Abstract
The shoot apical meristem (SAM), initially formed during embryogenesis, gives rise to the aboveground portion of the maize (Zea mays) plant. The shootless phenotype (sml) described here is caused by disruption of SAM formation due to the synergistic interaction of mutations at two genetic loci. Seedlings must be homozygous for both sml (shootmeristemless), and the unlinked dgr (distorted growth) loci for a SAM-less phenotype to occur. Seedlings mutant only for sml are impaired in their morphogenesis to different extents, whereas the dgr mutation alone does not have a recognisable phenotype. Thus, dgr can be envisaged as being a dominant modifier of sml and the 12 (normal):3 (distorted growth):1 (shoot meristemless) segregation observed in the F2 of the double heterozygote is the result of the interaction between the sml and dgr genes. Other segregation patterns were also observed in the F2, suggesting instability of the dgr gene. Efforts to rescue mutant embryos by growth on media enriched with hormones have been unsuccessful so far. However, mutant roots grow normally on medium supplemented with kinetin at a concentration that suppresses wild-type root elongation, suggesting possible involvement of the mutant in the reception or transduction of the kinetin signal or transport of the hormone. The shootless mutant appears to be a valuable tool with which to investigate the organization of the shoot meristem in monocots as well as a means to assay the origins and relationships between organs such as the scutellum, the coleoptile, and leaves that are initiated during the embryogenic process.
Embryogenesis is a developmental process leading to the formation of a complex structure, the embryo, which gives rise to the seedling following germination. The maize (Zea mays) embryo consists of an embryonic axis and a surrounding scutellum. After an asymmetric first division of the zygote, the fertilized egg goes through a series of cell divisions leading to a structure consisting of the embryo proper and the suspensor. The embryo subsequently acquires bilateral asymmetry, establishes a structural axis, and initiates the organs and tissues that the adult plant will elaborate. In the succeeding phase, a period of maturation and differentiation of up to six leaf primordia takes place prior to seed dormancy. The organs of the seedling, namely the leaves, stem, and primary root, derive from groups of cells organized as shoot and apical meristems, the activities of which continue during the entire developmental cycle of the plant. The shoot apical meristem (SAM) is first recognizable when the embryonic axis is established and appears as an organized structure consisting of two overlapping layers, the tunica and the corpus. Asymmetry is established with the initials of the SAM appearing laterally at the adaxial surface of the embryo and the abaxial portion of the embryo enlarging to form the scutellum. At the same time, the ring-shaped coleoptile primordium separates the meristem from the scutellum and ultimately encloses the SAM and the leaf primordia.
Meristems are the source of stem cells as well as the sites of organ formation. Establishment of the SAM during embryogenesis is a key event in plant development because the architecture of the plant relies on its function. SAM organization and maintenance are therefore of fundamental interest in understanding plant development. A useful tool with which to study how the SAM is established and to determine its role in regulating plant organ differentiation is the analysis of mutants affected in meristem function and organization. In this regard, mutants have been obtained in several species that result from failure of the SAM to form correctly during embryogenesis (Evans and Barton, 1997). In Arabidopsis, the stm (shootmeristemless) mutation results in the lack of SAM formation during embryogenesis, whereas other embryonic organs such as the cotyledons, hypocotyls, and radicles develop normally (Barton and Poethig, 1993). The STM gene encodes a KNOTTED1 (KN1)-type homeodomain protein. kn1 is a marker of meristem activity with a pattern of expression very similar to that of STM (Kerstetter and Hake, 1997). Being a homeodomain protein, STM may promote shoot meristem formation by regulating other genes. Supporting this view is the observation that the expression of the UNUSUAL FLOWER ORGANS (UFO) gene is dependent on STM function because stm mutant embryos fail to express UFO (Bowman and Eshed, 2000). The SAM-less phenotype in Arabidopsis is also observed in seedlings homozygous for other mutations such as topless or pinhead and in one specific case is only manifest in a double mutant (Aida et al., 1999). Similarly, in petunia the no apical meristem (nam) mutation seems to be associated with the determination of SAM position (Souer et al., 1996). In cereals, shootless mutants affected in SAM initiation have been described in rice (Hong et al., 1995; Satoh et al., 1999) and mutants with similar phenotypes have been found in maize (Sheridan and Clark, 1993), although they have not been well characterized. A maize defective kernel mutant, ed-41v, disrupted in shoot differentiation was shown to be impaired in kinetin metabolism by embryo rescue experiments on enriched media (Racchi et al., 1996). In maize and other species, knotted-like homeobox (knox) genes related by sequence to KNOTTED1 constitute a gene family. These genes are expressed in meristems but not in developing organ primordia. Loss-of-function mutations implicate class 1 knox genes in the determination of cell fate and patterning in meristems. The study of grass embryo mutants may well be important not only for elucidating the genetic program leading to SAM initiation but also for unraveling the interrelationships between organs such as the scutellum, coleoptile, and leaf initials formed during embryogenesis.
RESULTS
Origin and Phenotype of a Shootless Maize Mutant
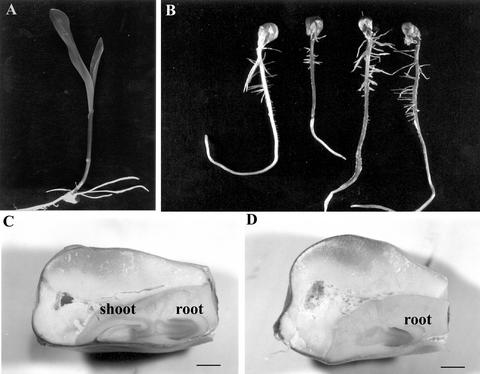
The shootless maize mutant described herein was originally observed in an F2 family obtained by Steve L. Dellaporta (Department of Biology, Yale University, New Haven, CT) from a large transposon mutagenesis experiment. This family, segregating for the Ac transposon and r-scm:3 (the latter monitors the presence of Ac by eliciting a change in aleurone pigmentation from colorless to spotted), was one of several families selected on the basis of the segregation of presumed germination impaired mutants following sand bench screening. However, when a sample of seeds was germinated on filter paper, three-fourths were normal (Fig. 1A) and one-fourth produced a normal primary root with normal lateral roots but failed to form a shoot (Fig. 1B), indicating that the apparent failure to germinate on the sand bench resulted from the lack of shoot production and that the shootless trait was likely due to the segregation of a single gene mutant. Analysis of longitudinal sections of rehydrated mature seeds (Fig. 1D) showed that one-fourth were totally devoid of shoot primordia, whereas root primordia were identical in all cases to those observed in wild-type sibling embryos (Fig. 1C).
Figure 1.
Effect of the sml mutation on seedling and embryo development. A, Normal seedling. B, Homozygous sml seeds are easily identified after germination because they form an apparently normal primary root but no shoot. C and D, Longitudinal sections of wild-type and sml seeds removed from the same ear. Mutant embryos (D) are easily recognizable due to the absence of the shoot primordium present in their wild-type siblings (C). Bars = 1 mm.
Lack of Shoot Formation Is Due to a Block in SAM Organization
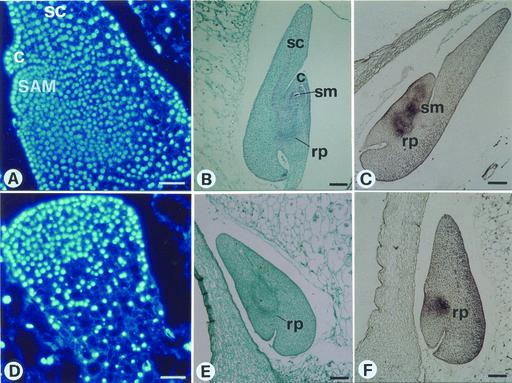
To find out if early events during embryogenesis can account for the lack of shoot formation in the mutant, wild-type and mutant embryos were compared (Fig. 2). Longitudinal sections obtained from immature (9 DAP) seeds excised from a segregating ear were stained with DAPI. In wild-type coleoptilar stage embryos (Fig. 2A), the shoot/root axis is evident on the anterior side, showing at its apical pole the shoot meristem composed of small cells containing large nuclei organized into an epidermal layer that is clonally distinct from the interior cells because of anticlinal divisions. At the transition stage, a stage just prior to that at which the SAM first becomes histologically distinguishable, mutant sibling embryos (Fig. 2D) are similar to wild-type embryos. No evidence was found for cells having the characteristics of SAM cells, nor were signs of scutellum elongation and coleoptile differentiation found. At 17 DAP, mutant embryos (Fig. 2E) differ from wild type (Fig. 2B) in their lack of a shoot primordium. Concordant with this, when mutant embryos (17 DAP) were hybridized in situ with a probe corresponding to the maize homeobox gene knotted1 (kn1; Jackson et al., 1994), a marker of meristematic activity expressed in the SAM but not in the scutellum nor in the coleoptile (Smith et al., 1995), no positive signal was observed at the position where the SAM is normally located. However, knotted1 expression was evident at the root pole (compare Fig. 2, C with F). Control reactions using labeled sense RNA probes revealed no signal or only a very low level of nonspecific hybridization (data not shown). Therefore, the cytological observations and in situ hybridization data concord with the lack of appearance of histological features corresponding to the apical meristem. The mutation was duly designated sml.
Figure 2.
The sml mutant is impaired in SAM organization. Median longitudinal sections through normal (A–C) and sml (D–F) immature sibling embryos obtained by selfing a Sml/sml plant. A and D, Sections of immature (12 d after pollination [DAP]) sibling embryos stained with 4,6-diamidino-2-phenylindole (DAPI). SAM is present in wild-type (A) and absent in mutant (D) embryos; bars = 50 μm. B and E, Sections of immature (17 DAP) embryos stained with safranin-fast green. Shoot and root primordia are visible in normal siblings (B), whereas mutant embryos (E) only have root primordial. Bars = 200 μm. C and F, In situ hybridization of wild-type (C) embryos (17 DAP) with a kn1 probe shows a positive signal on shoot and root primordial, whereas in the mutant (F) kn1 mRNA is detectable only in the root initials. Bars = 200 μm. c, Coleoptile; rp, root primordium; sc, scutellum; sm, shoot meristem.
Mapping of sml
Heterozygous Sml/sml females were crossed with heterozygous or hyperploid B-A translocation males with the aim of establishing the chromosomal arm location of sml. The F1 that revealed the mutant was obtained from crosses involving the TB-10L male parent, thus indicating that sml lies on the long arm of chromosome 10. A more refined position for sml was achieved by analysis of simple sequence repeat (SSR) marker distribution in a segregating population obtained by crossing heterozygous Sml/sml females with B73 inbred male parents. A polymorphism for the marker UMC 1084 established a distance of about 21 cM (13 recombinants out of 62) between this marker and sml. Further mapping data are needed to establish its centromeric proximal or distal position.
Different Hypotheses Can Be Proposed to Account for the sml Phenotype
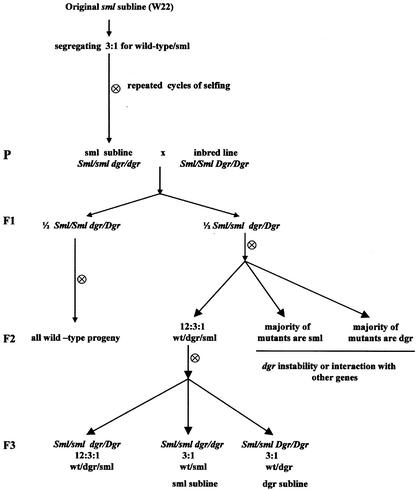
In its original background (the W22 inbred line), the shootless mutant segregates in the selfed progeny of Sml/sml heterozygous parents in a one-fourth shootless, three-fourths normal seedling ratio as would be expected if the basis of this trait were a single monogenic mutation. This segregation pattern is maintained in repeated cycles of selfing. However, an unexpected result was obtained when Sml/sml parents were outcrossed either to W22 or different inbred lines and the progeny of the selfed F1 was germinated. In addition to normal and shootless seedlings, the F2 families yielded a new class of seedlings exhibiting distorted growth, referred to as dgr (Fig. 3). This pattern of segregation was again observed in the F3 generation and yielded a 12:3:1 (normal:distorted growth:shootless) ratio consistent with the occurrence of an interaction of mutations at two independent genetic loci, sml and dgr (Fig. 4). According to this hypothesis, the shootless phenotype (sml) is recovered when both dgr and sml are homozygous, whereas the distorted growth phenotype (dgr) could be explained by assuming that the loss of the Sml function in Dgr/-sml/sml individuals is partially compensated by the activity of Dgr. The unexpected recovery of dgr seedlings in the progeny of outcrosses of Sml/sml W22 heterozygotes to different inbred lines thus would reflect a different constitution at the dgr locus in the inbred lines employed in the outcrosses compared with that of the original line. To be specific, the parental genotypes would thus be: Sml/sml dgr/dgr × Sml/Sml Dgr/Dgr, whereas the three phenotypes recovered in the F2 in the 12:3:1 ratio would have the genetic constitution: Dgr/-Sml/- and dgr/dgr Sml/- (12/16) normal; Dgr/- sml/sml (3/16) distorted growth, and dgr/dgr sml/sml (1/16) shootless (Table I). This conclusion was corroborated by analysis of the F3 generation that led to the establishment of two lines yielding mainly sml or dgr segregants as a result of fixation of dgr or sml in the homozygous condition. Similarly, the observation that the original line segregates sml but not dgr can be expected if we assume that its propagation by selfing and selection of families segregating for sml led to the establishment of homozygosity for dgr while maintaining heterozygosity at the sml locus (Fig. 4).
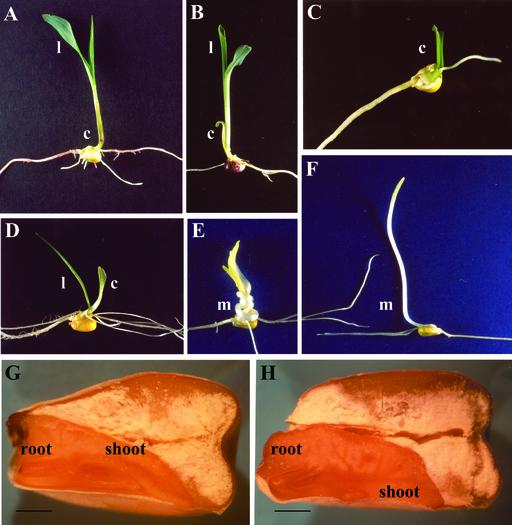
Figure 3.
Spectrum of abnormalities attributable to the dgr phenotype conditioned by the Dgr/- sml/sml genotype. A, Wild-type seedling. B, Mutant seedling with an open coleoptile and an apparently normal first leaf. C, Mutant seedling with an empty coleoptile. D, Mutant seedling with abnormal coleoptile and abnormal leaf morphology. Mutant seedling grown in darkness developed a twisted mesocotyl (E). F, Wild-type seedling grown in darkness. G and H, Longitudinal sections of wild-type and dgr seeds removed from the same ear. Mutant embryos (H) are easily recognizable due to the asymmetry of the shoot primordium. l, Leaf; c, coleoptile; m, Mesocotyl. Bars = 1.5 mm.
Figure 4.
Pedigree diagram showing the genetics of the sml mutation and how the sml and dgr sublines were generated.
Table I.
Segregation of mutant phenotypes observed in the F2 obtained by selfing the progeny of male parents heterozygous for sml to different inbred lines
| Coded No. | Outcrossed to | Segregation of Selfed
Outcrosses (F2)
|
||||
|---|---|---|---|---|---|---|
| wt | dgr | sml | χ2 value | P | ||
| R4-70 | B73 | 237 | 47 | 16 | 2.56 | 0.28 |
| R4-83 | B73 | 377 | 85 | 29 | 0.85 | 0.65 |
| 27 (2) | A344 | 115 | 30 | 8 | 0.31 | 0.86 |
| R53-2 | K6 | 253 | 60 | 16 | 1.22 | 0.54 |
| R19 (2) | W22 | 312 | 64 | 26 | 2.12 | 0.35 |
| Total | 1,294 | 286 | 95 | 4.53 | 0.10 | |
The segregation values of the two mutant phenotypes (dgr and sml) are indicative of two loci exhibiting a dominant epistatic interaction (12:3:1). wt, Wild type.
However, an alternative interpretation can be advanced to account for the observed pattern of segregation. The mutant, originally detected in a line segregating for Ac and r-scm:3 (an Ac reporter), could be due to an Ac-induced transposition of Ds to the Sml gene. Subsequent somatic excision of Ds from Sml in response to an active Ac would allow the mutant to achieve partial growth recovery leading to dgr seedlings. Such a phenotype should cosegregate with a functional Ac, a prediction not confirmed by the results reported in Table II.
Table II.
Test to verify the association between the occurrence of the dgr phenotype and the presence of an active Ac in the genome
| Coded No. | Segregation upon Selfing
|
Aleurone Color after the Cross to r-scm:3/r-scm:3 Plantsa | Ac Constitutionb | ||
|---|---|---|---|---|---|
| wt | dgr | sml | |||
| R28-29 | 23 | 7 | 0 | Colorless | – |
| R28-37 | 27 | 1 | 2 | Spotted | + |
| R28-26 | 23 | 6 | 1 | Spotted | + |
| R28-14 | 20 | 5 | 5 | Colorless | – |
| R28-9 | 26 | 1 | 3 | Colorless | – |
| R28-27 | 25 | 1 | 4 | Spotted | + |
| R28-24 | 24 | 3 | 3 | Spotted | + |
| R28-34 | 24 | 4 | 2 | Colorless | – |
| R28-38 | 28 | 2 | 0 | Colorless | – |
| R28-21 | 26 | 2 | 2 | Colorless | – |
Plants genotipically Sml/sml r/r, segregating or not for Ac, were selfed and mated to r-scm:3 Sml no Ac (colorless seed) females, a stock monitoring the presence of active Ac by inducing spotted kernels. The selfed progeny was germinated and scored for the presence of dgr and sml seedlings. wt, Wild type.
Colorless refers to ears yielding only colorless seeds, whereas spotted refers to ears with spotted and colorless seeds in a 1:1 ratio.
–, Absent; +, present.
Deviations from the Segregation Ratio Expected in the F2
The hypothesis that the sml phenotype results from the interaction of two independent gene mutations was based on results obtained by germinating a sample of seeds from individual F2 families. In this screening, some families were noted where either sml or dgr formed the great majority or the only kind of mutant phenotype detectable. In the selfed progeny of outcrosses of heterozygous sml/Sml male parents in the W22 background (where only sml mutants are recovered) to B73 females, segregating and nonsegregating families were typically recovered in a 1:1 ratio. Among segregating families (72/143), five yielded dgr only, 30 yielded either sml only or a majority of sml, and the remaining 37 produced both kinds of phenotypes in a 12:3:1 ratio. In addition, selfing selected progeny of families exhibiting a majority of one or other of the two mutant phenotypes led to the establishment of two sublines, where either dgr or sml is the main mutant phenotype. Upon selfing, the sml subline typically yields sml and dgr seedlings at a frequency of 21.5% and 3.5%, respectively, whereas in the dgr sublines those frequencies are 2% and 23%, respectively. The observation that about one-half of the F2 families deviate from the expected 12:3:1 ratio, showing prevalence of one of the two mutant phenotypes, is suggestive of instability of dgr or an interaction with other genes involved in SAM development.
The dgr Phenotype Is Highly Variable
Many developmental abnormalities are associated with the dgr phenotype (Fig. 3). After 7 d of germination in light, dgr seedlings can be divided into three classes: those with an open coleoptile and an apparently normal first leaf (Fig. 3B), those with an empty coleoptile or with a root protruding out of the coleoptile (Fig. 3C), and those with an abnormal coleoptile and abnormal first leaf (Fig. 3D). Individuals of these three classes appeared with frequencies of 37%, 27%, and 36%, respectively, in a sample of 200 dgr mutant seedlings scored. When grown in darkness, dgr seedlings frequently develop a twisted mesocotyl (Fig. 3E). Upon transfer to soil, the few surviving mutants initially show normal growth but later begin to bend toward the soil exhibiting asymmetry in their body architecture (data not shown). Longitudinal sections of mature dgr embryos revealed displacement of the plumule initial from the apical-basal axis of the embryo (Fig. 3H). It would appear that the dgr mutant upsets the bipolar embryonic axis by displacing the root primordium from the axis and thus introducing an asymmetry into the body plan that is further elaborated after seed germination and during plant development.
Northern analysis was performed to assay the expression of the kn1 gene in the shoot apices of dgr mutants. RNA gel blots (Fig. 5) revealed weaker kn1 transcript signals in dgr mutants compared with wild type.
Figure 5.
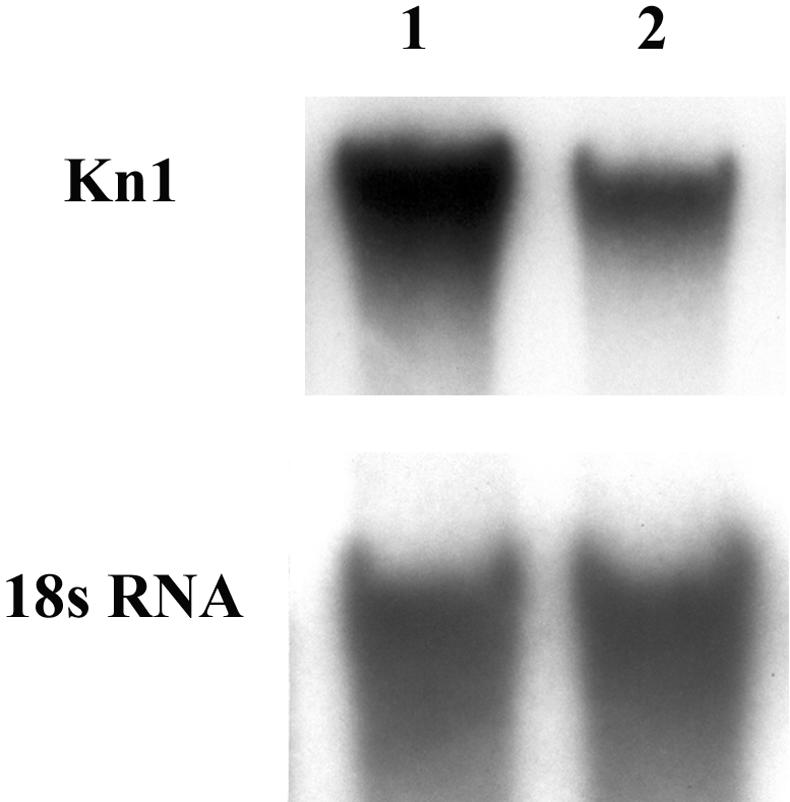
Upper, Northern analysis showing that the expression of knotted1 in dgr mutant apices (lane 2) is weaker than in wild type (lane 1). Twenty micrograms of total RNA from normal and dgr shoot apices was probed with the 559-bp KpnI-HindIII fragment of kn1 cDNA. Bottom, Hybridization with an 18S rRNA probe that served as a loading control.
Cytokinin Perception Altered in dgr Mutants
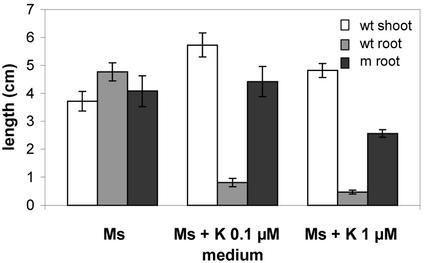
Cytokinins play a well-established role in the promotion of shoot elongation; hence, mutational suppression or reduction of their concentration may account for the sml phenotype. To test if the lack of this hormone is responsible for this phenotype, immature (14 DAP) excised embryos from a selfed Dgr/dgr Sml/sml parent were cultured on Murashige and Skoog medium with or without cytokinin and left to grow for 10 d under continuous light. As might have been expected, the addition of kinetin (6-furfurylamino-purine) did not rescue the mutant phenotype because at the time of its administration, root and shoot primordia are already formed. However, whereas nonmutant embryos grown in the presence of kinetins were promoted in their shoot growth and severely inhibited in their root elongation, mutant siblings, while remaining shootless, were only partially inhibited in root growth (Fig. 6), suggesting that the hormone is perceived in an altered fashion or impaired in its transport.
Figure 6.
Effect of kinetin on sml mutant growth. Elongation of wild-type shoots and roots and mutant roots after culturing immature (14 DAP) embryos on basic or supplemented (kinetin 0.1 μm and 1 μm) Murashige and Skoog medium for 10 d in continuous light at 25°C. sml mutant embryos, while impaired in shoot growth, appear to be less sensitive to the root-inhibiting effect exerted by the hormone than their wild-type counterparts. Error bars indicate ±se.
The possible involvement of other hormones was tested by culturing immature (18 DAP) embryos obtained from the selfed progeny of an sml subline in the presence of indolacetic acid (IAA), gibberellic acid (GA3), or absissic acid (ABA). Normal and mutant embryos, easily recognizable at this stage on the basis of their different sizes, were grown separately to confirm the validity of this classification. Each of the three hormones was administered at a concentration of 10 μ m. Both mutant and wild-type embryos were equally affected by the hormones (Table III). IAA caused inhibition of primary root elongation, GA3 stimulated root and shoot growth (the latter in wild type only), and ABA completely suppressed germination. None of these compounds promoted mutant embryo rescue. A further test with IAA was performed on embryos from a selfed dgr line to see if the dgr phenotype could be partially rescued by this hormone. This test, performed on 29-DAP embryos, also yielded negative results (data not shown).
Table III.
Effect of different hormones on sml embryo growth
| Growth Medium | Length
|
|||||
|---|---|---|---|---|---|---|
| n1 | wt
|
n2 | sml
|
|||
| Sa | Ra | S | R | |||
| cm | ||||||
| Murashige and Skoog Medium | 30 | 3.9 | 4.6 | 13 | – | 5.5 |
| Murashige and Skoog Medium + IAA | 43 | 3.5 | 1.4 | 16 | – | 2.0 |
| Murashige and Skoog Medium + GA | 55 | 12.6 | 6.7 | 30 | – | 6.0 |
| Murashige and Skoog Medium + ABA | 15 | – | – | 15 | – | – |
Early (18 DAP) embryos obtained by selfing plants (derived from B73 outcrosses) isolated as an sml subline were divided into normal and mutant on the basis of their embryo size and lack of a shoot, and transferred separately to basic (Murashige and Skoog medium + vitamins) or supplemented (contain the hormone IAA or GA or ABA, each at a concentration of 10 μm) media. Measurements of shoot and root elongation were made after 10 d of growth in continuous light at 25°C. The data refer to the pooled progeny of two selfed ears.
S, shoot; R, primary root.
DISCUSSION
The mutant described in this paper represents a useful tool with which to investigate the control exerted by the SAM on plant organogenesis and a means with which to analyze the origin of embryonic organs such as the scutellum and the coleoptile in relation to leaves. Both the histological analysis and the in situ hybridization results with knotted1, a marker of meristem differentiation (Smith et al., 1995), support the idea that the sml gene product is required for establishment of the SAM. The genetics of the shootless mutant indicates that failure to form the apical meristem is observed when two independent mutations, sml and dgr, are simultaneously present in the homozygous condition, whereas a Dgr/-sml/sml genotype results in a distorted growth phenotype. This apparent genetic redundancy in the control of the establishment of the apical meristem is not unexpected if we consider the fundamental role of this structure in establishing the plant body and could explain why these kinds of mutants have escaped observation so far. Similar situations have already been reported in other species (Aida et al., 1999). Evidence for the segregation of two genes was obtained when the heritability of the shootmeristemless trait was assayed by crossing plants exhibiting monogenic inheritance of this trait to a range of standard maize inbred lines. Self-pollination of the F1 revealed the two-gene segregation ratio. A similar situation has been reported in the study of a recently isolated loss-of-function knl allele (Vollbrecht et al., 2000) that reveals a novel embryonic shoot phenotype consisting of plants arrested as seedlings and referred to as “limited shoot.” Penetrance of this phenotype is background dependent and correlates with meristem size. The authors suggest that the background effect could be explained by assuming that the loss of kn1 function is compensated by the activity of a duplicate locus whose activity is variable in different inbred lines or that the redundant function is supplied by a redundant class 1 knox gene involved in meristem maintenance and/or correct organization of lateral organ primordia or by genes unrelated to kn1 (Reiser et al., 2000). Even though these explanations could equally as well be applied to the situation described here, with one gene (sml) affecting SAM establishment or maintenance and a second independent gene (dgr) supplying a partially redundant function, our results also imply an intrinsic instability of dgr. Similar instabilities have been observed and extensively analyzed in the study of allelic interactions at different loci controlling anthocyanin pigmentation in various plant tissues (Chandler et al., 2000). A further possibility to account for these results would be the assumption that the expression of sml is affected by one or more quantitative trait loci, the genetic constitution of which is background dependent. However, the variable phenotype of dgr needs to be addressed in subsequent studies.
The weak expression of Knotted 1 detected by northern analysis of the shoot meristem of immature dgr embryos suggests that their SAM consists of a reduced number of meristematic cells or it is reduced in size or it is developed ectopically. Alternatively, the expression of the kn1 gene could simply be reduced in the mutant. The histology of mutant seedlings at different developmental times should help to unravel the origin and constitution of the SAM. Analysis of the dgr/dgr sml/sml double mutant could also shed light on the origin of the scutellum and the leaf-like coleoptile in relation to the SAM, a long-debated issue. Studies by different authors suggest that the scutellum and coleoptile do not arise from the SAM. Elster et al. (2000) analyzed the distribution pattern of a protoderm marker, LPT2, in wild-type embryos. Its absence in the L1 layer of the SAM and the epidermis of leaf primordia but its co-expression in the outer cell layer of the coleoptile and the scutellum led them to conclude that the coleoptile is not derived from the SAM but is instead an appendix of the scutellum, the single cotyledon in maize. This idea gained further support from the observation that the ns (narrow sheath) maize mutant affects all foliar organs that develop from the main SAM by reducing their margins but does not affect either the scutellum or the coleoptile (Scanlon and Freeling, 1998). The conclusion that cotyledons and meristem comprise separate developmental compartments when they are determined is corroborated by similar observations in other species. In Arabidopsis, the stm1 mutation prevents meristem formation without eliminating cotyledon development. Similarly, clonal analysis in cotton (Gossypium hirsutum; Christianson, 1986) suggests the existence of separate developmental compartments for cotyledons and the shoot meristem. The analysis of the dgr sml double mutant (this paper) indicates that disruption of a functional SAM in sml embryos is associated with the lack of the coleoptile and to a change of the scutellum from a spade-shaped to a shorter, more rounded structure. This observation may be interpreted as evidence that coleoptilar development requires a functional SAM, whereas the scutellum may differentiate separately from the shoot meristem even though its shape is somehow affected by the presence of the meristem. As to the partial lack of inhibition of mutant root elongation exerted by exogenous cytokinin application, it is interesting to note that the crr2 gene, a cytokinin response regulator expressed in meristem, is located in the same chromosomal region as sml (chromosome 10L, bin 10.07). Future work will try to clarify the relationship between these two genes.
MATERIALS AND METHODS
Mutant Isolation and Genetic Analysis
The shootless phenotype (sml) was originally detected as a putative non-germinating mutant in the selfed progeny of an F1 plant heterozygous for Ac and r-scm:3. The genetic background of the plant was that of the W22 inbred line. Ac is the autonomous component of the Ac/Ds system residing at the P locus, where it confers a variegated pericarp phenotype. r-scm3 is an allele of r, a gene controlling anthocyanin biosynthesis, originally obtained by insertion of Ds into R-sc and causing suppression of aleurone pigmentation. r-scm3 monitors the presence of Ac by inducing a spotted aleurone as a result of somatic excision of Ds. Extensive germination of F2 and F3 families confirmed that the sml phenotype is due to a single recessive gene mutant. The mutant originally referred to as emb7190 (Giulini et al., 1998) was renamed sml. Efforts to find cosegregation of an Ac homologous fragment with the mutant phenotype have been unsuccessful so far (data not shown).
Mapping sml
Crosses of heterozygous Sml/sml females to a stock carrying the TB-A translocation (Beckett, 1978) were used to position sml on the long arm of chromosome 10. sml was then mapped in segregating F1 populations using SSRs. F1 seedlings were obtained from the cross (W22 Sml/sml × A188) × B73. DNA was prepared from leaf samples obtained from each individual in this F1 population (Dellaporta et al., 1983). F1 plants were then self-pollinated to produce F2 progeny ears and 30 seeds were germinated to establish the genetic constitution, either Sml/Sml or Sml/sml, of F1 plants. PCR was performed using SSR primers defined in the Maize Database corresponding to chromosome 10L. Reaction conditions and gel running were carried out as described in the SSR Methods Manual by the Missouri Maize Project (http://www.agron.missouri.edu/ssr.html).
Light Microscopy
Mature dry seeds were soaked in water for 24 h and subsequently fixed in 25% (v/v) glutaraldehyde for 24 h under vacuum and then cut longitudinally to examine embryos by stereomicroscopy (Carl Zeiss GmbH, Jena, Germany). For histological analysis, 9 and 17 DAP immature seeds were dissected from cobs and immediately fixed in freshly prepared 4% (w/v) p-formaldehyde in phosphate-buffered saline (130 mm NaCl, 7 mm Na2HPO4, and 3 mm Na2HPO4) for 12 h. The fixed material was placed in 70% (v/v) ethanol and stored at 4°C until processed. Embedding procedures were performed as previously described (Procissi et al., 1997). Longitudinal sections (8 mm) were stained with DAPI (1 mm in phosphate-buffered saline for 10 min) or with safranin-fast green. Slides were examined using an Axioskop light microscope (Carl Zeiss). Photomicrographs were taken with Ektachrome 64T (in situ; Eastman Kodak, Rochester, NY) and 320T (DAPI) films. For in situ hybridization, sections were processed as detailed below.
In Situ Hybridization
In situ hybridization experiments were carried out as described by Jackson (1991). Digoxygenin-labeled RNA probes for Knotted1 (Jackson et al., 1994), hybridization, washes, blocking, and antibody incubation and detection were done according to the same protocol and following the instructions of the manufacturer (Boehringer Mannheim, Germany). Digoxygenin-labeled hybrids were viewed using bright-field microscopy and photographed using Kodak Ektachrome 64T film.
RNA Gel-Blot Analysis
Total RNA was extracted from 1 g of frozen shoot tissue prepared from a segregating population of sml in a B73 background using the method described by Van Tunen et al. (1988). The dgr and normal siblings were identified by phenotype after 5 d of growth. Twenty micrograms of total RNA for each sample was electrophoresed on a 1.5% (w/v) agarose-formaldehyde gel (Sambrook et al., 1989). Hybond-NT membrane (Amersham Biosciences AB, Uppsala) was used for blotting. Hybridization was performed at 65°C (high stringency) in 5× SSC, 5× Denhardt's solution, 0.5% (w/v) SDS, and 100 μg ml−1 salmon sperm DNA. Blots were washed twice in 2× SSC and 1% (w/v) SDS for 20 min at 65°C for high stringency, with 0.1× SSC and 0.1% (w/v) SDS for 15 min at 65°C. The hybridization probes used were the 559-bp KpnI-HindIII fragment of Kn1 cDNA (Jackson, 1991) and an Arabidopsis 18S rRNA. The probes were 32P labeled using random hexamer primers (Feinberg and Volgelstein, 1983).
Embryo Culture
Ears were harvested 9 or 18 DAP, surface sterilized with 5% (w/v) sodium hypochlorite for 15 min and then rinsed in sterile, distilled water. Embryos were removed aseptically and transferred to Murashige and Skoog medium (pH 5.6) containing 3% (w/v) Suc, solidified with 0.8% (w/v) agar, and supplemented or not with hormones. Cultures were incubated in a growth chamber at 25°C in continuous light for 14 d. Seedling growth was expressed as shoot and primary root elongation. se of mean values, not reported, were less than 10%.
ACKNOWLEDGMENTS
We wish to thank Steve L. Dellaporta for providing the original stock carrying sml and David Jackson for kindly providing knotted1 cDNA clone.
Footnotes
This work was supported by EC-BIOTECH (grant no. BIO4–CT96–0210 to G.G.) and by Ministero dell'Università e della Ricerca Scientifica e Tecnologica-Cofin 1998 (to G.G.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010767.
LITERATURE CITED
- Aida M, Ishida T, Tasaka M. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development. 1999;126:1563–1570. doi: 10.1242/dev.126.8.1563. [DOI] [PubMed] [Google Scholar]
- Barton MK, Poethig RS. Formation of shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development. 1993;119:823–831. [Google Scholar]
- Beckett JB. B-A translocations in maize: I. Use in locating genes by chromosome arm. J Hered. 1978;69:27–36. [Google Scholar]
- Bowman JL, Eshed Y. Formation and maintenance of the shoot apical meristem. Trends Plant Sci. 2000;5:110–115. doi: 10.1016/s1360-1385(00)01569-7. [DOI] [PubMed] [Google Scholar]
- Chandler VL, Eggleston WB, Dorweiler JE. Paramutation in maize. Plant Mol Biol. 2000;43:121–145. doi: 10.1023/a:1006499808317. [DOI] [PubMed] [Google Scholar]
- Christianson ML. Fate map of the organizing shoot apex in Gossypium. Ann J Bot. 1986;73:947–958. [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Elster R, Bommert P, Sheridan WF, Werr W. Analysis of four embryo-specific mutants in Zea maysreveals that incomplete radial organization of the pro-embryo interferes with subsequent development. Dev Genes Evol. 2000;210:300–310. doi: 10.1007/pl00008189. [DOI] [PubMed] [Google Scholar]
- Evans MMS, Barton HK. Genetics of angiosperm shoot apical meristem development. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:673–701. doi: 10.1146/annurev.arplant.48.1.673. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Volgelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giulini A, Busti E, Consonni G, Dolfini S, Furini A, MacCabe AP, Gavazzi G. Maize mutants defective in embryogenesis. Maize Genet Coop Newslett. 1998;72:58–60. [Google Scholar]
- Hong SK, Aoki T, Kitano H, Satoh H, Nagato Y. Phenotypic diversity of 188 rice embryo mutants. Dev Genet. 1995;16:298–310. [Google Scholar]
- Jackson DP. In situ hybridization in plants. In: Gurr SJ, McPherson M, Bowles DJ, editors. Molecular Plant Pathology: A Practical Approach. Oxford: Oxford University Press; 1991. pp. 163–174. [Google Scholar]
- Jackson DP, Veit B, Hake S. Expression of Knotted1related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. [Google Scholar]
- Kerstetter RA, Hake S. Shoot meristem formation in vegetative development. Plant Cell. 1997;9:1001–1010. doi: 10.1105/tpc.9.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procissi A, Dolfini S, Ronchi A, Tonelli C. Light-dependent spatial and temporal expression of pigment regulatory genes in developing maize seeds. Plant Cell. 1997;9:1–12. doi: 10.1105/tpc.9.9.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racchi ML, Chiusi AP, Bagnoli F, Manzocchi LA. Characterization of maize defective kernel (dek) mutants affecting seed germination. Maydica. 1996;41:271–277. [Google Scholar]
- Reiser L, Sanchez-Baracaldo P, Hakes S. Knots in the family tree: evolutionary relationships and functions of knoxhomeobox genes. Plant Mol Biol. 2000;42:151–156. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Satoh N, Hong S-K, Nishimura A, Matsuoka M, Kitano H, Nagato Y. Initiation of shoot apical meristem in rice: characterization of four SHOOTLESSgenes. Development. 1999;126:3629–3636. doi: 10.1242/dev.126.16.3629. [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Freeling M. The narrow sheath leaf domain deletion: a genetic tool used to reveal developmental homologies among modified maize organs. Plant J. 1998;13:547–561. [Google Scholar]
- Sheridan WF, Clark JK. Mutational analysis of morphogenesis of the maize embryo. Plant J. 1993;3:347–358. [Google Scholar]
- Smith LG, Jackson D, Hake S. Expression of knotted1marks shoot meristem formation during maize embryogenesis. Dev Genet. 1995;16:344–348. [Google Scholar]
- Souer E, Vanhouwelingen A, Kloos D, Mol J, Koes R. The NO APICAL MERISTEM gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell. 1996;85:159–170. doi: 10.1016/s0092-8674(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Van Tunen AJ, Koes RE, Spelt CE, van Der Krol AR, Stuitje AR, Mol JNM. Cloning of two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light regulated and differential expression of flavonoid genes. EMBO J. 1988;7:1257–1263. doi: 10.1002/j.1460-2075.1988.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht E, Reiser L, Hake S. Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted 1. Development. 2000;127:3161–3172. doi: 10.1242/dev.127.14.3161. [DOI] [PubMed] [Google Scholar]