Abstract
Angiogenesis, the process by which new blood vessels are formed from preexisting vasculature, is critical for vascular remodeling during development and contributes to the pathogenesis of diseases such as cancer. Prior studies from our laboratory demonstrate that the EphA2 receptor tyrosine kinase is a key regulator of angiogenesis in vivo. The EphA receptor-mediated angiogenic response is dependent on activation of Rho family GTPase Rac1 and is regulated by phosphatidylinositol 3-kinase. Here we report the identification of Vav2 and Vav3 as guanine nucleotide exchange factors (GEFs) that link the EphA2 receptor to Rho family GTPase activation and angiogenesis. Ephrin-A1 stimulation recruits the binding of Vav proteins to the activated EphA2 receptor. The induced association of EphA receptor and Vav proteins modulates the activity of Vav GEFs, leading to activation of Rac1 GTPase. Overexpression of either Vav2 or Vav3 in primary microvascular endothelial cells promotes Rac1 activation, cell migration, and assembly in response to ephrin-A1 stimulation. Conversely, loss of Vav2 and Vav3 GEFs inhibits Rac1 activation and ephrin-A1-induced angiogenic responses both in vitro and in vivo. In addition, embryonic fibroblasts derived from Vav2−/− Vav3−/− mice fail to spread on an ephrin-A1-coated surface and exhibit a significant decrease in the formation of ephrin-A1-induced lamellipodia and filopodia. These findings suggest that Vav GEFs serve as a molecular link between EphA2 receptors and the actin cytoskeleton and provide an important mechanism for EphA2-mediated angiogenesis.
Angiogenesis, the process by which new blood vessels are formed from preexisting vasculature, is critical for vascular remodeling during development and contributes to the pathogenesis of diseases such as cancer. Two critical steps in this process are endothelial cell migration and assembly into new tubules. Over the last decade, a diverse array of molecular regulators that participate in the process of angiogenesis has been identified (4, 47). The Eph family of receptor tyrosine kinases is one such family of angiogenic regulators that plays a prominent role in endothelial cell assembly and migration.
The Eph receptors belong to the largest family of receptor tyrosine kinases in the genome, with 16 receptors and 9 ligands identified to date in vertebrates (28, 38). Based on binding specificity and structural properties, the Eph receptors are divided into two subclasses, class A and class B (23). In general, EphA receptors bind to glycosylphosphatidylinositol-linked ephrin-A ligands, while EphB receptors bind to transmembrane ephrin-B ligands. Gene targeting studies have established several class B Eph family members as key regulators of embryonic vascular development (2, 24, 46). In contrast, class A Eph receptors have been shown to regulate postnatal angiogenesis in adults. Ephrin-A1 stimulates endothelial cell migration and assembly in culture (15, 34) and induces corneal angiogenesis in vivo (37). More recently, Eph receptors have been detected in tumor blood vessel endothelial cells (reviewed in references 8 and 9). Inhibition of class A Eph receptor signaling by soluble EphA2-Fc or EphA3-Fc receptors decreased tumor volume, tumor angiogenesis, and metastatic progression in vivo (6, 13, 18). A main target of soluble EphA receptors appears to be EphA2, as EphA2-deficient endothelial cells fail to migrate and assemble in vitro (7), and loss of EphA2 receptor resulted in impaired tumor growth and metastasis in vivo (10). These data support the crucial role for Eph receptor-mediated regulation of angiogenesis.
Investigation of ephrin/Eph receptor-mediated signal transduction mechanisms that regulate cellular responses in various cell types has been centered on Rho-family GTPases (33). In vascular smooth muscle cells, for example, the EphA4 receptor stimulates RhoA activity via direct interaction with Vsm-RhoGEF (35), while ephrin-A1 stimulation inhibits Rac1 and p21-activated kinase (PAK) activity (17). In endothelial cells, however, EphA2 receptor-mediated cell migration is dependent on Rac1 GTPase activation (7). Ephrin-A1 stimulation induces activation of the Rac1 GTPase, and a dominant negative N17 Rac1 mutant inhibits ephrin-A1-induced endothelial cell motility. Rac1 activity also appears to be regulated by phosphatidylinositol 3 kinase (PI3K). PI3K-specific inhibitors, wortmannin, LY294002, or a dominant negative p85 subunit of PI3K, block ephrin-A1-induced Rac1 activation and endothelial cell migration. These data suggest that the EphA2 receptor controls endothelial cell motility by regulating Rac1 GTPase activity.
The molecular mechanism by which the EphA2 receptor regulates the activity of Rac1 GTPase in endothelial cells remains to be elucidated. The Vav family of guanine nucleotide exchange factors (GEFs), which includes Vav1, Vav2, and Vav3, has been shown to modulate activity of Rho, Rac, and/or Cdc42 to elicit changes in cytoskeletal organization (27, 41, 48). In addition, Vav proteins can interact with the PI3K lipid product phosphatidylinositol-3,4,5-triphosphate in activation of Rac1 (16, 26). While the majority of studies on these proteins have focused on functions in the immune system (45), Vav2 and Vav3 display a broader tissue expression profile and therefore likely regulate cytoskeletal dynamics in other cells types (27). Vav2 and Vav3 are expressed in heart and other highly vascularized organs, including placenta, lung, and kidney (44, 48). As EphA2-mediated Rac1 activation in endothelial cells is PI3K-dependent (7), these data suggest that Vav proteins may link the EphA2 receptor to Rac1 directly and/or through PI3K.
Through a yeast two-hybrid screen, we identified the Vav3 GEF as a binding partner of EphA2. In this study, we report that both Vav3 and Vav2 GEFs are recruited to phosphorylated EphA2 receptors in mammalian cells. Endothelial cells deficient in both Vav2 and Vav3 exhibit impaired activation of Rac1 GTPase and endothelial cell migration and assembly in response to ephrin-A1 ligand. In addition, loss of both Vav2 and Vav3 inhibits ephrin-A1-induced angiogenesis in vivo. We propose that regulation of Rac1 GTPase signaling by modulation of Vav2/3 activity may underlie endothelial responses to ephrin-A1.
MATERIALS AND METHODS
Yeast two-hybrid screening.
The mouse EphA2 cytoplasmic domain was cloned into pBridge-LexA (BD Biosciences) (pSGS2) as a bait to screen a human placenta library consisting of 3.5 × 106 independent clones (Clontech). Briefly, yeast strain L40 [MATa his3Δ200 trp1-901 leu2-3,112 ade2 LYS2::(lexAop)4-HIS3 URA3::(lexAop)8-lacZ GAL4] was transformed with pSGS2 and the placenta cDNA library. The resulting transformants were screened for histidine prototrophy and expression of LacZ. The His+ LacZ+ clones that did not interact with lamin C were subjected to PCR and restriction analyses to eliminate duplicate clones. Among 14 unique His+ LacZ+ clones, two overlapping clones encompassing the SH2-SH3 domains of the Vav3 gene were identified.
Antibodies.
Antibodies used for immunoblotting include the following: anti-myc (1:1,000; BD Biosciences); anti-EphA2 (1:2,500) and phosphotyrosine antibodies (1:600; Santa Cruz Biotechnology); anti-β-galactosidase (1:500; Chemicon); anti-tubulin (1:1,000; Sigma-Aldrich); anti-Rac1 and anti-cdc42 antibodies (1:1,000 and 1:500, respectively; BD Bioscience); and anti-Vav3 (1:3,000; Upstate Biotechnology). A mixture of polyclonal anti-EphA2 (0.5 μg; C-20; Santa Cruz Biotechnology) and monoclonal anti-EphA2 (1 μg; D7; Upstate Biotechnology) antibodies were used to immunoprecipitate EphA2 from endothelial cell lysates. Anti-Vav2 antibodies have been previously described (14).
In vitro binding assay.
MBP-EphA2, the fusion of the intracellular portion of mouse EphA2 and maltose-binding protein (MBP), was expressed in pMAL-c2X (New England Biolabs) and purified on amylose resin according to the manufacturer's instructions. Escherichia coli lysate containing glutathione S-transferase (GST)-Vav3 SH3-SH2-SH3 domains was incubated with amylose-bound MBP-EphA2. After extensive washing, bound proteins were eluted and subjected to Western blot analyses using anti-EphA2 and anti-Vav3 antibodies.
Coimmunoprecipitation and Western blot analyses.
COS7 cells were cotransfected with 1 μg each of T7-tagged Vav2 or myc-tagged Vav3 and EphA2 per well in a six-well dish using Lipofectamine 2000. Cells were lysed in Brij buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 0.88% Brij, 0.125% NP-40 plus protease inhibitors). T7-agarose (20 μl of beads/ml of lysate; Novagen) or Myc-agarose (20 μl of beads/ml of lysate; Sigma-Aldrich) was used to immunoprecipitate Vav2 or Vav3, respectively. The resulting proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to Western blotting using anti-EphA2. A mixture of anti-EphA2 antibodies (D7 at 1:1,000 and sc-924 at 1:500) was also used in immunoprecipitation, and precipitated proteins were subjected to SDS-PAGE and Western blot analysis by Vav2 or Vav3 antibodies.
Fibroblast morphology assays.
A total of 100,000 wild-type or Vav2−/− Vav3−/− embryonic fibroblasts were plated on glass slides precoated with ephrin-A1, immunoglobulin G (IgG), or fibronectin by placing 1 ml/per well (six-well dish) of the protein solution at 10 μg/ml in the dish overnight and subsequently blocking in Dulbecco's modified Eagle's medium containing 2% bovine serum albumin for 30 min. Five hours after plating, cells were fixed in 4% paraformaldehyde, permeabilized in 0.4% Triton X-100, and stained with fluorescein isothiocyanate-conjugated phalloidin to detect F-actin. Experiments were repeated three times, and an average of 100 cells were examined and quantified for each experiment.
Guanine nucleotide exchange assays.
For Rac1 and cdc42 activation assays, cells were serum starved for 24 h in OptiMEM, followed by stimulation with ephrin-A1 (1 μg ml−1). Lysates were prepared and incubated with PAK1 p21-binding domain (PBD)-GST beads (Upstate Biotechnology) as described by the manufacturer's protocol to pull down GTP-bound Rac1 and/or cdc42. Activated Rac1 and cdc42 (or total Rac1 and cdc42 in lysates) were detected by immunoblotting using anti-Rac1 or anti-cdc42 antibodies (BD Transduction Labs). Relative levels of GTP-bound Rac1 and cdc42 were quantified by densitometry using Scion Image 1.62c software analysis.
Endothelial cells.
Primary murine pulmonary microvascular endothelial cells (MPMEC) were isolated from 1- to 3-month-old wild-type or Vav2/Vav3 dual-deficient mice (14) and maintained in EGM-2 medium (Clonetics), as previously described (7, 39). Endothelial cell purity was greater than 95% in these cultures, as determined by expression of CD31, an endothelial cell marker (data not shown). For transfection experiments, primary bovine pulmonary microvascular endothelial cells (BPMEC) were seeded into six-well plates and transfected with 1 μg of plasmid DNA per well using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Migration assay.
Migration assays using 6.5-mm, 8-μm-pore-size Transwells (Costar) were performed as described previously (7). Both sides of the transwell were coated with a 1/20 dilution of growth factor-reduced Matrigel and blocked with 1% bovine serum albumin. After 24 h of starvation in OptiMEM, cells (100,000/well) were seeded into the upper chamber of transwells in the presence or absence of ephrin-A1 (2.5 μg ml−1) in the lower chamber. After 5 h, cells on the lower surface were fixed, stained with crystal violet, and counted in three random fields from each well, with triplicate samples per condition.
Assembly assay.
In vitro vascular assembly assays were performed as described previously (7). Briefly, 12-well plates were coated with 100 μl of growth factor-reduced Matrigel (Becton-Dickinson). After 24 h of starvation in OptiMEM, 25,000 MPMEC were plated in wells in the presence or absence of ephrin-A1 (1.5 μg ml−1; R&D Systems) and photodocumented after 9 h. Images were acquired on an Olympus CK40 inverted microscope through an Optronics DEI-750C charge-coupled-device video camera using Scion Image capture software, version 1.62c. Degree of assembly was quantified by measuring branch length, i.e., the distance from branching point to the tip of assembled cells. Only assembled cells consisting of at least three cells were measured. The branch length in assembled endothelial cell networks was expressed as arbitrary units per field (at a magnification of ×10) in four random fields from each well, with triplicate samples per condition, using Scion Image 1.62c software for analysis.
In vivo angiogenesis assay.
Sponge assays for angiogenesis were performed as described previously (7). Briefly, gel foam sponges (Pharmacia and Upjohn) were cut into small pieces (2.5 to 3 mm wide by 5 mm long) and soaked with 100 μl of phosphate-buffered saline containing 10 μg of ephrin-A1 or IgG. The sponges were then implanted into the subcutaneous dorsal flank of recipient mice. Each recipient received one ephrin-A1-treated sponge and one control IgG sponge implanted in the opposite flank. After 7 days, the mice were injected with a 2% tetramethyl rhodamine isothiocyanate (TRITC)-dextran-phosphate-buffered saline solution to label host blood vessels (6), and the sponges were collected and analyzed. Whole-mount images were acquired on an Olympus CK40 inverted microscope through an Optronics DEI-750C charge-coupled-device video camera using Scion Image, capture software version 1.62c. Density of blood vessels within the sponges was quantified by fluorescence intensity of rhodamine-dextran using Scion Image software. The density of fluorescent pixels within each field (magnification, ×10) was determined and compared in Vav2−/− Vav3−/− mice with that in wild-type controls. Data are a representation of results from five independent sponges in each genotype. Statistical significance was determined by a two-tailed, paired Student's t test.
RESULTS
Activated EphA2 receptor recruits Vav2 and Vav3 GEFs.
To identify EphA2-interacting proteins that function to regulate endothelial cell migration, we performed a yeast two-hybrid screen. The bait construct consisted of the intracellular portion of mouse EphA2 fused to the DNA binding domain of LexA. Upon screening a cDNA library from human placenta, we obtained two independent but overlapping interacting clones that encoded the Vav3 GEF. To verify the interaction between EphA2 and Vav3, the SH3-SH2-SH3 portion of Vav3 was expressed as a GST fusion, and the EphA2 cytoplasmic domain was expressed as an MBP fusion. Soluble GST-Vav3 was incubated with MBP-EphA2 that was linked to amylose beads. After extensive washing, only GST-Vav3 and MBP-EphA2 were eluted from the column (Fig. 1A). While MBP-EphA2 was phosphorylated and bound to GST-Vav3, MBP alone failed to interact with GST-Vav3 (Fig. 1B), suggesting that the binding is specific to EphA2 in vitro.
FIG. 1.
Interaction between EphA2 and Vav proteins. (A) The MBP-EphA2 cytoplasmic domain fusion protein and the GST-Vav3 SH3-SH2-SH3 domain fusion protein were expressed in E. coli. Soluble GST-Vav3 was added to the MBP-EphA2-amylose column, and bound proteins were eluted and analyzed by SDS-PAGE, followed by silver staining. (B) Western blot of eluted fractions as described in panel A and from control resin with MBP alone. (C) EphA2 and myc-tagged Vav3 or vectors alone were cotransfected into COS7 cells. Cells were stimulated with ephrin-A1 at indicated times, and cell lysates were immunoprecipitated with anti-myc-conjugated resin, followed by Western blot analysis with anti-EphA2 antibodies. Blots were stripped and reprobed for expression of Vav3. (D) A431 cell lysates were added to GST-Vav3 or control GST resin, and bound proteins were eluted and analyzed by Western blot analysis using anti-EphA2 antibodies. (E and F) T7-tagged Vav2 or vector was transfected into COS7 cells and stimulated in the presence or absence of ephrin-A1 for 5 min. Lysates were immunoprecipitated by anti-EphA2 (F) or anti-T7 (E), followed by Western blot analyses with anti-Vav2 (F) or anti-EphA2 (E). IP, immunoprecipitation; IB, immunoblotting.
The observation that Vav3 interacted with the cytoplasmic domain of EphA2 in yeast and in vitro raised the possibility that Vav3 and EphA2 interact in mammalian cells. To test this possibility, we transfected COS7 cells with a myc-tagged Vav3 and immunoprecipitated cell lysates with Myc-conjugated agarose beads. As shown in Fig. 1C, the EphA2 protein was readily detected in anti-Myc immunoprecipitates when the cells were stimulated with ephrin-A1 but not in unstimulated cells. The coimmunoprecipitation of EphA2 with the anti-Myc antibody was dependent on the expression of Vav3 and was undetectable in immunoprecipitates in which a control vector was expressed. In addition, GST-Vav3 but not control GST could also bind to endogenously expressed EphA2 in response to ephrin-A1 ligand stimulation (Fig. 1D). These findings indicate that Vav3 and EphA2 interact when they are coexpressed in mammalian cells and that this interaction is dependent on activation of the EphA2 receptor by ephrin-A1 ligand.
As Vav2 and Vav3 exchange factors are closely related and both are broadly expressed in many tissue types, we investigated whether Vav2 GEF also binds to EphA2 receptor. A T7-tagged Vav2 GEF or control vector was transfected into COS7 cells. Cells were stimulated in the presence or absence of ephrin-A1 ligand. As shown in Fig. 1E, T7-conjugated beads efficiently pulled down phosphorylated EphA2 in Vav2-transfected cells but not in control vector-transfected cells. In the reverse direction, Vav2 was readily detected in anti-EphA2 immunoprecipitates from cells transfected with Vav2 but not in cells harboring control vector (Fig. 1F). Although Vav2 appeared to bind to EphA2 in both stimulated and nonstimulated cells, the intensity of the Vav2 band was stronger in cells stimulated with ephrin-A1 ligand, suggesting that more Vav2 was recruited to the activated EphA2 receptor. Taken together, these results demonstrate that phosphorylated EphA2 receptor can bind to Vav2 or Vav3 GEFs in mammalian cells.
Mapping of interaction domains between EphA2 and Vav3.
To identify the domains within Vav3 that mediate the interaction with the EphA2 receptor, a panel of Vav3 deletion mutant constructs was generated and tested in the yeast two-hybrid system for their interaction with EphA2 (Fig. 2A). Since the original yeast two-hybrid-interacting clones of Vav3 contained both SH2 and SH3 domains, we expressed the C-terminal SH2 or SH3 domain or the N-terminal portion (containing calponin homology, GEF, pleckstrin homology [PH], and C1 domains) of Vav3 and tested which domain(s) was sufficient to maintain the interaction with EphA2 in the yeast two-hybrid assay. Neither the N-terminal portion (containing calponin homology, GEF, PH, and C1 domains) nor the C-terminal SH3 domain of Vav3 was able to bind to EphA2. In contrast, the SH2 domain alone was sufficient to mediate an interaction with EphA2. These data suggest that the SH2 domain of the Vav3 GEF binds to phosphorylated tyrosine residue(s) on the EphA2 receptor.
FIG. 2.
Domain mapping between EphA2 and Vav3. (A) The EphA2 cytoplasmic domain was coexpressed in the yeast two-hybrid assay with wild-type or various deletion mutants of Vav3. NT, not tested. (B) The SH3-SH2-SH3 region of Vav3 was coexpressed in the yeast two-hybrid assay with wild-type or various mutants of the EphA2 cytoplasmic domain. D738N is a kinase-dead mutation. (C) Analysis of the Vav3 and wild-type or mutant EphA2 interaction by coimmunoprecipitation. Vav3 and either wild-type or mutant constructs were transfected into COS7 cells. Cell lysates were immunoprecipitated with anti-myc-conjugated resin, followed by Western blot analysis using anti-EphA2 antibodies. SAM Y/F, Y-to-F mutations in the SAM domain; FF, a double Y-to-F mutation (Y587F/Y593F). EE, a double mutation of Y to E (Y587E/Y593E); juxt, juxtamembrane domain; WT, wild type; CH, calponin homology domain.
We next sought to determine the Vav3 binding site on the EphA2 receptor. To narrow the search, we began with a panel of EphA2 deletion mutants (Fig. 2B). Deletion of the kinase domain or a kinase-dead point mutation (D738N) (21) completely eliminated the ability to bind to Vav3, suggesting that EphA2 kinase activity was essential for recruitment of Vav3. This result, together with the data shown in Fig. 2A, indicated a phosphorylated tyrosine as the binding site for Vav3. To determine whether Vav3 binds to the two tyrosine residues in the juxtamembrane (JM) domain, we examined Vav3 binding to EphA2 mutants carrying a deletion of the JM domain or bearing a double Y-to-F point mutation, Y587F/Y593F (FF). Interestingly, deleting the JM did not affect binding in yeast, but the FF mutation completely abolished binding to Vav3. The failure of Vav3 to interact with the FF mutant could suggest a possible direct interaction with the phosphorylated JM tyrosines; however, the FF mutation also leads to a loss of kinase activity (5, 49, 50). To determine whether Vav3 can interact with the JM tyrosines or if loss of binding results from a loss of kinase activity, we tested an EphA2 mutant (Y587E/Y593E) that retains normal tyrosine kinase activity but cannot be phosphorylated at the JM tyrosines (50). As shown in Fig. 2B and C, the double mutation of Y to E significantly inhibited binding to Vav3, suggesting that Vav3 may directly interact with the phosphorylated tyrosines in the JM.
In addition to the two tyrosines in the JM domain, the other region of EphA2 that appeared to affect Vav3 binding was the sterile alpha motif (SAM) domain. Deletion of the SAM domain significantly reduced binding to Vav3 (Fig. 2B). To test if phosphorylated tyrosines in the SAM can serve as binding sites, we analyzed the ability of Vav3 to bind to three mutations of Y to F (Y921F, Y929F, and Y959F) in the EphA2 SAM (Fig. 2C). As shown in Fig. 2C, a mutation of Y to F in any of these three tyrosine residues moderately inhibited binding to Vav3, suggesting that the SAM domain provides additional binding sites for Vav3.
Regulation of EphA2-dependent Rac1 activation by Vav GEFs.
Having established that Vav2/Vav3 and EphA2 interact in a phosphorylation-dependent manner, we sought to determine whether the activated EphA2 receptor regulated Rho family GTPases via Vav GEFs. As a first step, we examined the ability of EphA receptors to regulate the activities of Vav2 and Vav3 in primary murine embryonic fibroblasts (MEFs). Since MEFs do not express Vav1, they provide a suitable background to analyze Vav2 and Vav3 activities specifically. Wild-type or Vav2−/− Vav3−/− MEFs were plated on ephrin-A1-, IgG-, or fibronectin-coated surfaces. Both wild-type and Vav2−/− Vav3−/− MEFs attached and spread on a fibronectin-coated surface, while none of them spread on an IgG-coated surface after 45 min of plating (data not shown). In contrast, when the wild-type or Vav2−/− Vav3−/− MEFs were plated onto ephrin-A1-coated surfaces, both adhered, but only the wild-type cells exhibited a spreading phenotype (Fig. 3A).
FIG. 3.
Characterization of Vav GEF activity in Vav2−/− Vav3−/− MEFs. Wild-type or Vav2−/− Vav3−/− MEFs were plated on fibronectin-, IgG-, or ephrin-A1-coated surfaces. (A) Wild-type cells were completely attached to an ephrin-A1-coated surface 45 min after plating, while Vav2−/− Vav3−/− cells were still rounded. (B) Cells were stained with fluorescein isothiocyanate-phalloidin to detect F-actin 5 h after plating. Cells with phenotypes of membrane ruffle, microspike, ruffle and microspike, or branched were shown and quantified in panel C. Experiments were repeated three times, and an average of 100 cells were examined each time. *, P < 0.05; **, P < 0.01. (D) Active GTP-bound forms of Rac1 and Cdc42 were analyzed by PAK1 PBD pull-down assay followed by immunoblotting in lysates from wild-type or Vav2−/− Vav3−/− MEFs stimulated with ephrin-A1. Total Rac1 and Cdc42 levels within the lysate prior to PBD pull-down were detected by immunoblotting. WT, wild type; KO, knockout.
We also observed the effects of Vav deficiency on the morphology of fibroblasts to assess the distinct morphological changes elicited by RhoA, Cdc42, and Rac1. Specifically, RhoA induces stress fiber formation, Cdc42 induces filopodia extensions (a microspike phenotype), and Rac1 induces lamellipodia formation and membrane ruffling (25). There was no significant stress fiber formation in either wild-type or Vav2−/− Vav3−/− MEFs at 5 h postplating. Ephrin-A1 induced the formation of membrane ruffles, microspikes, and a combination of both in approximately 70% of wild-type cells. In contrast, ruffles and microspikes were significantly reduced in Vav2−/− Vav3−/− MEFs (Fig. 3B and C). The majority of Vav2−/− Vav3−/− MEFs exhibited a “branched” phenotype in which cells adhered but did not spread. No morphological differences between wild-type and Vav2−/− Vav3−/− MEFs were observed when they were plated on IgG- or fibronectin-coated surfaces (Fig. 3C). Furthermore, ephrin-A1 stimulation activated both Rac1 and Cdc42 GTPases in wild-type MEFs, whereas GTP-bound Rac1 and Cdc42 levels were reduced in ephrin-A1-stimulated Vav2−/− Vav3−/− MEFs (Fig. 3D). Taken together, these data suggest that ephrin-A1 activates Rac1 and Cdc42 GTPases through Vav2 and/or Vav3.
To directly test the activation state of Rac1 GTPase in endothelial cells, we employed the PBD pull-down assay in cells stimulated with ephrin-A1 or Fc control. Vav2 or Vav3 was overexpressed in BPMEC. Cells were stimulated with ephrin-A1, and activated GTP-bound Rac was isolated from lysates by precipitation with PAK1 PBD-GST fusion proteins. As shown in Fig. 4A, consistent with our previous findings (7), ephrin-A1 induced Rac1 activation in mock-transfected cells. Vav2- and Vav3-transfected cells exhibited elevated basal levels of Rac activation, and Rac activity was moderately increased in response to ephrin-A1 stimulation. To determine whether endogenous Vav2 and Vav3 are required for ephrin-A1-induced activation of Rac1 in endothelial cells, we used primary microvascular endothelial cells isolated from either Vav2−/− Vav3−/− mice or wild-type control mice. Endothelial cells isolated from Vav2−/− Vav3−/− mice expressed no detectable Vav2 or Vav3 proteins, but EphA2 expression and phosphorylation were not affected in these mice (Fig. 4B). Ephrin-A1 stimulation induced elevation of Rac1-GTP levels at 2.5 and 5 min in wild-type endothelial cells, but this induction of Rac1 activity was impaired in Vav2−/− Vav3−/− cells (Fig. 4C). Taken together, these results indicate that both Vav2 and Vav3 are activated by EphA receptor forward signaling to promote Rac1 GTPase activity.
FIG. 4.
Regulation of ephrin-A1-induced Rac1 activation by Vav GEFs in endothelial cells. (A) Active GTP-bound Rac1 was analyzed by PAK1 PBD pull-down assay using lysates from ephrin-A1-stimulated BPMEC transfected either with vector, Vav2, or Vav3, expression constructs (top). Total Rac1 levels within the lysate prior to PBD pull-down were detected by immunoblotting. Results were quantified using Scion Image software and expressed as means ± standard deviations of six independent experiments (bottom). (B) Loss of Vav2 and Vav3 expression in Vav2−/− Vav3−/− endothelial cells was confirmed by Western blot analysis (left). EphA2 expression and phosphorylation were not affected in Vav2−/− Vav3−/− cells, as judged by immunoprecipitation and Western blot analysis (right). (C) Active GTP-bound forms of Rac1 were analyzed by PAK1 PBD pull-down assay followed by immunoblotting using lysates from wild-type or Vav2−/− Vav3−/− MPMEC stimulated with ephrin-A1 (top). Total Rac1 within the lysates prior to PBD pull-down was detected by immunoblotting. Results were quantified using Scion Image software and expressed as means ± standard deviations of four independent experiments (bottom). WT, wild type.
Vav family GEFs mediate ephrin-A1-induced endothelial cell migration and assembly.
As ephrin-A/EphA forward signaling induces the activation of Rac1 GTPase via Vav2 and/or Vav3 exchange factors and as EphAs are known to be critical for adult angiogenesis (7), we investigated whether Vav proteins might also be necessary for ephrin-A1-induced angiogenic responses. We first assessed vascular assembly responses in BPMEC transiently transfected with Vav2 or Vav3. As shown in Fig. 5A, consistent with our previous findings (7), ephrin-A1 induced vascular assembly in mock-transfected cells. In Vav2- or Vav3-transfected cells, basal levels of assembly were increased, and endothelial cell assembly into capillary-like structures was significantly increased in response to ephrin-A1 stimulation (Fig. 5A and C). Vav2- or Vav3-transfected cells were incorporated into endothelial cellular networks, as judged by anti-T7 (Vav2) or anti-Myc (Vav3), whereas green fluorescent protein-transfected control cells remained isolated in the dish (Fig. 5B). Furthermore, ephrin-A1-induced endothelial cell migration is significantly increased in BPMEC transfected with either Vav2 or Vav3, compared to mock-transfected cells (Fig. 5D). These data suggest a functional link between Vav family GEFs and ephrin-A1-mediated vascular endothelial cell migration and assembly.
FIG.5.
Overexpression of Vav2 and Vav3 promotes ephrin-A1-induced endothelial cell assembly and migration. (A) BPMEC were transfected with Vav2, Vav3, or vector and assayed for their ability to assemble into capillary-like structures on growth factor-reduced Matrigel in response to ephrin-A1. Phase-contrast view of BPMEC transfected with Vav2, Vav3, or vector 9 h after plating on Matrigel. Lower magnification view shows that an equal number of cells was plated. (B) Immunofluorescence shows that transfected cells are incorporated into cellular networks, as judged by anti-T7 (Vav2) or anti-Myc (Vav3) staining. (C) Quantification of the results shown in panel A. Branch length in assembled endothelial cell networks was measured. Four fields per culture were quantified for each condition and experiments were repeated four times. (D) Migration of BPMEC transfected with Vav2 or Vav3 in response to ephrin-A1 was quantified by transwell assay. The number of endothelial cells that had migrated to the lower surface of the transwell in 5 h was counted. Three fields per transwell were quantified for each condition in triplicate samples, and data are means ± standard deviations of three independent experiments. GFP, green fluorescent protein.
FIG. 5—
Continued.
To assess whether endogenous Vav2 and Vav3 are required for endothelial cell assembly and migration, we performed migration and assembly assays in MPMEC that are deficient in both Vav2 and Vav3 proteins. Cultured wild-type and Vav2−/− Vav3−/− endothelial cells displayed similar morphology and growth rates (data not shown). When stimulated with ephrin-A1 ligand, migration of Vav2−/− Vav3−/− MPMEC was significantly impaired relative to wild-type control cells (Fig. 6A). However, the migration response to serum was not affected in Vav2−/− Vav3−/− MPMEC (Fig. 6A), indicating that the phenotype in these cells was not due to generalized migration defects. When these cells were placed on Matrigel, endothelial cell assembly into capillary-like structures was significantly inhibited in Vav2−/− Vav3−/− MPMEC compared to wild-type control cells (Fig. 6B and C). Taken together, these data indicate that Vav family GEFs are necessary for ephrin-A1-mediated angiogenic responses.
FIG. 6.
Impaired cell migration and assembly in Vav2−/− Vav3−/− endothelial cells. (A) Migration of MPMEC derived from wild-type or Vav2−/− Vav3−/− mice in response to ephrin-A1 or serum was quantified by transwell assay. The number of endothelial cells that had migrated to the lower surface of the transwell in 5 h was counted. Three fields per transwell were quantified for each condition in triplicate samples, and data are means ± standard deviations of three independent experiments. (B) MPMEC isolated from wild-type or Vav2−/− Vav3−/− mice were plated on a thin layer of growth factor-reduced Matrigel in the presence or absence of ephrin-A1 to examine and quantify vascular assembly. After 9 h, the endothelial cells were photographed. (C) Branch length in assembled endothelial cell networks was measured. Four fields per culture were quantified for each condition, and experiments were repeated three times. KO, knockout.
Impaired ephrin-A1-induced angiogenesis in Vav2−/− Vav3−/− mice.
Based on the defects in assembly and migration of Vav2−/− Vav3−/− endothelial cells ex vivo, we wished to determine if Vav2−/− Vav3−/− mice display impaired angiogenesis in vivo. To test this, we implanted surgical sponges impregnated with control IgG or ephrin-A1 subcutaneously into the dorsal flank of Vav2−/− Vav3−/− mice or wild-type controls. After 7 days, we injected the mice intravenously with a TRITC-dextran solution to visualize host blood vessels associated with sponges. In wild-type control mice, we observed a marked increase in surface vessels associated with ephrin-A1-treated sponges relative to control IgG sponges (Fig. 7A). In contrast, Vav2−/− Vav3−/− mice showed a significant reduction in vascular density in response to ephrin-A1-treated sponges (Fig. 7A and B). Though host vessels were observed in the skin surrounding IgG-treated sponges, no infiltration of these vessels into the control sponges was detected (data not shown). These data suggest that Vav2/3 GEFs are required for ephrin-A1-mediated angiogenic remodeling in vivo.
FIG. 7.
Vav2/3-deficiency impairs ephrin-A1-induced angiogenesis in vivo. (A) Sponges impregnated with ephrin-A1 or IgG were subcutaneously implanted into the dorsal flank of Vav2−/− Vav3−/− mice or wild-type controls. After 7 days, mice were injected intravenously with TRITC-dextran to visualize host blood vessels associated with sponges. Fewer surface vessels were associated with ephrin-A1-treated sponges in −/− animals relative to +/+ controls. Scale bar, 5 mm. Arrowheads indicate surface blood vessels covering sponges. (B) Density of blood vessels within the sponges was quantified by fluorescence intensity of rhodamine-dextran using Scion Image software. The density of fluorescent pixels within each field (magnification, ×10) was determined and compared in Vav2−/− Vav3−/− mice or wild-type controls. Data are a representation of results from five independent sponges in each genotype. Statistical significance was determined by a two-tailed, paired Student's t test.
DISCUSSION
Vav GEFs link Eph receptors to Rho family GTPase activation.
Investigation of ephrin/Eph receptor-mediated signal transduction mechanisms that regulate cellular responses in various cell types has been centered on Rho-family GTPases. We have recently shown that EphA2 receptor-dependent endothelial cell migration and assembly require the activation of Rac1 GTPase (7). However, the mechanisms responsible for EphA2 receptor-mediated Rac1 GTPase activation remain undefined. In this study, we show that when ephrins bind to Ephs, a Rho family GEF, Vav2 or Vav3, is recruited to the EphA2 receptor, leading to increased Rac1 activity in both primary endothelial cells and embryonic fibroblasts. In addition, we observed that ephrin-A1-induced activation of Rac1 is impaired in Vav2−/− Vav3−/− endothelial cells and MEFs, implicating Vav proteins as critical Rho family GEFs downstream of Eph receptors.
Our findings indicate that Vav2 and/or Vav3 are required for ephrin-A1-induced endothelial cell migration/assembly and Rac1 activation. It remains unclear whether ephrin-A1 stimulation of Vav proteins leads to a direct activation of Rac1 or whether ephrin-A1-induced Rac1 activation occurs indirectly via the Vav-dependent activation of another GEF pathway. Although Vav2/3 can function directly as exchange factors (1, 32, 42), it is interesting that Vav proteins contain multiple SH2/SH3 adaptor motifs that may mediate the recruitment of other Rac- or cdc42-specific GEFs to the activated Eph receptor, as observed in the immune system (W. Swat et al., unpublished observation). In the future, it will be important to determine whether Vav proteins serve to directly activate Rac1 or whether Vav proteins are recruited to the activated Eph receptor to serve an adaptor function.
Regulation of Vav GEF activation.
A wealth of evidence has demonstrated that phosphorylation of highly conserved tyrosine residues in the acidic domain of Vav proteins regulates the GDP/GTP exchange activity (45). In the absence of tyrosine phosphorylation, the Vav acidic domain binds to the catalytic Dbl homology domain, blocking access to Rho family GTPases. This inhibition is released upon phosphorylation of these tyrosine residues, resulting in activation of GDP/GTP exchange activity. We observed constitutive phosphorylation of Vav2 and Vav3 when they were overexpressed in COS7 cells (data not shown). However, we were unable to detect phosphorylation of either endogenous Vav2 or Vav3 in primary endothelial cells. The failure to detect tyrosine phosphorylation could be due to the relatively low abundance of Vav proteins in these cells or to a low stoichiometry of total Vav protein phosphorylation. Alternatively, Vav proteins may not be significantly phosphorylated upon ephrin-A1 stimulation. Aoki et al. observed that although Vav2/3 phosphorylation increased upon epidermal growth factor stimulation of PC12 cells, Vav phosphorylation was not increased in response to nerve growth factor, even though depletion of Vav2/3 significantly inhibited NGF-induced Rac1 activation and neurite outgrowth (3).
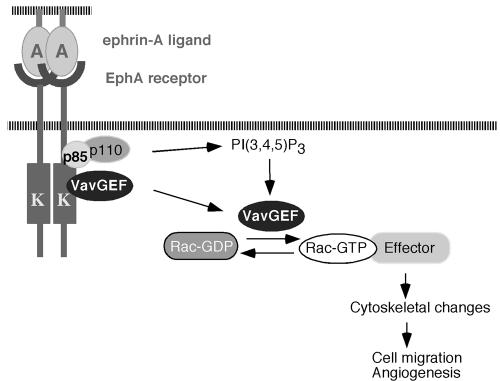
Aside from phosphorylation-dependent activation, Vav proteins may also be activated through their PH domains via a PI3K-dependent mechanism. Vav1 was shown to be activated in vitro by PtdIns(3,4,5)P3 (PIP3) (26) and the unphosphorylated Vav1 Dbl homology-PH domains can bind to Rac1 in the presence of PIP3 (16). More recently, both Vav2 and Vav3 have been shown to be activated by PI3K-dependent translocation to the membrane through their PH domains (3). We have previously shown that ephrin-A1-induced Rac1 GTPase activation is dependent on PI3K activity (7). In our yeast two-hybrid screen, we identified p85 as an EphA2-interacting protein (data not shown), consistent with observations described by Pandey et al. (36). In addition, Vav3 and p85 have been shown to interact upon ligand stimulation both in yeast two-hybrid assays (data not shown) and in mammalian cells (48). Thus, it is possible that ephrin-A1 could activate Vav GEFs through at least two mechanisms. Activated Eph receptors could directly recruit Vav GEFs through the SH2 domain, allowing subsequent phosphorylation and activation of Vav proteins either directly or indirectly. In addition, through the recruitment of p85, Eph receptors could also upregulate PIP3 levels and enhance Vav GEF activity through the PH domain (Fig. 8).
FIG. 8.
A model for how Vav GEFs may mediate ephrin-induced angiogenesis. Upon binding to ephrins, EphA2 receptor is tyrosine phosphorylated. Activated EphA2 receptor recruits Vav GEFs through the SH2 domains, resulting in subsequent activation of Vav proteins, possibly through tyrosine phosphorylation of their acidic domains. In addition, through the recruitment of the p85 subunit of PI3K, EphA2 receptor up-regulates phospholipid PIP3 levels, which in turn recruit and activate Vav GEFs through their PH domains. Activated Vav GEFs subsequently increase Rac1-GTP levels and promote endothelial cell migration and angiogenesis.
Role of Vav GEFs in cell spreading and cytoskeletal reorganization.
Ephrin-A1 and EphA2 receptor have been shown to play a critical role in the spreading and reorganization of the cytoskeleton in both NIH 3T3 cells and primary MEFs (12). Ephrin-A1-induced actin reorganization requires focal adhesion kinase (FAK) and p130Cas (Crk-associated substrate), as FAK−/− or p130Cas−/− MEFs fail to spread on an ephrin-A1-coated surface (12). Although the mechanism by which EphA signals to FAK and p130Cas remains unclear, it likely involves Rho family small GTPases, which are the principle regulators of F-actin assembly in most cell types (33, 40). Since Vav2 and/or Vav3 are involved in the ephrin-A-induced spreading process as well, it will be important to understand the relationship between Vav2/Vav3, FAK, and p130Cas. Vav proteins may function as upstream activators, which is the case in NIH 3T3 cells where Vav3 expression induces the phosphorylation of FAK (41). However, it is also possible that Vav activation of Rac1 may function in a parallel signaling pathway independent of FAK and p130Cas and act downstream of EphA receptors to coordinately regulate actin cytoskeletal dynamics, as is the case with Vav1 in Syk nonreceptor tyrosine kinase-mediated lamellipodia formation and cell spreading (30).
Vav GEFs are required for angiogenesis.
The function of Vav proteins has been best characterized downstream of immune response receptors. Targeted disruption of Vav1 leads to severe defects in T lymphocyte functions (11) and impairs FcR-induced degranulation and cytokine production in mast cells (29). There are distinct defects in T- and B-cell function in Vav1−/− Vav2−/− and Vav1−/− Vav3−/− mice, with loss of Vav2 enhancing the B cell phenotype and loss of Vav3 enhancing the T-cell phenotype (19, 22, 43). Deletion of all Vav family members prevents production of all mature T and B cells (22). More recently, Vav family proteins were also shown to be activated downstream of several growth factor receptors in nonimmune cells (31, 48). Cowan et al. reported that Vav family GEFs play a critical role in ephrin/Eph receptor-mediated axon guidance in retinal ganglion cells during development (10). In this study, we provide several lines of evidence that Vav GEFs are required for ephrin-A1-induced angiogenesis. First, ephrin-A1 stimulation increased levels of GTP-bound active Rac1 proteins in wild-type endothelial cells, and this increase was absent in Vav2−/− Vav3−/− endothelial cells. Second, overexpression of either Vav2 or Vav3 in primary bovine endothelial cells led to enhanced cell migration and assembly in response to ephrin-A1. Conversely, loss of Vav2 and Vav3 in murine primary endothelial cells resulted in inhibition of ephrin-A1-induced endothelial cell migration and assembly. Finally, ephrin-A1-induced angiogenesis in vivo is significantly impaired in Vav2−/− Vav3−/− mice.
In addition to the role of EphA2 receptor in angiogenesis, class B Eph receptors and ephrin-Bs are involved in vascular remodeling in both embryogenesis (2, 24, 46) and cancer (20). We did not detect significant interaction between the EphB1 receptor and Vav3 GEF in our yeast two-hybrid screen (data not shown). It is also unlikely that Vav2 and Vav3 proteins mediate signaling downstream of the EphB4 receptor, as Vav2−/− Vav3−/− mice are viable, whereas EphB4-deficient mice are embryonic lethal due to impaired vascular remodeling.
While our data suggest that defects in Vav2−/− Vav3−/− cells may be due to disruption of Vav-dependent Eph signaling, it is important to note that Vav proteins are activated downstream of several growth factor receptors, some of which have been shown to cross-talk with Eph receptors (38). Defective signaling downstream of one or several of these various receptors could contribute to the disruption of cell migration and assembly. With this in mind, we considered the possibility that the defects in Vav2−/− Vav3−/− cell spreading, migration, and Rac1 activation in response to ephrin-A1 stimulation could be due to an ephrin-A-independent role for Vav proteins in actin cytoskeletal remodeling. However, we find that the Vav2−/− Vav3−/− cells effectively spread on fibronectin-coated surfaces (Fig. 3C) and migrate toward serum in a transwell assay (Fig. 6A), suggesting that the failure of ephrin-A1 to induce migration and assembly in these cells is more specific to defects in ephrin-A-induced actin cytoskeletal remodeling. We also considered the possibility that the defects in Vav2−/− Vav3−/− cells could be due to changes in Eph receptor expression or activation. However, we observed no obvious differences between wild-type and Vav2−/− Vav3−/− cells in either the EphA2 expression levels or phosphorylation status (Fig. 4B). Taken together, our findings support the possibility that Vav2/Vav3 GEFs are required for regulating EphA receptor-mediated angiogenic responses.
Although distinct functions have been attributed to different Vav family members in certain cell types (11), our results suggest that Vav2 and Vav3 may play overlapping roles in regulating endothelial cell function downstream of Eph receptors, as overexpression of either Vav2 or Vav3 enhances angiogenic responses in BPMEC. The contribution of Vav1 in Eph-mediated angiogenic responses is, however, presently unclear. Although initially identified as a hematopoietic cell-specific exchange factor, Vav1 can be detected in endothelial cells (W. Swat, personal communication), suggesting that it may contribute to ephrin-A-induced cellular responses. Regardless, our findings indicate that Vav1 is not sufficient to compensate for the loss of Vav2 and Vav3 in the Vav2−/− Vav3−/− MPMEC, establishing the critical role of Vav2/Vav3 in ephrin-A1-induced cell migration and assembly.
In summary, we find that Vav family GEFs are required for ephrin-A1-induced migration and assembly of endothelial cells, a process that is dependent on Rac1 activation. These findings suggest an important role for Vav proteins as regulators of angiogenesis in vivo. It will be important to assess the role of Vav proteins in both normal angiogenesis and in tumor neovascularization where EphA2 receptors are known to function (7). In the future, the analysis of the Vav2−/− Vav3−/− mutant mice should provide an excellent model system in which to investigate the function of Vav GEFs in tumor angiogenesis.
Acknowledgments
We thank Takamune Takahashi, Nobuo Tsuboi, and Shreevrat Goenka for providing the human placenta cDNA library and for advice on the yeast two-hybrid screen, as well as Donna Hicks, Paul Holcomb, and Yoonha Hwang for their expert administrative and technical assistance. We also thank Michael Greenberg for sharing reagents and data prior to publication.
This work was supported by National Institutes of Health grants CA95004 and CA114301 to J.C., NIH postdoctoral fellowship GM072461 to S.H., and Department of Defense postdoctoral fellowship DAMD17-03-1-0379 to D.B.-S. This work was also supported by core facilities grant 2P30CA68485 to the Vanderbilt-Ingram Cancer Center.
REFERENCES
- 1.Abe, K., K. L. Rossman, B. Liu, K. D. Ritola, D. Chiang, S. L. Campbell, K. Burridge, and C. J. Der. 2000. Vav2 is an activator of Cdc42, Rac1, and RhoA. J. Biol. Chem. 275:10141-10149. [DOI] [PubMed] [Google Scholar]
- 2.Adams, R. H., G. A. Wilkinson, C. Weiss, F. Diella, N. W. Gale, U. Deutsch, W. Risau, and R. Klein. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 3:295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, K., T. Nakamura, K. Fujikawa, and M. Matsuda. 2005. Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells. Mol. Biol. Cell 16:2207-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergers, G., and L. E. Benjamin. 2003. Tumorigenesis and the angiogenic switch. Nat Rev. Cancer 3:401-410. [DOI] [PubMed] [Google Scholar]
- 5.Binns, K. L., P. P. Taylor, F. Sicheri, T. Pawson, and S. J. Holland. 2000. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol. Cell. Biol. 20:4791-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brantley, D. M., N. Cheng, E. J. Thompson, Q. Lin, R. A. Brekken, P. E. Thorpe, R. S. Muraoka, D. P. Cerretti, A. Pozzi, D. Jackson, C. Lin, and J. Chen. 2002. Soluble EphA receptors inhibit tumor angiogenesis and progression in vivo. Oncogene 21:7011-7026. [DOI] [PubMed] [Google Scholar]
- 7.Brantley-Sieders, D., J. Caughron, D. Hicks, A. Pozzi, J. C. Ruiz, and J. Chen. 2004. EphA2 receptor tyrosine kinase regulates endothelial cell migration and assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J. Cell Sci. 117:2037-2049. [DOI] [PubMed] [Google Scholar]
- 8.Brantley-Sieders, D., and J. Chen. 2004. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis 7:17-28. [DOI] [PubMed] [Google Scholar]
- 9.Brantley-Sieders, D., S. Schmidt, M. Parker, and J. Chen. 2004. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr. Pharm. Des. 10:3431-3442. [DOI] [PubMed] [Google Scholar]
- 10.Brantley-Sieders, D. M., W. B. Fang, D. Hicks, T. Koyama, Y. Shyr, and J. Chen. 2005. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 19:1884-1886. [DOI] [PubMed] [Google Scholar]
- 11.Bustelo, X. R. 2000. Regulatory and signaling properties of the Vav family. Mol. Cell. Biol. 20:1461-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter, N., T. Nakamoto, H. Hirai, and T. Hunter. 2002. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat. Cell Biol. 4:565-573. [DOI] [PubMed] [Google Scholar]
- 13.Cheng, N., D. Brantley, H. Liu, W. Fanslow, D. P. Cerretti, A. D. Reith, D. Jackson, and J. Chen. 2003. Inhibition of VEGF-dependent multi-stage carcinogenesis by soluble EphA receptors. Neoplasia 5:445-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan, C. W., Y. R. Shao, M. Sahin, S. M. Shamah, M. Z. Lin, P. L. Greer, S. Gao, E. C. Griffith, J. S. Brugge, and M. E. Greenberg. 2005. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron 46:205-217. [DOI] [PubMed] [Google Scholar]
- 15.Daniel, T. O., E. Stein, D. P. Cerretti, P. L. St. John, B. Robert, and D. R. Abrahamson. 1996. ELK and LERK-2 in developing kidney and microvascular endothelial assembly. Kidney Int. Suppl. 57:S73-S81. [PubMed] [Google Scholar]
- 16.Das, B., X. Shu, G. J. Day, J. Han, U. M. Krishna, J. R. Falck, and D. Broek. 2000. Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J. Biol. Chem. 275:15074-15081. [DOI] [PubMed] [Google Scholar]
- 17.Deroanne, C., V. Vouret-Craviari, B. Wang, and J. Pouyssehur. 2003. EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 116:1367-1376. [DOI] [PubMed] [Google Scholar]
- 18.Dobrzanski, P., K. Hunter, S. Jones-Bonlin, H. Chang, C. Robinson, S. Pritchard, H. Zhao, and B. Ruggeri. 2004. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 64:910-919. [DOI] [PubMed] [Google Scholar]
- 19.Doody, G. M., S. E. Bell, E. Vigorito, E. Clayton, S. McAdam, R. Tooze, C. Fernandez, I. J. Lee, and M. Turner. 2001. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2:542-547. [DOI] [PubMed] [Google Scholar]
- 20.Erber, R., U. Eichelsbacher, V. Powajbo, T. Korn, V. Djonov, J. Lin, H. P. Hammes, R. Grobholz, A. Ullrich, and P. Vajkoczy. 2006. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 25:628-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang, W. B., D. M. Brantley-Sieders, M. A. Parker, A. D. Reith, and J. Chen. 2005. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene 24:7859-7868. [DOI] [PubMed] [Google Scholar]
- 22.Fujikawa, K., A. V. Miletic, F. W. Alt, R. Faccio, T. Brown, J. Hoog, J. Fredericks, S. Nishi, S. Mildiner, S. L. Moores, J. Brugge, F. S. Rosen, and W. Swat. 2003. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J. Exp. Med. 198:1595-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale, N. W., S. J. Holland, D. M. Valenzuela, A. Flenniken, L. Pan, T. E. Ryan, M. Henkemeyer, K. Strebhardt, H. Hirai, and D. G. Wilkinson. 1996. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 17:9-19. [DOI] [PubMed] [Google Scholar]
- 24.Gerety, S. S., H. U. Wang, Z. F. Chen, and D. J. Anderson. 1999. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell 4:403-414. [DOI] [PubMed] [Google Scholar]
- 25.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-513. [DOI] [PubMed] [Google Scholar]
- 26.Han, J., K. Luby-Phelps, B. Das, X. Shu, Y. Xia, R. D. Mosteller, U. M. Krishna, J. R. Falck, M. A. White, and D. Broek. 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279:558-560. [DOI] [PubMed] [Google Scholar]
- 27.Hornstein, I., A. Alcover, and S. Katzav. 2004. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal. 16:1-11. [DOI] [PubMed] [Google Scholar]
- 28.Kullander, K., and R. Klein. 2002. Mechanisms and functions of Eph and ephrin signaling. Nat. Rev. Mol. Cell. Biol. 3:475. [DOI] [PubMed] [Google Scholar]
- 29.Manetz, T. S., C. Gonzalez-Espinosa, R. Arudchandran, S. Xirasagar, V. Tybulewicz, and J. Rivera. 2001. Vav1 regulates phospholipase Cγ activation and calcium responses in mast cells. Mol. Cell. Biol. 21:3763-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranti, C. K., L. Leng, P. Maschberger, J. S. Brugge, and S. J. Shattil. 1998. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr. Biol. 8:1289-1299. [DOI] [PubMed] [Google Scholar]
- 31.Moores, S. L., L. M. Selfors, J. Fredericks, T. Breit, K. Fujikawa, F. W. Alt, J. S. Brugge, and W. Swat. 2000. Vav family proteins couple to diverse cell surface receptors. Mol. Cell. Biol. 20:6364-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Movilla, N., and X. R. Bustelo. 1999. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell. Biol. 19:7870-7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noren, N. K., and E. B. Pasquale. 2004. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 16:655-666. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa, K., R. Pasqualini, R. A. Lindberg, R. Kain, A. L. Freeman, and E. B. Pasquale. 2000. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene 19:6043-6052. [DOI] [PubMed] [Google Scholar]
- 35.Ogita, H., S. Kunimoto, Y. Kamioka, H. Sawa, M. Masuda, and N. Mochizuki. 2003. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ. Res. 93:23-31. [DOI] [PubMed] [Google Scholar]
- 36.Pandey, A., D. F. Lazar, A. R. Saltiel, and V. M. Dixit. 1994. Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J. Biol. Chem. 269:30154-30157. [PubMed] [Google Scholar]
- 37.Pandey, A., H. Shao, R. M. Marks, P. J. Polverini, and V. M. Dixit. 1995. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-α-induced angiogenesis. Science 268:567-569. [DOI] [PubMed] [Google Scholar]
- 38.Pasquale, E. B. 2005. Developmental Cell Biology: Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell. Biol. 6:462-475. [DOI] [PubMed] [Google Scholar]
- 39.Pozzi, A., P. E. Moberg, L. A. Miles, S. Wagner, P. Soloway, and H. A. Gardner. 2000. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. USA 97:2202-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridley, A. J. 2001. Rho GTPases and cell migration. J. Cell Sci. 114:2713-2722. [DOI] [PubMed] [Google Scholar]
- 41.Sachdev, P., L. Zeng, and L. H. Wang. 2002. Distinct role of phosphatidylinositol 3-kinase and Rho family GTPases in Vav3-induced cell transformation, cell motility, and morphological changes. J. Biol. Chem. 277:17638-17648. [DOI] [PubMed] [Google Scholar]
- 42.Schuebel, K. E., N. Movilla, J. L. Rosa, and X. R. Bustelo. 1998. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 17:6608-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedford, K., L. Nitschke, I. Girkontaite, A. Charlesworth, G. Chan, V. Sakk, M. Barbacid, and K. D. Fischer. 2001. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2:548-555. [DOI] [PubMed] [Google Scholar]
- 44.Trenkle, T., M. McClelland, K. Adlkofer, and J. Welsh. 2000. Major transcript variants of VAV3, a new member of the VAV family of guanine nucleotide exchange factors. Gene 245:139-149. [DOI] [PubMed] [Google Scholar]
- 45.Turner, M., and D. D. Billadeau. 2002. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2:476-486. [DOI] [PubMed] [Google Scholar]
- 46.Wang, H. U., Z. F. Chen, and D. J. Anderson. 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93:741-753. [DOI] [PubMed] [Google Scholar]
- 47.Yancopoulos, G. D., S. Davis, N. W. Gale, J. S. Rudge, S. J. Wiegand, and J. Holash. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242-248. [DOI] [PubMed] [Google Scholar]
- 48.Zeng, L., P. Sachdev, L. Yan, J. L. Chan, T. Trenkle, M. McClelland, J. Welsh, and L. H. Wang. 2000. Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol. Biol. Cell 20:9212-9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zisch, A. H., M. S. Kalo, L. D. Chong, and E. B. Pasquale. 1998. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene 16:2657-2670. [DOI] [PubMed] [Google Scholar]
- 50.Zisch, A. H., C. Pazzagli, A. L. Freeman, M. Schneller, M. Hadman, J. W. Smith, E. Ruoslahti, and E. B. Pasquale. 2000. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene 19:177-187. [DOI] [PubMed] [Google Scholar]