pyruvate, 1× minimal essential medium nonessential amino acids, 0.5× minimal essential medium amino acids, and 50 μg/ml penicillin-streptomycin (all from Invitrogen, Carlsbad, CA). BCg3R-1d cells were stimulated with CD40 ligand for 18 h as previously described (44) to ensure the presence of functionally active Bright DNA-binding complexes and were transfected with 5 μg of DNA including a GFP reporter plasmid by using program T27 with the Cell Line Nucleofector kit T (Amaxa) according to the manufacturer's directions. Cos-7 transfections were performed using calcium phosphate precipitation. Briefly, 10 μg of each plasmid DNA was mixed with 450 μl of warm water and 50 μl of CaCl2 (2.5 M) and was added dropwise to 500 μl of prewarmed 2× HEPES-buffered saline containing 0.28 M NaCl, 0.05 M HEPES, 1.5 mM NA2HPO4 (pH 7.5). This solution was vortexed and added to 75%-confluent Cos-7 cells. Medium was changed after 4 to 6 h. Transfected cells were sorted 48 h later for GFP expression using a MoFlo cell sorter (Cytomation, Inc., Fort Colllins, CO) by either the Oklahoma Medical Research Foundation or the University of Oklahoma Health Sciences Center Flow Cytometry Core Facility. The purity of sorted cells was typically 90 to 97%.
Real-time and semiquantitative PCR.
Approximately 200,000 GFP-positive cells were collected for RNA isolation. RNA was isolated using Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH) and immediately treated with DNase I according to the manufacturer's protocol (Ambion, Austin, TX). Quantities were measured by spectrophotometry using a Unicam UV1 spectrophotometer (Spectronic Unicam). Reactions to generate cDNA included 300 ng RNA, a 1 mM concentration of a deoxynucleoside triphosphate mix, 25 pM V1-specific or random primer (Integrated DNA Technologies, Coralville, IA), 40 units RNasin (Promega, Madison, WI), and 200 units SuperScript II RNase H reverse transcriptase (RT) (Invitrogen). Reactions without RT were performed in parallel as negative controls. Semiquantitative PCR of μ heavy-chain mRNA from the BCg3R-1d cell line was assessed using a forward primer from the V1 variable region (5′-GTATCCAGTGTGAGGTGAAGC-3′) and a reverse primer from exon 1 of Cμ (5′-GAGCTTCCCATCCTTTAGCCA-3′) after 40 cycles of 93°C for 30 s, 59°C for 25 s, and 72°C for 40 s. Levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were amplified with forward (5′-TTAGCACCCCTGGCCAAGG-3′) and reverse (5′CTTACTCCTTGGAGGCCATG-3′)primers to assess relative mRNA levels among samples. Intensities of the PCR products were determined using LumiAnalyst 3.0 software (Roche, Indianapolis, IN).
Real-time quantitative RT-PCR was performed with specific TaqMan primers and probes (Integrated DNA Technologies) that spanned the first intron of the V1 gene as described previously (32). Reactions were performed with 96-well plates using the following conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 55°C for 1 min. Standards were run with every experiment for consistency and quantification of the amplified DNA. Standard curves were generated by averaging cycle threshold (CT) values for triplicate reactions performed with 10-fold serial dilutions (from 10−4 ng to 20 ng) of plasmid containing the V1 gene. Data from triplicate samples were averaged and converted to ng of product by using the standard curve and analyzed using ABI Prism 7700 SDS software (Applied Biosystems). GAPDH transcripts were measured to ensure that the transcription of other genes was not affected by the transfected proteins, and results are reported as average CT values.
Western blotting and immunoprecipitation.
Whole-cell extracts were prepared from transfected Cos-7 cells 36 h posttransfection using hypotonic lysis as previously described (43). Briefly, cells were scraped from flasks using a cell scraper (Costar, Corning, NY) and washed twice in phosphate-buffered saline containing protease inhibitors (500 μM dithiothreitol, 500 μM phenylmethylsulfonyl fluoride, 21 μM leupeptin, 750 nM aprotinin) before suspension in extraction buffer (20 mM HEPES [pH 7.9], 420 mM NaCl, 1 mM MgCl2, 0.2 mM EDTA, 20% glycerol). Cells were homogenized with pestles, and lysates were collected after 15 min of 4°C centrifugation at 12,000 × g. Lysates were dialyzed at room temperature for 2 h in storage buffer (20 mM HEPES [pH 7.9], 100 mM KCl, 0.2 mM EDTA, 20% glycerol) containing protease inhibitors. Total protein was measured by a modified Bradford assay (Bio-Rad, Richmond, CA).
Whole-cell lysates from transfected Cos-7 cells (200 μg) were precleared with protein A-Sepharose beads for 30 min. Precleared protein extracts were added to anti-glutathione S-transferase (GST; Amersham Biosciences), anti-myc (Invitrogen), or isotype control (anti-Sp1) antibodies in phosphate-buffered saline containing protease inhibitors (phenylmethylsulfonyl fluoride [5 × 10−5 M], leupeptin [1 × 10−2 mg/ml], aprotinin [5 × 10−3 mg/ml], and sodium vanadate [30 mM]) and rocked at 4°C for 2 h before the addition of protein A-Sepharose (25 μl; 1:1 slurry) and further incubation at 4°C for 12 h. Immunoprecipitates were washed five times with wash buffer (100 mM Tris-Cl, 500 mM NaCl, 0.1% Tween 20, pH 8) containing protease inhibitors, and proteins were eluted by the addition of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer and boiling for 5 min. Samples were run on 7.5% SDS-polyacrylamide gels using standard protocols and transferred to nitrocellulose membranes (Protran; Schleicher & Schuell, Keene, NH). CL01 cells were stimulated with phorbol myristate acetate (PMA; 10 μg/ml) for 48 h to induce the Bright complex prior to use in immunoprecipitation assays. Bright and Btk were detected with rabbit anti-Bright (gift of P. Tucker) and with anti-Btk C-20 (Santa Cruz Biotechnology, Santa Cruz, CA), respectively, and alkaline phosphatase-labeled goat anti-rabbit Ig (Southern Biotech, Birmingham, AL). TFII-I was detected with goat anti-peptide TFII-I or goat anti-GST (Amersham Biosciences, Piscataway, NJ) and alkaline phosphatase-labeled rabbit anti-goat Ig (Southern Biotech). All blots were visualized using alkaline phosphatase conjugate substrate development reagents (Bio-Rad).
Probes and EMSAs.
Electrophoretic mobility shift assays (EMSAs) were performed with 4% nondenaturing polyacrylamide gels as previously described (43). The prototypic Bright binding site (a 150-bp BamHI-FokI fragment called bf150) from the S107 V1 5′ flanking sequence (43) was labeled with γ-32P and used as a probe. Five micrograms of protein was added to the probe and incubated for 15 min prior to electrophoresis. In some cases, antibodies were added to the samples for 5 min prior to incubation with the probe. Recombinant Bright and TFII-I were prepared as previously described (28, 37). In vitro-translated Bright was produced with TNT-coupled rabbit reticulocyte lysates (Promega).
Antibody-facilitated DNA precipitation.
The BCg3R-1d B-cell line stimulated for 20 h with 20 μg/ml lipopolysaccharide to induce Bright activity (44) or a negative-control T hybridoma cell line, KD3B5.8 (a gift of D. Farris, Oklahoma Medical Research Foundation), was subjected to cross-linking and immunoprecipitation as previously described (32). Polyclonal anti-peptide TFII-I (32) or control goat Ig was incubated overnight at 4°C with precleared sonicated extracts. Immunoprecipitated DNA was phenol-chloroform purified and subjected to PCR at 95°C for 40 s, 60°C for 1 min, and 72°C for 2 min for 40 cycles with a 10-min extension at 72°C using primers (forward, 5′-CTAGATCCACATGTATGATTT-3′; reverse, 5′-GTCTTTCAGACAATAGATTGG-3′) that amplify a 150-bp Bright-binding region of the V1 gene (43).
RESULTS
A TFII-I-related protein associates with the Bright DNA-binding complex.
Our previous data suggested that a third protein, related to TFII-I, associated with Bright (32). To determine if TFII-I associated with Bright in B lymphocytes, mobility shift assays were performed using the prototypic Bright binding site (a 150-bp BamHI fragment called bf150) from the V1 S107 5′ flanking sequence and nuclear extracts from BCg3R-1d cells (Fig. 1). When anti-peptide antibodies to the amino terminus of Bright (anti-p29), Btk, and TFII-I were added to the reaction mixtures, they supershifted the Bright DNA complexes (lanes 2 to 5), supporting the idea that a protein serologically related to TFII-I was a component of the Bright complex. Because the Bright complex is large, and supershifting with anti-TFII-I was not complete at any available concentration (lanes 4 to 6), an in vitro-translated, amino-terminal-deletion form of Bright (Δ216) (15) was also assessed for reactivity with these antibodies. While anti-p29 no longer reacted with the complex (lane 9), both the anti-Btk and anti-TFII-I antibodies produced clear supershifted bands (lanes 8 and 10 to 12). Previous data showed that Btk was present in reticulocyte lysates and that TFII-I is ubiquitously expressed (44). These data confirm earlier experiments indicating the presence of Btk in the Bright complex (32, 44) and indicate that the amino-terminal portion of Bright is not required for interactions with Btk or with the TFII-I-related protein.
FIG. 1.
Bright DNA-bound complexes are supershifted by antibodies to TFII-I. Mobility shift assays were performed with the prototypic Bright binding site (bf150) and nuclear extracts from the BCg3R-1d B-cell line (lanes 1 to 6) or with rabbit reticulocyte lysates containing an amino-terminally truncated form of in vitro-translated mouse Bright, Δ216 (lanes 7 to 12). Antisera to Btk, TFII-I, or a peptide in the amino-terminal domain of Bright (p29) were added to the extracts as indicated. Dilutions of the anti-TFII-I serum (1:2 and 1:10) were also used. Supershifted bands are indicated with an arrow.
Endogenous Bright, Btk, and TFII-I are associated in a human B-cell line.
Immunoprecipitation of protein extracts from human CL01 B cells with anti-Btk, anti-TFII-I, and anti-Bright (lanes 2 to 4) indicated that complexes containing all three proteins exist in these cells (Fig. 2). Control goat immunoglobulin (lane 1) did not precipitate any of the three proteins. Each of the three antibodies precipitated both the 120 and 128 isoforms of TFII-I as previously observed (5, 29, 45). These results support earlier data showing an association of Btk and TFII-I in activated human B cells (45) and allow us to conclude that the TFII-I-related protein in the Bright complex is TFII-I.
FIG. 2.
Bright, TFII-I, and Btk coprecipitate from the human B-cell line CL01. Whole-cell extracts from PMA-stimulated CL01 cells were immunoprecipitated (IP) with goat anti-Btk (C20), anti-TFII-I, anti-Bright, or control goat Ig (Anti-GtIg) and then immunoblotted (IB) for the presence of the indicated proteins. The first lane (load) contains 5% of the total protein used for each precipitation reaction. Relative molecular weights are indicated.
TFII-I and Bright associate with the promoter of a heavy-chain gene in a B-cell line.
The EMSA and immunoprecipitation assay suggest that TFII-I can associate with the Bright complex but do not address whether these proteins act together on the Ig locus in B cells. To address this issue, CHIP assays were performed on the B-cell line BCg3R-1d, which expresses the V1 heavy-chain gene from a transfected Ig gene containing two Bright binding sites, including the prototypic Bright site necessary for Bright-induced transcription in vitro (32, 42). Figure 3 shows that both anti-Bright and anti-TFII-I precipitated the bf150 Bright binding site from the B-cell line but not from a T-cell line that contains but does not express the V1 gene. These data suggest that Bright and TFII-I associate in vivo to regulate the V1 gene in B cells.
FIG. 3.
Bright and TFII-I associate in a DNA-bound complex on the V1 promoter in a B-cell line. Antibody-facilitated chromatin immunoprecipitation was performed with stimulated BCg3R-1d B cells or the T-cell hybridoma KD3B5.8, using anti-Bright, anti-TFII-I, or goat Ig (Anti-GtIg). Immunoprecipitated DNA was PCR amplified at final dilutions of 1:100, 1:500, and 1:1,000 (represented by triangles) for the presence of the IgH V1 promoter. Ten percent of the DNA used for each immunoprecipitation was used as a positive control (Input).
Discrete regions of the Bright protein interaction domain are required for association with TFII-I.
In order to determine which regions of Bright are important for interactions with TFII-I, a series of deletion mutants of human Bright was produced and is depicted in Fig. 4a. Because EMSA data from Fig. 1 suggested that the acidic amino-terminal domain of Bright was not required for either DNA-binding activity or an association with TFII-I and Btk, no additional mutants were produced for that domain. A point mutation in human Bright (Y324A) homologous to the point mutation that resulted in a dominant negative form of mouse Bright (27) was introduced in the DNA-binding domain. In addition, mutants with deletions of the activation domain, the helix-turn-helix regions of the protein interaction domain, and the carboxyl terminus were generated (Fig. 4a).
FIG. 4.
Bright-TFII-I interactions require amino acids in the protein interaction domain of Bright. (a) A schematic diagram shows the protein domains and motifs of wild-type human Bright (WT) and the mutant constructs produced. The Y324A point mutation acts as a dominant negative (DN). Δ216 is a mouse protein construct. The boxes in the protein interaction domain denote helices 1 and 2. Deletions to individual amino acids are noted. (b) An EMSA was performed using wild-type and mutant forms of Bright (Br). Lane 1 contains only probe, while lane 2 contains wild-type Bright (arrow). (c) Quantities of Bright proteins used in panel b are shown by Western blotting. (d) Anti-Bright, anti-TFII-I, or an isotype-matched control antibody (GtIg) was used for immunoprecipitation (IP) of protein extracts from transfected Raji cells. Blots were developed (immunoblotted [IB]) for Bright and/or TFII-I as indicated. GtIg, goat Ig.
Mobility shift assays of extracts from Cos-7 cells transfected with mutant Bright were used to determine if the mutations affected DNA-binding activity (Fig. 4b). As predicted from the murine Bright data (27), mutation of tyrosine 324 to alanine resulted in a protein that failed to interact with DNA. Deletion of the carboxyl-terminal domain and 25 amino acids of the protein interaction domain (Δ531) resulted in a protein that no longer bound DNA. Likewise, deletion of most of the activation domain, including the two helix-turn-helix regions of the protein interaction domain (ΔHelix-1,2), resulted in proteins that failed to bind DNA. On the other hand, mutants lacking the activation domain and only the first of the helix-turn-helix regions (ΔHelix-1) retained DNA-binding activity, suggesting that only helix 2 is necessary for DNA-binding activity. Similarly, deletion of up to 544 amino acids of the carboxyl end (Δ544) resulted in proteins capable of binding DNA (not shown). Mutant protein levels used for the EMSAs are shown by Western blotting (Fig. 4c). Together, these data suggest that tyrosine 324 in the ARID domain and amino acids 459 to 544 of the protein interaction domain of human Bright are critical for DNA-binding activity.
Earlier studies demonstrated that monomers of Bright failed to bind DNA and led to identification of the protein interaction domain in mouse Bright (15). These data were confirmed in studies showing the presence of dimeric forms of Bright in nuclear extracts and by data showing that mutant proteins with point mutations in the ARID domain of mouse Bright that interfered with DNA-binding activity acted as dominant negative proteins when expressed with wild-type Bright (27). Therefore, each of the human mutants that failed to bind DNA by EMSA was assessed for its ability to coprecipitate as a dimer with wild-type Bright. myc-tagged full-length or mutant forms of Bright that altered the size of the proteins relative to the nontagged forms of the proteins were used for these experiments. Full-length Bright was coprecipitated by anti-myc antibodies with the tagged wild-type protein and each of the tagged Bright mutants tested except the Δ531 mutant (data not shown). The results of these experiments are summarized in Table 1 and define the 85 amino acids between positions 459 and 544 as critical for human Bright dimerization, consistent with observations for mouse Bright.
TABLE 1.
Activities of Bright mutants
| Bright protein | Presence or absence of:
|
||
|---|---|---|---|
| DNA-binding activity | Dimerizationb | TFII-I association | |
| Wild type | + | + | + |
| Dominant negative | − | + | + |
| Δ216a | + | + | + |
| Δ374 | + | + | + |
| ΔHelix-1 | + | + | + |
| ΔHelix-1,2 | − | + | − |
| Δ549 | + | ND | + |
| Δ544 | + | ND | + |
| Δ531 | − | − | − |
Data were obtained from EMSAs using murine Bright.
ND, not done.
Immunoprecipitation experiments were performed with the human Bright mutants to determine which regions of Bright are critical for an association of Bright with TFII-I. In this case, the human B-cell line Raji, which does not express endogenous Bright DNA-binding complexes (28), was used for expression of the Bright mutants. Figure 4d shows that each of the mutants that bound DNA by EMSA (Fig. 4b) also coprecipitated with TFII-I with either anti-Bright or anti-TFII-I antibodies. However, the Δ531 mutant did not coprecipitate with TFII-I. While the ΔHelix-1 mutant retained the ability to interact with TFII-I, the ΔHelix-1,2 mutant did not precipitate with TFII-I (not shown). Results are summarized in Table 1. These data indicate that amino acids 459 to 544 are critical for an association of Bright with TFII-I.
Wild-type Bright interacts with several mutants of TFII-I.
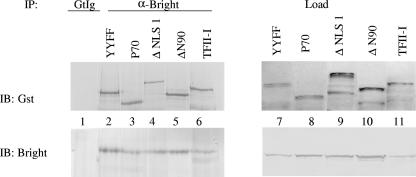
Multiple mutant forms of TFII-I have already been described, and some of those lack function as a transcription factor (8). As an initial step to determine if mutants of TFII-I could be used to assess whether TFII-I contributed functionally to Bright activity, Cos-7 cells were transfected with wild-type Bright as well as wild-type and mutated forms of TFII-I, GST tagged to distinguish those proteins from endogenous TFII-I. Immunoprecipitation experiments were performed using anti-Bright antibodies (Fig. 5). Wild-type TFII-I; the nuclear localization sequence mutant (ΔNLS1); a mutant lacking the amino-terminal leucine zipper domain (ΔN90); a dominant negative protein lacking the carboxyl terminus of the protein, including I region repeats 5 and 6 (p70); and a protein with mutations of two tyrosines previously shown to be targets for Btk phosphorylation, YY248/249FF (YYFF) (8, 12, 37), all coprecipitated with wild-type Bright as shown by immunoblotting with anti-GST antibodies. Control goat Ig failed to precipitate wild-type Bright or TFII-I under any conditions, as shown in lane 1. Similarly, Bright coprecipitated with each of the mutants when anti-GST antibodies were used to immunoprecipitate the proteins (not shown). These data indicate that Bright's interaction with TFII-I does not require the leucine zipper, carboxyl terminus, or I region repeats 5 and 6 (8). Moreover, these data suggest that these mutants of TFII-I can be used to assess the functional contribution of TFII-I to Bright-enhanced transcription of an Ig reporter gene.
FIG. 5.
The amino- and carboxyl-terminal domains of TFII-I are not required for interactions with Bright. Wild-type Bright and several GST-tagged TFII-I mutants (lanes 2 to 6) were coexpressed in Cos-7 cells and immunoprecipitated (IP) with anti-Bright and immunoblotted (IB) with anti-GST and anti-Bright antibodies (left panel). Control goat Ig (GtIg) did not precipitate Bright or TFII-I, as shown for the Bright-plus-wild-type TFII-I transfectant (lane 1). Protein levels prior to IP (Load) are shown in the right panel.
TFII-I activity is necessary for enhanced transcription of an Ig reporter gene by Bright and Btk.
We previously designed an Ig reporter vector that exhibits Bright- and Btk-dependent enhanced transcription from a Bright binding site upstream of the basal Ig promoter (32). Because TFII-I is ubiquitously expressed, it is not clear from those data whether endogenous TFII-I might also be required for transcriptional activation in that system. Therefore, real-time PCR was used to quantify the amount of Ig transcription in Cos-7 cells cotransfected with the reporter plasmid, Bright, Btk, and wild-type or dominant negative (p70) TFII-I (Fig. 6). Transfected cells were sorted by flow cytometry to ensure that the data were derived from viable and equivalently transfected cell populations. The reporter plasmid and empty vectors corresponding to the same amount of DNA used for the expression plasmids were used in control samples. Quantities of IgH mRNA were determined from the average of triplicate CT values by using a standard curve. Addition of wild-type TFII-I to Bright-plus-Btk-transfected cultures resulted in an approximately 2.5-fold increase in Ig transcription. On the other hand, addition of dominant negative TFII-I (p70) to Bright-plus-Btk-transfected cells dramatically decreased Bright-enhanced transcription (Fig. 6). GAPDH levels were assessed to demonstrate that transcription of genes not dependent on Bright was not affected by the transfected genes, and Bright proteins did not differ significantly among the different transfectants. The mRNA half-life of GAPDH was reported for several cell lines and ranges from 8.5 to 24 h (4, 25). The experiments shown in Fig. 6 were performed at 48 h and did not reveal significant differences in GAPDH levels between wild-type TFII-I- and dominant negative TFII-I-transfected cells. CT values relative to baseline are given in the legend for Fig. 6. These data suggest that TFII-I is critically important for Bright-induced transcription in this model system.
FIG. 6.
TFII-I contributes to Bright-activated transcription of an IgH reporter plasmid. (a) Cos-7 cells were transfected with an IgH reporter plasmid and wild-type Bright (Br), Btk, and either a wild-type or dominant negative (p70) TFII-I expression vector. Appropriate empty expression vectors were used to maintain equivalent levels of DNA in the control (Ctl) transfected cells. IgH mRNA levels were quantified from GFP-positive sorted transfectants using triplicate real-time PCR samples and a standard curve relating IgH mRNA quantities to average CT values. Data represent a minimum of three experiments. Standard error bars are shown. GAPDH levels for the control transfectants gave an average CT value of 21, while the Bright-Btk-p70-transfected cells gave a value of 22 and were within a cycle for each of the other transfectants. Baseline values averaged 37 to 40. (b) Western blots of proteins from transfected cells used in panel a demonstrate equivalent levels of Bright and TFII-I proteins in the transfected cells. IB, immunoblotting.
Tyrosine mutants of TFII-I fail to induce Ig mRNA in the presence of Bright and Btk.
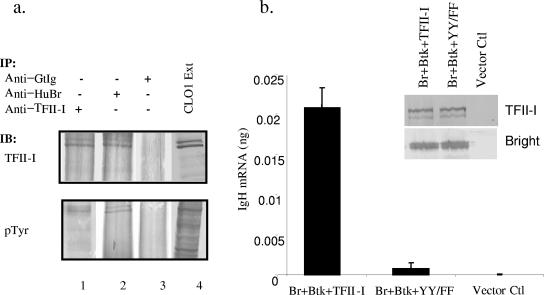
Earlier studies indicated that the kinase activity of Btk was critically required for Bright-induced transcription of the Ig reporter gene (32). However, Bright itself was not appreciably phosphorylated. Both anti-Bright and anti-TFII-I antibodies precipitated TFII-I from the human CL01 B-cell line (Fig. 7a). When immunoblotted with antiphosphotyrosine-specific antibodies, both isoforms of TFII-I were phosphorylated. More importantly, transfection of YY248/249FF TFII-I with Bright and Btk produced less transcription of the Ig reporter gene than when wild-type TFII-I was used (Fig. 7b), suggesting that, in this model system, the YY/FF mutant functions as a dominant negative protein. Together, these experiments support previous data demonstrating that TFII-I is a substrate for Btk (12, 37) and suggest a relationship between the phosphorylation state of TFII-I and Bright functional activity.
FIG. 7.
Tyrosines within TFII-I are important for its contribution to Bright activity. (a) Extracts (Ext) from the human B-cell line CL01 (lane 4) were immunoprecipitated (IP) with anti-TFII-I (lane 1), anti-human Bright (Anti-HuBr) (lane 2), or control goat Ig (Anti-GtIg) (lane 3), Western blotted (immunoblotted [IB]), and developed with anti-pTyr or anti-TFII-I antibodies. The 120 and 128 isoforms of TFII-I are identified. (b) Quantitative real-time PCR was used to measure IgH mRNA in sorted GFP+ cells transfected with a reporter plasmid and expression vectors for Bright, Btk, and either wild-type TFII-I or the YY248/249FF (YY/FF) mutant form of TFII-I. Control cells (Ctl) were transfected with the same quantity of DNA but used empty vectors with the reporter plasmid. GAPDH CT values from triplicate experiments varied from 23 to 25 among the three transfected groups. Data were taken from three independent experiments. Standard error bars are indicated.
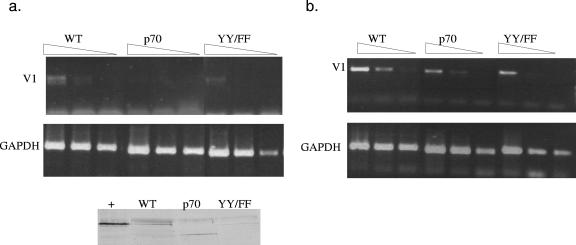
Inhibition of TFII-I activity in a B-cell line affects immunoglobulin heavy-chain transcript levels. The CHIP data in Fig. 2 suggested that endogenous Bright, Btk, and TFII-I bound 5′ of the V1 promoter in activated BCg3R-1d cells. Unlike plasma cell lines that express the V1 gene, this cell line continues to express endogenous Btk. To determine if the Bright complex in these cells was susceptible to inhibition by dominant negative forms of TFII-I, similarly to the reporter gene used in Fig. 6 and 7, BCg3R-1d cells were transfected with a GFP expression construct and either wild-type TFII-I, p70 dominant negative TFII-I, or the tyrosine YYFF mutant. Transfected cells were identified by GFP expression and isolated by flow cytometry. Semiquantitative PCR was performed for both GAPDH and the V1-containing IgM heavy chain. Figure 8 shows the results of two of three experiments performed. Both the p70 and tyrosine mutants of TFII-I inhibited immunoglobulin transcript levels relative to wild-type TFII-I levels. These data are consistent with the results obtained using the Cos-7 model system and suggest that Bright activation of the V1 immunoglobulin gene in this B-cell line requires TFII-I.
FIG. 8.
Dominant negative TFII-I reduces immunoglobulin mRNA levels in a B-cell line. Serial dilutions of cDNA prepared from wild-type (WT), p70, or YYFF TFII-I transfected BCg3R-1d cells were PCR amplified for the V1 μ heavy chain and GAPDH. The results of two individual experiments are shown (a and b). Quantification of the V1 levels relative to the GAPDH level in each lane indicated threefold reductions relative to WT levels for p70 transcripts in both experiments and threefold and twofold reductions for the YYFF mutant in panels a and b, respectively. A Western blot indicates relative transfected protein levels in the lower portion of panel a. The positive control in the first lane (+) is an extract from Cos-7 transfected cells containing wild-type TFII-I.
Exogenous TFII-I enhances Bright DNA-binding activity.
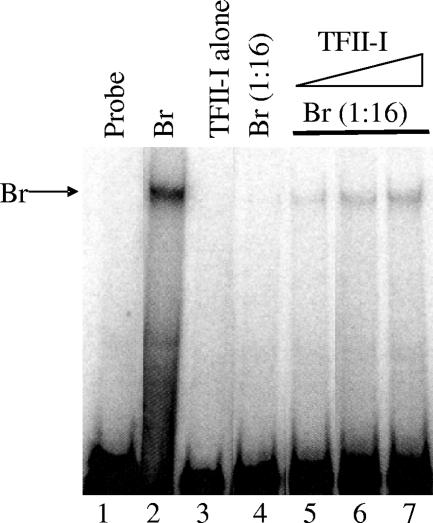
Although the previous experiments suggest that TFII-I is critical for Bright function as a transcription activator, the mechanism(s) by which TFII-I contributes to increased transcription is unknown. Previously, we reported that exogenous Btk appeared to stabilize Bright DNA-binding activity by EMSA (32). To determine if TFII-I also contributes to the stability of the Bright complex, purified TFII-I was used in an EMSA with Cos-7 extracts containing suboptimal levels of Bright (Fig. 9). Suboptimal levels of Bright (diluted 1:16 in lane 4) showed little to no binding activity alone. Similarly, TFII-I alone did not show any significant binding to the probe under these conditions (lane 3). However, when increasing amounts of TFII-I were added to Bright (lanes 5 to 7), the Bright DNA-binding activity was increased in a concentration-dependent fashion. These data suggest that TFII-I contributes to the stability of the Bright complex but does not bind to the 150-bp fragment of DNA containing the Bright binding site with enough stability to be detected by EMSA.
FIG. 9.
Recombinant TFII-I enhances Bright DNA-binding activity. Mobility shift assay mixtures containing extracts from Bright (Br)-expressing Cos-7 cells (neat in lane 2 or at a suboptimal 1:16 dilution in lanes 4 to 7) were incubated with 2, 3, and 4 μg (lanes 5, 6, and 7, respectively) of purified TFII-I prior to addition of the bf150 Bright binding site probe. Probe alone was assayed in lane 1. An arrow indicates the Bright complex.
DISCUSSION
Our earlier studies showing that kinase-active Btk was required for Bright activity in a model system using an Ig reporter assay suggested that a Btk substrate was critical for transcription activation in this assay (32). Data presented here show that TFII-I is a component of the Bright DNA-binding complex and that it is associated with endogenous Bright in a human B-cell line. A region of 85 amino acids highly conserved between mouse and human Bright was required for the association of Bright and TFII-I. Experiments using dominant negative forms of TFII-I, including one that was not efficiently phosphorylated by Btk, indicated that TFII-I was critically required for Bright-induced activation of an Ig reporter gene. Furthermore, dominant negative forms of TFII-I also effectively inhibited Ig transcript levels in a B-cell line. These data extend our previous studies indicating an important role for Btk in Bright function and provide functional evidence that supports other studies suggesting a link between Btk-induced phosphorylation of TFII-I and human B-lymphocyte activation (45).
Mobility supershift, CHIP, and immunoprecipitation experiments indicate that TFII-I is a component of the Bright complex. Results from Fig. 2 show that endogenous forms of Bright, Btk, and TFII-I proteins associate in a human B-cell line. Both the 120- and 128-kDa isoforms of TFII-I coprecipitated with Bright. Dimerization of TFII-I has been reported, and several isoforms are expressed in multiple cell lines (5, 8). Previous data showed coprecipitation of 120 and 128 isoforms with Btk from activated human B cells (45). These data are consistent with earlier data identifying Btk as a functionally important component of the Bright complex (32). They further suggest that Btk, Bright, and TFII-I form a protein complex that is recruited to the Ig locus.
The domains important for mouse Bright activity have been described previously (15) and are predicted to be similar in the human protein due to the high homology between mouse and human Bright/ARID3a. Because Btk defects result in human disease that differs phenotypically from the murine disease by exhibiting earlier blocks in B-lymphocyte development (9), it was important to examine the structural properties of human Bright as well as its association with Btk and TFII-I. Mobility shift data demonstrate that a point mutation within the ARID domain of human Bright is sufficient to interfere with DNA binding, as has been observed for murine Bright (27). Furthermore, the data presented in Fig. 4 and Table 1 support previous findings, suggesting that DNA-binding activity and the ability to form dimers are strongly correlated (15, 27) and extend those studies to better define the amino acids involved in human Bright. Finally, we show that an 85-amino-acid sequence within the protein interaction domain that partially overlaps the region required for Bright dimerization is critical for TFII-I interactions. Immunoprecipitation data from transfected Cos-7 cells (Fig. 5) suggest that TFII-I can associate with human Bright in the absence of Btk. The domains required for association of Btk with Bright have not been mapped because most systems also contain endogenous TFII-I. Indeed, Btk may associate with Bright only through its interactions with TFII-I.
The functional significance of TFII-I in the Bright complex was demonstrated using an in vitro model system previously shown to be dependent on both Bright DNA-binding activity and Btk kinase activity (32). Although TFII-I is ubiquitously expressed, addition of exogenous TFII-I further enhanced Bright-dependent activation of the Ig reporter gene over twofold (Fig. 6). These results are similar to findings of Hirano et al., in whose work enforced expression of TFII-I led to increased transcriptional induction of a reporter by Btk and the activation domain of BAM11 (16). However, gene targets for BAM11 have not yet been identified. Expression of the p70 dominant negative form of TFII-I that lacks an activation domain inhibited Vβ promoter activity in a T-cell system (7). Dominant negative TFII-I expression in our model system resulted in only 25% of the transcription induced by Bright and Btk alone and only 10% of the activity observed when wild-type TFII-I was expressed with Bright and Btk. However, transcription of the housekeeping gene GAPDH was only modestly affected by either form of TFII-I, suggesting that the effects observed with the Ig reporter were not due to general effects on all transcription. These data indicate that TFII-I is critically required for Bright-induced Ig transcription in this model system.
TFII-I was originally identified as a substrate for Btk in B cells (45). TFII-I is a large protein and has multiple potential phosphorylation sites (6, 8) which may play important roles in many intracellular pathways. Indeed, most cells contain phosphorylated forms of TFII-I, and the TFII-I that coprecipitated with Bright in CL01 cells was no exception (Fig. 7). Several tyrosines toward the amino terminus of TFII-I were suggested to be phosphorylated by Btk (12, 37). Bright-dependent transcription activation in our in vitro model system was totally dependent upon tyrosines at positions 248 and 249 of TFII-I (Fig. 7), suggesting that the mutations in TFII-I resulted in a dominant negative protein. These data are consistent with previous experiments showing a critical requirement for Btk kinase activity for Bright-induced transcription activation (32).
Although we have not addressed whether the requirement for TFII-I in Bright-induced transcription extends to all Bright target genes, V1 Ig gene expression in the B-cell line BCg3R-1d was inhibited by both the p70 form of TFII-I and the YYFF tyrosine mutant (Fig. 8). Transfection of wild-type TFII-I did not affect Ig transcription in those cells. These data support the findings from the in vitro model system and suggest that endogenous Bright and Btk can be inhibited by dominant negative forms of TFII-I. However, it is not clear whether TFII-I/Bright/Btk complexes are important for the transcription of all immunoglobulin heavy-chain genes, as some VH promoter-flanking regions do not contain obvious Bright binding sites (14). Nonetheless, a model for Bright-enhanced activation of at least some Ig genes involving Bright DNA-binding activity—interaction of Bright with TFII-I/Btk and subsequent phosphorylation of TFII-I by Btk—can be envisioned.
The precise mechanism by which TFII-I contributes to transcription activation remains unclear, but data from Fig. 9 suggest that one function of TFII-I is to stabilize Bright DNA-binding activity. Our chromatin-facilitated immunoprecipitation data indicate that Bright and TFII-I are recruited to the Ig promoter of the V1 gene. However, these experiments do not imply that TFII-I binds directly to the Bright binding motif, or even nearby sequences. Indeed, TFII-I alone failed to bind the 150-bp fragment containing the prototype Bright site by EMSA (Fig. 9). In other systems, TFII-I has been shown to bind upstream of basal reporter sequences (17, 21, 30). Therefore, sequences between the Bright site and the transcription start site may bind TFII-I. It is less likely that sequences upstream of the Bright sequence are important for Ig activation, because such sequences are absent from the Ig reporter construct. On the other hand, TFII-I was originally suggested to play an important role in activating TATA-less promoters through sequences near the transcription start site and was even shown to play a role in the transcription of immunoglobulin promoters (7, 11, 39). The V1 reporter system used here lacks a typical consensus TATA box and contains several putative initiator-like sequences. Additional experiments will be required to determine if TFII-I binds directly to the V1 promoter region and which additional promoter sequences are critical for Bright-dependent TFII-I activation.
The data presented here are the first to show that TFII-I associates with the transcription factor Bright and that this association is functionally significant for Ig transcription in vitro. In addition, these data extend findings demonstrating that Btk is also a key component of the Bright regulatory complex and suggest that its major role in this system is to phosphorylate TFII-I. Other cytoplasmic roles for Btk contribute significantly to events in B-cell activation (1, 3, 19, 31, 38), but the functional significance of the nuclear Btk reported by our lab and others (26, 44) was not clear. Our data now suggest that nuclear Btk, Bright, and TFII-I may be important for Ig transcription in vivo.
Defects in Btk have long been associated with blocks in B-lymphocyte development and subsequent failure to produce serum Ig (10, 33, 40). The data presented here do not address whether the Bright/TFII-I/Btk complex plays a role in B-cell development through regulation of the Ig locus. Nor do they rule out additional roles for Btk that do not require Bright and/or TFII-I. However, it is interesting to speculate that failure to upregulate surface Ig in antigen-stimulated B cells due to inappropriate functioning of the Bright complex could result in blocks in B-cell differentiation. Alternatively, other critically important gene targets for Bright/Btk/TFII-I may contribute to B-cell differentiation. Additional experiments using Bright-deficient systems are in progress to explore these questions.
Acknowledgments
We thank A. Windell and T. D. Ashworth for technical assistance, S. Hutchinson for TFII-I purification, X.-H. Sun for critically reading the manuscript, and K. Humphrey for help in its preparation.
This work was supported by the USIDNET and NIH grants AI044215 (C.F.W.) and AI45150 (A.L.R.). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program grant C06 RR14570-01 from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.Aoki, Y., K. J. Isselbacher, and S. Pillai. 1994. Bruton tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proc. Natl. Acad. Sci. USA 91:10606-10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, Y., M. Matsushita, Y. Matsuda, J. Inazawa, T. Yamadori, S. Hashimoto, T. Kishimoto, and S. Tsukada. 1999. Assignment of SH3BP5/Sh3bp5 encoding Sab, an SH3 domain-binding protein which preferentially associates with Bruton's tyrosine kinase, to human chromosome 1q43 and mouse chromosome 14B by in situ hybridization. Cytogenet. Cell Genet. 87:221-222. [DOI] [PubMed] [Google Scholar]
- 3.Cariappa, A., M. Tang, C. Parng, E. Nebelitskiy, M. Carroll, K. Georgopoulos, and S. Pillai. 2001. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity 14:603-615. [DOI] [PubMed] [Google Scholar]
- 4.Chaplet, M., C. Detry, C. Deroanne, L. W. Fisher, V. Castronovo, and A. Bellahcene. 2004. Zoledronic acid up-regulates bone sialoprotein expression in osteoblastic cells through Rho GTPase inhibition. Biochem. J. 384:591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheriyath, V., and A. L. Roy. 2000. Alternatively spliced isoforms of TFII-I—complex formation, nuclear translocation, and differential gene regulation. J. Biol. Chem. 275:26300-26308. [DOI] [PubMed] [Google Scholar]
- 6.Cheriyath, V., Z. P. Desgranges, and A. L. Roy. 2002. c-Src-dependent transcriptional activation of TFII-I. J. Biol. Chem. 277:22798-22805. [DOI] [PubMed] [Google Scholar]
- 7.Cheriyath, V., C. D. Novina, and A. L. Roy. 1998. TFII-I regulates Vβ promoter activity through an initiator element. Mol. Cell. Biol. 18:4444-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheriyath, V., and A. L. Roy. 2001. Structure-function analysis of TFII-I—roles of the N-terminal end, basic region, and I-repeats. J. Biol. Chem. 276:8377-8383. [DOI] [PubMed] [Google Scholar]
- 9.Conley, M. E. 2002. Early defects in B cell development. Curr. Opin. Allergy Clin. Immunol. 2:517-522. [DOI] [PubMed] [Google Scholar]
- 10.Conley, M. E., A. Broides, V. Hernandez-Trujillo, V. Howard, H. Kanegane, T. Miyawaki, and S. A. Shurtleff. 2005. Genetic analysis of patients with defects in early B-cell development. Immunol. Rev. 203:216-234. [DOI] [PubMed] [Google Scholar]
- 11.Du, H., A. L. Roy, and R. G. Roeder. 1993. Human transcription factor USF stimulates transcription through the initiator elements of the HIV-1 and the Ad-ML promoters. EMBO J. 12:501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egloff, A. M., and S. Desiderio. 2001. Identification of phosphorylation sites for Bruton's tyrosine kinase within the transcriptional regulator BAP/TFII-I. J. Biol. Chem. 276:27806-27815. [DOI] [PubMed] [Google Scholar]
- 13.Fruman, D. A., A. B. Satterthwaite, and O. N. Witte. 2000. Xid-like phenotypes: a B cell signalosome takes shape. Immunity 13:1-3. [DOI] [PubMed] [Google Scholar]
- 14.Goebel, P., A. Montalbano, N. Ayers, E. Kompfner, L. Dickinson, C. F. Webb, and A. J. Feeney. 2002. High frequency of matrix attachment regions and cut-like protein x/CCAAT-displacement protein and B cell regulator of IgH transcription binding sites flanking Ig V region genes. J. Immunol. 169:2477-2487. [DOI] [PubMed] [Google Scholar]
- 15.Herrscher, R. F., M. H. Kaplan, D. L. Lelsz, C. Das, R. Scheuermann, and P. W. Tucker. 1995. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes Dev. 9:3067-3082. [DOI] [PubMed] [Google Scholar]
- 16.Hirano, M., Y. Kikuchi, S. Nisitani, A. Yamaguchi, A. Satoh, T. Ito, H. Iba, and K. Takatsu. 2004. Bruton's tyrosine kinase (Btk) enhances transcriptional co-activation activity of BAM11, a Btk-associated molecule of a subunit of SWI/SNF complexes. Int. Immunol. 16:747-757. [DOI] [PubMed] [Google Scholar]
- 17.Hong, M., M. Lin, J. M. Huang, P. Baumeister, S. Hakre, A. L. Roy, and A. S. Lee. 2005. Transcriptional regulation of the Grp78 promoter by endoplasmic reticulum stress: role of TFII-I and its tyrosine phosphorylation. J. Biol. Chem. 280:16821-16828. [DOI] [PubMed] [Google Scholar]
- 18.Humphries, L. A., C. Dangelmaier, K. Sommer, K. Kipp, R. M. Kato, N. Griffith, I. Bakman, C. W. Turk, J. L. Daniel, and D. J. Rawlings. 2004. Tec kinases mediate sustained calcium influx via site-specific tyrosine phosphorylation of the phospholipase C γ Src homology 2-Src homology 3 linker. J. Biol. Chem. 279:37651-37661. [DOI] [PubMed] [Google Scholar]
- 19.Khan, W. N. 2001. Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase. Immunol. Res. 23:147-156. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi, Y., M. Hirano, M. Seto, and K. Takatsu. 2000. Identification and characterization of a molecule, BAM11, that associates with the pleckstrin homology domain of mouse Btk. Int. Immunol. 12:1397-1408. [DOI] [PubMed] [Google Scholar]
- 21.Kim, D. W., V. Cheriyath, A. L. Roy, and B. H. Cochran. 1998. TFII-I enhances activation of the c-fos promoter through interactions with upstream elements. Mol. Cell. Biol. 18:3310-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, D. W., and B. H. Cochran. 2000. Extracellular signal-regulated kinase binds to TFII-I and regulates its activation of the c-fos promoter. Mol. Cell. Biol. 20:1140-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, D. W., and B. H. Cochran. 2001. JAK2 activates TFII-I and regulates its interaction with extracellular signal-regulated kinase. Mol. Cell. Biol. 21:3387-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahajan, S., A. Vassilev, N. Sun, Z. Ozer, C. Mao, and F. M. Uckun. 2001. Transcription factor STAT5A is a substrate of Bruton's tyrosine kinase in B cells. J. Biol. Chem. 276:31216-31228. [DOI] [PubMed] [Google Scholar]
- 25.Maret, D., M. B. Boffa, D. F. Brien, A. E. Nesheim, and A. L. Koschinsky. 2004. Role of mRNA transcript stability in modulation of expression of the gene encoding thrombin activable fibrinolysis inhibitor. J. Thromb. Haemost. 2:1969-1979. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed, A. J., L. Vargas, B. F. Nore, C. M. Backesjo, B. Christensson, and C. I. Smith. 2000. Nucleocytoplasmic shuttling of Bruton's tyrosine kinase. J. Biol. Chem. 275:40614-40619. [DOI] [PubMed] [Google Scholar]
- 27.Nixon, J. C., J. Rajaiya, and C. F. Webb. 2004. Mutations in the DNA-binding domain of the transcription factor Bright act as dominant negative proteins and interfere with immunoglobulin transactivation. J. Biol. Chem. 279:52465-52472. [DOI] [PubMed] [Google Scholar]
- 28.Nixon, J. C., J. B. Rajaiya, N. Ayers, S. Evetts, and C. F. Webb. 2004. The transcription factor, Bright, is not expressed in all human B lymphocyte subpopulations. Cell. Immunol. 228:42-53. [DOI] [PubMed] [Google Scholar]
- 29.Novina, C. D., S. Kumar, U. Bajpai, V. Cheriyath, K. M. Zhang, S. Pillai, H. H. Wortis, and A. L. Roy. 1999. Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton's tyrosine kinase. Mol. Cell. Biol. 19:5014-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker, R., T. Phan, P. Baumeister, B. Roy, V. Cheriyath, A. L. Roy, and A. S. Lee. 2001. Identification of TFII-I as the endoplasmic reticulum stress response element binding factor ERSF: its autoregulation by stress and interaction with ATF6. Mol. Cell. Biol. 21:3220-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petro, J. B., and W. N. Khan. 2001. Phospholipase C-γ2 couples Bruton's tyrosine kinase to the NF-κB signaling pathway in B lymphocytes. J. Biol. Chem. 276:1715-1719. [DOI] [PubMed] [Google Scholar]
- 32.Rajaiya, J., M. Hatfield, J. C. Nixon, D. J. Rawlings, and C. F. Webb. 2005. Bruton's tyrosine kinase regulates immunoglobulin promoter activation in association with the transcription factor Bright. Mol. Cell. Biol. 25:2073-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlings, D. J., D. C. Saffran, S. Tsukada, D. A. Largaespada, J. C. Grimaldi, L. Cohen, R. N. Mohr, J. F. Bazan, M. Howard, N. G. Copeland, N. A. Jenkins, and O. N. Witte. 1993. Mutation of the unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261:358-361. [DOI] [PubMed] [Google Scholar]
- 34.Roy, A. L. 2001. Biochemistry and biology of the inducible multifunctional transcription factor TFII-1. Gene 274:1-13. [DOI] [PubMed] [Google Scholar]
- 35.Roy, A. L., H. Du, P. D. Gregor, C. D. Novina, E. Martinez, and R. G. Roeder. 1997. Cloning of an Inr- and E-box-binding protein, TFII-I, that interacts physically and functionally with USF1. EMBO J. 16:7091-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy, A. L., M. Meisterernst, P. Pognonec, and R. G. Roeder. 1991. Cooperative interaction of an initiator-binding transcription initiation factor and the helix-loop-helix activator USF. Nature 354:245-248. [DOI] [PubMed] [Google Scholar]
- 37.Sacristan, C., M. I. Tussie-Luna, S. M. Logan, and A. L. Roy. 2004. Mechanism of Bruton's tyrosine kinase-mediated recruitment and regulation of TFII-I. J. Biol. Chem. 279:7147-7158. [DOI] [PubMed] [Google Scholar]
- 38.Satterthwaite, A. B., and O. N. Witte. 2000. The role of Bruton's tyrosine kinase in B-cell development and function: a genetic perspective. Immunol. Rev. 175:120-127. [PubMed] [Google Scholar]
- 39.Tantin, D., M. I. Tussie-Luna, A. L. Roy, and P. A. Sharp. 2004. Regulation of immunoglobulin promoter activity by TFII-I class transcription factors. J. Biol. Chem. 279:5460-5469. [DOI] [PubMed] [Google Scholar]
- 40.Thomas, J. D., P. Sideras, C. I. E. Smith, I. Vorechovsky, V. Chapman, and W. E. Paul. 1993. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261:355-358. [DOI] [PubMed] [Google Scholar]
- 41.Tsukada, S., M. I. Simon, O. N. Witte, and A. Katz. 1994. Binding of beta gamma subunits of heterotrimeric G proteins to the PH domain of Bruton tyrosine kinase. Proc. Natl. Acad. Sci. USA 91:11256-11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb, C. F., C. Das, R. L. Coffman, and P. W. Tucker. 1989. Induction of immunoglobulin μ mRNA in a B cell transfectant stimulated with interleukin-5 and a T-dependent antigen. J. Immunol. 143:3934-3939. [PubMed] [Google Scholar]
- 43.Webb, C. F., C. Das, S. Eaton, K. Calame, and P. W. Tucker. 1991. Novel protein-DNA interactions associated with increased immunoglobulin transcription in response to antigen plus interleukin-5. Mol. Cell. Biol. 11:5197-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb, C. F., Y. Yamashita, N. Ayers, S. Evetts, Y. Paulin, M. E. Conley, and E. A. Smith. 2000. The transcription factor Bright associates with Bruton's tyrosine kinase, the defective protein in immunodeficiency disease. J. Immunol. 165:6956-6965. [DOI] [PubMed] [Google Scholar]
- 45.Yang, W. Y., and S. Desiderio. 1997. BAP-135, a target for Bruton's tyrosine kinase in response to B cell receptor engagement. Proc. Natl. Acad. Sci. USA 94:604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]