Abstract
The Ca2+/calmodulin-dependent phosphatase calcineurin promotes yeast survival during environmental stress. We identified Slm1 and Slm2 as calcineurin substrates required for sphingolipid-dependent processes. Slm1 and Slm2 bind to calcineurin via docking sites that are required for their dephosphorylation by calcineurin and are related to the PXIXIT motif identified in NFAT. In vivo, calcineurin mediates prolonged dephosphorylation of Slm1 and Slm2 during heat stress, and this response can be mimicked by exogenous addition of the sphingoid base phytosphingosine. Slm proteins also promote the growth of yeast cells in the presence of myriocin, an inhibitor of sphingolipid biosynthesis, and regulation of Slm proteins by calcineurin is required for their full activity under these conditions. During heat stress, sphingolipids signal turnover of the uracil permease, Fur4. In cells lacking Slm protein activity, stress-induced endocytosis of Fur4 is blocked, and Fur4 accumulates at the cell surface in a ubiquitinated form. Furthermore, cells expressing a version of Slm2 that cannot be dephosphorylated by calcineurin display an increased rate of Fur4 turnover during heat stress. Thus, calcineurin may modulate sphingolipid-dependent events through regulation of Slm1 and Slm2. These findings, in combination with previous work identifying Slm1 and Slm2 as targets of Mss4/phosphatidylinositol 4,5-bisphosphate and TORC2 signaling, suggest that Slm proteins integrate information from a variety of signaling pathways to coordinate the cellular response to heat stress.
Calcineurin is a Ca2+- and calmodulin-regulated serine/threonine protein phosphatase that is highly conserved from unicellular eukaryotes, such as the budding yeast Saccharomyces cerevisiae, to humans and plays a critical role in Ca2+-dependent signaling in these organisms (2). In mammalian cells, calcineurin mediates a plethora of physiological processes, including T-cell activation, skeletal and cardiac muscle development, long-term potentiation, and neural outgrowth, as well as pathophysiological processes such as cardiac hypertrophy (7, 11, 28, 34). Specific inhibitors of calcineurin, FK506 and cyclosporine A, are in wide clinical use as immunosuppressants. In yeast cells, calcineurin is dispensable for growth under standard laboratory conditions. However, calcineurin activity becomes crucial for survival during specific stress conditions (15). Indeed, calcineurin is activated in mutants with cell wall defects and by a variety of environmental stresses, including high concentrations of Na+, Li+, and Mn2+ ions; high pH; heat stress; and prolonged exposure to mating factor (reviewed in reference 15). Calcineurin is a heterodimer consisting of a large catalytic subunit (A subunit; 59 kDa), encoded in S. cerevisiae by two functionally redundant genes, CNA1 and CNA2, and a small regulatory subunit (B subunit; 19 kDa), encoded by CNB1 (16, 17, 48, 53). Extracellular stresses promote a transient rise in the cytosolic Ca2+ concentration and subsequent activation of calcineurin through binding of Ca2+/calmodulin (reviewed in reference 15). A major role of calcineurin is to regulate gene expression via the transcription factor Crz1, which, upon dephosphorylation by calcineurin, accumulates in the nucleus and directs the transcription of genes that promote cell survival during stress (56, 65, 66, 74).
An important feature of calcineurin signaling is its direct interaction with substrates and regulators via a conserved docking site that is distinct from sites of dephosphorylation. This property was first characterized for NFAT (nuclear factor of activated T cells), a family of mammalian transcription factors (1, 55). A small motif with the consensus amino acid sequence PXIXIT is responsible for calcineurin binding to NFAT proteins and is required for their dephosphorylation (1). Furthermore, this docking site is conserved in other calcineurin-interacting proteins (18, 67). In yeast, calcineurin binds to Crz1 via the PXIXIT-related sequence PIISIQ, and this site is required for its regulation by calcineurin (10).
We searched for novel calcineurin substrates by identifying calcineurin-interacting proteins. One of these proteins is Slm2, which was found to interact with the A subunit of calcineurin in a genome-wide yeast two-hybrid screen (69). In this study, we show that Slm2 as well as its homologue, Slm1, binds calcineurin via a PXIXIT-related motif and that both proteins are dephosphorylated by calcineurin in vivo and in vitro. In each case, this dephosphorylation requires the calcineurin-docking site.
Recent studies provide insights into the physiological functions of Slm1 and Slm2 (4, 27). SLM1 and SLM2 form an essential gene pair, and each gene product contains a conserved pleckstrin homology domain (PH domain) that binds phosphatidylinositol 4,5-bisphosphate (PIP2) (75). The essential lipid kinase Mss4 synthesizes PIP2 in the plasma membrane (19, 40), and this PIP2 production recruits Slm1 and Slm2 to the cell periphery (75). Slm proteins seem to be critical downstream effectors of Mss4/PIP2, as slm1Δ is synthetically lethal with mss4ts, and like Mss4, Slm1 and Slm2 are required for polarization of the actin cytoskeleton (4, 27). In addition, Slm1 is phosphorylated by TORC2, a Tor2 kinase-containing complex that regulates actin cytoskeleton organization; this phosphorylation is required for its localization to the cell periphery (4). Furthermore, an avo3ts mutant which lacks an essential subunit of the TORC2 complex is suppressed by SLM1 overexpression (39). Thus, Slm proteins are downstream effectors of both PIP2 and TORC2 signaling.
In addition, Slm proteins participate in the cellular response to heat stress. Heat stress causes a transient increase in PIP2 levels as well as a transient depolarization of the actin cytoskeleton (19). The temporal pattern of Slm1 phosphorylation is modulated in response to heat stress and parallels the depolarization/repolarization of the cytoskeleton (4). Furthermore, slm1ts slm2Δ cells are suppressed by mutations that activate the cell integrity pathway, a key component of cell tolerance to elevated temperature (4, 50). In this study we show that high temperature leads to increased phosphorylation of Slm1 and Slm2 by protein kinases, which is counteracted by their dephosphorylation by calcineurin.
Another component of the heat stress response is the production of sphingolipids. When cells are exposed to high temperature, levels of dihydrosphingosine and phytosphingosine, the major sphingoid bases in yeast, increase within minutes; these increased levels are required for tolerance to heat stress (21, 44). One consequence of phytosphingosine accumulation is inhibition of nutrient uptake via turnover of permeases, such as the uracil permease, Fur4 (13, 14, 30). Recently, the PDK1-related kinases Pkh1 and Pkh2 were identified as signaling effectors of phytosphingosine (12, 29, 77). These kinases are required to maintain cell integrity and for endocytosis and act in part through activation of downstream kinases including Pkc1, Ypk1, and Ypk2 (29, 41, 61). Interestingly, overexpression of YPK1 confers resistance to myriocin, a potent inhibitor of the de novo sphingolipid biosynthesis pathway (68).
Here, we demonstrate a novel role for Slm1 and Slm2 in sphingolipid signaling and/or metabolism. We show that the Slm proteins are required for growth of yeast cells in the presence of myriocin and for endocytosis of Fur4 during heat stress. Both of these aspects of Slm function are altered by mutations that abrogate the ability of calcineurin to dephosphorylate Slms, suggesting that calcineurin acts through Slm1 and Slm2 to regulate sphingolipid-dependent events. Thus, Slm1 and Slm2 apparently coordinate TORC2, Mss4/PIP2, sphingolipid, and Ca2+/calcineurin signaling to drive actin polarization and promote endocytosis of a nutrient permease during heat stress.
MATERIALS AND METHODS
Yeast strains and media.
Yeast media and culture conditions were essentially as previously described (62), except that twice the levels of amino acids and nucleotides were used in synthetic media. Yeast transformations were performed with the lithium acetate method (5). Yeast strains are listed in Table 1; strains from the yeast deletion collection were purchased from Open Biosystems (Huntsville, AL).
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| PJ69-4A | MATatrp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ | 43 |
| YPH499 | MATaura3-52 lys2-801 ade2-101 trp1 Δ63 his3-Δ200 leu2-Δl | 63 |
| DD12 | As for YPH499 except cnb1::URA3::hisG | 17 |
| BY4741 | MATaleu2Δ ura3Δ met15Δ his3Δ | Open Biosystems |
| VHY61 | MATα leu2Δ ura3Δ met15Δ his3Δ slm2Δ::KANR | This study |
| VHY66 | As for BY4741; slm1Δ::KANR | This study |
| GBY059 | As for VHY61; slm1Δ::NATR + pRS316-Slm2 | This study |
| SEY6210.1 | MATaleu2-3 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | 4 |
| AAY1602 | As for SEY6210.1; slm1Δ::HIS3 | 4 |
| AAY1610 | As for SEY6210.1; slm2Δ::HIS3 | 4 |
| AAY1623.2 | As for SEY6210.1; slm1Δ::HIS3 slm2Δ::HIS3 + pRS415-slm1-2 (LEU2 CEN6 slm1-2) | 4 |
| LBY66 | As for YPH499 except 4× CDRE::lacZ::TRP1 | Laboratory stock |
| JK9-3da | MATaleu2-3,112 trp1 ura3 rme1 his4 HMLa | 49 |
| SH121 | As for JK9-3daade2 tor2::ADE2/YPplac111::tor2-21 | 38 |
| NY13 | MATa ura3-52 gal2 | 33 |
| NY279 | MATa act1-3 ura3-52 gal2 | 33 |
| FY56 | MATα his4-912δ lys2-128δ ura3-52 | Fred Winston |
| FW1808 | MATα rsp5-1 his4-912δ lys2-128δ ura3-52 | Fred Winston |
| GBY030 | YPH499 + pRD56-SLM2 | This study |
| GBY031 | YPH499 + pRD56-SLM1 | This study |
| GBY032 | DD12 + pRD56-SLM2 | This study |
| GBY033 | DD12 + pRD56-SLM1 | This study |
| GBY115 | As for VHY61; slm1Δ::NATR + pRD56-SLM2 | This study |
| GBY116 | As for VHY61; slm1Δ::NATR + pRD56-SLM2ΔPEFYIE | This study |
| GBY117 | As for VHY61; slm1Δ::NATR + pRD56-SLM1 | This study |
| GBY118 | As for VHY61; slm1Δ::NATR + pRD56-SLM1ΔPNIYIQ | This study |
| GBY152 | As for GBY059 + pRS313 | This study |
| GBY153 | As for GBY059 + pRS313-SLM2 | This study |
| GBY154 | As for GBY059 + pRS313-SLM2ΔPEFYIE | This study |
| GBY155 | As for GBY059 + pRS313-SLM1 | This study |
| GBY156 | As for GBY059 + pRS313-SLM1ΔPNIYIQ | This study |
| GBY100 | As for VHY61; slm1Δ::NATR + pRS313-SLM2 | This study |
| GBY101 | As for VHY61; slm1Δ::NATR + pRS313-SLM2ΔPEFYIE | This study |
| GBY102 | As for VHY61; slm1Δ::NATR + pRS313-SLM1 | This study |
| GBY103 | As for VHY61; slm1Δ::NATR + pRS313-SLM1ΔPNIYIQ | This study |
| GBY104 | As for VHY61; slm1Δ::NATR + pUG34-SLM2 | This study |
| GBY105 | As for VHY61; slm1Δ::NATR + pUG34-SLM2ΔPEFYIE | This study |
| GBY106 | As for VHY61; slm1Δ::NATR + pUG34-SLM1 | This study |
| GBY107 | As for VHY61; slm1Δ::NATR + pUG34-SLM1ΔPNIYIQ | This study |
| GBY160 | BY4741 + pRS313 | This study |
| GBY161 | BY4741 + pRS313-SLM2 | This study |
| GBY162 | BY4741 + pRS313-SLM1 | This study |
| GBY179 | SEY6210.1 + pVTu-FUR4 | This study |
| GBY180 | AAY1623.2 + pVTu-FUR4 | This study |
| GBY181 | GBY115 + pVTl-FUR4 | This study |
| GBY182 | GBY116 + pVTl-FUR4 | This study |
| GBY183 | GBY117 + pVTl-FUR4 | This study |
| GBY184 | GBY118 + pVTl-FUR4 | This study |
VHY61 is a haploid segregant of BY4743, slm2Δ::KANR (deletion collection). To create GBY59, the SLM1 open reading frame (ORF) of VHY61 transformed with pRS316-SLM2 was replaced by the natMX4 cassette via PCR, using pAG25 as the template (32). This strain was then transformed with all other Slm alleles expressed from different plasmids. The pRS316-Slm2 plasmid was lost by streaking out the transformants on selective medium supplemented with 5-fluorootic acid (5FOA; U.S. Biochemicals, Swampscott, MA).
The plasmids used in this study are reported in Table 2. Recombinant DNA procedures were performed as previously described (5). All genes were initially cloned by amplification of genomic DNA with Vent polymerase or with Taq high-fidelity polymerase (Invitrogen, Beverly, MA) to create fragments flanked by restriction sites. Cloned genes were subcloned in different expression vectors by PCR amplification of plasmid DNA with PfuUltra HotStart polymerase (Stratagene, Cedar Creek, TX). All clones were sequenced by Sequetech Corporation (Mountain View, CA).
TABLE 2.
Plasmids used in this study
| Plasmid | Vector | Insert/ORFa | Source or reference |
|---|---|---|---|
| pGBT9 | 6 | ||
| pACT2 | 35 | ||
| pRD56 | Gift from R. Deshaies | ||
| pRS316 | 63 | ||
| pRS313 | 63 | ||
| pUG34 | Gift from U Güldener and J. H. Hegemann | ||
| pVT102-U | 71 | ||
| BJP2014 | pGBT9 | GAL4DBD-CNA1 | 45 |
| pVH7 | pGBT9 | GAL4DBD-CNA2 | 36 |
| pGB003 | pACT2 | GAL4AD-SLM2 | This study |
| pGB004 | pACT2 | GAL4AD-SLM1 | This study |
| pGB005 | pACT2 | GAL4AD-SLM2ΔPEFYIE | This study |
| pGB006 | pACT2 | GAL4AD-SLM1ΔPNIYIQ | This study |
| pGB007 | pRD56 | GST-SLM2 | This study |
| pGB008 | pRD56 | GST-SLM2ΔPEFYIE | This study |
| pGB009 | pRD56 | GST-SLM1 | This study |
| pGB010 | pRD56 | GST-SLM1ΔPNIYIQ | This study |
| pGB013 | pRS316 | SLM2 | This study |
| pGB017 | pRS313 | SLM2 | This study |
| pGB018 | pRS313 | SLM2ΔPEFYIE | This study |
| pGB019 | pRS313 | SLM1 | This study |
| pGB020 | pRS313 | SLM1ΔPNIYIQ | This study |
| pGB029 | pUG34 | GFP-SLM2 | This study |
| pGB030 | pUG34 | GFP-SLM2ΔPEFYIE | This study |
| pGB031 | pUG34 | GFP-SLM1 | This study |
| pGB032 | pUG34 | GFP-SLM1ΔPNIYIQ | This study |
| pfF | pJDB207 | FUR4 | 72 |
| pGB047 | pVT102-U | FUR4 | This study |
DBD, DNA-binding domain; AD, activation domain.
For yeast two-hybrid studies, the pGBT9 and pACT2 vectors were used to create GAL4-binding and GAL4 activation domain fusions, respectively. All full-size and truncated versions of SLM1 and SLM2 were cloned as NcoI/XhoI fragments into pACT2 and as XmaI/SalI fragments into pGBT9. A PCR-based deletion strategy was used to delete codons 639 to 644 of SLM2, encoding PEFYIE, and codons 672 to 677 of SLM1, encoding PNIYIQ. All glutathione S-transferase (GST) and green fluorescent protein (GFP) expression plasmids were constructed by cloning SLM1 and SLM2 as XmaI/XhoI fragments. For pRS316 and pRS313 plasmids, we first cloned the promoters of SLM1 and SLM2 (500-bp sequences upstream of the respective ORFs) as SpeI/XmaI fragments. We then cloned the different SLM alleles as XmaI/XhoI fragments. FUR4 was subcloned in the pVT102-U overexpression plasmid as an XbaI/XhoI fragment amplified by PCR, using pfF as the template (70).
Chemical compounds.
Myriocin (Sigma, St. Louis, MO) was dissolved in methanol at a stock concentration of 1 mg/ml and was stored at 4°C. Aureobasidin A (Takara Mirus Bio, Madison, WI) was dissolved in ethanol at a stock concentration of 1 mg/ml and was stored at 4°C. Phytosphingosine (Sigma) was dissolved in ethanol at a stock concentration of 20 mM and was stored at −20°C. All these stock solutions were put at room temperature 30 min prior to their addition to media. FK506 (LC Laboratories, Woburn, MA) was dissolved in a mixture of 90% ethanol and 10% Tween 20 at a stock concentration of 20 mg/ml and stored at 4°C. FK506 was used at a final concentration of 2 μg/ml.
Spot assays.
Growth of various strains was assessed by spotting serial dilutions of stationary-phase yeast cultures on yeast-peptone-dextrose (YPD) plates containing various drugs or selective medium (for yeast two-hybrid analysis). Cultures were diluted to an optical density at 600 nm (OD600) of 2.0, and five or six fivefold serial dilutions were prepared and transferred to the plates. Plates were incubated at 30°C for 2 to 3 days unless otherwise indicated.
Yeast two-hybrid analysis.
Yeast two-hybrid assays were performed by introducing combinations of Gal4p activation and binding domain fusions into strain PJ69-4A, which contains a GAL1 promoter-HIS3 reporter gene, a GAL2 promoter-ADE2 reporter gene, and a GAL7 promoter-lacZ reporter gene (42, 43). Strains were grown in selective medium and spotted onto synthetic medium containing and lacking either histidine or adenine. β-Galactosidase activity was determined in exponentially growing yeast cells at 30°C as follows. Cells were harvested and washed once and the cell pellets were frozen. Cells were broken using glass bead lysis in breaking buffer (100 mM Tris [pH 8], 20% glycerol, 1 mM dithiothreitol [DTT]) plus protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM benzamidine, 2 μg/ml leupeptin, 2 μg/ml aprotinin). β-Galactosidase activity was measured at 30°C in a microtiter plate using 10 μl of total protein extract, 90 μl of Z-buffer (100 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 0.027% β-mercaptoethanol), and 20 μl of 4 mg/ml ONPG (O-nitrophenyl-β-d-galactopyranoside; Sigma). Protein concentrations of all cell extracts were determined using a Bio-Rad protein assay (Bio-Rad, Richmond, CA) and were used to calculate the specific β-galactosidase activity. Values result from averages of two independent extracts, each measured in triplicate.
Isolation of GST-tagged proteins.
Yeast cells expressing GST-Slm1 or GST-Slm2 were grown at 30°C in synthetic medium lacking uracil with 4% raffinose as the carbon source. Cells were diluted to an OD600 of 0.2 in synthetic medium lacking uracil with 4% raffinose as the carbon source. After 4 h, expression of the GST fusion proteins was induced by the addition of 2% galactose for 4 h. When indicated in Fig. 2, 3, and 5, FK506 (2 μg/ml) was added 30 min prior to the addition of galactose. Cells were harvested by centrifugation, and cell pellets were frozen at −80°C prior to the preparation of extracts. Cells were broken by using glass bead lysis in modified radioimmunoprecipitation assay buffer (20 mM Tris-HCl [pH 7.4], 2 mM EDTA, 750 mM NaCl, 1% Triton X-100, 1 mM DTT), supplemented with protease inhibitors (1 mM PMSF, 1 mM benzamidine, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 1 μg/ml aprotinin) and phosphatase inhibitors (5 mM sodium phosphate, 10 mM sodium molybdate, 20 mM sodium fluoride, 10 mM sodium pyrophosphate, 5 mM EGTA, and 5 mM EDTA). Protein concentrations of all cell extracts were determined using a Bio-Rad protein assay. Protein extracts (150 to 350 μg) were diluted fivefold in dilution buffer (20 mM Tris-HCl [pH 7.4], 2 mM EDTA, 150 mM NaCl, 1 mM DTT, 1 mM PMSF, 1 mM benzamidine, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 1 μg/ml aprotinin) and incubated for 2 h with glutathione-Sepharose beads preequilibrated with dilution buffer at 4°C. Beads were washed three times in wash buffer (20 mM Tris-HCl [pH 7.4], 2 mM EDTA, 150 mM NaCl, 0.5% Triton X-100, 1 mM DTT, 1 mM PMSF, 1 mM benzamidine, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 1 μg/ml aprotinin). GST fusion proteins were eluted in sodium dodecyl sulfate (SDS) sample buffer, separated on a 6.75% SDS-polyacrylamide gel electrophoresis (PAGE) gel, and transferred to a nitrocellulose membrane. Blots were incubated either with the rabbit polyclonal antiphosphorylated proteins (Pan) antibody (diluted 1/250; Zymed Laboratories Inc., South San Francisco, CA) or with the mouse 4C10 monoclonal anti-GST (diluted 1/1,000; Covance/Berkeley Antibody Co., Richmond, CA) and then with horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin (Amersham, Arlington Heights, IL).
FIG. 2.
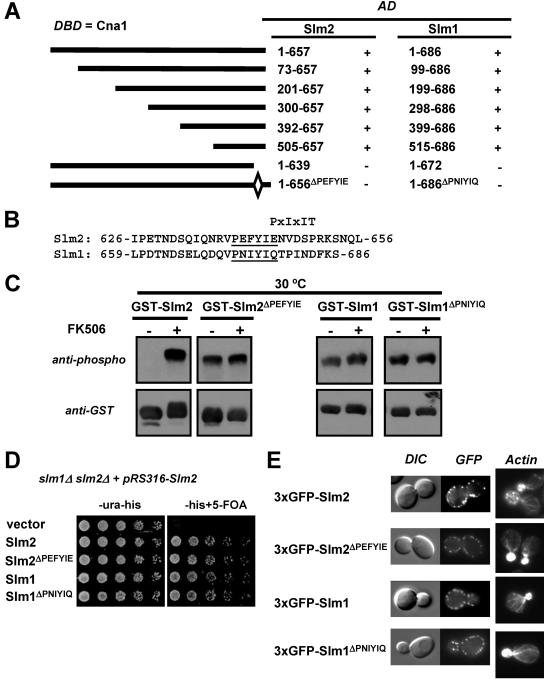
Calcineurin interacts with Slm1 and Slm2 via a C-terminal PXIXIT-related motif. (A) Mapping of the calcineurin-docking site on Slm1 and Slm2 via yeast two-hybrid assays. The PJ69-4A strain containing the GAL4 DNA-binding domain fusion of CNA1 (DBD) was transformed with different truncations or internal deletions of SLM1 and SLM2. Positive interactions were identified as growth on synthetic medium lacking histidine (3 days at 30°C). GAL4 activation domain fusions (AD) that interacted with Cna1 are indicated as “+”, and those that did not interact are indicated as “−.” (B) Relevant parts of the amino acid sequences of Slm2 and Slm1 with the conserved calcineurin-docking sites, PXIXIT-related motifs, underlined. (C) Deletion of the calcineurin-docking site abolishes the calcineurin-dependent dephosphorylation of Slm1 and Slm2. Wild-type GST-SLM2 (GBY115) and GST-SLM1 (GBY117) and their counterparts lacking the calcineurin-docking site, GST-SLM2ΔPEFYIE (GBY116) and GST-SLM1ΔPNIYIQ (GBY118), were expressed in a strain deleted for slm1Δ and slm2Δ. Cells were grown in selective synthetic medium supplemented with 4% raffinose. One culture was treated with FK506 (as indicated). GST fusion proteins were purified from cell extracts, subjected to SDS-PAGE, and Western blotted with anti-phospho and anti-GST antibodies. (D) Deletion of the calcineurin-docking site in SLM1 or SLM2 does not impair growth. An slm1Δ slm2Δ strain expressing SLM2 under the control of its own promoter on a pRS316 plasmid (GBY059) was transformed with either pRS313 (vector; GBY152), pRS313-SLM2 (Slm2; GBY153), pRS313-SLM2ΔPEFYIE (Slm2 ΔPEFYIE; GBY154), pRS313-SLM1 (Slm1; GBY155), or pRS313-SLM1ΔPNIYIQ (Slm1ΔPNIYIQ; GBY156). Serial dilutions of saturated cell cultures were plated on synthetic medium lacking uracil and histidine (-ura-his) and on synthetic medium lacking histidine but supplemented with 5FOA (-his+5-FOA). Cells were grown for 3 days at 30°C. (E) Localization of Slm proteins and actin cytoskeleton stabilization by Slm proteins are not dependent on the calcineurin-docking site. slm1Δ slm2Δ cells expressing GFP-SLM2 (GBY104), GFP-SLM2ΔPEFYIE (GBY105), GFP-SLM1 (GBY106), or GFP-SLM1ΔPNIYIQ (GBY107) were grown to log phase and visualized by differential interference microscopy (DIC) and by fluorescence microscopy (GFP). Actin cytoskeleton was visualized with Texas Red-X phalloidin in formaldehyde-fixed cells.
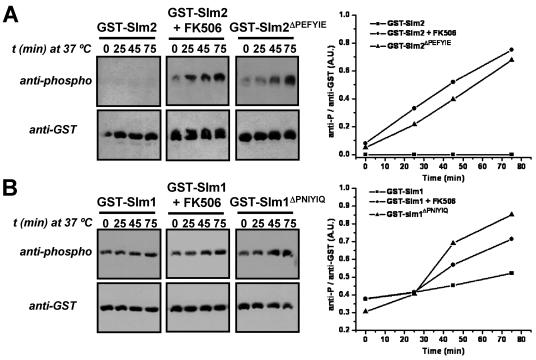
FIG. 3.
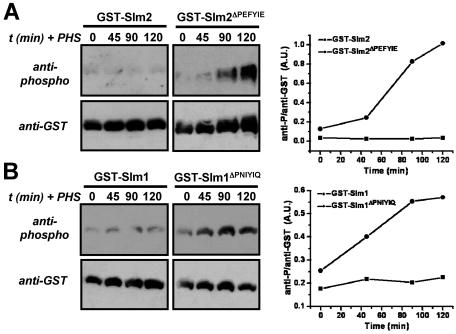
Phosphorylation of Slm1 and Slm2 is increased during heat stress when their calcineurin-dependent dephosphorylation is compromised. (A) GST-SLM2 (GBY115) and GST-SLM2ΔPEFYIE (GBY116) were expressed in a strain lacking slm1 and slm2. Cells were grown at 25°C in selective synthetic medium and expression was induced with 2% galactose. One culture was treated with FK506 (2 μg/ml) as indicated. Cells were shifted to 37°C and samples were taken at indicated time points (t). GST fusion proteins were purified from cell extracts and subjected to SDS-PAGE and Western blotting with anti-phospho and anti-GST antibodies. Immunoreactive bands were quantified using ImageJ software. The ratio of the anti-phospho signal over the anti-GST signal was plotted in arbitrary units (A.U.) as a function of time. (B) An approach similar to that described for panel A was taken for GST-SLM1 (GBY117) and GST-SLM1ΔPNIYIQ (GBY118).
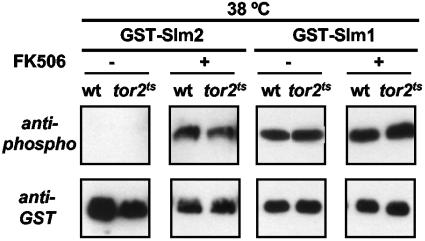
FIG. 5.
The heat stress-induced increase in Slm1 and Slm2 phosphorylation is independent of Tor2p. Overnight cultures of wild-type (wt; JK9-3da) and tor2ts (SH121) cells expressing either GST-Slm2 or GST-Slm1 were diluted to an OD600 of 0.2 and grown at 25°C for 4 h. To one set of cultures, FK506 (final concentration, 2 μg/ml) or solvent (90% ethanol/10% Tween 20) was added and cells were shifted to 38°C. After 30 min, galactose (2%) was added to induce protein expression and cells were grown for another 4 h at 38°C. GST fusion proteins were purified from cell extracts and subjected to SDS-PAGE and Western blotting with anti-phospho and anti-GST antibodies.
Detection and quantification of immunoreactive bands by immunoblotting.
Immunoreactive bands on Western blots were visualized with an ECL kit (Pierce, Rockford, IL) and subsequent exposure to Biomax MR film (VWR, San Francisco, CA). For each blot, multiple exposures, ranging from 6 seconds to several minutes, were taken. Each exposure was scanned to obtain a digital image, and immunoreactive bands were quantified using ImageJ (version 1.34n) software (http://rsb.info.nih.gov/ij/). Data presented in the figures represent analysis of a single optimal exposure for each blot. This optimal exposure was determined empirically; an exposure for which the signal from the most intense band was ≤30% of its observed maximum was used, thus ensuring that quantification was not distorted by signal saturation. In each case, from two to four independent experiments were conducted; the data displayed in the figures depict results from a single representative experiment.
In vitro phosphatase assay.
DD12 cells expressing GST-Slm1 or GST-Slm2 were grown for 4 h in synthetic medium lacking uracil and methionine with 4% raffinose as the carbon source. Expression of the GST fusion proteins was induced by the addition of 2% galactose. Lysis of the cells and purification of the GST fusion proteins were performed as described above. GST fusion proteins bound to the glutathione-Sepharose 4B beads were washed twice in wash buffer containing protease inhibitors and phosphatase inhibitors, twice in wash buffer containing protease inhibitors only, and twice in CP buffer (50 mM Tris-HCl [pH 7.5], 1 mM MgCl2, 1 mM DTT) containing protease inhibitors. Washed beads were divided among four tubes prior to the administration of different phosphatase treatments. Phosphatase assays were performed in a total of 100 μl in CP buffer for calcineurin and in the supplied buffer for λ-phosphatase (New England Biolabs, Beverly, MA); 500 U of recombinant human calcineurin (Calbiochem, San Diego, CA), 2,600 U of calmodulin (Calbiochem), or 10 μl of λ-phosphatase (New England Biolabs) was used per assay. Where indicated, CaCl2 was added to a final concentration of 40 mM and EGTA to 10 mM. Phosphatase assays were incubated at 30°C for 30 min; the supernatant was then removed, and SDS-PAGE sample buffer was added to the beads. Samples were analyzed by SDS-PAGE (7.5% gels) and blotted as described above. Immunoreactive bands were visualized as described above.
Fluorescence microscopy.
The localization of GFP-Slm2, GFP-Slm2ΔPEFYIE, GFP-Slm1, and GFP-Slm1ΔPNIYIQ was examined in strains GBY104 through GBY107. Liquid cultures were set up in synthetic medium lacking histidine and supplemented with methionine (75 mg/liter). Log-phase cultures (OD600, 1.0) were concentrated by brief centrifugation and mounted on microscope slides. Cells expressing a single GFP fusion were visualized with a Nikon Eclipse E600 microscope with fluorescence optics and an HB100 mercury lamp. Fluorescein filter sets (Chroma Technology Co., Brattleboro, VT) were used to visualize GFP. Photos were taken with a Hamamatsu 47420-95 digital charge-coupled-device camera and QED software (QED Imaging). Actin cytoskeleton staining was performed on cells grown to log phase. Cells were then fixed for 30 min at room temperature by the direct addition of 37% formaldehyde stock to a final concentration of 5%. Fixed cells were collected and washed twice with phosphate-buffered saline supplemented with 1 mg/ml bovine serum albumin. Then, 0.04 U/μl of Texas Red-X phalloidin (Molecular Probes, Eugene, OR) was added for 30 min at room temperature to stain actin. Cells were washed three times with phosphate-buffered saline supplemented with 1 mg/ml bovine serum albumin. Cells were resuspended in mounting medium and visualized as described above, except that a rhodamine filter was used. Only those cells in which the bud was significantly smaller than the mother cell were analyzed.
Fur4 turnover.
Wild-type cells and slm1ts slm2Δ cells transformed with pVT102u-Fur4 (pGB047) were grown at 25°C in selective synthetic medium lacking uracil to log phase. Cells were shifted to 40°C after OD600 was measured, and at the indicated time points, 3 OD600 units of yeast cells (i.e., 3 ml of culture at an OD600 of 1.0 or the equivalent) was removed. Cells were harvested and resuspended in H2O, yielding a total volume of 500 μl. Cells were lysed for 10 min on ice by adding 50 μl 1.85 M NaOH supplemented with 300 mM β-mercaptoethanol. Proteins were precipitated on ice for 10 min by adding 50 μl of 50% trichloroacetic acid. Proteins were collected by centrifugation and the protein pellets were dissolved in 120 μl urea-SDS sample buffer (8 M urea, 5% SDS, 200 mM Tris-HCl [pH 6.8], 0.1 mM EDTA [pH 8], 0.1% bromophenol blue, 100 mM DTT) supplemented with 100 mM Tris. Samples were heated for 10 min at 37°C just before being loaded onto a 4 to 15% SDS-PAGE gel (Bio-Rad, Richmond, CA). After Western blotting to nitrocellulose, membranes were incubated with the rabbit polyclonal anti-Fur4 (1/15,000 dilution) and with the mouse monoclonal 22C5 anti-Pgk1p (1/5,500 dilution; Molecular Probes). After image analysis, the anti-Fur4 signal was corrected for the Pgk1p loading control, and all values were normalized to the 0-min time point. A similar approach was taken for determining the Fur4 turnover rate in cells expressing GST-Slm2, GST-Slm2ΔPEFYIE, GST-Slm1, or GST-Slm1ΔPNIYIQ, except that pfF was used for the overexpression of Fur4.
Uracil uptake assay.
Exponentially growing wild-type or slm1ts slm2Δ cells transformed with pVT102u-Fur4 (pGB047) and grown in selective synthetic medium lacking uracil at 25°C were preshifted 10 min at 40°C. At time zero, cells were treated with cycloheximide (10 μg/ml). The uracil uptake was measured as previously described (31). Yeast cultures (1 ml) were incubated with 5 μM [14C]uracil (Amersham, Arlington Heights, IL) for 20 s at 30°C and quickly filtered through Whatman GF/C filters. Filters were washed twice with ice-cold water and assessed for radioactivity.
Membrane preparation for detection of ubiquitinated Fur4.
Exponentially growing yeast cells transformed with pVT102u-Fur4 (pGB047) and grown in selective synthetic medium lacking uracil at 25°C were shifted to 40°C. Ten minutes after heat shock, cycloheximide (100 μg/ml) was added for 50 min. Yeast cells (8.5 OD600 units) were harvested before (0 min) and after (60 min) the temperature shift by centrifugation in the presence of 10 mM sodium azide, washed once in distilled water plus 10 mM sodium azide, and used to prepare membrane-enriched fractions, as described in reference 23. Washed cells were transferred to a conical 1.5-ml Eppendorf tube and suspended in 150 μl of lysis buffer (100 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 5 mM EDTA plus protease inhibitors and 25 mM freshly prepared N-ethylmaleimide to prevent artifactual deubiquitination). All subsequent steps were carried out at 4°C. Chilled glass beads (150 μl) were added, and the cells were lysed by vigorous vortex mixing seven times for 1 min each, separated by 1-min intervals. The homogenate was collected and centrifuged at 3,000 rpm for 2 min to remove unbroken cells and debris. The total protein extract was centrifuged for 45 min at 16,100 × g. This pellet was washed by suspension in 100 ml lysis buffer supplemented with 5 M urea, incubated for 30 min on ice, and centrifuged for 45 min at 16,100 × g. The resulting pellet was suspended in 100 μl of lysis buffer, and trichloroacetic acid was added to 10% to precipitate proteins and to prevent proteolysis by residual endogenous proteases. Protein precipitates were collected by sedimentation (16,100 × g, 45 min). The protein precipitates were suspended in 40 μl urea-SDS sample buffer supplemented with 100 mM Tris, and 8 μl of this mixture was analyzed on a 4 to 15% SDS-PAGE gel (Bio-Rad, Richmond, CA). After the Western blotting procedure, membranes were incubated with anti-Fur4, developed, stripped with Restore Western blot stripping solution (Pierce, Rockford, IL), and reprobed with anti-ubiquitin (P4D1 monoclonal; Santa Cruz Biotechnology, Santa Cruz, CA).
RESULTS
Slm1 and Slm2 interact with and are physiological substrates of calcineurin.
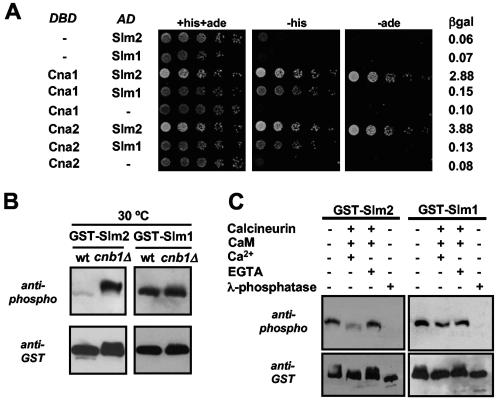
Genome-wide yeast two-hybrid analyses demonstrated an interaction between the A subunit of calcineurin and the ORF YNL047C, characterized as SLM2. We used yeast two-hybrid assays to confirm this observation and to investigate whether the A subunit of calcineurin was also able to interact with Slm1, which is 53% identical to Slm2 at the protein level. Cna1 and Cna2 were fused in frame with the Gal4 DNA-binding domain, whereas Slm1 and Slm2 were fused in frame with the Gal4 activation domain. Combinations of these constructs were expressed in strain PJ69-4A, which contains three different reporter genes, each driven by a different promoter. The GAL1 promoter-HIS3 reporter is activated by low- and high-affinity interactions, whereas the more stringent GAL2 promoter-ADE2 reporter and GAL7 promoter-lacZ reporter are activated only by high-affinity interactions. As indicated in Fig. 1A, both Slm1 and Slm2 interacted with Cna1 as well as Cna2 and allowed growth of strains on medium lacking histidine. However, only Slm2 was able to activate the ADE2 and lacZ reporter genes, suggesting that Slm2 interacts with Cna1 and Cna2 with an affinity higher than that of Slm1. We believe these differences in reporter activation reflect differences in affinity, since the Slm1- and Slm2-Gal4 activation domain fusion proteins are expressed at similar levels and show identical interactions in two-hybrid assays with other gene products, such as Avo2, a subunit of the TORC2 protein kinase complex (data not shown).
FIG. 1.
Slm1 and Slm2 interact with and are dephosphorylated by calcineurin. (A) Slm1 and Slm2 interact with Cna1 and Cna2 in yeast two-hybrid assays. A strain (PJ69-4A) containing GAL1-HIS3, GAL2-ADE2, and GAL7-lacZ reporter genes was transformed with combinations of GAL4 DNA-binding domain fusions of CNA1 and CNA2 (DBD) and GAL4 activation domain fusions of SLM1 and SLM2 (AD), as indicated. Serial dilutions of saturated cultures were plated on synthetic medium containing histidine and adenine (+his+ade) or lacking histidine (-his) or adenine (-ade), and incubated for 3 days at 30°C. β-Galactosidase (βgal) activity in the different strains was measured in liquid cultures as described in Materials and Methods and reported as units/μg of protein. (B) Slm1 and Slm2 are both dephosphorylated in vivo by calcineurin. Wild-type cells (wt; YPH499) and cells lacking the B subunit of calcineurin (cnb1Δ; DD12) expressing GST-SLM2 (GBY030 and GBY032) or GST-SLM1 (GBY031 and GBY033) were grown to log phase. GST fusion proteins were purified from extracts, subjected to SDS-PAGE, and Western blotted with anti-phospho or anti-GST antibody. (C) Slm1 and Slm2 are both dephosphorylated in vitro by calcineurin. GST-Slm1 (GBY032) and GST-Slm2 (GBY033) purified from cnb1Δ cells were treated with recombinant calcineurin, calmodulin (CaM), CaCl2, EGTA, or λ-phosphatase as indicated in the left-hand lanes (see Materials and Methods). These samples, together with untreated GST-Slm1 and GST-Slm2 purified from cnb1Δ cells, were then subjected to SDS-PAGE and Western blotted with anti-phospho and anti-GST antibodies.
Since Slm1 and Slm2 interacted with both calcineurin A subunits, we determined whether they were substrates of calcineurin in vivo (Fig. 1B). Functional N-terminal GST fusions of Slm1 and Slm2 were expressed in wild-type and calcineurin-null cells (cnb1Δ, lacking the calcineurin regulatory B subunit). After purification of GST-Slm1 and GST-Slm2 on glutathione-Sepharose 4B beads, we examined their electrophoretic mobilities by Western blotting with anti-GST antibodies. In addition, we determined the extents of phosphorylation of Slm1 and Slm2 by Western blotting with a mixture of antibodies that recognizes phosphorylated serine, threonine, and tyrosine residues (see Materials and Methods). We will refer to this as the phospho-antibody. As shown in Fig. 1B, Slm2 displayed a calcineurin-dependent change in phosphorylation, as is evident from the shift in electrophoretic mobility (anti-GST blot) and from the dramatic difference in the extents of phosphorylation (anti-phospho blot) in extracts of wild-type cells versus cnb1Δ cells. Slm1 also showed calcineurin-dependent changes in electrophoretic mobility and in the extent of phosphorylation; however, the differences were more subtle. Quantification of the signal obtained with the phospho-antibody showed an ∼20 to 30% increase in the phosphorylation of GST-Slm1 in cells lacking calcineurin activity that was consistently and reproducibly observed. As we will show (see Fig. 3), the dephosphorylation of Slm1 by calcineurin becomes more evident under specific stress conditions (such as heat stress), leading to hyperphosphorylation of GST-Slm1 when calcineurin function is compromised. These data indicate that both Slm1 and Slm2 display calcineurin-dependent changes in phosphorylation in vivo.
We then used an in vitro dephosphorylation assay to show that Slm1 and Slm2 are direct substrates of calcineurin. GST-Slm1 and GST-Slm2, purified from calcineurin-deficient cells, showed increased mobilities and reduced extents of phosphorylation when incubated with purified Ca2+/CaM-activated calcineurin (Fig. 1C). These changes were abolished by inhibiting calcineurin with EGTA, a Ca2+ chelator (Fig. 1C). Calcineurin was able to perform a robust dephosphorylation of GST-Slm2 (>80% reduction in phosphorylation in comparison to untreated GST-Slm2) and had a partial effect on the phosphorylation state of GST-Slm1 (∼30% reduction in phosphorylation in comparison to untreated GST-Slm1). In agreement with these changes in the extent of phosphorylation, the protein samples incubated with active purified calcineurin showed increased electrophoretic mobility (anti-GST blot). As a control, both proteins were completely dephosphorylated by λ-phosphatase treatment.
Hence, both Slm1 and Slm2 are calcineurin substrates. However, both the in vivo and in vitro experiments show that while the majority of Slm2 phosphorylation is subject to dephosphorylation by calcineurin, only some of the phosphorylated sites of Slm1 are dephosphorylated by calcineurin.
Calcineurin binds to a conserved C-terminal PXIXIT-related motif in Slm1 and Slm2.
To further examine the physiological relevance of the calcineurin-dependent dephosphorylation of Slm1 and Slm2, we mapped the calcineurin-binding site in Slm1 and Slm2. A series of N-terminal deletion constructs of Slm1 and Slm2 was tested for interaction with Cna1 by use of the yeast two-hybrid method (Fig. 2A). All N-terminally truncated proteins interacted with calcineurin, suggesting that the calcineurin-docking site was located in the C-terminal protein of Slm1 and Slm2. Many proteins interact with calcineurin via a docking motif with a consensus PXIXIT sequence. We found a potential PXIXIT-related motif in the C-terminal tail of Slm2 (PEFYIE) that had a similar counterpart in Slm1 (PNIYIQ) (Fig. 2B). Slm1 and Slm2 lacking their C-terminal tails (amino acids [aa] 1 to 672 and aa 1 to 639, respectively) failed to interact with Cna1, as did mutant constructs in which only the PXIXIT motifs were deleted (Slm1, aa 1 to 686ΔPNIYIQ, and Slm2, aa 1 to 657ΔPEFYIE) (Fig. 2A). Similar results were obtained for interactions with Cna2 (data not shown). In contrast, deletion of the PXIXIT motif had no effect on Slm1 or Slm2 interaction with Avo2, as measured by use of the two-hybrid technique (data not shown). Thus, for both Slm1 and Slm2, we defined a small PXIXIT-related motif required specifically for their interaction with calcineurin.
For other substrates, eliminating the calcineurin-docking site abolishes their calcineurin-dependent dephosphorylation. Hence, we investigated the effect of deleting the PXIXIT motif in Slm1 and Slm2. We expressed Slm1ΔPNIYIQ and Slm2ΔPEFYIE as GST fusion proteins in cells lacking endogenous Slm1 and Slm2 (Fig. 2C). In contrast to what was seen for the wild-type GST-Slm2 and GST-Slm1, GST-Slm2ΔPEFYIE and GST-Slm1ΔPNIYIQ showed no difference in electrophoretic mobilities or extents of phosphorylation in extracts of cells containing or lacking calcineurin. Furthermore, it was evident that in contrast to GST-Slm2, GST-Slm2ΔPEFYIE accumulated in the phosphorylated form even in cells containing calcineurin. We observed a similar behavior for GST-Slm1ΔPNIYIQ; however, the differences were less striking due to the partial effects of calcineurin on Slm1 dephosphorylation. We conclude from these findings that calcineurin binding to Slm1 and Slm2 via their PXIXIT motifs is required for calcineurin-dependent dephosphorylation of these proteins and that GST-Slm1ΔPNIYIQ and GST-Slm2ΔPEFYIE are hyperphosphorylated in vivo.
Next, we tested whether the PXIXIT-related motif was required for Slm protein function or localization. SLM1 and SLM2 are redundant for an essential function (4). Therefore, to test the abilities of different SLM alleles to support growth, we introduced them into an slm1Δ slm2Δ strain that also contained a URA3 CEN plasmid expressing SLM2 from its own promoter (pGB013). We then tested whether the different strains could grow in the absence of pGB013 (i.e., on plates containing 5FOA) (Fig. 2D). As expected, cells expressing the vector alone were not able to grow, confirming that SLM1 and SLM2 form an essential gene pair. Wild-type SLM1 and SLM2 as well as their PXIXIT-deleted counterparts were able to promote growth with similar levels of efficiency. This shows that calcineurin-dependent regulation of Slm1 and Slm2 is dispensable for growth under standard conditions. Calcineurin is essential for yeast growth under certain stress conditions, including ionic stress (Na+, Li+, Mn2+, Ca2+), high pH, and the presence of cell wall-disrupting compounds (calcofluor white and Congo red). However, no growth defects were observed for the strains expressing PXIXIT-deleted products of SLM1 and SLM2 alleles under any of these stress conditions (data not shown). Slm1 and Slm2 localize to punctate patches at the cell surface (4, 27), and GFP-Slm proteins lacking the PXIXIT motif displayed an identical localization (Fig. 2E). In addition, Slm1 and Slm2 are required for polarization of the actin cytoskeleton. We used Texas Red-phalloidin to visualize cellular actin in budded yeast cells and observed no differences in actin cytoskeleton organization between cells expressing either wild-type Slm1 or Slm2 or the corresponding mutant proteins lacking the calcineurin-docking site (Fig. 2E).
Thus, calcineurin-dependent regulation of Slm1 and Slm2 is not required for their abilities to support growth, to localize to the cell periphery, or to polarize the actin cytoskeleton.
Slm1 and Slm2 are dephosphorylated by calcineurin during heat stress and after addition of sphingolipids.
During heat stress, Slm1 is transiently dephosphorylated and subsequently rephosphorylated (4). Hence, we tested the effect of calcineurin on Slm protein phosphorylation under these conditions. We expressed Slm1 and Slm2 and their PXIXIT-deleted counterparts, Slm1ΔPNIYIQ and Slm2ΔPEFYIE, as GST fusion proteins in the strain lacking endogenous Slm1 and Slm2 (Fig. 3). Shifting cells from 25°C to 37°C induced no detectable change in the phosphorylation of GST-Slm2 and resulted in a slight increase in the net phosphorylation of GST-Slm1 after 75 min (20% increase). However, the effect of heat stress on phosphorylation of GST-Slm1 and GST-Slm2 became much more apparent when calcineurin activity was inhibited with the specific inhibitor FK506 or when the calcineurin-docking site of GST-Slm1 or that of GST-Slm2 was deleted. After we shifted the temperature from 25°C to 37°C, we found a time-dependent increase in the phosphorylation of GST-Slm2 purified from cells treated with FK506 and of purified GST-Slm2ΔPEFYIE (∼10-fold increase in both cases) (Fig. 3A). Phosphorylation of GST-SLM1 purified from cells treated with FK506 and of purified GST-Slm1ΔPNIYIQ also increased after a shift to high temperature, although the magnitude of the change was smaller (approximately two- to threefold) (Fig. 3B).
Heat stress transiently increases biosynthesis of sphingoid bases (phytosphingosine and dihydrosphingosine), which in turn activate downstream kinases (21, 44). Therefore, we tested whether the addition of phytosphingosine could mimic the effect of elevated temperature to promote phosphorylation of GST-Slm1ΔPNIYIQ and GST-Slm2ΔPEFYIE (Fig. 4). Indeed, as observed for heat stress (Fig. 3), the addition of 20 μM phytosphingosine to cells at 25°C increased the phosphorylation of GST-Slm2ΔPEFYIE (∼10-fold increase) (Fig. 4A) and that of GST-Slm1ΔPNIYIQ (∼2-fold increase) (Fig. 4B). Together, these results indicate that through increased synthesis of phytosphingosine, heat stress triggers an increase in Slm1 and Slm2 phosphorylation that is largely counteracted in vivo by calcineurin-mediated dephosphorylation.
FIG. 4.
Phytosphingosine mimics the heat stress-induced phosphorylation of Slm1 and Slm2. GST-SLM2 (GBY115), GST-SLM2ΔPEFYIE (GBY116), GST-SLM1 (GBY117), and GST-SLM1ΔPNIYIQ (GBY118) were expressed in a strain lacking slm1 and slm2. Cells were grown at 25°C in selective synthetic medium and expression was induced with 2% galactose. Samples were taken just before the addition of 20 μM phytosphingosine (0 min) and at time points (t) 45, 90, and 120 min after the addition of phytosphingosine. GST fusion proteins were purified from cell extracts and subjected to SDS-PAGE and Western blotting with anti-phospho and anti-GST antibodies. Immunoreactive bands were quantified as described in Materials and Methods. The ratio of the anti-phospho signal over the anti-GST signal was plotted in arbitrary units (A.U.).
These data suggest that both elevated temperature and addition of phytosphingosine lead to activation of calcineurin in vivo and consequently to increased Slm protein dephosphorylation. We therefore tested the effects of heat stress and phytosphingosine on calcineurin by monitoring the activity of Crz1, a calcineurin-regulated transcription factor. We measured expression of a 4× CDRE::lacZ reporter gene which is specifically activated by Crz1 (65). Table 3 shows that elevated temperature as well as the addition of phytosphingosine (20 μM) increased Crz1 activity and that this increased activity was completely inhibited by FK506. Thus, the increase in Crz1 activity reflects activation of calcineurin in response to heat stress or incubation with phytosphingosine.
TABLE 3.
Heat stress and phytosphingosine causes calcineurin-dependent 4× CDRE::lacZ expressiona
| Stress condition | β-Galactosidase activity (U/μg protein) in:
|
|
|---|---|---|
| Absence of FK506 | Presence of FK506 | |
| Control (30°C) | 0.29 ± 0.05 | 0.29 ± 0.04 |
| Heat stress (25-37°C) | 1.37 ± 0.145 | 0.11 ± 0.06 |
| Phytosphingosine (25°C) | 3.93 ± 0.178 | 0.38 ± 0.12 |
| Phytosphingosine (30°C) | 7.51 ± 0.51 | 0.29 ± 0.11 |
Strain LBY66 containing the lacZ reporter gene under the control of four copies of the 24-bp calcineurin downstream response element in its genome was grown in YPD under different conditions in the absence or presence of FK506 (2 μg/ml). After reaching log phase, cells either were not triggered (no stress; control) or were triggered with a heat stress for 3 h or with 20 μM phytosphingosine for 3 h at the indicated temperatures; then, β-galactosidase activity was assayed in cell lysates. Values represent the mean specific β-galactosidase activity obtained from two independent experiments each performed in triplicate.
A previous study showed that Slm1 is phosphorylated by the TORC2 protein kinase (4). If heat stress activates phosphorylation of Slm1 and Slm2 by TORC2 and this TORC2-mediated phosphorylation is reversed by calcineurin, then in tor2ts cells we should observe no increase in Slm1 or Slm2 phosphorylation following exposure to high temperature even in cells devoid of calcineurin activity. To test this hypothesis, we shifted wild-type or tor2ts cells to 37°C and then induced synthesis of either GST-Slm1 or GST-Slm2. As we observed earlier (Fig. 3), GST-Slm2 phosphorylation was undetectable in wild-type or tor2ts cells at 25°C, and GST-Slm1 displayed basal levels of phosphorylation that were identical in wild-type and tor2ts cells (Fig. 5). We also examined GST-Slm1 and GST-Slm2 phosphorylation in cells shifted to high temperature in the presence of the calcineurin inhibitor FK506. As expected, phosphorylation of both proteins increased as a result of exposure to high temperature, and we observed the same level of phosphorylation in wild-type and tor2ts FK506-treated cells (Fig. 5). Thus, these findings suggest that heat stress causes an increase in Slm1 and Slm2 phosphorylation that is Tor2p-independent and counteracted by calcineurin.
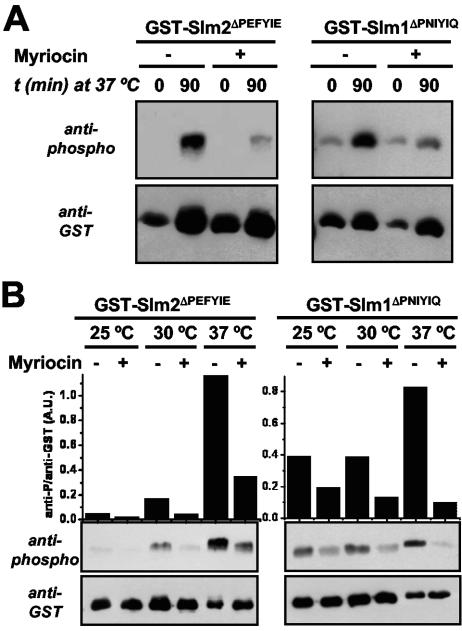
Because heat stress-induced phosphorylation of Slm1 and Slm2 was mimicked by the addition of phytosphingosine (Fig. 4), we tested whether the increased phosphorylation of GST-Slm1ΔPNIYIQ and GST-Slm2ΔPEFYIE at high temperature was prevented by the addition of myriocin, a potent inhibitor of the serine palmitoyltransferase responsible for catalyzing the first step in the biosynthesis of sphingolipids. Indeed, we found that more than 90% of the heat stress-induced phosphorylation of GST-Slm1ΔPNIYIQ and GST-Slm2ΔPEFYIE was inhibited by myriocin (Fig. 6A), indicating that this increase in phosphorylation of Slm proteins is dependent on de novo sphingolipid biosynthesis. In addition, these results suggest that sphingolipid-dependent protein kinases are responsible for the increase in Slm1 and Slm2 phosphorylation observed (Fig. 3). We also investigated the effect of myriocin on the steady-state levels of GST-Slm1ΔPNIYIQ and GST-Slm2ΔPEFYIE phosphorylation in cells grown at three different temperatures: 25°C, 30°C, and 37°C (Fig. 6B). First, we observed that the overall levels of both GST-Slm1ΔPNIYIQ and GST-Slm2ΔPEFYIE phosphorylation increased with temperature. Phosphorylation of GST-Slm2ΔPEFYIE was extremely low at 25°C and increased dramatically with increasing temperature. GST-Slm1ΔPNIYIQ was phosphorylated at all temperatures but showed increased phosphorylation with increased temperature, especially 37°C. Second, at each temperature where phosphorylation was detected, it was decreased by the addition of myriocin. We made similar observations for GST-Slm1 and GST-Slm2 in myriocin-treated cells lacking calcineurin (cnb1Δ) (data not shown). Thus, under all conditions tested, phosphorylation of Slm1 and Slm2 is dependent on de novo biosynthesis of sphingoid bases.
FIG. 6.
Myriocin inhibits phosphorylation of Slm1 and Slm2 during heat stress and steady-state growth conditions. GST-SLM2ΔPEFYIE (GBY116) and GST-SLM1ΔPNIYIQ (GBY118) were expressed in an slm1Δ slm2Δ strain. (A) Cells were grown at 25°C in selective synthetic medium for 4 h and protein expression was induced with 2% galactose. Thirty minutes before the shift from 25°C to 37°C, myriocin (2 μg/ml) was added to one set of cultures and solvent (methanol) to the other. Samples of the different cultures were taken at time points (t) just before the temperature shift and 90 min after the shift in temperature. GST fusion proteins were purified from cell extracts and subjected to SDS-PAGE and Western blotting with either an anti-phospho or an anti-GST antibody. (B) Cells were grown at either 25°C, 30°C, or 37°C in selective synthetic medium. Myriocin (2 μg/ml) was added 30 min before the cells were incubated with 2% galactose for 4 h. GST fusion proteins were purified from cell extracts and analyzed via Western immunoblotting with either an anti-phospho or an anti-GST antibody. Immunoreactive bands were quantified as described in Materials and Methods. A.U., arbitrary units.
Slm1 and Slm2 activity determines sensitivity to myriocin.
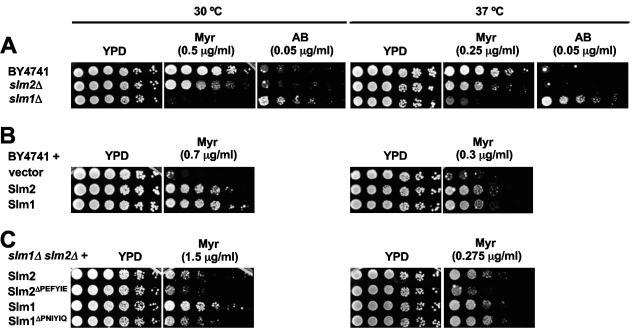
We then investigated whether Slm1 and Slm2 play a physiological role in sphingolipid signaling and/or metabolism. Sphingolipids are essential for yeast cell growth and survival. Here, we used myriocin to investigate growth phenotypes related to deletion or overexpression of SLM1 and SLM2 (Fig. 7). Because Slm1 and Slm2 phosphorylation levels varied with temperature, we also examined growth at two different temperatures: 30°C and 37°C. First, we found that all strains tested were more sensitive to growth inhibition by myriocin at 37°C than at 30°C, which necessitated the use of lower myriocin concentrations at the high temperature (Fig. 7A, B, and C; compare left and right panels). Second, we found that strains lacking either SLM1 or SLM2 showed increased sensitivity to myriocin at both temperatures (Fig. 7A). A stronger effect was observed with deletion of SLM1, which is more highly expressed than SLM2 (4). In contrast, we found that slm1Δ cells were more resistant to aureobasidin A (Fig. 7A), which inhibits Aur1, an enzyme responsible for the production of inositol phosphorylceramide, a complex sphingolipid (37). Incubation of cells with aureobasidin A can lead to the cellular accumulation of intermediates in the sphingolipid biosynthesis pathway, including phytosphingosine and ceramide (59). We also increased Slm1 and Slm2 levels by expressing SLM1 or SLM2 from a CEN-based plasmid under the control of their own promoters. We found that increasing SLM1 and SLM2 copy number conferred resistance to myriocin at either 30°C or 37°C (Fig. 7B). Taken together, these data implicate Slm1 and Slm2 in sphingolipid metabolism and/or signaling. We then tested whether the presence or the absence of the calcineurin-binding site on Slm1 and Slm2 affected resistance to myriocin (Fig. 7C). At 30°C, cells expressing SLM1ΔPNIYIQ were more sensitive to myriocin than cells expressing SLM1, while at 37°C, surprisingly, there was no difference in the growth rates of these two strains over a range of myriocin concentrations (Fig. 7B and data not shown). Similarly, cells expressing SLM2ΔPEFYIE were more sensitive to myriocin than cells expressing SLM2. In this case, however, the difference in growth was more dramatic at 37°C than at 30°C. We observed equivalent effects on growth using cells that expressed GFP-tagged versions of these proteins and determined that the expression level of the GFP-tagged proteins was unaffected by the presence or absence of the calcineurin-binding site (data not shown). Thus, we conclude that the growth differences described above reflect calcineurin-dependent differences in Slm protein activity in vivo.
FIG. 7.
Slm1 and Slm2 modulate growth of yeast in the presence of drugs that perturb sphingolipid biosynthesis. (A) Saturated cultures of wild-type cells (BY4741) and cells lacking either slm1 (slm1Δ; VHY66) or slm2 (slm2Δ; VHY61) were serially diluted and spotted onto YPD, YPD plus myriocin (Myr), or YPD plus aureobasidin A (AB) and grown for 2 to 4 days at either 30°C or 37°C. (B) Wild-type cells containing either pRS313 (vector; GBY160) or pRS313 expressing Slm2 (GBY161) and Slm1 (GBY162) under the control of their own promoters were grown to saturation in selective synthetic medium. Saturated cultures were serially diluted and were spotted onto YPD with or without myriocin. Plates were incubated at either 30°C or 37°C for 2.5 days. (C) slm1Δ slm2Δ cells expressing SLM2 (GBY100), SLM2ΔPEFYIE (GBY101), SLM1 (GBY102), or SLM1ΔPNIYIQ (GBY103) under the control of their own promoters from pRS313 were grown to saturation in selective synthetic medium. Saturated cultures were serially diluted and were spotted onto YPD or YPD supplemented with 1.5 μg/ml myriocin. Cells were grown for 3 to 4 days at either 30°C or 37°C as indicated.
These data show that Slm protein activity becomes rate limiting for yeast growth when cellular levels of sphingolipids are reduced and suggest that dephosphorylation of Slm1 and Slm2 by calcineurin is necessary for these proteins to be fully functional.
Slm proteins may act downstream of phytosphingosine.
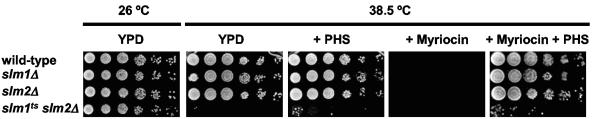
The observation that slm1Δ cells are both more sensitive to myriocin and more resistant to aureobasidin A than wild-type cells suggests that Slm proteins may be effectors of an intermediate in the sphingolipid biosynthesis pathway, such as phytosphingosine. However, our results do not rule out a role for Slm proteins in regulating sphingolipid levels. In order to address the potential role of Slm proteins upstream of sphingolipids, we investigated whether an external source of phytosphingosine promotes cell growth in the absence of functional Slm proteins. Addition of phytosphingosine (up to 20 μM) to the growth medium did not overcome the growth defect of slm1ts slm2Δ cells at the nonpermissive temperature (38.5°C) (Fig. 8), suggesting that these cells are defective in sphingolipid-mediated cellular processes rather than in sphingolipid production. In contrast, exogenously added phytosphingosine overcame the myriocin-induced growth defect of wild-type cells at 38.5°C (Fig. 8), indicating that phytosphingosine is able to promote growth in the absence of de novo sphingolipid biosynthesis at this high temperature. In addition, we observed that phytosphingosine restores the myriocin-induced growth inhibition in slm1Δ cells as well as in slm2Δ cells, indicating that either Slm1 or Slm2 protein can mediate the growth-promoting effect of phytosphingosine. Taken together, these results are consistent with a role for Slm proteins acting downstream of phytosphingosine.
FIG. 8.
Slm proteins are downstream of phytosphingosine. Saturated cultures of wild-type (SEY6210), slm1Δ (AAY1602), slm2Δ (AAY1610), and slm1ts slm2Δ (AAY1623.2) cells were serially diluted and spotted onto YPD, YPD plus 20 μM phytosphingosine (PHS), YPD plus 0.4 μg/ml myriocin, or YPD plus 0.4 μg/ml myriocin plus 20 μM phytosphingosine and grown for 2.5 days at the indicated temperatures. All plates contained 0.05% NP-40, which was used for obtaining even distributions of phytosphingosine in the plates.
Slm proteins are required for heat stress-induced endocytosis of the Fur4 uracil permease.
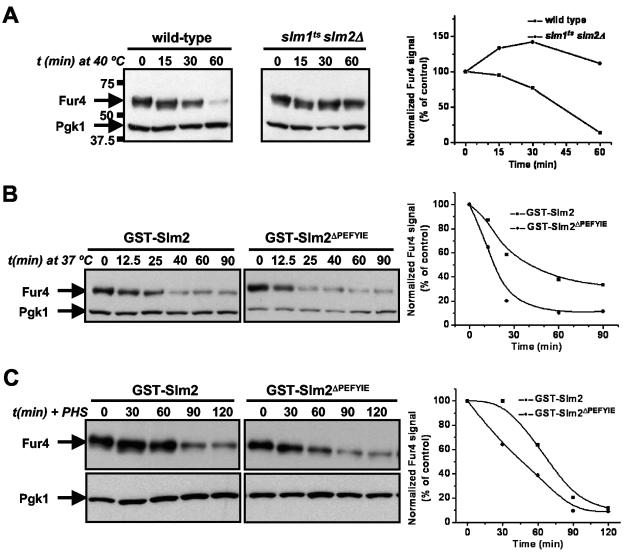
Increased phytosphingosine levels disrupt yeast cell growth by inhibiting nutrient uptake, including the import of tryptophan, uracil, leucine, and histidine (14). The mechanism of this inhibition has been elucidated for the uracil permease, Fur4, which undergoes ubiquitin-dependent endocytosis and transport to the vacuole, where it undergoes proteolysis. This degradation of Fur4 also occurs in response to heat stress (73) and is mediated by a specific increase in phytosphingosine (13). We compared Fur4 turnover in wild-type cells (SEY6210.1) to that in slm1ts slm2Δ cells (AAY1623.2) during heat stress (Fig. 9). Since endogenous Fur4 levels are too low to detect by Western blotting, we overexpressed Fur4 in these cells. Cells were grown to log phase and shifted from 25°C to 40°C. This temperature was used as the heat stress condition in these experiments because Fur4 turnover was more dramatic at 40°C than at 37°C in this strain background. However, we observed similar trends in Fur4 turnover at both temperatures (data not shown). In wild-type cells, heat stress induced a time-dependent decrease in Fur4 protein levels, which is consistent with previous results (13, 73). In contrast, Fur4 levels were stable in slm1ts slm2Δ cells during prolonged incubation at high temperature (Fig. 9A). This demonstrates that Slm proteins are required for Fur4 turnover induced by heat stress. Then, we tested whether this process was modulated by the presence or absence of the calcineurin-binding site of Slm1 or Slm2. We compared the Fur4 turnover rate for slm1Δ slm2Δ cells expressing GST-Slm2 to that for slm1Δ slm2Δ cells expressing GST-Slm2ΔPEFYIE and found that Fur4 was degraded more rapidly in cells expressing GST-Slm2ΔPEFYIE (Fig. 9B). A similar result was obtained when cells were incubated with phytosphingosine (Fig. 9C). This indicates that heat stress-induced dephosphorylation of Slm2 by calcineurin slows the rate of Fur4 turnover. The observed difference in turnover rates may reflect an alteration in one or more steps in the ubiquitin-dependent endocytosis and trafficking of Fur4 to the vacuole. These findings suggest that calcineurin can regulate sphingolipid-mediated processes through dephosphorylation of Slm2. However, we observed no difference in Fur4 turnover rates in cells expressing GST-Slm1 versus GST-Slm1ΔPNIYIQ (data not shown).
FIG. 9.
Slm function is required for heat shock-induced Fur4 turnover. (A) Wild-type cells (SEY6210.1) and slm1ts slm2Δ cells (AAY1623.2) carrying pVTu-FUR4 (GBY179 and GBY180, respectively) were shifted from 25°C to 40°C and samples were taken at time points (t) just prior to (0 min) and 15, 30, and 60 min after heat shock. Cell lysates were analyzed by SDS-PAGE and immunoblotting with anti-Fur4 and anti-PGK antibodies. Pgk1 was used as a loading control, which also shows the absence of nonspecific protein degradation by heat shock. The numbers to the left of the gels indicate the molecular masses of the protein standards. Immunoreactive bands were quantified as described in Materials and Methods. The ratio of the anti-Fur4 signal to the anti-Pgk1p signal is shown as a function of time. The value at 0 min was used as the reference value and set at 100%. (B) slm1Δ slm2Δ cells expressing either GST-SLM2 or GST-SLM2ΔPEFYIE and overexpressing FUR4 (GBY115 and GBY116, respectively) were shifted from 25°C to 40°C and sampled at the indicated time points. Cell lysates were analyzed by immunoblotting and quantified as described for panel A. (C) GBY115 and GBY116 were grown at 30°C, incubated with 20 μM phytosphingosine, and sampled at the indicated time points. Lysates were analyzed and signals quantified as described for panel A.
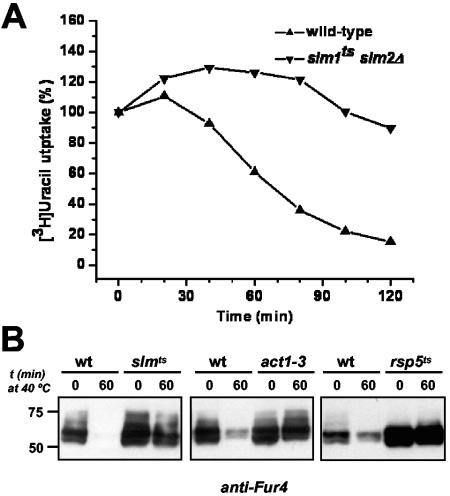
Next, we examined in more detail the accumulation of Fur4 in slmts cells. We included cycloheximide during the incubation at high temperature to follow only the fate of presynthesized Fur4 (73). We also assayed Fur4 levels by monitoring [14C]-uracil uptake (Fig. 10A), which allows sensitive quantification of cell surface Fur4, as any Fur4 in intracellular compartments will not contribute to uracil transport. As previously described, after a shift to high temperature, uracil uptake rapidly decreased in wild-type cells (73). In contrast, uracil transport activity remained high in slmts cells. These observations establish that internalization of Fur4 is blocked in slmts cells at restrictive temperature. It has been shown that, as for many membrane proteins, ubiquitination of Fur4 precedes and is required for its internalization (31). Using the anti-Fur4 antibody, we showed that in plasma membrane fractions of slmts cells exposed to high temperature, Fur4 accumulates in high-molecular-weight forms (Fig. 10B). These slow-migrating forms of Fur4 were also seen in plasma membrane fractions of act1-3 cells at restrictive temperature, which are defective for endocytosis and have been shown to accumulate ubiquitinated forms of Fur4 (reference 31 and Fig. 10B). In contrast, high-molecular-weight forms of Fur4 were not observed in mutants defective for the Rsp5 ubiquitin protein ligase, which is responsible for cell surface ubiquitylation of Fur4 and many other plasma membrane proteins (reference 31 and Fig. 10B). Membrane fractions of slmts and act1-3 cells incubated at restrictive temperature also accumulated high-molecular-weight species that reacted with an anti-ubiquitin antibody (data not shown). Therefore, we conclude that in slmts cells, Fur4 accumulates at the plasma membrane in a ubiquitinated form and that internalization is inhibited at a postubiquitination step.
FIG. 10.
Fur4 is stabilized at the plasma membrane in the ubiquitinated form in cells lacking Slm protein activity. Wild-type cells (SEY6210.1) and slm1ts slm2Δ cells (AAY1623.2) carrying pVTu-FUR4 (GBY179 and GBY180, respectively) were shifted to 40°C for 10 min before the addition of cycloheximide (100 μg/ml). (A) Uracil uptake was measured at the times indicated. Results are percentages of initial activities. (B) Plasma membrane fractions were prepared from cycloheximide-treated cells before and 60 min after a shift from 25°C to 40°C and analyzed for uracil permease (anti-Fur4) by Western immunoblotting. Strains are as follows: left panel, wild type (wt) (SEY6210.1) and slmts (AAY1623.2); middle panel, wild type (NY13) and act1-3 (NY279); right panel, wild type (FY56) and rsp5ts (FW1808).
DISCUSSION
The major finding of this study is that Slm1 and Slm2 are novel calcineurin substrates that promote yeast cell growth under sphingolipid-limiting conditions and endocytosis of a nutrient permease in response to heat stress. Interfering with calcineurin-dependent dephosphorylation of Slm proteins alters their activity in vivo. Thus, these studies identify a mechanism by which calcineurin may modulate sphingolipid-dependent events and establish the potential for cross talk between these two signaling pathways.
Slm1 and Slm2 are novel calcineurin substrates.
In this study, we identify Slm1 and Slm2 as targets of the phosphatase calcineurin and show that, like other calcineurin substrates (1, 3, 18, 36, 66), Slm1 and Slm2 each contain a PXIXIT-related docking site that mediates its interaction with the A subunit of calcineurin. This interaction is essential for dephosphorylation of Slm1 and Slm2 by calcineurin, and by creating SLM1 and SLM2 alleles lacking their respective PXIXIT motifs, we were able to assess the contribution of calcineurin signaling to Slm1 and Slm2 function in vivo.
Slm1 and Slm2 bind to calcineurin with different affinities. In vivo yeast two-hybrid assays suggest that full-length Slm2 associates more tightly with calcineurin than Slm1 does, and in vitro assays confirm this difference (J. Roy, H. Li, P. G. Hogan, and M. S. Cyert, unpublished results). Furthermore, the affinities of Slm1 and Slm2 for calcineurin correlate with the extents to which they are dephosphorylated by this phosphatase. Slm2 is completely dephosphorylated by calcineurin in vivo and in vitro, while Slm1 is only partially dephosphorylated by calcineurin.
The physiological consequences of calcineurin-mediated Slm dephosphorylation have yet to be fully elucidated. SLM1 and SLM2 are redundant for an essential function, which does not require calcineurin, as SLM1ΔPNIYIQ and SLM2ΔPEFYIE each support viability. In cells expressing a single SLM allele, however, abrogating Slm2 regulation by calcineurin modifies its function: the ability of Slm2ΔPEFYIE to promote growth in the presence of myriocin is compromised, and expression of this protein causes faster turnover of the Fur4 permease during heat stress. Disruption of Slm1 regulation by calcineurin also diminishes its ability to support myriocin resistance at 30°C but has no effect on Fur4 turnover. Taken together, these observations indicate that calcineurin can modify Slm function. However, in wild-type cells, which express both Slm1 and Slm2 and in which Slm1 is the predominant protein (4), the physiological significance of this regulation is somewhat unclear. Calcineurin, which is activated under conditions of environmental stress, may modify a specific aspect of Slm function or a restricted population of Slm proteins under these conditions. Further investigation is required to establish the biochemical mechanism by which Slm proteins act and, similarly, to fully appreciate the consequences of their dephosphorylation by calcineurin.
Slm proteins may act downstream of sphingoid bases.
Sphingolipids are produced via a pathway that is conserved from yeast to mammals (20, 60). They consist of a long-chain base, which is usually a linear alkane of 18 or 20 carbons having hydroxyls on C-1 and C-3 and an amino group on C-2. Yeast makes two types of long-chain bases, dihydrosphingosine and phytosphingosine. These sphingoid bases rapidly and transiently accumulate during heat stress but are not incorporated into ceramides or complex sphingolipids (60). Rather, they act as signals to promote cellular stress responses. Importantly, addition of exogenous sphingoid bases to yeast at low temperature induces several aspects of the heat stress response, including trehalose accumulation, the expression of stress element (STRE)-controlled stress response genes, cell cycle arrest, and the stimulation of ubiquitin-dependent protein turnover (13, 21, 44, 58, 76). Sphingoid bases exert their effects at least in part by activating protein phosphorylation (12, 41, 29).
Our data suggest a possible role for Slm proteins as mediators of sphingolipid-dependent signaling. First, phosphorylation of Slm1 and Slm2 is modulated by sphingoid bases in vivo. Second, Slm proteins are required for heat stress-induced turnover of the Fur4 permease, which is induced by sphingoid bases (13). Third, Slm1 and Slm2 modulate cell growth in the presence of drugs that perturb sphingolipid biosynthesis. Fourth, in contrast to lcb1-100 cells, which are defective in the first step of sphingolipid biosynthesis (76), the lethality of cells lacking Slm protein activity is not overcome by providing an external source of phytosphingosine, suggesting that Slm proteins act downstream of this mediator.
Phosphorylation of Slm proteins is modulated by sphingolipids.
The biochemical function of Slm1 and Slm2 is yet to be elucidated; however, it is clear that their activity and phosphorylation are highly regulated in vivo. Our studies examine modulation of Slm protein phosphorylation by sphingolipids and the protein phosphatase calcineurin during both heat stress and growth at constant temperature. During heat stress, a major consequence of increased levels of phytosphingosine and dihydrosphingosine is activation of protein kinases. Pkh1 and Pkh2, the yeast homologs of mammalian PDK1 (12), are activated by phytosphingosine and in turn phosphorylate and activate downstream kinases, including Ypk1, Ypk2, Pkc1, and Sch9 (12, 41, 61). Some of these kinases (Ypk1, Ypk2, Sch9) may also be stimulated directly by phytosphingosine (52). Heat stress and exogenous addition of sphingoid bases also stimulate calcineurin activity (reference 78 and Table 3). The mechanism for this activation must still be uncovered but is likely to involve stimulation of Ca2+-influx systems, as exposure of yeast cells to sphingosine l-phosphate, a sphingolipid produced in mammalian cells, stimulates Ca2+ accumulation (8).
When cells are shifted to high temperature, the net level of Slm1 and Slm2 phosphorylation changes little over the next 75 min. However, during this time, there is enhanced sphingolipid-dependent phosphorylation of Slm proteins that is counteracted by calcineurin-mediated dephosphorylation. Thus, during heat stress, the rate at which Slm1 and Slm2 cycle between their phosphorylated and dephosphorylated states must increase. Currently, it is unclear what consequence these changes in phosphorylation state have on Slm protein function. Perhaps, as for small GTP-binding proteins, the switch between two different states is an integral aspect of Slm protein activity.
Additional studies are required to establish which kinase or kinases are responsible for the heat stress-induced phosphorylation of Slm proteins. Kinases that are activated by phytosphingosine directly (Pkh1, Pkh2, Ypk1, Ypk2, and Sch9) or indirectly (Pkc1) are attractive candidates. A recent study showed that myriocin treatment abrogates Mss4-dependent PIP kinase activity (47), which might in turn reduce recruitment of Slm proteins to the plasma membrane, which depends on PIP2 binding to the PH domain in each protein (4). Thus, sphingolipids may also affect Slm protein phosphorylation indirectly by modulating their localization and access to kinases that reside at the cell periphery, such as Tor2p. Consistent with this possibility, we observed an increase in cytosolic localization of GFP-Slm1 and GFP-Slm2 in myriocin-treated cells (data not shown).
A previous study showed that TORC2 phosphorylates Slm1 in vitro (4). Furthermore, pulse-chase analysis of Slm1 revealed its rapid dephosphorylation after exposure to high temperature, followed by TOR2-dependent rephosphorylation. The timing of these events parallels that of the transient delocalization of the actin cytoskeleton in response to heat stress. In contrast, by analyzing steady-state phosphorylation of the entire cellular pool of Slm1 and Slm2, we observe a heat stress-induced increase in phosphorylation that is independent of Tor2p and occurs over a prolonged time period (≥75 min) when calcineurin is inactivated. These kinetics are more similar to that of downstream events such as endocytosis of Fur4, which continues ≥120 min following a shift to high temperature. It is likely, then, that multiple phosphorylation events occur, with both Tor2p and sphingolipid-regulated kinases contributing to total phosphorylation of Slm proteins during heat stress and potentially playing quite different roles in Slm regulation. Sphingolipid-dependent phosphorylation of Slm1 and Slm2 that is counteracted by calcineurin is also observed in cells grown under constant conditions, with the extent of Slm phosphorylation increasing with temperature. Thus, similar processes may underlie Slm protein phosphorylation during both normal and stress conditions.
Slm proteins are required for heat stress-induced Fur4 endocytosis.
Sphingolipids inhibit nutrient import during heat stress by promoting degradation of nutrient permeases, including the uracil permease, Fur4, and the general amino acid permease, Gap1 (13, 14). This degradation depends on the de novo biosynthesis of sphingolipids and is mimicked by the addition of exogenous phytosphingosine (13). Slm proteins are required for heat stress-induced Fur4 turnover, which occurs through ubiquitination, internalization, and trafficking of the permease to the vacuole (9, 25, 26, 30, 31). Ubiquitinated Fur4 accumulates at the surface of slm1ts cells, indicating that Slm proteins are required for internalization of Fur4. The actin cytoskeleton is required for the internalization step of endocytosis; many mutants with defects in actin organization or actin-binding proteins are unable to carry out endocytosis and accumulate ubiquitylated forms of Fur4 at the cell surface (31, 46, 57). Furthermore, mutants that lack components of the endocytosis machinery, such as the amphiphysin homologs, Rvs161 and Rvs167, are defective for actin polarization (54). slm1ts cells show defects in both actin cytoskeleton polarization (4) and endocytosis and may therefore participate directly in either or both of these processes.
A role for sphingolipid signaling in endocytosis has been clearly demonstrated by studies of lcb1-100 cells, which, at restrictive temperature, are unable to carry out the first step in sphingolipid biosynthesis. These cells display defects in endocytosis and actin organization which are remediated by addition of phytosphingosine or overexpression of sphingolipid-regulated kinases (29, 76). The functions of Lcb1 and Slm proteins in endocytosis, however, seem distinct. In contrast to lcb1-100 cells, slm1ts cells are able to accumulate lucifer yellow-carbohydrazide at restrictive temperature, indicating that Slm proteins are not generally required for endocytosis (data not shown). In addition, lcb1-100 cells, at least under some growth conditions, display no defect in stress-induced endocytosis of Fur4 (24). Thus, the defect in Fur4 endocytosis observed in slm1ts cells may reflect a specific requirement for the Slm proteins in stress-induced sphingolipid-dependent protein turnover. The possibility that calcineurin may modulate this process through dephosphorylation of Slm proteins is particularly intriguing, as calcineurin regulates endocytosis in higher eukaryotes by dephosphorylating dynamin, a PH domain-containing protein, and its associated proteins (51, 64). It is possible that there are parallels between the function of yeast Slm proteins and some of these proteins.
Slm proteins are involved in sphingolipid-dependent growth.
Sphingolipids are essential for yeast cell growth (22), and drugs that inhibit different steps of the sphingolipid biosynthesis pathway act as potent antifungal compounds (60). Myriocin inhibits the first step of sphingolipid biosynthesis, whereas aureobasidin A acts later in the pathway, blocking the inositol phosphorylceramide synthase and causing depletion of complex sphingolipids, such as mannosylated inositol phosphorylceramides, and accumulation of sphingolipid intermediates, such as phytosphingosine. We demonstrate that modifying SLM gene copy number has pronounced effects on cell growth at both standard (30°C) and elevated (37°C) temperatures when sphingolipid biosynthesis is inhibited. These findings suggest that Slm proteins modify the levels of sphingolipids in cells and/or are required to carry out sphingolipid-dependent cellular functions. A full understanding of the role of Slm proteins in sphingolipid signaling and metabolism awaits further characterization of the biochemical mechanism by which Slms function. However, because addition of phytosphingosine fails to rescue the growth of slm1ts slm2Δ cells at restrictive temperature, we propose that Slm1 and Slm2 are downstream mediators of sphingolipid function. Furthermore, the finding that slm1Δ mutants are both sensitive to myriocin and more resistant than wild-type cells to aureobasidin A is consistent with a role for Slm proteins acting downstream of sphingolipid bases rather than complex sphingolipids.
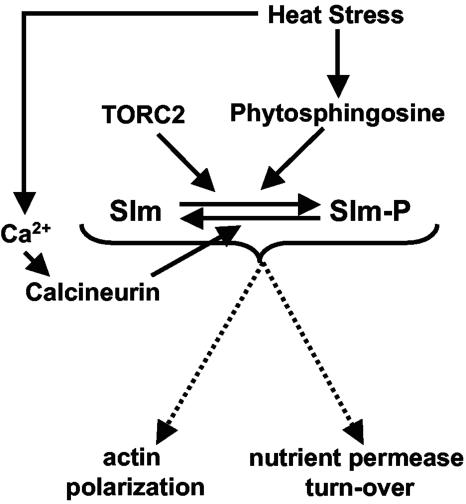
A model for Slm function during heat stress.
During stress, cell survival depends on integrating the responses of a variety of signaling pathways, each of which monitors a distinct aspect of cell physiology. While there remain many questions concerning the mechanism by which Slm proteins act, they seem to represent a key regulatory step (Fig. 11). During heat stress, Mss4 activity and concomitant PIP2 levels are increased, thereby recruiting Slm proteins to the cell periphery, where they are phosphorylated by the TORC2 kinase complex, which further stabilizes their localization. We show here that Slm proteins are similarly affected by heat stress and sphingoid bases, which rapidly and transiently accumulate during heat stress. Both thermal stress and phytosphingosine cause increased phosphorylation of Slm proteins (via unknown kinases) and simultaneously activate their dephosphorylation by calcineurin. All of these regulatory pathways, i.e., Mss4/PIP2, TORC2, sphingolipids, and calcineurin, independently contribute to cell survival during stress and impinge on Slm protein function. Furthermore, Slm proteins are required for polarization of the actin cytoskeleton (4, 27) and for endocytosis of the uracil permease, Fur4, during heat stress. A direct role for Slm1 and Slm2 in either actin organization or endocytosis could explain both of these defects. However, we cannot rule out the possibility that these defects occur as an indirect consequence of disrupting Slm function. Future experiments will aim to elucidate the biochemical function of these proteins and to determine the role that phosphorylation plays in modulating their activity.
FIG. 11.
Regulation of actin cytoskeleton organization and nutrient permease turnover by Slm protein functioning in response to heat stress. See the text for a discussion of the model.
Acknowledgments
We thank Scott Emr for generously providing slm1ts cells, for helpful discussion, and for communicating experimental results prior to publication. We thank F. Roelants and J. Thorner for helpful discussions and all members of the Cyert laboratory for both technical advice and useful discussions. We thank J. Roy for critically reading the manuscript.
Funding for this work was provided by NIH research grant GM-48729 to M.S.C. G.B. is a postdoctoral fellow of the Fund for Scientific Research Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen) and was the recipient of a postdoctoral fellowship for biomedical research in the United States funded by the D. Collen Research Foundation vzw and the Belgian American Educational Foundation. Work performed in R.H.-T.'s laboratory is supported by the Centre National de la Recherche Scientifique (CNRS), by a grant from the Association pour la Recherche contre le Cancer (ARC) (grant no. 3298), and by a special grant from the Ministère de la Recherche (ACI BSMC).
REFERENCES
- 1.Aramburu, J., F. Garcia-Cozar, A. Raghavan, H. Okamura, A. Rao, and P. G. Hogan. 1998. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol. Cell 1:627-637. [DOI] [PubMed] [Google Scholar]
- 2.Aramburu, J., A. Rao, and C. B. Klee. 2000. Calcineurin: from structure to function. Curr. Top. Cell. Regul. 36:237-295. [DOI] [PubMed] [Google Scholar]
- 3.Aramburu, J., M. B. Yaffe, C. Lopez-Rodriguez, L. C. Cantley, P. G. Hogan, and A. Rao. 1999. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129-2133. [DOI] [PubMed] [Google Scholar]
- 4.Audhya, A., R. Loewith, A. B. Parsons, L. Gao, M. Tabuchi, H. Zhou, C. Boone, M. N. Hall, and S. D. Emr. 2004. Genome-wide lethality screen identifies new PI4,5P(2) effectors that regulate the actin cytoskeleton. EMBO J. 23:3747-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel, F. M, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 6.Bartel, P. L., C.-T. Chien, R. Sternglanz, and S. Fields. 1993. Using the two-hybrid system to detect protein-protein interactions, p. 153-179. In D. A. Hartley (ed.), Cellular interactions in development: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 7.Bassel-Duby, R., and E. N. Olson. 2003. Role of calcineurin in striated muscle: development, adaptation, and disease. Biochem. Biophys. Res. Commun. 311:1133-1141. [DOI] [PubMed] [Google Scholar]
- 8.Birchwood, C. J., J. D. Saba, R. C. Dickson, and K. W. Cunningham. 2001. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J. Biol. Chem. 276:11712-11718. [DOI] [PubMed] [Google Scholar]
- 9.Blondel, M. O., J. Morvan, S. Dupre, D. Urban-Grimal, R. Haguenauer-Tsapis, and C. Volland. 2004. Direct sorting of the yeast uracil permease to the endosomal system is controlled by uracil binding and Rsp5p-dependent ubiquitylation. Mol. Biol. Cell 15:883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boustany, L. M., and M. S. Cyert. 2002. Calcineurin-dependent regulation of Crz1 nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 16:608-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bultynck, G., E. Vermassen, K. Szlufcik, P. De Smet, R. A. Fissore, G. Callewaert, L. Missiaen, H. De Smedt, and J. B. Parys. 2003. Calcineurin and intracellular Ca2+-release channels: regulation or association? Biochem. Biophys. Res. Commun. 311:1181-1193. [DOI] [PubMed] [Google Scholar]
- 12.Casamayor, A., P. D. Torrance, T. Kobayashi, J. Thorner, and D. R. Alessi. 1999. Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr. Biol. 9:186-197. [DOI] [PubMed] [Google Scholar]
- 13.Chung, N., G. Jenkins, Y. A. Hannun, J. Heitman, and L. M. Obeid. 2000. Sphingolipids signal heat stress-induced ubiquitin-dependent proteolysis. J. Biol. Chem. 275:17229-17232. [DOI] [PubMed] [Google Scholar]
- 14.Chung, N., C. Mao, J. Heitman, Y. A. Hannun, and L. M. Obeid. 2001. Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae. J. Biol. Chem. 276:35614-35621. [DOI] [PubMed] [Google Scholar]
- 15.Cyert, M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311:1143-1150. [DOI] [PubMed] [Google Scholar]
- 16.Cyert, M. S., R. Kunisawa, D. Kaim, and J. Thorner. 1991. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 88:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cyert, M. S., and J. Thorner. 1992. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol. Cell. Biol. 12:3460-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell'Acqua, M. L., K. L. Dodge, S. J. Tavalin, and J. D. Scott. 2002. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315-360. J. Biol. Chem. 277:48796-48802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desrivieres, S., F. T. Cooke, P. J. Parker, and M. N. Hall. 1998. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 273:15787-15793. [DOI] [PubMed] [Google Scholar]
- 20.Dickson, R. C., and R. L. Lester. 2002. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1583:13-25. [DOI] [PubMed] [Google Scholar]
- 21.Dickson, R. C., E. E. Nagiec, M. Skrzypek, P. Tillman, G. B. Wells, and R. L. Lester. 1997. Sphingolipids are potential heat stress signals in Saccharomyces. J. Biol. Chem. 272:30196-30200. [DOI] [PubMed] [Google Scholar]
- 22.Dickson, R. C., G. B. Wells, A. Schmidt, and R. L. Lester. 1990. Isolation of mutant Saccharomyces cerevisiae strains that survive without sphingolipids. Mol. Cell. Biol. 10:2176-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupre, S., and R. Haguenauer-Tsapis. 2001. Deubiquitination step in the endocytic pathway of yeast plasma membrane proteins: crucial role of Doa4p ubiquitin isopeptidase. Mol. Cell. Biol. 21:4482-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupre, S., and R. Haguenauer-Tsapis. 2003. Raft partitioning of the yeast uracil permease during trafficking along the endocytic pathway. Traffic 4:83-96. [DOI] [PubMed] [Google Scholar]
- 25.Dupre, S., D. Urban-Grimal, and R. Haguenauer-Tsapis. 2004. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim. Biophys. Acta 1695:89-111. [DOI] [PubMed] [Google Scholar]
- 26.Dupre, S., C. Volland, and R. Haguenauer-Tsapis. 2001. Membrane transport: ubiquitylation in endosomal sorting. Curr. Biol. 11:R932-R934. [DOI] [PubMed] [Google Scholar]
- 27.Fadri, M., A. Daquinag, S. Wang, T. Xue, and J. Kunz. 2005. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol. Biol. Cell 16:1883-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feske, S., H. Okamura, P. G. Hogan, and A. Rao. 2003. Ca2+/calcineurin signalling in cells of the immune system. Biochem. Biophys. Res. Commun. 311:1117-1132. [DOI] [PubMed] [Google Scholar]
- 29.Friant, S., R. Lombardi, T. Schmelzle, M. N. Hall, and H. Riezman. 2001. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 20:6783-6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galan, J. M., and R. Haguenauer-Tsapis. 1997. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 16:5847-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galan, J. M., V. Moreau, B. Andre, C. Volland, and R. Haguenauer-Tsapis. 1996. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem. 271:10946-10952. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein, A. L., and J. H. McCusker. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541-1553. [DOI] [PubMed] [Google Scholar]
- 33.Goud, B., A. Salminen, N. C. Walworth, and P. J. Novick. 1988. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53:753-768. [DOI] [PubMed] [Google Scholar]
- 34.Groth, R. D., R. L. Dunbar, and P. G. Mermelstein. 2003. Calcineurin regulation of neuronal plasticity. Biochem. Biophys. Res. Commun. 311:1159-1171. [DOI] [PubMed] [Google Scholar]
- 35.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 36.Heath, V. L., S. L. Shaw, S. Roy, and M. S. Cyert. 2004. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot. Cell 3:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidler, S. A., and J. A. Radding. 1995. The AUR1 gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent aureobasidin A (LY295337). Antimicrob. Agents Chemother. 39:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helliwell, S. B., I. Howald, N. Barbet, and M. N. Hall. 1998. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148:99-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho, H. L., Y. S. Shiau, and M. Y. Chen. 2005. Saccharomyces cerevisiaeTSC11/AVO3 participates in regulating cell integrity and functionally interacts with components of the Tor2 complex. Curr. Genet. 47:273-288. [DOI] [PubMed] [Google Scholar]
- 40.Homma, K., S. Terui, M. Minemura, H. Qadota, Y. Anraku, Y. Kanaho, and Y. Ohya. 1998. Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J. Biol. Chem. 273:15779-15786. [DOI] [PubMed] [Google Scholar]
- 41.Inagaki, M., T. Schmelzle, K. Yamaguchi, K. Irie, M. N. Hall, and K. Matsumoto. 1999. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol. 19:8344-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James, P. 2001. Yeast two-hybrid vectors and strains. Methods Mol. Biol. 177:41-84. [DOI] [PubMed] [Google Scholar]
- 43.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins, G. M., A. Richards, T. Wahl, C. Mao, L. Obeid, and Y. Hannun. 1997. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J. Biol. Chem. 272:32566-32572. [DOI] [PubMed] [Google Scholar]
- 45.Jiang, B., and M. S. Cyert. 1999. Identification of a novel region critical for calcineurin function in vivo and in vitro. J. Biol. Chem. 274:18543-18551. [DOI] [PubMed] [Google Scholar]
- 46.Kaksonen, M., C. P. Toret, and D. G. Drubin. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123:305-320. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi, T., H. Takematsu, T. Yamaji, S. Hiramoto, and Y. Kozutsumi. 2005. Disturbance of sphingolipid biosynthesis abrogates the signaling of Mss4, phosphatidylinositol-4-phosphate 5-kinase, in yeast. J. Biol. Chem. 280:18087-18094. [DOI] [PubMed] [Google Scholar]
- 48.Kuno, T., H. Tanaka, H. Mukai, C. D. Chang, K. Hiraga, T. Miyakawa, and C. Tanaka. 1991. cDNA cloning of a calcineurin B homolog in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 180:1159-1163. [DOI] [PubMed] [Google Scholar]
- 49.Kunz, J., R. Henriquez, U. Schneider, M. Deuter-Reinhard, N. R. Movva, and M. N. Hall. 1993. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 73:585-596. [DOI] [PubMed] [Google Scholar]
- 50.Levin, D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:262-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu, J. P., A. T. Sim, and P. J. Robinson. 1994. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science 265:970-973. [DOI] [PubMed] [Google Scholar]
- 52.Liu, K., X. Zhang, R. L. Lester, and R. C. Dickson. 2005. The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. J. Biol. Chem. 280:22679-22687. [DOI] [PubMed] [Google Scholar]
- 53.Liu, Y., S. Ishii, M. Tokai, H. Tsutsumi, O. Ohki, R. Akada, K. Tanaka, E. Tsuchiya, S. Fukui, and T. Miyakawa. 1991. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol. Gen. Genet. 227:52-59. [DOI] [PubMed] [Google Scholar]
- 54.Lombardi, R., and H. Riezman. 2001. Rvs161p and Rvs167p, the two yeast amphiphysin homologs, function together in vivo. J. Biol. Chem. 276:6016-6022. [DOI] [PubMed] [Google Scholar]
- 55.Luo, C., K. T. Shaw, A. Raghavan, J. Aramburu, F. Garcia-Cozar, B. A. Perrino, P. G. Hogan, and A. Rao. 1996. Interaction of calcineurin with a domain of the transcription factor NFAT1 that controls nuclear import. Proc. Natl. Acad. Sci. USA 93:8907-8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreau, V., J. M. Galan, G. Devilliers, R. Haguenauer-Tsapis, and B. Winsor. 1997. The yeast actin-related protein Arp2p is required for the internalization step of endocytosis. Mol. Biol. Cell 8:1361-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munn, A. L., and H. Riezman. 1994. Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J. Cell Biol. 127:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagiec, M. M., E. E. Nagiec, J. A. Baltisberger, G. B. Wells, R. L. Lester, and R. C. Dickson. 1997. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J. Biol. Chem. 272:9809-9817. [DOI] [PubMed] [Google Scholar]
- 60.Obeid, L. M., Y. Okamoto, and C. Mao. 2002. Yeast sphingolipids: metabolism and biology. Biochim. Biophys. Acta 1585:163-171. [DOI] [PubMed] [Google Scholar]
- 61.Roelants, F. M., P. D. Torrance, N. Bezman, and J. Thorner. 2002. Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol. Biol. Cell 13:3005-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 63.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smillie, K. J., and M. A. Cousin. 2005. Dynamin I phosphorylation and the control of synaptic vesicle endocytosis. Biochem. Soc. Symp. 72:87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stathopoulos-Gerontides, A., J. J. Guo, and M. S. Cyert. 1999. Yeast calcineurin regulates nuclear localization of the Crz1 transcription factor through dephosphorylation. Genes Dev. 13:798-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun, L., H. D. Youn, C. Loh, M. Stolow, W. He, and J. O. Liu. 1998. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity 8:703-711. [DOI] [PubMed] [Google Scholar]
- 68.Sun, Y., R. Taniguchi, D. Tanoue, T. Yamaji, H. Takematsu, K. Mori, T. Fujita, T. Kawasaki, and Y. Kozutsumi. 2000. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol. Cell. Biol. 20:4411-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uetz, P., L. Giot, G. Cagney, T. A. Mansfield, R. S. Judson, J. R. Knight, D. Lockshon, V. Narayan, M. Srinivasan, P. Pochart, A. Qureshi-Emili, Y. Li, B. Godwin, D. Conover, T. Kalbfleisch, G. Vijayadamodar, M. Yang, M. Johnston, S. Fields, and J. M. Rothberg. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:623-627. [DOI] [PubMed] [Google Scholar]
- 70.Urban-Grimal, D., B. Pinson, J. Chevallier, and R. Haguenauer-Tsapis. 1995. Replacement of Lys by Glu in a transmembrane segment strongly impairs the function of the uracil permease from Saccharomyces cerevisiae. Biochem. J. 308:847-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vernet, T., D. Dignard, and D. Y. Thomas. 1987. A family of yeast expression vectors containing the phage f1 intergenic region. Gene 52:225-233. [DOI] [PubMed] [Google Scholar]
- 72.Volland, C., C. Garnier, and R. Haguenauer-Tsapis. 1992. In vivo phosphorylation of the yeast uracil permease. J. Biol. Chem. 267:23767-23771. [PubMed] [Google Scholar]
- 73.Volland, C., D. Urban-Grimal, G. Geraud, and R. Haguenauer-Tsapis. 1994. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J. Biol. Chem. 269:9833-9841. [PubMed] [Google Scholar]
- 74.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1 signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277:31079-31088. [DOI] [PubMed] [Google Scholar]
- 75.Yu, J. W., J. M. Mendrola, A. Audhya, S. Singh, D. Keleti, D. B. DeWald, D. Murray, S. D. Emr, and M. A. Lemmon. 2004. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell 13:677-688. [DOI] [PubMed] [Google Scholar]
- 76.Zanolari, B., S. Friant, K. Funato, C. Sutterlin, B. J. Stevenson, and H. Riezman. 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19:2824-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang, X., R. L. Lester, and R. C. Dickson. 2004. Pil1p and Lsp1p negatively regulate the 3-phosphoinositide-dependent protein kinase-like kinase Pkh1 and downstream signaling pathways Pkc1 and Ypk1. J. Biol. Chem. 279:22030-22038. [DOI] [PubMed] [Google Scholar]
- 78.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]