Abstract
The C-terminal binding protein (CtBP) family includes four proteins (CtBP1 [CtBP1-L], CtBP3/BARS [CtBP1-S], CtBP2, and RIBEYE) which are implicated both in transcriptional repression and in intracellular trafficking. However, the precise mechanisms by which different CtBP proteins are targeted to different subcellular regions remains unknown. Here, we report that the nuclear import of the various CtBP proteins and splice isoforms is differentially regulated. We show that CtBP2 contains a unique nuclear localization signal (NLS) located within its N-terminal region, which contributes to its nuclear accumulation. Using heterokaryon assays, we show that CtBP2 is capable of shuttling between the nucleus and cytoplasm of the cell. Moreover, CtBP2 can heterodimerize with CtBP1-L and CtBP1-S and direct them to the nucleus. This effect strongly depends on the CtBP2 NLS. PXDLS motif-containing transcription factors, such as BKLF, that bind CtBP proteins can also direct them to the nucleus. We also report the identification of a splice isoform of CtBP2, CtBP2-S, that lacks the N-terminal NLS and localizes to the cytoplasm. Finally, we show that mutation of the CtBP NADH binding site impairs the ability of the proteins to dimerize and to associate with BKLF. This reduces the nuclear accumulation of CtBP1. Our results suggest a model in which the nuclear localization of CtBP proteins is influenced by the CtBP2 NLS, by binding to PXDLS motif partner proteins, and through the effect of NADH on CtBP dimerization.
Human CtBP1, the founding member of the C-terminal binding protein (CtBP) family, was originally identified as a partner of the adenovirus E1A protein (36) and derives its names from its ability to bind the sequence at the E1A C-terminal Pro-X-Asp-Leu-Ser (PXDLS). Subsequently, a second highly related factor, CtBP2, was identified in vertebrates (26, 51). It now appears that CtBP1 is the first in a new family of corepressors that mediate the repression activity of a large number of transcription factors (13, 52). The corepression activity of CtBP1 and CtBP2 relies on the formation of a multiprotein complex containing the essential components for coordinated histone modifications, such as the histone deacetylases HDAC-1 and HDAC-2, the histone methyltransferase G9a, and the histone demethylase LSD1 (42, 43). Moreover, the CtBP family proteins share a high degree of homology with NAD+-dependent 2-hydroxy acid dehydrogenases (37), and it has been postulated that CtBP possesses intrinsic enzymatic activity (28). The significance of this is not yet fully understood.
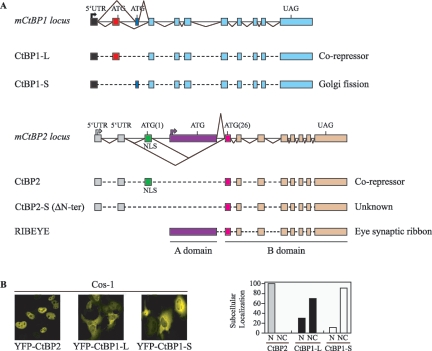
At present, four CtBP protein isoforms that are generated from the two distinct mammalian genes, CtBP1 and CtBP2, have been described (13). The CtBP1 protein and the CtBP3/brefeldin A-dependent ADP ribosylation substrate (BARS) protein (now termed CtBP1-L [L for long form] and CtBP1-S [S for short form], respectively) (12) are encoded by the CtBP1 gene locus, being generated by alternative splicing, while CtBP2 and RIBEYE are produced from the CtBP2 locus and are generated by differential promoter usage (Fig. 1A). CtBP1-L and CtBP1-S are splice isoforms that differ only in their N termini, a reflection of the fact that they are derived from mRNA with distinct AUG-containing first coding exons (12, 17, 46). CtBP1-S was first reported in the rat (47), whereas CtBP1-L was reported in human cells (36), but an examination of databases has confirmed that the CtBP1-S-specific exon is present in several mouse and human expressed sequence tag sequences, indicating that CtBP1-S is not an isoform exclusive to the rat (46). CtBP2 and RIBEYE are formed by differential promoter usage (39). Analysis of the human, rat, and bovine amino acid sequences of RIBEYE has revealed that RIBEYE is composed of a C-terminal B domain (420 residues) that is identical to the C terminus of CtBP2 (39) (Fig. 1A). This RIBEYE B domain contains the full-length CtBP2 sequence except the 20 N-terminal amino acids of CtBP2. RIBEYE also contains a large N-terminal A domain (565 residues) that is encoded by a unique exon. In contrast, CtBP2 is generated from an upstream promoter and a separate unique 5′ first coding exon (Fig. 1A). The shared C-terminal sequences are contained within eight common 3′ exons (33, 39).
FIG. 1.
Cellular localization of exogenously expressed CtBP1-L, CtBP2, and CtBP1-S. (A) Schematic representation (not to scale) of the exon structure of the genomic locus and splicing pattern, relative to mouse CtBP1 and mouse CtBP2, adapted from references 12, 17, 24, 33, and 39. Exons used exclusively for CtBP1-L (red box), CtBP2 (green box), CtBP2-S (CtBP2 ΔN-ter) (pink box), CtBP1-S (dark blue box), and RIBEYE (purple box) are color coded. The unique NLS of CtBP2 is indicated. (B) CtBP2 localizes into the nucleus of the cells, CtBP1-L is distributed throughout the nucleus and the cytoplasm, and CtBP1-S is predominantly cytoplasmic. Cos-1 cells were transfected with the indicated expression vectors, and the expressed proteins were visualized by confocal microscopy. The images shown in each panel are representative of all of the transfected cells. The graph depicts the quantitative data obtained from three independent experiments. In each experiment, more than 100 cells were counted in each group, with <10% standard deviation. N, exclusive nuclear staining; NC, cytoplasmic and/or nuclear staining.
CtBP1-L and CtBP2 can homo- and heterodimerize (and, more probably, multimerize) and, although it is well established that they copurify in transcriptional corepressor complexes (43), numerous studies have suggested broader roles for CtBP proteins in different cellular compartments. CtBP1-S was identified during a search for cytosolic factors that control Golgi tubulation (47) and was later shown to be a component of Golgi tubule-fissioning machinery in vitro (55) and to mediate mitotic Golgi partitioning (9). In line with its subcellular localization, CtBP1-S has also been implicated in the control of fission and basolateral transport from the Golgi to the plasma membrane and in fluid phase endocytosis in the dynamin-independent endocytic pathway (7). Initially, in in vitro studies, CtBP1-S was thought to be associated with a lysophosphatidic acid-specific acyltransferase activity that was proposed to play a role in the fissioning process (55). However, more recently, it has been reported that this activity is not essential for fission (7, 9, 56). This aspect of fissioning remains controversial, because recently it has been shown that the lysophosphatidic acid-acyltransferase activity associated with CtBP1-S (and also with the fissioning protein endophilin [38]) could result from copurification with an associated acyltransferase (20). The CtBP family member RIBEYE is a major constituent of the photoreceptor ribbon synapse (39). Interestingly, CtBP1-L is also a ribbon-associated molecule, suggesting that RIBEYE and CtBP1-L may be involved in tethering synaptic vesicles to the ribbon and in preparing them for exocytosis (50). While CtBP1-L, CtBP1-S, and RIBEYE appear to have functions in the cytoplasm, there is as yet little evidence that CtBP2 has significant functions outside the nucleus.
The mechanisms that direct CtBP proteins to different cellular compartments have not been elucidated. It has been noted that CtBP proteins can bind NAD+/NADH, and it has been proposed that CtBP1-S also binds acyl coenzyme A and that these bindings are mutually exclusive and may be involved in switching CtBPs from the nucleus to the cytoplasm and from transcriptional corepression to membrane fission (30). In accordance with this hypothesis, the interaction with CtBP1-S and the GTPase-activating protein ARFGAP1 appeared to be oppositely regulated by p-coenzyme A and NADH, with NADH inhibiting the release of the core complex of coat protein I (COPI)-coated vesicles from Golgi membranes (56). Moreover, there is evidence that the binding of CtBP proteins to NADH significantly increases their affinity for PXDLS motif-containing nuclear transcription factors, and so it is possible that this serves to drive them to the nucleus (58). In fact, CtBP proteins are believed to act as redox sensors in transcriptional repression, thus providing a link between gene expression and metabolism (44). However, the effect of altering the NAD+/NADH ratio on CtBP cellular localization has not been investigated.
There is evidence that CtBP activity and its cellular localization are regulated by a variety of effectors in different cell types. To date, most studies have focused on CtBP1-L, and it is clear that its cellular localization can be regulated. For example, exogenously expressed CtBP1-L was found to be exclusively cytoplasmic in Cos-7 cells but remains mainly nuclear in Saos2 cells (15). However, when the PXDLS motif-containing transcription factor Net was coexpressed with CtBP1-L in Cos-7 cells, CtBP1-L was found to be mainly nuclear (15), suggesting that interactions with nuclear transcription factors can redirect CtBP1-L. Similarly, in U2OS and Cos-1 cells, the expression of the transcription factor HIC-1 resulted in the relocalization of CtBP1-L into HIC-1-positive nuclear dots (18). Likewise, CtBP1-L and CtBP2 have been found to partially colocalize with Pc2 in polycomb nuclear domains (PcG bodies) (25, 41). Moreover, the homeodomain-interacting protein kinase 2 (HIPK2) has recently been reported to phosphorylate CtBP1-L at Ser422, an important modification that marks CtBP1-L for clearance via a proteasome-dependent pathway and thus promotes apoptosis (59).
Additionally, binding of neuronal nitric oxide synthase (nNOS) to CtBP1-L, by virtue of its interaction with the C terminus DQL-containing sequence of CtBP1-L, redirects CtBP1-L from the nucleus to the cytosol (34). CtBP1-L is also subject to sumoylation at Lys 428, and mutation of this residue shifts CtBP1-L from the nucleus to the cytoplasm (29). Interestingly, sumoylation of CtBP1-L is inhibited by the PDZ domain of nNOS, consistent with the known inhibitory effect of nNOS on the nuclear accumulation of CtBP1-L (29, 34). Finally, the p21-activated kinase 1 (Pak1) has recently been shown to phosphorylate CtBP1-L selectively on Ser 158, triggering CtBP1-L cellular redistribution to the cytoplasm and blocking CtBP1-L corepressor functions (3).
Nevertheless, there is strong evidence that CtBP1-L and CtBP2 are found in transcriptional complexes in the nucleus, indicating that CtBP proteins must be imported into the nucleus from the cytoplasm. However, the regions within CtBP1-L and CtBP2 that serve as signals for their nuclear or cytoplasmic localization, and the proteins that enable CtBP1-L and CtBP2 to become localized in the nucleus, are unknown. In this report, we provide a study of the signals that mediate the nuclear localization of CtBP1-L and CtBP2 proteins. We show that the unique first coding exon of CtBP2, which represents one of the most divergent regions between CtBP1-L and CtBP2, contains a nuclear localization signal (NLS). This observation fits with reports that CtBP2 is involved primarily in nuclear gene repression functions (49, 51). However, by using a heterokaryon assay, we show that CtBP2 not only localizes to the nucleus but also is capable of shuttling between the nucleus and the cytoplasm. Furthermore, it was observed that the expression of a CtBP2 isoform containing this NLS can direct CtBP1-L and CtBP1-S to the nucleus, as can nuclear transcription factors that bind CtBPs. We also describe a splice variant of CtBP2, CtBP2-S, that lacks the NLS and is consequently not localized to the nucleus. Finally, our results support the view that NADH stimulates CtBP dimerization. This result is of interest, as the nuclear localization of CtBP1 proteins, in some circumstances, may depend on dimerization with CtBP2. Furthermore, the ability of NADH to enhance binding of transcriptional repressors may reflect CtBP oligomerization and the presentation of multiple PXDLS-binding clefts. In conclusion, the data obtained suggest that the subcellular distribution of CtBP proteins is controlled by multiple mechanisms and that the nonconserved N-terminal regions of CtBPs are critical determinants in regulating the biological functions of the various CtBP proteins.
MATERIALS AND METHODS
Plasmid constructs.
pMT3-YFP and pMT3-CFP were generated by ligating NsiI/NotI PCR fragments from pEYFP-C1 and pECFP-C1 (Clontech), respectively, into PstI/NotI sites of pMT3 vector (derived from pMT2). pMT3-YFP-CtBP1-L, pMT3-CFP-CtBP1-L, pMT3-YFP-CtBP1-L (amino acids [aa] 20 to 440), pMT3-YFP-CtBP1-L (75 to 440) and pMT3-YFP-CtBP1-L G181V/L182V/G183V were obtained by ligating NotI/EcoRI PCR fragments from pCMV5-Flag-hCtBP1-L (25), kindly provided by D. Wotton (Department of Biochemistry and Molecular Genetics, Center for Cell Signaling, University of Virginia, Charlottesville, Va.), using appropriate oligonucleotides and in frame into NotI/EcoRI sites of pMT3-YFP or pMT3-CFP vectors. For the C-terminal Flag-tagged and yellow fluorescent protein (YFP)-tagged CtBP1-L constructs, full-length hCtBP1-L cDNA was ligated into NotI and SalI sites of pMT3 vector in frame with either Flag epitope or YFP ligated downstream into SalI/EcoRI sites of pMT3. The same procedure was followed for constructing C-terminal Flag-tagged and YFP-tagged CtBP2 and CtBP1-S constructs.
The same procedure was followed for constructing pMT3-YFP-CtBP1-S and pMT3-CFP-CTBP1-S. Briefly, NotI/SalI PCR fragments from pcDNA3-rCtBP3/BARS (47) were ligated in frame to the 3′ end of pMT3-YFP or pMT3-CFP digested by NotI/SalI.
pMT3-YFP-CtBP2, pMT3-CFP-CtBP2, pMT3-YFP-CtBP2 K10A/R11A, pMT3-CFP-CtBP2 K10A/R11A, pMT3-YFP-CtBP2 ΔNter (aa 26 to 445), and pMT3-YFP-CtBP2 Nter (1 to 25) were generated by ligating NotI/SalI PCR fragments from pcDNA3-mCtBP2 (51) into NotI/SalI sites of pMT3-YFP or pMT3-CFP. pECFP-BKLF was constructed by ligating the EcoRI/BamHI PCR fragment from pMT2-mBKLF (16) in frame to the 3′ end of the sequence encoding cyan fluorescent protein (CFP) in pECFP-C1 (Clontech). pMT3-YFP-Nter CtBP2-CtBP1-L (aa 20 to 440) and pMT3-YFP-Nter CtBP2 K10A/R11A-CtBP1-L (20 to 440) chimeras were generated as follows: N-ter wild-type and N-ter K10A/R11A CtBP2 NotI fragments (aa 1 to 25) were generated by PCR and ligated in frame into pMT3-YFP-CtBP1-L (20 to 440) digested by NotI. pMT2-HA-CtBP2 has been described previously (53).
Full-length human CtBP1 (hCtBP1-L), hCtBP1-L (aa 75 to 440), and hCtBP1-L G181V/L182V/G183V were amplified by PCR, and the products were cloned into EcoRI/SalI pGBT9 or pGAD10(new) (derived from pGAD10) vectors (Clontech). The resulting constructs were used in the yeast experiments described in the legend to Fig. 6. pGBT9-CtBP2, pGAD10(new)-CtBP2, and pGAD10(new)-BKLF (aa 1 to 268) have been previously described (51, 53). All plasmids have been verified by sequencing. Detailed information is available upon request. The plasmid pB-rRE-EGFP (RIBEYE B domain) was a gift from F. Schmitz (University of Saarland, Homburg, Saar, Germany) (39).
FIG. 6.
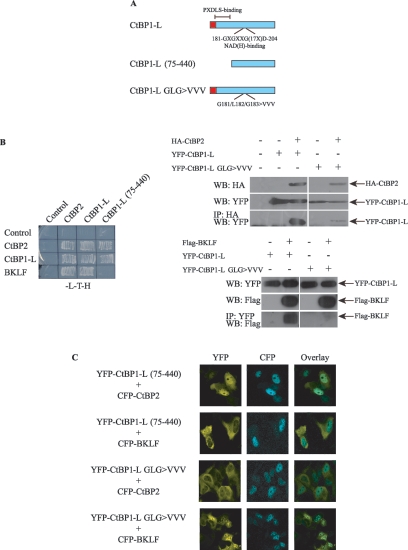
NADH regulates CtBP oligomerization. (A) Schematic illustration of CtBP1-L and mutants. The PXDLS-binding region and the NADH binding site are indicated. (B) CtBP1-L derivatives were tested for their ability to homodimerize, to heterodimerize with CtBP2, and to interact with a recognized PXDLS-motif-containing partner protein, BKLF. Left panel, yeast growth was monitored and the results reflecting association of the proteins are shown. Right panel, immunoprecipitation analysis. Cos-1 cells were transfected with YFP-CtBP1-L, YFP-CtBP1-L GLG>VVV, HA-CtBP2, and Flag-BKLF as indicated. Cell lysates were prepared and HA-CtBP2 or YFP-CtBP1-L protein complexes isolated using anti-HA and anti-YFP agarose affinity resin, respectively. Proteins in the cellular lysates and immunoprecipitated (IP) proteins were subsequently analyzed by Western blot (WB) analysis using the indicated antibodies. (C) Cos-1 cells were cotransfected with the indicated CFP and YFP expression vectors, and localization of the expressed proteins was visualized by confocal microscopy. The images shown in each panel are representative of all of the transfected cells.
Cell culture and transfections.
Cos-1, mouse erythroleukemia (MEL), and HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin, streptomycin, and glutamine. Cos-1 cells were transfected with 500 ng of pMT3-YFP or pMT3-CFP constructs complemented with pMT3 to achieve a total amount of 1 μg of DNA, using the transfection reagent FuGENE 6 (Roche Diagnostics) following the manufacturer's instructions.
Detection of YFP and CFP immunofluorescence.
Cells were grown on two-well chamber slides (Lab-Tek). Twenty-four hours after transfection, living cells were washed extensively with phosphate-buffered saline (PBS) and directly analyzed by confocal microscopy. We used a Leica TCS SP2 (AOBS) confocal microscope operating with a 40-mW argon laser. The laser was tuned to lines of 458 nm and 514 nm. Cells were examined with a 40×, 1.3-numerical-aperture objective. The gain of photomultiplier tubes and the emission intervals were adjusted to eliminate cross talk between the CFP (470 to 500 nm) and YFP (530 to 600 nm) channels. Quantitative estimates of nuclear/cytoplasmic distribution of pMT3-YFP-CtBP1-L, pMT3-YFP-CtBP2, and pMT3-YFP-CtBP1-S (Fig. 1B) were obtained by analyzing at least 100 cells in three different experiments.
To observe endogenous CtBP1-L and CtBP2, Cos-1 and HeLa cells were seeded on two-well chamber slides (Lab-Tek) and grown to 70 to 80% confluence in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Cells were washed extensively with PBS, fixed for 20 min at room temperature with 4% paraformaldehyde in PBS, permeabilized with 0.05% Triton X-100 in PBS for 5 min, and incubated for 15 min with PBS containing 1% bovine serum albumin. Cells were then incubated overnight at 4°C with the primary antibody at the appropriate dilution. The antibodies used were monoclonal anti-mCtBP1 and monoclonal anti-mCtBP2 (1:200; BD Transduction Laboratories, Clontech). Cells were then washed with PBS, incubated for 15 min with 1% bovine serum albumin in PBS and incubated for a further 60 min with the secondary antibody, fluorescein-conjugated goat anti-mouse immunoglobulin G (1:200, Pierce). After extensive washing with PBS, cell monolayers were mounted in 10 mM Tris-HCl (pH 9) containing 60% glycerol and were examined by confocal microscopy.
Heterokaryon assays.
Interspecies heterokaryons of Cos-1 and mouse NIH 3T3 cells were formed as follows. Briefly, Cos-1 cells were transfected with 500 ng of the YFP-CtBP2 expression vector. Twenty-four hours posttransfection, Cos-1 cells were seeded on two-well chamber slides (Lab-Tek) with an equal number of untransfected NIH 3T3 cells. The coculture was incubated overnight in the presence of 50 μg/ml of cycloheximide. After being fused with 50% Hybrimax polyethylene glycol (Sigma) for 2 min at 37°C, cells were washed and returned to medium containing 100 μg/ml of cycloheximide for a further 4 h. Cells were then fixed in 4% paraformaldehyde in PBS for 20 min and washed in PBS. Cell monolayers were mounted in 10 mM Tris-HCl (pH 9) containing 60% glycerol and stained in 1 μg/ml of Hoechst 33258 (Sigma) to allow identification of mouse NIH 3T3 and monkey Cos-1 nuclei. Samples were analyzed by confocal microscopy.
Western blot and immunoprecipitation analysis.
Cells were fractionated with NE-PER nuclear and cytoplasmic extraction reagent (Pierce Chemical Co.) according to the manufacturer's instructions. The extracts were used immediately or stored at −70°C. Ten micrograms of cytoplasmic and nuclear proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10 to 12% polyacrylamide gels and transferred on to a Biotrace nitrocellulose blotting membrane (Pall Gelman Sciences, Ann Arbor, Mich.) in a TE series Transphor electrophoresis unit (Hoefer), at 100 mA overnight at 4°C.
For immunoprecipitation, Cos-1 cells were transfected with 1 μg of pMT3-YFP-CtBP1-L, pMT3-YFP-CtBP1-L G181V/L182V/G183V, pMT2-HA-CtBP2, or pMT3-Flag-BKLF (aa 1 to 268) vectors, as indicated in Fig. 6B. At 48 h after transfection, cells were harvested and lysed in NP-40 cell lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40) containing protease inhibitor cocktails. Cell lysates were subjected to immunoprecipitation with 20 μl of protein G agarose (Roche) and 10 μg of the various antibodies (as shown in Fig. 6). Antibody-protein G agarose-bound protein complexes were extensively washed with NP-40 cell lysis buffer. After washing, protein G-antibody-bound proteins were separated by SDS-PAGE, transferred onto nitrocellulose membrane, and probed with primary antibodies as shown in Fig. 6.
For Western blotting, the membrane was washed once in 50 mM Tris-HCl (pH 7.5) containing 150 mM NaCl and 0.05% Tween-20 (Tris-NaCl-Tween) and then incubated at room temperature in skim milk powder solution (5% [wt/vol] in Tris-NaCl-Tween) for 1 h. The membrane was rinsed in Tris-NaCl-Tween and incubated for 1 h with gentle shaking in 10 ml of Tris-NaCl-Tween containing 10 μg of primary antibody. After five washes with 150 ml of Tris-NaCl-Tween, the secondary antibody solution was added, and incubation was continued for 1 h. The membrane was washed for 1 h in several changes of Tris-NaCl-Tween. Detection was carried out using Renaissance Chemiluminescence Reagent Plus (NEN Life Sciences, Boston, Mass.), and the signal was detected on X-ray film (Eastman Kodak Company, Rochester, N.Y.) and developed using Kodak reagents. Anti-CtBP2, anti-CtBP1, and anti-BD Living Colors A.v. (anti-YFP, JL-8) mouse monoclonal antibodies were obtained from BD Transduction Laboratories, Clontech. Anti-HA monoclonal antibody (12CA5) was provided by Roche Corporation, Germany, and anti-Flag M2 was provided by Sigma.
RT-PCR.
Total RNA from murine fetal livers at 12.5 days postcoitum (dpc) was isolated as outlined in the TRI-reagent protocol (Sigma) and reverse transcribed using a SuperScript first-strand synthesis system (Invitrogen). Reverse transcription (RT)-PCRs were then carried out for 30 cycles using 1 μl of cDNA template and recombinant Taq Pfu DNA polymerase. Cycling parameters were denaturation at 92°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 3 min. PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining. The primer sequences were designed to target the 5′ untranslated regions (UTRs) and the first three coding exons of mCtBP2 (GenBank accession number NM_009980). A first round of RT-PCR was performed with sense primer 5′-CTGGGAGCCATGCCGCGCTGAGAG-3′ (5′ UTR1) and antisense primer 5′-GAGCTCGCCAGCAGCCTTGATGTCCACGTT-3′ (Exon 3) and then nested with sense primer 5′-CTTCATCACTGTAAATGGTTGCAAGCCGAC (5′ UTR2) and antisense primer 5′-CAGGATGGGCATCTCCACAGTGCA (Exon 2). See Fig. 1A and 7B for more details.
FIG. 7.
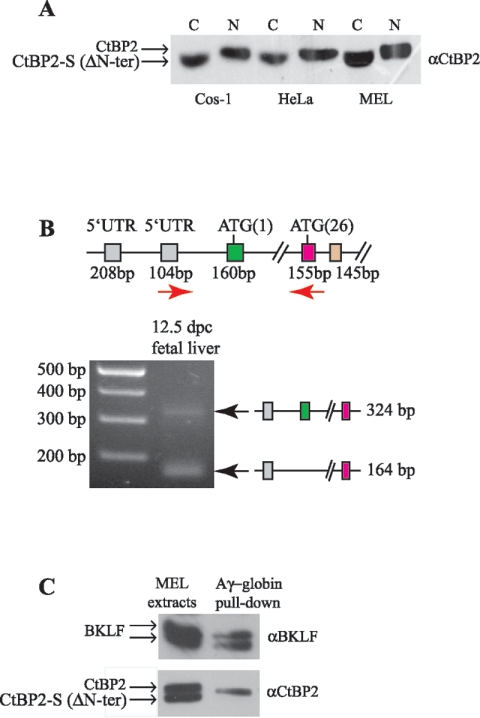
Identification of a new CtBP2 splice form. (A) Cytoplasmic (C) and nuclear (N) protein extracts of Cos-1, HeLa, and MEL cells were subjected to Western blot analysis with anti-CtBP2 antibodies. A faster migrating band is consistently found only in the cytoplasmic fraction and likely represents a splice form of CtBP2 without the first coding exon containing the NLS. Full-length CtBP2 and CtBP2-S (ΔN-ter) are indicated (arrows). (B) Expression of mouse CtBP2. RT-PCR was performed with cDNA templates from mouse fetal liver at 12.5 dpc. PCR products were electrophoresed on agarose gels and visualized by ethidium bromide staining. The genomic organization of the mCtBP2 locus is indicated as in Fig. 1A, and the primers used for RT-PCR are indicated by red arrows. Two differentially expressed bands are detected, the upper one (324 bp) corresponding to CtBP2 containing both exon 1 (methionine M1, green) and exon 2 (methionine M26, pink) and the lower one (164 bp) corresponding to the CtBP2 isoform missing the first coding exon (methionine M1, 160 bp). The two splice forms are indicated at right. (C) A magnetic bead-DNA pull-down assay showing that full-length CtBP2, but not CtBP2-S (ΔN-ter), is able to localize to the proximal Aγ-globin promoter. MEL cell extract was incubated with DNA containing the BKLF binding sites (CACCC boxes) coupled to magnetic beads. Complexes were blotted with antibodies against BKLF and CtBP2.
Yeast two-hybrid assay.
A Clontech two-hybrid system was used according to the manufacturer's instructions. Test proteins were expressed in HF7c yeast as either Gal4DBD (pGBT9) vector or Gal4AD fusions [pGAD10(new)] vector. Transformed colonies were selected on Leu/Trp-deficient plates and patched onto His/Leu/Trp-deficient (−L−T−H) plates. Growth was scored following 72 h of incubation.
Biotinylated DNA oligonucleotide precipitation.
Biotin-labeled sense and unlabeled antisense oligonucleotides (200 pmol/mg beads), encompassing 350 bp of the human Aγ globin proximal promoter, including the CACCC boxes that bind BKLF, were annealed and coupled to Dynabeads M-280 Streptavidin (Dynal) according to the manufacturer's instructions. For pull-down assays, MEL cell whole extract (250 μg) was incubated with 125 μg of oligonucleotide-coupled beads in 500 μl of binding buffer (20 mM Tris-HCl [pH 7.9], 5 mM EDTA, 50 mM KCl, 2.5 mM MgCl2, 0.05% Nonidet P-40, 10% glycerol, 1 mM dithiothreitol) with protease inhibitors for 3 h at 4°C. After extensive washing, bound proteins were run on SDS-PAGE and detected by Western blotting with either anti-CtBP2 monoclonal mouse antibody (BD Transduction Laboratories, Clontech) or anti-BKLF (16).
RESULTS
Subcellular localization of CtBP proteins.
Although CtBP proteins are well known as nuclear transcriptional corepressors, the mechanisms governing their nuclear import have not been extensively investigated. Therefore, we first set out to define regions of CtBP1-L and CtBP2 involved in nuclear import. To visualize the cellular localization of CtBP proteins by confocal laser microscopy, we first linked the gene encoding YFP upstream of the full-length CtBP1-L and CtBP2 cDNAs. These YFP constructs were transfected into Cos-1 cells, and the cellular localization of CtBP1-L and of CtBP2 was determined. As shown in Fig. 1B, the YFP-CtBP2 fusion protein is found exclusively in the nucleus. In contrast, YFP-CtBP1-L appears localized to both the nucleus and the cytoplasm. In around 30% of cells, CtBP1-L was found predominantly in the nucleus, while in the remaining 70%, it was present in both the nucleus and the cytoplasm (Fig. 1B). In addition, we found that rat CtBP1-S, the CtBP1 isoform previously implicated in Golgi fission (7, 47, 55), is found predominantly in the cytoplasm but also appeared in the nucleus in a minority of cells (Fig. 1B). Therefore, despite their strong sequence homology, CtBP1-L, CtBP2, and CtBP1-S show different patterns of subcellular distribution in transfected Cos-1 cells.
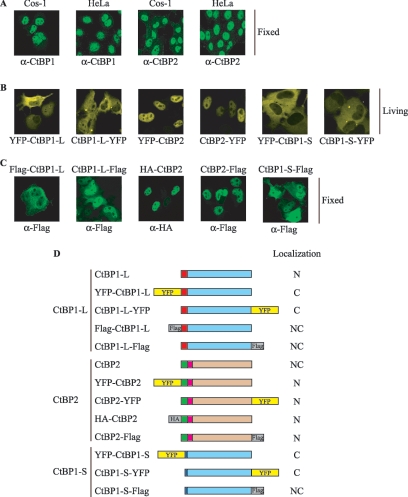
CtBP1-L has been variably reported as entirely nuclear or as partly cytoplasmic (1, 3, 15, 18, 19, 27, 29, 34, 35, 41). Thus, the observed localization of CtBP1-L may depend on the cell type, antibody, or detection methods used. Indeed, when the cellular localization of endogenous CtBP1-L was analyzed by indirect fluorescent microscopy in fixed Cos-1 and HeLa cells, in contrast to YFP-CtBP1-L (Fig. 1B), CtBP1-L was found predominantly in the nucleus (Fig. 2A). Interestingly, endogenous CtBP2 localized mainly to the nucleus, in a pattern similar to that exhibited by YFP-CtBP2, but was also found in the cytoplasm (Fig. 2A).
FIG. 2.
Divergent effects of N-terminal and C-terminal Flag tag or YFP tag on CtBP cellular localization. (A to C) Endogenous CtBP1-L is mainly nuclear in Cos-1 and HeLa cells. Cos-1 and HeLa cells were either nontransfected (A) or transfected with the indicated expression vectors (B and C), and the expressed proteins were visualized by fluorescence microscopy either directly (B, yellow) or by using the indicated primary mouse antibodies and a fluorescein isothiocyanate-labeled secondary antibody (A and C, green). Cells were fixed with formaldehyde (A and C); living cells (B) were used directly for microscopic analysis. (D) Schematic representation of the fusion proteins used in this study. The constructs were generated as Flag-tagged (gray boxes), HA-tagged (gray boxes), and YFP fusion (yellow boxes) proteins as indicated. The unique N-terminal regions of CtBP1-L, CtBP2, and CtBP1-S are color coded as in Fig. 1A. Subcellular localization of each construct is summarized at right. C, predominantly cytoplasmic; N, predominantly nuclear; NC, nuclear and cytoplasmic.
To exclude the possibility that the observed cytoplasmic localization of the CtBP fluorescent proteins was due to aberrant conformation generated by N- or C-terminal YFP fusions, we examined the localization by immunofluorescence after transfecting Cos-1 cells with eukaryotic expression vectors expressing YFP-tagged CtBPs (YFP-CtBP1-L, CtBP1-L-YFP, YFP-CtBP1-S, CtBP1-S-YFP, YFP-CtBP2, CtBP2-YFP) or Flag-tagged (Flag-CtBP1-L, CtBP1-L-Flag, CtBP1-S-Flag, CtBP2-Flag) or HA-tagged (HA-CtBP2) CtBPs fused to either the N-terminal or the C-terminal end. While all of the tagged CtBP2 constructs display a similar consistent nuclear pattern, YFP or Flag tags interfere with CtBP1-L and CtBP1-S cellular localization (Fig. 2B and C). In particular, YFP-tagged proteins appear preferentially localized in the cytoplasm, while Flag-tagged fusions are more diffuse with both cytoplasmic and nuclear staining. The reason for this unexpected result is not known, but these experiments strongly suggested that a tag fused to the N-terminal end or the C-terminal end of CtBP1-L or CtBP1-S does not completely recapitulate the endogenous localization. Our data are, however, in agreement with previous reports (1, 3, 15, 18, 19, 27, 29, 34, 35, 41) and suggest that CtBP2 is predominantly a nuclear protein, while CtBP1-L and CtBP1-S may shuttle between the nucleus and the cytoplasm.
The unique N-terminal region in CtBP2 contains an NLS.
One of the most significant regions of diversity between CtBP1-L, CtBP2, and CtBP1-S is in the N-terminal stretch (Fig. 3A), where these proteins differ in sequence and length. Although this region is small (∼15 amino acids), it is likely to have significant regulatory or targeting functions. We noted a potential nuclear localization signal KRQR in the unique N-terminal portion of CtBP2 (Fig. 3A) and postulated that this may contribute to the unique nuclear localization of CtBP2 compared to CtBP1-L or CtBP1-S. Interestingly, this motif is identical to known NLSs in other nuclear proteins, such as NF-κB p50 (Fig. 3A).
FIG. 3.
Characterization of the CtBP2 NLS. (A) Alignment of the N-terminal region of CtBP family members with the box indicating the conserved NLS. Prefix r, rat; h, human; m, mouse; b, Bos taurus; d, Drosophila sp.; z, zebra fish; x, Xenopus sp. The start of the CtBP2-S (ΔN-ter CtBP2) isoform is indicated by an arrow. Shown at the lower part of the panel is the sequence comparison between NF-κB family members, highlighting the homology to the CtBP2 NLS, adapted from reference 40. (B to D) Cellular localization of YFP-CtBP fusion proteins. Cos-1 cells were transfected with the indicated expression vectors, and the expressed proteins were visualized by confocal microscopy. The images shown in each panel are representative of all of the transfected cells. Schematic illustrations of the constructs used are indicated below each microscopic image.
To test the possibility that this motif contributes to CtBP2 localization to the nuclei, we either deleted the N terminus of CtBP2 (i.e., the translational initiation began at the second methionine) (Fig. 1A) or mutated this putative KRQR NLS to AAQR. Confocal analysis of transfected Cos-1 cells revealed that the deletion of the 25 amino acids at the N-terminal end of CtBP2 (YFP-CtBP2 [aa 26 to 445] and CtBP2 (26 to 445)-YFP) or the mutation of KRQR to AAQR (YFP-CtBP2 K10A/R11A) results in localization to the cytoplasm (Fig. 3B and C). In accordance with this result, the B domain of RIBEYE that corresponds to CtBP2 missing the first 20 amino acids, and thus the NLS, was also found diffusely present throughout the cytoplasm (Fig. 3B) (39). This result suggested that the unique first coding exon of CtBP2 does indeed contain a functional NLS.
To confirm this finding, we fused this region to CtBP1-L, generating a CtBP2-CtBP1-L chimera linked to YFP, where the N-terminal portion of CtBP1-L has been replaced by the N-terminal portion of CtBP2 containing the NLS. As shown in Fig. 3C, and in contrast to wild-type YFP-CtBP1-L (Fig. 1B), the fusion protein YFP-CtBP2 (aa 1 to 25)-CtBP1-L (20 to 440) is now localized to the nucleus, in a pattern similar to that exhibited by wild-type YFP-CtBP2. As a control, the fusion YFP-CtBP2 (1 to 25) K10A/R11A-CtBP1-L (20 to 440), which contains a mutation in the NLS, is found predominantly in the cytoplasm, similar to wild-type YFP-CtBP1-L. Taken together, these experiments suggest that the unique first coding exon of CtBP2 encodes a domain that contains a functional NLS and contributes to CtBP2's nuclear localization.
To further determine the contribution of the first 25 residues of CtBP2 to nuclear targeting, we examined this region's ability to direct a heterologous protein, YFP, to the nucleus. Figure 3D shows that this domain alone (YFP-CtBP2 [aa 1 to 25]) does not enhance nuclear targeting of YFP. Consequently, we concluded that the unique N-terminal domain of CtBP2 is necessary but not sufficient for nuclear targeting and that other components present in CtBP proteins must also play a role.
CtBP2 is a nucleocytoplasmic shuttling protein.
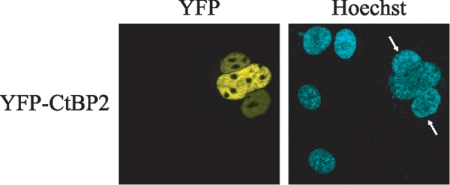
The finding that CtBP2 is detected predominantly in the nucleus does not exclude the possibility that the protein shuttles to the cytoplasm. To test this possibility, we performed a heterokaryon assay, in which monkey Cos-1 cells expressing YFP-CtBP2 were fused to an equivalent number of mouse NIH 3T3 cells to form interspecies heterokaryons, in the presence of the protein synthesis inhibitor cycloheximide. Cells were then stained with Hoechst 33258 to differentiate mouse and monkey nuclei and were examined for the presence of YFP-CtBP2 in the mouse nuclei (Fig. 4). Fusion of the YFP-CtBP2-expressing cells to the mouse cells clearly resulted in a number of mouse cell nuclei containing the YFP-CtBP2 fusion protein (Fig. 4), suggesting that, despite its predominant nuclear localization, CtBP2 is capable of shuttling between the nucleus and the cytoplasm of the cell.
FIG. 4.
CtBP2 is capable of nuclear shuttling. Heterokaryon assays were carried out between untransfected mouse NIH 3T3 cells and monkey Cos-1 cells expressing YFP-CtBP2. Mouse cells (arrows) were identified by their speckled nuclei when stained with Hoechst 33258.
The NLS in CtBP2 can act in trans to localize CtBP1-L and CtBP1-S to the nucleus.
Since CtBP1 and CtBP2 can form heterodimers (2, 43), we were prompted to investigate whether CtBP2 might be able to influence the subcellular localization of CtBP1. Cotransfection of CtBP2 fused to YFP or CFP resulted in the nuclear accumulation of CFP-CtBP1-L and YFP-CtBP1-S (Fig. 5). Most importantly, the increase of CtBP1 localization in the nucleus requires an intact CtBP2 nuclear localization signal. Indeed, cotransfection of CFP-CtBP2 K10A/R11A carrying a mutant NLS did not result in nuclear translocation of CtBP1 (Fig. 5). Significantly, NLS-deleted CtBP2 and CtBP1 were observed to colocalize in cytoplasmic structures, suggesting that CtBP1 and CtBP2 can also associate in the cytoplasm but that the absence of the NLS prevents the CtBP1/CtBP2 complex from being imported into the nucleus.
FIG. 5.
CtBP1-L and CtBP1-S become nuclear when cells are cotransfected with CtBP2 or BKLF. The NLS of CtBP2 is required for directing the nuclear localization of CtBP1-L and CtBP1-S. Cos-1 cells were cotransfected with the indicated CFP- and YFP-containing expression vectors, and localization of the expressed proteins was visualized by confocal microscopy. The images shown in each panel are representative of all of the transfected cells.
Having observed that CtBP2 could direct CtBP1 to the nucleus, we also tested whether another nuclear protein that binds CtBP1, the transcription factor BKLF, could also direct CtBP1 to the nucleus. BKLF contains a typical PXDLS CtBP contact motif and can bind both CtBP1 and CtBP2 (32, 51). We indeed found that BKLF can relocalize CtBP1-L and CtBP1-S into dense nuclear structures (Fig. 5). Taken together, these results suggest that CtBP1 itself does not have an NLS but that it can be transported into the nucleus, either by a heterologous NLS (Fig. 3C) or by one of its partners (CtBP2 or BKLF).
Regulation of nuclear localization by NADH.
It was recently proposed that NADH may stimulate not only the interaction between CtBP proteins and PXDLS motif-containing DNA-binding repressors but also CtBP oligomerization (2, 49, 58). This led us to ask whether disrupting NADH binding alters the cellular localization of CtBP proteins (Fig. 6). For this purpose, we constructed CtBP1-L mutant proteins that either lack the N-terminal region encompassing the PXDLS-binding cleft (CtBP1-L [aa 75 to 440]) or are mutated in the conserved NADH binding site (G181V/L182V/G183V) (Fig. 6A). First, we investigated whether these two mutations affect BKLF recruitment and CtBP dimerization. As expected (15, 41), deletion of the PXDLS-binding cleft in the yeast two-hybrid assay abolishes the CtBP1-L/BKLF interaction without interfering with homo- or heterodimerization (Fig. 6B, left panel). An additional experiment (immunoprecipitating CtBP1-L with anti-YFP and Western blotting with anti-Flag to detect BKLF) also indicated that mutating the NADH binding site strongly reduced CtBP1-L/BKLF interaction (Fig. 6B, lower right panel). Moreover, when dimerization was tested in the context of mammalian cells, and HA-CtBP2 was recovered with HA antibody, CtBP1-L but not CtBP1-L GLG>VVV was efficiently retained, as revealed by Western blotting against YFP (Fig. 6B, right upper panel). This result indicated that, as expected, dimerization is also significantly impaired by the NADH binding site mutation.
Next, we tested whether full-length CtBP1-L with a defective NADH binding site can be targeted to the nucleus upon exogenous expression of CtBP2 and BKLF. Remarkably, coexpression of CFP-BKLF but not CFP-CtBP2 promoted nuclear accumulation of YFP-CtBP1-L GLG>VVV (Fig. 6C), suggesting that CtBP1-L is still carried to the nucleus by the PXDLS motif-containing transcription factor, BKLF, despite the reduced binding observed between CtBP1-L GLG>VVV and BKLF. The CtBP1-L with a defective NADH binding site is, however, no longer carried into the nucleus by its heterodimeric partner CtBP2. Conversely, and in accordance with the yeast results, the CtBP1-L mutant carrying an N-terminal deletion is targeted to the nucleus by CtBP2 but not by BKLF, as predicted, since it retains the ability to dimerize but can no longer bind BKLF. In summary, these results indicate that direct binding of BKLF or CtBP2 proteins to CtBP1-L can affect its dynamic nucleocytoplasmic shuttling and suggest that NADH plays an important role in CtBP1-L's heterodimerization and PXDLS motif binding and, hence, its subcellular localization.
CtBP2-S (CtBP2 ΔN-ter) is a natural splice form of CtBP2.
We next investigated why endogenous CtBP2 was localized to both the cytoplasm and the nucleus (Fig. 2A), although it possesses the newly identified NLS (Fig. 3). Independent evidence that CtBP2 is also localized in the cytoplasm is provided by Western blot experiments (Fig. 7A). In agreement with the immunofluorescence localization data, endogenous CtBP2 is found in both the cytoplasmic and nuclear fractions of Cos-1, HeLa, and MEL cells (Fig. 7A). Remarkably, a closer inspection indicates that the cytoplasmic form of CtBP2 migrates more rapidly than its nuclear counterpart, suggesting that it represents a different species. To further characterize the cytoplasmic form of CtBP2, a specific peptide antibody against the 25 first amino acids of CtBP2 was raised that recognized only the full-length nuclear form of CtBP2. Unfortunately, endogenous nuclear CtBP2 was barely detectable with the antibody, preventing a faithful identification of the CtBP2 cytoplasmic form (data not shown). Nevertheless, a previous report indicates that the Ctbp2 message is likely to be subject to alternative splicing at the 5′ end (24) and that this could delete the first coding exon and first ATG [ATG (1)] in (Fig. 1A). Since a second potential start codon [ATG (26)] is present in the second coding exon, one explanation is that the cytoplasmic form of CtBP2 arises from a natural splice form missing the NLS. RT-PCR analysis supports this possibility (Fig. 7B). We amplified mCtBP2 from murine fetal liver at 12.5 dpc, using primers located in the 5′UTR and exon 2 (Fig. 7B). Such amplifications resulted in two PCR products with different amplicon sizes. The amplicon of 324 bp is indicative of the normal mCtBP2 transcript containing the first coding exon and the NLS, and the amplicon of 164 bp is specific for the mCtBP2-S (mCtBP2 ΔNter) variant. Automated sequencing was employed to confirm that the two bands contained the correct spliced sequences. Taken together, these results strongly suggest that the short form of CtBP2 is a natural splice variant that might play a cytoplasmic function, as do CtBP1-L and CtBP1-S.
To further decipher the role of the CtBP2 ΔNter variant, CtBP2-S, the ability of the two CtBP2 isoforms to assemble in a transcription complex on a natural promoter was examined (Fig. 7C). We have previously found that BKLF can potently repress the Aγ-globin promoter in a CtBP2-dependent manner (32, 53). This result suggests that BKLF and CtBP2 colocalize at the Aγ promoter. To directly test this possibility, we used magnetic beads coupled to Aγ promoter DNA. A 350-bp biotinylated oligonucleotide probe containing the proximal Aγ promoter DNA and including the BKLF-binding CACCC boxes was used to retrieve endogenous protein complexes from MEL cell lysates. As expected, a characteristic doublet at ∼46 kDa, corresponding to BKLF, was found to associate with the Aγ promoter DNA (Fig. 7C, upper panel). Remarkably, only the long form of CtBP2, corresponding to the nuclear form containing the NLS (Fig. 7A), was found to associate with the Aγ promoter DNA (Fig. 7C, lower panel). As a control, we tested whether both forms of CtBP2 interact with BKLF in immunoprecipitation assays and found that both forms can associate with BKLF in the absence of DNA (data not shown). Taken together, these results suggest that the long form of CtBP2 preferentially associates with DNA-bound transcription complexes and that the short CtBP2-S (CtBP2 ΔN-ter) form does not localize to the Aγ promoter, consistent with its potentially distinct cytoplasmic functions.
DISCUSSION
The identification and functional evaluation of protein domains that are responsible for targeting transcriptional factors and coregulators to subcellular compartments is central to understanding the regulation of many genetic programs (10). This point is well illustrated by the CtBP family, whose members play at least three entirely different cellular functions in the nucleus, in Golgi membranes, and in synaptic ribbons (7, 11, 13, 14, 52).
In this study, we confirm that despite their high degree of similarity, CtBP1-L and CtBP1-S have intracellular localizations different from that of full-length CtBP2. In particular, we have provided evidence that the unique N-terminal region of CtBP2 contains an NLS which contributes to its nuclear localization. One conclusion from the data presented could be that CtBP2 is the CtBP primarily responsible for transcriptional repression, while CtBP1-L and CtBP1-S could be predominantly cytoplasmic proteins responsible for the regulation of membrane dynamics (7, 9, 50). Knockout studies have revealed that CtBP1-null mice are viable, while CtBP2-null embryos die by embryonic day 10.5 (24). This shows that CtBP1 cannot compensate for CtBP2's functions. Whether the lack of compensation by CtBP1 centers on the fact that CtBP1 lacks an NLS remains to be assessed.
CtBP1-L has been variably reported to be entirely nuclear or partly cytoplasmic (1, 3, 15, 18, 19, 27, 29, 34, 35, 41). One explanation is that CtBP1-L is subject to regulation by active nucleocytoplasmic trafficking. Since we observed that overexpressed CtBP1-L, in contrast to the native protein, is more abundant in the cytoplasm than in the nucleus, the results presented herein alternatively demonstrate that a YFP or Flag tag may interfere with the normal endogenous localization of CtBP1-L. Our data are consistent with previous reports. For example, a CFP-CtBP1-L construct was reported to be present in both the nucleus and the cytoplasm of Cos-1 cells (25). Moreover, a Flag-tagged CtBP1-L was found diffusely in the nucleus and cytoplasm, while endogenous CtBPs displayed diffuse localization, predominantly in the nuclei (35).
Nuclear localization signal in CtBP2.
We show that the N-terminal region of CtBP2, encompassing amino acids 1 to 25, is necessary for fully localizing CtBP2 to the nucleus. Indeed, the deletion of the N-terminal 25-amino-acid sequence causes CtBP2 to be completely retained in the cytoplasm. More specifically, our mutagenesis data indicated that the positively charged amino acids K10 and R11 are critical for the function of the NLS (Fig. 3). An inspection of the sequence reveals that these two amino acids are conserved in other organisms, such as in Drosophila sp. (dCtBP) (Fig. 3A). Interestingly, Drosophila carries a single copy of the dCtBP gene, and dCtBP is detected primarily in the nuclei in the early embryo (31), also consistent with the view that these residues are critical for nuclear localization. Moreover, this stretch of four basic amino acids is similar to a sequence found in the Rel/NF-κB family (40) that has been shown to be a functional NLS in the v-rel oncogene product (21), in p50 (5, 23), and p65 (57), further indicating that they define a functional NLS within CtBP2. This result is consistent with other recent reports that the N-terminal region of CtBP2 plays a role in nuclear targeting (60). However, the analysis of the isolated N-terminal region of CtBP2 with respect to its ability to target YFP into the nucleus has shown that this region does not enhance nuclear targeting (Fig. 3D). This result is surprising, since this same region, when tethered in cis to CtBP1-L, is sufficient to efficiently target CtBP1-L into the nucleus (Fig. 3C). This result suggests that the 25 N-terminal amino acids of CtBP2 are necessary but not sufficient for targeting and that other sequences in CtBP family proteins are involved in nuclear entry or retention. Alternatively, we cannot exclude the fact that this YFP fusion protein is partially unfolded and thus not competent for nuclear import.
Interestingly, by using heterokaryon assays, we show that CtBP2 is capable of shuttling between the nucleus and cytoplasm (Fig. 4). One possibility is that CtBP2 shuttles between the two compartments using its NLS and an intrinsic nuclear export signal (NES). We noted that CtBP proteins have a potential NES (data not shown) that conforms to the NES consensus motif (6, 22). However, leptomycin B treatment did not influence the cellular localization of CtBP proteins, raising the possibility that they are not exported into the cytoplasm through a CRM1-dependent pathway (data not shown).
Regulation of CtBP1-L and CtBP1-S nuclear localization.
We observed that overexpressed CtBP1-L and CtBP1-S are more abundant in the cytoplasm than in the nucleus and can be transferred to the nucleus upon overexpression of one of their partners. These results suggest that CtBP1-L and CtBP1-S do not have an NLS and enter the nucleus only with NLS-containing partners. We also show that this effect depends on the formation of CtBP1/CtBP2 heterodimers or CtBP1/BKLF complexes. This finding is in agreement with previously published data (15, 18, 41) and suggests that interactions with nuclear proteins are important in relocating CtBP1. Homo- or heterodimerization has been proposed as one of the typical modes of regulating nucleocytoplasmic transport. An example that follows this scheme is the nuclear import of HDAC4 by complex formation with MEF2C (8). Similar regulatory interactions that specifically affect the subcellular localization of proteins have been described, for example, for TAF10 (45) and PREP1 (4).
Interestingly, both CtBP1 and CtBP2 were found in the core CtBP corepressor complex (43), indicating that they might function as homodimers or heterodimers or higher order multimers. One might speculate that the presence of CtBP2 is required to target CtBP1-L into the nucleus and, thus, is critical for the functional assembly of the complex. Alternatively, posttranslational modifications of CtBP1-L may also regulate its nuclear localization. For instance, CtBP1-L is subjected to sumoylation at Lys 428 (29), and mutation of this residue shifted CtBP1-L from the nucleus to the cytoplasm. Interestingly, due to a lack of a SUMO consensus (54), the corresponding residue within CtBP2 is only weakly modified (25, 29). When combined with our data, these results suggest that CtBP1-L and CtBP2 have evolved differently with respect to the regulation of their nuclear localization and that the lack of an NLS in CtBP1 might be subsumed by other mechanisms, such as sumoylation.
Integration of the cytoplasmic and nuclear actions of CtBP proteins.
An aspect remaining unresolved is whether CtBP1-L, CtBP1-S, and CtBP2 each serve a specific role in the cell or whether they act at both the nuclear and cytosolic levels. Several lines of evidence are in favor of the second possibility. Firstly, it is conceivable that, due to their high degree of homology, these CtBP proteins could interchangeably modulate fission as well as transcription. Secondly, in addition to RIBEYE, CtBP1-L was identified as a component of synaptic ribbons (50). We have identified an isoform of CtBP2 (CtBP2-S or CtBP2 ΔN-ter) that lacks the NLS. It is found in the cytoplasm and is unable to assemble with the DNA-binding protein BKLF at the Aγ-globin promoter, suggesting that this isoform of CtBP2 may have cytoplasmic rather than nuclear functions. In contrast, we found that CtBP1-S is localized in both the cytoplasm and the nucleus (Fig. 1 and 2) and can repress transcription when tethered to a heterologous DNA-binding domain (data not shown). Finally, CtBP1-L and CtBP1-S are targeted to the nucleus upon dimerization with CtBP2 or BKLF. An interesting question for the future is whether the cytoplasmic form of CtBP2 can substitute for CtBP1 in the membrane fission and transport pathway. Because the three CtBP proteins are likely to be coexpressed in many cells, we propose that the sum total of CtBP activity is dependent on the ratio of the individual isoforms coupled with both their inherent ability to form homo- and heterodimers and their ability to interact with cytoplasmic and nuclear partners. In strong accordance with this hypothesis, binding of nNOS changes the localization of CtBP1-L from the nucleus to the cytosol (34), while CtBP2 and BKLF can induce CtBP1 nuclear localization (this study).
It has been reported that CtBP binding to transcriptional repressors is stimulated by NADH (58). Additionally, NADH was found to stimulate CtBP dimerization (2, 30, 49). Here, we show that mutation in the NADH binding site abolishes the nuclear accumulation of CtBP1-L driven by CtBP2 but has no detectable effect on recruitment into the nucleus by the PXDLS motif-containing transcription factor BKLF (Fig. 6). We favor the interpretation that the ability of NADH to stimulate CtBP dimerization contributes indirectly to the enhanced binding of PXDLS motif-containing transcription factors. This interpretation is supported by the crystal structure of CtBP, which indicates that NADH binding can promote the formation of a closed dimeric form of CtBP, with the PXDLS consensus peptide-binding sites located remotely at the two N-terminal regions (30). Importantly, the NADH binding site is about 25 Å apart from the PXDLS-binding cleft, suggesting that NADH and PXDLS binding may be uncoupled. Such dimeric assembly may be instrumental in CtBP1 corepressor action, since it provides a stable and bivalent aggregation nucleus for multimeric protein complexes that can bridge repressors and their targets (13, 52). In accordance, mutation of the NADH binding site has been shown to be critically important for the transcriptional repression activity of CtBP proteins (28, 48, 49). Our results also indicate that CtBP1 can be targeted to the nucleus by transcription factors, such as BKLF, even when CtBP1's NADH binding site is mutated, consistent with the view that PXDLS binding is not strictly dependent on an intact NADH binding site and dimerization.
In summary, our studies suggest that the unique N-terminal region of CtBP2 encompasses an NLS that is important for the nuclear retention of CtBP2 and its function as a transcriptional corepressor. The lack of an NLS in CtBP1-L and CtBP1-S might be subsumed by other mechanisms, such as heterodimerization with CtBP2 or interaction with PXDLS-containing factors. Our results also suggest that NADH may regulate the cellular localization of CtBP proteins by stimulating both dimerization and PXDLS binding.
Acknowledgments
We thank D. Wotton and F. Schmitz for their generous gifts of reagents. We are indebted to Louise Cole for help with confocal microscopy analyses and Alister Funnell for help with RT-PCR.
This work was supported by an Australian NHMRC grant and a National Institutes of Health grant (HL073443) to M.C. and by grants from AIRC (Italian Association for Cancer Research) and Telethon (Italy) to D.C.
REFERENCES
- 1.Alpatov, R., G. C. Munguba, P. Caton, J. H. Joo, Y. Shi, Y. Shi, M. E. Hunt, and S. P. Sugrue. 2004. Nuclear speckle-associated protein Pnn/DRS binds to the transcriptional corepressor CtBP and relieves CtBP-mediated repression of the E-cadherin gene. Mol. Cell. Biol. 24:10223-10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramanian, P., L. J. Zhao, and G. Chinnadurai. 2003. Nicotinamide adenine dinucleotide stimulates oligomerization, interaction with adenovirus E1A and an intrinsic dehydrogenase activity of CtBP. FEBS Lett. 537:157-160. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, C. J., R. K. Vadlamudi, S. K. Mishra, R. H. Jacobson, F. Li, and R. Kumar. 2003. Functional inactivation of a transcriptional corepressor by a signaling kinase. Nat. Struct. Biol. 10:622-628. [DOI] [PubMed] [Google Scholar]
- 4.Berthelsen, J., C. Kilstrup-Nielsen, F. Blasi, F. Mavilio, and V. Zappavigna. 1999. The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 13:946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank, V., P. Kourilsky, and A. Israel. 1991. Cytoplasmic retention, DNA binding and processing of the NF-kappa B p50 precursor are controlled by a small region in its C-terminus. EMBO J. 10:4159-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogerd, H. P., R. A. Fridell, R. E. Benson, J. Hua, and B. R. Cullen. 1996. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol. Cell. Biol. 16:4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonazzi, M., S. Spano, G. Turacchio, C. Cericola, C. Valente, A. Colanzi, H. S. Kweon, V. W. Hsu, E. V. Polishchuck, R. S. Polishchuck, M. Sallese, T. Pulvirenti, D. Corda, and A. Luini. 2005. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 7:570-580. [DOI] [PubMed] [Google Scholar]
- 8.Borghi, S., S. Molinari, G. Razzini, F. Parise, R. Battini, and S. Ferrari. 2001. The nuclear localization domain of the MEF2 family of transcription factors shows member-specific features and mediates the nuclear import of histone deacetylase 4. J. Cell Sci. 114:4477-4483. [DOI] [PubMed] [Google Scholar]
- 9.Carcedo, C. H., M. Bonazzi, S. Spano, G. Turacchio, A. Colanzi, A. Luini, and D. Corda. 2004. Mitotic Golgi partitioning is driven by the membrane-fissioning protein CtBP3/BARS. Science 305:93-96. [DOI] [PubMed] [Google Scholar]
- 10.Carmo-Fonseca, M. 2002. The contribution of nuclear compartmentalization to gene regulation. Cell 108:513-521. [DOI] [PubMed] [Google Scholar]
- 11.Chinnadurai, G. 2003. CtBP family proteins: more than transcriptional corepressors. Bioessays 25:9-12. [DOI] [PubMed] [Google Scholar]
- 12.Chinnadurai, G. 2006. CtBP family proteins: unique transcriptional regulators in the nucleus with diverse cytosolic functions. In G. Chinnadurai (ed.), CtBP family proteins. Landes Biosciences, Georgetown, Tex.
- 13.Chinnadurai, G. 2002. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell 9:213-224. [DOI] [PubMed] [Google Scholar]
- 14.Corda, D., A. Colanzi, and A. Luini. 2006. The multiple activities of CtBP/BARS proteins: the Golgi view. Trends Cell Biol. 16:167-173. [DOI] [PubMed] [Google Scholar]
- 15.Criqui-Filipe, P., C. Ducret, S. M. Maira, and B. Wasylyk. 1999. Net, a negative Ras-switchable TCF, contains a second inhibition domain, the CID, that mediates repression through interactions with CtBP and de-acetylation. EMBO J. 18:3392-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crossley, M., E. Whitelaw, A. Perkins, G. Williams, Y. Fujiwara, and S. H. Orkin. 1996. Isolation and characterization of the cDNA encoding BKLF/TEF-2, a major CACCC-box-binding protein in erythroid cells and selected other cells. Mol. Cell. Biol. 16:1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Grange, P., M. Dutertre, N. Martin, and D. Auboeuf. 2005. FAST DB: a website resource for the study of the expression regulation of human gene products. Nucleic Acids Res. 33:4276-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deltour, S., S. Pinte, C. Guerardel, B. Wasylyk, and D. Leprince. 2002. The human candidate tumor suppressor gene HIC1 recruits CtBP through a degenerate GLDLSKK motif. Mol. Cell. Biol. 22:4890-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes, I., Y. Bastien, T. Wai, K. Nygard, R. Lin, O. Cormier, H. S. Lee, F. Eng, N. R. Bertos, N. Pelletier, S. Mader, V. K. Han, X. J. Yang, and J. H. White. 2003. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol. Cell 11:139-150. [DOI] [PubMed] [Google Scholar]
- 20.Gallop, J. L., P. J. Butler, and H. T. McMahon. 2005. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature 438:675-678. [DOI] [PubMed] [Google Scholar]
- 21.Gilmore, T. D., and H. M. Temin. 1988. v-rel oncoproteins in the nucleus and in the cytoplasm transform chicken spleen cells. J. Virol. 62:703-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson, B. R., and A. Eleftheriou. 2000. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp. Cell Res. 256:213-224. [DOI] [PubMed] [Google Scholar]
- 23.Henkel, T., U. Zabel, K. van Zee, J. M. Muller, E. Fanning, and P. A. Baeuerle. 1992. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell 68:1121-1133. [DOI] [PubMed] [Google Scholar]
- 24.Hildebrand, J. D., and P. Soriano. 2002. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol. Cell. Biol. 22:5296-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagey, M. H., T. A. Melhuish, and D. Wotton. 2003. The polycomb protein Pc2 is a SUMO E3. Cell 113:127-137. [DOI] [PubMed] [Google Scholar]
- 26.Katsanis, N., and E. M. Fisher. 1998. A novel C-terminal binding protein (CTBP2) is closely related to CTBP1, an adenovirus E1A-binding protein, and maps to human chromosome 21q21.3. Genomics 47:294-299. [DOI] [PubMed] [Google Scholar]
- 27.Kegel, K. B., A. R. Meloni, Y. Yi, Y. J. Kim, E. Doyle, B. G. Cuiffo, E. Sapp, Y. Wang, Z. H. Qin, J. D. Chen, J. R. Nevins, N. Aronin, and M. DiFiglia. 2002. Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription. J. Biol. Chem. 277:7466-7476. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, V., J. E. Carlson, K. A. Ohgi, T. A. Edwards, D. W. Rose, C. R. Escalante, M. G. Rosenfeld, and A. K. Aggarwal. 2002. Transcription corepressor CtBP is an NAD+-regulated dehydrogenase. Mol. Cell 10:857-869. [DOI] [PubMed] [Google Scholar]
- 29.Lin, X., B. Sun, M. Liang, Y. Y. Liang, A. Gast, J. Hildebrand, F. C. Brunicardi, F. Melchior, and X. H. Feng. 2003. Opposed regulation of corepressor CtBP by SUMOylation and PDZ binding. Mol. Cell 11:1389-1396. [DOI] [PubMed] [Google Scholar]
- 30.Nardini, M., S. Spano, C. Cericola, A. Pesce, A. Massaro, E. Millo, A. Luini, D. Corda, and M. Bolognesi. 2003. CtBP/BARS: a dual-function protein involved in transcription co-repression and Golgi membrane fission. EMBO J. 22:3122-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nibu, Y., H. Zhang, and M. Levine. 1998. Interaction of short-range repressors with Drosophila CtBP in the embryo. Science 280:101-104. [DOI] [PubMed] [Google Scholar]
- 32.Perdomo, J., A. Verger, J. Turner, and M. Crossley. 2005. Role for SUMO modification in facilitating transcriptional repression by BKLF. Mol. Cell. Biol. 25:1549-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piatigorsky, J. 2001. Dual use of the transcriptional repressor (CtBP2)/ribbon synapse (RIBEYE) gene: how prevalent are multifunctional genes? Trends Neurosci. 24:555-557. [DOI] [PubMed] [Google Scholar]
- 34.Riefler, G. M., and B. L. Firestein. 2001. Binding of neuronal nitric-oxide synthase (nNOS) to carboxyl-terminal-binding protein (CtBP) changes the localization of CtBP from the nucleus to the cytosol: a novel function for targeting by the PDZ domain of nNOS. J. Biol. Chem. 276:48262-48268. [DOI] [PubMed] [Google Scholar]
- 35.Sasai, N., E. Matsuda, E. Sarashina, Y. Ishida, and M. Kawaichi. 2005. Identification of a novel BTB-zinc finger transcriptional repressor, CIBZ, that interacts with CtBP corepressor. Genes Cells 10:871-885. [DOI] [PubMed] [Google Scholar]
- 36.Schaeper, U., J. M. Boyd, S. Verma, E. Uhlmann, T. Subramanian, and G. Chinnadurai. 1995. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc. Natl. Acad. Sci. USA 92:10467-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaeper, U., T. Subramanian, L. Lim, J. M. Boyd, and G. Chinnadurai. 1998. Interaction between a cellular protein that binds to the C-terminal region of adenovirus E1A (CtBP) and a novel cellular protein is disrupted by E1A through a conserved PLDLS motif. J. Biol. Chem. 273:8549-8552. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt, A., M. Wolde, C. Thiele, W. Fest, H. Kratzin, A. V. Podtelejnikov, W. Witke, W. B. Huttner, and H. D. Soling. 1999. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature 401:133-141. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz, F., A. Konigstorfer, and T. C. Sudhof. 2000. RIBEYE, a component of synaptic ribbons: a protein's journey through evolution provides insight into synaptic ribbon function. Neuron 28:857-872. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz, M. L., T. Henkel, and P. A. Baeuerle. 1991. Proteins controlling the nuclear uptake of NF-kappa B, Rel and dorsal. Trends Cell Biol. 1:130-137. [DOI] [PubMed] [Google Scholar]
- 41.Sewalt, R. G., M. J. Gunster, J. van der Vlag, D. P. Satijn, and A. P. Otte. 1999. C-Terminal binding protein is a transcriptional repressor that interacts with a specific class of vertebrate Polycomb proteins. Mol. Cell. Biol. 19:777-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi, Y., F. Lan, C. Matson, P. Mulligan, J. R. Whetstine, P. A. Cole, R. A. Casero, and Y. Shi. 2004. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941-953. [DOI] [PubMed] [Google Scholar]
- 43.Shi, Y., J. Sawada, G. Sui, B. Affar el, J. R. Whetstine, F. Lan, H. Ogawa, M. P. Luke, Y. Nakatani, and Y. Shi. 2003. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422:735-738. [DOI] [PubMed] [Google Scholar]
- 44.Shi, Y., and Y. Shi. 2004. Metabolic enzymes and coenzymes in transcription—a direct link between metabolism and transcription? Trends Genet. 20:445-452. [DOI] [PubMed] [Google Scholar]
- 45.Soutoglou, E., M. A. Demeny, E. Scheer, G. Fienga, P. Sassone-Corsi, and L. Tora. 2005. The nuclear import of TAF10 is regulated by one of its three histone fold domain-containing interaction partners. Mol. Cell. Biol. 25:4092-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spano, S., C. H. Carcedo, and D. Corda. 2006. CtBP3/BARS and membrane fission. In G. Chinnadurai (ed.), CtBP family proteins. Landes Biosciences, Georgetown, Tex.
- 47.Spano, S., M. G. Silletta, A. Colanzi, S. Alberti, G. Fiucci, C. Valente, A. Fusella, M. Salmona, A. Mironov, A. Luini, D. Corda, and S. Spanfo. 1999. Molecular cloning and functional characterization of brefeldin A-ADP-ribosylated substrate. A novel protein involved in the maintenance of the Golgi structure. J. Biol. Chem. 274:17705-17710. [DOI] [PubMed] [Google Scholar]
- 48.Sutrias-Grau, M., and D. N. Arnosti. 2004. CtBP contributes quantitatively to Knirps repression activity in an NAD binding-dependent manner. Mol. Cell. Biol. 24:5953-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thio, S. S., J. V. Bonventre, and S. I. Hsu. 2004. The CtBP2 co-repressor is regulated by NADH-dependent dimerization and possesses a novel N-terminal repression domain. Nucleic Acids Res. 32:1836-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.tom Dieck, S., W. D. Altrock, M. M. Kessels, B. Qualmann, H. Regus, D. Brauner, A. Fejtova, O. Bracko, E. D. Gundelfinger, and J. H. Brandstätter. 2005. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J. Cell Biol. 168:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner, J., and M. Crossley. 1998. Cloning and characterization of mCtBP2, a co-repressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J. 17:5129-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner, J., and M. Crossley. 2001. The CtBP family: enigmatic and enzymatic transcriptional co-repressors. Bioessays 23:683-690. [DOI] [PubMed] [Google Scholar]
- 53.Turner, J., H. Nicholas, D. Bishop, J. M. Matthews, and M. Crossley. 2003. The LIM protein FHL3 binds basic Kruppel-like factor/Kruppel-like factor 3 and its co-repressor C-terminal-binding protein 2. J. Biol. Chem. 278:12786-12795. [DOI] [PubMed] [Google Scholar]
- 54.Verger, A., J. Perdomo, and M. Crossley. 2003. Modification with SUMO: a role in transcriptional regulation. EMBO Rep. 4:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weigert, R., M. G. Silletta, S. Spano, G. Turacchio, C. Cericola, A. Colanzi, S. Senatore, R. Mancini, E. V. Polishchuk, M. Salmona, F. Facchiano, K. N. Burger, A. Mironov, A. Luini, and D. Corda. 1999. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402:429-433. [DOI] [PubMed] [Google Scholar]
- 56.Yang, J. S., S. Y. Lee, S. Spano, H. Gad, L. Zhang, Z. Nie, M. Bonazzi, D. Corda, A. Luini, and V. W. Hsu. 2005. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 24:4133-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zabel, U., T. Henkel, M. S. Silva, and P. A. Baeuerle. 1993. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 12:201-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, Q., D. W. Piston, and R. H. Goodman. 2002. Regulation of corepressor function by nuclear NADH. Science 295:1895-1897. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Q., Y. Yoshimatsu, J. Hildebrand, S. M. Frisch, and R. H. Goodman. 2003. Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell 115:177-186. [DOI] [PubMed] [Google Scholar]
- 60.Zhao, L. J., T. Subramanian, Y. Zhou, and G. Chinnadurai. 2006. Acetylation by p300 regulates nuclear localization and function of the transcriptional corepressor CtBP2. J. Biol. Chem. 281:4183-4189. [DOI] [PubMed] [Google Scholar]