Abstract
To investigate the control of the γ-globin gene during development, we produced transgenic mice in which sequences of the β-gene promoter were replaced by equivalent sequences of the γ-gene promoter in the context of a human β-globin locus yeast artificial chromosome (βYAC) and analyzed the effects on globin gene expression during development. Replacement of 1,077 nucleotides (nt) of the β-gene promoter by 1,359 nt of the γ promoter resulted in striking inhibition of the γ-promoter/β-gene expression in the adult stage of development, providing direct evidence that the expression of the γ gene in the adult is mainly controlled by autonomous silencing. Measurements of the expression of the γ promoter/β-globin gene as well as the wild γ genes showed that gene competition is also involved in the control of γ-gene expression in the fetal stage of development. We conclude that autonomous silencing is the main mechanism controlling γ-gene expression in the adult, while autonomous silencing as well as competition between γ and β genes contributes to the control of γ to β switching during fetal development.
The β-globin locus spans at least 100 kb on chromosome 11 and consists of a distal enhancer, called the locus control region (LCR), and five functional genes, ɛ, Gγ, Aγ, δ, and β, organized in the order of their developmental expression (reviewed in reference 23). The ɛ-globin gene is the first gene expressed during ontogeny, and its expression is restricted to the blood islands of the yolk sac. At approximately 6 to 8 weeks of gestation, the ɛ-globin gene is silenced and the γ-globin genes are activated. Around birth, β- and δ-globin gene activity dramatically increases and the γ-globin gene expression progressively declines to about 0.5% of the total globin expression. These developmental changes in human globin gene activity are recapitulated in transgenic mice carrying human β-locus yeast artificial chromosome (βYAC) constructs, except for the fact that in this murine model the human γ-globin genes display both embryonic and fetal expression (12, 18, 20, 24).
Currently, the silencing of the γ gene is considered to be autonomous and independent of the presence of other globin genes in cis (4). The molecular mechanism of autonomous silencing is unclear, but it is likely that it is complex. Early transgenic studies have shown that a DNA fragment containing the entire γ-globin gene, but no sequences of the LCR, expresses the γ gene only in the embryonic erythroid cells (2, 13). Although the γ gene was not expressed in the fetal erythroid cells where it is normally expressed, these results have been considered evidence that the γ gene itself contains the information required for silencing in definitive erythropoiesis. The level of γ-gene transcription in these transgenic mice was extremely low (2, 13). When enhanced by a truncated LCR (designated μLCR), the γ gene is expressed in the embryonic but also the fetal and adult stages (7). The γ gene is partially silenced in adult transgenic mice carrying one or two copies of a cosmid construct containing the entire LCR and the γ gene but not in transgenic mice carrying multiple copies of that construct (4). The gene is completely silenced in human β-locus YAC in adult transgenic mice (18). Sequences involved in activation as well as in γ gene silencing are located in the γ-gene promoter. The minimal promoter of the galago γ gene, which is expressed exclusively in embryonic erythroid cells, suppresses the expression of the human γ gene in the definitive erythropoiesis of transgenic mice (27). The elements responsible for this silencing activity have been localized in the CACCC and CCAAT boxes of the galago γ-gene promoter (14). However, mutations of the human γ-gene promoter have shown that each of these boxes also is indispensable for γ-gene activation in the adult (5, 9, 22). In addition to the globin gene promoters, the LCR has a role in developmental regulation of the globin genes, and individual DNase I-hypersensitive sites of this regulatory element appear to have gene specificity (11, 15). The hypersensitive site 3 (HS3) of the LCR preferentially enhances the expression of the γ gene, and deletion of the core sequences of the HS3 abolishes γ-gene expression in the fetal stage of erythropoiesis (16).
To investigate the control of γ-globin gene during development, we replaced the β-gene promoter with its γ-gene counterpart, in the context of β-globin locus YAC constructs, and analyzed the effects on globin gene expression during development of transgenic mice. We obtained evidence that the silencing of the γ gene in the adult stage of development is controlled through sequences residing in the γ-gene promoter. Measurements of the expression of the hybrid γ promoter/β gene as well as of the expression of the wild γ genes located in the same YAC provided evidence that gene competition is also involved in the control of γ-gene expression during the fetal stage of development. We conclude that autonomous silencing is the main mechanism that controls γ-gene expression in the adult, and autonomous silencing as well as competition between the γ- and the β-globin genes controls the γ to β switch during fetal development.
MATERIALS AND METHODS
βYAC constructs.
A yeast interacting plasmid (YIP) containing a BglII/RcaI fragment (coordinates 60577 to 64029; GenBank humhbb accession number U01317) with 1,080 bp of the β-globin gene promoter (coordinates 61110 to 62189) replaced with 1,404 bp of the γ-globin gene promoter (coordinates 38066 to 39469) was made by standard molecular cloning techniques. After linearizing with PmlI, the plasmid was transformed into the yeast containing 155 kb of βYAC using the Clontech yeast transformation kit following the manufacturer's protocol. The transformants were selected on uracil dropout media and PCRs were performed to select the correct clones, which were confirmed by Southern hybridization analysis. Spontaneous excision of the YIP was induced by overnight growth in nonselective rich medium (yeast peptone dextrose). The yeast cells were plated on the 5-fluoroorotic acid plate to select for loss of a Ura3 gene residing on the YIP vector, which results in 5-fluoroorotic acid resistance. The replacement of 1,080 bp of the β-gene promoter with the 1,359-bp γ-gene promoter was confirmed by Southern hybridization. This YAC was designated 1.36kb γpr-βYAC.
To delete the Gγ and Aγ genes from 1.36kb γpr-βYAC, a loxP-ura3-loxP deletion cassette was prepared. A 348-bp fragment 5′ to the Gγ gene (coordinates 33170 to 33517) was inserted upstream of the first loxP site, and a 861-bp fragment 3′ to the Aγ gene (coordinates 41308 to 42168) was inserted downstream of the second loxP site. The construct sequence was released from the plasmid backbone with restriction enzymes and transformed into the yeast containing 1.36kb γpr-βYAC as described above. The transformants were selected on the dropout media lacking uracil, and PCR was performed to check the replacement of the 7.8 kb of the γ genes by the loxP-Ura3-loxP cassette. The positive clones were then transformed with a cytomegalovirus-Cre plasmid. The transformants were plated on 5-fluoroototic acid plates and further selected on dropout media lacking lysine and tryptophan. PCR was performed to screen the correct clones in which excision had happened between the loxP sites. The deletion was further confirmed by sequencing. This YAC was designated (1.36kb γpr-β)(ΔGγAγ) YAC.
Transgenic mice.
YACs were separated from the yeast chromosomes by pulsed-field gel electrophoresis. The agarose gel block containing the YAC was cut into small pieces, equilibrated with the β-agarase buffer, and digested with β-agarase (New England BioLabs). Undigested agarose was removed by centrifugation. Purified DNA fragments were injected into fertilized mouse eggs (B6/C3F1) and then transferred to pseudopregnant foster mothers (B6/D2F1). Founder animals were identified by slot blotting with an HS3 probe. F1 progeny were obtained by breeding founder animals with nontransgenic mice (B6/D2F1) and were used for structural analysis. To study the developmental pattern of human globin gene expression, staged pregnancies were interrupted at day 12, 14, or 16, and samples from blood, yolk sac, and fetal liver were collected.
Structural analysis of YAC transgenes.
Intactness of YAC transgenes in transgenic mice was examined as previously described (16). Briefly, agarose plugs prepared from mouse liver cell suspensions were digested overnight with SfiI, and the DNA was fractionated by pulsed-field gel electrophoresis. The gel was capillary blotted overnight to nylon membranes. Individual lanes were cut out, and each one was hybridized overnight to a different probe. After washing, the membrane was reassembled and an autoradiograph or a phosphorimage was made.
Copy number measurement.
DNA from fetal brain or carcass of F2 progeny in each line was isolated by standard procedures. At least three DNA samples were obtained from each line. Individual samples were then restricted with HindIII. Ten micrograms of DNA from each enzyme reaction performed on samples from a given line was loaded onto a 0.8% agarose gel, and DNA fragments were resolved by electrophoresis. Southern blots were consecutively hybridized with four probes encompassing the HS3 or ɛ-, γ-, or β-globin gene sequence. Signals were quantitated with a PhosphorImager. Copy numbers were determined by comparing the signal from a given transgenic line with those of human genomic DNA. In cases in which the computed value was not an integer, the copy number was rounded to the nearest integer in standard fashion.
RNA analysis.
Total RNA was prepared from the yolk sacs, embryonic and fetal blood, fetal liver, and adult blood. RNA samples were separately isolated from three or more transgenic individuals for each time point. The human ɛ-, γ-, and β-globin mRNAs and murine α- and ζ-globin mRNAs were detected by RNase protection assay and quantified by a PhosphorImager. To minimize experimental error, samples from individual animals were quantified independently and multiple measurements were performed by RNase protection. Copy number-corrected globin mRNA levels were expressed as human γ mRNA/γ copy number/[(murine ζ-globin mRNA/2) + (murine α-globin mRNA/4)]. Mean values and standard deviations are shown in Tables 1 to 5. For t test calculations, the mean values of expression calculated from multiple measurements of multiple individuals were used for each transgenic line.
TABLE 1.
ɛ-Globin gene expression in 1.36kb γpr-βYAC transgenic micea
| Line | Copy no. | Gene expressionb (mean ± SD) in:
|
||||
|---|---|---|---|---|---|---|
| Day 12 blood | Day 12 YS | Day 12 FL | Day 14 blood | Day 14 FL | ||
| A | 4 | 20.0 ± 4.6 | 23.5 ± 3.7 | 1.0 ± 0.3 | 1.6 ± 0.6 | 0.1 ± 0.0 |
| B | 2 | 30.7 ± 3.3 | 31.6 ± 4.7 | 7.8 ± 0.8 | 5.7 ± 2.2 | 1.6 ± 0.6 |
| C | 2 | 18.5 ± 2.5 | 18.5 ± 4.5 | 2.1 ± 0.0 | 1.7 ± 0.5 | 0.4 ± 0.0 |
| Avg | 23.0 ± 6.7 | 24.5 ± 6.6 | 3.6 ± 3.6 | 3.0 ± 2.4 | 0.7 ± 0.8 | |
| Αmt of ɛ gene expression in wt YAC | 15.3 ± 1.7 | 16.5 ± 2.2 | 3.3 ± 1.4 | 2.6 ± 0.7 | 1.7 ± 0.3 | |
YS, yolk sac; FL, fetal liver; wt, wild type.
Percentage of murine α-like globin genes per copy.
TABLE 5.
Expression of 1.36kb γpr-β gene in (1.36kb γpr-β)(ΔGγAγ) YAC transgenic micea
| Line | Copy no. | Gene expressionb (mean ± SD) in:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 12 blood | Day 12 YS | Day 12 FL | Day 14 blood | Day 14 FL | Day 16 blood | Day 16 FL | Ad blood | ||
| A | 2 | 20.8 ± 2.7 | 29.1 ± 5.2 | 39.3 ± 0.2 | 33.2 ± 2.7 | 21.7 ± 8.3 | 24.7 ± 3.9 | 24.2 ± 4.3 | 4.3 ± 0.5 |
| B | 1 | 16.4 ± 2.9 | 29.2 ± 9.3 | 12.6 ± 8.1 | 12.6 ± 1.8 | 9.5 ± 5.0 | 1.2 ± 0.9 | ||
| C | 2 | 23.4 ± 1.7 | 35.4 ± 2.6 | 20.6 ± 3.3 | 14.4 ± 0.9 | 12.4 ± 4.4 | 8.9 ± 0.8 | 12.7 ± 0.8 | 0.9 ± 0.0 |
| D | 1 | 33.3 ± 3.7 | 37.6 ± 8.2 | 55.5 ± 26.9 | 32.2 ± 6.2 | 23.5 ± 8.1 | 32.1 | 26.6 | 2.7 ± 0.8 |
| E | 2 | 28.4 ± 3.4 | 52.4 ± 13.8 | 55.2 ± 0.8 | 35.6 ± 4.5 | 37.9 ± 12.2 | 37.1 ± 2.8 | 8.9 ± 1.1 | |
| Avg | 24.5 ± 6.6 | 36.8 ± 9.5 | 36.7 ± 19.7 | 25.6 ± 11.2 | 21.0 ± 11.2 | 25.7 ± 12.3 | 21.2 ± 7.4 | 3.6 ± 3.3 | |
| Αmt of γ gene expression in wt YAC | 29.4 ± 2.0 | 35.8 ± 10.3 | 13.2 ± 2.9 | 5.6 ± 1.3 | 2.8 ± 0.4 | 1.9 ± 0.5 | 1.2 ± 0.3 | <0.1 | |
YS, yolk sac; FL, fetal liver; wt, wild type; Ad, adult.
Percentage of murine α-like globin genes per copy.
RESULTS
γ-Gene promoter sequences control γ-globin gene silencing in the adult stage of development.
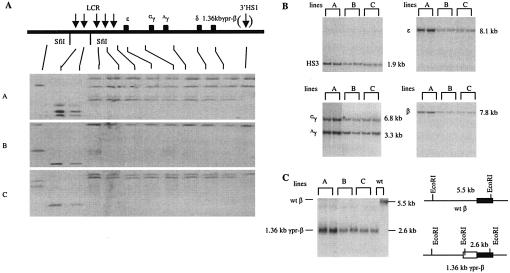
To investigate the role of the γ-globin gene promoter on γ-gene silencing, we replaced the 1,077-bp β-gene promoter sequence 5′ to the translation initiation codon with the corresponding 1,359-bp sequence (subsequently referred to as 1.36kb) of the Aγ promoter in the context of a 155-kb β-globin locus YAC. The transitional point from the γ-gene sequence to the β-gene sequence was at the NcoI restriction site that contains the ATG starting codon in both genes. Figure 1 shows the structural analysis of 1.36kb γpr-βYAC in three established transgenic lines. The continuity of the transgene integrated in the mouse genome was determined by pulsed-field gel electrophoresis (Fig. 1A). Single-cell suspensions of transgenic livers were embedded in agarose gel and digested with SfiI. SfiI sites are located upstream of 5′HS5, between 5′HS3 and 5′HS4, and downstream of 3′HS1. After electrophoresis and Southern blotting, each blot was hybridized with 12 DNA probes spanning from the HOR2 gene (which is located upstream of the 5′HS6) to the downstream DNase I-hypersensitive site, 3′HS1 (which is located about 22 kb 3′ to the β-globin gene). All lines contained continuous SfiI fragments from 5′HS3 to 3′HS1. The next upstream SfiI fragment, which contains 5′HS4 to 5′HS6, was also intact. Two of the four copies of the transgene in line A and one of the two copies in line C lacked the 3′HS1 hybridization band; however, the absence of the 3′HS1 sequences is not expected to affect proper regulation of the globin genes in transgenic mice (4). The copy numbers of the transgenes were determined by conventional Southern hybridization analysis. The blots of HindIII-restricted mouse genomic DNA were hybridized with four DNA probes (HS3, ɛ, γ, and β), and as shown in Fig. 1B, each probe produced the expected fragment with the correct sizes. Copy numbers were estimated to be 4, 2, and 2 for lines A, B, and C, respectively.
FIG. 1.
Structural analysis of the 1.36kb γpr-βYAC transgene. (A) Examination of continuity of the βYAC transgene integrated in the mouse genome. The line diagram on the top represents the human β-globin locus. The five vertical arrows are DNase I-hypersensitive sites of the LCR. 3′HS1 is bracketed because it is not formed in transgenic mice. Five globin genes (ɛ, Gγ, Aγ, δ, and β) are shown as filled boxes. Agarose-embedded mouse genomic DNA from lines A to C was restricted with SfiI, fractionated by pulsed-field gel electrophoresis, and Southern blotted onto nylon membrane. Blot strips were hybridized with 12 probes spanning from HOR2 (which is located upstream of the LCR) to 3′HS1. Reassembled blots are shown. The thin lines link the probes used in each strip to the corresponding positions in the β-globin locus. (B) Measurement of copy number of the 1.36kb γpr-βYAC transgenes. Mouse genomic DNA was digested with HindIII and analyzed with Southern hybridization using four different probes encompassing HS3, ɛ, γ, and β genes. Sizes of the hybridization bands are indicated on the right side of each blot. (C) Confirmation of the replacement of the β-gene promoter with its γ-gene counterpart. The mouse genomic DNA was restricted with EcoRI and hybridized with a β-gene probe. The right line diagram shows that the wild-type βYAC produces a 5.5-kb band, while the 1.36kb γpr-βYAC produces a 2.6-kb band. The closed boxes indicate the coding sequence of the β gene. The open box is the 1.36kb γ-gene promoter, which introduces a new EcoRI site. wt, wild type.
The replacement of the β-gene promoter by the γ-gene promoter was verified by Southern hybridization analysis of transgenic mouse DNA. In the wild-type βYAC, the EcoRI fragment encompassing the β gene is 5.5 kb long. The replacement of the β-gene promoter with the 1.36-kb γ-gene promoter introduces an additional EcoRI site, generating a new fragment 2.6 kb long. As shown in Fig. 1C, all three transgenic lines contained the new 2.6-kb fragment, indicating that the replacement mutation was intact. Taken together, these analyses suggest that the 1.36kb γpr-βYAC constructs were integrated as intact copies in the mouse genome.
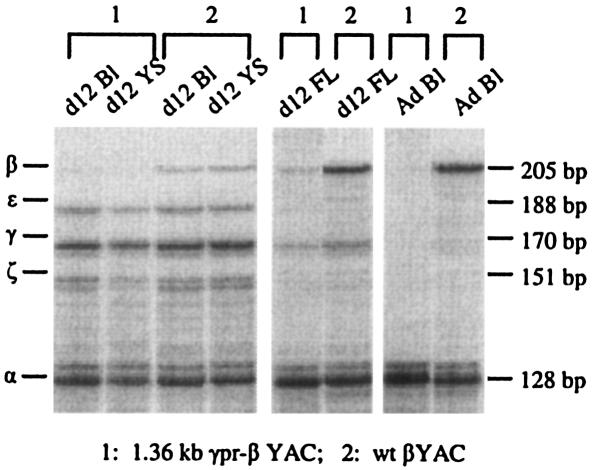
The expression of the 1.36kb γpr-β gene was measured by RNase protection assays. A representative result is shown in Fig. 2. The protected mRNA fragments of the human β-, γ-, and ɛ-globin gene along with the endogenous murine α- and ζ-globin genes (which serve as the internal controls) are shown. The samples from day 12 blood and day 12 yolk sac are composed almost exclusively of nucleated erythroid cells of the embryonic erythropoiesis; the day 12 fetal liver as well as days 14 and 16 blood and fetal liver contain enucleated erythroid cells of the definitive erythropoiesis of fetal liver; the adult blood contains enucleated erythroid cells from the adult erythropoiesis.
FIG. 2.
Globin gene expression in 1.36kb γpr-βYAC transgenic mice by RNase protection assay. Representative RNase protection assays (line A of Tables 1 to 3) showing the protected fragments of murine α and ζ as well as human ɛ, γ, and β mRNA. The corresponding sizes of the protected band are shown on the right side. Bl, blood; YS, yolk sac; FL, fetal liver; wt, wild type.
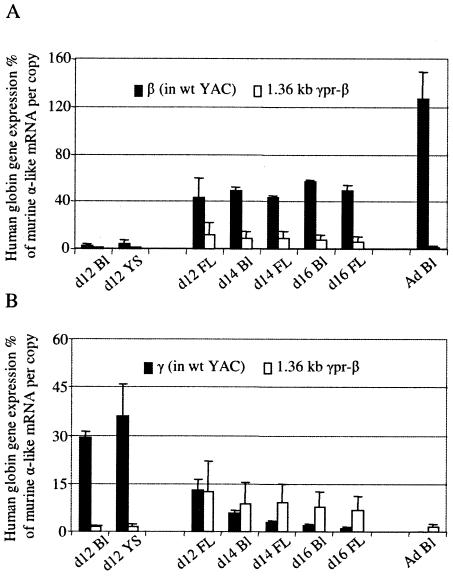
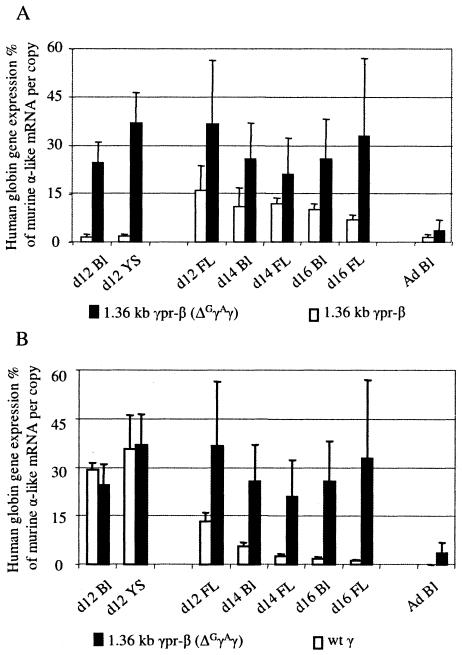
Detailed results of globin gene expression at the different stages of development in the three transgenic mouse lines are presented in Tables 1 to 3. The mean expression levels of the 1.36kb γpr-β transgene are compared, in Fig. 3A, with the levels of β-gene expression in the wild-type βYAC transgenic mice. The wild-type β gene is expressed in adult blood at 126.9% ± 23.0% of murine α-like globin mRNA per copy; in contrast, the level of expression of the 1.36kb γpr-β gene in the adult transgenic mice is 1.7% ± 1.1% per copy (range, 0.5% to 2.5%), providing evidence that the sequences responsible for γ-gene silencing in adult erythropoiesis are located in the 1,359-bp γ-gene promoter. The fact that the γ/β hybrid construct is still expressed although at these low levels at the adult stage suggests that remaining β-globin gene regulatory elements, like the 3′ enhancer and intronic enhancer, are able to communicate with basal promoter elements in the gamma promoter to direct expression of the hybrid gene.
TABLE 3.
Expression of the 1.36kb γpr-β gene in 1.36kb γpr-βYAC transgenic micea
| Line | Copy no. | Gene expressionb (mean ± SD) in:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 12 blood | Day 12 YS | Day 12 FL | Day 14 blood | Day 14 FL | Day 16 blood | Day 16 FL | Ad blood | ||
| A | 4 | 1.4 ± 1.0 | 1.8 ± 0.7 | 19.1 ± 4.7 | 9.9 ± 4.2 | 12.8 ± 1.8 | 11.7 ± 2.6 | 10.2 ± 2.3 | 0.5 ± 0.1 |
| B | 2 | 2.4 ± 0.2 | 2.4 ± 0.5 | 21.7 ± 6.9 | 17.1 ± 2.4 | 12.5 ± 1.1 | 10.2 ± 0.0 | 9.0 ± 0.0 | 2.2 ± 0.4 |
| C | 2 | 0.8 ± 0.1 | 0.8 ± 0.1 | 7.6 ± 2.2 | 5.7 ± 0.4 | 9.6 ± 1.4 | 8.3 ± 1.5 | 7.1 ± 2.3 | 2.5 ± 0.4 |
| Avg | 1.5 ± 0.8 | 1.7 ± 0.8 | 16.1 ± 7.5 | 10.9 ± 5.8 | 11.7 ± 1.8 | 10.1 ± 1.7 | 8.8 ± 1.5 | 1.7 ± 1.1 | |
| Αmt of β gene expression in wt YAC | 2.9 ± 1.2 | 5.1 ± 2.0 | 43.9 ± 15.7 | 49.2 ± 3.6 | 43.4 ± 2.1 | 56.1 ± 2.8 | 49.6 ± 4.7 | 126.9 ± 23.0 | |
YS, yolk sac; FL, fetal liver; wt, wild type; Ad, adult.
Percentage of α-like globin genes per copy.
FIG. 3.
Levels of globin mRNA species during the development of 1.36kb γpr-βYAC transgenic mice. (A) Mean mRNA levels of the 1.36kb γpr-β gene in transgenic lines A to C of Tables 1 to 3 (white columns) are compared to the expression levels of the β gene in the wild-type βYAC mice (black columns). Expression levels are corrected by the endogenous α-like mRNA and copy number. (B) Mean mRNA levels of the 1.36kb γpr-β gene (white columns) are compared to the expression levels of the γ gene in the wild-type βYAC mice (black columns). Ad Bl, adult blood; YS, yolk sac; FL, fetal liver; wt, wild type; d12, day 12.
Competition by the wild γ-globin genes inhibits the expression of the hybrid γ promoter/β gene in embryonic erythropoiesis.
For studies of embryonic erythropoiesis, RNase protection assays were done using cells from day 12 yolk sacs and day 12 blood of 1.36kb γpr-βYAC mice. As shown in Fig. 2 and 3A and B, the expression of the 1.36kb γpr-β gene was strikingly reduced in the embryonic cells of the transgenic mice. The level of γ mRNA of the wild-type βYAC embryos was 29.4% in the day 12 blood and 37.8% in the day-12 yolk sac. In contrast, the levels of the β mRNA in the blood and the yolk sac of day 12 embryos carrying the 1.36kb γpr-β gene were barely detectable, i.e., 1.5% and 1.7%, respectively. These findings were unexpected, because the 1.36kb γpr-β transgene was driven by a promoter identical to the wild γ-gene promoter. The most likely explanation of these results was that competition by the upstream Gγ and Aγ genes was decreasing the probability of interaction between the 1.36kb γpr-β gene and the LCR, resulting in the unexpectedly low expression of that gene in embryonic cells. Previous studies have shown that the two γ genes compete with each other in the yolk sac erythropoiesis (26).
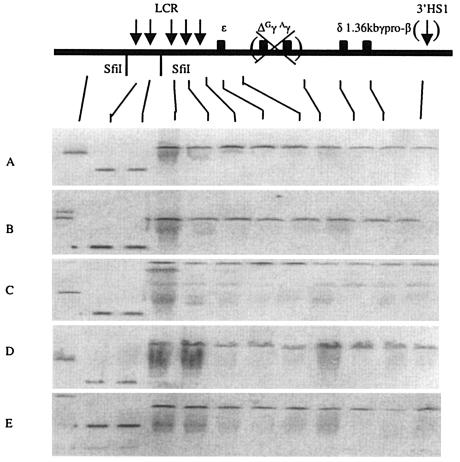
To test this hypothesis, we deleted the Gγ and Aγ genes in the context of the 1.36kb γpr-βYAC construct, producing the (1.36kb γpr-β)(ΔGγAγ) YAC. Five transgenic lines carrying intact copies of this construct were established. Structural analysis by pulsed-field gel electrophoresis showed that all lines contained intact YAC constructs (Fig. 4). Separate Southern hybridization analyses (data not shown) revealed that either one or two copies of the construct have integrated into the genome of the five transgenic lines.
FIG. 4.
Structural analysis of the 1.36kb γpr-β transgene in the (1.36kb γpr-β)(ΔGγAγ) YAC transgenic mice. The data show continuity of the transgene integrated in the mouse genome in all five lines. The distance between LCR (starting from the middle between HS2 and HS3) and the γ-pr-β gene was approximately 50 kb before the “Gγ-Aγ” deletion and 42 kb after the “Gγ-Aγ” deletion. See the legend to Fig. 1 for details.
Globin gene expression of the five lines is presented in Tables 4 and 5. Notice that the expression of the ɛ gene of the (1.36kb γpr-β)(ΔGγAγ) YAC mice was about 50% higher than that of the ɛ gene in the wild βYAC mice, but the difference was not statistically significant. Also notice that there was robust expression of the 1.36kb γpr-β gene in the yolk sac and the embryonic blood (Tables 4 and 5). In Fig. 5A we compare the levels of expression of the 1.36kb γpr-β gene in mice with or without the Gγ and Aγ gene deletion, and in Fig. 5B we compare the levels of expression of the 1.36kb γpr-β gene with the expression of the wild γ gene in wild βYAC mice (Fig. 5B). In contrast to the barely detectable expression when the Gγ and Aγ genes were present, the 1.36kb γpr-β gene was expressed at levels of 24.5% and 36.8% of murine α-like mRNA per copy in the embryonic cells when the Gγ and Aγ genes were deleted; these levels are very close to the level of γ-gene expression in the embryonic cells of the wild-type βYAC mice (Tables 4 and 5). Apparently, the suppression of transcription of the 1.36kb γpr-β gene in the embryonic stage shown in Fig. 2 and 3 is due to competition by the actively transcribed upstream γ-globin genes. When this competition is eliminated, as in the (1.36kb γpr-β)(ΔGγAγ) YAC mice, the γ-gene promoter of the 1.36kb γpr-β gene is able to drive that gene to the expected (for the embryonic erythropoiesis) γ-gene transcription level.
TABLE 4.
ɛ-Globin gene expressions in (1.36kb γpr-β)(ΔGγAγ) YAC transgenic micea
| Line | Copy no. | Gene expressionb (mean ± SD) in:
|
||||
|---|---|---|---|---|---|---|
| Day 12 blood | Day 12 YS | Day 12 FL | Day 14 blood | Day 14 FL | ||
| A | 2 | 17.9 ± 1.7 | 19.3 ± 1.7 | 2.2 ± 0.6 | 1.1 ± 0.1 | 1.1 ± 1.0 |
| B | 1 | 8.3 ± 1.1 | 9.5 ± 2.0 | 0.9 ± 0.5 | 4.1 ± 0.1 | 1.2 ± 0.3 |
| C | 2 | 23.5 ± 2.6 | 23.2 ± 2.3 | 3.3 ± 0.8 | 2.8 ± 0.7 | 0.8 ± 0.1 |
| D | 1 | 32.2 ± 4.6 | 34.9 ± 4.3 | 4.3 ± 0.9 | 2.8 ± 1.0 | 0.8 ± 0.8 |
| E | 2 | 29.0 ± 2.8 | 37.2 ± 7.4 | 2.9 ± 0.6 | 2.8 ± 1.9 | 0.5 ± 0.3 |
| Avg | 22.2 ± 9.5 | 24.8 ± 11.4 | 2.7 ± 1.3 | 2.7 ± 1.1 | 0.9 ± 0.3 | |
| Amt of ɛ gene expression in wt YAC | 15.3 ± 1.7 | 16.5 ± 2.2 | 3.3 ± 1.4 | 2.6 ± 0.7 | 1.7 ± 0.3 | |
YS, yolk sac; FL, fetal liver; wt, wild type.
Percentage of α-like globin genes per copy.
FIG. 5.
Levels of gene expression during the development of the (1.36kb γpr-β)(ΔGγAγ) YAC mice. (A) Comparison of the mean expression of the 1.36kb γpr-β gene in the ΔGγAγ transgenic mice (black columns) with that of the 1.36kb γpr-β gene in the 1.36kb γpr-βYAC transgenic mice (white columns). Notice that the level of 1.36kb γpr-β gene expression in embryonic cells is restored when the GγAγ genes are deleted (black columns). (B) Comparisons of the expression of the 1.36kb γpr-β gene of the ΔGγAγ transgenic mice to the expression of the wild γ gene in wild βYAC mice (white columns). Ad Bl, adult blood; YS, yolk sac; FL, fetal liver; wt, wild type; d12, day 12.
Presence of the β gene is required for silencing of the γ gene during fetal erythropoiesis.
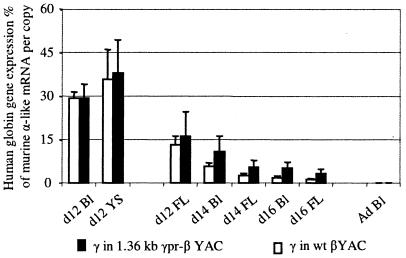
Globin gene expression in the fetal stage of erythropoiesis was evaluated using cells from day 12 fetal liver and day 14 and -16 fetal liver and blood. As shown in Fig. 3B, in the day 12 fetal liver the expression of the 1.36kb γpr-β gene was very close to that of the γ gene of wild βYAC. In day 16 fetal liver, the levels of γ-gene expression in the wild βYAC mice was 1.2% ± 0.3%, while that of the 1.36kb γpr-β gene was 8.8% ± 1.5%. Thus, the activity of the γ genes of the wild YAC declined by 11-fold between days 12 and 16, while the activity of the 1.36kb γpr-β gene declined by only 1.9-fold. The rate of the switch of the wild γ genes of the 1.36kb γpr-β YAC was also affected. As shown in Tables 1 to 3 and Fig. 6, the expression of the wild γ gene in these mice declined by 5-fold by day 16, compared to the 11-fold decline of expression of the γ gene of the wild βYAC mice.
FIG. 6.
Comparison of levels of expression of the wild γ genes of the 1.36kb γpr-βYAC (black columns) to the wild γ genes of a wild βYAC (white columns). Ad Bl, adult blood; YS, yolk sac; FL, fetal liver; wt, wild type; d12, day 12.
When the upstream Gγ and Aγ genes were absent, expression of the 1.36kb γpr-β gene in the day-12 fetal liver was almost three times higher than that of the γ gene of the wild βYAC mice (Fig. 5B). Importantly, the expression of the 1.36kb γpr-β gene remained high throughout fetal liver erythropoiesis (Fig. 5) so that between days 12 and 16, the expression of the 1.36kb γpr-β gene declined only by 0.6-fold (from 36.7% to 21.2%) compared to the 11-fold decline of the wild γ gene.
The common denominator of the 1.36kb γpr-βYAC and the (1.36kbγ pr-β)(ΔGγAγ) YAC transgenes is the absence of a β-globin gene promoter. We interpret these data to suggest that absence of competition by the β promoter results in the diminished turning off of the hybrid γ/β genes as well as the wild γ genes of the mutant YACs. These results indicate that competition between γ and β genes plays a role in the control of the switch from γ- to β-gene expression during fetal erythropoiesis.
DISCUSSION
Our results provide new evidence that the primary mechanism of control of γ-globin gene expression in the adult is autonomous silencing. Sequences of the γ-gene promoter replaced equivalent sequences of the β-gene promoter in the context of a β-locus YAC, and the effect of this manipulation on the expression of the γ promoter/β gene was assessed. The γ-gene promoter severely suppressed β-gene expression in adult erythropoiesis, providing new evidence that γ-gene silencing in the adult is an intrinsic property of the γ-gene promoter. Previously, hybrid genes containing sequences of the human γ-gene promoter as well as sequences of the galago γ-gene promoter have been used to investigate γ-gene silencing (14). This approach is informative because the galago γ gene, in contrast to the human γ gene, behaves as a strictly embryonic gene (25), and the embryonic restriction of the galago γ gene is recapitulated in transgenic mice (27). Expression studies in transgenic mice carrying hybrid galago γ/human γ promoters with various promoter mutations showed that primarily the γCACCC box, but also the γCCAAT box, is the main contributor to γ-gene silencing in adult erythropoiesis (14). A direct repeat element which is collocated with the γ-gene CCAAT box binds a transcriptional complex that contributes to γ-gene silencing (17). The role of the γ-gene promoter in γ-globin silencing is also shown by the finding that silencing is severely impaired by the substitution of the β-spectrin promoter for the γ-gene promoter in a cosmid construct (21).
The decline of γ- to β-globin gene expression during fetal development was initially attributed solely to competition between the γ- and β-globin genes for interaction with the LCR (1, 8). The initial experiments were done in transgenic mice carrying either LCRγ or LCRβ constructs or LCR constructs in which the γ and β genes were in their natural location (1, 8). While the γ and the β genes lose developmental control when each is alone in a construct, they display the expected developmental regulation when they are placed together in LCRγβ cosmids (1, 8). Subsequent studies showing γ-gene silencing in adult transgenic mice (4) challenged the contribution of gene competition on the control of γ- to β-gene globin switching, and they were interpreted to indicate that autonomous silencing is the sole mechanism of γ to β switching during development. As we show here, autonomous silencing is the principal mechanism of the control of γ-gene expression in the adult, but competition appears to play a role in the switch from γ- to β-gene expression during fetal development. This is surmised from the impaired rate of γ-gene switching during the fetal erythropoiesis of the γpr-β hybrid genes and of the wild γ genes in the βYAC transgenics which lack a β-globin gene. The most likely explanation for the essential lack of silencing (Fig. 5B) or the impaired silencing (Fig. 3B) of the γpr-β genes and the impaired silencing (Fig. 6) of the wild γ gene contained in the mutant YACs is the lack of competition by the β gene. A decreased rate of decline of γ-gene expression in the fetal stage has also been observed before in transgenic mouse lines lacking β-globin genes (19). Collectively, these data indicate that gene competition as well as autonomous silencing contributes to the control of the γ genes during the fetal stage of development.
Competition may also contribute to a smaller degree to γ-gene silencing in the adult. As shown here, the 1.36kb γ-gene promoter does not completely silence γ-gene expression in the adult, but the γpr-β gene is still expressed to 1.7% of the level of murine α mRNA in the adult blood. Significantly, thalassemia mutants carrying deletions of the β-gene promoter are characterized by elevated γ-gene expression in the adult heterozygotes (23), which is expected if competition also contributes to γ-gene silencing in the adult.
Several studies have provided unequivocal evidence for the role of gene competition in the control of gene activity during development. The gene competition hypothesis was first proposed by Engel and colleagues to interpret the mutually exclusive expression of the chicken adult βA- and embryonic ɛ-globin genes which are developmentally regulated by a shared enhancer residing between the two genes (3). Strong evidence in support of the hypothesis was provided by the finding that expression of the human β gene is suppressed when the upstream ɛ and γ genes are active during embryonic and fetal erythropoiesis (1, 6, 8, 10). Furthermore, experiments using RNA fluorescence in vivo hybridization have shown that the human γ and β genes are alternatively expressed in the fetal liver of transgenic mice, suggesting that the LCR is able to interact with only one of these genes at a given time (28). A striking example of the role of competition in the control of gene expression during development is provided in our study by the expression of the hybrid γ promoter/β gene in embryonic erythropoiesis. The expression of the wild γ-globin gene in the wild βYAC mice is very robust in embryonic erythroid cells. Surprisingly, the expression of the γ promoter/β gene was very low in the embryonic state in spite of the fact that this gene was driven by the γ-gene promoter. The most likely explanation of this finding is that competition by the upstream Gγ and Aγ genes decreased the probability of interaction of the hybrid γ promoter/β gene with the locus control region. Indeed, deletion from the βYAC of the upstream Gγ and Aγ genes restored the expression of the γ promoter/β gene in the embryonic cells, providing a clear-cut example of the contribution of gene competition in the control of gene expression during development.
TABLE 2.
γ-Globin gene expression in 1.36kb γpr-βYAC transgenic micea
| Line | Copy no. | Gene expressionb (mean ± SD) in:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 12 blood | Day 12 YS | Day 12 FL | Day 14 blood | Day 14 FL | Day 16 blood | Day 16 FL | Ad blood | ||
| A | 4 | 27.3 ± 4.0 | 40.5 ± 6.2 | 18.1 ± 5.1 | 11.6 ± 0.6 | 7.3 ± 0.4 | 5.6 ± 1.2 | 4.9 ± 0.9 | <0.1 |
| B | 2 | 26.3 ± 1.0 | 29.8 ± 7.2 | 23.4 ± 8.5 | 15.5 ± 2.0 | 6.2 ± 1.8 | 7.1 ± 0.0 | 2.7 ± 0.0 | <0.1 |
| C | 2 | 34.6 ± 14.0 | 43.1 ± 18.1 | 7.2 ± 0.7 | 5.3 ± 0.7 | 2.6 ± 0.3 | 2.9 ± 1.1 | 2.2 ± 1.3 | <0.1 |
| Avg | 29.4 ± 4.5 | 37.8 ± 11.5 | 16.2 ± 8.2 | 10.8 ± 5.2 | 5.4 ± 2.5 | 5.2 ± 2.1 | 3.3 ± 1.4 | <0.1 | |
| Αmt of γ gene expression in wt YAC | 29.4 ± 2.0 | 35.8 ± 10.3 | 13.2 ± 2.9 | 5.6 ± 1.3 | 2.8 ± 0.4 | 1.9 ± 0.5 | 1.2 ± 0.3 | <0.1 | |
YS, yolk sac; FL, fetal liver; wt, wild type; Ad, adult.
Percentage of murine α-like globin genes per copy.
Acknowledgments
We thank Xin Ye and Mary Stafford for skillful technical help.
This study was supported by National Institutes of Health grants DK61805 and HL73439 to Q.L. and DK45365 to G.S.
REFERENCES
- 1.Behringer, R. R., T. M. Ryan, R. D. Palmiter, R. L. Brinster, and T. M. Townes. 1990. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 4:380-389. [DOI] [PubMed] [Google Scholar]
- 2.Chada, K., J. Magram, and F. Costantini. 1986. An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature 319:685-689. [DOI] [PubMed] [Google Scholar]
- 3.Choi, O. R., and J. D. Engel. 1988. Developmental regulation of beta-globin gene switching. Cell 55:17-26. [DOI] [PubMed] [Google Scholar]
- 4.Dillon, N., and F. Grosveld. 1991. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature 350:252-254. [DOI] [PubMed] [Google Scholar]
- 5.Duan, Z. J., X. Fang, A. Rohde, H. Han, G. Stamatoyannopoulos, and Q. Li. 2002. Developmental specificity of recruitment of TBP to the TATA box of the human gamma-globin gene. Proc. Natl. Acad. Sci. USA 99:5509-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel, J. D. 1993. Developmental regulation of human beta-globin gene transcription: a switch of loyalties? Trends Genet. 9:304-309. [DOI] [PubMed] [Google Scholar]
- 7.Enver, T., A. J. Ebens, W. C. Forrester, and G. Stamatoyannopoulos. 1989. The human beta-globin locus activation region alters the developmental fate of a human fetal globin gene in transgenic mice. Proc. Natl. Acad. Sci. USA 86:7033-7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enver, T., N. Raich, A. J. Ebens, T. Papayannopoulou, F. Costantini, and G. Stamatoyannopoulos. 1990. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature 344:309-313. [DOI] [PubMed] [Google Scholar]
- 9.Fang, X., H. Han, G. Stamatoyannopoulos, and Q. Li. 2004. Developmentally specific role of the CCAAT box in regulation of human gamma-globin gene expression. J. Biol. Chem. 279:5444-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, P., and F. Grosveld. 1998. Locus control regions, chromatin activation and transcription. Curr. Opin. Cell Biol. 10:361-365. [DOI] [PubMed] [Google Scholar]
- 11.Fraser, P., S. Pruzina, M. Antoniou, and F. Grosveld. 1993. Each hypersensitive site of the human beta-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev. 7:106-113. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman, R. M., C. T. Pham, and T. J. Ley. 1999. Transgenic analysis of a 100-kb human beta-globin cluster-containing DNA fragment propagated as a bacterial artificial chromosome. Blood 94:3178-3184. [PubMed] [Google Scholar]
- 13.Kollias, G., N. Wrighton, J. Hurst, and F. Grosveld. 1986. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell 46:89-94. [DOI] [PubMed] [Google Scholar]
- 14.Li, Q., X. Fang, H. Han, and G. Stamatoyannopoulos. 2004. The minimal promoter plays a major role in silencing of the galago g-globin gene in adult erythropoiesis. Proc. Natl. Acad. Sci. USA 101:8096-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Q., and J. A. Stamatoyannopoulos. 1994. Position independence and proper developmental control of gamma-globin gene expression require both a 5′ locus control region and a downstream sequence element. Mol. Cell. Biol. 14:6087-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navas, P. A., K. R. Peterson, Q. Li, E. Skarpidi, A. Rohde, S. E. Shaw, C. H. Clegg, H. Asano, and G. Stamatoyannopoulos. 1998. Developmental specificity of the interaction between the locus control region and embryonic or fetal globin genes in transgenic mice with an HS3 core deletion. Mol. Cell. Biol. 18:4188-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omori, A., O. Tanabe, J. D. Engel, A. Fukamizu, and K. Tanimoto. 2005. Adult stage gamma-globin silencing is mediated by a promoter direct repeat element. Mol. Cell. Biol. 25:3443-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson, K. R., C. H. Clegg, C. Huxley, B. M. Josephson, H. S. Haugen, T. Furukawa, and G. Stamatoyannopoulos. 1993. Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes. Proc. Natl. Acad. Sci. USA 90:7593-7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson, K. R., Q. L. Li, C. H. Clegg, T. Furukawa, P. A. Navas, E. J. Norton, T. G. Kimbrough, and G. Stamatoyannopoulos. 1995. Use of yeast artificial chromosomes (YACs) in studies of mammalian development: production of beta-globin locus YAC mice carrying human globin developmental mutants. Proc. Natl. Acad. Sci. USA 92:5655-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porcu, S., M. Kitamura, E. Witkowska, Z. Zhang, A. Mutero, C. Lin, J. Chang, and K. M. Gaensler. 1997. The human beta globin locus introduced by YAC transfer exhibits a specific and reproducible pattern of developmental regulation in transgenic mice. Blood 90:4602-4609. [PubMed] [Google Scholar]
- 21.Sabatino, D. E., A. P. Cline, P. G. Gallagher, L. J. Garrett, G. Stamatoyannopoulos, B. G. Forget, and D. M. Bodine. 1998. Substitution of the human β-spectrin promoter for the human Aγ-globin promoter prevents silencing of a linked human β-globin gene in transgenic mice. Mol. Cell. Biol. 18:6634-6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent, T. G., and J. A. Lloyd. 2001. The human gamma-globin TATA and CACCC elements have key, distinct roles in suppressing beta-globin gene expression in embryonic/fetal development. J. Biol. Chem. 276:41817-41824. [DOI] [PubMed] [Google Scholar]
- 23.Stamatoyannopoulos, G., and F. Grosveld. 2001. Hemoglobin switching, p. 135-182. In G. Stamatoyannopoulos, P. W. Majerus, R. M. Perlmutter, and H. Varmus (ed.), Molecular basis of blood diseases, 3rd ed. W. B. Saunders Publishing Company, Philadelphia, Pa.
- 24.Strouboulis, J., N. Dillon, and F. Grosveld. 1992. Developmental regulation of a complete 70-kb human beta-globin locus in transgenic mice. Genes Dev. 6:1857-1864. [DOI] [PubMed] [Google Scholar]
- 25.Tagle, D. A., B. F. Koop, M. Goodman, J. L. Slightom, D. L. Hess, and R. T. Jones. 1988. Embryonic epsilon and gamma globin genes of a prosimian primate (Galago crassicaudatus). Nucleotide and amino acid sequences, developmental regulation and phylogenetic footprints. J. Mol. Biol. 203:439-455. [DOI] [PubMed] [Google Scholar]
- 26.Tanimoto, K., Q. Liu, J. Bungert, and J. D. Engel. 1999. Effects of altered gene order or orientation of the locus control region on human beta-globin gene expression in mice. Nature 398:344-348. [DOI] [PubMed] [Google Scholar]
- 27.TomHon, C., W. Zhu, D. Millinoff, K. Hayasaka, J. L. Slightom, M. Goodman, and D. L. Gumucio. 1997. Evolution of a fetal expression pattern via cis changes near the gamma globin gene. J. Biol. Chem. 272:14062-14066. [DOI] [PubMed] [Google Scholar]
- 28.Wijgerde, M., F. Grosveld, and P. Fraser. 1995. Transcription complex stability and chromatin dynamics in vivo. Nature 377:209-213. [DOI] [PubMed] [Google Scholar]