Abstract
Embryogenic cultures of Norway spruce (Picea abies) are composed of pro-embryogenic masses (PEMs) and somatic embryos of various developmental stages. Auxin is important for PEM formation and proliferation. In this report we show that depletion of auxin blocks PEM development and causes large-scale cell death. Extracts of the media conditioned by embryogenic cultures stimulate development of PEM aggregates in auxin-deficient cultures. Partial characterization of the conditioning factor shows that it is a lipophilic, low-molecular-weight molecule, which is sensitive to chitinase and contains GlcNAc residues. On the basis of this information, we propose that the factor is a lipophilic chitin oligosaccharide (LCO). The amount of LCO correlates to the developmental stages of PEMs and embryos, with the highest level in the media conditioned by developmentally blocked cultures. LCO is not present in nonembryogenic cultures. Cell death, induced by withdrawal of auxin, is suppressed by extra supply of endogenous LCO or Nod factor from Rhizobium sp. NGR234. The effect can be mimicked by a chitotetraose or chitinase from Streptomyces griseus. Taken together, our data suggest that endogenous LCO acts as a signal molecule stimulating PEM and early embryo development in Norway spruce.
The involvement of extracellular signal molecules in somatic embryogenesis has been reported in several plant species. Already in 1980 it was shown that when nonembryogenic cultures were treated with growth medium conditioned by highly embryogenic cultures, the cultures became embryogenic (Hari, 1980). Several components in the conditioned growth medium have been found to promote somatic embryogenesis. These components include chitinases (De Jong et al., 1992; Egertsdotter et al., 1993) and arabinogalactan proteins (AGPs; Kreuger and Van Holst, 1993, 1995; Egertsdotter and von Arnold, 1995; Thompson and Knox, 1998; Chapman et al., 2000). It has been suggested that oligosaccharides released from AGPs by a chitinase act as signal molecules stimulating somatic embryogenesis (Van Hengel et al., 2001).
Oligosaccharides with signaling functions, oligosaccharins (Darvill et al., 1992), are involved in the regulation of the plant growth and development (for review, see Spiro et al., 1998). Oligosaccharins of endogenous nature are suggested as activating factors in differentiation of tracheary elements in zinnia (Zinnia elegans; Roberts et al., 1997; Groover and Jones, 1999), Fucus sp. embryo patterning (Bouget et al., 1998) and regeneration of roots or flowers in thin-cell-layer explants of tobacco (Nicotiana tabacum; Eberhard et al., 1989). In addition, endogenous oligosaccharins (McCabe et al., 1997b; Van Hengel et al., 2001) and Nod factors, oligosaccharins isolated from Rhizobium, promote embryogenesis in plants (De Jong et al., 1993; Egertsdotter and von Arnold, 1998; Dyachok et al., 2000).
Nod factors are a family of lipo-chitooligosaccharide (LCO) signals uniformly consisting of an oligosaccharide backbone of β-1,4-linked GlcNAc residues varying in length between three and five sugar units, with an N-linked fatty acid moiety replacing the N-acetyl group on the nonreducing end. Because of their lipophilicity, Nod factors are generally isolated by the reverse-phase extraction, followed by purification by reverse-phase thin-layer chromatography or HPLC (Spaink et al., 1991; Price et al., 1992). In the absence of a suitable chromophore, LCOs are often labeled by metabolic incorporation of d-[1-14C] glucosamine into the chitin oligomeric backbone (Price and Carlson, 1995). Sensitivity to chitinases and a positive reaction to the modified Morgan-Elson assay are further evidences for the presence of a β-1,4-linked GlcNAc backbone. In addition, LCOs are further defined by their ability to induce certain morphological responses on plants.
It has long been known that Nod factors produced by rhizobia induce cell divisions in the root cortex of the host legume, leading to the formation of nodules (Spaink et al., 1991; Truchet et al., 1991). Furthermore, in Norway spruce (Picea abies), Nod factors can substitute for auxin and cytokinin to promote cell division (Dyachok et al., 2000). They also promote the development of pro-embryogenic masses (PEMs) from small cell aggregates in Norway spruce (Egertsdotter and von Arnold, 1998; Dyachok et al., 2000). In carrot (Daucus carota), Nod factors stimulate somatic embryos to proceed to the late globular stage (De Jong et al., 1993). Both in carrot and Norway spruce embryogenic systems, bacterial Nod factors can substitute for chitinases in their effect on early somatic embryo development (De Jong et al., 1992; Egertsdotter and von Arnold, 1998).
A homolog of the early nodulin gene ENOD40, OsENOD40, has been isolated from rice (Oryza sativa; Kouchi et al., 1999). In transgenic soybean (Glycine max), the OsENOD40 is expressed in peripheral nodule cells, suggesting that OsENOD40 and legume ENOD40 have similar functions in plants. Furthermore, the expression of another early nodulin gene from legume, ENOD12, in transgenic rice is stimulated by rhizobial Nod factors (Reddy et al., 1998). This demonstrates that the perception and transduction machinery required for the activation of this leguminous promoter by Nod factors is present in a non-legume.
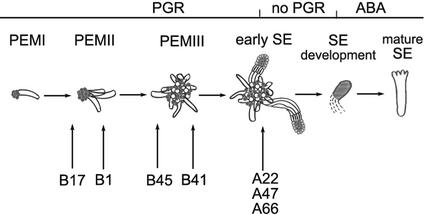
Somatic embryogenesis in Norway spruce is a multistep regeneration process, which starts with development of PEMs, followed by somatic embryo formation, maturation, desiccation, and plant regeneration. Embryogenic cultures contain a large number of PEMs that have reached various developmental stages, as well as early somatic embryos. Plant growth regulators (PGRs) auxin and cytokinin are required for the proliferation of PEMs, which includes subsequent development of PEMI through PEMII into PEMIII (Fig. 1). At stages of PEMI and PEMII the cell aggregates lack the organization and size that are needed for the differentiation of somatic embryos, whereas PEMIII is the stage when somatic embryos differentiate. Depletion of PGRs stimulates differentiation of somatic embryos from PEMIII (Filonova et al., 2000b). The rhizobial Nod factor and chitinases stimulate PEM development but not further embryo development (Egertsdotter and von Arnold, 1998; Dyachok et al., 2000).
Figure 1.
Schematic overview of somatic embryogenesis in Norway spruce (adapted from Filonova et al., 2000b). Proliferating cultures supplemented with the PGR cytokinin and auxin contain PEMs and early somatic embryos (SEs). PGRs are required for proliferation of PEMs, which includes subsequent development of PEMI through PEMII into PEMIII. Trans-differentiation of SEs from PEMIII is stimulated by withdrawal of PGRs. Abscisic acid (ABA) is required for development of mature SEs. Arrows under the developmental path indicate the most developed stage present in different cell lines under proliferation conditions in the presence of PGRs. In normal cell lines (A22, A47, and A66) SEs start to differentiate in the presence of PGRs. In cell lines B41 and B45, no SEs are formed but PEMIII are present. Cell line B41 contains more PEMIII than B45. Cell lines B1 and B17 contain only PEMI and PEMII. Cell line B1 contains more PEMII than B17.
In this work, we describe a biologically active Nod-factor-like compound that is present in conditioned medium from embryogenic cultures of Norway spruce. We show that endogenous LCO and rhizobial Nod factor stimulate early stages of somatic embryogenesis in Norway spruce. We also suggest a possible mechanism of LCO's action by demonstrating that LCOs suppress death of embryogenic cells.
RESULTS
Isolation of Extracellular Lipophilic Chitooligosaccharides from Embryogenic Cultures of Norway Spruce
When fractionated embryogenic cells were cultured in medium supplemented with the culture filtrate from embryogenic suspension cultures, the proliferation of PEMs was stimulated (data not shown). Based on previous work (Dyachok et al., 2000), we assumed that the stimulatory activity was related to LCO(s).
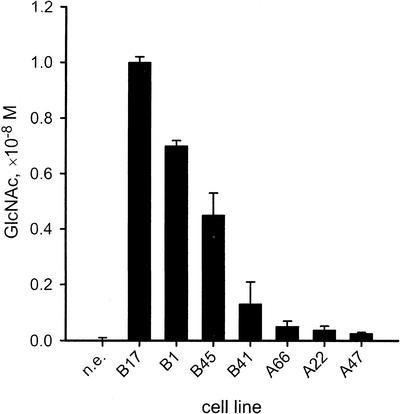
Lipophilic compounds were extracted from culture filtrates of embryogenic suspensions of different cell lines. Extracts were analyzed for the presence of GlcNAc-containing compounds using Morgan-Elson assay. Morgan-Elson positive compounds were found in lipophilic extracts from embryogenic suspension cultures but not in lipophilic extracts from nonembryogenic cultures (Fig. 2). The amount of Morgan-Elson positive compounds present in embryogenic cultures correlated to the developmental stage of PEMs and somatic embryos present in the cell line (Fig. 1). The content was higher in cell lines consisting of only PEMI and PEMII (B17 and B1), and lower in cell lines with developed somatic embryos (A66, A22, and A47). Addition of the chitinase inhibitor, allosamidin, at 10−6 m to the cell line B17 increased the content of Morgan-Elson positive compounds significantly from 1.1 × 10−8 m to 1.6 × 10−8 m (estimated using Student t test at P ≤ 0.05).
Figure 2.
Content of GlcNAc in Norway spruce suspension cultures. A nonembryogenic (n.e.) cell line and cell lines of group B (B17, B1, B45, and B41) and A (A66, A22, and A47) were cultured for 2 weeks. Lipophilic compounds were extracted from 500 mL of culture filtrates, and the amount of Morgan-Elson positive compounds was determined in the extracts. The data represent means ± se of two to three independent measurements per cell line.
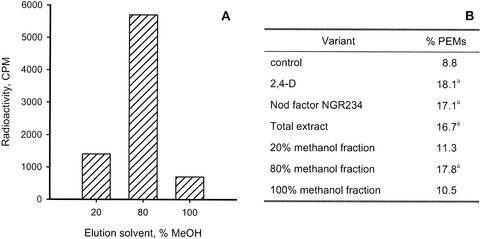
The lipophilic extract from embryogenic cultures of cell line B1 grown in the presence of sodium [1-14C]acetate was fractionated using the elution of the reverse-phase cartridge by successively increasing concentrations of methanol in water. The highest radioactivity was found in a fraction eluted by 80% (v/v) methanol (Fig. 3A). Eluates from the reverse-phase cartridge upon extraction of nonradiolabeled cultures (2 L) were further tested for their effect on PEM proliferation. In the absence of auxin, 9% of the PEMs proliferated into PEM aggregates as compared with 18% for those cultured with 2,4-D (Fig. 3B). Addition of the NGR234 Nod factor stimulated PEM proliferation. Similar stimulation was obtained when the nonseparated 100% (v/v) methanol extract or the 80% (v/v) methanol fraction was added.
Figure 3.
Metabolic labeling and biological activity of lipophilic compounds in culture filtrates from embryogenic cultures of Norway spruce. Suspension cultures (20 mL) of cell line B1 were grown in the presence of sodium [1-14C] acetate for 7 d. A larger volume (2 L) of this cell line also was cultured without radiolabeled precursor. Lipophilic compounds extracted by reverse-phase cartridge were eluted in one portion with 100% (v/v) methanol (total extract) or in sequential fractions with 20% (v/v), 80% (v/v), and 100% (v/v) methanol in water. A, Fractions of radiolabeled extract assayed for 14C radioactivity. B, Effect of different fractions on PEM proliferation. Total extract (amount corresponded to 10−8 m GlcNAc) or equivalent aliquots of fractions were added to the 80- to 160-μm fraction of A22 cultures. As an alternative, 9 × 10−6 m 2,4-D or 10−8 m Nod factor NGR234 were added to the fractionated cell cultures. The frequency of proliferating PEMs was determined after 3 weeks. The data are based on 2,000 PEMs per trial. aSignificantly different from the control as estimated using Z-test (P ≤ 0.05).
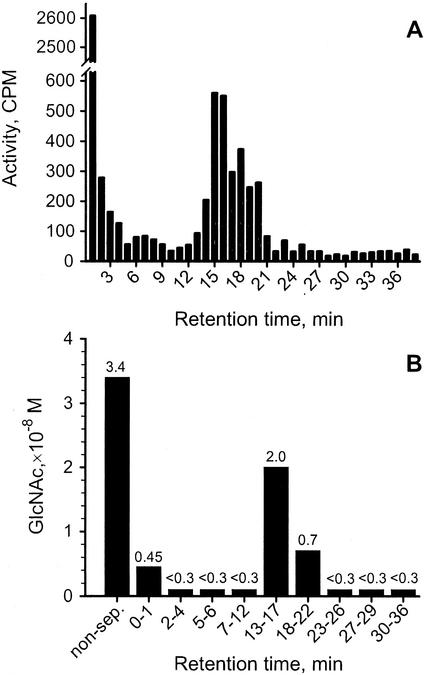
The 80% (v/v) methanol fraction from B1 cultures grown in the presence of N-acetyl-d-[1-14C]glucosamine was further separated using HPLC (Fig. 4A). The HPLC separation was repeated three times with different culture filtrates. In all cases, the fractions with the retention times (Rt) 13 to 22 min contained 14C-labeled compounds. However, most radioactivity of the culture filtrate extract was found in the injection peak (Rt 0–1 min). In some culture filtrates there was also incorporation of radioactivity from N-acetyl-d-[1-14C]glucosamine into fractions with Rt 25, 27 to 28, 30 to 31, and 35 to 39 min (data not shown). A large volume of nonradiolabeled culture filtrate was separated using HPLC and assayed for Morgan-Elson positive compounds (Fig. 4B). Morgan-Elson positive compounds were detected in fractions with Rt 0 to 1 min and 13 to 22 min (Fig. 4B). Fractions that contained Morgan-Elson positive compounds and fractions labeled with N-acetyl-d-[1-14C]glucosamine had the same Rt (Fig. 4, A and B). The experiment was repeated twice with similar results.
Figure 4.
HPLC separation of lipophilic compounds from embryogenic cultures of Norway spruce. Suspension cultures (20 mL) of cell line B1 were grown in the presence of N-acetyl-d-[1-14C] glucosamine for 14 d. A larger volume (2 L) of this cell line was also cultured without radiolabeled precursor. Lipophilic compounds were extracted by reverse-phase cartridge and separated by reverse-phase HPLC. A, HPLC fractions of radiolabeled extract assayed for 14C radioactivity. B, Amount of GlcNAc measured in extract before separation (non-sep), and in aliquot of fractions with Rts: 0 to 1, 2 to 4, 5 to 6, 7 to 12, 13 to 17, 18 to 22, 23 to 26, 27 to 29, and 30 to 36 min.
Culture filtrate fractionated by HPLC was analyzed by gas chromatography-mass spectrometry (GC-MS) for constituent monosaccharides. A GC peak corresponding to GlcN was obtained in fractions with the Rt 0 to 4 min (fraction A), 5 to 9 min (fraction B), and 13 to 17 min (fraction C), but not in the later fractions (Table I). The experiment was repeated three times with similar results. HPLC fractions were further tested for their ability to stimulate PEM proliferation. Fractions A and C stimulated PEM proliferation in a similar way as the nonseparated extract (Table I). MALDI-TOF mass spectra of the isolated fraction C revealed low-Mr compound(s) (Mr approximately 700) (data not shown).
Table I.
Effect of GlcN-positive lipophilic compounds on PEM proliferation
| Variant | GlcN | PEMs |
|---|---|---|
| ×10−8m | % | |
| Control | 8.5 | |
| 2,4-D | 17.6a | |
| 80% (v/v) methanol extract, nonseparated | 2.0 | 14.7a |
| Fraction A | 0.5 | 13.6a |
| Fraction B | 0.2 | 10.8 |
| Fraction C | 0.8 | 15.9a |
| Fraction D | <0.1 | 7.5 |
Lipophilic extracts from culture filtrates of cell line B1 were separated by HPLC. The content of GlcN was measured by GC-MS in the nonseparated 80% (v/v) methanol extract and in fractions with retention times (min): fraction A, 0 to 4; fraction B, 5 to 9; fraction C, 13 to 17; fraction D, 18 to 26. Equivalent aliquots of the nonseparated extract or fractions A, B, C, and D were added to the 80- to 160-μm fraction of A22 cultures. As an alternative, 9 × 10−6 m 2,4-D was added to the fractionated cultures. The frequency of proliferating PEMs was determined after 3 weeks. The data are based on 2,000 PEMs per trial.
Significantly different from the control as estimated using Z-test (P ≤ 0.05).
To test if the biological activity of fraction C is related to chitin containing compounds, we assayed the sensitivity to treatment with chitinase from Streptomyces griseus (Table II). In cultures supplemented with concentrated compounds from fraction C of the nontreated extract, the frequency of proliferating PEMs was significantly higher compared with the control (Table II). Pretreatment of the extract with chitinase significantly decreased the amount of GlcN in fraction C and resulted in the loss of the stimulatory effect of fraction C on PEM proliferation (Table II).
Table II.
Effect of chitinase treatment on lipophilic compounds from embryogenic cell line B1 of Norway spruce
| Variant | Pretreatment with Chitinase | GlcN | PEMs |
|---|---|---|---|
| ×10−8m | % | ||
| Control | 8.00 | ||
| 2,4-D | 18.20a | ||
| Nod factor NGR234 | 14.10a | ||
| 80% (v/v) methanol extract, nonseparated | − | 1.6 | 17.50a |
| Fraction C | − | 0.7 | 16.15a |
| Fraction C | + | <0.1 | 6.90 |
Equivalent aliquots of the nonseparated 80% (v/v) methanol extract were subjected to incubation without (−) or with (+) chitinase from S. griseus and thereafter separated by HPLC. Radiolabeled compounds were used as markers for HPLC separation. The GlcN content of the fractions coeluting with 14C radioactivity was measured by GC-MS. Equivalent aliquots of the nonseparated extract, fraction C of the non-digested extract, or fraction C of the extract treated with chitinase were added to the 80- to 160-μm fraction of A22 cultures. As an alternative, 9 × 10−6 m 2,4-D or 10−8 m Nod factor NGR234 was added to the fractionated cell cultures. The frequency of proliferating PEMs was determined after 3 weeks. The data are based on 2,000 PEMs per trial.
Significantly different from the control as estimated using Z-test (P ≤ 0.05).
We thus found that embryogenic cultures of spruce produce a compound that stimulates PEM proliferation in a similar way as rhizobial Nod factor. The biologically active compound is lipophilic, contains GlcNAc, is sensitive to chitinase, and has a low Mr.
Influence of Chitin Oligosaccharides and Chitinase on Embryogenic Cultures
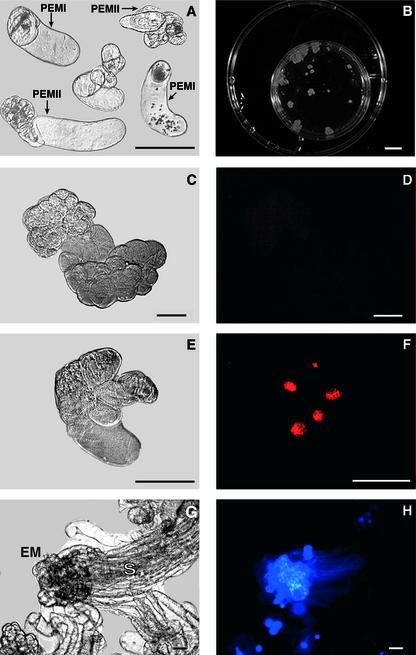
The 80- to 160-μm fraction of the cell line A22 consists of single cells and small PEMs (PEMI and PEMII; Fig. 5A). When cultured in medium containing benzyladenine (BA) and 2,4-D for 5 d, some PEMs proliferated while others died (Fig. 5C and E). Proliferating PEMs consisted of two types of cells, small meristematic cells and vacuolated cells (Fig. 5C). Most of the cells in proliferating PEMs were fluorescein diacetate (FDA)-positive, which indicated their viability (data not shown). Very few TUNEL-positive cells were present in proliferating PEMs (Fig. 5D). On the contrary, dead structures consisted of cells expressing morphological features of cell death, such as condensed and shrunken cytoplasm, and small and condensed nucleus (Fig. 5E). Most of the dead cells excluded FDA but were TUNEL-positive (Fig. 5F). Fractionated cultures proliferated and formed large cell aggregates consisting of PEMIII (>600 μm) and somatic embryos after 3 weeks (Fig. 5, B and G). The newly formed embryos could be distinguished by the presence of the embryonal mass consisting of densely packed small meristematic cells and the suspensor formed by the long vacuolated cells (Fig. 5, G and H).
Figure 5.
Development of PEMs in proliferation medium. Cell suspensions of cell line A22 were fractionated, and the 80- to 160-μm fraction consisting of single cells and small PEMs (PEMI and PEMII) was transferred to the medium supplemented with 9 × 10−6 m 2,4-D and 4.4 × 10−6 m BA. A, Day 0 (light microscopy). B, Examples of PEMI and PEMII at d 21. C to F, Examples of PEM aggregates after 5 d. Examples of proliferating (C and D) and dead (E and F) PEMs. C and E, Light microscopy. D and F, Labeling with TUNEL. Notice poor labeling in proliferating PEM and bright fluorescence in dead PEM. G and H, d 21. Example of a somatic embryo. G, Light microscopy. EM, Embryonal mass. S, Suspensor. H, Labeling with 4,6-diamidino-2-phenylindole. Notice bright fluorescence in the EM region consisting of small, densely packed cells. Bars, 100 μm in A and in C to H; 1 cm in B.
Three-day-old cultures were fractionated using nylon mesh, and the 80- to 160-μm cell fraction was transferred to medium supplemented with BA but free from 2,4-D. Cell death was assayed in cultures 5 d after fractionating. In the control cultures 70% of the cell aggregates were dead (Table III). Addition of 2,4-D significantly decreased the number of dead cell aggregates to 25%. The absence of 2,4-D could be compensated for by addition of fraction C, Nod factor, or chitotetraose. The effect could be mimicked by addition of chitinase. In contrast, addition of chitobiose did not significantly influence the number of dead cell aggregates.
Table III.
Effects of 2,4-D, Nod factor NGR234, LCO fraction, chitin oligosaccharides, and chitinase on survival and proliferation of PEMs and on differentiation of somatic embryos
| Variant | Dead Aggregates | PEMs | Embryos per 100 str |
|---|---|---|---|
| % | |||
| Control | 70.7 | 7.8 | 18.8 |
| 2,4-D | 25.5a | 20.9a | 37.5a |
| Nod factor NGR234 | 37.0a | 15.7a | 26.8a |
| Fraction C | 40.0a | 16.3a | 26.6a |
| Chitobiose | 64.8 | 8.5 | 16.2 |
| Chitotetraose | 44.7a | 16.7a | 25.0a |
| Chitinase | 27.4a | 22.1a | 47.0a |
Lipophilic compounds were extracted from culture filtrates of 14-d-old cultures of the B1 cell line. Extract was separated by reverse-phase HPLC, and the fraction corresponding to the retention times 13 to 17 min (fraction C) was collected. The GlcN content of the fraction was measured by GC-MS. 2,4-D (9 × 10−6 m), Nod factor (10−8 m), fraction C (10−8 m GlcN), chitobiose (10−8 m), chitotetraose (10−8 m), or chitinase from S. griseus (5.7 × 10−3 units mL−1) were added to the 80- to 160-μm fraction of A22 cultures. The frequency of dead cell aggregates was determined after 5 d. The frequency of proliferating PEMs and somatic embryos was determined after 3 weeks. The data are based on 2,000 PEMs per trial.
Significantly different from corresponding controls as estimated using Z-test (P ≤ 0.05).
After 3 weeks of growth, fractionated cultures were analyzed for proliferation and presence of somatic embryos. In the control cultures, 8% of small PEMs had proliferated (Table III). However, proliferation frequency increased to 21% when 2,4-D was added to the medium. In the absence of 2,4-D, proliferation was enhanced significantly by the addition of fraction C, Nod factor, chitotetraose, or chitinase but not by chitobiose (Table III). Regression analysis revealed negative correlation between the frequency of dead structures and the percentage of proliferating PEMs (r2 = 0.951).
The formation of somatic embryos in control cultures was poor (Table III). However, somatic embryo formation was stimulated when 2,4-D was added to the medium. Addition of fraction C, Nod factor, chitotetraose, or chitinase significantly enhanced formation of somatic embryos in the absence of 2,4-D (Table III). Addition of chitobiose did not significantly influence formation of somatic embryos. A positive correlation (r2 = 0.836) was seen between the frequency of proliferating PEMs and the number of somatic embryos.
We thus found that the frequency of cell death decreases upon addition of 2,4-D with simultaneous increase in PEM and embryo formation, and that addition of fraction C of culture filtrate, Nod factor, chitotetraose, or chitinase, but not chitobiose, could substitute for 2,4-D suppressing cell death and promoting PEM and embryo formation.
DISCUSSION
Embryogenic Cultures of Norway Spruce Produce LCO
To identify endogenous LCO in embryogenic cultures of Norway spruce, we screened cultures for β-1,4-GlcNAc-containing lipophilic compounds with pronounced biological activities. A similar approach was previously used to isolate LCO Nod factors from Rhizobium spp. (Spaink et al., 1991; Truchet et al., 1991). Reverse-phase extracts of the media conditioned by embryogenic cultures contain several lipophilic compounds. This is reflected by the presence of several absorbance peaks in the HPLC profile (not shown). We used degradation of the crude extract by chitinase in combination with the bioassay to test whether biological activity in the lipophilic fractions is related to chitin derivatives (Price et al., 1992). This method allowed preliminary identification of at least one fraction, fraction C (Rt 13–17 min), in which the decrease in GlcN content was accompanied by the loss of the ability to stimulate PEM formation. This fraction was also labeled by N-acetyl-d-[1-14C]glucosamine (Price and Carlson, 1995) and was positive in the Morgan-Elson assay for N-acetylated aminosugars (Chaplin, 1994). Furthermore, sugar analysis revealed the presence of GlcN. Mass-spectra analysis identified a low-Mr compound. These data indicate that the biological activity of the compound in fraction C with Rt 13 to 17 min is related to LCO.
Oligosaccharide isolated from the conditioned medium of embryogenic cultures of Norway spruce can be assigned to a group of oligosaccharins, the oligosaccharides with signaling function (Darvill et al., 1992). To date, biologically active oligosaccharins have been obtained by enzymatic degradation of cell wall polysaccharides, and their presence in planta is still questionable. It has been suggested that plants produce an endogenous oligosaccharin similar in structure to rhizobial Nod factors (Staehelin et al., 1994). Finding endogenous LCO in embryogenic cultures of Norway spruce supports this possibility.
The Content of LCO Is Developmentally Regulated
Embryogenic suspension cultures of Norway spruce contain PEMs, at different developmental stages, as well as early somatic embryos (Fig. 1). PEMs and embryos of a certain degree of development predominate in each of the cell lines we used in this study. Type B cell lines with only PEMI and PEMII have the highest content of extracellular LCOs, corresponding to 10−8 m GlcNAc. In type A cultures, which also contain PEMIII and somatic embryos, the extracellular LCO concentration is significantly lower. It is interesting that LCOs were not detected in nonembryogenic cultures. These findings indicate that LCOs are stimulating early processes during somatic embryogenesis. In consistence, Rhizobium sp. NGR234 Nod factor was previously found to stimulate PEM formation but not further embryo development (Egertsdotter and von Arnold, 1998; Dyachok et al., 2000). Inhibiting chitinase with allosamidin increases LCO content in embryogenic cultures. We have previously shown that cells in embryogenic cultures secrete chitinases and that there is a close correlation between the presence of specific chitinase and the developmental stage of PEMs and somatic embryos (Mo et al., 1996). At present we do not know if secreted chitinases can degrade LCOs in a similar way as plant chitinases hydrolyze the rhizobial Nod factors (Staehelin et al., 1994, 1995). However, it is tempting to assume that the 28-kD chitinase secreted in type A cultures but not in type B cultures degrades LCOs with the result that the LCO content is lower in type A cultures.
Other data suggest that chitinases are involved in the production of plant signal molecules, similar to the rhizobial Nod factors. The effect of EP3 endochitinase promoting somatic embryo development in carrot could be mimicked by rhizobial LCOs (De Jong et al., 1993). The EP3 chitinase colocalizes with AGPs in developing seeds, and it was shown to cleave AGPs in vitro (Van Hengel et al., 2001). Extracellular AGPs stimulate somatic embryogenesis in carrot. The stimulatory effect is enhanced if AGPs are first treated with EP3 (Van Hengel et al., 2001). These results suggest that AGPs are a substrate for EP3. This is supported by the finding that the nurse cells in carrot embryogenic cultures have specific AGP epitopes and release an embryogenesis promoting carbohydrate compound into the medium (McCabe et al., 1997b). Furthermore, in this study we show that chitinase from S. griseus stimulates PEM growth in a similar way as endogenous LCO. In accordance, endochitinase of class IV from sugar beet was found to stimulate early embryo development in Norway spruce in a similar way as LCO Nod factors from Rhizobium (Egertsdotter and von Arnold, 1998; Dyachok et al., 2000). Taken together these data indicate that different chitinases regulate embryogenesis in different ways. Some chitinases, such as the 28-kD chitinase specifically secreted by type A cultures, degrade LCOs, whereas others, such as the S. griseus chitinase and EP3, are involved in the formation of LCOs. It has been previously shown that enzymes that form and degrade oligosaccharins are largely responsible for when and where oligosaccharins are active in plant tissues (Albersheim et al., 1994). Chitinases might therefore be part of such a regulatory mechanism involving production and degradation of LCO oligosaccharins.
LCOs and Chitinase Stimulate Survival and Growth of PEMs
Embryogenic capacity of PEMs is closely related to the stage of development. According to their morphology and size, PEMs were divided into three groups, I, II, and III (Filonova et al., 2000b). PEMIII have an average size greater than 600 μm and after withdrawal of PGR give rise to somatic embryos. PEMs of smaller sizes, PEMI and PEMII, cannot differentiate somatic embryos. We used fractionated cultures consisting of PEMI and PEMII for the bioassay to examine specifically the effect of endogenous LCO and chitinase from S. griseus on early PEM development. Nod factor from Rhizobium sp. NGR234 and chitin fragments were tested for reference. Our data show that in Norway spruce endogenous and rhizobial LCOs, chitotetraose, and chitinase from S. griseus stimulate proliferation of PEMs. These results are consistent with previous data showing that Nod factors from Rhizobium and endochitinase from sugar beet stimulate the early stages of somatic embryogenesis in Norway spruce by promoting division of embryogenic cells (Egertsdotter and von Arnold, 1998; Dyachok et al., 2000). Stimulated PEM proliferation results in formation of PEMIII, large-sized PEMs giving rise to somatic embryos. We therefore propose that the increased number of embryos is a result of stimulating cell division and subsequent growth of PEMIII in cultures.
Chitotetraose but not chitobiose mimics the promotive effect of LCOs on PEM development. This indicates that the size of the chitin oligosaccharide is crucial. In accordance, synthetic chitin oligosaccharides inducing cortical cell division in a host plant always contain a carbohydrate core of four or more GlcNAc residues (Schlaman et al., 1997). Chitin oligosaccharides higher than trisaccharides are necessary to induce alkalinization response in cultures of tomato (Baureithel et al., 1994). The lipid part of the molecule does not appear to be required for stimulating somatic embryo development in Norway spruce. Similarly, chitin pentaose induces transient expression of the early nodulin gene ENOD40 in soybean roots when applied externally (Minami et al., 1996), and the chitin core devoid of the lipid part is sufficient to induce the mitogenic response once the molecule is delivered inside the cell (Schlaman et al., 1997). This indicates the crucial role of the chitin core for the activity of chitin oligosaccharides. However, the lipid moiety may still be important for the signaling function of oligosaccharins in planta.
Differentiation of somatic embryos from PEMIII in Norway spruce is accompanied by a large-scale programmed cell death (PCD; Filonova et al., 2000a). PCD is induced in embryogenic cultures of Norway spruce by withdrawal of PGRs (Filonova et al., 2000a). In this study we have shown that withdrawal of auxin induces PCD in PEMI and PEMII. Addition of LCOs, chitotetraose, or chitinase suppresses PCD. Similarly, carrot cells cultured at low density activate a PCD pathway that can be prevented by addition of cell free conditioned medium (McCabe et al., 1997a). It has previously been postulated that PCD occurs by default unless a constant supply of signal molecules released by the other cells keep it suppressed (Raff, 1992; Jacobson et al., 1997). β-d-glucosyl Yariv phenylglycoside, a chemical that specifically binds AGPs, inhibited growth of suspension-cultured cells of rose (Serpe and Nothnagel, 1994) and Arabidopsis (Gao and Showalter, 1999), implicating AGPs or their derivates as signaling molecules suppressing cell death. Similarly, we showed that chitin oligosaccharides and chitinase substitute for auxin in suppressing cell death in embryogenic cultures of Norway spruce.
In conclusion, our findings support the hypothesis that LCOs analogous to the rhizobial Nod factors do occur in plants. We have identified such an LCO in embryogenic cultures of Norway spruce. The amount of LCO is developmentally regulated. The endogenous LCO suppresses cell death in embryogenic cultures of Norway spruce in a similar manner as the bacterial Nod factors do. The future work will address the precise structure of endogenous compounds and their role in plant morphogenesis.
MATERIALS AND METHODS
Embryogenic and Nonembryogenic Cultures
Embryogenic suspension cultures of Norway spruce (Picea abies [L.] Karst) were maintained in LP medium as described earlier (Egertsdotter and von Arnold, 1993). Cell lines A22, A47, A66, B1, B17, B41, and B45 were used in these studies. The A cell lines were subcultured into fresh one-half-strength LP medium weekly, and the B cell lines were subcultured at 2-week intervals.
Somatic embryos were present in group A cell lines A22, A47, and A66, whereas group B cell lines B41, B45, B1, and B17 contained PEMs only (Fig. 1). The B cell lines were rated in order of their degree of development based on the size and organization of PEMs: B41>B45 >B1> B17. Cell line B41 contains large PEMs corresponding to PEMIII, whereas cell line B17 contains small PEMs corresponding to PEMI and PEMII (Fig. 1; Filonova et al., 2000b).
Nonembryogenic cultures were maintained as described earlier (Egertsdotter and von Arnold, 1995). The cell line NE1 was used in this study. The nonembryogenic suspension cultures were subcultured weekly.
Identification of LCO in Embryogenic Cultures of Norway Spruce
Radiolabeling and Extraction of LCO Fraction
Embryogenic cultures of cell line B1 (20 mL) were labeled with N-acetyl-d-[1-14C]glucosamine or sodium [1-14C]acetate (1 μCi mL−1), for 2 weeks.
Extraction of the LCO fraction was achieved by passing culture filtrates through a C18 reverse-phase silica cartridge (Chromabond, Chromos Express Ltd., Macclesfield, Cheshire, UK). After washing the cartridge with five volumes of water, compounds bound to the cartridge were eluted with 3-mL volume of 100% (v/v) methanol. As an alternative, cartridge bound compounds were eluted sequentially with 3-mL volumes of 20% (v/v) methanol in water, 80% (v/v) methanol in water, and 100% (v/v) methanol. Methanol fractions were evaporated to dryness under an airflow. The radioactivity in each fraction was assayed by scintillation counting (liquid scintillation counter 1209 Rackbeta, LKB, Uppsala, Sweden).
HPLC
The radiolabeled samples of culture filtrate extract were redissolved in aqueous 20% (v/v) acetonitrile. Aliquots of radiolabeled extract (20 μL, 5–10 nCi) were separated on an analytical C18 reverse-phase LiChrospher column (4 × 125 mm) using isocratic conditions of aqueous 20% (v/v) acetonitrile for 10 min, followed by a linear gradient to 100% (v/v) acetonitrile within 30 min at a flow rate of 2 mL min−1. The eluate was monitored at 206 nm, and the radioactivity in fractions was measured by scintillation counting.
Morgan-Elson Assay
Culture filtrates (0.5–2.0 L) were extracted by passing through a reverse-phase cartridge. After washing the cartridge with 5 volumes of water, bound compounds were eluted with a volume of 100% (v/v) methanol. The free GlcNAc content of the extract was measured by the Morgan-Elson reaction, using the procedure of Chaplin (1994). The glycosidic linkages in GlcNAc chains were hydrolyzed with trifluoroacetic acid:acetic acid:water (1:15:4, v/v) for 2 h at 100°C before Morgan-Elson analysis. Hydrolyzed samples were evaporated to dryness, redissolved in water, and assayed for free GlcNAc.
Compositional and Structural Analysis of LCO
MALDI-TOF mass spectra were recorded in positive detection mode on a Linear LDI 1700 XS mass spectrometer, using a dihydroxybenzoic acid matrix (100 mm dihydrobenzoic acid in 50% [v/v] methanol in water). GC-MS was performed on a Hewlett-Packard 5989B instrument (Hewlett-Packard, Palo Alto, CA). Samples were initially hydrolyzed with trifluoroacetic acid (2 m, 0.5 mL, 1 h, 121°C). Hydrolyzed sugars were reduced with 3 mg of sodium borohydride in 300 μL of 1 m ammonium hydroxide. After 1 h at room temperature the reaction was stopped by the drop-wise addition of acetic acid. Peracetylation of the alditols was completed by heating with 0.2 mL of pyridine and 0.2 mL of acetic anhydride (121°C, 30 min). The alditol acetates were then extracted into chloroform. For the quantitative estimation of GlcN, the instrument was calibrated using GlcNAc (Sigma, St. Louis). The GlcNAc content of the samples was determined from the areas of peaks co-eluting with an GlcNAc standard (Rt 29.6 min) and having the electron impact mass spectral fragmentation pattern predicted for GlcNAc alditol acetate. These data were then recalculated to give the initial GlcN content.
Manipulating Content of Morgan-Elson Positive Compounds by the Specific Chitinase Inhibitor Allosamidin
Allosamidin from Streptomyces sp. 1713 (Sakuda et al., 1986) was a kind gift of Dr. S. Sakuda (Dept. Applied Biological Chemistry, University of Tokyo). Allosamidin was previously reported to inhibit endochitinase activities in Pinus sylvestris roots (Hodge et al., 1996). Allosamidin was dissolved in 0.1 m acetic acid, diluted with growth media, filter sterilized, and added to the suspension cultures of Norway spruce at 10−6 m. After 1 week, the GlcNAc content of culture filtrates was determined by Morgan-Elson assay.
Isolation of Partially Purified LCO from Culture Filtrate of Embryogenic Cultures of Norway Spruce
Preparative scale volumes (up to 100 L) of culture filtrates were extracted by passing through C18 reverse-phase silica cartridges. The culture filtrate was extracted in portions of 2.5 to 3.0 L per cartridge. The cartridges were then washed, as described above for the radiolabeled cultures, with 5 volumes of water and eluted sequentially with volumes of 20% (v/v), 80% (v/v), and 100% methanol in water. The methanol phases from all cartridges were pooled and evaporated under an airflow. The residue was redissolved in aqueous 20% (v/v) acetonitrile. Radiolabeled compounds were used as markers for the isolation procedures. Aliquots of extract (370 μL) were separated by analytical reverse-phase HPLC as described in the “HPLC” section. Further separation was achieved using 20% (v/v) acetonitrile in an aqueous 20 mm ammonium acetate buffer (pH 5.8) for 10 min, followed by a linear gradient to 60% (v/v) acetonitrile within 15 min at a flow rate of 2 mL min−1. The amount of GlcNAc or GlcN in fractions co-eluting with radiolabeled compounds was determined by the Morgan-Elson reaction or by GC-MS, respectively. The nonseparated reverse-phase extract and fractions containing Morgan-Elson- or GlcN-positive compounds were used in bioassays.
Somatic Embryogenesis Bioassay
The bioassay was established to test the effect of conditioning factors on somatic embryo development in Norway spruce. The bioassay makes it possible to follow the development of small PEMs (PEMI and PEMII). The survival of PEMs was analyzed in parallel with the proliferation of PEMs and embryo formation. Suspension cultures of cell line A22 were fractionated by sequential sieving through nylon meshes with pore sizes of 160 and 80 μm. The fraction from 80 to 160 μm in size (80- to 160-μm fraction) was collected. This fraction consisted of single cells, PEMI and PEMII (Fig. 5A).
The recovered cells were washed thoroughly in liquid one-half-strength LP medium containing 4.4 × 10−6 m BA and then resuspended in the same medium. The number of PEMI and PEMII per mL of the 80- to 160-μm fraction was counted microscopically and adjusted to approximately 800 PEMs mL−1. Aliquots of 2.5 mL of the suspensions were mixed 1:1 with the medium containing the nonseparated reverse-phase extract or its fractions, Nod factor from Rhizobium sp. NGR234, tetra-N-acetyl-chitotetraose (chitotetraose, Sigma), N,N′-diacetylchitobiose (chitobiose, Sigma), or chitinase from Streptomyces griseus (Sigma). Purified broad host range Nod factor NGR234 was a kind gift from Prof. W.J. Broughton (Universite de Geneve, Switzerland). All compounds tested were dissolved in 80% (v/v) methanol in water, except for the chitinase, which was dissolved in water and added to a final volume of 20 μL per assay. Controls were supplied with 20 μL of corresponding solvent. As an alternative, 2.5 mL of the suspensions were mixed 1:1 with the medium supplemented with 2,4-D at 1.8 × 10−5 m.
Cultures were plated in agarose medium for the analysis of PEM formation, or grown in liquid medium for the analysis of the cell death and embryo formation. For the analysis of PEM development, the suspensions were mixed 1:1 with the medium containing 1.2% (w/v) low temperature melting agarose, 2-mL aliquots were plated in 60-mm Petri dishes. Cultures were kept under high relative humidity by placing the Petri dishes with cultures within 90-mm Petri dishes containing 5 mL of sterile water. The external dishes were sealed with plastic tape and kept in the dark at 22°C. After 3 to 4 weeks without subculturing, proliferating PEMs had protruded through the agarose layer and formed aggregates of PEMs on the surface of the solid medium (Fig. 5B). During this period, cultures were analyzed for changes in morphology. The frequency of proliferating PEMs was determined as the proportion of the initial PEMI and PEMII that proliferated and formed PEM aggregates (>600 μm) 3 to 4 weeks after plating.
For the in situ detection of the cell death associated with DNA fragmentation (TUNEL assay) and for the analysis of somatic embryo development, the suspensions containing tested compounds were mixed 1:1 with the liquid medium and cultured in 25-mL Erlenmeyer flasks. The suspensions (5 mL) were sampled for TUNEL assay after 5 d. The preparations were fixed in 4% (w/v) paraformaldehyde as previously described (Filonova et al., 2000a) and labeled with the in situ cell death detection kit, tetramethyl-rhodamine-dUTP (TMR)-red (Roche, Basel). As an alternative, the suspensions (5 mL) were sampled for staining with FDA (Sigma) and 4,6-diamidino-2-phenylindole (Boehringer Mannheim, Basel) at d 6, 10, 14, 17 and 21. Samples were examined using a Microphot FXA fluorescent microscope (Nikon, Tokyo).
ACKNOWLEDGMENTS
We thank Prof. W.J. Broughton for purified broad host range Nod factor NGR234 and Dr. S. Sakuda for allosamidin from Streptomyces sp. 1713. We also thank Dr. Lada Filonova for helping with TUNEL assay and for critical reading of manuscript and Dr. Steven Footit for helpful discussions.
Footnotes
This work was supported by the Royal Swedish Academy of Forestry and Agriculture (to J.V.D.) and by the Swedish International Development Cooperation Agency (to M.W.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010547.
LITERATURE CITED
- Albersheim P, An JH, Freshour G, Fuller MS, Guillen R, Ham KS, Hahn MG, Huang J, O'Neill M, Whitcombe A et al. Structure and function studies of plant cell wall polysaccharides. Biochem Soc Trans. 1994;22:374–378. doi: 10.1042/bst0220374. [DOI] [PubMed] [Google Scholar]
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes: competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Bouget FY, Berger F, Brownlee C. Position dependent control of cell fate in the Fucusembryo: role of intercellular communication. Development. 1998;125:1999–2008. doi: 10.1242/dev.125.11.1999. [DOI] [PubMed] [Google Scholar]
- Chaplin MF. Monosaccharides. In: Chaplin MF, Kennedy JF, editors. Carbohydrate Analysis: A Practical Approach. Ed 2. Oxford: IRL Press; 1994. pp. 1–7. [Google Scholar]
- Chapman A, Blervacq AS, Vasseur J, Hilbert JL. Arabinogalactan-proteins in Cichoriumsomatic embryogenesis: effect of beta-glucosyl Yariv reagent and epitope localization during embryo development. Planta. 2000;211:305–314. doi: 10.1007/s004250000299. [DOI] [PubMed] [Google Scholar]
- Darvill A, Augur C, Bergmann C, Carlson RW, Cheong JJ, Eberhard S, Hahn MG, Lo VM, Marfa V, Meyer B et al. Oligosaccharins: oligosaccharides that regulate growth, development and defense responses in plants. Glycobiology. 1992;2:181–198. doi: 10.1093/glycob/2.3.181. [DOI] [PubMed] [Google Scholar]
- De Jong AJ, Cordewener J, Lo Shiavo F, Terzi M, Vandekerckhove J, Van Kammen A, De Vries SC. A carrot somatic embryo mutant is rescued by chitinase. Plant Cell. 1992;4:425–433. doi: 10.1105/tpc.4.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong AJ, Heidstra R, Spaink HP, Hartog MV, Meijer EA, Hendriks T, Lo Shiavo F, Terzi M, Bisseling T, Van Kammen A et al. Rhizobiumlipooligosaccharides rescue a carrot somatic embryo mutant. Plant Cell. 1993;5:615–620. doi: 10.1105/tpc.5.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachok JV, Tobin AE, Price NPJ, von Arnold S. Rhizobial Nod factors stimulate somatic embryo development in Picea abies. Plant Cell Rep. 2000;19:290–297. doi: 10.1007/s002990050015. [DOI] [PubMed] [Google Scholar]
- Eberhard S, Doubrava N, Marfa V, Mohnen D, Southwick A, Darvill A, Albersheim P. Pectic cell wall fragments regulate tobacco thin-cell-layer explant morphogenesis. Plant Cell. 1989;1:747–755. doi: 10.1105/tpc.1.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertsdotter U, Mo LH, von Arnold S. Extracellular proteins in embryogenic suspension cultures of Norway spruce (Picea abies) Physiol Plant. 1993;88:315–321. [Google Scholar]
- Egertsdotter U, von Arnold S. Importance of arabinogalactan proteins for the development of somatic embryos of Norway spruce (Picea abies) Physiol Plant. 1995;93:334–345. [Google Scholar]
- Egertsdotter U, von Arnold S. Development of somatic embryos in Norway spruce. J Exp Bot. 1998;49:155–162. [Google Scholar]
- Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S. Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci. 2000a;113:4399–4411. doi: 10.1242/jcs.113.24.4399. [DOI] [PubMed] [Google Scholar]
- Filonova LH, Bozhkov PV, von Arnold S. Developmental pathway of somatic embryogenesis in Picea abiesas revealed by time-lapse tracking. J Exp Bot. 2000b;51:249–264. doi: 10.1093/jexbot/51.343.249. [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM. Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J. 1999;19:321–331. doi: 10.1046/j.1365-313x.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary wall synthesis. Plant Physiol. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari V. Effect of cell density changes and conditioned media on carrot somatic embryogenesis. Z Pflanzenphysiol. 1980;96:227–231. [Google Scholar]
- Hodge A, Gooday GW, Alexander IJ. Inhibition of chitinolytic activities from tree species and associated fungi. Phytochemistry. 1996;41:77–84. doi: 10.1016/0031-9422(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Kouchi H, Takane KI, So RB, Ladha JK, Reddy PM. Rice ENOD40: isolation and expression analysis in rice and transgenic soybean root nodules. Plant J. 1999;18:121–129. doi: 10.1046/j.1365-313x.1999.00432.x. [DOI] [PubMed] [Google Scholar]
- Kreuger M, Van Holst GJ. Arabinogalactan-proteins are essential in somatic embryogenesis of Daucus carotaL. Planta. 1993;189:243–248. [Google Scholar]
- Kreuger M, Van Holst GJ. Arabinogalactan-proteins epitopes in somatic embryogenesis of Daucus carotaL. Planta. 1995;197:135–141. [Google Scholar]
- McCabe PF, Levine A, Meijer PJ, Tapon NA, Pennell RI. A programmed cell death pathway activated in carrot cells cultured at low density. Plant J. 1997a;12:267–280. [Google Scholar]
- McCabe PF, Valentine TA, Forsberg LS, Pennell RI. Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell. 1997b;9:2225–2241. doi: 10.1105/tpc.9.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami E, Kouchi H, Cohn JR, Ogawa T, Stacey G. Expression of the early nodulin, ENOD40, in soybean roots in response to various lipo-chitin signal molecules. Plant J. 1996;10:23–32. doi: 10.1046/j.1365-313x.1996.10010023.x. [DOI] [PubMed] [Google Scholar]
- Mo LH, Egertsdotter U, von Arnold S. Secretion of specific extracellular proteins by somatic embryos of Picea abiesis dependent on embryo morphology. Ann Bot. 1996;77:143–152. [Google Scholar]
- Price NPJ, Carlson RW. Rhizobial lipo-oligosaccharide nodulation factors: multidimensional chromatographic analysis of symbiotic signals involved in the development of legume root nodules. Glycobiology. 1995;5:233–242. doi: 10.1093/glycob/5.2.233. [DOI] [PubMed] [Google Scholar]
- Price NPJ, Relic B, Talmont F, Lewin A, Prome P, Pueppke SG, Maillet F, Denarie J, Prome JC, Broughton WJ. Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulfated. Mol Microbiol. 1992;6:3575–3584. doi: 10.1111/j.1365-2958.1992.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Reddy PM, Ladha JK, Ramos MC, Maillet F, Hernandez RJ, Torrizo LB, Oliva NP, Datta SK, Datta K. Rhizobial lipochitooligosaccharide nodulation factors activate expression of the legume early nodulin gene ENOD12 in rice. Plant J. 1998;14:693–702. [Google Scholar]
- Roberts AW, Donovan SG, Haigler CH. A secreted factor induces cell expansion and formation of metaxylem-like tracheary elements in xylogenic suspension cultures of zinnia. Plant Physiol. 1997;115:683–692. doi: 10.1104/pp.115.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuda S, Isogai A, Matsumoto S, Suzuki A. The structure of allosamidin, a novel insect chitinase inhibitor, produced by Streptomycessp. Tetrahedron Lett. 1986;27:2475–2478. [Google Scholar]
- Schlaman HRM, Gisel AA, Quaedvlieg NEM, Bloemberg GV, Lugtenberg BJJ, Kijne JW, Potrykus I, Spaink HP, Sautter C. Chitin oligosaccharides can induce cortical cell division in roots of Vicia sativawhen delivered by ballistic microtargeting. Development. 1997;124:4887–4895. doi: 10.1242/dev.124.23.4887. [DOI] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA. Effects of Yariv phenylglycosides on Rosacell suspensions: evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta. 1994;193:542–550. [Google Scholar]
- Spaink HP, Sheeley DM, Van Brussel AAN, Glushka J, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg BJJ. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991;354:125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Spiro MD, Ridley BL, Eberhard S, Kates KA, Mathieu Y, O'Neill MA, Mohnen D, Guern J, Darvill A, Albersheim P. Biological activity of reducing-end-derivatized oligogalacturonides in tobacco tissue cultures. Plant Physiol. 1998;116:1289–1298. doi: 10.1104/pp.116.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin C, Schultze M, Kondorosi E, Kondorosi A. Lipo-chitooligosacharide nodulation signals from Rhizobium melilotiinduce their rapid degradation by the host plant alfalfa. Plant Physiol. 1995;108:1607–1614. doi: 10.1104/pp.108.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin C, Schultze M, Kondorosi E, Mellor RB, Boller T, Kondorosi A. Structural modifications in Rhizobium melilotiNod factors influence their stability against hydrolysis by root chitinases. Plant J. 1994;5:319–330. [Google Scholar]
- Thompson HJM, Knox JP. Stage-specific responses of embryogenic carrot cell suspension cultures to arabinogalactan protein-binding beta-glucosyl Yariv reagent. Planta. 1998;205:32–38. [Google Scholar]
- Truchet G, Roche P, Lerouge P, Vasse J, Camut S, De Billy F, Prome J-C, Denarie J. Sulfated lipo-oligosaccharide signals of Rhizobium melilotielicit root nodule organogenesis in alfalfa. Nature. 1991;351:670–673. [Google Scholar]
- Van Hengel AJ, Tadesse Z, Immerzeel P, Schols H, Van Kammen A, De Vries SC. N-acetylglucosamine and glucosamine-containing arabinogalactan proteins control somatic embryogenesis. Plant Physiol. 2001;125:1880–1890. doi: 10.1104/pp.125.4.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]