Abstract
Abscisic acid (ABA) is one of the plant hormones involved in the interaction between plants and pathogens. In this work, we show that tomato (Lycopersicon esculentum Mill. cv Moneymaker) mutants with reduced ABA levels (sitiens plants) are much more resistant to the necrotrophic fungus Botrytis cinerea than wild-type (WT) plants. Exogenous application of ABA restored susceptibility to B. cinerea in sitiens plants and increased susceptibility in WT plants. These results indicate that ABA plays a major role in the susceptibility of tomato to B. cinerea. ABA appeared to interact with a functional plant defense response against B. cinerea. Experiments with transgenic NahG tomato plants and benzo(1,2,3)thiadiazole-7-carbothioic acid demonstrated the importance of salicylic acid in the tomato-B. cinerea interaction. In addition, upon infection with B. cinerea, sitiens plants showed a clear increase in phenylalanine ammonia lyase activity, which was not observed in infected WT plants, indicating that the ABA levels in healthy WT tomato plants partly repress phenylalanine ammonia lyase activity. In addition, sitiens plants became more sensitive to benzo(1,2,3)thiadiazole-7-carbothioic acid root treatment. The threshold values for PR1a gene expression declined with a factor 10 to 100 in sitiens compared with WT plants. Thus, ABA appears to negatively modulate the salicylic acid-dependent defense pathway in tomato, which may be one of the mechanisms by which ABA levels determine susceptibility to B. cinerea.
Upon pathogen attack, infected plant cells generate signaling molecules to initiate defense mechanisms in surrounding cells to limit pathogen spread. The role of the plant hormones salicylic acid (SA), jasmonic acid (JA), and ethylene in this process is supported by well-documented observations and molecular characterization (Hammond-Kosack and Jones, 1996). This kind of information is not available for another plant hormone, abscisic acid (ABA), which participates in several processes. The role of ABA in developmental programs, such as seed dormancy, root geotropism, opening of stomata through stomatal guard cells, and dormancy of buds, has been most extensively documented (Walton, 1980). Furthermore, ABA is involved in the wound response (WR) activated upon insect feeding (Birkenmeier and Ryan, 1998).
Regarding plant-pathogen interactions, information on ABA involvement is mainly based on indirect observations. Increased endogenous ABA levels were observed in response to infection with viruses, bacteria, and fungi (Whenham et al., 1986; Steadman and Sequeira, 1970; Kettner and Dörffling, 1995). In addition, it is generally found that application of exogenous ABA increases the susceptibility of plants to fungal pathogens (Henfling et al., 1980; Ward et al., 1989; McDonald and Cahill, 1999). ABA also seems to interact with pathogen associated plant defense. In soybean (Glycine max), ABA suppressed Phe ammonia lyase (PAL) activity and transcription of PAL mRNA in hypocotyls inoculated with the incompatible pathogen Phytophthora megasperma f.sp. glycinea (Ward et al., 1989). Moreover, physiological ABA concentrations down-regulate β-1,3-glucanase at the level of transcription in tobacco (Nicotiana tabacum) cell cultures. β-1,3-Glucanases have been implicated in responses to stress, wounding, and pathogen infection (Rezzonico et al., 1998). However, these observations only give a fragmentary picture and provide few or indirect clues for the mechanistic basis of the involvement of ABA in plant defense toward pathogens.
To study the role of plant hormones such as SA, JA, and ethylene in plant defense to pathogens, mutants impaired in the perception or biosynthesis of these hormones have been successfully used in Arabidopsis and tomato (Lycopersicon esculentum Mill. cv Moneymaker; Lund et al., 1998; Thomma et al., 1998). To our knowledge, ABA mutants have not previously been used in plant-pathogen interaction studies, with the exception of the work by Kettner and Dörffling (1995). The ABA-deficient flacca tomato mutant was used in their study on biosynthesis and metabolism of ABA in tomato leaves infected with Botrytis cinerea. To further elucidate the role of ABA in plant-pathogen interactions, we used ABA-negative sitiens tomato mutants. Sitiens mutants have a residual ABA level of 8% of the WT plants and are unable to increase their ABA levels upon elicitation by wounding, heat, or electrical current (Herde et al., 1999). Sitiens tomato mutants are defective in the structural gene for ABA-aldehyde oxidase, the enzyme that converts ABA-aldehyde to ABA.
The present study shows that ABA-negative sitiens tomato plants are much more resistant to B. cinerea than WT plants, indicating that ABA increases susceptibility of tomato to B. cinerea. In a first attempt to elucidate the mechanistic basis for this observation, we studied the potential cross talk between the plant hormone ABA- and SA-associated plant defenses. Results suggest that ABA negatively regulates SA-dependent defense signaling, which in turn appears to be an effective plant defense mechanism against B. cinerea.
RESULTS
ABA Increases Susceptibility of Tomato to B. cinerea
To analyze the role of ABA in the interaction between tomato and B. cinerea, leaves of sitiens tomato mutants (Linforth et al., 1987), impaired in the biosynthesis of ABA, and wild-type (WT) leaves (tomato cv Moneymaker) were infected in a comparative assay. Because B. cinerea typically needs a nutrient supply to initiate an infection (Van Den Heuvel, 1981), several inoculation solutions composed of different Glc and phosphate concentrations were tested on detached WT tomato leaves (Fig. 1A) based on the infection methods described by Van Den Heuvel (1981) and Von Tiedemann (1997).
Figure 1.
Effect of Glc and phosphate concentrations on the infection of B. cinerea on tomato. Detached tertiary leaves (A) or tertiary leaves of intact plants (B) were infected by a droplet solution. Ten droplets each containing 4 μL of spore suspension were placed on a tomato leaf surface. The infection was evaluated at several time points after infection by counting the number of B. cinerea lesions spreading out of the initial inoculation droplets on each leaf. The inoculation solutions tested in this work were: ●, 0.1 m Glc, 67 mm KH2PO4 (pH 5), and 106 spores mL−1; ▪, 0.05 m Glc, 33 mm KH2PO4 (pH 5), and 106 spores mL−1; and ▴, 0.01 m Glc, 6.7 mm KH2PO4 (pH 5), and 106 spores mL−1. White signs show the infection development without adding phosphate to the inoculation solutions. Data are means of three experiments containing 12 leaves per treatment.
When using the infection solution composed of 106 spores mL−1, 0.1 m Glc, and 67 mm KH2PO4 (pH 5), all inoculations resulted in brownish spreading lesions colonizing the whole leaf area (Fig. 1A). When a very mild infection solution was used (106 spores mL−1 + 0.01 m Glc), none of the infection droplets resulted in a spreading lesion. This interaction is considered to be resistant because further fungal spread was not observed and the fungus did not colonize the leaf. In this resistant interaction, B. cinerea development was restricted to a few black spots under the inoculation droplet, indicating a clear reduction of pathogen growth. Because we wanted to use assay conditions that would result in a moderately aggressive infection, an inoculation solution containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4 (pH 5) was selected. This solution produced a moderate number of spreading B. cinerea lesions in WT detached leaves (Fig. 1A). This infection allowed us to detect both increases and decreases in disease severity.
To eliminate an effect of leaf detachment, infection solutions were tested on intact plants (Fig. 1B). These experiments showed that the infection developed similarly on intact plants and on detached leaves, although fewer lesions spread out of the initial inoculum droplet when the infection was carried out on intact plants. Because the uniformity of infection was higher on detached leaves than on intact plants, it was decided to perform further infections using detached leaves.
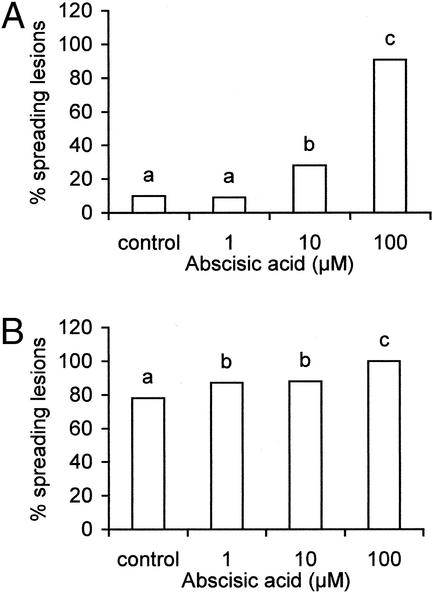
In the comparative assay, sitiens leaves appeared to be much more resistant to B. cinerea than WT leaves because a considerable decrease in the number of spreading B. cinerea lesions was observed (Fig. 2, A–C). Experiments were performed subsequently to determine whether exogenous ABA applied to petioles of sitiens leaves could restore the susceptibility observed in WT plants. Results presented in Figure 3A clearly illustrate that concentrations from 10 to 100 μm ± cis-trans ABA increased the susceptibility of sitiens leaves to B. cinerea. These results also demonstrate that a threshold concentration of ABA is necessary to induce the susceptible response in sitiens tomato to B. cinerea because 1 μm ABA did not induce susceptibility in sitiens leaves. Finally, applying ABA to WT leaves further increased the susceptibility to B. cinerea, although in these experiments no threshold concentration to increase susceptibility was observed (Fig. 3B). These experiments clearly illustrate the importance of ABA, at levels present in WT tomato plants, in the interaction with B. cinerea.
Figure 2.
A, Influence of endogenous ABA concentrations on spreading of B. cinerea in tomato 4 d after infection. Tertiary WT (tomato cv Moneymaker) and sitiens leaves (tomato cv Moneymaker) were detached from 5-week-old tomato plants and infected with 10 droplets of a 4-μL spore suspension containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4. Infection was evaluated 4 d after infection by counting the number of spreading lesions on each leaf. Data are means of three experiments containing 12 leaves per treatment. B shows an infected WT leaf (tomato cv Moneymaker) with four spreading lesions of six lesions. C shows a resistant sitiens leaf (tomato cv Moneymaker) with no spreading lesions. Bars with different letters are significantly different with P = 0.05 after a logistic regression.
Figure 3.
Effect of exogenous ABA fed to petioles of 5-week-old of sitiens (A) and WT (B) leaves (tomato cv Moneymaker) on an infection with B. cinerea. Tertiary leaves were detached and placed for 16 h in ABA solutions varying from 1 to 100 μm. ABA solutions were prepared from 1 mL of 10 mm stock solution of ABA in ethanol. Ethanol concentrations varied from 0.1% to 0.001% (v/v), respectively, in the final solutions. Control leaves were fed with water containing 0.1% (v/v) ethanol. Inoculation solutions contained 0.01 m Glc, 6.7 mm KH2PO4, and 106 spores mL−1. Infection was scored 4 d after inoculation by counting the number of spreading B. cinerea lesions on each leaf. Data are means of three experiments containing 12 leaves per treatment. Bars with different letters are significantly different with P = 0.05 after a logistic regression.
Basis of Increased Resistance in sitiens Plants to B. cinerea
Several hypotheses were tested in search of an explanation for the altered resistance of sitiens plants to B. cinerea. First, we had to exclude the possibility that the increased resistance of sitiens mutants to B. cinerea was an artifact resulting from the use of detached leaves in our infection assays. It is known that endogenous ABA levels can increase in WT plants upon wounding, whereas sitiens mutants do not show this increase (Herde et al., 1999). Therefore, we infected intact sitiens and WT with B. cinerea and still observed increased resistance in sitiens plants compared with WT plants (Fig. 4).
Figure 4.
Influence of endogenous ABA concentrations on spreading of B. cinerea in intact tomato plants. Tertiary leaves of WT (tomato cv Moneymaker) and sitiens (tomato cv Moneymaker) plants were infected with 10 droplets of 4 μL of spore suspension containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4. Data are means of two experiments containing five plants (10 leaves) per treatment. Infections were evaluated 4 d after inoculation by counting the number of spreading B. cinerea lesions on each leaf. Bars with different letters are significantly different with P = 0.05 after a logistic regression.
To investigate a potential direct effect of ABA on fungal growth, B. cinerea was plated on potato dextrose agar (Oxoid, Drongen, Belgium) medium containing 1 to 100 μm ± cis-trans ABA. None of the ABA concentrations stimulated fungal growth. Moreover, in vivo experiments illustrated that lesions in sitiens plants grew at the same rate in WT and sitiens plants, indicating that ABA present in WT plants did not stimulate directly the growth rate of B. cinerea (data not shown). These results suggest that ABA does not directly influence the pathogen or its interaction with the host but rather modulates the defense mechanism of the host to the pathogen.
JA-Dependent Plant Signaling Defense Is Not Involved in Defense of Tomato to B. cinerea
Because we wanted to investigate further the possible involvement of ABA in the defense response of tomato to B. cinerea, we first had to know which defense signaling pathways play a role in the tomato-B. cinerea interaction. Because it is known that JA-dependent defense is important in the Arabidopsis-B. cinerea interaction (Thomma et al., 1998, 1999), we investigated whether the same was true in tomato. For this purpose, the JA biosynthesis tomato mutant def 1 was used (Howe et al., 1996). Def 1 is mutated in the conversion of hydroxyperoxylinolenic acid to oxy-phytodieonic acid. We infected WT plants (tomato cv Castlemart) and def1 mutants (tomato cv Castlemart) with B. cinerea and found no effect of JA on the resistance level of tomato to B. cinerea (Fig. 5A). Moreover, applying concentrations of exogenous JA from 5 up to 100 μm to the tomato leaf petiole did not affect the resistance level of WT tomato to B. cinerea (Fig. 5B). In tomato, JA apparently does not play an important role in the basal defense to B. cinerea.
Figure 5.
A, Effect of endogenous JA concentrations on the infection of B. cinerea in tomato. Tertiary leaves of WT (tomato cv Castlemart) and def1 tomato mutants (tomato cv Castlemart) were infected with 10 droplets of 4 μL of spore suspension containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4. Data are means of two experiments containing 10 leaves per treatment. Infections were evaluated 4 d after inoculation by counting the number of spreading B. cinerea lesions on each leaf. Bars with different letters are significantly different with P = 0.05 after a logistic regression. B, Effect of exogenous ± JA feeding through petioles of tomato leaves from 5-week-old plants. Leaves were infected with 10 droplets of 4 μL of spore suspension containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4. Data are means of two experiments containing 10 leaves per treatment. Bars with different letters are significantly different with P = 0.05 after a logistic regression.
SA-Dependent Resistance Controls B. cinerea in Tomato
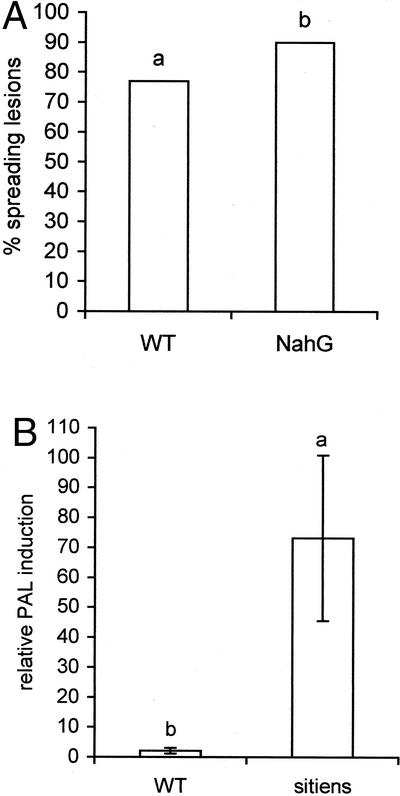
Because De Meyer et al. (1999a, 1999b) showed that resistance to B. cinerea in bean (Phaseolus vulgaris) could be induced via the SA-dependent defense pathway, the role of this pathway in the tomato-B. cinerea interaction was investigated. For this purpose, we used transgenic NahG tomato plants that cannot accumulate SA because they express a bacterial SA hydroxylase that converts SA to catechol (Brading et al., 2000). NahG leaves were slightly more susceptible to B. cinerea than WT leaves (Fig. 6A), suggesting a role for SA in the basal defense of tomato to B. cinerea. In addition, PAL activity in sitiens leaves increased severalfold 16 h after infection, whereas in WT leaves, only a small increase could be observed (Fig. 6B). Basal PAL activity, however, was lower in sitiens leaves than in WT leaves (118 μkats kg−1 protein for WT and 18 μkats kg−1 protein for sitiens). Mock-inoculated sitiens and WT leaves did not show an increased PAL activity. Because PAL is a major enzyme in the phenylpropanoid pathway, which is also involved in SA synthesis (Pallas et al., 1996; Mauch-Mani and Slusarenko, 1996; Smith-Becker et al., 1998), these results indicate that SA-dependent defense in sitiens plants was activated to a higher extent than in WT plants.
Figure 6.
A, Influence of endogenous SA on spreading of B. cinerea in the detached leaf assay. Tertiary WT (tomato cv Moneymaker) and NahG leaves (tomato cv Moneymaker) were infected with 10 droplets of 4 μL of spore suspension containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4. Data are means of two experiments each composed of 10 leaves per treatment. Infection was evaluated 4 d after inoculation by counting the number of spreading B. cinerea lesions on each leaf. Bars with different letters are significantly different with P = 0.05 after a logistic regression. B, Relative induction of PAL activity in 5-week-old WT (tomato cv Moneymaker) and sitiens leaves (tomato cv Moneymaker) 16 h after infection with B. cinerea with an infection solution of 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4. Values for PAL activity were obtained by dividing values for PAL activity 16 h after infection by values for PAL activity of control leaves. Bars represent the average of 10 individual tomato leaves of sitiens and WT plants. Data represent two independent experiments. Data were analyzed by an ANOVA analysis. Bars with different letters are significantly different with P = 0.05.
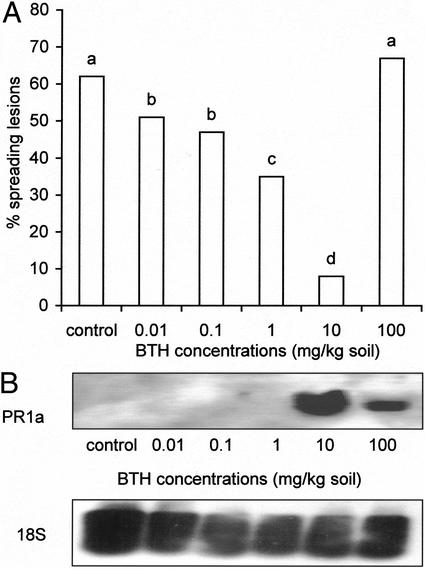
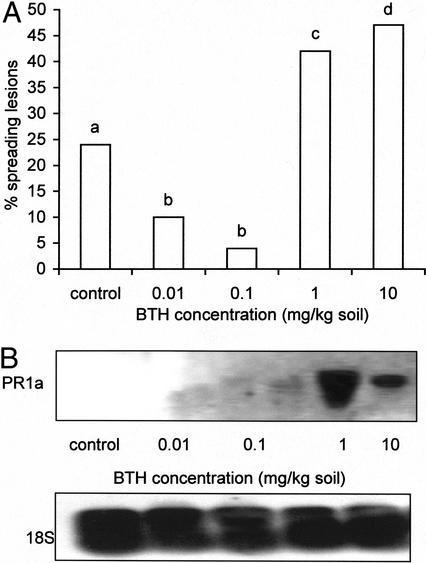
To confirm the role of SA-dependent defense in the control of B. cinerea, the plant defense activator and SA-analog benzo(1,2,3)thiadiazole-7-carbothioic acid (BTH, BION; Novartis, Basel) was applied to the soil at the time of sowing and 10 d later when seedlings were transferred. Application of BTH at several concentrations (0.01, 0.1, 1, and 10 mg kg−1 soil) induced resistance to B. cinerea (Fig. 7A) in WT leaves, whereas PR1a gene expression was detected only at 10 mg kg−1 soil (50 μmol kg−1 soil; Fig. 7B). However, when applying higher concentrations of BTH, e.g. 100 mg kg−1 soil, resistance declined, whereas PR1a was still expressed. Applying such high concentrations was rather dramatic with regard to the morphology of tomato plants. Leaves turned dark green and became shrunken compared with control leaves (not shown). In addition, applying 100 mg kg−1 BTH to roots of tomato plants probably induced plant defense to a high extent because this concentration resulted in the development of spontaneous necrotic lesions. Although 10 mg kg−1 did not induce spontaneous lesions, minor changes in morphology, resulting in lengthening of internodes and shrinking of leaves, were observed. Applying 10 mg kg−1 BTH not only induces PR1a gene expression but initiates changes in plant morphology and plant resistance. As a consequence,10 mg kg−1 could be considered as a threshold concentration.
Figure 7.
A. Effect of BTH (BION) root application on spreading of B. cinerea lesions on the third pair of 5-week-old WT tomato leaves (tomato cv Moneymaker). Leaves were infected with 10 droplets of 4 μL of spore suspension containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4 25 d after the last BTH treatment. Data are means of three experiments with each 12 leaves per treatment. Infections were evaluated 4 d after inoculation by counting the number of spreading B. cinerea lesions on each leaf. Bars with different letters are significantly different with P = 0.05 after a logistic regression. B, Induction of PR1a gene expression in 5-week-old leaves of WT tomato plants after BTH root application. Each lane contains 30 μg of total RNA and PR1a was detected via digoxygenin (DIG)-labeled cDNA probes. BTH concentrations are represented in milligrams per kilogram. Detection limit of PR1a was determined to be 0.5 pg. An 18S rRNA probe was used as a constitutive probe to verify for equal RNA loading and transfer.
There is no clear link between PR1 gene expression and induced resistance by BTH to B. cinerea in tomato. Moreover, activating plant defense too extensively leads to necrotic lesions, which may serve as a nutritional source for B. cinerea. From these results, it can be concluded that inducing SA-dependent plant defense mechanisms in tomato can lead to an enhanced resistance to the necrotrophic fungus B. cinerea.
SA-Dependent Resistance Induced by BTH Is Modulated by Endogenous Plant-ABA
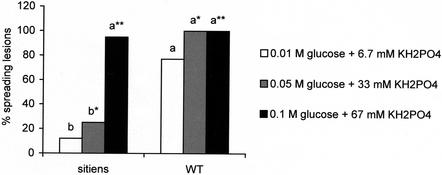
Because it was shown that BTH, which activates the SA-dependent pathway, induced resistance to the necrotrophic fungus B. cinerea in WT plants, we wanted to test whether the SA-dependent response was altered or enhanced in sitiens plants. BTH was applied at several concentrations to sitiens roots as described above to assay for induction of resistance. Because sitiens leaves displayed a high basal level of resistance (Fig. 2), more aggressive inoculation solutions were tested on sitiens leaves (Fig. 8). The infection solution with 0.05 m Glc and 33 mm KH2PO4 resulted in 100% spreading lesions in WT leaves (Fig. 1, A and B), whereas in sitiens leaves the infection was only moderately aggressive (Fig. 8). This solution was used in the subsequent study.
Figure 8.
Influence of inoculum aggressiveness on the resistance of sitiens leaves (tomato cv Moneymaker) to B. cinerea. Tertiary leaves of WT and sitiens plants were infected with 10 droplets of 4 μL of spore suspension containing different Glc and phosphate concentrations. Data are means of three experiments with each 12 leaves per treatment. Infections were evaluated 4 d after infection by counting the number of spreading B. cinerea lesions on each leaf. Bars with different letters are significantly different with P = 0.05 after a logistic regression.
Results shown in Figure 9A clearly demonstrate the induction of resistance by applying BTH to roots of sitiens tomato plants. However, in sitiens plants, PR1a expression was induced at a BTH concentration of 1 mg kg−1 (Fig. 9B), in contrast to the 10 mg kg−1 BTH, which was needed to induce these effects in WT plants. In addition, spontaneous necroses and stimulation of B. cinerea infection were already observed at a concentration of 1 mg kg−1, whereas 100 mg kg−1 was needed to induce these effects on WT plants. These results indicate that sitiens plants are sensitized to respond to the chemical plant activator BTH and suggest that ABA levels in WT plants negatively interfere with the SA-dependent defense pathway in tomato.
Figure 9.
A, Effect of BTH root application on spreading of B. cinerea lesions on the third pair of sitiens tomato leaves (tomato cv Moneymaker). Leaves were infected with 10 droplets of 4 μL of spore suspension containing 106 spores mL−1, 0.05 m Glc, and 33 mm KH2PO4 25 d after the last BTH treatment. Data are means of three experiments with each 12 leaves per treatment. Infections were evaluated 4 d after infection by counting the number of spreading B. cinerea lesions on each leaf. Bars with different letters are significantly different with P = 0.05 after a logistic regression. B, Induction of PR1a gene expression in 5-week-old leaves of sitiens tomato plants after BTH root application. Each lane contains 30 μg of total RNA and PR1a was detected via DIG-labeled cDNA probes. An 18S rRNA probe was used as a constitutive probe to verify for equal RNA loading and transfer.
DISCUSSION
In this study, we developed a reliable and reproducible infection method to study the B. cinerea-tomato interaction. By using this method we have shown that ABA-negative sitiens plants are much less susceptible to B. cinerea infection than WT plants. Moreover, in sitiens leaves, susceptibility could be restored by exogenous application of ABA at concentrations above 10 μm. Applying ABA also increased the level of susceptibility of WT tomato leaves to B. cinerea.
Several studies have shown that exogenously applied ABA can increase susceptibility of various plants species toward various pathogens such as Phytophthora infestans and Cladosporium cucumerinum on potato (Solanum tuberosum) slices (Henfling et al., 1980), P. megasperma f. sp. glycinea (P. sojae) on soybean (Ward et al., 1989), Peronospora tabacina on tobacco (Salt et al., 1986), and B. cinerea on tomato (Kettner and Dörffling, 1995). In addition, Kettner and Dörffling (1995) showed that B. cinerea infection resulted in increased ABA levels in infected tomato plants by at least four processes: stimulation of fungal ABA biosynthesis by the host, release of ABA or its precursor by the fungus, stimulation of biosynthesis of plant ABA by the fungus, and inhibition of its metabolism by the fungus. However, they did not mention a decline in susceptibility of ABA-negative flacca plants toward B. cinerea compared with WT plants like we observed in sitiens plants. We inoculated flacca tomato mutants (Marin and Marion-Poll, 1997) with B. cinerea using a moderately aggressive infection solution (0.01 m Glc and 6.7 mm KH2PO4) and found the same level of resistance as for sitiens plants (data not shown). The infection assay used by Kettner and Dörffling (1995) may have been too aggressive to visualize changes in resistance levels. Infecting sitiens plants with a very aggressive infection solution (0.1 m Glc and 67 mm KH2PO4) resulted in almost 100% spreading lesions, which was comparable with the number of spreading lesions in the WT plants (Fig. 8). This indicates that an aggressive infection method masks a possible defense mechanism against B. cinerea.
Because at least 10 μm exogenous ABA is needed to induce susceptibility to B. cinerea in sitiens tomato, a threshold ABA concentration appears to be necessary for a susceptible response of tomato toward B. cinerea. This threshold concentration is apparently higher than ABA levels present in sitiens (ABA levels 8% of WT) and flacca (ABA levels 21% of WT) plants (Herde et al., 1999). Herde et al. (1999) also suggested the presence of a threshold level of ABA within the plant must be reached for early events in electrical signaling and for proteinase inhibitor II gene expression upon wounding.
We subsequently studied how ABA induces susceptibility to B. cinerea. ABA did not have an effect on the growth of B. cinerea on plates or on the plant, indicating that ABA interferes with the plant response and not with the pathogen. Based on other plant hormone studies, two major possibilities for the interaction of ABA with plant defense could be proposed. One consists of an ABA-dependent defense signal transduction pathway. Alternatively, ABA could modulate one of the well-described plant defense responses dependent on the plant hormones SA, ethylene, or JA (Sticher et al., 1997; Thomma et al., 1998).
The presence of an ABA-dependent defense-signaling pathway has not been documented yet, to our knowledge. Dammann et al. (1997) illustrated the presence of an organ-specific ABA signal transduction pathway distinct from the classical JA-dependent WR signaling in potato, but its function and physiological relevance is not clear. Therefore, we suggest that the effects observed in the present study are more likely because of a modulation of a functional plant defense pathway.
Because it is not known which plant defense pathways are involved in the tomato-B. cinerea interaction, we initially tried to characterize a functional defense response. Based on previous work by Thomma et al. (1998) in Arabidopsis, we investigated the role of JA in defense of tomato to B. cinerea. However, exogenous JA application or elimination of JA in def1 mutants did not affect the response of tomato to B. cinerea. These results appear to be contradictory to results obtained by Thomma et al. (1998, 1999) who demonstrated a clear role for JA in resistance of Arabidopsis against B. cinerea using JA-insensitive coi1–1 plants. However, WT Arabidopsis has a very strong basal level of resistance to various isolates of B. cinerea (Thomma et al., 1998, 1999) including the B. cinerea isolate used in the present study (K. Audenaert, unpublished data). The most aggressive infection solution used in the present study (0.1 m Glc and 67 mm KH2PO4) did not give any spreading lesions in Arabidopsis. This indicates that the B. cinerea-Arabidopsis interaction approaches a non-host response that is completely different from the highly susceptible response of tomato to B. cinerea. In the Arabidopsis response, both JA and ethylene play an important role (Thomma et al., 1998). The strong basal level of resistance to B. cinerea observed in Arabidopsis is based on different mechanisms than the compatible interaction between tomato and B. cinerea.
In the present work, we show that in tomato, SA-dependent defense is a potential defense mechanism against B. cinerea. NahG tomato plants were more susceptible than WT plants. Furthermore, BTH treatment rendered WT plants more resistant than control plants. Again, this appears to be in contradiction with results obtained by Thomma et al. (1998) in Arabidopsis, where NahG plants were not more susceptible to B. cinerea than WT plants. However, Zimmerli et al. (2001) recently showed that NahG Arabidopsis plants were more susceptible to B. cinerea than WT Arabidopsis Columbia-0 plants and that a soil drench application of BTH drastically slowed down the B. cinerea infection on Arabidopsis, which is in agreement with our observations on tomato. Zimmerli et al. (2001) explained the discrepancy in their results and the results obtained by Thomma et al. (1998) by the fact that in their experiments the Arabidopsis plants were kept in constant high air humidity, which strongly favored the infection process. This indicates that in conditions that favor infection, SA-dependent signaling also contributes to restrict B. cinerea infection in Arabidopsis.
In addition, we could observe a dual modulation of the SA-dependent defense response by ABA. First, sitiens leaves showed a clear increase in PAL activity 16 h after infection with B. cinerea, which was not observed in WT leaves. These results suggest that PAL activity is partially repressed by ABA levels present in WT tomato leaves. A correlation between PAL and resistance to B. cinerea was previously described in bean plants (De Meyer et al., 1999a). Moreover, in soybean, exogenously applied ABA suppressed PAL activity and synthesis of PAL mRNA in the incompatible interaction of soybeans with P. megasperma f. sp. glycinea (Ward et al., 1989). Second, sitiens turned out to be hyper-responsive to BTH treatment. Threshold values for induction of PR1a gene expression and toxicity declined by a factor of 10 to 100 in sitiens leaves compared with WT leaves (Figs. 7 and 9).
It is possible that the higher BTH concentrations needed to induce PR1a in WT are because of the fact that ABA, at levels present in WT leaves, can directly influence expression of the PR1a gene promoter, which contains a negative-acting ABA-responsive element TAACAAA (for review, see Giraudat et al., 1994). This could lead to a transcriptional down-regulation of PR1a. Earlier studies by Rezzonico et al. (1998) illustrated down-regulation by ABA of β-1,3-glucanase (βGLU I) genes but not of chitinase (CHN) genes in cultured tobacco pith cells. It was suggested that the differential effect of ABA on βGLU I and CHN expression could be because of the absence of the ABA-responsive element in the CHN I gene, whereas distal and proximal copies of the TAACAAA box were present in the promoter of βGLU I (Rezzonico et al., 1998). We therefore suggest that the presence of the ABA-responsive element could result in transcriptional down-regulation of PR1a. However, expression of PR1a was not correlated with resistance of tomato to B. cinerea in our model system.
An attractive hypothesis to explain the interference of ABA with SA-dependent defense signaling originates from results in NIM1 overexpressing Arabidopsis mutants. NIM1, also called NPR1, is one of the first characterized proteins in SA-signaling downstream of SA (Cao et al., 1994). NIM1 overexpressing lines became more responsive to SA and were hyperresponsive to BTH, suggesting a direct or indirect interaction of BTH with the NIM1 protein (Friedrich et al., 2001). Because ABA-negative sitiens leaves show the same type of responsiveness to BTH as NIM1-overexpressing Arabidopsis lines, it is possible that ABA levels present in WT plants suppress NPR1 activity either directly or indirectly.
Because we have shown that ABA negatively modulates SA-dependent defense responses, it is interesting to notice that some B. cinerea strains produce high amounts of ABA in culture and that in vitro ABA production has also been observed for several other phytopathogenic and mycorrhizal fungi (Dörffling et al., 1984; Crocoll et al., 1991; Danneberg et al., 1993). In addition, it is known that endogenous ABA levels can rise upon pathogen infection (Kettner and Dörffling, 1995). ABA was also found at a considerably higher level in maize plants colonized with arbuscular mycorrhiza than in control plants (Bothe et al., 1994). It is tempting to speculate that these fungi produce ABA and/or induce endogenous ABA production in the plant to suppress SA-dependent defense mechanisms. Zimmerli et al. (2001) observed that B. cinerea fails to induce a strong SAR response in Arabidopsis and the suppression of plant defense responses seems to be a widespread phenomenon in associations between plants and endomycorrhizal fungi (Harrison, 1999). It remains to be investigated, however, whether the B. cinerea strains used in this study and the study of Zimmerli et al. (2001) produce ABA and/or stimulate ABA biosynthesis by the plant and whether increased endogenous ABA levels lead to a stronger suppression of SA-dependent defense mechanisms.
Although our results show an interaction of ABA with the SA-dependent disease response, which is functional in the control of B. cinerea, one cannot exclude an involvement of other plant hormones in the increased resistance of sitiens to B. cinerea. Where JA levels are unchanged in ABA-negative tomato plants (Herde et al., 1999), aminocyclopropane carboxylate levels (the direct precursor of ethylene) were 2-fold higher in ABA-negative tomato plants compared with WT plants (Sharp et al., 2000). The role of ethylene with respect to resistance of plants to B. cinerea is not well established. Thomma et al. (1999) reported the participation of ethylene in the defense of Arabidopsis against B. cinerea. In tomato, however, ethylene production during a B. cinerea infection was correlated with the development of necrosis (Elad, 1990). In addition, exposure of strawberries (Fragaria ananassa) to ethylene increased B. cinerea development (El Kazzaz et al., 1983). Recently, the role of ethylene in the tomato-B. cinerea interaction was extensively studied by using ethylene perception blockers and tomato mutants impaired in the biosynthesis or perception of ethylene (A.T. Have, J. Díaz, and J.A.L. van Kan, personal communication).
In conclusion, we have shown that ABA-negative sitiens tomato plants are more resistant to B. cinerea than WT plants, indicating that endogenous ABA levels present in WT plants increase susceptibility of tomato to B. cinerea. In a first attempt to elucidate the mechanistic basis for this observation, our results suggest that cross talk occurs between the plant hormone ABA- and SA-induced defenses. Hence, we suggest that negative modulation of SA-dependent signaling is probably one of the mechanisms by which ABA determines susceptibility of tomato to B. cinerea.
MATERIALS AND METHODS
Plant Material
Tomato (Lycopersicon esculentum Mill.) sitiens mutants (Taylor et al., 1988; Taylor et al., 2000), tomato cv Moneymaker NahG transgenes, and tomato cv Castlemart def1 mutants (Howe et al., 1996) were grown in potting compost soil (Klassmann Substrat 4, Hesepe, Germany). Maarten Koornneef (University of Wageningen, The Netherlands) provided seeds of tomato sitiens plants, Gregg Howe (Washington State University, Pullman) provided def1 seeds, and Jonathan Jones (John Innes Centre, Norwich, UK) provided seeds of NahG-tomato plants. Plant material was thereafter propagated by seed multiplication. Plants were grown for 5 weeks under greenhouse conditions (24°C ± 3) with a 16-h-light photoperiod and high humidity to prevent the sitiens plants from wilting.
Chemical Treatment of Plant Material
Tomato seeds were treated with BTH, or BION (Novartis), before planting by dipping them in a BTH solution of 0.01, 0.1, 1, or 10 mg L−1. Seeds were sown subsequently in soil containing already 0.1, 1, and 10 mg kg−1 BTH. Ten days after sowing, roots of seedlings were dipped in BTH solutions and transferred to pots containing BTH-treated soil in concentrations as mentioned above.
Treatments with ABA were performed by dipping petioles of 5-week-old tomato leaves in a solution containing 1 to 100 μm of cis,trans-ABA (Sigma, Bornem, Belgium) during 16 h before infection with B. cinerea. ABA solutions were prepared from a 1 mm stock solution containing 1% (v/v) ethanol. In accordance, final ethanol concentrations were, respectively, 0.1 to 0.001 in the 100 to 1 μm ABA solutions. Control leaves were dipped in water containing 0.1% (v/v) ethanol.
Treatments with JA were performed by dipping petioles of 5-week-old tomato leaves in a solution of 5 to 100 μm of ± JA (Sigma) during 16 h before infection with B. cinerea.
B. cinerea Infection
B. cinerea isolate R16 resulting from the cross SAS56 × SAS405 (Faretra and Pollastro, 1991) was grown on tomato leaf agar (Salinas and Schot, 1987) under a light regime of UV/dark (12 h/12 h). After 10 d, spores were washed from the plates with distilled water containing 0.01% (v/v) Tween 20. After removing mycelial debris, spores were counted and added to the inoculation solution in the proper concentration.
Tertiary leaves of 5-week-old tomato plants were excised by cutting the petioles near the stem. The petiole was immediately wrapped in wet absorbing paper. Leaves were transferred to trays and placed on a plastic lattice supported by glass rods. The wrapped petioles were put through the lattice to touch several layers of wet absorbing paper on the bottom of the trays. Finally, each tomato leaf composed of five leaflets was infected by putting 10 droplets of 4 μL of inoculation solution containing 106 spores mL−1, Glc, and KH2PO4 (pH 5) on the leaf surface. The amount of Glc and phosphate was dependent on the inoculation conditions used in the experiment. In experiments on induced resistance, WT leaves were infected with a solution containing 106 spores mL−1, 0.01 m Glc, and 6.7 mm KH2PO4 unless mentioned otherwise. Sitiens leaves were infected using 106 spores mL−1, 0.05 m Glc, and 33 mm KH2PO4 unless mentioned otherwise. Trays were covered with plastic folium to guarantee a relative humidity of 95% to 100%. Four days after inoculation, infection was evaluated by counting the number of spreading lesions on each leaf. Data were statistically analyzed as a dichotomous variable by logistic regression.
RNA Extraction, Gel-Blot Hybridization, and Enzyme Activity
Leaf material of 5-week-old plants was frozen in liquid N2 and ground to a fine powder with a mortar and a pestle. Total RNA was extracted by the phenol-SDS method as described by Ausubel et al. (1993). Fifteen micrograms of total RNA was loaded to a formaldehyde-denatured 1% (w/v) agarose gel and then transferred to a nylon membrane (Hybond N+, Amersham Pharmacia Biotech, Antwerpen, Belgium). Hybridization took place at 65°C. Nonradioactive labeled DIG probes (Boehringer Mannheim, Brussels) were prepared by random labeling using DIG-High-Prime (Boehringer Mannheim). The PR1a-probe was kindly provided by Pierre de Wit (University of Wageningen, Wageningen, The Netherlands). Stringency washes were performed for 1 h at room temperature in 2× SSC and for 1 h in 0.5× SSC at 65°C with 0.1% (w/v) SDS each. All RNA samples for WT and sitiens plants were loaded on the same gel, blotted on the same membrane, and hybridized in the same tube to eliminate differences in the gene expression pattern in WT and sitiens plants. To verify for equal amounts of RNA, hybridization was performed with an 18S rRNA probe.
PAL activity was measured as described by Edwards and Kessmann (1992). Three leaves, infected with 30 droplets each containing 2 μL of B. cinerea inoculum, were ground to powder in liquid nitrogen and extracted with 50 mm Tris-HCl (pH 8.5) containing 14 mm mercapto-ethanol and 5% (w/v) polyvinylpyrollidone. After centrifugation, protein levels were measured with bovine serum albumin as a standard. PAL activity was determined in 50 mm Tris-HCl (pH 8.5 containing 10 mm l-Phe) at 40°C with a UVIKON922 spectrometer (Kontron, B.R.S., Anderlecht, Belgium; A290), indicating the conversion of l-Phe to trans cinnamic acid. d-Phe was used as a blank. The increase in PAL activity upon B. cinerea was measured 16 h after infection by dividing PAL-activity in infected leaves by PAL activity in noninfected leaves. Data were statistically analyzed by an ANOVA analysis (Posthoc: Duncan).
ACKNOWLEDGMENTS
We thank Maarten Koornneef for providing sitiens tomato seeds, Jonathan Jones for providing NahG tomato seeds, Gregg Howe for providing def1 seeds, and Pierre de Wit for providing cDNA probes for PR1a detection. We thank Ilse Delaere and Valerie Rijckaert for technical assistance and Bart Kersschot for help with plant experiments.
Footnotes
This work was supported by the Flemish Institute for the Stimulation of Scientific-Technological Research in Industry (IWT, Belgium; specialization fellowship to K.A.) and by a grant from the Fund for Scientific Research-Flanders (FWO, Belgium).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010605.
LITERATURE CITED
- Ausubel FM, Brent R, Kingston ER. Current Protocols in Molecular Biology. Brooklyn, NY: Current Protocols; 1993. [Google Scholar]
- Birkenmeier GF, Ryan CA. Wound signaling in tomato plants, evidence that ABA is not a primary signal for defense gene activation. Plant Physiol. 1998;117:687–693. doi: 10.1104/pp.117.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe H, Klingner A, Kaldorf M, Schmitz O, Esch H, Hundeshagen B, Kernebeck H. Biochemical approaches to the study of plant-fungal interactions in arbuscular mycorrhiza. Experientia. 1994;50:919–925. [Google Scholar]
- Brading PA, Hammond-Kosack KE, Parr A, Jones JDG. Salicylic acid is not required for Cf-2 and Cf-9-dependent resistance of tomato to Cladosporium fulvum. Plant J. 2000;23:305–318. doi: 10.1046/j.1365-313x.2000.00778.x. [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocoll C, Kettner J, Dörffling K. Abscisic acid in saprophytic and parasitic species of fungi. Phytochemistry. 1991;30:1059–1060. [Google Scholar]
- Dammann C, Rojo E, Sánchez-Serrano JJ. Abscisic acid and jasmonic acid activate wound-inducible genes in potato through separate, organ-specific signal transduction pathways. Plant J. 1997;11:773–782. doi: 10.1046/j.1365-313x.1997.11040773.x. [DOI] [PubMed] [Google Scholar]
- Danneberg G, Latus Z, Zimmer W, Hundeshagen B, Schneiderpoetsch H, Bothe H. Influence of vesicular-arbuscular mycorrhiza on phytohormone balances in maize (Zea maysL.) J Plant Physiol. 1993;141:33–39. [Google Scholar]
- De Meyer G, Audenaert K, Höfte M. P. aeruginosa 7NSK2-induced systemic resistance in tobacco depends on in plantasalicylic acid accumulation but is not associated with PR1a gene-expression. Eur J Plant Pathol. 1999a;105:513–517. [Google Scholar]
- De Meyer G, Capieau K, Audenaert K, Buchala A, Métraux J-P, Höfte M. Nanogram amounts of salicylic acid produced by the rhizobacterium P. aeruginosa7NSK2 activate the systemic acquired resistance pathway in bean. Mol Plant-Microbe Interact. 1999b;12:450–458. doi: 10.1094/MPMI.1999.12.5.450. [DOI] [PubMed] [Google Scholar]
- Dörffling K, Petersen W, Sprecher E, Urbasch I, Hanssen HP. Abscisic acid in phytopathogenic fungi of the genera Botrytis, Ceratocystis, Fusarium, and Rhizoctonia. Z Naturforsch C. 1984;39:683–684. [Google Scholar]
- Edwards R, Kessmann H. Isoflavonoid phytoalexins and their biosynthetic enzymes. In: Gurr S, McPherson M, Bowles D, editors. Molecular Plant Pathology: A Practical Approach. Vol. 2. Oxford: Oxford University Press; 1992. pp. 45–62. [Google Scholar]
- Elad Y. Production of ethylene by tissues of tomato, pepper, French bean and cucumber in response to infection by Botrytis cinerea. Physiol Mol Plant Pathol. 1990;36:277–287. [Google Scholar]
- El Kazzaz MK, Sommer NF, Fortlage RJ. Effect of different atmospheres on postharvest decay and quality of fresh strawberries. Phytopathology. 1983;73:282–285. [Google Scholar]
- Faretra F, Pollastro S. Genetic bases of resistance to benzimidazole anddicarboximide fungicides in Botryotinia fuckeliana (Botrytis cinerea) Mycol Res. 1991;8:943–951. [Google Scholar]
- Friedrich L, Lawton K, Dietrich R, Willits M, Cade R, Ryals J. NIM1overexpression in Arabidopsis potentiates plant disease resistance and results in enhanced effectiveness of fungicides. Mol Plant-Microbe Interact. 2001;14:1114–1124. doi: 10.1094/MPMI.2001.14.9.1114. [DOI] [PubMed] [Google Scholar]
- Giraudat J, Parcy F, Bertauche N, Gosti F, Leung J, Morris P-C, Bouvier-Durand M, Vartanian N. Current advances in abscisic acid action and signaling. Plant Mol Biol. 1994;26:1557–1577. doi: 10.1007/BF00016490. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ. Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:361–389. doi: 10.1146/annurev.arplant.50.1.361. [DOI] [PubMed] [Google Scholar]
- Henfling JWDM, Bostock R, Kuc J. Effects of abscisic acid on rishitin and lubimin accumulation and resistance to Phythopthora infestans and Cladosporium cucumerinumin potato tuber tissue slices. Phytopathology. 1980;70:1074–1078. [Google Scholar]
- Herde O, Peña-Cortés H, Wasternack C, Willmitzer L, Fisahn J. Electric signaling and Pin2gene expression on different abiotic stimuli depend on a distinct threshold level of endogenous abscisic acid in several abscisic acid-deficient tomato mutants. Plant Physiol. 1999;119:213–218. doi: 10.1104/pp.119.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for plant defense against insect attack. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner J, Dörffling K. Biosynthesis and metabolism of abscisic acid in tomato leaves infected with Botrytis cinerea. Planta. 1995;196:627–634. [Google Scholar]
- Linforth R, Bowman W, Griffin D, Marples B, Taylor I. 2-trans-ABA alcohol accumulation in the wilty tomato mutants flacca and sitiens. Plant Cell Environ. 1987;10:599–606. [Google Scholar]
- Lund S, Stall RE, Klee HJ. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin E, Marion-Poll A. Tomato flaccamutant is impaired in ABA aldehyde oxidase and xanthine dehydrogenase activities. Plant Physiol Biochem. 1997;35:369–372. [Google Scholar]
- Mauch-Mani B, Slusarenko A. Production of salicylic acid precursors is a major function of phenylalanine ammonia lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KL, Cahill DM. Influence of abscisic acid and the abscisic acid biosynthesis inhibitor, norflurazon, on interactions between Phytophthora sojae and soybean (Glycine max) Eur J Plant Path. 1999;105:651–658. [Google Scholar]
- Pallas J, Paiva N, Lamb C, Dixon R. Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 1996;10:281–293. [Google Scholar]
- Rezzonico E, Flury N, Meins F, Beffa R. Transcriptional down-regulation by abscisic acid of pathogenesis-related β-1,3-glucanase genes in tobacco cell cultures. Plant Physiol. 1998;117:585–592. doi: 10.1104/pp.117.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas J, Schot CP. Morphological and physiological aspects of B. cinerea. Mededelingen van de Faculteit Landbouwwetenschappen, Rijksuniversiteit Gent. 1987;52:771–776. [Google Scholar]
- Salt SD, Tuzun S, Kuć J. Effects of β-ionone and abscisic acid on the growth of tobacco and resistance to blue mold: mimicry of effects of stem infection by Peronospora tabacinaAdam. Physiol Mol Plant Biol. 1986;28:287–297. [Google Scholar]
- Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot. 2000;51:1575–1584. doi: 10.1093/jexbot/51.350.1575. [DOI] [PubMed] [Google Scholar]
- Smith-Becker J, Marois E, Huguet EJ, Midland SL, Sims JJ, Keen NT. Accumulation of salicylic acid and 4-hydroxybenzoic acid in phloem fluids of cucumber during systemic acquired resistance is preceded by a transient increase in phenylalanine ammonia lyase activity in stems and petioles. Plant Physiol. 1998;116:231–238. doi: 10.1104/pp.116.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman JR, Sequeira L. Abscisic acid in tobacco plants: tentative identification and its relation to stunting induced by Pseudomonas solanacearum. Plant Physiol. 1970;45:691–697. doi: 10.1104/pp.45.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux J-P. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Taylor IB, Burbidge A, Thompson AJ. Control of abscisic acid biosynthesis. J Exp Bot. 2000;62:1563–1574. doi: 10.1093/jexbot/51.350.1563. [DOI] [PubMed] [Google Scholar]
- Taylor IB, Linforth RST, Al-Naieb RJ, Bowman WR, Marples BA. The wilty tomato mutants flacca and sitiensare impaired in the oxidation of ABA-aldehyde to ABA. Plant Cell Environ. 1988;11:739–745. [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broeckaert W. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsisare essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel J. Effect of inoculum composition on infection of French bean leaves by conidia of Botrytis cinerea. Neth J Phytopathol. 1981;87:55–64. [Google Scholar]
- Von Tiedemann AV. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol Mol Plant Pathol. 1997;50:151–166. [Google Scholar]
- Walton DC. Biochemistry and physiology of abscisic acid. Annu Rev Plant Physiol. 1980;31:453–489. [Google Scholar]
- Ward EWB, Cahill DM, Bhattacharyya M. Abscisic acid suppression of phenylalanine ammonia lyase activity and mRNA, and resistance of soybeans to Phytophthora megasperma f. sp. Glycinea. Plant Physiol. 1989;91:23–27. doi: 10.1104/pp.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whenham RJ, Fraser RSS, Brown LP, Payne JA. Tobacco mosaic virus-induced increase in abscisic acid concentration in tobacco leaves: intracellular location in light and darkgreen areas, and relationship to symptom development. Planta. 1986;168:592–598. doi: 10.1007/BF00392281. [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Métraux J-P, Mauch-Mani B. β-aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol. 2001;126:517–523. doi: 10.1104/pp.126.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]