Abstract
Proliferating cells have a higher metabolic rate than quiescent cells. To investigate the role of metabolism in cell cycle progression, we examined cell size, mitochondrial mass, and reactive oxygen species (ROS) levels in highly synchronized cell populations progressing from early G1 to S phase. We found that ROS steadily increased, compared to cell size and mitochondrial mass, through the cell cycle. Since ROS has been shown to influence cell proliferation and transformation, we hypothesized that ROS could contribute to cell cycle progression. Antioxidant treatment of cells induced a late-G1-phase cell cycle arrest characterized by continued cellular growth, active cyclin D-Cdk4/6 and active cyclin E-Cdk2 kinases, and inactive hyperphosphorylated pRb. However, antioxidant-treated cells failed to accumulate cyclin A protein, a requisite step for initiation of DNA synthesis. Further examination revealed that cyclin A continued to be ubiquitinated by the anaphase promoting complex (APC) and to be degraded by the proteasome. This antioxidant arrest could be rescued by overexpression of Emi1, an APC inhibitor. These observations reveal an intrinsic late-G1-phase checkpoint, after transition across the growth factor-dependent G1 restriction point, that links increased steady-state levels of endogenous ROS and cell cycle progression through continued activity of APC in association with Cdh1.
Maintenance of cell size during cellular division requires the coordinated regulation of the cell cycle machinery and cell growth (31, 36). Metabolism or reactive oxygen species (ROS) has been proposed to stimulate cell cycle progression as an intrinsic cellular signal (9, 66); however, a direct role for growth or metabolism in regulating the cell cycle machinery has remained elusive. Tumor cells and transformed cells are often characterized by a robust metabolic rate, including increased glucose utilization, increased lipid synthesis, and increased levels of ROS (20, 29, 40, 74). Jones et al. (35) recently reported a G1 cell cycle checkpoint monitoring glucose availability that links the AMP-activated protein kinase and the p53 tumor suppressor. In addition, members of the retinoblastoma tumor suppressor (pRb) family have been shown to regulate the expression of genes involved in cell cycle progression as well as metabolism and mitochondrial biogenesis (10). Furthermore, three groups (8, 28, 43) have provided important insight into the von Hippel-Lindau/hypoxia-inducible factor pathway, mutated in some cancers, that determines how cells sense and respond to changes in oxygen availability. In low oxygen, mitochondria produce increased ROS levels that stabilize Hif-1α, resulting in the transcription of genes involved in glucose transport and glycolytic enzymes. Together, these observations suggest that cells inherently possess a mechanism to monitor and control cellular metabolism and that this regulation is important for proliferation and tumorigenesis.
ROS has been reported to be involved in a number of cellular processes. High levels of ROS have been shown to cause cellular damage, oxidative stress, and DNA damage, whereas low endogenous ROS levels play a role in redox signaling pathways in cellular biology (9, 66). For example, nitric oxide (NO) is used as a cell-to-cell signaling molecule (5), demonstrating that cells utilize endogenous ROS for important biological functions. Low physiologic levels of ROS (H2O2) have been shown to stimulate cell proliferation in multiple cell types including fibroblast, prostate, macrophage, endothelial, and smooth muscle (16, 52, 60, 64, 78). Likewise, reducing intracellular ROS levels by the addition of catalase, vitamins (E, C and A), or N-acetyl-l-cysteine decreases cellular proliferation (34, 45, 47, 58, 59, 76).
Growth factor stimulation by platelet-derived growth factor, epidermal growth factor, and insulin-like growth factor results in an increase in intracellular ROS (65). This ROS production can inactivate phosphatases at the cell membrane (46), activate kinases, and activate transcription factors (65) leading to cell cycle progression. Lambeth and others have shown that many nonphagocytic cells express homologues of the NADPH oxidase that produce ROS at the cell membrane (39). Overexpression of Nox1, the catalytic subunit of the NADPH oxidase, causes an increase in the intracellular H2O2 concentration, cellular transformation, and tumor growth in mice (40, 49). Furthermore, fibroblasts transformed with constitutively active Ras or Rac1 expressed dramatically higher levels of ROS, and antioxidant treatment reverses the phenotype (49, 73). Taken together, these observations suggest that ROS plays a role in intracellular signaling and cell proliferation, potentially influencing transformation and tumor progression.
Progression of cells through early G1, across the restriction point into late G1 and then into S phase requires the coordinated regulation of multiple positive and negative factors (31). Cyclin D-Cdk4/6 complexes promote early G1 progression, but cyclin E (or cyclin A)-Cdk2 (or Cdk1) activity (2) is required to inactive pRb by hyperphosphorylation to transit the restriction point into late G1 phase. pRb inactivation results in release of E2F transcription factors and induction of late-G1-specific genes, including dihydrofolatereductase (DHFR), Emi1, and cyclin A (21, 33). Cyclin A-associated kinase activity is required to initiate DNA synthesis, prevent rereplication, and enter mitosis (14, 80). Although cyclin A is transcriptionally induced by E2Fs at the restriction point, cyclin A protein does not accumulate until the late G1/S phase transition due to ubiquitination by the anaphase promoting complex (APC) and subsequent proteolysis by the 26S proteasome (33). APC is active throughout G1 phase by association with Cdh1 (APCCdh1), an activator that confers substrate specificity (22). Prior to initiation of S phase, APCCdh1 is inactivated by the binding of Emi1 to Cdh1, resulting in stabilization of cyclin A (33), activation of cyclin A-associated kinase activity, and subsequent inactivation of Cdh1 by phosphorylation (42, 71). Thus, tight regulation of cyclin E- and A-associated kinase activity results in a coordinated G1 cell cycle progression. Here, we find that an increase in the steady-state levels of endogenous ROS is required to inactivate APCCdh1, allow cyclin A accumulation, and transition into S phase. These observations point to a novel intrinsic late G1/S phase checkpoint that coordinates cellular ROS production and possibly metabolism with cell cycle progression.
MATERIALS AND METHODS
Cell culture and reagents.
Human foreskin fibroblasts (a kind gift from M. Haas, University of California-San Diego), NIH 3T3 fibroblasts, and Rat1a fibroblasts were maintained in Dulbecco's modified Eagle's medium high glucose (Life Technologies), 10% fetal bovine serum (FBS; Sigma), and penicillin-streptomycin. Human Jurkat T cells and T98G human glioblastoma cells were obtained from ATCC and maintained in RPMI 1640 medium or minimal essential medium (Life Technologies), respectively, supplemented with 5% FBS, 1× penicillin-streptomycin. All cells were grown at 37°C in 5% CO2. Stocks (1 M) of tempol (Calbiochem), a free radical scavenger/spin trap (48, 67), and diethyldithiocarbamic acid (DDC; Sigma) (51), a superoxide dismutase peptidyl mimetic, in water were used at final concentrations of 1 to 5 mM and 10 to 100 μM, respectively. Other antioxidants or reducing agents used were the following: pyrrolidine dithiocarbamate (11, 12, 73), dimethylthiourea (65), N-acetyl-l-cysteine (73), vitamins E and C (69), and catalase (59). Cyclin A-expressing retrovirus was made by inserting human cyclin A-hemagglutinin (HA) cDNA into pCX4bsr retrovirus vector (1), followed by packaging in BOSC23 cells. Cycloheximide was purchased from Sigma, while MG132 and MG115 were purchased from Calbiochem.
Cell cycle synchronization.
Centrifugal elutriations of G1 and G2/M phase cell populations were performed as previously described using human Jurkat T cells (41). T98G, NIH 3T3, and Rat1a cells were serum deprived for 72 h, followed by restimulation with 5% serum (T98G cells) and 10% serum (NIH 3T3 Rat1a cells). Human foreskin fibroblast cells were synchronized by contact inhibition at high density (6 × 106 cells/10-cm dish) in 10% serum for 48 h and then replated at low density (1 × 106 cells/10-cm dish). G2/M enriched NIH 3T3s were serum starved and restimulated until the majority of the population reached G2/M (20 h). For transfection experiments cells were thymidine (Sigma) blocked for 20 h, released, and then transfected using Effectene (QIAGEN) for 4 h. Cells were harvested and analyzed by fluorescence-activated cell sorting (FACS) analysis 22 h later. For cyclin A [35S]methionine pulse-chase experiments, T98G cells were synchronized as before, treated with medium without methionine for 30 min, then pulsed with 200 μCi/ml Pro-Mix L[35S] in vitro cell labeling mix (Amersham) for 15 min, and chased with medium containing nonradiolabeled methionine. Cells were lysed in radioimmunoprecipitation assay buffer and precleared with protein A-Sepharose beads (Amersham), and cyclin A was immunoprecipitated (H432; Santa Cruz Biotech) and analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and phosphorimaging. Densitometry was done using Imagequant, software, version 1.1 (Molecular Dynamics).
Immunoblotting and kinase assays.
Cells were lysed in radioimmunoprecipitation assay buffer, and immunoblotting was performed as previously described (21) using anti-cyclin E1 (HE12), anti-cyclin A2 (H432), anti-cyclin B1 (245), anti-Cdk2 (M20 and D12), anti-p27 (C19), anti-p21 (C19), anti-Skp2 (H435), anti-CDC6 (H-304), anti-CDC27 (AF3.1), anti-Cdc20 (8358), anti-Myc (9e10), anti-E2F1 (KH95), anti-p107 (C18), anti-p130 (C20), anti-actin (I19; Santa Cruz Biotech); anti-pRb (554136; BD Biosciences); anti-alpha-tubulin (Sigma); anti-CDH1 (DH01; Neomarkers); anti-UbcH10 (Boston Biochem); and anti-Emi1 and anti-Plk1 (Zymed) antibodies. Immunoprecipitation kinase assays were performed as previously described (21) using glutathione transferase (GST) C terminus pRb substrate for Cdk4/6 and histone H1 (Calbiochem) substrate for cyclin E and cyclin A and anti-Cdk6 (C-21), anti Cdk4 (C-22), anti-cyclin E (C-19), and anti-cyclin A antibodies (H-432; Santa Cruz Biotech).
RT-PCR.
RNA was purified using an RNeasy kit (QIAGEN). Reverse transcription-PCR (RT-PCR) was performed using a QIAGEN Omniscript RT kit with the minimum number of PCR cycles to detect a signal using the following primers: human cyclin A2 (GGCCGAAGACGAGACGGGTTGCACC and CAGGCCAGCTTTGTCCCGTGAC), 20 cycles; human DHFR (ATGCCTTTCTCCTCCTGG and CGCTAAACTGCATCGTCGC), 25 cycles; human Emi1 (GCCTCCTGGAGGAGAATTTCGG and CCTTTCTGATCACCTTGATTGG), 30 cycles; and human beta actin (TGAACCCCAAGGCCAACCGCGAGAA and AAGCAGCCGTGGCCATCTCTTG), 20 cycles.
Flow cytometry analysis.
Cell cycle progression was assayed by DNA content using propidium iodide and flow cytometry as previously described (21). ROS levels and mitochondrion content were measured by flow cytometry using H2DCFDA (2′,7′-dichlorofluorescein diacetate) (53, 55) and MitoTracker Green (Molecular Probes), respectively. T98G cells deprived of serum for 72 h were restimulated with 10% serum and pulsed with bromodeoxyuridine (BrdU; Amersham Biosciences) at 16 to 20 h and assayed by flow cytometry as previously described (41). Relative cell size was measured by forward scatter flow cytometry.
APC immunoprecipitation and in vitro ubiquitination assay.
Cells were lysed in buffer A (20 mM Tris-Hcl, pH 7.5, 100 mM NaCl, 10% glycerol, 0.2% NP-40, EDTA-free complete protease inhibitor cocktail [Roche]) (77), spun at 10,000 × g for 10 min, and precleared with protein A-Sepharose beads (Amersham). APC was immunoprecipitated using anti-Cdc27 AF3.1 antibody (sc-9972; Santa Cruz Biotech) conjugated to protein A-Sepharose beads. APC beads were then washed three times, aliquoted, and frozen at −80°C until use. For in vitro ubiquitination assay, 2.5 mM UbcH10-His, 1 mM E1-GST, 10 mg/ml ubiquitin, 1× energy regeneration system (Boston Biochem), immunoprecipitated APC beads, and 1 ml of in vitro translated 35S-labeled cyclin A-HA (Promega) were incubated at 30°C for 1 h. The reaction was stopped with the addition of SDS sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis and phosphorimaging. Densitometry was done using Imagequant software, version 1.1 (Molecular Dynamics).
RESULTS
Endogenous intracellular ROS increases throughout the cell cycle.
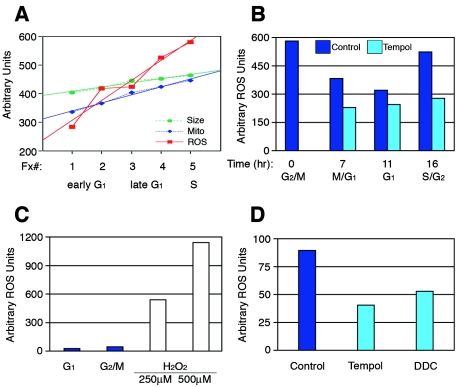
Prior to division into two new daughter cells, cells must double their cellular and mitochondrial mass each cell cycle. To further understand the relationship between cellular growth, metabolism, and cell cycle progression, we examined various parameters of highly synchronized cell cycle fractions obtained by centrifugal elutriation of asynchronously dividing Jurkat leukemic T cells. As expected, both cell size and mitochondrion content increased linearly with similar slopes in fractions from early G1 to S phase (Fig. 1A), while mitochondrial potential did not fluctuate in a cell cycle-dependent manner (not shown). Unexpectedly, endogenous levels of ROS increased as cells progressed from early G1 to S phase (Fig. 1A). Consistent with the increase in ROS from early G1 to S phase, ROS levels also decreased as cells transited from mitosis into early G1 and then increased as they progressed into S phase (Fig. 1B). In addition to Jurkats, we also detected ROS levels increasing throughout the cell cycle in G1 phase-synchronized T98G human glioblastoma cells and primary human fibroblasts (data not shown). Cells treated with antioxidants demonstrated decreased ROS levels, whereas cells treated with concentrations of hydrogen peroxide known to cause DNA damage demonstrated dramatically increased ROS levels (Fig. 1B to D) (3). These observations show that physiologic, intracellular ROS accumulates and oscillates in a cell cycle-dependent manner, suggesting a potential role for ROS in proliferative signaling.
FIG. 1.
ROS levels increase throughout the cell cycle and oscillate each cell division. (A) Early G1 to S phase fractions of Jurkat cells were synchronized by centrifugal elutriation and then analyzed for cell size (forward scatter), mitochondrial content (Mitofluor green), and ROS levels (H2DCFDA). ROS levels increase from early G1 to S phase considerably in comparison to cell size and mitochondrial content. (B) G2/M Jurkat cells were synchronized by centrifugal elutriation, replated, and either not treated or treated with the antioxidant tempol. Cells were then analyzed for ROS levels at 0, 7, 11, and 16 h. ROS levels were highest in the G2/M population, then dropped as control cells transited into early G1, and increased as they progressed towards S phase. (C) ROS levels in untreated G1- and G2/M-synchronized T98G cells in comparison to the dramatic difference in ROS levels known to cause DNA damage. (D) Asynchronous T98G cells treated with the antioxidants tempol or DDC show a reduction in ROS levels in comparison to control ROS levels.
Increase in the ROS steady-state level is required for entry into S phase.
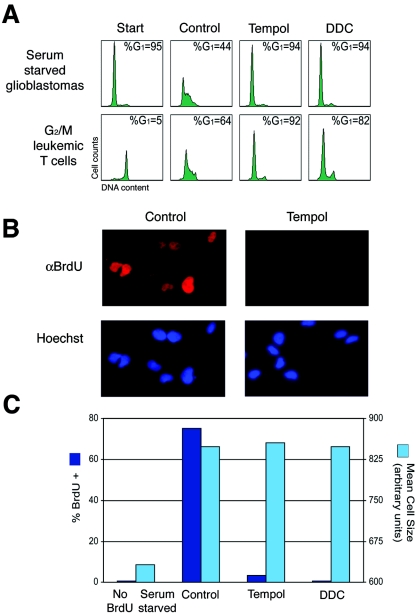
To determine if the observed ROS accumulation was required for cell cycle progression, we treated cells with antioxidants to prevent the increase in intracellular ROS. Treatment of serum-deprived and restimulated G1-synchronized T98G cells with tempol or DDC antioxidants reduced intracellular ROS levels and induced a strong G1 cell cycle arrest (Fig. 2A). Untreated control cells exhibited an increase in ROS levels and progressed into S/G2/M phases. Similarly, serum-deprived NIH 3T3 cells and contact inhibited-released primary fibroblasts treated with antioxidants also arrested in G1 (see Fig. S1A in the supplemental material), while control cells progressed into S and G2/M phases. Furthermore, G2/M elutriated Jurkat cells treated with antioxidants progressed from G2/M into G1 but failed to enter S phase, while untreated control cells progressed through G1 into S phase (Fig. 2A). Likewise, antioxidant-treated G2/M NIH 3T3 cells exited mitosis and arrested in G1, whereas control cells progressed into S phase (not shown). Consistent with these observations, treatment of primary human fibroblasts, immortalized rodent (NIH 3T3 and Rat1a) fibroblasts, transformed human keratinocytes (HaCat cells), T98G glioblastoma, and Jurkat leukemic T cells with various antioxidants (DDC, dimethylthiourea, pyrrolidine dithiocarbamate, tempol, vitamins E and C, and catalase) also induced a G1 cell cycle arrest (not shown). Taken together, these observations suggest that an increase in endogenous ROS levels is necessary for cell cycle progression through the G1 phase of the cell cycle.
FIG. 2.
(A) Human T98G glioblastoma cells were serum starved for 72 h, restimulated with 10% FBS with or without antioxidants, and harvested at 20 h (top panel). G2/M human leukemic T cells (Jurkats) were synchronized by centrifugal elutriation, replated, and either not treated or treated with antioxidants. Zero and 16-h ROS levels in Fig. 1B correspond to DNA in the bottom panel. Cells were analyzed for DNA content by propidium iodide and FACS analysis. (B) T98G cells serum starved and restimulated with 10% FBS with or without antioxidants were pulsed with BrdU for 4 h to detect DNA replication and examined by immunofluorescence. (C) T98G cells treated as above and analyzed for cell size (forward scatter) and BrdU incorporation by FACS analysis.
To exclude the possibility that antioxidant-treated cells had arrested just after initiation of DNA synthesis (a difficult position to detect by propidium iodide-FACS analysis), G1-synchronized T98G cells were restimulated with 10% FBS, treated with antioxidants, and pulsed with BrdU from 16 to 20 h. By immunofluorescence microscopy, antioxidant-treated cells contained only background levels of BrdU-positive cells, confirming that they arrest before initiation of DNA synthesis (Fig. 2B). In strong agreement with these results, FACS analysis of BrdU-pulsed cells showed background levels of BrdU incorporation, whereas control cells had ∼80% BrdU-positive cells (Fig. 2C). Although antioxidant-treated cells failed to initiate DNA synthesis, cells remained viable and continued to grow in size with kinetics similar to that of control cells progressing into S/G2/M phases (Fig. 2C). Furthermore, upon removal of antioxidants, cells progressed into S phase and continued to divide normally (not shown). Taken together, these observations demonstrate that failure to accumulate a physiologic threshold level of ROS results in a G1 phase arrest; however, cells continue to grow in size.
Failure to accumulate endogenous ROS results in a late G1 cell cycle arrest.
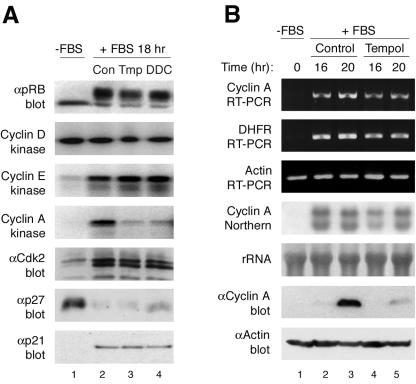
We next assessed molecular markers to define where antioxidant-treated cells had arrested in G1 phase. Control and antioxidant (tempol or DDC)-treated G1-synchronized T98G cells contained active mitogen-activated protein kinase (not shown), active cyclin D-Cdk4/6, active cyclin E-Cdk2, and similar p21 and p27 levels (Fig. 3A). Consistent with transition across the restriction point and the presence of active cyclin E-Cdk2 (21), both antioxidant-treated and control cells contained the slower-migrating inactive, hyperphosphorylated form of pRb (Fig. 3A). Control cells contained active cyclin A-Cdk2 complexes and progressed into S phase. In contrast, antioxidant-treated cells failed to activate cyclin A-Cdk2 (Fig. 3A). Similar results were observed in antioxidant-treated primary human fibroblasts (not shown). These observations suggest that antioxidant-treated cells transit across the growth factor restriction point from early G1 into late G1, but due to a failure to accumulate sufficient levels of intracellular ROS, arrest in late G1 phase.
FIG. 3.
Failure to accumulate endogenous ROS results in a late G1 cell cycle arrest due to the absence of cyclin A protein. (A) T98G cells serum starved and restimulated with 5% FBS and either untreated or treated with antioxidants were assayed for pRb phosphorylation; cyclin D, cyclin E, and cyclin A kinase activity; Cdk2 phosphorylation status (immunoprecipitation, Cdk2; blotting, Cdk2); and levels of Cdk inhibitors p27 and p21. (B) Serum-starved and restimulated T98G cells either untreated or treated with tempol were examined at 16 h (late G1) and 20 h (late G1/S phase) as follows: for cyclin A, DHFR, and actin mRNA levels by reverse transcription-PCR; for cyclin A RNA levels by Northern blotting; and for cyclin A and actin protein levels by immunoblotting.
Antioxidant-arrested cells fail to accumulate cyclin A protein.
Cyclin A protein accumulation and subsequent activation of Cdk2 or Cdk1 (2) are critical for initiation of DNA synthesis (14, 26, 54, 63, 80). Consequently, cyclin A-Cdk2 activity is tightly regulated by transcription and proteolysis of the cyclin and phosphorylation and dephosphorylation of the Cdk subunit (70, 80). Antioxidant-treated cells contained Thr-160 phosphorylated Cdk2, assayed for by mobility shift and phospho-specific antibody immunoblotting (Fig. 3A and data not shown). Therefore, we focused our attention on cyclin A regulation. Cyclin A is an E2F-responsive gene (30, 68) and, consistent with inactive pRb, both RT-PCR and Northern blot analysis of antioxidant-treated and control cells showed similar levels and kinetics of cyclin A mRNA induction (Fig. 3B). DHFR, also an E2F responsive gene, showed a similar pattern of mRNA induction to that of cyclin A in both control and antioxidant-treated cells (Fig. 3B). Taken together, these observations suggest that E2F-dependent transcription was not altered in antioxidant-treated cells and that preventing the accumulation of physiologic ROS results in a late G1 cell cycle arrest that regulates cyclin A by a posttranscriptional mechanism.
We next directly examined cyclin A protein levels. Antioxidant-treated cells failed to accumulate cyclin A protein, whereas untreated control cells contained cyclin A protein (Fig. 3B). Similar results of hyperphosphorylated pRB and absence of cyclin A protein were observed in primary human fibroblasts (see Fig. S1B in the supplemental material), indicating that these cells were arrested at the same point as the T98G cells. We next examined whether cyclin A translation was affected by preventing the increase in endogenous ROS. Sucrose density gradient polyribosome profiles revealed that cyclin A mRNA was present in the highly translated polysomal fractions in both antioxidant-treated and control cells (B. Maedge and S. F. Dowdy, unpublished data). Moreover, 35S incorporation experiments demonstrated that cyclin A was translated at a similar rate in both antioxidant-treated and control cells (see Fig. S1C in the supplemental material). These observations demonstrate that cyclin A was transcribed and translated in antioxidant-treated cells, yet cyclin A protein failed to accumulate.
Expression and phosphorylation of proteins involved in the pRb pathway occur normally in antioxidant-treated cells.
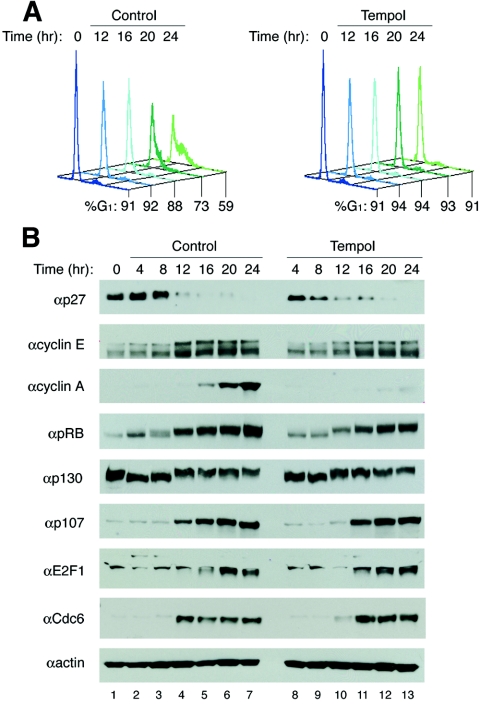
To further examine the antioxidant induced arrest, a detailed time course was conducted from 0 to 24 h (Fig. 4A). Proteins previously implicated in ROS or stress-sensing pathways such as Bcl2, c-Myc, p38, and AMP-activated protein kinase had the same expression patterns, with the same levels of phosphorylation for the latter two (15, 18, 35, 75 and data not shown). At 4 and 8 h after the addition of serum, both control and antioxidant-treated cells were in early G1, characterized by high levels of p27, low levels of cyclin E, and active hypophosphorylated pRb and p130 (Fig. 4B). Equivalent kinetics of pRb phosphorylation were also observed as both populations contained inactive hyperphosphorylated pRb (and p130) by 12 h, the same time when E2F-responsive genes were expressed. pRb inactivation, transition across the restriction point, and entry into late G1 resulted in expression of E2F responsive genes, such as cyclin E, E2F1, Cdc6, and p107, along with down-regulation of p27 in both antioxidant-treated and control cells. Conversely, cyclin A protein was absent and failed to accumulate in arrested cells, even in the presence of cyclin A transcript (Fig. 3B and 4B). Thus, preventing an increase in the steady-state level of physiologic ROS levels leads to a late G1 phase arrest where derepression of E2F-responsive genes occurs normally, but cyclin A protein fails to accumulate, suggesting that cyclin A protein stability may be affected.
FIG. 4.
Detailed analysis of expression and phosphorylation of proteins involved in the pRb/E2F pathway occur normally in antioxidant-treated cells. (A) T98G cells were synchronized by serum starvation and restimulated with 5% FBS with or without tempol and analyzed for DNA content by FACS analysis at 0 and 12 to 24 h. (B) Immunoblot analysis of the same T98G cells as above every 4 h from 0 to 24 h.
Failure to accumulate intracellular ROS results in continued targeting of cyclin A for degradation by APCCdh1.
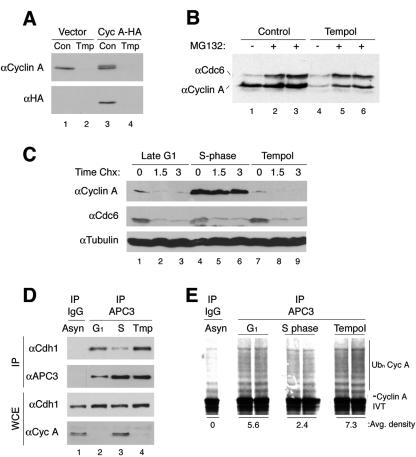
Cyclin A is ubiquitinated by APC and targeted for subsequent degradation by the 26S proteasome in G1 and mitosis (56). In late G1 phase, cyclin A transcript levels increase after inactivation of pRb, but the protein remains unstable due to APCCdh1 ubiquitination until S phase, when APCCdh1 is inactivated by phosphorylation and dissociation of Cdh1. To examine protein stability, T98G cells were treated with cycloheximide to inhibit translation in late G1 (16 h) or S phase (20 h) and tempol (20 h). Cycloheximide treatment resulted in rapid turnover of cyclin A protein in late G1 and antioxidant-treated cells (Fig. 5C). In contrast, cyclin A was stable in control S phase cells (Fig. 5C). 35S pulse-chase experiments demonstrated that cyclin A was translated at similar rates in control and tempol-treated cells; however, cyclin A was highly unstable in antioxidant-treated cells (see Fig. S1C in the supplemental material). Consistent with cyclin A instability in antioxidant-treated cells, inhibition of the 26S proteasome with MG-132 or MG-115 from 18 to 20 h post-serum restimulation resulted in the accumulation of cyclin A protein in antioxidant-treated cells (Fig. 5B and not shown). Cdc6 was also stabilized by treatment with proteasome inhibitors. Furthermore, similar results from cycloheximide and proteasome inhibitor experiments were obtained using primary human fibroblasts (see Fig. S1B in the supplemental material). These observations suggest that inhibition of an increase in steady-state levels of physiologic ROS by antioxidants results in continued degradation of cyclin A protein.
FIG. 5.
Failure to accumulate intracellular ROS results in continued targeting of cyclin A for degradation by APCCdh1. (A) NIH 3T3 cells were infected with retrovirus-expressing HA-tagged cyclin A, serum starved, and then restimulated with or with tempol for 20 h. Cyclin A proteinfails to accumulate, due to degradation, when expressed from a long terminal repeat promoter. (B) T98G cells restimulated with or without tempol for 18 h were treated with either dimethyl sulfoxide or the proteasome inhibitor MG-132 for 1.5 h. Cdc6 was used as a control for the MG-132. Inhibition of the proteasome by MG-132 causes accumulation of cyclin A in antioxidant-treated cells. (C) T98G cells were synchronized as before and treated with cycloheximide at 16 (late G1) or 20 h (tempol and S phase). Cyclin A protein is stable in S phase cells but rapidly degraded in late G1 or antioxidant-treated cells. (D) Coimmunoprecipitation analysis of APC with APC3 (Cdc27) antibody shows high levels of Cdh1 associated with APC in G1 and antioxidant-treated cells. (E) In vitro APC ubiquitination assays where APC was immunoprecipitated from late G1, S phase, or tempol treated cells (as above in panel D). APC beads were incubated for 1 h at 30°C with 35S-labeled, in vitro-translated cyclin A, E2 (UbcH10), GST-E1, ubiquitin, and an ATP regenerating system. APCCdh1 remains highly active in G1 and antioxidant-treated cells.
In G1 phase, APC activity is regulated by the presence of Cdh1, an adapter protein that confers substrate binding specificity (22, 56). Dissociation of Cdh1 is necessary for APCCdh1 inactivation, accumulation of cyclin A protein, and progression into S phase (42, 71, 72). Coimmunoprecipitation analysis of APC3 (Cdc27), an APC subunit, confirmed that Cdh1 was associated with APC in G1 cells and dissociated in S phase cells (Fig. 5D). However, antioxidant-treated cells showed continued association of Cdh1 with APC3, suggesting that APCCdh1 remained active in antioxidant-treated cells. Moreover, APC ubiquitination assays showed that immunoprecipitated APC from G1 and antioxidant-treated samples was able to highly ubiquitinate in vitro translated 35S-labeled cyclin A, compared to S phase or control samples without APC (Fig. 5E). Consistent with these observations and similar to cyclin A, Skp2, also a G1 target of APCCdh1 (4, 79), was present at high levels in control cells and at reduced levels in antioxidant-treated cells (Fig. 6A). These observations demonstrate that preventing the accumulation of endogenous ROS results in a late in G1 phase arrest characterized by continued APCCdh1 activity and degradation of its substrates.
FIG. 6.
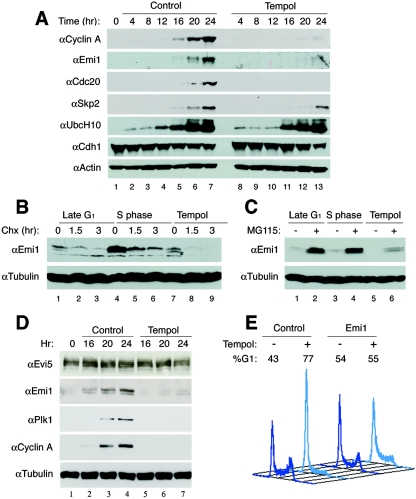
Antioxidant arrest is overcome by overexpression of Emi1. (A) T98G cells were synchronized by serum starvation for 72 h, restimulated with 5% FBS with or without tempol, harvested every 4 h from 0 to 24 h (as described in the legend of Fig. 4) and examined for expression of proteins involved in inactivation of APC. (B) T98G cells were synchronized as before and treated with cycloheximide at 16 (late G1) or 20 h (tempol and S phase), and harvested at 0, 1.5, or 3 h after the addition of cycloheximide; cells were then analyzed by immunoblotting for protein stability of Emi1. (C) Cells were synchronized as before, treated with MG115 in late G1 (16 h) or S phase (20 h) or tempol (20 h) and harvested after 1.5 h; cells were then analyzed by immunoblotting for protein levels of Emi1. (D) Cells were synchronized as before and either untreated or treated with tempol and examined for proteins that affect Emi1 stability. (E) Cells were thymidine blocked, released, transfected with an empty vector or pCS2-Myc-Emi1, and either untreated or treated with tempol and analyzed by FACS analysis 22 h later.
Antioxidant arrest is overcome by expression of Emi1.
We next sought to rescue the arrest caused by a failure to accumulate intracellular ROS and drive cells into S phase. Ectopic expression of wild-type cyclin A in antioxidant-treated cells by retroviral infection or transfection failed to accumulate cyclin A protein or drive cells into S phase (Fig. 5A and data not shown). Similarly, cyclin A-containing mutations in (13) or deletion of (17) the destruction box failed to accumulate or cause S phase entry (not shown). These results are consistent with the literature, as cyclin A has been shown to contain the conserved destruction box as well as a complex extended destruction motif that can also target it for degradation (17, 25). Moreover, expressing deletion constructs of the cyclin A N terminus, the portion of cyclin A containing the destruction motifs (either Δ97 [17] or Δ174) resulted in very low expression levels, failure to cause S phase progression, and frequent cell death (not shown). These results are in agreement with the literature that overexpression or constitutive expression of cyclin A is highly cytotoxic (32, 50). These data show that ectopic expression of cyclin A or various cyclin A mutants failed to rescue the antioxidant arrest.
A number of different mechanisms have been reported to inactivate or contribute to the inactivation of APCCdh1 at the late G1/S transition. Cyclin A-associated kinase has been shown to phosphorylate Cdh1, causing dissociation from and inactivation of APC (42, 71, 74). Yet, because cyclin A protein is extremely unstable in late G1 and in the antioxidant arrest, it cannot accumulate and phosphorylate Cdh1 as the initial Cdh1 inactivation mechanism. The ubiquitin conjugating enzyme UbcH10 was recently reported to be autoubiquitinated at the G1/S transition, allowing for accumulation of cyclin A and phosphorylation of Cdh1 (61). However, upon restimulation after serum deprivation, UbcH10 levels are absent and then increase at the G1/S transition (Fig. 6A) (81), demonstrating that UbcH10 levels do not correlate with APC activity or cyclin A levels.
Emi1, an E2F-responsive gene induced at the same time as cyclin A, binds to Cdh1 to prevent substrate binding, thereby inactivating APCCdh1 in late G1 phase. This initial inactivation is thought to allow accumulation of cyclin A protein, phosphorylation of Cdh1, and initiation of DNA synthesis (33). Consistent with Hsu et al. (33), Emi1 mRNA was absent in early G1 and present in both control and antioxidant-arrested cells at 16 and 20 h (see Fig. S1D in the supplemental material). However, Emi1 protein failed to accumulate in antioxidant-treated cells, mirroring the cyclin A results (Fig. 6A; see Fig. S1D in the supplemental material). Therefore, we examined Emi1 protein stability with cycloheximide treatment, as before, and found that Emi1 protein was highly unstable in antioxidant-treated cells (Fig. 6B). In agreement with Emi1 instability, inhibition of the proteasome with MG-115 or MG-132 showed accumulation of Emi1 protein in antioxidant-treated cells, as well as in late G1 and S phase cells (Fig. 6C and data not shown). Similar Emi1 results were obtained using primary human fibroblasts (see Fig. S1B in the supplemental material). Other APC substrates such as cyclin B, Cdc20, and Polo-like kinase (Plk1) also failed to accumulate in antioxidant-treated cells. Recently, Evi5 was shown to protect Emi1 from phosphorylation by Plk1 and ubiquitination by SCFβTrCP (19). Yet, even in the presence of Evi5 and absence of Plk1, Emi1 protein is rapidly degraded (Fig. 6D). These observations demonstrate that Emi1 protein is inherently unstable in antioxidant-treated, late G1 and S phase cells.
Overexpression of Emi1 has been found to inactivate APC, shorten G1, and increase the number of cells in S phase (33). To test if Emi1 could rescue the antioxidant-induced arrest, T98G cells were transfected with Myc-tagged Emi1. Unlike cyclin A, overexpression of Emi1 did not induce cytotoxicity. Ectopic expression of Myc-tagged Emi1 in antioxidant-treated cells rescued the late G1 arrest, driving cells into S and G2/M phases (Fig. 6E). Taken together, these observations suggest that accumulation of physiologic ROS levels plays a role in the accumulation or stabilization of Emi1 protein and/or inactivation of APCCdh1 at the late G1/S phase transition, allowing accumulation of APCCdh1 substrates necessary for S phase initiation, including cyclin A.
DISCUSSION
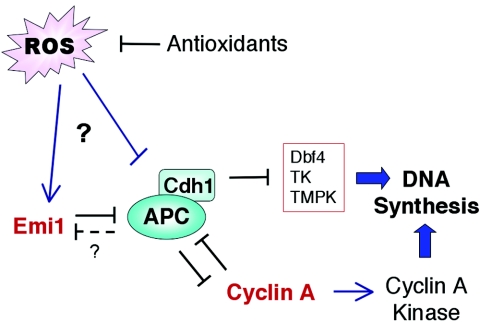
ROS is known to cause oxidative stress and DNA damage at high levels; however, at low physiologic levels, ROS can stimulate proliferation (9, 66) and regulate protein function (24). Here, we show that endogenous physiologic ROS levels increase in a cell cycle-dependent manner and oscillate with every cell division. Preventing the accumulation in steady-state ROS levels by antioxidant treatment results in activation of a novel late G1 phase checkpoint after transition across the restriction point that is characterized by active cyclin E-Cdk2 complexes, inactive hyperphosphorylated pRb, and induction of E2F target genes, including cyclin A and Emi1. However, the cells arrest in late G1 with continued APCCdh1 activity targeting cyclin A for degradation and preventing S phase entry (Fig. 7). In addition to cyclin A, other proteins necessary for DNA replication, such as Dbf4 (23), thymidylate kinase, and thymidine kinase (37), have been identified as APC substrates.
FIG. 7.
Model of ROS inhibition of APCCdh1 activity.
Emi1, an APC inhibitor, also failed to accumulate in antioxidant-arrested cells. Upon closer examination, Emi1 protein accumulated after Emi1 transcript in late G1 and was stabilized by proteasome inhibitors, similar to cyclin A. Emi1 is phosphorylated by Plk1 (51), ubiquitinated by SCFβTrCP, and degraded by the proteasome at the onset of mitosis (27, 44). However, Plk1, also an APCCdh1 substrate (56), is not expressed in late G1 or antioxidant-arrested cells; therefore, Emi1 degradation is likely due to another mechanism. Furthermore, Evi5 was recently shown to bind to and stabilize Emi1 by preventing SCFβTrCP association in S and G2 phases, allowing accumulation of Emi1 and APCCdh1 inactivation (19). However, even in the presence of Evi5, Emi1 protein is unstable. Emi1 is a zinc binding protein (62) that contains many cysteine residues, and therefore, it is plausible that Emi1 or the Emi1-Evi5 interaction could be redox regulated. Alternatively, Emi1 also contains KEN and D-box motifs, known to target substrates to APC (57), suggesting that Emi1 could function as a substrate or inhibitor of APCCdh1, depending on redox modification. Yet it is also conceivable that APCCdh1 itself could be redox sensitive. APC contains at least 12 subunits with many phosphorylation sites (38), and it is plausible that ROS-mediated activation of a kinase, inactivation of a phosphatase, or structural change could trigger a decrease in APCCdh1 activity to initiate the feed-forward loop of APCCdh1 inactivation. Extensive studies into redox regulation of Emi1 and APCCdh1 will ultimately be required to dissect these pathways.
ROS can affect the activity of phosphatases, kinases, and transcription factors (46, 66), influencing protein activity of downstream pathways. SUMOylation, a posttranslational modification affecting protein activity, is also inhibited in the presence of low physiologic ROS levels (6). Direct redox modification of cysteine residues in proteins by the formation of disulfide bonds, cyclic sulfonamides, S-hydroxylation, S-nitrosylation, and S-glutathiolation can affect protein activity in a manner similar to the removal or addition of a phosphate group (24). However, it has not yet been defined how ROS affects APCCdh1 or Emi1.
Here, we demonstrate that an increase in endogenous ROS steady-state levels from late G1 to S phase affects APCCdh1 activity or Emi1 protein stability to allow initiation of DNA replication. There are several potential sources that generate endogenous ROS including mitochondria, peroxisomes, and NADPH oxidase (66). Interestingly, cells without functional mitochondria (ρo [11, 12]) or cells treated with small interfering RNA against NADPH oxidase subunits still proliferate (7) but at a reduced rate. However, it remains unclear as to whether a single species or source or multiple species from multiple sources of ROS are involved in regulating APCCdh1 activity.
In summary, we find that a failure to achieve a critical level of intracellular ROS activates a previously uncharacterized late G1 phase arrest, and we propose that this is an intrinsic late G1 phase checkpoint that monitors the cellular metabolic state prior to replication of the genome. This arrest occurs after transition across the growth factor restriction point and either directly or indirectly (through Emi1) regulates APCCdh1 activity. APCCdh1 inactivation is necessary for accumulation of substrates essential for DNA replication, including cyclin A (72), Dbf4 (23), thymidylate kinase, thymidine kinase (37), Skp2, and Cks1 (4). We also found that an antioxidant-induced G1 arrest occurs in budding yeast (M.V. Wagner and S. F. Dowdy, unpublished observations), suggesting that this may be an intrinsic evolutionary conserved feedback mechanism to monitor the status of cellular metabolism prior to commitment of DNA synthesis. These observations reveal an intrinsic late G1 phase checkpoint that links cellular ROS production, and possibly metabolism, with cell cycle progression via APCCdh1-mediated protein degradation.
Supplementary Material
Acknowledgments
We thank P. K. Jackson for pCS2-Emi1, Emi1 antibody, and Evi5 antibody; P. Kaldes and J. Pines for cyclin A plasmids; and A. Wynshaw-Boris for critical input.
N.Y. was funded by JSPS Research Fellowships for Young Scientists. This work was supported by the Howard Hughes Medical Institute (S.F.D.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Akagi, T., T. Shishido, K. Murata, and H. Hanafusa. 2000. v-Crk activates the phosphoinositide 3-kinase/AKT pathway in transformation. Proc. Natl. Acad. Sci. USA 97:7290-7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleem, E., H. Kiyokawa, and P. Kaldis. 2005. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7:831-836. [DOI] [PubMed] [Google Scholar]
- 3.Barnouin, K., M. L. Dubuisson, E. S. Child, S. Fernandez de Mattos, J. Glassford, R. H. Medema, D. J. Mann, and E. W. Lam. 2002. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J. Biol. Chem. 277:13761-13770. [DOI] [PubMed] [Google Scholar]
- 4.Bashir, T., N. V. Dorrello, V. Amador, D. Guardavaccaro, and M. Pagano. 2004. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428:190-193. [DOI] [PubMed] [Google Scholar]
- 5.Blaise, G. A., D. Gauvin, M. Gangal, and S. Authier. 2005. Nitric oxide, cell signaling and cell death. Toxicology 208:177-192. [DOI] [PubMed] [Google Scholar]
- 6.Bossis, G., F. Melchior. 2006. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21:349-357. [DOI] [PubMed] [Google Scholar]
- 7.Brar, S. S., T. P. Kennedy, A. B. Sturrock, T. P. Huecksteadt, M. T. Quinn, A. R. Whorton, and J. R. Hoidal. 2002. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am. J. Physiol. Cell Physiol. 282:C1212-C1224. [DOI] [PubMed] [Google Scholar]
- 8.Brunelle, J. K., E. L. Bell, N. M. Quesada, K. Vercauteren, V. Tiranti, M. Zeviani, R. C. Scarpulla, and N. S. Chandel. 2005. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1:409-414. [DOI] [PubMed] [Google Scholar]
- 9.Burdon, R. H. 1995. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 18:775-794. [DOI] [PubMed] [Google Scholar]
- 10.Cam, H., E. Balciunaite, A. Blais, A. Spektor, R. C. Scarpulla, R. Young, Y. Kluger, and B. D. Dynlacht. 2004. A common set of gene regulatory networks links metabolism and growth inhibition. Mol. Cell. 16:399-411. [DOI] [PubMed] [Google Scholar]
- 11.Chandel, N. S., E. Maltepe, E. Goldwasser, C. E. Mathieu, M. C. Simon, and P. T. Schumacker. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 95:11715-11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandel, N. S., and P. T. Schumacker. 2000. Cellular oxygen sensing by mitochondria: old questions, new insight. J. Appl. Physiol. 88:1880-1889. [DOI] [PubMed] [Google Scholar]
- 13.Chibazakura, T., S. G. McGrew, J. A. Cooper, H. Yoshikawa, and J. M. Roberts. 2004. Regulation of cyclin-dependent kinase activity during mitotic exit and maintenance of genome stability by p21, p27, and p107. Proc. Natl. Acad. Sci. USA 101:4465-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coverley, D., H. Laman, and R. A. Laskey. 2002. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 4:523-528. [DOI] [PubMed] [Google Scholar]
- 15.Dang, C. V., F. Li, and L. A. Lee. 2005. Could MYC induction of mitochondrial biogenesis be linked to ROS production and genomic instability? Cell Cycle 4:1465-1466. [DOI] [PubMed] [Google Scholar]
- 16.del Bello, B., A. Paolicchi, M. Comporti, A. Pompella, and E. Maellaro. 1999. Hydrogen peroxide produced during gamma-glutamyl transpeptidase activity is involved in prevention of apoptosis and maintenance of proliferation in U937 cells. FASEB J. 13:69-79. [DOI] [PubMed] [Google Scholar]
- 17.den Elzen, N., and J. Pines. 2001. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153:121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng, X., F. Gao, and W. S. May, Jr. 2003. Bcl2 retards G1/S cell cycle transition by regulating intracellular ROS. Blood 102:3179-3185. [DOI] [PubMed] [Google Scholar]
- 19.Eldridge, A. G., A. V. Loktev, D. V. Hansen, E. W. Verschuren, J. D. R. Reimann, and P. K. Jackson. 2006. The evi5 oncogene regulates cyclin accumulation by stabilizing the anaphase-promoting complex inhibitor emi1. Cell 124:367-380. [DOI] [PubMed] [Google Scholar]
- 20.Esteban, M. A., and P. H. Maxwell. 2005. HIF, a missing link between metabolism and cancer. Nat. Med. 11:1047-1048. [DOI] [PubMed] [Google Scholar]
- 21.Ezhevsky, S. A., A. Ho, M. Becker-Hapak, P. K. Davis, and S. F. Dowdy. 2001. Differential regulation of retinoblastoma tumor suppressor protein by G1 cyclin-dependent kinase complexes in vivo. Mol. Cell. Biol. 21:4773-4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang, G., H. Yu, and M. W. Kirschner. 1998. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2:163-171. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira, M. F., C. Santocanale, L. S. Drury, and J. F. Diffley. 2000. Dbf4p, an essential S phase-promoting factor, is targeted for degradation by the anaphase-promoting complex. Mol. Cell. Biol. 20:242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filomeni, G., G. Rotilio, and M. R. Ciriolo. 2005. Disulfide relays and phosphorylative cascades: partners in redox-mediated signaling pathways. Cell Death Differ. 12:1555-1563. [DOI] [PubMed] [Google Scholar]
- 25.Geley, S., E. Kramer, C. Gieffers, J. Gannon, J. M. Peters, and T. Hunt. 2001. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard, F., U. Strausfeld, A. Fernandez, and N. J. Lamb. 1991. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 67:1169-1179. [DOI] [PubMed] [Google Scholar]
- 27.Guardavaccaro, D., Y. Kudo, J. Boulaire, M. Barchi, L. Busino, M. Donzelli, F. Margottin-Goguet, P. K. Jackson, L. Yamasaki, and M. Pagano. 2003. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell 4:799-812. [DOI] [PubMed] [Google Scholar]
- 28.Guzy, R. D., B. Hoyos, E. Robin, H. Chen, L. Liu, K. D. Mansfield, M. C. Simon, U. Hammerling, and P. T. Schumacker. 2005. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1:401-408. [DOI] [PubMed] [Google Scholar]
- 29.Hatzivassiliou, G., F. Zhao, D. E. Bauer, C. Andreadis, A. N. Shaw, D. Dhanak, S. R. Hingorani, D. A. Tuveson, and C. B. Thompson. 2005. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8:311-321. [DOI] [PubMed] [Google Scholar]
- 30.Henglein, B., X. Chenivesse, J. Wang, D. Eick, and C. Brechot. 1994. Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc. Natl. Acad. Sci. USA 91:5490-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho, A., and S. F. Dowdy. 2002. Regulation of G1 cell-cycle progression by oncogenes and tumor suppressor genes. Curr. Opin. Genet. Dev. 12:47-52. [DOI] [PubMed] [Google Scholar]
- 32.Hoang, A. T., K. J. Cohen, J. F. Barrett, D. A. Bergstrom, and C. V. Dang. 1994. Participation of cyclin A in Myc-induced apoptosis. Proc. Natl. Acad. Sci. USA 91:6875-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu, J. Y., J. D. Reimann, C. S. Sorensen, J. Lukas, and P. K. Jackson. 2002. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat. Cell Biol. 4:358-366. [DOI] [PubMed] [Google Scholar]
- 34.Irani, K., Y. Xia, J. L. Zweier, S. J. Sollott, C. J. Der, E. R. Fearon, M. Sundaresan, T. Finkel, and P. J. Goldschmidt-Clermont. 1997. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275:1649-1652. [DOI] [PubMed] [Google Scholar]
- 35.Jones, R. G., D. R. Plas, S. Kubek, M. Buzzai, J. Mu, Y. Xu, M. J. Birnbaum, and C. B. Thompson. 2005. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18:283-293. [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen, P., and M. Tyers. 2004. How cells coordinate growth and division. Curr. Biol. 14:R1014-R1027. [DOI] [PubMed] [Google Scholar]
- 37.Ke, P. Y., Y. Y. Kuo, C. M. Hu, and Z. F. Chang. 2005. Control of dTTP pool size by anaphase promoting complex/cyclosome is essential for the maintenance of genetic stability. Genes Dev. 19:1920-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraft, C., F. Herzog, C. Gieffers, K. Mechtler, A. Hagting, J. Pines, and J. M. Peters. 2003. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22:6598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambeth, J. D. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4:181-189. [DOI] [PubMed] [Google Scholar]
- 40.Lim, S. D., C. Sun, J. D. Lambeth, F. Marshall, M. Amin, L. Chung, J. A. Petros, and R. S. Arnold. 2005. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 62:200-207. [DOI] [PubMed] [Google Scholar]
- 41.Lissy, N. A., P. K. Davis, M. Irwin, W. G. Kaelin, and S. F. Dowdy. 2000. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature 407:642-645. [DOI] [PubMed] [Google Scholar]
- 42.Lukas, C., C. S. Sorensen, E. Kramer, E. Santoni-Rugiu, C. Lindeneg, J. M. Peters, J. Bartek, and J. Lukas. 1999. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401:815-818. [DOI] [PubMed] [Google Scholar]
- 43.Mansfield, K. D., R. D. Guzy, Y. Pan, R. M. Young, T. P. Cash, P. T. Schumacker, and M. C. Simon. 2005. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 1:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margottin-Goguet, F., J. Y. Hsu, A. Loktev, H. M. Hsieh, J. D. Reimann, and P. K. Jackson. 2003. Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4:813-826. [DOI] [PubMed] [Google Scholar]
- 45.Mason, B., N. Ghanee, W. G. Haigh, S. P. Lee, and D. Oda. 1999. Effect of vitamins A, C and E on normal and HPV-immortalized human oral epithelial cells in culture. Anticancer Res. 19:5469-5474. [PubMed] [Google Scholar]
- 46.Meng, T. C., T. Fukada, and N. K. Tonks. 2002. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9:387-399. [DOI] [PubMed] [Google Scholar]
- 47.Menon, S. G., E. H. Sarsour, D. R. Spitz, R. Higashikubo, M. Sturm, H. Zhang, and P. C. Goswami. 2003. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 63:2109-2117. [PubMed] [Google Scholar]
- 48.Mitchell, J. B., S. Xavier, A. M. DeLuca, A. L. Sowers, J. A. Cook, M. C. Krishna, S. M. Hahn, and A. Russo. 2003. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic. Biol. Med. 34:93-102. [DOI] [PubMed] [Google Scholar]
- 49.Mitsushita, J., J. D. Lambeth, T. Kamata. 2004. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 64:3580-3585. [DOI] [PubMed] [Google Scholar]
- 50.Morgan, D. O. 1992. Cell cycle control in normal and neoplastic cells. Curr. Opin. Genet. Dev. 2:33-37. [DOI] [PubMed] [Google Scholar]
- 51.Moshe, Y., J. Boulaire, M. Pagano, and A. Hershko. 2004. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl. Acad. Sci. USA 101:7937-7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murrell, G. A., M. J. Francis, and L. Bromley. 1990. Modulation of fibroblast proliferation by oxygen free radicals. Biochem. J. 265:659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oyama, Y., A. Hayashi, T. Ueha, and K. Maekawa. 1994. Characterization of 2′,7′-dichlorofluorescin fluorescence in dissociated mammalian brain neurons: estimation on intracellular content of hydrogen peroxide. Brain Res. 635:113-117. [DOI] [PubMed] [Google Scholar]
- 54.Pagano, M., R. Pepperkok, F. Verde, W. Ansorge, and G. Draetta. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pani, G., R. Colavitti, B. Bedogni, R. Anzevino, S. Borrello, and T. Galeotti. 2000. A redox signaling mechanism for density-dependent inhibition of cell growth. J. Biol. Chem. 275:38891-38899. [DOI] [PubMed] [Google Scholar]
- 56.Peters, J. M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9:931-943. [DOI] [PubMed] [Google Scholar]
- 57.Pfleger, C. M., and M. W. Kirschner. 2000. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14:655-665. [PMC free article] [PubMed] [Google Scholar]
- 58.Policastro, L., B. Molinari, F. Larcher, P. Blanco, O. L. Podhajcer, C. S. Costa, P. Rojas, and H. Duran. 2004. Imbalance of antioxidant enzymes in tumor cells and inhibition of proliferation and malignant features by scavenging hydrogen peroxide. Mol. Carcinog. 39:103-113. [DOI] [PubMed] [Google Scholar]
- 59.Preston, T. J., W. J. Muller, and G. Singh. 2001. Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J. Biol. Chem. 276:9558-9564. [DOI] [PubMed] [Google Scholar]
- 60.Rao, G. N., and B. C. Berk. 1992. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ. Res. 70:593-599. [DOI] [PubMed] [Google Scholar]
- 61.Rape, M., and M. W. Kirschner. 2004. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 432:588-595. [DOI] [PubMed] [Google Scholar]
- 62.Reimann, J. D., E. Freed, J. Y. Hsu, E. R. Kramer, J. M. Peters, and P. K. Jackson. 2001. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105:645-655. [DOI] [PubMed] [Google Scholar]
- 63.Resnitzky, D., L. Hengst, and S. I. Reed. 1995. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol. Cell. Biol. 15:4347-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruiz-Gines, J. A., S. Lopez-Ongil, M. Gonzalez-Rubio, L. Gonzalez-Santiago, M. Rodriguez-Puyol, and D. Rodriguez-Puyol. 2000. Reactive oxygen species induce proliferation of bovine aortic endothelial cells. J. Cardiovasc. Pharmacol. 35:109-113. [DOI] [PubMed] [Google Scholar]
- 65.Sauer, H., B. Klimm, J. Hescheler, and M. Wartenberg. 2001. Activation of p90RSK and growth stimulation of multicellular tumor spheroids are dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. FASEB J. 15:2539-2541. [DOI] [PubMed] [Google Scholar]
- 66.Sauer, H., M. Wartenberg, and J. Hescheler. 2001. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 11:173-186. [DOI] [PubMed] [Google Scholar]
- 67.Schubert, R., L. Erker, C. Barlow, H. Yakushiji, D. Larson, A. Russo, J. B. Mitchell, and A. Wynshaw-Boris. 2004. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 13:1793-1802. [DOI] [PubMed] [Google Scholar]
- 68.Schulze, A., K. Zerfass, D. Spitkovsky, S. Middendorp, J. Berges, K. Helin, P. Jansen-Durr, and B. Henglein. 1995. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc. Natl. Acad. Sci. USA 92:11264-11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sen Gupta, R., E. Sen Gupta, B. K. Dhakal, A. R. Thakur, and J. Ahnn. 2004. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol. Cell 17:132-139. [PubMed] [Google Scholar]
- 70.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 71.Sorensen, C. S., C. Lukas, E. R. Kramer, J. M. Peters, J. Bartek, and J. Lukas. 2001. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A-Cdk2 during cell cycle progression. Mol. Cell. Biol. 21:3692-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorensen, C. S., C. Lukas, E. R. Kramer, J. M. Peters, J. Bartek, and J. Lukas. 2000. Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol. Cell. Biol. 20:7613-7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sundaresan, M., Z. X. Yu, V. J. Ferrans, K. Irani, and T. Finkel. 1995. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296-299. [DOI] [PubMed] [Google Scholar]
- 74.Szatrowski, T. P., and C. F. Nathan. 1991. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51:794-798. [PubMed] [Google Scholar]
- 75.Torres, M., and H. J. Forman. 2003. Redox signaling and the MAP kinase pathways. Biofactors 17:287-296. [DOI] [PubMed] [Google Scholar]
- 76.Turley, J. M., F. W. Ruscetti, S. J. Kim, T. Fu, F. V. Gou, and M. C. Birchenall-Roberts. 1997. Vitamin E succinate inhibits proliferation of BT-20 human breast cancer cells: increased binding of cyclin A negatively regulates E2F transactivation activity. Cancer Res. 57:2668-2675. [PubMed] [Google Scholar]
- 77.Vodermaier, H. C., C. Gieffers, S. Maurer-Stroh, F. Eisenhaber, and J. M. Peters. 2003. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 13:1459-1468. [DOI] [PubMed] [Google Scholar]
- 78.Wartenberg, M., H. Diedershagen, J. Hescheler, and H. Sauer. 1999. Growth stimulation versus induction of cell quiescence by hydrogen peroxide in prostate tumor spheroids is encoded by the duration of the Ca(2+) response. J. Biol. Chem. 274:27759-27767. [DOI] [PubMed] [Google Scholar]
- 79.Wei, W., N. G. Ayad, Y. Wan, G. J. Zhang, M. W. Kirschner, and W. G. Kaelin, Jr. 2004. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428:194-198. [DOI] [PubMed] [Google Scholar]
- 80.Woo, R. A., and R. Y. Poon. 2003. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2:316-324. [PubMed] [Google Scholar]
- 81.Yamanaka, A., S. Hatakeyama, K. Kominami, M. Kitagawa, M. Matsumoto, and K. Nakayama. 2000. Cell cycle-dependent expression of mammalian E2-C regulated by the anaphase-promoting complex/cyclosome. Mol. Biol. Cell. 11:2821-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.