Abstract
Cyclin-dependent kinase 4 (CDK4) is a master integrator of mitogenic and antimitogenic extracellular signals. It is also crucial for many oncogenic transformation processes. Various molecular features of CDK4 activation remain poorly known or debated, including the regulation of its association with D-type cyclins, its activating Thr172 phosphorylation, and the roles of Cip/Kip CDK “inhibitors” in these processes. Thr172 phosphorylation of CDK4 was reinvestigated using two-dimensional gel electrophoresis in various experimental systems, including human fibroblasts, canine thyroid epithelial cells stimulated by thyrotropin, and transfected mammalian and insect cells. Thr172 phosphorylation of CDK4 depended on prior D-type cyclin binding, but Thr172 phosphorylation was also found in p16-bound CDK4. Opposite effects of p27 on cyclin D3-CDK4 activity observed in different systems depended on its stoichiometry in this complex. Thr172-phosphorylated CDK4 was enriched in complexes containing p21 or p27, even at inhibitory levels of p27 that precluded CDK4 activity. Deletion of the p27 nuclear localization signal sequence relocalized cyclin D3-CDK4 in the cytoplasm but did not affect CDK4 phosphorylation. Within cyclin D3 complexes, T-loop phosphorylation of CDK4, but not of CDK6, was directly regulated, identifying it as a determining target for cell cycle control by extracellular factors. Collectively, these unexpected observations indicate that CDK4-activating kinase(s) should be reconsidered.
Cyclin-dependent kinase 4 (CDK4) and CDK6 act in G1 phase as a master integrator of various mitogenic and antimitogenic signals (76, 80). They phosphorylate and inactivate the cell cycle/tumor suppressor proteins of the pRb family (p105Rb, p107, and p130Rb2) (6, 21, 22, 39, 49, 92) and Smad3 (55). CDK4 activity is deregulated in many human tumors (61, 77) and was recently found to be crucial for various oncogenic transformation processes (43, 56, 84, 88). Understanding CDK4 regulation is thus of fundamental importance.
As initially considered, mitogens activate CDK4/6 by inducing at least one D-type cyclin (D1, D2, and D3) to concentrations allowing an inhibitory threshold imposed by INK4 CDK4/6 inhibitory proteins to be overcome (76). These proteins (p15, p16, p18, and p19) bind to the catalytic domain of the isolated CDK4/6, preventing cyclin association and thus its activation (25, 65, 78). The functions of CDK inhibitors of the CIP/KIP family (p21Cip1, p27Kip1, and p57Kip2) in the activation of D-type cyclin-CDK complexes are more complex and debated. Their down-regulation by mitogenic factors and/or their titration by D-type cyclin-CDK complexes participates in cyclin E/A-CDK2 activation (70, 78, 79). Mostly in in vitro experiments, p21 and p27 were initially observed to similarly inhibit CDK4 activity (26, 40, 67). Nevertheless, p21 is transiently induced in G1 by mitogenic factors in different cell systems (42, 51, 93). Moreover, p21 and p27 were found to be associated with a pRb kinase activity (7, 11, 44, 83), to stabilize cyclin D1/3-CDK4 complexes in vitro or in cotransfected cells (44), and to target these complexes to the nucleus (1, 18, 44, 69). These CDK “inhibitors” were shown to be essential for these functions (9). Nevertheless, this conclusion has been tempered by other authors, who showed that p21 and p27 are not absolutely required for the assembly of cyclin D3-CDK4 (3) and cyclin D1-CDK4 (85) and that only the minor fraction of cyclin D3-CDK4 complexes devoid of CIP/KIP proteins are active as pRb kinases (4). Whether phosphorylations of p27 and p21 (8, 31, 71, 75, 90) could affect their different functions in CDK4 complexes has not been addressed.
Phosphorylation is the least studied level of regulation of CDK4. An inhibitory phosphorylation of CDK4 on Tyr17 was observed in UV irradiation-induced G1 arrest (87) or during cell arrest in quiescence (33) or in response to transforming growth factor β (TGF-β) (30). Moreover, by analyzing human D-type cyclin-CDK4 expressed in insect cells through baculoviral infection, Kato et al. demonstrated that the activity of CDK4 requires its phosphorylation on Thr172 (41) within the activation loop. Furthermore, that group showed that mammalian cell extracts also possess a CDK4-activating kinase activity which was attributed to cyclin H-CDK7 (CAK) on the basis of the immunodepletion of this in vitro activity by a polyclonal CDK7 antibody (53). The complex role of p27 in CDK4 activation is exemplified by the opposite cell cycle controls by cyclic AMP (cAMP) in different systems. G1 arrest by cAMP in mouse macrophages is associated with p27 up-regulation, which would inhibit cyclin D1-CDK4 activity by impeding Thr172 phosphorylation of CDK4 by CAK (40), as described for other CDK complexes (2, 36, 78). By contrast, in the cAMP-dependent cell cycle progression induced by thyrotropin (TSH) in dog thyroid epithelial cells (15), an apparently similar elevation of p27 concentration might facilitate the nuclear import of cyclin D3-CDK4, its activity, and the phosphorylation of CDK4 on an undefined site (11).
To the best of our knowledge, only two figures in published articles have shown the Thr phosphorylation of endogenously expressed mammalian CDK4, by means of metabolic 32P incorporation followed by tryptic peptide mapping and/or phosphoamino acid analysis (30, 40). In the present study, we exploited the high-resolution power of two-dimensional (2D) gel electrophoresis combined with a new Thr172-phospho-specific antibody to compare and reevaluate the impact of D-type cyclins and CDK “inhibitors,” including p27, on the phosphorylation of CDK4 and the regulation of its activity in various native, as well as reconstituted, experimental systems.
MATERIALS AND METHODS
Cloning and mutagenesis.
For production of recombinant baculoviruses, cDNAs encoding human hemagglutinin (HA)-tagged CDK4, human cyclin D3, and dog p27 were subcloned by PCR in baculovirus transfer vector (pBlueBacHis2 for cyclin D3 and pBlueBac4.5/V5-His for p27 and CDK4-HA) for further recombination with the viral DNA (Bac-N-Blue DNA) (Invitrogen, Paisley, United Kingdom). Cyclin D3 presents as an N-terminal tag the X-press epitope and six histidine residues (His6). CDK4 and p27 are C-terminally tagged with an HA epitope and His6 for CDK4 and a V5 epitope and His6 for p27. For CHO cell transfections, cDNAs were subcloned into mammalian expression vectors (pcDNA3.1His for cyclin D3-X-press and pcDNA3.1Myc-His [Invitrogen] for CDK4-HA and p27-V5). Site-directed mutagenesis of wild-type CDK4 (wtCDK4) to CDK4T172A and CDK4T172E was performed by two-step PCR using oligonucleotide primers containing the desired mutation. The mutant p27 lacking the C-terminal 47-amino-acid portion comprising the nuclear localization signal (p27-NLS) was generated by PCR using a reverse oligonucleotide primer situated upstream of the p27 nuclear localization signal. All the inserts were verified by sequencing.
Cell culture, transfections, and infections.
Primary cultures of dog thyroid follicular cells were obtained as described previously (72). Naturally quiescent cells cultured in monolayer in the control medium (Dulbecco modified Eagle medium [DMEM] plus Ham's F-12 medium plus MCDB104 medium [2:1:1 by volume] supplemented with bovine insulin [Sigma, St. Louis, MO; 5 μg/ml], ascorbic acid [40 μg/ml], and antibiotics) were induced to progress into the cell cycle by bovine TSH (Sigma; 1 mU/ml), forskolin (10−5 M) (Calbiochem), or a combination of TSH, epidermal growth factor (EGF) (Sigma; 25 ng/ml), and 10% fetal bovine serum (FBS) (72). Human diploid fibroblasts (IMR-90) and T98G human gliosarcoma cells (both from the American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with antibiotics and 10% fetal calf serum as described previously (12, 62, 68). After starvation in 0.2% FBS for 3 days, quiescent cells were growth stimulated by addition of FBS (20% for IMR-90 cells and 15% for T98G cells). CHO (Chinese hamster ovary) cells cultured in Ham's F-12 medium supplemented with 10% FBS and antibiotics were transfected using Fugene (Roche Diagnostics, Mannheim, Germany) with 6 μg of each pcDNA3 construct (or empty vector to a total of 18 μg DNA) and harvested at 48 h after transfection. Sf9 (Spodoptera frugiperda) cells (Invitrogen) were cultured in monolayers in Grace's insect medium supplemented with 10% FBS and antibiotics. The baculovirus transfer vectors encoding proteins of interest were cotransfected with linearized baculovirus DNA into Sf9 cells. Recombinant viruses were isolated by plaque assay with color selection and amplified. The expression of recombinant proteins was detected by Western blotting. For protein production, Sf9 cells cultured in six-well plates were infected with combinations of recombinant baculoviruses and harvested 64 h after infection.
Indirect immunofluorescence.
Double-labeling immunofluorescent detection from transfected CHO cells was performed exactly as described previously (5, 11). Cyclin D3 or V5-tagged p27 were detected using DCS-22 (hybridoma supernatant kindly provided by J. Bartek) or the V5 monoclonal antibody from Invitrogen, respectively, followed by a biotinylated anti-mouse immunoglobulin antibody and fluorescein-conjugated streptavidin. CDK4 was simultaneously revealed using a selected batch of the C-22 polyclonal antibody from Santa Cruz Biotechnology (Santa Cruz, CA), followed by a Texas Red-conjugated anti-rabbit immunoglobulin antibody.
Immunoprecipitations.
For the analysis of protein complexes and pRb kinase activity, cells were lysed and homogenized in 1 ml NP-40 lysis buffer as described previously (11). Precleared cellular lysates were incubated at 4°C for 3 h with protein A-Sepharose (Amersham Biosciences, Uppsala, Sweden) which had been preincubated overnight with 2 μg of the following antibodies: polyclonal antibodies against CDK4 (C-22) or p21 (C-19) (Santa Cruz) or monoclonal antibodies against cyclin D1 (DCS-11), cyclin D3 (DCS-28), or p16 (DCS-50) (all from Neomarkers, Fremont, CA), V5 epitope (Invitrogen), HA epitope (Santa Cruz), or a mixture of the K25020 anti-p27 monoclonal antibody from BD-Transduction Laboratories and the C-15 p27 polyclonal antibody (Santa Cruz).
pRb kinase assay.
The pRb kinase assay was performed exactly as described previously (11). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes, and the phosphorylation of the pRb fragment was detected using the phospho-specific pRb (Ser780) antibody from Cell Signaling Technology. Membranes were then reprobed using the antibodies described above, except that the cyclin D1 DCS-6 (kindly provided by J. Bartek) was used. Protein A (Pierce, Perbio Science, Erembodegem, Belgium) or an anti-mouse immunoglobulin antibody (Amersham Biosciences), both coupled to horseradish peroxidase, was used for detection by enhanced chemiluminescence (Western Lightning; Perkin-Elmer, Boston, MA).
Gel electrophoresis and Western blotting.
For 2D gel electrophoresis separations, immunoprecipitated proteins were denatured in a buffer containing 7 M urea and 2 M thiourea. Proteins were separated by isoelectric focusing using the IPGphor apparatus from Amersham Biosciences after active in-gel rehydration as described previously (11) on immobilized linear pH gradient (pH 3 to 10) strips (Amersham Biosciences). After loading onto SDS-polyacrylamide slab gels (12.5%) for separation according to molecular mass, proteins were transferred to PVDF membranes. CDK4 was immunodetected using a sample of a noncommercialized phospho-specific-CDK4 (Thr172) antibody from Cell Signaling Technology (Beverly, MA) (produced by immunizing rabbits with a keyhole limpet hemocyanin-coupled peptide antigen to T172-phosphorylated human CDK4 and purified by protein A- and immunogen-based affinity column separation), the DCS-156 monoclonal antibody (Cell Signaling Technology), or the C-22 polyclonal antibody. CDK6 was detected using the DCS-83 monoclonal antibody (NeoMarkers). p27 was immunodetected using the polyclonal phospho-specific-p27(Ser10) or C-15 p27 antibodies (Santa Cruz) or the V5 monoclonal antibody for V5-tagged p27. Membranes detected with the phospho-specific antibodies were reprobed for detection of total CDK4 or p27.
Whole-cell extract proteins were separated according to molecular mass by SDS-PAGE (7, 10, or 12%) and immunoblotted. The polyclonal and monoclonal antibodies were as described above, except that the cyclin D3 antibody DCS-22 (NeoMarkers) was used. Cyclin H, CDK7, and Mat1 were detected using the C-18 polyclonal, C-4 monoclonal, and F-6 monoclonal antibodies, respectively, from Santa Cruz.
Metabolic 32P labeling of CDK4.
One hour before arrest, cells in 9-cm petri dishes were rinsed twice in phosphate-free DMEM and incubated in 3 ml phosphate-free DMEM supplemented with nonessential amino acids. [32P]phosphate (1.7 mCi/ml) was then added for the last 40 min. 32P-labeled CDK4 was immunoprecipitated, separated by 2D gel electrophoresis, and transferred to PVDF membranes exactly as described above and was detected by film autoradiography (2 weeks of exposure). The positions of CDK4 in these membranes were then defined by their immunodetection by enhanced chemiluminescence as described above.
In vitro complex formation in crude cell lysates.
Sf9 cells were coinfected with baculoviruses encoding cyclin D3 and CDK4 or p27 and lysed in the NP-40 lysis buffer. The approximate concentration of the recombinant protein(s) in the lysate was estimated by Coomassie blue staining after SDS-PAGE performed with known amounts of bovine serum albumin. The binding assay was performed by mixing the lysate containing about 0.5 μg of recombinant cyclin D3-CDK4 complexes with various amounts of the p27 lysate (containing from 0.5 μg to 17 ng of recombinant p27) in a total volume of 50 μl of lysis buffer. After 1 h of incubation at 23°C, the mixture was subjected to immunoprecipitation and pRb kinase assay to assess the association of p27 with preformed cyclin D3-CDK4 complexes and the activity of cyclin D3-CDK4-p27 complexes.
In vitro activation of cyclin D3-CDK4/6 complexes by CAK.
Cyclin D3 complexes containing CDK4 and CDK6 from serum-starved T98G cells were immunoprecipitated as described above in NP-40 lysis buffer. The immunoprecipitates were washed three times with NP-40 lysis buffer and then three times with CAK buffer (80 mM β-glycerophosphate [pH 7.3], 15 mM MgCl2, 20 mM EGTA, and 5 mM dithiothreitol) (53). The beads were resuspended in 50 μl of CAK buffer containing protease and phosphatase inhibitors with or without 1.4 μg of recombinant CDK7-cyclin H-MAT1 complex (Upstate, Charlottesville, Virginia). After addition of either 1 mM ATP or 40 μCi of [γ-32P]ATP in the presence of 50 μM ATP, the suspensions were incubated at 30°C for 30 min. After six washes in the appropriate buffer, the immunoprecipitated proteins were either prepared for 2D gel electrophoresis analysis of CDK4 and CDK6 or assayed for pRb kinase activity.
Crude-lysate CAK assay using recombinant CDK2.
Dog thyrocytes or T98G cells were scraped on ice in CAK buffer containing protease and phosphatase inhibitors and sonicated at 4°C. Cleared cell extracts from 5 × 105 to 2 × 106 cells were mixed with 0.2 μg of human glutathione S-transferase (GST)-tagged CDK2 (Abnova Corporation, Taiwan) in a final volume of 50 μl and incubated at 30°C for 30 min with 40 μCi of [γ-32P]ATP in the presence of 50 μM ATP. As a positive control, the cell extract was replaced by 1.4 μg of recombinant CDK7-cyclin H-MAT1 complex diluted in CAK buffer. At the end of the reaction, the mixture was diluted with 1 ml of cold NP-40 buffer and incubated for 1 h at 4°C with 30 μl of glutathione-Sepharose beads (Amersham). After three washes, the pellets were resuspended in Laemmli buffer, radiolabeled proteins were detected by autoradiography after SDS-PAGE separation and blotting transfer, and recovered GST-CDK2 was detected using the PSTAIRE antibody (Santa Cruz).
All the experiments were reproduced at least two times with very similar results.
RESULTS
Two-dimensional gel electrophoresis identification of Thr172 phosphorylation of CDK4.
Thr172 phosphorylation does not affect the electrophoretic migration of CDK4 in SDS-polyacrylamide gels, and no phospho-specific antibody has been described so far for this phosphorylation. Since phosphorylations add negative charges and thus predictably shift the isoelectric points of proteins towards more acidic pHs, CDK4 was immunoprecipitated from normal human fibroblasts or dog thyroid primary cultures that were metabolically labeled with 32Pi, and its different forms were resolved by isoelectric focusing followed by SDS-PAGE and then detected by Western blotting using different CDK4 antibodies (Fig. 1A). In control quiescent cells, CDK4 was resolved as three spots (spots 0, 1, and 2) with similar apparent molecular weights but different isoelectric points. The mitogenic stimulation of IMR-90 fibroblasts with serum and of dog thyrocytes with TSH and EGF led to the appearance of a more negatively charged form (spot 3) (Fig. 1A). Only this form and a very minor one (spot 4) incorporated 32Pi (Fig. 1A), even after prolonged labeling periods (up to 20 h) (not shown). A sample of a new phospho-CDK4(T172) antibody still under development by Cell Signaling Technology also detected only spots 3 and 4 of CDK4 from both fibroblasts and thyrocytes (Fig. 1A). On the other hand, antiphosphotyrosine antibodies (PY20 and 4G10) failed to detect CDK4 in these experiments or after mitogenic inhibition of dog thyrocytes by TGF-β (negative data not shown).
FIG. 1.
Two-dimensional gel electrophoresis identification of Thr172-phosphorylated CDK4. (A) 32P-metabolically labeled extracts from human IMR-90 fibroblasts stimulated for 16 h with serum (16 h) and from dog thyrocytes stimulated for 20 h with TSH plus EGF (ET) were immunoprecipitated with a CDK4 antibody, separated by 2D gel electrophoresis, and electroblotted. The membranes were exposed for autoradiographic detection of phosphorylated forms of CDK4 (32P). CDK4 phosphorylated on Thr172 or total CDK4 was then immunodetected from the same membranes by using enhanced chemiluminescence (P-T172-CDK4 and CDK4, respectively). As indicated, the polyclonal C-22 (Santa Cruz) or monoclonal DCS-156 antibodies were used. Unstimulated quiescent cells are shown for comparison (Cont). The different spots of CDK4 are numbered; only forms 3 and 4 are phosphorylated, including on Thr172. (B) Extracts of CHO cells transfected with vectors encoding CDK4-HA (K4), CDK4T172A-HA (K4T172A), CDK4T172E-HA (K4T172E), CDK4-HA plus cyclin D3 (K4+D3), CDK4T172A-HA plus cyclin D3 (K4T172A+D3), or CDK4T172E-HA plus cyclin D3 (K4T172E+D3) were immunoprecipitated (IP) with anti-HA or anti-cyclin D3 antibodies, separated by 2D gel electrophoresis, and electroblotted. K4T172A+D3 + K4+D3 is the 1:1 mixture of K4T172A+D3 and K4+D3 samples before 2D gel separation. CDK4 phosphorylated on Thr172 or total CDK4 was immunodetected from the same membranes (P-T172-CDK4 and CDK4, respectively). (C) The same CHO cell extracts were immunoprecipitated with anti-HA (for CDK4) or anti-cyclin D3 antibodies, assayed for pRb kinase activity, separated by SDS-PAGE, and immunoblotted. CDK4 was detected using anti-HA antibody, and the pRb fragment phosphorylated in vitro at Ser780 (P-Rb-780) were detected using a phospho-specific antibody.
To confirm the identity of the major phosphorylated form 3 of CDK4 and the specificity of the prototypic phospho-CDK4(T172) antibody, we next compared the 2D gel patterns of CDK4 obtained from CHO cells transfected with wtCDK4 or with CDK4 in which T172 was replaced by a neutral nonphosphorylatable alanine (CDK4T172A) or a negatively charged phospho-mimetic glutamate (CDK4T172E). Immunoprecipitated wtCDK4, CDK4T172A, or CDK4T172E displayed only form 1 (and minor spot 0) (Fig. 1B). In the T172E mutant, the isoelectric point was shifted to a more acidic pH (Fig. 1B). The cotransfection of CDK4 with cyclin D3 induced the appearance of the more negatively charged form 3 in wtCDK4 but not in CDK4T172A, which comigrated perfectly with the form 1 of wtCDK4 (Fig. 1B). This form 3 was bound to cyclin D3 and specifically recognized by the phospho-CDK4(T172) antibody (Fig. 1B). By contrast, the phospho-CDK4(T172) antibody did not recognize CDK4T172A and CDK4T172E cotransfected with cyclin D3 at all.
CDK4wt cotransfected with cyclin D3 in CHO cells, but not CDK4wt transfected alone, displayed a very strong pRb kinase activity. By contrast, CDK4T172A was even more expressed and associated with cyclin D3 but displayed a very weak activity (Fig. 1C). A moderate pRb kinase activity was also associated with cyclin D3-CDK4T172E, showing that this mutation could mimic in part the activating Thr172 phosphorylation (Fig. 1C). Altogether, these experiments confirm that the activity of CDK4 requires its activating Thr172 phosphorylation, which depends on D-type cyclin binding (41). They establish the specificity of the phospho-CDK4(T172) antibody and identify CDK4 phosphorylated form (phosphoform) 3 as the fraction of activated CDK4 phosphorylated on Thr172.
The phospho-CDK4(T172) antibody also recognized the most negatively charged form of CDK6 (see Fig. 7B and 8) but not the T160-phosphorylated forms of CDK2 abundantly associated with cyclin A in IMR-90 fibroblasts (not shown). This is explained by the high conservation of the T-loop phosphorylation domains in the highly related CDK4 (YSYQMALTPVVVTLWY) and CDK6 (YSFQMALTSVVVTLWY).
FIG. 7.
Thr172 phosphorylation of CDK4 is regulated. (A) Extracts from dog thyrocytes stimulated for 24 h with forskolin (Forsk) or forskolin plus TGF-β (2 ng/ml) were immunoprecipitated (IP) with anti-cyclin D3 antibody, assayed for pRb kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3, CDK4 and its activating Thr172 phosphorylation (P-T172-CDK4), p27, and the pRb fragment phosphorylated in vitro at Ser780 (P-Rb-780) were detected using specific antibodies. In the right panel, similar coimmunoprecipitates from dog thyrocytes stimulated with TSH or TSH plus TGF-β (2 ng/ml) were separated by 2D gel electrophoresis and electroblotted for detection of CDK4 with an anti-CDK4 antibody. (B) Extracts from quiescent serum-starved T98G cells (Cont) or cells stimulated by 15% FBS for 10 h were immunoprecipitated with anti-cyclin D3 or anti-p27 antibodies, assayed for pRb kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3, CDK4, CDK6, CDK2, p27, and the pRb fragment phosphorylated in vitro at Ser780 (P-Rb-780) were detected using specific antibodies. In the right panel, the same coimmunoprecipitates were separated by 2D gel electrophoresis and electroblotted for detection of CDK4 and CDK6 with anti-CDK4 and anti-CDK6 antibodies. In the bottom panel, similar cyclin D3 coimmunoprecipitates from serum-starved (Cont) and stimulated (10 h) T98G cells were separated by 2D gel electrophoresis and electroblotted for detection using the phospho-CDK4(T172) antibody (PT-172). Total CDK4 and CDK6 were then detected from the same membrane by using anti-CDK4 and anti-CDK6 antibodies. Arrows in panels A and B indicate the regulated Thr172-phosphorylated form of CDK4 and the corresponding, but unregulated, phosphorylated (Thr177) form of CDK6. (C) CAK expression and activity in the same cultures of dog thyrocytes and T98G cells as in panels A and B. Cyclin H (Cyc H), CDK7, and Mat1 were detected from whole-cell extracts of T98G cells (serum starved and stimulated for 10 h) or dog thyrocytes treated as in panel A and from anti-cyclin H immunoprecipitates (IP Cyc H) of dog thyrocytes. In the right panel, CAK activity was assessed by the incubation of GST-CDK2 in the presence of [32P]ATP, with (+) or without (α) recombinant cyclin H-CDK7-Mat1 complex (CAK) or with crude extracts of T98G cells (serum starved [Cont] or stimulated for 10 or 26 h) or of dog thyrocytes (stimulated or not [Cont] with forskolin and TGF-β). GST-CDK2 was recovered using glutathione-Sepharose, put on SDS-polyacrylamide gels, and electroblotted, and its phosphorylation was detected by autoradiography (32P-GST-CDK2). GST-CDK2 was then immunodetected using the PSTAIRE antibody. The lower band of the 32P-GST-CDK2 doublet reflects the T-loop phosphorylation.
FIG. 8.
CAK phosphorylates CDK6 more readily than CDK4 in vitro. Inactive cyclin D3 complexes containing CDK4 or CDK6 were immunoprecipitated using the anti-cyclin D3 antibody (cyclin D3 IP) from serum-starved T98G cells. The immunoprecipitated complexes were incubated in the presence of 15 mM Mg2+ and 1 mM ATP or 50 μM [32P]ATP, without (−) or with (+) recombinant cyclin H-CDK7-Mat1 complex (CAK) or with CAK and 100 mM EDTA. The cyclin D3 coimmunoprecipitates were then separated by 2D gel electrophoresis and electroblotted for immunodetection using the phospho-CDK4(T172) antibody (PT-172). Total CDK4 and CDK6 were then detected from the same membrane using anti-CDK4 and anti-CDK6 antibodies. In the case of incubation with [32P]ATP, 32P labeling was revealed by autoradiography after electroblotting (P-CDK6 and P-CDK4 indicate the positions of phosphorylated forms of CDK6 and CDK4 as detected by CDK4 and CDK6 antibodies). Arrows indicate the Thr172-phosphorylated form of CDK4 and the corresponding phosphorylated (Thr177) form of CDK6. In the bottom panel, the cyclin D3 coimmunoprecipitates were assayed for pRb kinase activity (PRb-780) after incubation with or without (α) CAK.
Phospho-Thr172 CDK4 is associated with both D-type cyclins and CDK inhibitors.
We have compared in IMR-90 fibroblasts the 2D gel electrophoresis patterns of total CDK4 and CDK4 coimmunoprecipitated with cyclin D1 and cyclin D3 or with the CDK inhibitors p21Cip1, p27Kip1, and p16Ink4A in response to mitogenic stimulation by serum. In these experiments, the CDK4 level increased slightly after stimulation for 16 h or 24 h (Fig. 2A). Cyclin D1 and cyclin D3 were detected in quiescent cells, and their expression was induced by serum. The p21 level was transiently enhanced 16 h after stimulation. By contrast the presence of p27 was markedly reduced after cell stimulation, whereas the level of p16 was unchanged (Fig. 2A). Serum stimulated the appearance of Thr172 phosphoform 3 in total CDK4 and in the different CDK4 complexes (Fig. 2B). Compared to in total CDK4, phosphoform 3 was enriched in CDK4 complexed not only to cyclin D1 or cyclin D3 but also to p21 or p27 (Fig. 2B). As in total CDK4, phosphoform 3 was also, surprisingly, present in CDK4 associated with p16 from serum-stimulated cells (Fig. 2B). On the other hand, the nonphosphorylated form 2 was weakly detected in CDK4 bound to cyclins D1/D3 and p21 or p27, but it was strongly associated with p16 (Fig. 2B).
FIG. 2.
Phospho-Thr172 CDK4 is associated with both D-type cyclins and CDK inhibitors. (A to C) IMR-90 fibroblasts; (D to F) dog thyrocytes. (A) Western blotting analyses of CDK4, cyclin D3, cyclin D1, p21, p27, and p16 from whole-cell extracts of quiescent unstimulated IMR-90 cells (Cont) or cells stimulated by 20% serum for 16 or 24 h. p38 mitogen-activated protein kinase (MAPK) was detected as a loading control. (B) CDK4 separated by 2D gel electrophoresis was detected by Western blotting with a CDK4 antibody from (co)immunoprecipitates (IP) of cyclin D1, cyclin D3, p21, p27, or p16 from quiescent unstimulated IMR-90 cells (Cont), cells stimulated with 20% serum for 16 h (16 h), or cells stimulated for 16 h and treated for 3 h with the protein synthesis inhibitor cycloheximide (100 μg/ml) in the presence of serum (16 h + 3 h Cx). In the inset, controls of cycloheximide impact on the presence of proteins in whole-cell extracts are shown; D1-bound CDK4 and p16-bound CDK4 are CDK4 coimmunoprecipitated using cyclin D1 and p16 antibodies, respectively. (C and D) Extracts from IMR-90 cells (C) stimulated or not (Cont) by 20% serum for 16 h or dog thyrocytes (D) stimulated or not (Cont) by forskolin (Forsk) were immunoprecipitated with anti-p16, anti-cyclin D1, anti-cyclin D3, anti-p21, or anti-p27 antibodies or a control immunoglobulin (IgG), assayed for pRb kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3, CDK4 and its activating Thr172 phosphorylation (P-T172-CDK4), cyclin D1, p16, p21, p27, and the pRb fragment phosphorylated in vitro at Ser780 (P-Rb-780) were detected using specific antibodies. (E) Western blotting analyses of CDK4 separated by 2D gel electrophoresis from coimmunoprecipitates of p27 from quiescent unstimulated dog thyrocytes (Cont) or cells stimulated with TSH (1 mU/ml) or TSH plus EGF plus 10% serum (ETS) for 20 h. (F) Extracts from dog thyrocytes stimulated for 20 h with TSH were immunoprecipitated with p27 antibodies (p27 IP) or a phospho-specific (Ser10) p27 antibody (P-S10-p27 IP), separated by 2D gel electrophoresis, and electroblotted. Immunodetections were performed using antibodies against total p27 or p27 phosphorylated on Ser10 (P-S10-p27) or with a mixture of CDK4 and p27 antibodies. In panels B, E, and F, arrows 3 indicate the main Thr172-phosphorylated form of CDK4.
In parallel, the relative abundances, compositions, and activities of the different CDK4 complexes were investigated (Fig. 2C). p16-CDK4 complexes were by far the most abundant ones, in both quiescent and serum-stimulated cells (Fig. 2C). Thus, even though only a minority of p16-bound CDK4 was phosphorylated on Thr172 in serum-stimulated cells (Fig. 2B), a significant proportion (possibly the majority) of total Thr172-phospho-CDK4 must be associated with p16. Whether the small quantity of cyclin D1 detected in p16 complexes (Fig. 2C) could suffice to support the Thr172 phosphorylation of p16-bound CDK4 is unclear. Thr172 phosphorylation of CDK4 appeared to be relatively stable, which could allow the reassociation with p16 of some phosphorylated CDK4 released by the rapid turnover of labile D-type cyclins. Indeed, some Thr172 phosphorylation persisted in CDK4 bound to either p16 or p27, even after disappearance of cyclin D1 (and cyclin D3 [not shown]) induced by a 3-hour incubation of serum-stimulated IMR-90 cells with the protein synthesis inhibitor cycloheximide (Fig. 2B).
Despite the presence of the Thr172 phosphorylation and cyclin D1, p16-bound CDK4 was inactive in serum-stimulated cells, as expected (Fig. 2C). By contrast, the enrichment of Thr172-phosphoform 3 in the p21 coimmunoprecipitate, exactly as found in CDK4 bound to cyclins D1 and D3 (Fig. 2B), correlated with similar stimulated pRb kinase activities of CDK4 associated with cyclin D1 and p21 (Fig. 2C). At variance with the strong pRb kinase activity associated with p21, only a weak activity was detected in p27 coimmunoprecipitates from serum-stimulated fibroblasts (Fig. 2C), despite a similar enrichment of the Thr172-phosphoform 3 in p27-bound CDK4 (Fig. 2B). This could be explained by the low abundance of this complex due to the reduction of p27 levels induced by serum (Fig. 2A and C). Indeed, in dog thyrocytes, in which the cAMP-dependent mitogenic stimulation by TSH is associated with an increased expression of p27 (15), p27 as cyclin D3 supported both the Thr172 phosphorylation and the pRb kinase activity of CDK4 in response to TSH (Fig. 2D). In these cells, p27 would be unlikely to prevent the Thr172 phosphorylation of CDK4, as illustrated by the fact that most (about 80%) of p27-bound CDK4 was phosphorylated on Thr172 in response to a maximal mitogenic stimulation (TSH plus EGF plus serum) (Fig. 2E).
Therefore, at least at their endogenous levels increased by mitogenic factors in intact cells, p21 and p27 were indistinguishable from D-type cyclins in their association with both the activating phosphorylation and pRb kinase activity of CDK4.
Ser10 phosphorylation of p27 does not affect Thr172 phosphorylation of CDK4.
Most in vitro studies showing the CDK4-inhibitory activity of p27 have used bacterially produced p27, which is presumably unphosphorylated. One possibility would be that some phosphorylations of p27 could interfere with its CDK4-inhibitory activity. Since a large proportion of p27 is generally considered to be associated with D-type cyclin-CDK4 in G1-progressing cells (79), only stoichiometrically important phosphorylations of p27 might influence its impact on CDK4. Ser10 is by far the major phosphorylation site of p27 (32). It is also the closest to the p27 domain of interaction with cyclins and CDKs. This phosphorylation is essential for nuclear export of p27 by binding to CRM1 (31) and for subsequent Thr157 phosphorylation within the NLS by protein kinase B, which prevents p27 nuclear reimport (82), but other authors concluded that Ser10-phosphorylated p27 remains nuclear (14). In dog thyrocytes stimulated by TSH or the adenylyl cyclase activator forskolin, up-regulated p27 is mostly nuclear, even in cells progressing in G1 and S phases (15), but about 50% of p27 was phosphorylated on Ser10, as shown by 2D gel separation and detection with a phospho-p27(Ser10) antibody (Fig. 2F). A similar proportion of Thr172-phosphoform 3 and the nonphosphorylated form 1 of CDK4 was codetected with these different forms of p27 in p27 coimmunoprecipitates (Fig. 2F). However, Thr172-phosphorylated CDK4 was not specifically associated with Ser10-phosphorylated p27 in forskolin-stimulated dog thyrocytes, since identical proportions of Thr172-phospho-CDK4 versus nonphosphorylated CDK4 were coimmunoprecipitated by the antibodies against total p27 and the phospho-p27(Ser10) antibody (Fig. 2F). Similar results were obtained with CHO cells cotransfected with CDK4, cyclin D3, and p27 (not shown). Contrary to our hypothesis, the main phosphorylation of p27 is thus unlikely to have a major impact on the Thr172 activating phosphorylation of CDK4.
Different impacts of p27 stoichiometry on Thr172 phosphorylation and activity of CDK4.
p21 and p27 have been suggested to inhibit or support the kinase activity of D-type cyclin-CDK4 complexes (and cyclin E/A-CDK2 in the case of p21 [28, 94]) depending on the stoichiometry of p21 or p27 relative to the cyclin (7, 44, 60). However, this remains controversial (3, 29). Moreover, LaBaer et al. (44) reported that p21 but not p27 can support the activity of CDK4 complexes, while Blain et al. (7) found the opposite. p21 and p27 were suggested to inhibit CDK4 activity in part by preventing its Thr172 activating phosphorylation. Nevertheless, the impact of their binding stoichiometry on CDK4 phosphorylation has never been investigated. p21 and p27 might also interfere with CDK4 activity by inserting into the catalytic cleft of the kinase, thus competing with ATP, as suggested by the crystal structure of the cyclin A-CDK2-p27 complex (73). In the present experiments, pRb kinase activity was assayed in the presence of a more physiological concentration of ATP (2 mM), which was higher than the ATP concentrations (10 to 50 μM) used in all of the previous studies. Moreover, to reevaluate the impact of p27 stoichiometry within cyclin D3-CDK4 complexes, we have expressed these different proteins by baculoviral infection of Sf9 cells or by transfection of CHO cells. Most of the canine p27 produced in these cells was phosphorylated on Ser10 (Fig. 3A). Additional phosphorylations were also evident, as deduced from the abundant presence of more negatively charged forms detected using the phospho-p27(Ser10) antibody (Fig. 3A). A p27 mutant in which the nuclear localization sequence (containing the Thr157, Thr187, and Thr198 phosphorylation sites) was removed (p27-NLS) presented a reduced number of forms, with only one major form phosphorylated on Ser10 (Fig. 3A).
FIG. 3.
Different impacts of p27 stoichiometry on activity and Thr172 phosphorylation of CDK4. (A) Extracts of CHO cells transfected with vectors encoding V5-tagged wild-type p27 or V5-tagged p27 lacking its C-terminal NLS region, or of insect Sf9 cells transduced with baculovirus encoding p27-V5, were immunoprecipitated (IP) with anti-V5 antibody, separated by 2D gel electrophoresis, and electroblotted. p27 phosphorylated on Ser10 or total p27 was immunodetected from the same membranes (P-S10-p27 and p27, respectively). (B) Extracts of Sf9 cells or CHO cells transduced/transfected with baculoviruses or plasmids encoding cyclin D3, CDK4-HA plus cyclin D3, or CDK4-HA plus cyclin D3 plus p27-V5 or untagged p27 (p27) (and dilutions of p27[-V5] vectors to 1/5, 1/7, 1/10, and 1/20) were immunoprecipitated with anti-cyclin D3, anti-p27, or anti-V5 antibodies, assayed for pRb kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3, p27, and the pRb fragment phosphorylated in vitro at Ser780 (P-Rb-780) were detected using their specific antibodies; CDK4-HA and p27-V5 were detected using anti-HA and anti-V5 antibodies, respectively. (C) Extracts of Sf9 or CHO cells transduced/transfected as for panel B were immunoprecipitated with anti-HA (for CDK4), anti-cyclin D3, or anti-V5 antibodies, separated by 2D gel electrophoresis, and electroblotted for detection of CDK4 with an anti-CDK4 antibody. Arrows indicate the Thr172-phosphorylated form of CDK4.
In Sf9 and CHO cells, coexpressed cyclin D3 and CDK4 assembled in the absence of p27 and formed very active complexes (Fig. 3B). The coexpressed p27 also efficiently associated with cyclin D3-CDK4, as judged from the coimmunoprecipitation of similar amounts of cyclin D3 and CDK4 by the cyclin D3 and p27 (V5) antibodies (Fig. 3B). As observed by others (4, 9), p27 increased the abundance of cyclin D3-CDK4 complexes (Fig. 3B). This could be due to an enhanced stability of these complexes in both Sf9 and CHO cells, but in CHO cells this was also associated with increased levels of cyclin D3 and CDK4 in whole-cell extracts (data not shown), possibly resulting from stabilization of these proteins within the complexes. At high relative expression levels of p27 (i.e., at levels exceeding the amount of p27 that could be engaged in cyclin D3 complexes), cyclin D3-CDK4 complexes containing p27 were totally inactive in Sf9 and CHO cells (Fig. 3B). When p27 expression was gradually decreased by dilution of the p27 expression vectors (1/5, 1/10, and 1/20 in Sf9 cells and 1/5, 1/7, and 1/10 in CHO cells), the catalytic activity associated with cyclin D3 was progressively restored (Fig. 3B). Moreover, at reduced concentrations at which p27 was almost completely recruited into cyclin D3-CDK4 complexes (1/20 in Sf9 cells and 1/10 in CHO cells), the pRb kinase activity was present in the p27 immunoprecipitate (Fig. 3B). Similar results were obtained using V5-tagged p27 (immunoprecipitated with the V5 antibody) or untagged p27 (Fig. 3B). These results confirm, with p27 processed and phosphorylated in different higher eukaryote cells, that the opposite impacts of p27 on the activity of cyclin D3-CDK4 depend on its stoichiometry of binding to these complexes.
In the same experiments, we compared the 2D gel electrophoresis patterns of total CDK4 and of CDK4 coimmunoprecipitated with cyclin D3 or p27 at the different levels of p27 production (Fig. 3C). The coexpression of cyclin D3 strongly increased the Thr172 phosphorylation of CDK4 in Sf9 and CHO cells (Fig. 3C). In CHO cells, this was impaired in part by p27 only under conditions of very large excess of relative p27 expression (“nondiluted” p27 cotransfection; Fig. 3C). Under the other conditions of p27 coexpression, CDK4 was largely phosphorylated on Thr172, and a similar proportion of Thr172-phosphoform 3 was coimmunoprecipitated by cyclin D3 and p27 (V5) antibodies, in both Sf9 and CHO cells (Fig. 3C). p27 did not inhibit the Thr172 phosphorylation of CDK4 (Fig. 3C) in situations in which the pRb kinase activity of cyclin D3-CDK4-p27 complexes was strongly inhibited (Fig. 3B) (1/10 p27 in Sf9 cells or 1/5 in CHO cells). This confirms that the inhibition of CDK4 phosphorylation is not the main mechanism by which p27 can inhibit the activity of cyclin D3-CDK4 complexes.
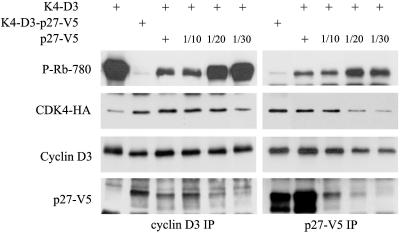
These conclusions and the importance of p27 binding stoichiometry were verified by the in vitro incubation of active phosphorylated cyclin D3-CDK4 complexes (preformed by their coexpression in Sf9 cells) with different amounts of p27-V5 separately produced in Sf9 cells (Fig. 4). High relative levels of p27-V5 strongly inhibited the pRb kinase activity of CDK4 complexes (Fig. 4), as previously reported by others using bacterially produced p27 (20, 40, 44). Nevertheless, when lower p27-V5 concentrations were added, cyclin D3-CDK4 complexes bound to p27-V5 were clearly active (Fig. 4).
FIG. 4.
Various amounts of p27 differently affect cyclin D3-CDK4 activity in vitro. Cyclin D3-CDK4-HA complexes formed by their coexpression in infected Sf9 cells were incubated in vitro with decreasing amounts of p27-V5 (1, 1/10, 1/20, and 1/30 dilutions) produced in Sf9 cells. This mixture and cyclin D3-CDK4-HA-p27-V5 complexes formed by their coexpression in Sf9 cells were immunoprecipitated (IP) using anti-cyclin D3 or anti-V5 antibodies, assayed for pRb kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3 and the pRb fragment phosphorylated in vitro at Ser780 (P-Rb-780) were detected using specific antibodies; CDK4 and p27 were detected with anti-HA and anti-V5 antibodies.
A stoichiometric regulation of cyclin D3-CDK4 activity by p27, as suggested by these different experiments, implies the binding of several p27 molecules to one complex of cyclin D3-CDK4 and thus the existence of different p27 binding sites on either CDK4 or cyclin D3. We have thus reevaluated the existence of binary p27-CDK4 or p27-cyclin D3 complexes by baculoviral infection of Sf9 cells or by CHO cell transfection. Surprisingly, the amount of CDK4 coimmunoprecipitated by the p27 (V5) antibody from the CDK4/p27 coinfection was very similar to the amount of CDK4 coimmunoprecipitated by the cyclin D3 antibody from the CDK4/cyclin D3 infection (Fig. 5A). Similarly, most of CDK4 was found to bind to V5-tagged p27 or untagged p27 in CHO cells cotransfected without cyclin D3 (Fig. 5A). On the other hand, only a faint association of p27 with cyclin D3 was detected in the absence of CDK4 in Sf9 cells (not shown). CDK4 can thus massively bind to p27 in the absence of a D-type cyclin, producing a totally inactive binary p27-CDK4 complex (Fig. 5A), which did not permit the phosphorylation of CDK4 (Fig. 5B).
FIG. 5.
p27 can associate with cyclin-free CDK4. (A) Extracts of CHO cells transfected with vectors encoding CDK4-HA or CDK4-HA plus p27-V5 or untagged p27 (p27), or Sf9 cells infected with baculoviruses encoding cyclin D3 plus CDK4-HA or p27-V5 plus CDK4-HA, were immunoprecipitated (IP) with anti-cyclin D3, anti-HA, anti-p27, or anti-V5 antibodies, assayed for pRb kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3 and the pRb fragment phosphorylated in vitro at Ser780 (P-Rb-780) were detected using specific antibodies. CDK4 and p27 were detected using, respectively, anti-HA and anti-V5 (left and middle panels) or anti-p27 (right panel) antibodies. (B) The same CHO or Sf9 cell extracts were immunoprecipitated with anti-HA or anti-V5 antibodies, separated by 2D gel electrophoresis, and electroblotted for detection of CDK4 with an anti-CDK4 antibody. Arrows indicate the form of CDK4 phosphorylated on Thr172.
Subcellular localization of CDK4 does not affect its Thr172 phosphorylation.
In mammalian cells, the major CAK activity consists of cyclin H, CDK7, and Mat1, which are also subunits of transcription factor II H (27). These different subunits localize to the cell nucleus, and, as generally accepted, progression through the cell cycle and mitogenic stimulations have little effect on their expression, localization, and activity (13, 53, 86). The activating phosphorylation of CDK4 should thus depend on its nuclear (trans)location, as proposed by others (18) and also reported for the analogous Thr160 phosphorylation of CDK2 (19). Whereas D-type cyclins and CDK4 do not contain a nuclear localization signal, their nuclear location has been shown to depend on their association with Cip/Kip family proteins which possess a classical bipartite nuclear localization signal (44, 69). We have thus transfected CHO cells with different combinations of vectors expressing cyclin D3, CDK4, p27, or a truncated p27 with its NLS C-terminal portion deleted (p27-NLS). As determined by indirect immunofluorescence, cyclin D3 and CDK4 transfected alone or together had the same overall diffuse cellular localization (Fig. 6A). Transfected p27 was located mostly in the nucleus in the majority of cells, whereas p27-NLS was predominantly cytoplasmic in all the cells (Fig. 6A). Consistent with their association in binary complexes (Fig. 5), cotransfected CDK4 and p27 or p27-NLS perfectly colocalized, irrespective of the coexpression of cyclin D3 (Fig. 6A and B). Intact p27 retained CDK4 in the nucleus, whereas p27-NLS retained CDK4 in the cytoplasm (Fig. 6A and B). In the triple cotransfections (cyclin D3/CDK4/p27 [or cyclin D3/CDK4/p27-NLS]) (Fig. 6B), cyclin D3 localization exactly followed the localization of CDK4 determined by p27 (or p27-NLS). When p27 expression was decreased by dilution of the p27-plasmid (Fig. 6B), the nuclear translocations of CDK4 and cyclin D3 (not shown) were incomplete in many cells. p27 can thus determine the subcellular localization of both cyclin-free CDK4 and cyclin D3-CDK4 complexes.
FIG. 6.
The subcellular localization of CDK4 does not affect its Thr172 phosphorylation. CHO cells were transfected as indicated with different combinations of vectors encoding cyclin D3, HA-tagged CDK4 (K4), V5-tagged wild-type p27 (p27) or V5-tagged p27 lacking its C-terminal NLS region (p27-NLS). (A and B) Cells were fixed 48 h after transfection and processed for double indirect immunofluorescent staining with either mouse monoclonal anti-cyclin D3 and rabbit polyclonal anti-CDK4 antibodies or mouse monoclonal anti-V5 (p27) and rabbit polyclonal anti-CDK4 antibodies. Nuclei were counterstained with Hoechst dye. Images were recorded using a 100× immersion lens and the SPOT RT camera (Diagnostic Instrument, Inc.). To demonstrate the colocalizations of cyclin D3 and CDK4 or of CDK4 and p27 or p27-NLS, green and red fluorescent images were merged using the Adobe Photoshop program. (A) Single and double transfections. (B) Triple transfections. In some transfections, the p27 or p27-NLS expression plasmids were diluted. Only the 10-fold dilution of the p27 plasmid is illustrated (K4+D3 + 1/10p27). (C) Extracts from the same transfection experiment as in panels A and B were immunoprecipitated (IP) with anti-cyclin D3 or anti-V5 antibodies, separated by 2D gel electrophoresis, and electroblotted. CDK4 was detected using anti-CDK4 antibody. In some transfections, the plasmids encoding p27 or p27-NLS were diluted as indicated (1/5 and 1/10). Arrows indicate the form of CDK4 phosphorylated on Thr172.
In the same CHO cell cotransfection experiments, we compared the 2D gel electrophoresis patterns of cyclin D3-bound CDK4 and of CDK4 coimmunoprecipitated with p27 or p27-NLS at different levels of p27 production (Fig. 6C). Contrary to our expectation, mostly nuclear CDK4 bound to p27 and predominantly cytoplasmic CDK4 bound to p27-NLS displayed very similar proportions of the Thr172-phosphoform 3. The activating phosphorylation of CDK4 was thus independent of its subcellular location, which seemed to be inconsistent with the generally observed nuclear compartmentation of cyclin H-CDK7-Mat1 (which was verified in these CHO cells [data not shown]). Even though shuttling in and out the nucleus could allow some cytoplasmic accumulation of CDK4 that had been phosphorylated in the nucleus, this experiment indicates that nuclear translocation of CDK4 is not the rate-limiting step of CDK4 phosphorylation by nuclear CAK.
Thr172 phosphorylation of CDK4 is regulated.
Since CAK seems to be constitutively active, the Thr172 phosphorylation of CDK4 is generally assumed to passively result from the induction of D-type cyclins and the assembly of nuclear cyclin-CDK4 complexes (53). Figure 7 illustrates two examples where the Thr172 phosphorylation of CDK4 and its association with cyclin D3 were dissociated in response to extracellular regulation. In dog thyrocytes, TGF-β prevents the cAMP-dependent mitogenic response and pRb phosphorylation elicited by TSH or forskolin without reducing the levels of cyclin D3 and CDK4 and the formation of cyclin D3-CDK4 complexes (16). As shown, using both the phospho-CDK4(T172) antibody and 2D gel separation of CDK4, TGF-β inhibited the stimulated pRb kinase activity of cyclin D3-CDK4 at least in part by inhibiting the Thr172 phosphorylation of cyclin D3-bound CDK4 (Fig. 7A). In quiescent T98G human glioblastoma cells, the massive cell cycle reentry induced by serum was accompanied by a very strong stimulation of the pRb kinase activity supported by cyclin D3 (Fig. 7B). However, the high basal presence of cyclin D3 and its constitutive association with CDK4, CDK6, or CDK2 were not significantly increased by serum in these p16-defective cells (Fig. 7B). Instead, the Thr172 phosphorylation of CDK4, but not the similar Thr177 phosphorylation of CDK6, was markedly stimulated by serum in both cyclin D3 and p27 immunoprecipitations (Fig. 7B). Indeed, the phospho-CDK4(T172) antibody also specifically detected the most negatively charged Thr177-phosphorylated form of CDK6 in the same membranes from 2D gel separation of cyclin D3 coimmunoprecipitates (Fig. 7B). Whereas only a small minority of cyclin D3-bound CDK6 was phosphorylated in both quiescent and serum-stimulated T98G cells, the Thr172 phosphorylation of CDK4 was markedly stimulated by serum (Fig. 7B). In various cell systems, the activating phosphorylation of CDK4 (but not of CDK6 in T98G cells) could thus be directly regulated by extracellular mitogenic or antimitogenic factors.
Of note, p27-bound CDK4 and CDK6 were almost completely inactive compared to cyclin D3 immunoprecipitates in serum-stimulated T98G cells (Fig. 7B), whereas similar pRb kinase activities were associated with p27 and cyclin D3 in stimulated dog thyrocytes (Fig. 2D). p27 thus appeared to support the pRb kinase activity of cyclin D3-CDK4 in dog thyrocytes and to prevent it in T98G cells. This discrepancy might be explained by very different relative concentrations of p27 and cyclin D3. Indeed, in TSH-stimulated dog thyrocytes, similar amounts of p27 and CDK4 were coimmunoprecipitated by cyclin D3 and p27 antibodies, but the p27 antibody coimmunoprecipitated only a fraction of the amount of cyclin D3 that could be precipitated using the cyclin D3 antibody (Fig. 2D). Conversely, in serum-stimulated T98G cells, the antibodies against p27 and cyclin D3 precipitated similar amounts of cyclin D3 and CDK4, but the cyclin D3 antibody coimmunoprecipitated only a small fraction of the p27 that was precipitated by the p27 antibody (Fig. 7B). Thus, even after its accumulation in response to TSH, p27 could be in limiting amounts for its association with cyclin D3-CDK4 in dog thyrocytes (Fig. 2D), whereas p27 still remained in excess for the formation of cyclin D3-CDK4/6-p27 complexes in T98G cells, even after its partial disappearance 10 h after serum stimulation (Fig. 7B). This confirms in native cell systems that the opposite effects of p27 on cyclin D3-CDK4 activity depend on its binding stoichiometry, as suggested from the ectopic expression of these proteins in CHO and Sf9 cells (Fig. 3 to 5). Moreover, as in these systems, p27 inhibited the activity of cyclin D3 complexes in T98G cells without impeding the serum-stimulated phosphorylation of CDK4, as judged from the 2D gel separation of p27-bound CDK4 (Fig. 7B).
Since the directly regulated phosphorylation of CDK4 but not of CDK6 appeared to be at variance with the general belief that mammalian CAK expression and activity are constitutive, we investigated CAK complex proteins in dog thyrocytes and T98G cells (Fig. 7C). Serum did not stimulate the accumulation of CDK7, cyclin H, and Mat1 in T98G cells (Fig. 7C), and TGF-β did not inhibit their expression and association in dog thyrocytes (Fig. 7C). We failed to detect a CAK activity in cell extracts by using a recombinant GST-CDK4 (Abnova Corporation, Taiwan) mixed with cyclin D3 produced in Sf9 cells, possibly due to their inefficient in vitro association (41) (negative data not shown). Since CAK (CDK7) can phosphorylate cyclin-free CDK2 in vitro (23, 36, 86), we next assessed CAK activity in crude extracts of dog thyrocytes and T98G cells by using GST-CDK2 as a substrate in a direct phosphorylation assay. Whereas GST-CDK2 phosphorylated by recombinant cyclin H-CDK7-Mat1 complex migrated as one band, phosphorylation of GST-CDK2 by thyrocyte extracts was detected as a doublet, in which the lower band is attributed to the Thr160 phosphorylation (the upper band should correspond to inhibitory phosphorylations) (47). The treatment of thyrocytes with forskolin and/or TGF-β did not affect their GST-CDK2 T-loop-phosphorylating activity (Fig. 7C). In this experiment, extracts of T98G cells (stimulated or not with serum) poorly phosphorylated GST-CDK2. Only the upper band corresponding to inhibitory phosphorylations was clearly generated by extracts of cells that were serum stimulated for 26 h (Fig. 7C), coincident with enhanced Tyr15 phosphorylation of endogenous CDK2 at this time point (unpublished data). Therefore, CAK presence and activity were not modulated in dog thyrocytes. In T98G cells, CAK activity could be weaker, which could be consistent with the weak phosphorylation of cyclin D3-bound CDK4 (in quiescent cells) and CDK6 (in both quiescent and stimulated cells).
CAK phosphorylates CDK6 more readily than CDK4 in vitro.
These results prompted us to reevaluate whether cyclin D3-bound CDK4 and CDK6 can be phosphorylated by recombinant CAK in vitro. Whereas Matsuoka and collaborators did activate cyclin D2-CDK4 by use of recombinant CAK (53), others were unable to phosphorylate and/or activate D-type cyclin-CDK4 by CAK under conditions that efficiently allowed the phosphorylation/activation of cyclin D3-CDK6 and cyclin A-CDK2 (36, 60). Cyclin D3 complexes from serum-deprived T98G cells were incubated with a large amount of recombinant cyclin H-CDK7-Mat1 complex in the presence of either 1 mM ATP or 50 μM [32P]ATP. This induced the pRb kinase activity of cyclin D3 complexes (Fig. 8). As detected by the phospho-CDK4(T172) antibody (as reprobed with CDK4 and CDK6 antibodies) after 2D gel separation, incubation with CAK in the presence of 1 mM ATP did increase the T-loop phosphorylation of both CDK4 and CDK6 in the cyclin D3 complexes (Fig. 8). By contrast, under the conditions of lower ATP required for 32P incorporation, CAK phosphorylated CDK6 but not CDK4, as shown by 32P autoradiography (Fig. 8). Moreover, the addition of 100 mM EDTA (in the presence of 15 mM Mg2+ and 1 mM ATP) during the incubation with CAK prevented CDK4 phosphorylation but not CDK6 phosphorylation (Fig. 8).
CDK4 and CDK6 complexed to cyclin D3 in quiescent T98G cells can thus be phosphorylated by CAK in vitro, indicating that the CDK4/6 phosphorylating activity could have been rate limiting in these cells. Whereas both CDK4 and CDK6 bound to cyclin D3 can be phosphorylated by a large amount of CAK, only cyclin D3-CDK6 could be phosphorylated by CAK under more stringent conditions (low ATP concentrations or reduction of the Mg-ATP concentration by EDTA), reconciling the divergent observations of Matsuoka et al. (53) and Kaldis et al. (36), who used different ATP concentrations. This preference of CAK for CDK6 over CDK4 under suboptimal in vitro conditions contrasts with the regulation by serum in intact cells, in the same cyclin D3 complexes, of the phosphorylation of CDK4 but not of CDK6.
DISCUSSION
Despite the fact that it is required for CDK4 activity (41), the activating Thr172 phosphorylation of CDK4 has been infrequently investigated because of lack of methodological tools. We have recently shown that the high-resolution power of the 2D gel electrophoresis allows one to separate several phosphorylated and nonphosphorylated forms of CDKs and to visualize their relative proportions (11, 12). In this study, we have identified the main phosphorylated form 3 and minor form 4 of CDK4 as comprising the activating Thr172 phosphorylation, by the analysis of the CDK4 T172A mutant, in vitro phosphorylation by recombinant CAK, as well as the utilization of the first Thr172-phospho-specific antibody. These tools were also useful for the analysis of the corresponding (Thr177) phosphorylation of the much-related CDK6.
The 2D gel electrophoresis profiles of CDK4 and CDK6 are less complex than the 2D gel pattern of CDK2 phosphorylations (12), in part due to the absence in CDK4/6 of a phosphorylated threonine residue analogous to Thr14 of CDK2. According to their relative isoelectric point shifts, the phosphoform 3 of CDK4 should contain only the Thr172 phosphorylation, while the very minor phosphoform 4 might also contain a second phosphorylation or another posttranslational modification. In CHO cells, the T172A mutation did not unmask any other abundant phosphorylated form of CDK4 which might have comigrated with Thr172-phosphorylated form 3. Thus, in the present cell systems and experimental conditions, no stoichiometrically (biologically) significant phosphorylated form of CDK4 (except the very minor form 4) could contain the undefined serine phosphorylation observed by Kato et al. (41) and by Iavarone and Massagué (30) but not by Terada et al. (87) or the inhibitory Tyr17 phosphorylation (30, 33, 87). In IMR-90 fibroblasts, the lack of Tyr17-phosphorylated CDK4 contrasts with the major phosphorylation of CDK2 on Tyr15 (12), implying that activation of the two CDKs must very differently depend on Cdc25 phosphatases. Sherr's group has also failed to detect tyrosine phosphorylation of CDK4 (41, 74), in agreement with the observation that CDK4, unlike CDK1 and CDK2, is not an in vitro substrate of the Wee1 CDK tyrosine kinase (91). Moreover, the nature of the two nonphosphorylated forms of CDK4 (forms 1 and 2) remains unclear. CDK4 form 2 associates preferentially with p16 but weakly with D-type cyclins and thus might well constitute a nonactivatable pool of CDK4. Its identification is complicated by the low abundance of endogenous CDK4 and the very weak presence of form 2 in overexpression systems.
Thr172-phosphorylated CDK4 is associated with cyclins and CDK inhibitors.
We confirm here that the activity of CDK4 critically depends on both binding to a D-type cyclin and subsequent Thr172 phosphorylation, whereas the T172A mutation of CDK4 does not affect its association with cyclin D3 (41). Consistently, the endogenous Thr172-phosphorylated CDK4 was markedly enriched in cyclin D1/D3 complexes of stimulated human fibroblasts, dog thyrocytes (11), and T98G cells. More unexpectedly, in the different cell systems used in this study, the Thr172 phosphorylation of CDK4 was also associated with the two classes of CDK inhibitors that were reported to prevent the activating phosphorylation of CDKs, including CDK4, by CAK (cyclin H-CDK7) (2, 34, 40, 66). It might be argued that the inhibitors could have bound CDK4 after its phosphorylation by CAK within the D-type cyclin complex. The observed accumulation of Thr172-phosphorylated CDK4 in the abundant inactive p16-CDK4 complexes of serum-stimulated IMR-90 cells most likely resulted from a reassociation of phosphorylated CDK4 with p16 after degradation of more labile D-type cyclins. This possibility is less likely to fully explain the strong enrichment of the phosphorylated form in CDK4 complexes containing p21 or p27 in all the presently investigated cell systems. Indeed, more than 80% of p27-bound CDK4 was phosphorylated in maximally stimulated dog thyrocytes or in transduced Sf9 cells and transfected CHO cells. In the present study, an inhibition of the activating phosphorylation of CDK4 by p27 was observed only in cotransfected CHO cells and required cellular concentrations of p27 exceeding those that inhibited cyclin D3-CDK4 activity. We thus conclude that Cip/Kip proteins do not prevent the activating phosphorylation of CDK4 in most cellular contexts.
Roles of p27 in CDK4 complexes.
Cip/Kip proteins have been described as inhibitors of CDKs, including CDK4 (3, 4, 26, 38, 40) (although p21/p27-containing CDK4 complexes can be active [83]) or as essential adaptors for the assembly and the nuclear localization of the D-type cyclin-CDK complexes (1, 9, 44, 69, 93). Blain et al. (7) have interestingly suggested that these opposite roles depend on the stoichiometry of p27 relative to a D-type cyclin (cyclin D2) in CDK4 complexes, as initially shown for the dual action of p21 on cyclin A-CDK2 (94). Nevertheless, structural studies indicate that cyclin A-CDK2 complexes can accommodate only one molecule of p21 or p27, which fully inhibits the activity (29, 45, 73). Finally, Bagui et al. recently claimed that only the minor fraction of cyclin D3-CDK4 complexes devoid of Cip/Kip proteins are active (3, 4). In our experiments, the roles of p27 depended on its expression levels relative to cyclin D3. When the p27 cellular concentration was limiting relative to the p27-binding capacity of cyclin D3-CDK4 complexes, as found in TSH-stimulated thyrocytes and with diluted amounts of p27 expression vectors in CHO and Sf9 cells, p27 supported the pRb kinase activity to levels approaching those associated with cyclin D3. At levels transiently increased by serum, p21 also supported cyclin D1/D3-CDK4 activity in human fibroblasts (Fig. 2C) and in T98G cells and thyrocytes (63). Conversely, excessive relative concentrations of p27 completely inhibited cyclin D3-CDK4 activity by a mechanism independent of CDK4 phosphorylation, as observed in T98G cells and with large amounts of p27 vectors in CHO and Sf9 cells. The CDK4-inhibitory activity of p27 appears to be independent of its phosphorylation status, since in the present experiments p27 was highly phosphorylated, including at Ser10, at variance with earlier studies using bacterially produced p27. Our results thus fully support the “stoichiometric” model of Blain et al. (7, 60), implying the existence of two types of cyclin D-CDK4-p27 complexes depending on relative p27 concentrations: the first shows a low-stoichiometry binding of p27 and displays a pRb kinase activity, and the second is inactive due to an additional p27 molecule(s). A third stage of p27 binding to cyclin D3-CDK4 complexes might be suggested by the inhibition of CDK4 phosphorylation observed with even higher concentrations of p27 exceeding those that sufficed to inhibit CDK4 activity (Fig. 3B and C). Whereas p27 is generally believed to stably associate only with complexed cyclins and CDKs (25, 45, 79), we demonstrate here, with both Sf9 and CHO cells, that p27 can avidly form binary complexes with CDK4 in the absence of a D-type cyclin. This is not an “overexpression artifact,” since we also observed persistence of p27-CDK4 complexes after the disappearance of labile D-type cyclins provoked by protein synthesis inhibition (Fig. 2B). This association of p27 with cyclin-free CDK4 in intact cells is consistent with the model of different p27-binding modes permitting the association of several p27 molecules with D-type cyclin-CDK4 complexes.
At variance with the results of LaBaer et al. (44), we were unable to detect any in vitro assembly activity of p27 with cyclin D3 and CDK4 (negative data not shown), even under conditions that allow the binding of p27 to preassembled cyclin D3-CDK4 complexes (Fig. 3D). This is in agreement with previous studies showing that abundant p27 in G0 cells is unable to assemble ectopically expressed cyclin D3 into CDK4 complexes in serum-starved fibroblasts (54) and, conversely, that some active cyclin D3-CDK4 and cyclin D1-CDK4 complexes are formed in fibroblasts derived from p27-p21-null mice (4, 85). On the other hand, consistent with similar conclusions by others (44, 69), the C-terminal NLS domain of p27 was crucial for the nuclear (trans)location of CDK4 (even in the absence of cyclin D3) and cyclin D3 complexed to CDK4. These results recapitulate our previous observations from the physiological model of TSH-stimulated dog thyrocytes (11, 16), in which p27 induced by TSH might facilitate the activation of cyclin D3-CDK4 complexes, not as their still-elusive assembly factor, but by determining their nuclear import.
The D-type cyclin/p27 ratio clearly contributes to determining the cell responsiveness to mitogens (46) or growth-inhibitory factors, which in turn act to modify this equilibrium to levels that allow, or prevent, the concerted activation of CDKs leading to S-phase entry. In some circumstances, such as in quiescent dog thyrocytes, which express large amounts of cyclin D3 (17) but low levels of p27 and p21, p27 (in response to TSH) or p21 (in response to growth factors) (63) rather than D-type cyclins may have to be up-regulated to facilitate CDK4 activation. Similarly in mammary gland and prostate of p27 null mice, epithelial cell proliferation was reported to be impaired, whereas p27 haplo-insufficiency accelerated cyclin D1-dependent transformation, which is prevented by normal p27 expression (24, 57, 58).
Thr172 phosphorylation of CDK4 is regulated by extracellular factors.
We have previously found that assembly of cyclin D3-CDK4-p27 holoenzyme (16, 17) and a subsequent phosphorylation of CDK4 that correlates with its activity depend on kinetically distinct cAMP actions in dog thyrocytes (64). This phosphorylation is now identified as the Thr172 phosphorylation. In thyrocytes, the activating phosphorylation of CDK4 thus integrates the opposite cell cycle controls by cAMP and TGF-β after formation of cyclin D3-CDK4 complexes. Similarly in T98G cells, serum strongly stimulated the activity of constitutively formed cyclin D3-CDK4 complexes at least in part by directly stimulating the Thr172 phosphorylation of CDK4. This generalizes the new concept that Thr172 phosphorylation could be the latest regulated step that determines the catalytic activity of CDK4, the phosphorylation of Rb family proteins, and thus the passage through the G1 phase restriction point. Recent studies have also pointed out the Thr160 phosphorylation of CDK2 as a direct target of treatments that prevent S-phase entry (10, 50, 59, 89). In some of these studies, the activation of CDK4 (10, 59) and/or the in vitro assayed activity of CDK7 (59, 89) remained unaffected, leading their authors to suggest the involvement of distinct CAK activities.
According to the generally accepted characteristics and substrate specificity of CDK7 complexes, CDK4 Thr172 phosphorylation could have been anticipated to depend on prior D-type cyclin binding (53), to be prevented by CDK inhibitors (36, 40), to occur in the nuclear compartment (86), and not to be subject to upstream regulatory control by mitogens (13, 53, 86). Only the first characteristic was verified in the present study, thus plausibly arguing for an implication of other activating kinases. At variance with nuclear cyclin H-CDK7, the cytoplasmic monomeric Cak1p of budding yeast (35) preferentially phosphorylates monomeric CDK2 and CDK6 in vitro, and CDK inhibitors do not block this activity (36). Several monomeric CAKs with distinct substrate specificities coexist with CDK7 orthologs in plants (81). In Drosophila melanogaster, CDK7 is required for the activation of CDK1 complexes but not for phosphorylation and activation of CDK2-cyclin E (48). In human cells, a small distinct CAK activity was enriched (37), and a candidate nuclear p42 CAK was recently cloned (52). The only direct evidence that CDK7 is the sole or main CDK4-activating kinase was based on the activation of cyclin D2-CDK4 by CDK7 immunoprecipitates and the presence in NIH 3T3 cell extracts of a CDK4-activating activity that could be immunodepleted by a polyclonal CDK7 antibody (53). As most often reported (13, 53, 86), we obtained no evidence of a regulation of CAK (CDK7) expression or activity in dog thyrocytes and T98G cells. Whereas CDK4 was phosphorylated in response to serum in cyclin D3 complexes of T98G cells, the much-related CDK6 was not. Nevertheless, the latter could be more readily phosphorylated by recombinant CAK in the same experiments, consistent with observations by others who succeeded in readily phosphorylating CDK2 and CDK6, but not different CDK4 preparations, by using purified human cyclin H-CDK7-Mat1 (36, 60). At variance with the corresponding phosphorylated Thr residues of CDK1, CDK2, and CDK6, the Thr172 of CDK4 is followed by a proline. Although CAK (CDK7) can unambiguously phosphorylate cyclin D3-bound CDK4 in vitro, another regulated (proline-directed?) CDK4-activating kinase(s) might thus remain to be discovered.
Acknowledgments
The phospho-specific-CDK4 (Thr172) antibody was a kind gift of Cell Signaling Technology Inc. (Beverly, MA). We thank J. Bartek and J. Lukas (Danish Cancer Society) for kindly providing several antibodies and plasmids and Sébastien Mauën and Marie-Louise Draps for advice and help with the baculoviral transduction of insect cells.
This study was supported by grants from the Belgian Federation against Cancer (to P.P.R.), the Communauté Française de Belgique—Actions de Recherches Concertées (to P.P.R. and Y.D.L.), the Belgian Fund for Scientific Medical Research (FRSM) (to P.P.R.), the National Fund for Scientific Research (FNRS, Belgium) (to Y.D.L.), Télévie (to P.P.R.), and the Fortis Bank Foundation (to Y.D.L.). L.B., H.K., and S.P. are fellows of the Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture (FRIA). F.L. and P.P.R. are Research Associates of the FNRS.
REFERENCES
- 1.Alt, J. R., A. B. Gladden, and J. A. Diehl. 2002. p21(Cip1) promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 277:8517-8523. [DOI] [PubMed] [Google Scholar]
- 2.Aprelikova, O., Y. Xiong, and E. T. Liu. 1995. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J. Biol. Chem. 270:18195-18197. [DOI] [PubMed] [Google Scholar]
- 3.Bagui, T. K., R. J. Jackson, D. Agrawal, and W. J. Pledger. 2000. Analysis of cyclin D3-cdk4 complexes in fibroblasts expressing and lacking p27(kip1) and p21(cip1). Mol. Cell. Biol. 20:8748-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagui, T. K., S. Mohapatra, E. Haura, and W. J. Pledger. 2003. p27Kip1 and p21Cip1 are not required for the formation of active D cyclin-cdk4 complexes. Mol. Cell. Biol. 23:7285-7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baptist, M., F. Lamy, J. Gannon, T. Hunt, J. E. Dumont, and P. P. Roger. 1996. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J. Cell. Physiol. 166:256-273. [DOI] [PubMed] [Google Scholar]
- 6.Bartek, J., J. Bartkova, and J. Lukas. 1996. The retinoblastoma protein pathway and the restriction point. Curr. Opin. Cell Biol. 8:805-814. [DOI] [PubMed] [Google Scholar]
- 7.Blain, S. W., E. Montalvo, and J. Massague. 1997. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 272:25863-25872. [DOI] [PubMed] [Google Scholar]
- 8.Boehm, M., T. Yoshimoto, M. F. Crook, S. Nallamshetty, A. True, G. J. Nabel, and E. G. Nabel. 2002. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J. 21:3390-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiariello, M., E. Gomez, and J. S. Gutkind. 2000. Regulation of cyclin-dependent kinase (Cdk) 2 Thr-160 phosphorylation and activity by mitogen-activated protein kinase in late G1 phase. Biochem. J. 349:869-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulonval, K., L. Bockstaele, S. Paternot, J. E. Dumont, and P. P. Roger. 2003. The cyclin D3-CDK4-p27kip1 holoenzyme in thyroid epithelial cells: activation by TSH, inhibition by TGFbeta, and phosphorylations of its subunits demonstrated by two-dimensional gel electrophoresis. Exp. Cell Res. 291:135-149. [DOI] [PubMed] [Google Scholar]
- 12.Coulonval, K., L. Bockstaele, S. Paternot, and P. P. Roger. 2003. Phosphorylations of cyclin-dependent kinase 2 revisited using two-dimensional gel electrophoresis. J. Biol. Chem. 278:52052-52060. [DOI] [PubMed] [Google Scholar]
- 13.Darbon, J. M., A. Devault, S. Taviaux, D. Fesquet, A. M. Martinez, S. Galas, J. C. Cavadore, M. Doree, and J. M. Blanchard. 1994. Cloning, expression and subcellular localization of the human homolog of p40MO15 catalytic subunit of cdk-activating kinase. Oncogene 9:3127-3138. [PubMed] [Google Scholar]
- 14.Delmas, C., N. Aragou, S. Poussard, P. Cottin, J. M. Darbon, and S. Manenti. 2003. MAP kinase-dependent degradation of p27Kip1 by calpains in choroidal melanoma cells. Requirement of p27Kip1 nuclear export. J. Biol. Chem. 278:12443-12451. [DOI] [PubMed] [Google Scholar]
- 15.Depoortere, F., J. E. Dumont, and P. P. Roger. 1996. Paradoxical accumulation of the cyclin-dependent kinase inhibitor p27kip1 during the cAMP-dependent mitogenic stimulation of thyroid epithelial cells. J. Cell Sci. 109:1759-1764. [DOI] [PubMed] [Google Scholar]
- 16.Depoortere, F., I. Pirson, J. Bartek, J. E. Dumont, and P. P. Roger. 2000. Transforming growth factor beta(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1). Mol. Biol. Cell 11:1061-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Depoortere, F., A. Van Keymeulen, J. Lukas, S. Costagliola, J. Bartkova, J. E. Dumont, J. Bartek, P. P. Roger, and S. Dremier. 1998. A requirement for cyclin D3-cyclin-dependent kinase (cdk)-4 assembly in the cyclic adenosine monophosphate-dependent proliferation of thyrocytes. J. Cell Biol. 140:1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehl, J. A., and C. J. Sherr. 1997. A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin-dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol. Cell. Biol. 17:7362-7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietrich, C., K. Wallenfang, F. Oesch, and R. Wieser. 1997. Translocation of cdk2 to the nucleus during G1-phase in PDGF-stimulated human fibroblasts. Exp. Cell Res. 232:72-78. [DOI] [PubMed] [Google Scholar]
- 20.Dong, F., D. Agrawal, T. Bagui, and W. J. Pledger. 1998. Cyclin D3-associated kinase activity is regulated by p27kip1 in BALB/c 3T3 cells. Mol. Biol. Cell 9:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewen, M. E., H. K. Sluss, C. J. Sherr, H. Matsushime, J. Kato, and D. M. Livingston. 1993. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell 73:487-497. [DOI] [PubMed] [Google Scholar]
- 22.Farkas, T., K. Hansen, K. Holm, J. Lukas, and J. Bartek. 2002. Distinct phosphorylation events regulate p130- and p107-mediated repression of E2F-4. J. Biol. Chem. 277:26741-26752. [DOI] [PubMed] [Google Scholar]
- 23.Fisher, R. P., and D. O. Morgan. 1994. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 78:713-724. [DOI] [PubMed] [Google Scholar]
- 24.Gao, H., X. Ouyang, W. Banach-Petrosky, A. D. Borowsky, Y. Lin, M. Kim, H. Lee, W. J. Shih, R. D. Cardiff, M. M. Shen, and C. Abate-Shen. 2004. A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. USA 101:17204-17209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall, M., S. Bates, and G. Peters. 1995. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene 11:1581-1588. [PubMed] [Google Scholar]
- 26.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 27.Harper, J. W., and S. J. Elledge. 1998. The role of Cdk7 in CAK function, a retro-retrospective. Genes Dev. 12:285-289. [DOI] [PubMed] [Google Scholar]
- 28.Harper, J. W., S. J. Elledge, K. Keyomarsi, B. Dynlacht, L. H. Tsai, P. Zhang, S. Dobrowolski, C. Bai, L. Connell-Crowley, and E. Swindell. 1995. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6:387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hengst, L., U. Gopfert, H. A. Lashuel, and S. I. Reed. 1998. Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1). Genes Dev. 12:3882-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iavarone, A., and J. Massague. 1997. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature 387:417-422. [DOI] [PubMed] [Google Scholar]
- 31.Ishida, N., T. Hara, T. Kamura, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277:14355-14358. [DOI] [PubMed] [Google Scholar]
- 32.Ishida, N., M. Kitagawa, S. Hatakeyama, and K. Nakayama. 2000. Phosphorylation at serine 10, a major phosphorylation site of p27(Kip1), increases its protein stability. J. Biol. Chem. 275:25146-25154. [DOI] [PubMed] [Google Scholar]
- 33.Jinno, S., S. C. Hung, and H. Okayama. 1999. Cell cycle start from quiescence controlled by tyrosine phosphorylation of cdk4. Oncogene 18:565-571. [DOI] [PubMed] [Google Scholar]
- 34.Kaldis, P. 1999. The cdk-activating kinase (CAK): from yeast to mammals. Cell Mol. Life Sci. 55:284-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaldis, P., Z. W. Pitluk, I. A. Bany, D. A. Enke, M. Wagner, E. Winter, and M. J. Solomon. 1998. Localization and regulation of the cdk-activating kinase (Cak1p) from budding yeast. J. Cell Sci. 111:3585-3596. [DOI] [PubMed] [Google Scholar]
- 36.Kaldis, P., A. A. Russo, H. S. Chou, N. P. Pavletich, and M. J. Solomon. 1998. Human and yeast cdk-activating kinases (CAKs) display distinct substrate specificities. Mol. Biol. Cell 9:2545-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaldis, P., and M. J. Solomon. 2000. Analysis of CAK activities from human cells. Eur. J. Biochem. 267:4213-4221. [DOI] [PubMed] [Google Scholar]
- 38.Kato, A., H. Takahashi, Y. Takahashi, and H. Matsushime. 1997. Inactivation of the cyclin D-dependent kinase in the rat fibroblast cell line, 3Y1, induced by contact inhibition. J. Biol. Chem. 272:8065-8070. [DOI] [PubMed] [Google Scholar]
- 39.Kato, J., H. Matsushime, S. W. Hiebert, M. E. Ewen, and C. J. Sherr. 1993. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7:331-342. [DOI] [PubMed] [Google Scholar]
- 40.Kato, J. Y., M. Matsuoka, K. Polyak, J. Massague, and C. J. Sherr. 1994. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 79:487-496. [DOI] [PubMed] [Google Scholar]
- 41.Kato, J. Y., M. Matsuoka, D. K. Strom, and C. J. Sherr. 1994. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol. Cell. Biol. 14:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kivinen, L., and M. Laiho. 1999. Ras- and mitogen-activated protein kinase kinase-dependent and -independent pathways in p21Cip1/Waf1 induction by fibroblast growth factor-2, platelet-derived growth factor, and transforming growth factor-beta1. Cell Growth Differ. 10:621-628. [PubMed] [Google Scholar]
- 43.Kozar, K., M. A. Ciemerych, V. I. Rebel, H. Shigematsu, A. Zagozdzon, E. Sicinska, Y. Geng, Q. Yu, S. Bhattacharya, R. T. Bronson, K. Akashi, and P. Sicinski. 2004. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118:477-491. [DOI] [PubMed] [Google Scholar]
- 44.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 45.Lacy, E. R., I. Filippov, W. S. Lewis, S. Otieno, L. Xiao, S. Weiss, L. Hengst, and R. W. Kriwacki. 2004. p27 binds cyclin-CDK complexes through a sequential mechanism involving binding-induced protein folding. Nat. Struct. Mol. Biol. 11:358-364. [DOI] [PubMed] [Google Scholar]
- 46.Ladha, M. H., K. Y. Lee, T. M. Upton, M. F. Reed, and M. E. Ewen. 1998. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol. Cell. Biol. 18:6605-6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larochelle, S., and R. P. Fisher. 2005. CDK-activating kinases: detection and activity measurements. Methods Mol. Biol. 296:279-290. [DOI] [PubMed] [Google Scholar]
- 48.Larochelle, S., J. Pandur, R. P. Fisher, H. K. Salz, and B. Suter. 1998. Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev. 12:370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leng, X., M. Noble, P. D. Adams, J. Qin, and J. W. Harper. 2002. Reversal of growth suppression by p107 via direct phosphorylation by cyclin D1/cyclin-dependent kinase 4. Mol. Cell. Biol. 22:2242-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lents, N. H., S. M. Keenan, C. Bellone, and J. J. Baldassare. 2002. Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. J. Biol. Chem. 277:47469-47475. [DOI] [PubMed] [Google Scholar]
- 51.Li, Y., C. W. Jenkins, M. A. Nichols, and Y. Xiong. 1994. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene 9:2261-2268. [PubMed] [Google Scholar]
- 52.Liu, Y., C. Wu, and K. Galaktionov. 2004. p42, a novel cyclin-dependent kinase-activating kinase in mammalian cells. J. Biol. Chem. 279:4507-4514. [DOI] [PubMed] [Google Scholar]
- 53.Matsuoka, M., J. Y. Kato, R. P. Fisher, D. O. Morgan, and C. J. Sherr. 1994. Activation of cyclin-dependent kinase 4 (cdk4) by mouse MO15-associated kinase. Mol. Cell. Biol. 14:7265-7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsushime, H., D. E. Quelle, S. A. Shurtleff, M. Shibuya, C. J. Sherr, and J. Y. Kato. 1994. D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 14:2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuura, I., N. G. Denissova, G. Wang, D. He, J. Long, and F. Liu. 2004. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430:226-231. [DOI] [PubMed] [Google Scholar]
- 56.Miliani de Marval, P. L., E. Macias, R. Rounbehler, P. Sicinski, H. Kiyokawa, D. G. Johnson, C. J. Conti, and M. L. Rodriguez-Puebla. 2004. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol. Cell. Biol. 24:7538-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muraoka, R. S., A. E. Lenferink, B. Law, E. Hamilton, D. M. Brantley, L. R. Roebuck, and C. L. Arteaga. 2002. ErbB2/Neu-induced, cyclin D1-dependent transformation is accelerated in p27-haploin-sufficient mammary epithelial cells but impaired in p27-null cells. Mol. Cell. Biol. 22:2204-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muraoka, R. S., A. E. Lenferink, J. Simpson, D. M. Brantley, L. R. Roebuck, F. M. Yakes, and C. L. Arteaga. 2001. Cyclin-dependent kinase inhibitor p27(Kip1) is required for mouse mammary gland morphogenesis and function. J. Cell Biol. 153:917-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagahara, H., S. A. Ezhevsky, A. M. Vocero-Akbani, P. Kaldis, M. J. Solomon, and S. F. Dowdy. 1999. Transforming growth factor beta targeted inactivation of cyclin E:cyclin-dependent kinase 2 (Cdk2) complexes by inhibition of Cdk2 activating kinase activity. Proc. Natl. Acad. Sci. USA 96:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obaya, A. J., I. Kotenko, M. D. Cole, and J. M. Sedivy. 2002. The proto-oncogene c-myc acts through the cyclin-dependent kinase (Cdk) inhibitor p27(Kip1) to facilitate the activation of Cdk4/6 and early G(1) phase progression. J. Biol. Chem. 277:31263-31269. [DOI] [PubMed] [Google Scholar]
- 61.Ortega, S., M. Malumbres, and M. Barbacid. 2002. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim. Biophys. Acta 1602:73-87. [DOI] [PubMed] [Google Scholar]
- 62.Pagano, M., R. Pepperkok, J. Lukas, V. Baldin, W. Ansorge, J. Bartek, and G. Draetta. 1993. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J. Cell Biol. 121:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paternot, S., T. Arsenijevic, K. Coulonval, L. Bockstaele, J. E. Dumont, and P. P. Roger. 2006. Distinct specificities of pRb phosphorylation by CDK4 activated by cyclin D1 or cyclin D3: differential involvement in the distinct mitogenic modes of thyroid epithelial cells. Cell Cycle 5:61-70. [DOI] [PubMed] [Google Scholar]
- 64.Paternot, S., K. Coulonval, J. E. Dumont, and P. P. Roger. 2003. Cyclic AMP-dependent phosphorylation of cyclin D3-bound CDK4 determines the passage through the cell cycle restriction point in thyroid epithelial cells. J. Biol. Chem. 278:26533-26540. [DOI] [PubMed] [Google Scholar]
- 65.Pavletich, N. P. 1999. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287:821-828. [DOI] [PubMed] [Google Scholar]
- 66.Polyak, K., J. Y. Kato, M. J. Solomon, C. J. Sherr, J. Massague, J. M. Roberts, and A. Koff. 1994. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8:9-22. [DOI] [PubMed] [Google Scholar]
- 67.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59-66. [DOI] [PubMed] [Google Scholar]
- 68.Ren, S., and B. J. Rollins. 2004. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 117:239-251. [DOI] [PubMed] [Google Scholar]
- 69.Reynisdottir, I., and J. Massague. 1997. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11:492-503. [DOI] [PubMed] [Google Scholar]
- 70.Reynisdottir, I., K. Polyak, A. Iavarone, and J. Massague. 1995. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9:1831-1845. [DOI] [PubMed] [Google Scholar]
- 71.Rodier, G., A. Montagnoli, L. Di Marcotullio, P. Coulombe, G. F. Draetta, M. Pagano, and S. Meloche. 2001. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 20:6672-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roger, P. P., P. Servais, and J. E. Dumont. 1987. Induction of DNA synthesis in dog thyrocytes in primary culture: synergistic effects of thyrotropin and cyclic AMP with epidermal growth factor and insulin. J. Cell Physiol. 130:58-67. [DOI] [PubMed] [Google Scholar]
- 73.Russo, A. A., P. D. Jeffrey, A. K. Patten, J. Massague, and N. P. Pavletich. 1996. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382:325-331. [DOI] [PubMed] [Google Scholar]
- 74.Sexl, V., J. A. Diehl, C. J. Sherr, R. Ashmun, D. Beach, and M. F. Roussel. 1999. A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene 18:573-582. [DOI] [PubMed] [Google Scholar]
- 75.Sheaff, R. J., M. Groudine, M. Gordon, J. M. Roberts, and B. E. Clurman. 1997. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11:1464-1478. [DOI] [PubMed] [Google Scholar]
- 76.Sherr, C. J. 1995. D-type cyclins. Trends Biochem. Sci. 20:187-190. [DOI] [PubMed] [Google Scholar]
- 77.Sherr, C. J., and F. McCormick. 2002. The RB and p53 pathways in cancer. Cancer Cells 2:103-112. [DOI] [PubMed] [Google Scholar]
- 78.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 79.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 80.Sherr, C. J., and J. M. Roberts. 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18:2699-2711. [DOI] [PubMed] [Google Scholar]
- 81.Shimotohno, A., C. Umeda-Hara, K. Bisova, H. Uchimiya, and M. Umeda. 2004. The plant-specific kinase CDKF;1 is involved in activating phosphorylation of cyclin-dependent kinase-activating kinases in Arabidopsis. Plant Cell 16:2954-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin, I., J. Rotty, F. Y. Wu, and C. L. Arteaga. 2005. Phosphorylation of p27Kip1 at Thr-157 interferes with its association with importin alpha during G1 and prevents nuclear re-entry. J. Biol. Chem. 280:6055-6063. [DOI] [PubMed] [Google Scholar]
- 83.Soos, T. J., H. Kiyokawa, J. S. Yan, M. S. Rubin, A. Giordano, A. DeBlasio, S. Bottega, B. Wong, J. Mendelsohn, and A. Koff. 1996. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 7:135-146. [PubMed] [Google Scholar]
- 84.Sotillo, R., P. Dubus, J. Martin, E. de la Cueva, S. Ortega, M. Malumbres, and M. Barbacid. 2001. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 20:6637-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sugimoto, M., N. Martin, D. P. Wilks, K. Tamai, T. J. Huot, C. Pantoja, K. Okumura, M. Serrano, and E. Hara. 2002. Activation of cyclin D1-kinase in murine fibroblasts lacking both p21(Cip1) and p27(Kip1). Oncogene 21:8067-8074. [DOI] [PubMed] [Google Scholar]
- 86.Tassan, J. P., S. J. Schultz, J. Bartek, and E. A. Nigg. 1994. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase). J. Cell Biol. 127:467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Terada, Y., M. Tatsuka, S. Jinno, and H. Okayama. 1995. Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet irradiation. Nature 376:358-362. [DOI] [PubMed] [Google Scholar]
- 88.Tetsu, O., and F. McCormick. 2003. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cells 3:233-245. [DOI] [PubMed] [Google Scholar]
- 89.Ukomadu, C., and A. Dutta. 2003. Inhibition of cdk2 activating phosphorylation by mevastatin. J. Biol. Chem. 278:4840-4846. [DOI] [PubMed] [Google Scholar]
- 90.Vlach, J., S. Hennecke, and B. Amati. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 16:5334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe, N., M. Broome, and T. Hunter. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14:1878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weinberg, R. A. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323-330. [DOI] [PubMed] [Google Scholar]
- 93.Weiss, R. H., A. Joo, and C. Randour. 2000. p21(Waf1/Cip1) is an assembly factor required for platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J. Biol. Chem. 275:10285-10290. [DOI] [PubMed] [Google Scholar]
- 94.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 8:1750-1758. [DOI] [PubMed] [Google Scholar]